Abstract
Activation of adenosine receptors in folliculostellate (FS) cells of the pituitary gland leads to the secretion of IL-6 and vascular endothelial growth factor (VEGF).
We investigated the action of adenosine A2 receptor agonists on IL-6 and VEGF secretion in two murine FS cell lines (TtT/GF and Tpit/F1), and demonstrated a rank order of potency, 5′-N-ethylcarboxamidoadenosine (NECA)>2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine>adenosine, suggesting mediation via the A2b receptor.
NECA-mediated IL-6 release was inhibited by the PLC inhibitor 1-[6-((17β-3-methoxyestra-1,3,5(10)-tiene-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione, the PI3 kinase inhibitor wortmannin and the PKC inhibitors bisindolylmaleimide 1 and bisindolymaleimide X1 HCl (Ro-32-0432).
NECA-mediated IL-6 release was attenuated (<50%) by the extracellular signal-regulated kinase MAPK inhibitor 2′-amino-3′-methoxyflavone, and completely (>95%) inhibited by the p38 MAPK inhibitor 4-(4-fluorophenyl)-2-(4-methylsulphinylphenyl)-5-(4-pyridyl)1H-imidazole.
NECA stimulates p38 MAPK phosphorylation that is inhibited by Ro-32-0432 but not by wortmannin.
Dexamethasone inhibits NECA-stimulated IL-6 and VEGF secretion.
These findings indicate that adenosine can stimulate IL-6 secretion in FS cells via the A2b receptor coupled principally to PLC/PKC and p38 MAPK; such an action may be important in the modulation of inflammatory response processes in the pituitary gland.
Keywords: Adenosine, A2b receptor, IL-6, folliculostellate cells, PKC, p38 MAPK, pituitary gland
Introduction
FS cells are star-shaped and possess long cellular processes that surround endocrine and endothelial cells, and comprise up to 10% of the anterior pituitary cell population (Allaerts et al., 1990; Matsumoto et al., 1993). They secrete cytokines and growth factors including IL-6, tumour necrosis factor α, VEGF and basic fibroblast growth factor (Gloddek et al., 1999; Turnbull & Rivier, 1999; Lohrer et al., 2000), and thus appear to regulate pituitary function by playing a supportive role for neighbouring endothelial and endocrine cells. These characteristics of FS cells and the fact that they possess receptors for bacterial lipopolysaccharide (LPS) (Lohrer et al., 2000) and complement C3a (unpublished observations) suggest that they coordinate the bidirectional exchange of information between the immune and endocrine systems (Besedovsky & del Ray, 1996; Sternberg, 1997). Maintaining this connection is important for homeostatic control and resistance to disease.
Recently, we described the presence of adenosine A2b receptors on the TtT/GF and Tpit/F1 murine cell lines, and showed that adenosine in this setting is a growth regulator (Rees et al., 2002). However adenosine, along with ATP and ADP, is ubiquitously expressed during normal metabolic activity and, under physiological states, adenosine levels are low and are regulated mainly by the intracellular enzyme adenosine kinase that phosphorylates it back to AMP. On the other hand, during ischaemia and inflammation, excessive ATP breakdown leads to accumulation of extracellular adenosine such that levels within the vicinity of the receptor reach as high as 30 μM (Van Belle et al., 1987; Matherne et al., 1990; Latini et al., 1999). In these circumstances, adenosine acts as an endogenous anti-inflammatory agent, the levels of which are controlled by extracellular enzymes such as ecto-ATPase (CD39) and ecto-5′ nucleotidase (CD73) that sequentially degrade ATP to adenosine and adenosine deaminase, which converts adenosine to inosine (Spychala, 2000).
During cellular ‘stress', it is conceivable that adenosine receptors on FS cells mediate the regulation of anti-inflammatory pituitary hormones either by direct activation or in synergy with other proinflammatory mediators (Gloddek et al., 1999; Lohrer et al., 2000; Kiriyama et al., 2001). The linking molecule between FS and endocrine cells may well be IL-6, particularly since it has been shown to regulate growth hormone, prolactin and adrenocorticotrophin (Naitoh et al., 1988; Spangelo et al., 1989) secretion as well as adrenal steroidogenesis (Turnbull & Rivier, 1999). FS cells, like corticotrophs and somatotrophs, also possess glucocorticoid receptors, suggesting their involvement in regulating stress and inflammatory responses (Ozawa et al., 1999). Adenosine mediates its actions through at least four G-protein-coupled, cell surface receptors that are linked either positively (A2a and A2b) or negatively (A1 and A3) to AC. These receptors can also signal through the mitogen-activated protein kinase (MAPK) family of molecules, and activation of all the four adenosine receptor subtypes can lead to phosphorylation of ERK 1/2 (Schulte & Fredholm, 2000).
In this report, we show that A2b receptors expressed in FS cells mediate the release of IL-6 via the PLC/PKC pathway that is coupled principally to p38 MAPK. These findings suggest that A2b receptors in the pituitary gland may mediate important actions in the hypothalamic pituitary adrenal (HPA) axis, thereby influencing inflammation, ischaemia and hypoxia.
Methods
Materials
Cell culture materials and reagents were obtained from Invitrogen (Paisley, U.K.), Autogen Bioclear (Calne, U.K.), Sarstedt Ltd (Leicester, U.K.) and Sigma-Aldrich (Poole, U.K.). Adenosine receptor agonists and antagonists and signal pathway inhibitors were either from Sigma-Aldrich or CN Biosciences (Nottingham, U.K.). ELISA kits for measuring IL-6 and VEGF were from R and D Systems (Abingdon, U.K.). Primary antisera to phosphorylated forms of p38 MAPK (V121C, lot 11485605), JNK (V793C, lot 12034104) and MAPK (V803C, lot 13490702) were from Promega (Southampton, U.K.). Anti-total p38 MAPK (SC 7149, lot H011) was from Santa Cruz Biotechnology (Santa Cruz, U.S.A.). The secondary conjugate, donkey anti-rabbit IgG (horseradish peroxidase, HRP)-linked whole antibody (NA 934, lot 198465) and Western blotting reagents were from Amersham Biosciences (Little Chalfont, U.K.). The murine-derived TtT/GF and Tpit/F1 FS cell lines were kindly provided by Professor Kinji Inoue (Department of Regulation Biology, Saitama University, Urawa, Japan).
Cells and culture conditions
TtT/GF (Inoue et al., 1992) and Tpit/F1 (Chen et al., 2000) cells were cultured as described previously, except that DMEM and 10% heat-inactivated (HI) FCS were used (Rees et al., 2002).
VEGF and IL-6 secretion
TtT/GF and Tpit/F1 cells (106 cells per plate) were seeded into 24-well plates and cultured as described above. Cells were grown to confluency and then incubated overnight in DMEM with 2% HI FCS. The compounds adenosine, NECA (universal adenosine receptor agonist) and CGS 21680 (selective A2a receptor agonist) were dissolved in fresh medium and added to the cultures at various concentrations for 6 h (TtT/GF) or 24 h (Tpit/F1) as indicated. In some experiments, adenosine receptor antagonists or signal transduction inhibitors were added 30 min prior to adding NECA. Cell culture supernatants were removed, centrifuged for 10 min at 10,000 × g, and stored at −85°C prior to assay for IL-6 and VEGF. The ELISA for IL-6 has a detection limit of <2 pg ml−1, and the intra-assay and interassay coefficients of variation are 3.4 and 6.4%, respectively. The VEGF ELISA recognises predominantly the mouse VEGF164 isoform, and has a detection limit of <2 pg ml−1, and the intra-assay and interassay coefficients of variation are 4.7 and 6.4%, respectively.
Western analysis
FS cells (106 cells per six-well plate) were cultured in DMEM supplemented with HI FCS as follows: TtT/GF (2% – 24 h, 0.1% – 16 h and 0% – 8 h) and Tpit/F1 (10% – 24 h, 2% – 16 h and 0% – 2 h). Replicate wells were exposed to 10 μM NECA in fresh serum-free medium at 37°C for the times indicated. After washing (3 ×) in ice-cold phosphate-buffered saline (PBS) containing 1 mM sodium orthovanadate, the cells were lysed in 0.2 ml electrophoresis sample (Laemmli) buffer. In some experiments, the PLC, PKC and PI3 kinase inhibitors U-73122, Ro-32-0432 and wortmannin were added 30 min prior to the addition of NECA. Aliquots (0.05 ml) of the samples were electrophoresed in 10% polyacrylamide and then electroblotted onto polyvinyldifluoride (PVDF) membranes. Blots were exposed overnight (4°C) to antisera to the phosphorylated forms of p38 MAPK (1 : 5000), JNK (1 : 2000) and MAPK (1 : 2000), and to total p38 MAPK (1 : 500). The secondary antisera conjugate was used at 1 : 5000 (1 h at room temperature) and visualised with ECL Plus reagent. The exposure time was 10–30 min.
Statistical analysis
The results were expressed as the mean±s.e.m. and compared by analysis of variance (ANOVA) with subsequent comparisons by the Tukey multiple comparison test. Four replicates were carried out for each treatment in each experiment, and all experiments were performed 3–4 times (n).
Results
Adenosine stimulates IL-6 secretion in FS cells
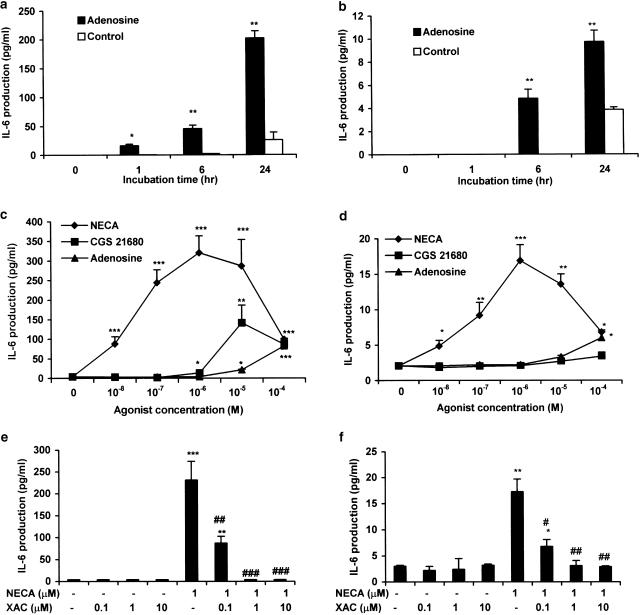
We have previously shown that adenosine can stimulate proliferation of FS cells via the A2b receptor (Rees et al., 2002). In this report, we investigated whether adenosine could modulate IL-6 and VEGF secretion. To demonstrate this, we firstly treated TtT/GF and Tpit/F1 cells with 100 μM adenosine, and measured the IL-6 production at different time points. In TtT/GF cells, IL-6 release under basal conditions was not detectable at early time points, but rose to 25±12 pg ml−1 after 24 h. Adenosine induced IL-6 secretion as early as 1 h (16±4 pg ml−1) and continued to rise to 200±12 pg ml−1 after 24 h (Figure 1a). Similar findings were obtained with Tpit/F1 cells, though the overall IL-6 levels were considerably lower and 100 μM adenosine only stimulated levels up to 8–12 pg ml−1 after 24 h (Figure 1b). In subsequent experiments, we used an incubation time of 6 h for TtT/GF cells and 24 h for Tpit/F1 cells. We did not observe any significant differences in cell number (Coulter counting of replicate cultures) between untreated and treated cells for both the TtT/GF and Tpit/F1 cell lines over the time periods studied.
Figure 1.
Effects of A2 receptor agonists on IL-6 secretion from TtT/GF and Tpit/F1 FS cell lines. TtT/GF (a, c, e) and Tpit/F1 (b, d, f) cells were preincubated with DMEM and 2% FCS for 24 h prior to experimentation, and then with adenosine, NECA and CGS 21680 (as indicated) for the times shown (a, b) and for 6 h (c, e) or 24 h (d, f). In the time course experiments (a, b), adenosine was used at a concentration of 100 μM. XAC was added 30 min prior to the addition of 1 μM NECA in (e, f). IL-6 was measured by ELISA. Data points represent the mean±s.e.m. from three (a, b, e, f) or four (c, d) experiments. *P<0.05, **P<0.01, ***P<0.001 when compared with basal values; #P<0.05, ♯♯P<0.01, ♯♯♯P<0.001 when compared with NECA-stimulated values.
Concentration–response curves for IL-6 production induced by adenosine A2 receptor agonists were performed in TtT/GF and Tpit/F1 cells. In TtT/GF cells, NECA, adenosine and CGS 21680 stimulated IL-6 secretion in a dose-dependent manner; detectable effects were seen at concentrations of 10 nM for NECA and ⩾1 μM for adenosine and CGS 21680 (Figure 1c). In addition, IL-6 secretion was maximal at 1 μM NECA (308±52 pg ml−1), in comparison with 20±8 and 10±8 pg ml−1, respectively, for similar concentrations of CGS 21680 and adenosine. We carried out similar experiments in Tpit/F1 cells. The pattern of IL-6 responses to adenosine, NECA and CGS 21680 in these cells (Figure 1d) paralleled those in TtT/GF cells (Figure 1c) though the degree of stimulation in Tpit/F1 cells was considerably lower (15–20 pg ml−1 for 1 μM NECA and a similar number of cells). The Tpit/F1 cells were additionally exposed to the agonists for 24 h before significant differences in IL-6 secretion could be detected.
The observed effect of NECA on IL-6 secretion (Figures 1c, d) also appeared to be biphasic, since NECA concentrations greater than 10 μM reduced the secretion of IL-6 although the levels were still clearly above basal. The adenosine receptor antagonist XAC (0.1–10 μM) also inhibited NECA-stimulated IL-6 secretion in both cell lines (Figures 1e, f). The relative potencies of CGS 21680 and NECA in these studies (Figures 1c, d) indicate that adenosine-stimulated IL-6 secretion is mediated via the A2b rather than the A2a receptor. This was further supported by our studies showing that alloxazine is a potent inhibitor (IC50<50 nM) of NECA-stimulated IL-6 secretion from TtT/GF cells (data not shown).
NECA stimulation of IL-6 secretion involves PLC, PI3 kinase and PKC
The major signal molecule for the A2b receptor is cAMP, and is likely to be a mediator for IL-6 secretion. Previously, we showed that adenosine and NECA stimulated cAMP production in primary pituitary cells and FS cells (Rees et al., 2002). Our current studies in which forskolin (1 μM) stimulated IL-6 secretion in TtT/GF cells (data not shown) concur with previous findings (Tatsuno et al., 1991; Matsumoto et al., 1993) indicating the involvement of AC in IL-6 secretion.
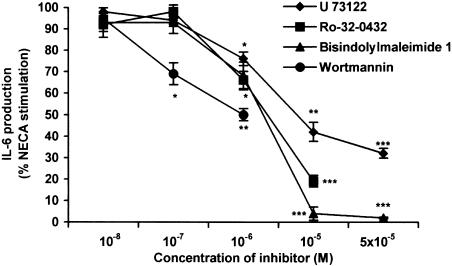
However, in addition to the AC pathway, the A2b receptor can also signal via PLC and Ca2+ (Feoktistov et al., 1999), and this may be cell type dependent (Fredholm et al., 2001). In our experiments, 100 μM 2′,5′-dideoxyadenosine (an adenylate cyclase inhibitor) only inhibited NECA-stimulated IL-6 secretion by 20% (data not shown), suggesting the involvement of other signal pathways. Figure 2 shows the dose-dependent inhibition of the PLC inhibitor U-73122 and the PKC inhibitors, bisindolylmaleimide 1 and Ro-32-0432, on NECA-stimulated IL-6 secretion in TtT/GF cells. Bisindolylmaleimide 1 and U-73122 also inhibited calcitonin-induced IL-6 secretion in TtT/GF cells with similar potencies (Kiriyama et al., 2001). We were, however, unable to use Ro-32-0432 at concentrations greater than 10 μM due to cell toxicity (cell death visible under light microscopy). We also showed that the PI3 kinase inhibitor wortmannin (Marquardt et al., 1996; Feoktistov et al., 1999) dose dependently inhibited NECA-mediated IL-6 secretion at the concentrations used by other authors (Figure 2). Due to concerns regarding the toxicity of all of these compounds, we were unable to perform similar experiments on the Tpit/F1 cells where a prolonged incubation (24 h) is necessary. Nevertheless, it is clear, at least in the TtT/GF cells, that NECA stimulation of IL-6 secretion involves mediation via the PLC/PKC and PI3 kinase pathways.
Figure 2.
Effect of PLC, PKC and PI3 kinase inhibitors on IL-6 secretion from TtT/GF cells. TtT/GF cells were preincubated in DMEM and 2% FCS for 24 h prior to experimentation, and then for 6 h with 1 μM NECA. Inhibitors were added 30 min prior to the addition of NECA. IL-6 was measured by ELISA. Data points represent the mean±s.e.m. of three experiments. *P<0.05, **P<0.01, ***P<0.001 when compared with NECA-stimulated values.
NECA stimulation of IL-6 secretion involves p38 MAPK
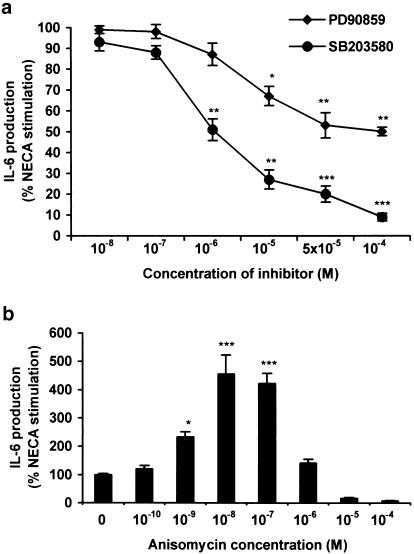
We also investigated the role of the ERK MAPK and the p38 MAPK pathways in mediating NECA-stimulated IL-6 secretion. In TtT/GF cells, PD90859 (MAPK inhibitor) and SB203580 (p38 MAPK inhibitor) dose dependently reduced NECA-stimulated IL-6 (up to 50 and 95%, respectively) (Figure 3a). SB203580, but not PD90859, also inhibited forskolin-mediated IL-6 secretion (data not shown). Both of these compounds also showed ‘toxicity' when incubated with Tpit/F1 cells for 24 h. We also showed that anisomycin (an activator of p38 MAPK) at concentrations up to 0.1 μM, although having no effects on basal secretion, did potentiate the effect of NECA-stimulated IL-6 in TtT/GF cells by up to 4–5-fold (Figure 3b). The observed effects of anisomycin are in fact biphasic, with concentrations greater than 1 μM totally inhibiting the stimulated IL-6 secretion, suggesting possible toxicity. These findings also indicate that stimulation of p38 MAPK alone is insufficient to drive IL-6 secretion and agonism at the A2b receptor (or via additional signal molecules) is also required.
Figure 3.
Effect of (a) MAPK and p38 MAPK inhibitors and (b) anisomycin (activator of p38 MAPK) on NECA-stimulated IL-6 secretion from TtT/GF cells. Cells were treated with inhibitors and anisomycin at the concentrations indicated for 30 min, prior to incubating with 1 μM NECA for 6 h. IL-6 was measured by ELISA. Data points represent the mean±s.e.m. of three experiments. *P<0.05, **P<0.01, ***P<0.001 when compared with basal values.
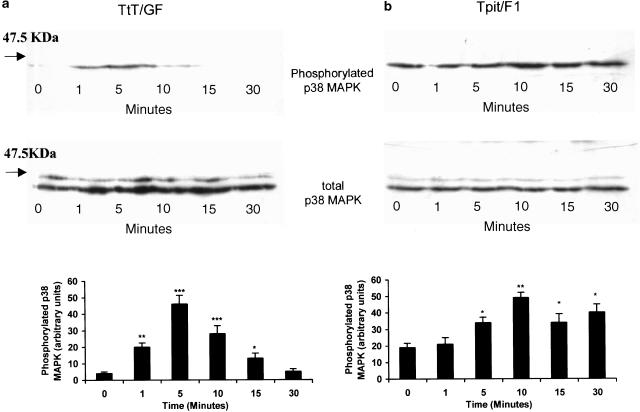
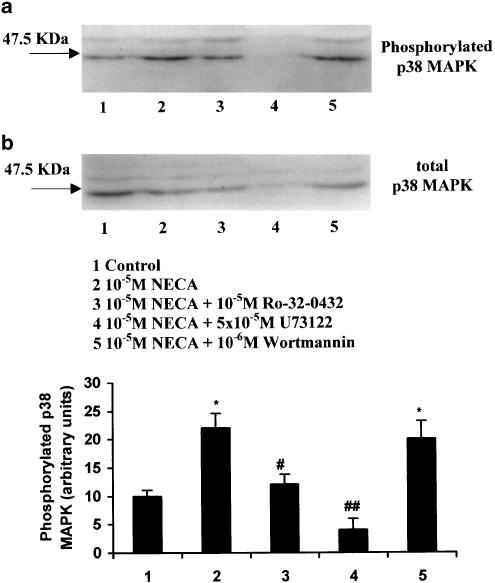
The role of p38 MAPK in NECA-mediated IL-6 secretion in FS cells was also supported by Western analysis. In TtT/GF and Tpit/F1 cells, p38 MAPK phosphorylation showed a time-dependent increase with a peak at between 5 and 10 min after agonist exposure (Figures 4a, b). The phosphorylation of p38 MAPK appeared to be more marked in the TtT/GF cells (5–10-fold) (Figure 4a) in comparison with Tpit/F1 cells (two-fold) (Figure 4b), where the phosphorylation appeared to be more sustained. Total p38 MAPK was similar, indicating equal sample loading. When the total p38 MAPK antibody was used, a higher molecular weight band, likely to be p38δ MAPK, was detected in all of the samples. There was, however, no indication that NECA could stimulate the phosphorylation of p38δ MAPK (Figure 4) in either TtT/GF or Tpit/F1 cells in our experiments. We also demonstrated that U-73122 and Ro-32-0432, but not wortmannin, inhibited NECA-stimulated p38 MAPK phosphorylation (Figure 5). U-73122 also appeared to inhibit the expression of total p38 MAPK. Using separate sample aliquots from these experiments, we were unable to detect the phosphorylated forms of ERK1/2 or JNK in either untreated or NECA-treated cultures (data not shown).
Figure 4.
Time course of phosphorylated and total p38 MAPK activation by NECA (10 μM) in the TtT/GF (a) and Tpit/F1 (b) cell lines. Cultures were depleted of serum for either 8 h (TtT/GF) or 2 h (Tpit/F1), and exposed to 10 μM NECA for the times indicated. The cells were then washed in PBS containing sodium orthovanadate and lysed in Laemelli electrophoresis buffer, and stored at −85°C. Replicate cell extracts were electrophoresed, blotted onto PVDF membranes and exposed to antisera to either p38 MAPK or phosphorylated p38 MAPK. Both antisera react with p38 and p38δ MAPK. Immunoreactive bands were visualised with ECL plus reagent. The molecular weight marker is indicated. The histograms show arbitary densitometric units for phosphorylated p38 MAPK, and are means±s.e.m. for three experiments. *P<0.05, **P<0.01, ***P<0.001 when compared with basal values.
Figure 5.
Effects of Ro-32-0432, U-73122 and wortmannin on NECA-stimulated p38 MAPK phosphorylation in TtT/GF cells. Cultures were depleted of serum for 8 h and exposed to Ro-32-0432 (10 μM), U-73122 (50 μM) and wortmannin (1 μM) for 30 min prior to the addition of 10 μM NECA for 5 min. The cells were then washed in PBS containing sodium orthovanadate and lysed in Laemelli electrophoresis buffer, and stored at −85°C. Replicate cell extracts were electrophoresed, blotted onto PVDF membranes and exposed to antisera to either p38 MAPK or phosphorylated p38 MAPK. Both antisera react with p38 and p38δ MAPK. Immunoreactive bands were visualised with ECL plus reagent. The histograms show arbitary densitometric units for phosphorylated p38 MAPK, and are means±s.e.m. for three experiments. *P<0.05 when compared with basal values, ♯P<0.05; ♯♯P<0.01 when compared with NECA-stimulated values.
NECA stimulates VEGF secretion in TtT/GF FS cells
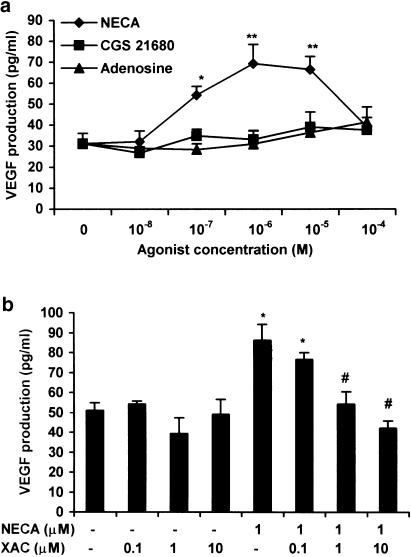
VEGF, like IL-6, is highly expressed during hypoxia and inflammation, and is also secreted by TtT/GF cells (Gloddek et al., 1999; Turnbull & Rivier, 1999). We thus investigated whether NECA had a similar effect on VEGF secretion in FS cells. In TtT/GF cells, basal VEGF (Gloddek et al., 1999), in contrast to IL-6, was easily measurable after 1 h, and levels increased linearly with time for at least 24 h (data not shown). The effects of NECA on VEGF secretion from TtT/GF cells paralleled those on IL-6 release (Figure 6a) though the magnitude of the response was much reduced (1–2-fold for VEGF and >100-fold for IL-6 at 1 μM NECA). Both adenosine and CGS 21680 over the dose range 10 nM–0.1 mM failed to have a significant effect on VEGF secretion. XAC, as expected, also inhibited NECA-stimulated VEGF secretion in TtT/GF cells (Figure 6b). Basal VEGF, however, was undetectable in Tpit/F1 cells, and NECA and adenosine both failed to stimulate its secretion.
Figure 6.
Effects of A2 receptor agonists on VEGF secretion from TtT/GF cells. Cultures were preincubated in DMEM and 2% FCS for 24 h, and then adenosine, NECA and CGS 21680 for 6 h as indicated (a, b). XAC was added 30 min prior to the addition of NECA (b). VEGF was measured by ELISA. Data points represent the mean±s.e.m. of three (b) or four (a) experiments. *P<0.05, **P<0.01 when compared with basal values; ♯P<0.05, when compared with NECA-stimulated values.
NECA-stimulated IL-6 and VEGF secretion is inhibited by dexamethasone
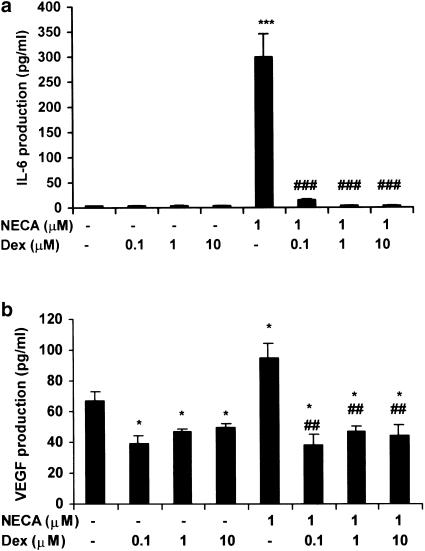
FS cells are known to possess glucocorticoid receptors and thus corticosteroids may also be involved in the modulation of adenosine receptor signalling. We therefore tested the effect of the synthetic glucocorticoid dexamethasone on basal and NECA-stimulated IL-6 and VEGF release from TtT/GF cells (Figures 7a, b). Dexamethasone (100 nM) was able to inhibit basal VEGF release and also completely abolish the NECA-mediated increase in IL-6 and VEGF production (Figures 7a, b).
Figure 7.
Effect of dexamethasone on NECA-stimulated IL-6 and VEGF secretion from TtT/GF cells. Cultures were preincubated in DMEM and 2% FCS for 24 h; dexamethasone was added 30 min prior to the addition of NECA for 6 h. Aliquots of conditioned media were measured for IL-6 (a) and VEGF (b). Data points represent the mean±s.e.m. of three experiments. *P<0.05, ***P<0.001 when compared with basal values; ♯♯P<0.01, ♯♯♯P<0.001 when compared with NECA-stimulated values.
Discussion
Adenosine has been shown to mediate the release of IL-6 both centrally and peripherally, where it regulates immune and inflammatory responses (Fiebich et al., 1996; Schwaninger et al., 1997; Sitaraman et al., 2001). We thus investigated whether adenosine would have a similar role in IL-6 and VEGF secretion in FS cells. Basal release of IL-6 over a 6-h period from TtT/GF and Tpit/F1 cells was low and sometimes undetectable, in agreement with Kiriyama et al. (2001). VEGF, on the other hand, was easily measurable in TtT/GF cells (Gloddek et al., 1999) but not in Tpit/F1 cells, although the latter clearly expressed VEGF mRNA (unpublished observations). This lack of measurable VEGF protein in Tpit/F1 cells may simply reflect an overall lower peptide expression when compared with TtT/GF cells. In TtT/GF cells, NECA had a much greater effect in stimulating IL-6 release when compared with VEGF, possibly suggesting that VEGF secretion may be mediated by an autocrine action of IL-6 (Gloddek et al., 1999).
A comparison of the concentration-related effects of NECA, CGS 21680 and adenosine on IL-6 secretion parallels that on cAMP generation in both TtT/GF and Tpit/F1 cells, indicating that the mediating receptor is likely to be of the A2b subtype (Rees et al., 2002). This is further supported by alloxazine being a potent inhibitor of NECA-mediated IL-6 secretion in our experiments. The relatively weak effects observed for adenosine on IL-6 secretion and cAMP expression may reflect its short half-life (<1 h under culture conditions, unpublished observations) and the fact that the A2b receptor is a low-affinity subtype (Fredholm et al., 2001). The short half-life of extracellular adenosine may be due to its rapid uptake and phosphorylation by adenosine kinase (Spychala, 2000), or its metabolism to inosine at the cell surface by the enzyme adenosine deaminase (Spychala, 2000). Furthermore, adenosine deaminase is often colocalised with adenosine receptors (Herrera et al., 2001), and may serve as an additional regulatory step by limiting the level of adenosine available for receptor binding.
The pattern of stimulated IL-6 secretion with peak values that occur at 10 μM NECA, followed by a reduction in secretion at higher concentrations of the agonist, parallels exactly that seen for cell proliferation (Rees et al., 2002). The reasons for this reduced secretion are unclear. It could, for example, reflect receptor desensitisation and internalisation as a result of arrestin binding (Mundell et al., 2000; Mathuru et al., 2001). The diverse actions of the adenosine A2b receptor as well as the other subtypes probably reflect the variety of signals downstream of the receptor itself. The A2b receptor couples to Gs, and in some cell types to Gq (Feoktistov et al., 1999; Fredholm et al., 2001). Our data in TtT/GF cells show that activation of PLC/PKC is important in the NECA regulation of IL-6 expression. The inhibitory effect of wortmannin, however, contrasts that seen in mast cells, where Marquardt et al. (1996) failed to demonstrate an inhibition of NECA-mediated IL-6 secretion.
NECA is a potent stimulus for TtT/GF cell growth (Rees et al., 2002), suggesting the involvement of the MAPK cascades. In our experiments, the MAPK inhibitor PD98059 only partially inhibited NECA- and calcitonin- (Kiriyama et al., 2001) stimulated secretion of IL-6, suggesting the involvement of other signal molecules. NECA stimulated p38 MAPK phosphorylation in both TtT/GF and Tpit/F1 cells, but failed to have a significant effect on ERK1/2 phosphorylation. The observed effect on p38 MAPK phosphorylation is, however, weak in comparison to that reported for LPS in TtT/GF cells (Lohrer et al., 2000). Furthermore, Ro-32-0432, in contrast to wortmannin, clearly inhibited NECA-mediated p38 MAPK phosphorylation. The findings with U-73122 were, however, difficult to interpret as it inhibited both total and phosphorylated p38 MAPK. Nevertheless, taken overall, our studies in FS cells indicate a close relationship between the A2b receptor, IL-6 secretion and p38 MAPK phosphorylation. In addition, it is clear that other signal molecules such as adenylate cyclase, PI3 kinase and the ERK MAPK family are also involved. Our findings and those of others (Feoktistov et al., 1999; Gao et al., 1999; Schulte & Fredholm, 2000) show that A2b receptors are linked to common signal molecules, but additional pathways may confer a specificity that is unique to individual cell types.
Subsequently, we showed that dexamethasone was able to completely inhibit both NECA-stimulated IL-6 and VEGF secretion as well as basal VEGF secretion in TtT/GF cells. Similar concentrations of dexamethasone also completely inhibited LPS and pituitary AC-activating polypeptide (PACAP)-mediated IL-6 release (Chen et al., 2000; Lohrer et al., 2000), and IL-6- and PACAP-stimulated VEGF release (Gloddek et al., 1999). These findings are consistent with glucocorticoid receptor expression in FS cells (Ozawa et al., 1999), and suggest that IL-6 and VEGF regulation by dexamethasone is important in the pituitary response to inflammation. The inhibitory action of dexamethasone may be mediated by several mechanisms, including the reduction of cAMP levels (Gerwins & Fredholm, 1991; Svenningsson & Fredholm, 1997), inhibition of p38 MAPK phosphorylation (Lasa et al., 2001;2002) and repression of the IL-6 gene via binding to a negative glucocorticoid response element (McKay & Cidlowski, 1999). Interestingly, it has been noted that the reduction of A2bR signalling by glucocorticoids in DDT1 MF-2 cells is accompanied by an upregulation in the number of coexpressed A1 receptors (Gerwins & Fredholm, 1991). A1 receptors are also coexpressed with A2b receptors in TtT/GF and Tpit/F1 cells (Rees et al., 2002).
In summary, our findings show that, in FS cells, adenosine stimulates IL-6 secretion via the A2b receptor that is also linked to the PLC/PKC pathways coupled principally to p38 MAPK. This coupling of adenosine (NECA) to p38 MAPK and IL-6 secretion, together with its suppression in the presence of corticosteroids, further indicates a role of adenosine and FS cells in inflammation, stress and hypoxia. Our findings are consistent with and extend the preliminary observations of Ritchie et al. (1997). We hypothesise that FS cells are able to respond to a variety of immune molecules and endotoxins that result in the accumulation of extracellular adenosine and an autocrine stimulation of IL-6 and VEGF.
Acknowledgments
This work is supported by a Clinical Endocrinology Trust Training Fellowship to D.A.R., the Wellcome Trust and the Welsh Office.
Abbreviations
- CGS 21680
2-p-(2-carboxyethyl)phenethylamino-5′-N-ethylcarboxamidoadenosine
- DMEM
Dulbecco's modified Eagle's medium
- EBSS
Earle's balanced salt solution
- ERK
extracellular signal-regulated kinase
- FCS
foetal calf serum
- FS
folliculostellate
- JNK
c-Jun N-terminal kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- NECA
5′-N-ethylcarboxamidoadenosine
- PACAP
pituitary AC-activating polypeptide
- PD90859
2′-amino-3′-methoxyflavone
- Ro-32-0432
bisindolylmaleimide X1 HCl
- SB203580
4-(4-fluorophenyl)-2-(4-methylsulphinylphenyl)-5-(4-pyridyl)1H-imidazole
- U-73122
1-[6-((17β-3-methoxyestra-1,3,5(10)-tiene-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione
- VEGF
vascular endothelial growth factor
- XAC
xanthine amine congener
References
- ALLAERTS W., CARMIELIET P., DENEF C. New perspectives in the function of pituitary folliculostellate cells. Mol. Cell. Endocrinol. 1990;71:73–81. doi: 10.1016/0303-7207(90)90244-3. [DOI] [PubMed] [Google Scholar]
- BESEDOVSKY H.O., DEL RAY A. Immune-neuro-endocrine interactions: facts and hypotheses. Endocr. Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- CHEN L., MURUYAMA D., SUGIYAMA M., SAKAI T., MOGI C., KATO M., KUROTANI R., SHIRASAWA N., TAKAKI A., RENNER U., KATO Y., INOUE K. Cytological characterization of a pituitary folliculo-stellate-like cell line, Tpit/F1, with special reference to adenosine triphosphate-mediated neuronal nitric oxide synthase expression and nitric oxide secretion. Endocrinology. 2000;141:3603–3610. doi: 10.1210/endo.141.10.7710. [DOI] [PubMed] [Google Scholar]
- FEOKTISTOV I., GOLDSTEIN A.E., BIAGGIONI I. Role of p38 mitogen-activated protein kinase and extracellular signal regulated protein kinase kinase in adenosine A2b receptor-mediated interleukin-8 production in human mast cells. Mol. Pharmacol. 1999;55:726–735. [PubMed] [Google Scholar]
- FIEBICH B.L., BIBER K., GYUFKO K., BERGER M., BAUER J., VAN CALKER D. Adenosine A2b receptors mediate an increase in interleukin (IL)-6 mRNA and IL-6 protein synthesis in human astroglioma cells. J. Neurochem. 1996;66:1426–1431. doi: 10.1046/j.1471-4159.1996.66041426.x. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., IJZERMAN A.P., JACOBSON K.A., KLOTZ K.-N., LINDEN J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- GAO Z., CHEN T., WEBER M.J., LINDEN J. A2b adenosine and P2Y2 receptors stimulate mitogen-activated protein kinase in human embryonic kidney-293 cells. Cross-talk between cAMP and protein kinase pathways. J. Biol. Chem. 1999;274:5972–5980. doi: 10.1074/jbc.274.9.5972. [DOI] [PubMed] [Google Scholar]
- GERWINS P., FREDHOLM B.B. Glucocorticoid receptor activation leads to up-regulation of adenosine A1 receptors and down-regulation of adenosine A2 responses in DDT1 MF-2 smooth muscle cells. Mol. Pharmacol. 1991;40:149–155. [PubMed] [Google Scholar]
- GLODDEK J., PAGOTTO U., PAEZ PEREDA M., ARTZ E., STALLA G.K. PACAP, IL-6 and glucocorticoids regulate the release of VEGF in pituitary folliculostellate cells. J. Endocrinol. 1999;160:483–490. doi: 10.1677/joe.0.1600483. [DOI] [PubMed] [Google Scholar]
- HERRERA C., CASADO V., CIRUELA F., SCHOFIELD P., MALLOL J., LLUIS C., FRANCO R. Adenosine A2b receptors behave as an alternative anchoring protein for cell surface adenosine deaminase in lymphocytes and cultured cells. Mol. Pharmacol. 2001;59:127–134. [PubMed] [Google Scholar]
- INOUE K., MATSUMOTO H., KOYAMA C., SHIBATA K., NAKAZATO Y., ITO A. Establishment of a folliculo-stellate cell line from a mouse thyrotropic pituitary tumor. Endocrinology. 1992;131:3110–3116. doi: 10.1210/endo.131.6.1446645. [DOI] [PubMed] [Google Scholar]
- KIRIYAMA Y., TSUCHIYA H., MURAKAMI T., SATOH K., TOKUMITSU Y. Calcitonin induces IL-6 production via both PKA and PKC pathways in the pituitary folliculostellate cell line. Endocrinology. 2001;142:3563–3569. doi: 10.1210/endo.142.8.8328. [DOI] [PubMed] [Google Scholar]
- LASA M., ABRAHAM S.M., BOUCHERON C., SAKLATVALA J., CLARK A.R. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 2002;22:7802–7811. doi: 10.1128/MCB.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASA M., BROOK M., SAKLATVALA J., CLARK A.R. Dexamethasone destabilizes cyclooxygenase 2 mRNA by inhibiting mitogen-activated protein kinase p38. Mol. Cell. Biol. 2001;21:771–780. doi: 10.1128/MCB.21.3.771-780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATINI S., BORDINI F., PEDATA F., CORRADETTI R. Extracellular adenosine concentrations during in vitro ischaemia in rat hippocampal slices. Br. J. Pharmacol. 1999;126:729–739. doi: 10.1038/sj.bjp.0702591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOHRER P., GLODDEK J., CARBIA NAGASHIMA A., KORALI Z., HOPFNER U., PAEZ PEREDA M., ARTZ E., STALLA G.K., RENNER U. Lipopolysaccharide directly stimulates the intrapituitary IL-6 production by folliculostellate cells via specific receptors and the p38alpha MAP kinase/NFkappaB pathway. Endocrinology. 2000;141:4457–4465. doi: 10.1210/endo.141.12.7811. [DOI] [PubMed] [Google Scholar]
- MARQUARDT D.L., ALONGI J.L., WALKER L.L. The phosphatidylinositol 3-kinase inhibitor wortmannin blocks mast cell exocytosis but not IL-6 production. J. Immunol. 1996;156:1942–1945. [PubMed] [Google Scholar]
- MATHERNE G.P., HEADRICK J.P., COLEMAN S.D., BERNE R.M. Interstitial transudate purines in normoxic and hypoxic immature and mature rabbit hearts. Pediatr. Res. 1990;28:348–353. doi: 10.1203/00006450-199010000-00010. [DOI] [PubMed] [Google Scholar]
- MATHURU A.L., MUNDELL S.J., BENOVIC J.L., KELLY E. Rapid agonist-induced desensitization and internalization of the A2b adenosine receptor is mediated by a serine residue close to the COOH terminus. J. Biol. Chem. 2001;276:30199–30207. doi: 10.1074/jbc.M010650200. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO H., KOYAMA C., SAWADA T., KOIKE K., HIROTA K., MIYAKE A., ARIMURA A., INOUE K. Pituitary folliculostellate cell line (TtT/GF) responds to novel hypophysiotropic peptide (PACAP) showing increased cAMP and IL-6 secretion and cell proliferation. Endocrinology. 1993;133:3150–3155. doi: 10.1210/endo.133.5.8404665. [DOI] [PubMed] [Google Scholar]
- MCKAY L.I., CIDLOWSKI J.A. Molecular control of immune/inflammatory responses: interactions between nuclear factor-κB and steroid receptor signaling pathways. Endocr. Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- MUNDELL S.J., MATHARU A.L., KELLY E., BENOVIC J.L. Arrestin isoforms dictate differential kinetics of A2b adenosine receptor trafficking. Biochemistry. 2000;39:12828–12836. doi: 10.1021/bi0010928. [DOI] [PubMed] [Google Scholar]
- NAITOH Y., FUKATA J., TOMINAGA T., NAKAKI Y., TAMAI S., MORI K., IMURA H. Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious free-moving rats. Biochem. Biophys. Res. Commun. 1988;155:1459–1463. doi: 10.1016/s0006-291x(88)81305-6. [DOI] [PubMed] [Google Scholar]
- OZAWA H., ITO T., OCHIAI I., KAWATA M. Cellular localization and distribution of glucocorticoid receptor immunoreactivity and the expression of glucocorticoid mRNA in rat pituitary gland. A combined double immunohistochemistry study and in situ hybridization histochemical analysis. Cell Tissue Res. 1999;295:207–214. doi: 10.1007/s004410051226. [DOI] [PubMed] [Google Scholar]
- REES D.A., LEWIS M.D., LEWIS B.M., SMITH P.J., SCANLON M.F., HAM J. Adenosine-regulated cell proliferation in pituitary folliculostellate and endocrine cells: Differential roles for the A1 and A2b adenosine receptors. Endocrinology. 2002;143:2427–2436. doi: 10.1210/endo.143.6.8837. [DOI] [PubMed] [Google Scholar]
- RITCHIE P.K., SPANGELO B.L., KRZYMOWSKI D.K., ROSSITER T.B., KURTH E., JUDD A.M. Adenosine increases interleukin 6 release and decreases tumour necrosis factor release from rat adrenal zona glomerulosa cells, ovary cells, anterior pituitary cells, and peritoneal macrophages. Cytokine. 1997;9:187–198. doi: 10.1006/cyto.1996.0153. [DOI] [PubMed] [Google Scholar]
- SCHULTE G., FREDHOLM B.B. Human adenosine A1, A2a, A2b, and A3 receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase1/2. Mol. Pharmacol. 2000;58:477–482. [PubMed] [Google Scholar]
- SCHWANINGER M., NEHER M., VIEGAS E., SCHNEIDER A., SPRANGER M. Stimulation of IL-6 secretion and gene transcription in primary astrocytes by adenosine. J. Neurochem. 1997;69:1145–1150. doi: 10.1046/j.1471-4159.1997.69031145.x. [DOI] [PubMed] [Google Scholar]
- SITARAMAN S.V., MERLIN D., WANG L., WONG M., GEWIRTZ A.T., SITAKAR M., MADARA J.L. Neurotrophil–epithelial cross-talk at the intestinal lumenal surface mediated by the reciprocal secretion of adenosine and IL-6. J. Clin. Invest. 2001;107:861–869. doi: 10.1172/JCI11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPANGELO B.L., JUDD A.M., ISAKSON P.C., MACLEOD R.M. Interleukin-6 stimulates anterior pituitary hormone release in vitro. Endocrinology. 1989;125:575–577. doi: 10.1210/endo-125-1-575. [DOI] [PubMed] [Google Scholar]
- SPYCHALA J. Tumor-promoting functions of adenosine. Pharm. Ther. 2000;87:161–173. doi: 10.1016/s0163-7258(00)00053-x. [DOI] [PubMed] [Google Scholar]
- STERNBERG E.M. Neuro-immune interactions in health and disease. J. Clin. Invest. 1997;100:2641–2647. doi: 10.1172/JCI119807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNINGSSON P., FREDHOLM B.B. Glucocorticoids regulate the expression of adenosine A1 but not A2a receptors in the rat brain. J. Pharm. Exp. Therap. 1997;280:1094–1101. [PubMed] [Google Scholar]
- TATSUNO I., SOMOGYVARI-VIGH A., MIZUNO K., GOTTSCHALL P.E., HIDAKA H., ARIMURA A. Neuropeptide regulation of IL-6 production from the pituitary: stimulation by PACAP and CGRP. Endocrinology. 1991;129:1797–1804. doi: 10.1210/endo-129-4-1797. [DOI] [PubMed] [Google Scholar]
- TURNBULL A.V., RIVIER C.L. Regulation of the hypothalamic pituitary–adrenal axis by cytokines: actions and mechanisms of action. Pharmacol. Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- VAN BELLE H., GOOSENS F., WYNANTS J. Formation and release of purine catabolites during hypoperfusion, anoxia and ischemia. Am. J. Physiol. 1987;252:H886–H893. doi: 10.1152/ajpheart.1987.252.5.H886. [DOI] [PubMed] [Google Scholar]