Abstract
Ginsenoside Rg3 (Rg3) isolated from Panax ginseng relaxes vessels and exerts a cytoprotective effect. In view of the fact that nitric oxide (NO) is involved in vascular hyporeactivity and immunostimulation, the effects of total ginsenosides (GS) and Rg3 on the vascular responses and the expression of inducible nitric oxide synthase (iNOS) were investigated.
Vasocontraction of endothelium-denuded aortic ring was induced by phenylephrine with or without GS or Rg3. The expression of iNOS was assessed by Western blot and RT–PCR analyses. NF-κB activation was monitored by gel shift, immunoblot and immunocytochemical analyses.
Incubation of the endothelium-denuded aortic ring with GS or Rg3 inhibited phenylephrine-induced vasocontraction, which was abrogated by NOS inhibition. GS or Rg3 increased NO production in aortic rings, but Rb1, Rc, Re and Rg1 had no effect.
Aortic rings obtained from rats treated with GS or Rg3 responded to phenylnephrine to a lesser extent, while producing NO to a larger extent, than those from control animals. GS or Rg3 induced iNOS in vascular smooth muscle.
Rg3 induced iNOS with increase in NO production in Raw264.7 cells. Rg3 increased NF-κB DNA binding, whose band was supershifted with anti-p65 and anti-p50 antibodies, and elicited p65 nuclear translocation, which was accompanied by phosphorylation and degradation of I-κBα. PKC regulated iNOS induction by Rg3.
In conclusion, Rg3 relaxes vessels as a consequence of NO production, to which iNOS induction contributes, and iNOS induction by Rg3 accompanied NF-κB activation, which involves phosphorylation and degradation of I-κBα and nuclear translocation of p65.
Keywords: Nuclear factor-κB, iNOS, ginsenosides, Rg3, Panax ginseng
Introduction
Panax ginseng has been popularly used as a herbal medicine in Asia for more than 2000 years and currently occupies an important place among the tonic remedies of oriental medicine. In North America, the ginseng species including P. quinquefolium represents an important industry for both domestic and export markets. There are a variety of reports explaining the pharmacological effects of P. ginseng against certain chronic disease states and aging, which may result from enhancement of vascular and immune functions.
Ginsenosides, which is considered to be a biologically active fraction of P. ginseng, is a mixture of triterpene glycosides. The major components of ginsenosides belong either to the protopanaxadiol or protopanaxatriol groups (Shibata et al., 1966). Ginsenoside Rg3, Rb1 and Rc represent protopanaxa-diols, whereas ginsenoside Rg1 and Re are protopanaxatriols. Ginsenosides from P. ginseng decrease the blood pressure in both experimental animals and hypertensive patients (Kim et al., 1994; Han et al., 1998; Jeon et al., 2000; Sung et al., 2000). It has also been shown that ginsenosides enhance vasodilatory response to perivascular nerve stimulation in monkey cerebral arteries (Toda et al., 2001). The antihypertensive effect of ginsenosides may result from their ability to inhibit vascular tone. Among the ginsenosides of the protopanaxatriol and protopanaxadiol groups, ginsenoside Rg3 is the most potent vasodilator. Previously, we showed that Rg3 inhibited calcium-induced vascular contraction (Kim et al., 1999a, 1999b).
Nitric oxide (NO) is a radical produced from L-arginine via nitric oxide synthase (NOS), and also serves as an important cellular second messenger (Palmer et al., 1988). NO plays a dual role as a beneficial or detrimental molecule in the inflammatory process. Inducible NOS (iNOS) produces a high output of NO during inflammation, whereas constitutively expressed NOS (cNOS) generates a physiologically active low level of NO (Kubes, 2000). A high level of NO may be associated with anticancer, bactericidal and antiparasitic effects, presumably through the formation of reactive radicals including peroxynitrite (Szabo & Ohshima, 1997). It has been shown that the mice treated with a NOS inhibitor or the iNOS-knockout mice were more susceptible to bacterial infection (e.g. Salmonella) (Zhou et al., 2002). Reactive oxygen and nitrogen intermediates produced in innate immune cells would be effective in the host-defense mechanism against microbial pathogens. Although the precise mechanism(s) by which NO antagonizes bacterial infection is not known, the mechanism may involve disruption of bacterial DNA, proteins, signaling, or induction of apoptosis of macrophages that harbor mycobacteria (Chan et al., 2001). In addition to the involvement of NO during the process of inflammation, the vascular hemodynamics is greatly affected by NO production.
It has been claimed that the polysaccharides, but not the triterpene-enriched aqueous fraction, prepared from P. ginseng stimulated NO production (Friedl et al., 2001). Conversely, Li et al. (2000) showed that ginsenosides enhanced NO production perhaps through the induction of NOS in endothelium. Previously, we showed that Rg3 induced the relaxation of rat aorta with endothelium, which occurred as a result of NO production by activation of endothelial nitric oxide synthase (eNOS) (Kim et al., 1999b). The content of Rg3 that is generally low in unprocessed P. ginseng markedly increased in the total ginsenosides or the semipurified fractions prepared from heat-activated P. ginseng root (Kim et al., 2000; Kwon et al., 2001). Thus, the content of Rg3 in the fraction of total ginsenosides varies depending on the preparative methods. If Rg3 is the biologically active component, the experimental data obtained with total ginsenosides may be variable depending on the Rg3 content in the preparation.
Given the effects of P. ginseng root on hemodynamics, vascular regulation and immune defense, and the conflicting report of the total ginsenosides on NO production, we were interested in whether Rg3 of ginsenosides might induce vascular relaxation and, if so, what the role of NO was in the aortic smooth muscle treated with Rg3. In the present study, we further evaluated the effect of Rg3 on the expression of NOS in macrophages. We revealed for the first time that total ginsenosides and Rg3, but not Rb1, Rc, Re and Rg1, relaxed the endothelium-denuded arotic rings stimulated by phenylephrine and increased NO production via NOS induction.
Methods
Materials
Rg3 was kindly gifted from Dr JI Park (Seoul National University, Seoul, Korea) and ginsenoside Rg1, Rb1, Rc and Re were provided by the Korea Ginseng and Tobacco Research Institute (Daejeon, Korea). The same lot of total ginsenosides was used throughout this study. The content of Rg3 in the total ginsenosides was ∼1/10th (Kwon et al., 2001). The chemical structure of Rg3 was confirmed by a variety of spectroscopic analyses (Kim et al., 1999a, 1999b; Kwon et al., 2001). The Limulus Amoebocyte Lysate test (i.e. an endotoxin test using the gel clot method) showed that either total ginsenosides or Rg3 was endotoxin-free, with a sensitivity limit of 0.03 EU ml−1. [γ-32P]ATP (3000 mCi mmol−1) was obtained from Amersham (Arlington Heights, IL, U.S.A.). Horseradish peroxidase-conjugated goat anti-rabbit IgG and 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium were obtained from Life Technologies (Gaithersburg, MD, U.S.A.). Alkaline phosphatase-conjugated goat anti-mouse and anti-rabbit antibodies were purchased from KPL (Gaithersburg, MD, U.S.A.). Anti-c-Rel (p65), anti-p50 and anti-I-κBα anti-phospho-I-κBα antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Anti-murine iNOS antibody was obtained from Transduction Laboratories (Lexington, KY, U.S.A.). Fluorescein isothiocyanate-conjugated anti-rabbit IgG was obtained from Zymed Laboratories Inc. (San Francisco, CA, U.S.A.). NG-nitro-L-arginine (NLA) was purchased from Aldrich Chemical (Milwaukee, WI, U.S.A.). PD98059 was obtained from Biomol (Plymouth Meeting, PA, U.S.A.). Indomethacin and other reagents in the molecular studies were supplied from Sigma Chemical (St Louis, MO, U.S.A.).
Organ chamber studies
Male Sprague–Dawley rats (270–330 g) were killed and thoracic aortas were removed and placed in a modified Krebs–Ringer-bicarbonate solution containing (in mM) NaCl, 118.3; KCl, 4.7; MgSO4, 1.2; KH2PO4, 1.2; CaCl2, 2.5; NaHCO3, 25.0; Ca2+EDTA, 0.016 and glucose, 11.1 (control solution). The aortas were cleaned of loose connective tissue and then cut into rings (2 mm wide), and the endothelium was removed mechanically. The rings of rat thoracic aorta without endothelium were incubated in MEM in the presence of ginsenosides (100 μg ml−1), Rb1 (100 μg ml−1), Rc (100 μg ml−1), Re (100 μg ml−1), Rg1 (100 μg ml−1) and Rg3 (10 μg ml−1) for 6 h at 37°C in the 5% CO2 incubator. To determine the in vivo effect of ginsenosides on the contraction of aortic rings induced by phenylephrine, rats were orally administered for 5 days with ginsenosides (100 mg kg−1) and the thoracic aortas were removed. The aortic rings were suspended horizontally between two stainless steel stirrups in the organ chambers filled with 10 ml of control solution (37°C, pH 7.4) and bubbled with 95% O2 and 5% CO2. One of the stirrups was anchored to the organ chamber and the other one was connected to a transducer coupler (Narco Bio-system) for the recording of isometric tension. The rings were stretched progressively to the optimal tension (2 g) before the addition of 90 mM KCl. Once the plateau of the contraction elicited by KCl was obtained, the aortic rings were rinsed three times with warm (37°C) control solution. Indomethacin (10 μM) was used to prevent the production of cyclooxygenase metabolites that are predominantly vasoconstrictors in this experimental setting. In some experiments, rings were incubated for 30 min with a NOS inhibitor (NLA, 10 μM; aminoguanidine (AG), 30 μM). After 30-min incubation, the cumulative concentration–contraction curve for phenylephrine (10−9–10−5 M) was obtained.
Citrulline assay
The activity of iNOS in the aorta was assayed as described previously (Chan et al., 1997), with a slight modification. The aortas obtained from the rats treated with ginsenosides were homogenized in 1 ml of 25 mM Tris-HCl buffer (pH 7.4) with a glass/glass potter, and centrifuged at 10,000 × g for 10 min. The supernatant was used as a crude fraction of iNOS. Enzyme activity was analyzed in the 50 μl reaction mixture, containing 1 mM β-NADPH, 4 μM tetrahydrobiopterin, 1 μM FAD, 1 μM FMN and 4 μM L-[U-14C]arginine in 25 mM Tris-HCl buffer (pH 7.4). The mixture was preincubated at 37°C for 3 min, and then 1 mg aortic homogenates were added to the mixture as the enzyme source. The samples were incubated for 15 min and the reaction was terminated by the addition of 50 mM HEPES-EDTA buffer (pH 5.5). A suspension of ion exchange resin (AG 50W-X8 resin; Bio-Rad, Hercules, CA, U.S.A.) was then added to the samples, which was transferred to the spin cup set in a new microtube, and centrifuged at 5500 × g for 1 min. Radioactivity in the filtrate was quantitated on a liquid scintillation analyzer (LSC-3500, Aloka, Tokyo, Japan). Disintegration per minute was converted to citrulline production and expressed in pmol mg−1 protein min−1.
Cell culture
Raw264.7 cells were obtained from American Type Culture Collection (ATCC, Rockville, MD, U.S.A.) and maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS), 50 U ml−1 penicillin and 50 μg ml−1 streptomycin at 37°C in a humidified atmosphere with 5% CO2. Raw264.7 cells were plated at a density of 5 × 106 per 10 cm2 dish and preincubated for 24 h at 37°C. For all experiments, cells were grown to 80–90% confluency, and were subjected to no more than 20 cell passages. To compare NO production, Raw264.7 cells were incubated with 1 μg ml−1 of LPS (Escherichia coli 026:B6; Difco, Detroit, MI, U.S.A.).
Assay of NO production
NO was monitored by measuring the nitrite content in culture medium. After mixing of the culture medium with Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine dihydrochloride and 2.5% phosphoric acid), absorbance was measured at 540 nm after incubation for 10 min.
Immunoblot analysis
Sodium dodecylsulfate (SDS)–polyacrylamide gel electropho-resis (PAGE) and immunoblot analysis were performed according to the previously published procedures (Kim et al., 1997). Cells were lysed in the buffer containing 20 mM Tris-HCl (pH 7.5), 1% Triton X-100, 137 mM sodium chloride, 10% glycerol, 2 mM EDTA, 1 mM sodium orthovanadate, 25 mM β-glycerophosphate, 2 mM sodium pyrophosphate, 1 mM phenylmethylsulfonylfluoride and 1 μg ml−1 leupeptin. Cell lysates were centrifuged at 10,000 × g for 10 min to remove debris. The proteins were fractionated using a 7.5% separating gel to assess the level of iNOS, whereas I-κBα and the phosphorylated form of I-κBα were resolved in a 12% SDS–PAGE. Briefly, the fractionated proteins were electrophoretically transferred to nitrocellulose paper. Cytosolic iNOS was immunoblotted with monoclonal anti-murine iNOS antibody, whereas polyclonal anti-I-κBα and antiphosphorylated I-κBα antibodies were used to assess I-κBα and its phosphorylated form, respectively. The secondary antibodies were alkaline phosphatase-conjugated anti-mouse and anti-rabbit antibodies, and the nitrocellulose paper was developed using 5-bromo-4-chloro-3-indolylphosphate/4-nitroblue tetrazolium chloride or developed using ECL chemiluminescence system (Amersham, Buckinghamshire, U.K.).
Reverse transcriptase–polymerase chain reaction (RT–PCR)
RT–PCR was carried out using the selective primers for the iNOS and GAPDH genes. Primers specific for iNOS (sense: 5′-ATGTCCGAAGCAAACATCAC-3′; antisense: 5′-TAATGTCCAGGAAGTAGGTG-3′) and GAPDH (sense: 5′-TCGTGGAGTCTACTGGCGT-3′; antisense: 5′-GCCTGCTTCACCACCTTCT-3′) were used, resulting in the amplified products of 449 and 510 bp, respectively. PCRs were performed for 30 cycles using the following conditions: denaturation at 95°C for 0.5 min, annealing at 49°C for 0.5 min and elongation at 68°C for 1.5 min. Band intensities of the amplified DNAs were compared after visualization on a UV transilluminator.
Preparation of nuclear extracts
Nuclear extracts were prepared essentially according to Schreiber et al. (1990). Briefly, the dishes were washed with ice-cold PBS. They were then scraped and transferred to microtubes. Cells were allowed to swell by adding 100 μl of lysis buffer (10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol and 0.5 mM phenylmethylsulfonylfluoride). Tubes were vortexed to disrupt the cell membranes. The samples were incubated for 10 min on ice and centrifuged for 5 min at 4°C. Pellets containing crude nuclei were resuspended in 50 μl of the extraction buffer containing 20 mM HEPES (pH 7.9), 400 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol and 1 mM phenylmethylsulfonylfluoride, and then incubated for 30 min on ice. The samples were centrifuged at 15,800 × g for 10 min to obtain the supernatant containing nuclear extracts. The nuclear extracts were stored at −70°C until use.
Gel retardation assay
A double-stranded DNA probe for the consensus sequence of nuclear factor-κB (NF-κB, 5′-AGTTGAGGGGACTTTCCCAGGC-3′) was used for gel shift analysis after endlabeling of the probe with [γ-32P]ATP and T4 polynucleotide kinase. The reaction mixture contained 2 μl of 5 × binding buffer containing 20% glycerol, 5 mM MgCl2, 250 mM NaCl, 2.5 mM EDTA, 2.5 mM dithiothreitol, 0.25 mg ml−1 poly dI-dC and 50 mM Tris-Cl (pH 7.5), 8 μg of nuclear extracts and sterile water in a total volume of 10 μl. Incubations were carried out at room temperature for 20 min by the addition of 1 μl probe (106 c.p.m.), following 10 min preincubations. In some experiments, 8 μg each of nuclear extracts was incubated with 2 μg of highly specific anti-p65 and/or anti-p50 antibody at room temperature for 1 h, according to the method described previously (Cho et al., 2002). Samples were loaded onto 4% polyacrylamide gels at 100 V. The gels were removed, fixed and dried, followed by autoradiography.
Immunocytochemistry of p65
Cells were grown on Lab-TEK chamber slides® (Nalge Nunc International Corp.) and incubated in serum-deprived medium for 24 h. The standard immunocytochemical method was used as described previously (Cho et al., 2002). For immunostaining, cells were fixed in 100% methanol for 30 min and washed three times with PBS. After blocking with 5% BSA in PBS for 1 h at room temperature or overnight at 4°C, cells were incubated for 1 h with polyclonal rabbit anti-p65 antibody (1 : 100) in PBS containing 0.5% BSA. Cells were incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (1 : 100) after serial washing with PBS. Counterstaining with propidium iodide (PI) verified the location and integrity of nuclei. Stained cells were washed and examined using a laser-scanning confocal microscope (Leica TCS NT, Leica Microsystems, Wetzlar, Germany).
Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) expression
Raw264.7 cells (1 × 107 cells) were incubated with 100 μg ml−1 ginsenosides, 10 μg ml−1 Rg3 or 1 μg ml−1 LPS for 24 h. After incubation, the levels of IL-1β and TNF-α in Raw264.7 cells were assayed by ELISA.
Data analysis
The data are expressed as means±s.e.m. or s.d. The contractile response is expressed in terms of the per cent of maximal contraction developed in response to 90 mM of KCl. Statistical significance was analyzed by Student's t-test procedure. The criterion for statistical significance was set at P<0.05 or P<0.01.
Results
In vitro inhibition of vascular contractility by Rg3
A previous study has shown that ginsenoside Rg3 inhibited calcium-induced concentration–contraction curves and the calcium influx in aortic rings incubated with a high concentration of KCl in a time- and concentration-dependent manner (Kim et al., 1999a). In the present study, the effect of ginsenosides and Rg3 on the vasoconstriction induced by phenylephrine, an α-adrenoceptor agonist, was determined by using endothelium-denuded aortic rings excised from rats.
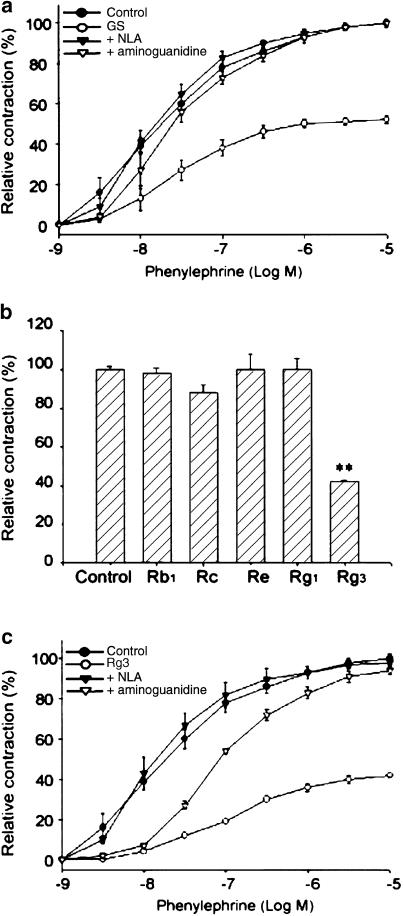
Contraction of aortic rings induced by phenylephrine (10 μM) was impaired after treatment with ginsenosides (100 μg ml−1) for 12 h (Figure 1a). Ginsenosides inhibited 50% phenylephrine-induced contraction of aortic rings (P<0.01), whereas ginsenosides in the presence of the nonspecific NOS inhibitor NLA (10 μM) or the selective iNOS inhibitor aminoguanidine (30 μM) failed to inhibit phenyleph-rine-induced vascular contraction (Figure 1a) (i.e. 98 vs 45%, P<0.01). Either NLA or AG alone exerted no effect. The suppression of phenylephrine-induced vascular reaction by ginsenosides was not affected by indomethacin (10 μM), suggesting that the production of prostaglandins might not be associated with the vascular hyporeactivity induced by ginsenosides (data not shown). Thus, it is highly likely that the inhibition of aortic contraction by ginsenosides may result from NO production.
Figure 1.
The inhibitory effects of ginsenosides or Rg3 on phenylephrine-induced contraction of rat thoracic aortic rings. (a) The concentration–response curves of vascular contraction by phenylephrine. Endothelium-denuded aortic rings were incubated in MEM with or without total ginsenosides (100 μg ml−1) for 12 h at 37°C in a 5% CO2 incubator. Endothelium-denuded aortic rings were anchored to the organ chamber and the contractile force in response to phenylephrine was monitored. To inhibit NOS activity, aortic rings were incubated with total ginsenosides in the presence or absence of NLA (10 μM) or AG (30 μM) for 30 min, and then exposed to phenylephrine. The data represent the mean±s.e.m. of five separate experiments. GS was significantly different from the respective control (P<0.01). GS+NLA was significantly different from the respective GS alone (P<0.05 at 3 nM phenylephrine; P<0.01 at 10 nM–10 μM phenylephrine). GS+AG was significantly different from the respective GS alone (P<0.05 at 10 nM phenyl-ephrine; P<0.01 at 30 nM–10 μM phenylephrine). GS, total ginsenosides. (b) The effects of Rb1, Rc, Re, Rg1 or Rg3 on phenylephrine (10 μM)-induced contraction of arotic rings. The data represent the mean±s.e.m. of five separate experiments (significant as compared to control, **P<0.01). (c) The effects of NOS inhibitors on the suppression of phenylephrine-induced aortic contraction by Rg3 (10 μg ml−1). The data (the mean±s.e.m. of five separate experiments) represent the extent (%) of contraction relative to the maximal contraction induced by 90 mM KCl. Rg3 was significantly different from the respective control (P<0.01). Rg3+NLA was significantly different from the respective Rg3 alone (P<0.01). Rg3+AG was significantly different from the respective Rg3 alone (P<0.01 at 30 nM–10 μM phenylephrine).
Our previous studies have shown that Rg3 caused relaxation of rat aortic rings at the micromolar concentrations, which was dependent on the production of NO in vascular endothelium (Kim et al., 1999b). Among the triterpene ginsenosides isolated from P. ginseng, Rg3 is a component that activates eNOS in the endothelium (Kim et al., 1999b). In the present study, we examined the effects of representative purified ginsenosides on the vascular responses, which included Rg3, Rb1, Rc, Re and Rg1. Phenylephrine-induced aortic constriction was significantly inhibited by Rg3 (10 μg ml−1, 10 μ), but not by other ginsenosides (Figure 1b). Rg3 inhibited phenylephrine-induced vascular contraction in a concentration-dependent manner (10−9–10−5 M). Incubation of the excised aortas with 10 μg ml−1 Rg3 for 6 and 12 h resulted in 35 and 58% inhibition of vessel contraction, respectively. The inhibition of phenylephrine-induced vascular contraction by Rg3 was also abolished completely by NLA (10 μM) or partially by AG (30 μM) (Figure 1c).
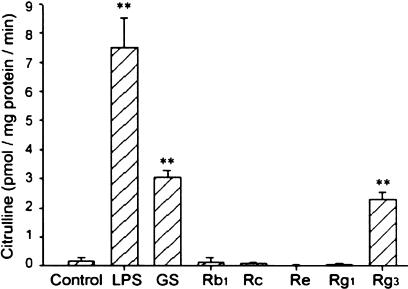
We then measured the extent of citrulline formation, which is generated from L-arginine with the production of NO, in the aortic rings that had been incubated with total or purified ginsenosides. The production of citrulline was greatly increased by treatment of aortic rings with total ginsenosides or Rg3 for 12 h, which was much smaller than that induced by LPS (1 μg ml−1, 12 h) (Figure 2). Rb1, Rc, Re or Rg1 did not increase citrulline production. Thus, total ginsenosides or Rg3 may induce NOS in the rat aorta.
Figure 2.
Production of citrulline in the rat aortic rings incubated with total or purified ginsenosides. Radiolabelled citrulline was measured in the homogenates prepared from the aortic ring incubated with total ginsenosides (100 μg ml−1), Rb1 (100 μg ml−1), Rc (100 μg ml−1), Re (100 μg ml−1), Rg1 (100 μg ml−1) or Rg3 (10 μg ml−1) for 12 h. The data represent the mean±s.e.m. of four separate experiments (significant as compared to control, **P<0.01). LPS, lipopolysaccharide; GS, total ginsenosides.
In vivo inhibition of vascular contractility by ginsenosides
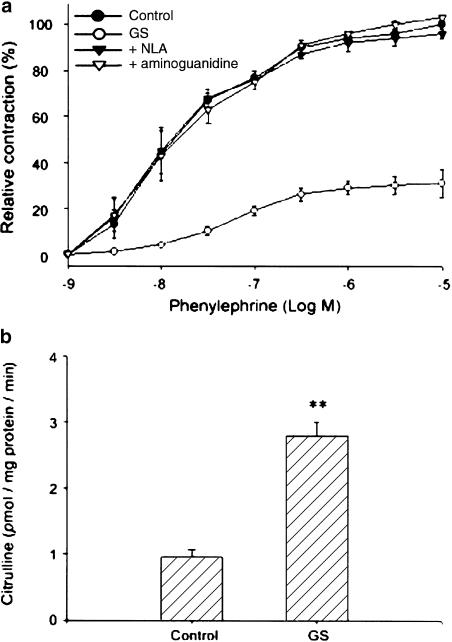
To determine whether ginsenosides inhibited vascular contraction through NO production, the reactivity of the aortas, excised from rats that had been orally administered with total ginsenosides (100 mg kg−1 per day, for 5 days), to phenyleph-rine was monitored. Phenylephrine-induced contraction of the aortic rings from ginsenoside-treated rats was significantly less than that from control animals (Figure 3a), resulting in ∼70% inhibition of the maximal contraction (P<0.01). Suppression of the aortic ring contraction was reversed completely by NLA (10 μM) or AG (30 μM) (Figure 3a).
Figure 3.
The in vivo effects of total ginsenosides on phenylephrine-induced contraction of aortic rings. (a) Phenylephrine-induced contraction of thoracic aortic rings. Endothelium-denuded aortic rings isolated from the rats treated with total ginsenosides (100 mg kg−1 per day, po) for 5 days were anchored to the organ chamber and the contractile force in response to phenylephrine was monitored. Aortic rings were preincubated with NLA (10 μM) or AG (30 μM) for 30 min for the inhibition of NOS activity, and then incubated with phenylephrine. The data were obtained as described in Figure 1a. (b) Citrulline production in the aorta excised from the rats treated with total ginsenosides. The radioactivity of [14C]-citrulline was measured by a liquid scintillation analyzer. The data represent the mean±s.e.m. with five separate experiments (significant as compared to control, **P<0.01). GS, total ginsenosides.
Next, we measured the levels of citrulline as an index of NOS activity in the aortic rings obtained from control or ginsenoside-treated animals. Production of citrulline, which parallels NO generation, was three-fold increased in the aortic rings excised from the rats treated with total ginsenosides, as compared to those from control animals (Figure 3b). These results showed that total ginsenosides or Rg3 causes vascular hyporesponsiveness to the vasoconstrictor via NO production. Immunoblot analysis verified that total ginsenosides or Rg3 induced NOS in rats (data not shown), which was in agreement with the increase in NO production.
Induction of iNOS by Rg3 in vascular smooth muscle
The effect of Rg3 on the expression of iNOS protein was determined in the vascular smooth muscle. Western blot analysis revealed that treatment of endothelium-denuded rat aortas with 100 μg ml−1 total ginsenosides for 8 h resulted in iNOS induction (Figure 4). We also observed that Rg3, but not Rb1, Rc, Re or Rg1, at the concentration of 100 μg ml−1 was active in inducing iNOS (Figure 4). iNOS was weakly induced by 10 μg ml−1 Rg3. The relatively weak induction of iNOS by 10 μg ml−1 Rg3 in the vascular smooth muscle may have resulted from dilution of the iNOS protein with other smooth muscle proteins (e.g. extracellular matrix and other types of cells). Nonetheless, the present data clearly demonstrated that total ginsenosides or Rg3 induced iNOS in the vascular smooth muscle. iNOS is induced in several types of cells including macrophages as well as smooth muscle cells. Since the mechanistic study of iNOS induction is difficult to accomplish in vascular smooth muscle isolated from animals, we used macrophage cells in the subsequent mechanistic experiments.
Figure 4.
iNOS induction by Rg3 in the vascular smooth muscle. The endothelium-denuded vascular smooth muscle excised from rats was incubated with total ginsenosides (100 μg ml−1), Rb1, Rc, Re, Rg1 (100 μg ml−1) or Rg3 (10–100 μg ml−1) for 8 h, and homogenated in 50 mM Tris-buffer (pH 7.5). The homogenates were centrifuged at 15,000 × g for 30 min to obtain the supernatant. The levels of iNOS in the cytosolic fractions were immunoblotted. Each lane was loaded with 30 μg of proteins. The results were confirmed by repeated experiments. GS, total ginsenosides.
Induction of iNOS by Rg3 in macrophage cells
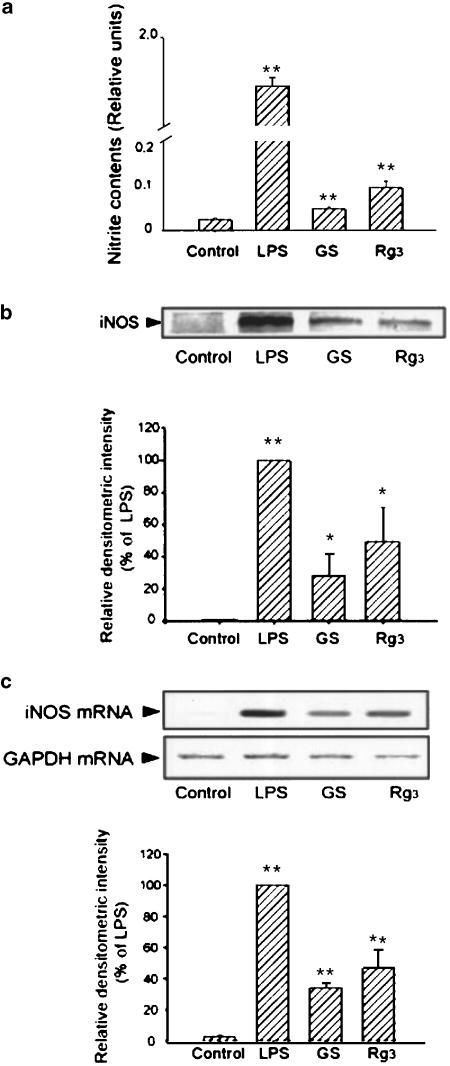
Production of nitrite was significantly increased in Raw264.7 cells incubated with total ginsenosides (100 μg ml−1, 24 h) or Rg3 (10 μg ml−1, 24 h), the extent of which, however, was smaller than that in LPS-treated cells (1 μg ml−1, 24 h) (Figure 5a). Western blot analysis was used to confirm that Rg3 induced iNOS in Raw264.7 cells. The expression of iNOS was increased in the cells treated with either ginsenosides or Rg3 (Figure 5b). The extent of iNOS induction by Rg3 was much less than that by LPS. RT–PCR analysis confirmed that the level of iNOS mRNA was increased 6 h after exposure of the cells to ginsenosides or Rg3 (10 μg ml−1) (Figure 5c). This was in parallel with the result of immunoblot analysis.
Figure 5.
iNOS induction by Rg3 in Raw264.7 cells. (a) NO production. Raw264.7 cells were incubated in the medium containing LPS (1 μg ml−1), total ginsenosides (100 μg ml−1) or Rg3 (10 μg ml−1) for 24 h, and the amount of nitrate and nitrite in the medium was assayed. (b) Immunoblot analysis for iNOS. The level of iNOS protein was immunochemically monitored in the lysates of the cells that had been treated with LPS, total ginsenosides or Rg3. Each lane was loaded with 30 μg of proteins. (c) RT–PCR analysis of iNOS mRNA. The expression of iNOS mRNA was assessed by RT–PCR analysis in the cells treated with LPS, total ginsenosides or Rg3 for 6 h. The levels of GAPDH mRNA were determined as control. The data represent the mean±s.d. of three separate experiments (significant as compared to control, *P<0.05, **P<0.01). LPS, lipopolysaccharide; GS, total ginsenosides.
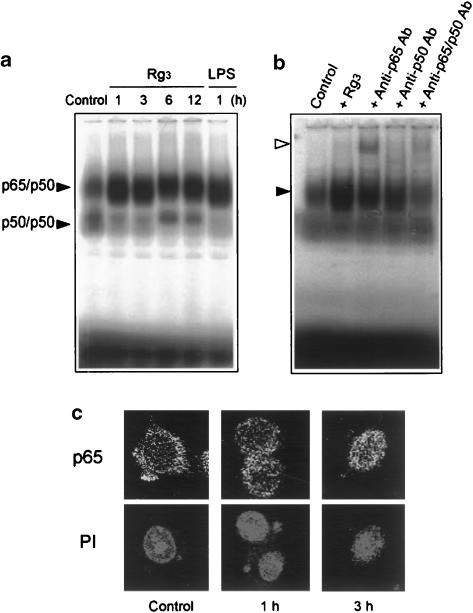
Activation of NF-κB by Rg3
To assess whether the induction of iNOS by Rg3 accompanied NF-κB activation, the nuclear extracts prepared from the Raw264.7 cells treated with Rg3 were probed with the radiolabeled NF-κB consensus oligonucleotide (Figure 6a). NF-κB was activated by Rg3 with the band of slow migrating complex being detected from 1 to 12 h. The intensity of the shifted band was identical to that obtained with the nuclear extracts from LPS-treated cells. Supershift analysis was carried out with anti-p65 and p50 antibodies. Addition of anti-p65 antibody to the reaction mixture induced supershift (Figure 6b). Either anti-p65 or anti-p50 antibody reduced the intensity of the retarded band. These data indicate that p65 and p50 proteins were the components active for the formation of NF-κB-binding complex. Since p65 was the major component of NF-κB activated by Rg3 in macrophages, immunocytochemistry was used to assess the nuclear translocation of p65 (Figure 6c). The Raw264.7 cells that had been incubated with Rg3 for 1–3 h were fixed and immunocytochemically stained. p65 protein was located predominantly in the cytoplasm of control cells. In contrast, the protein moved into the nucleus 3 h after Rg3 treatment. Nuclear integrity was confirmed using PI staining in the identical cells (Figure 6c).
Figure 6.
NF-κB activation by Rg3. (a) Gel shift analysis of NF-κB DNA binding. Gel shift analysis was performed with the nuclear extracts prepared from the Raw264.7 cells incubated with or without Rg3 (10 μg ml−1) for 1–12 h. Each lane contained 2 μg of nuclear extracts. LPS (1 μg ml−1, 1 h) was used for comparative purpose. (b) The specificity of NF-κB DNA binding was confirmed by a decrease in band intensity or supershift with anti-p65 or anti-p50 antibodies. The arrowheads show p65/p50 NF-κB complex (closed) and supershifted NF-κB (open). (c) The nuclear translocation of p65. Immunocytochemistry was performed with the Raw264.7 cells treated with Rg3 (10 μg ml−1) for 1–3 h. p65 migrated toward the nucleus or translocated into the nucleus at 1–3 h. The same fields were counterstained with propidium iodide (PI) to locate the nuclei. LPS, lipopolysaccharide.
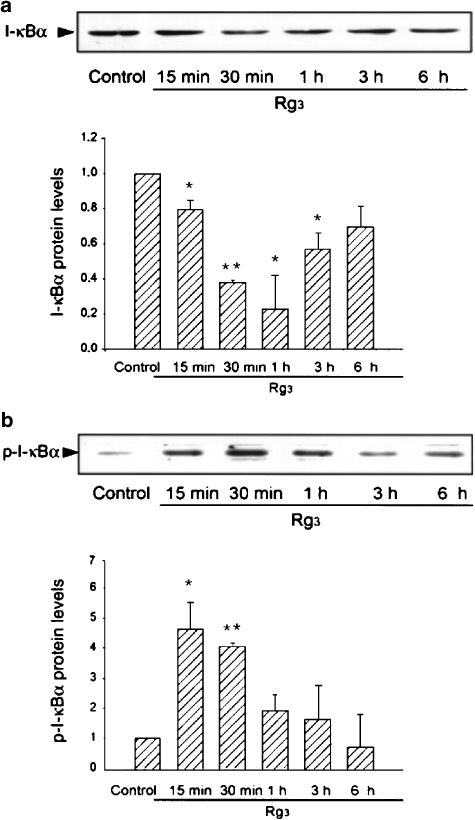
Phosphorylation of I-κBα by Rg3
Phosphorylation and proteolytic degradation of I-κBα precede the translocation of NF-κB to the nucleus. Studies were extended to determine whether NF-κB activation by Rg3 resulted from I-κBα degradation (Figure 7a). The levels of I-κBα were notably decreased 15 min–1 h after Rg3 treatment. Immunoblot analysis confirmed that Rg3 significantly induced phosphorylation of I-κBα from 15 to 30 min (Figure 7b). Thus, Rg3 increased I-κBα degradation via phosphorylation, which led to NF-κB activation.
Figure 7.
The effect of Rg3 on phosphorylation and degradation of I-κBα. (a) The levels of I-κBα. Immunoblots show the effects of Rg3 on the levels of I-κBα in Raw264.7 cells. The data represent the mean±s.d. of three separate experiments (significant as compared to control, *P<0.05, **P<0.01). (b) The levels of phosphorylated I-κBα in cells treated with Rg3. Immunoblots show the effects of Rg3 on the levels of phosphorylated I-κBα. A representative immunoblot is shown. p-I-κBα, phosphorylated I-κBα. The data represent the mean±s.e.m. of three separate experiments (significant as compared to control, *P<0.05, **P<0.01).
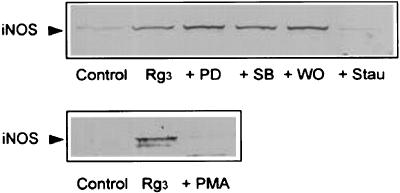
Role of protein kinase C (PKC) in the induction of iNOS by Rg3
Previous studies have shown that NF-κB activation is controlled by PKC (Shirakawa & Mizel, 1989; Kang et al., 2003). We next confirmed the role of PKC in iNOS induction by Rg3. Treatment of the Raw264.7 cells with staurosporine completely inhibited the induction of iNOS by Rg3 (Figure 8), whereas PD98059, SB203580 or wortmannin failed to inhibit iNOS induction. We have used the inhibitors in many Raw264.7 cell experiments and verified the inhibitory effects of PD98059, SB203580 or wortmannin on the respective kinases at the concentrations used (Cho et al., 2002, 2003; Kang et al., 2003; Lee et al., 2003). In addition, Rg3 did not induce iNOS in the cells pretreated with phorbol 12-myristate-13-acetate (PMA) for 18 h (i.e. PKC-depleted cells). These results indicated that PKC might play a role in iNOS induction by Rg3, presumably through NF-κB activation.
Figure 8.
The role of PKC in the induction of iNOS by Rg3. Raw264.7 cells were preincubated with PD98059 (PD, 50 μM), SB203580 (SB, 10 μM), wortmannin (WO, 250 nM) or staurosporine (Stau, 50 nM) for 1 h, and then further incubated with Rg3 (10 μg ml−1) for 18 h. The effect of Rg3 on the iNOS expression was also monitored in the cells that had been incubated with PMA (18 h) for PKC depletion. iNOS protein was immunoblotted in the cell lysates. Results were confirmed by repeated experiments and a representative blot is shown.
IL-1β and TNF-α expression in Raw264.7 cells
NF-κB is involved in the transcriptional activation of other genes including IL-1β and TNF-α (Baldwin, 1996). Studies were extended to determine whether total or purified ginsenosides were capable of inducing IL-1β and TNF-α in Raw264.7 cells. The levels of IL-1β or TNF-α were increased in the cells (1 × 107) treated with ginsenosides (100 μg ml−1) or Rg3 (10 μg ml−1), but not in those treated with Rb1, Rc, Re or Rg1 for 24 h (Table 1). The extents of IL-1β or TNF-α production by Rg3 were much smaller than that induced by LPS (1 μg ml−1). These results confirmed that Rg3 stimulates NF-κB-mediated gene expression.
Table 1.
The levels of IL-1β and TNF-α in the Raw264.7 cells treated with total or purified ginsenosides
| Treatment | Interleukin 1β (pg ml−1) | TNF-α (pg ml−1) |
|---|---|---|
| Control | 2.2±1.3 | 2.7±2.7 |
| LPS (1 μg ml−1) | 293.2±30.7** | 3187.9±266.6** |
| Ginsenosides (100 μg ml−1) | 29.7±0.7** | 147.6±67.0* |
| Rb1 (100 μg ml−1) | 0.5±0.5 | 3.3±2.1 |
| Rc (100 μg ml−1) | 0.3±0.3 | 3.3±3.3 |
| Re (100 μg ml−1) | 0.8±0.4 | 0 |
| Rg1 (100 μg ml−1) | 1.1±0.9 | 0 |
| Rg3 (10 μg ml−1) | 11.8±3.8* | 126.3±65.0* |
The levels of IL-1β and TNF-α in culture medium were assessed by ELISA 24 h after treatment of Raw264.7 cells with LPS or ginsenosides. Data represent the mean±s.e.m. (N=4) (significant as compared to control, *P<0.05, **P<0.01).
Discussion
Ginsenosides are considered to be the biologically active fraction of P. ginseng. In the present study, we report for the first time that ginsenoside Rg3, the level of which is increased after heat activation of P. ginseng (Kwon et al., 2001), served as the component active in relaxing the vessels contracted by phenylephrine, and that NO produced in the vessel was responsible for the vascular relaxation. The results of the present study clearly demonstrated that Rg3 specifically and completely reversed vascular contraction induced by phenyl-ephrine. In the present study, NLA or AG was used to inhibit iNOS activity. The slight difference in the inhibitory effect of AG on the vascular contraction induced by low concentrations of phenylephrine may have resulted from: (1) the effect of other ginsenoside(s) that may have the capability of producing NO to certain extents and/or (2) the relatively high variability of vessel contraction at the low concentrations of phenyl-ephrine.
NO production by Rg3 was, however, much smaller than that induced by LPS that interacts with membrane receptors including CD14 (mCD14) (Wright et al., 1990). The role of NO production in vascular relaxation by Rg3 is presumed to result from the induction of NOS in the vascular smooth muscle, which is supported by the decrease in contraction of the endothelium-denuded aortic rings from the rats treated with ginsenosides or Rg3, and also by the enhanced NO production in aortic vessels. Studies have shown that NOS is inducible in the aortic smooth muscle (Marczin et al., 1993; Sirsjo et al., 1994). We found that total ginsenosides, particularly Rg3, stimulated NO production in both vascular smooth muscle and macrophages, and caused iNOS induction with the increase in mRNA. Thus, Rg3 was capable of directly stimulating the expression of the iNOS gene.
Polysaccharides obtained from P. ginseng extracts induce the production of interferon-γ and TNF-α (Gao et al., 1996). NO production may contribute to the enhancement of host resistance due to ginseng treatment. In particular, the results of the present study show that Rg3 of those present in total ginsenosides serves as a component active in relaxing vessels. Conversely, NO also plays a role in pathologic mechanisms (Wong & Billiar, 1995). Excess NO activates the immune system with the production of inflammatory cytokines, and hence exerts proinflammatory effects (Andrew et al., 1999). In view of the implication of NO in immunomodulation, NOS induction by Rg3 may also contribute to the innate immune function.
The expression of the iNOS gene is regulated by the binding of transcriptional factors to several consensus sequences of the gene. The promoter region of the iNOS gene contains the binding sites for NF-κB, AP-1 and C/EBP. Of these, NF-κB is the essential component for the expression of the iNOS gene (Xie et al., 1993, 1994). NF-κB activation is induced by the signals controlled by PKC and phosphoinositide 3-kinase downstream of G proteins including Gαq and Gβγ, respectively (Rahman et al., 2002). In the present study, we found that the extent of NF-κB activation by Rg3 was comparable to that by LPS, although Rg3-induced iNOS expression was much smaller than that by LPS, suggesting that the signaling pathway(s) for the iNOS induction by Rg3 may be specific. We found that C/EBP DNA binding was not noticeably increased and that Rg3 failed to induce COX-2, the expression of which was increased primarily by C/EBP and potentially by NF-κB (Kang & Kim, unpublished data). Thus, the mechanism for iNOS induction by Rg3 appears to differ from that by LPS. The present study verified that Rg3, but not other representative purified ginsenosides, stimulates the IL-1β and TNF-α gene expression. Thus, Rg3 may stimulate a series of genes whose expression is activated by NF-κB.
NF-κB is controlled by the pathway involving Akt and IKKβ downstream of PKC. To explore a part of the mechanistic basis of Rg3-induced NF-κB activation, we immunocytochemically monitored the subcellular localization of p65 in macrophages. The nuclear translocation of p65 protein in response to Rg3 parallels the increase in the band intensity of NF-κB DNA binding. The level of I-κBα was also assessed to infer if activation of NF-κB resulted from degradation of I-κBα. Both gel shift and immunoblot analyses provide evidence that Rg3 stimulates I-κBα degradation with the increase in the phosphorylation of I-κBα.
The signaling pathways for the induction of iNOS have been intensely studied. PKC has been shown to play a role in a wide variety of cellular functions such as the regulation of receptor interaction with components of the signal transduction apparatus and gene expression (Nishizuka, 1988). Gene structures of PKC isozymes are classified as conventional (c) and novel (n) PKC. cPKCs require Ca2+ for activation, whereas nPKCs are independent of Ca2+. PKC is involved in the induction of iNOS in some other type of cells. Phorbol ester (i.e. PKC activator) potentiated the induction of iNOS in the cells primed with interferon-γ (Momose et al., 2000). Li et al. (2000) have shown that ginsenosides induce eNOS in porcine vascular endothelial cells, which was claimed to be possibly associated with the elevation of intracellular calcium. The induction of iNOS by Rg3 in macrophages was not inhibited by a calcium channel blocker verapamil or a competitor of calcium entry gadolinium chloride (data not shown). Thus, it is unlikely that iNOS induction by Rg3 results from the stimulation of calcium influx. The result of the present study revealed that Rg3-inducible iNOS expression was inhibited by PKC inhibition or depletion, raising the possibility that Rg3 interacts with membrane-associated physiological mediators for PKC activation presumably due to its amphiphilicity. It is also possible that Rg3 stimulates the protein assembly in the plasma membrane. We found that an MKK inhibitor PD98059, p38 kinase inhibitor SB203580 and phosphatidylinositol 3-kinase inhibitor wortmannin did not inhibit Rg3-inducible iNOS expression, which suggests that the molecular target of Rg3 be included in the pathway responsible for the activation of PKC.
Three distinct mammalian MAP kinase modules including c-Jun NH2-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38 mitogen-activated protein (MAP) kinase have been characterized (Cahill et al., 1996; Treisman, 1996). Among these kinases, ERK or p38 kinase is involved in the expression of iNOS by LPS or serum deprivation in macrophages (Ajizian et al., 1999; Liu et al., 2001). Phosphatidyl-inositol 3-kinase, which phosphorylates phosphatidylinositols at the 3 position of the inositol ring, is associated with the activation of cellular survival signals, mitogenesis and cell transformation (Daulhac et al., 1999). Phosphatidylinositol 3-kinase is involved in NO production presumably through p70S6 kinase as a downstream kinase (Salh et al., 1998). However, mitogen-activated protein kinases or the PI3-kinase inhibitor failed to alter iNOS induction by Rg3. Rg3 may act on the pathway involving PKC, but not on the other pathways.
Pharmacokinetic studies showed that Rg3 is rapidly absorbed in man and exhibits the first-order kinetic characteristics (Pang et al., 2001). Considering the physicochemical properties and the species difference in pharmacokinetics of the ginsenosides (e.g. lipophilicity and membrane distribution), the concentration used in this study (10 μg ml−1, 10 μM) seems to be relevant to clinical situations.
In conclusion, Rg3, a component present in total ginsenosides, induces vascular relaxation as a consequence of NO production, to which NOS induction contributes, and the induction of NOS by Rg3 accompanied NF-κB activation, which involves phosphorylation and degradation of I-κBα and nuclear translocation of p65.
Acknowledgments
This work was supported in part by The National Academy of Sciences, Korea, and in part by a grant 00-CH-14-0006 of the 2000' Good Health RND Project, Ministry of Health and Welfare, Republic of Korea.
Abbreviations
- AG
aminoguanidine
- iNOS
inducible nitric oxide synthase
- I-κB
inhibitor-κB
- NF-κB
nuclear factor-κB
- NLA
NG-nitro-L-arginine
- NO
nitric oxide
- PKC
protein kinase C
- PMA
phorbol 12-myristate-13-acetate
- Rg3
ginsenoside Rg3
- TNF-α
tumor necrosis factor-α
References
- AJIZIAN S.J., ENGLISH B.K., MEALS E.A. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-γ. J. Infect. Dis. 1999;179:939–944. doi: 10.1086/314659. [DOI] [PubMed] [Google Scholar]
- ANDREW P.J., HARANT H., LINDLEY I.J. Up-regulation of interleukin-1β-stimulated interleukin-8 in human keratinocytes by nitric oxide. Biochem. Pharmacol. 1999;57:1423–1429. doi: 10.1016/s0006-2952(99)00052-0. [DOI] [PubMed] [Google Scholar]
- BALDWIN A.S., Jr The NF-κB and I-κB proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- CAHILL M.A., JANKNECHT R., NORDHEIM A. Signalling pathways: jack of all cascades. Curr. Biol. 1996;6:16–19. doi: 10.1016/s0960-9822(02)00410-4. [DOI] [PubMed] [Google Scholar]
- CHAN E.D., CHAN J., SCHLUGER N.W. What is the role of nitric oxide in murine and human host defense against tuberculosis? Current knowledge. Am. J. Respir. Cell Mol. Biol. 2001;25:606–612. doi: 10.1165/ajrcmb.25.5.4487. [DOI] [PubMed] [Google Scholar]
- CHAN M.M., FONG D., HO C.T., HUANG H.I. Inhibition of inducible nitric oxide synthase gene expression and enzyme activity by epigallocatechin gallate, a natural product from green tea. Biochem. Pharmacol. 1997;54:1281–1286. doi: 10.1016/s0006-2952(97)00504-2. [DOI] [PubMed] [Google Scholar]
- CHO M.K., SUH S.H., KIM S.G. JunB/AP-1 and NF-kappa B-mediated induction of nitric oxide synthase by bovine type I collagen in serum-stimulated murine macrophages. Nitric Oxide. 2002;6:319–332. doi: 10.1006/niox.2001.0415. [DOI] [PubMed] [Google Scholar]
- CHO Y.H., LEE C.H., KIM S.G. Potentiation of lipopolysaccharide-inducible cyclooxygenase 2 expression by C2-ceramide via c-Jun N-terminal kinase-mediated activation of CCAAT/enhancer binding protein beta in macrophages. Mol. Pharmacol. 2003;63:512–523. doi: 10.1124/mol.63.3.512. [DOI] [PubMed] [Google Scholar]
- DAULHAC L., KOWALSKI-CHAUVEL A., PRADAYROL L., VAYSSE N., SEVA C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J. Biol. Chem. 1999;274:20657–20663. doi: 10.1074/jbc.274.29.20657. [DOI] [PubMed] [Google Scholar]
- FRIEDL R., MOESLINGER T., KOPP B., SPIECKERMANN P.G. Stimulation of nitric oxide synthesis by the aqueous extract of Panax ginseng root in Raw264.7 cells. Br. J. Pharmacol. 2001;134:1663–1670. doi: 10.1038/sj.bjp.0704425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO H., WANG F., LIEN E.J., TROUSDALE M.D. Immunostimulating polysaccharides from Panax notoginseng. Pharm. Res. 1996;13:1196–1200. doi: 10.1023/a:1016060119425. [DOI] [PubMed] [Google Scholar]
- HAN K.H., CHOE S.C., KIM H.S., SOHN D.W., NAM K.Y., OH B.H., LEE M.M., PARK Y.B., CHOI Y.S., SEO J.D., LEE Y.W. Effect of red ginseng on blood pressure in patients with essential hypertension and white coat hypertension. Am. J. Chin. Med. 1998;26:199–209. doi: 10.1142/S0192415X98000257. [DOI] [PubMed] [Google Scholar]
- JEON B.H., KIM C.S., PARK K.S., LEE J.W., PARK J.B., KIM K.J., KIM S.H., CHANG S.J., NAM K.Y. Effect of Korea red ginseng on the blood pressure in conscious hypertensive rats. Gen. Pharmacol. 2000;35:135–141. doi: 10.1016/s0306-3623(01)00096-9. [DOI] [PubMed] [Google Scholar]
- KANG K.W., CHOI S.Y., CHO M.K., LEE C.H., KIM S.G. Thrombin induces nitric oxide synthase via Gα12/13-coupled PKC-dependent I-κBα phosphorylation and JNK-mediated I-κBα degradation. J. Biol. Chem. 2003;278:17368–17378. doi: 10.1074/jbc.M300471200. [DOI] [PubMed] [Google Scholar]
- KIM N.D., KANG S.Y., KIM M.J., PARK J.H., SCHINI-KERTH V.B. The ginsenoside Rg3 evokes endothelium-independent relaxation in rat aortic rings: role of K+ channels. Eur. J. Pharmacol. 1999a;367:51–57. doi: 10.1016/s0014-2999(98)00899-1. [DOI] [PubMed] [Google Scholar]
- KIM N.D., KANG S.Y., PARK J.H., SCHINI-KERTH V.B. Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta: role of K+ channels. Eur. J. Pharmacol. 1999b;367:41–49. doi: 10.1016/s0014-2999(98)00898-x. [DOI] [PubMed] [Google Scholar]
- KIM N.D., KANG S.Y., SCHINI V.B. Ginsenosides evoke endothelium-dependent vascular relaxation in rat aorta. Gen. Pharmacol. 1994;25:1071–1077. doi: 10.1016/0306-3623(94)90121-x. [DOI] [PubMed] [Google Scholar]
- KIM S.G., NAM S.Y., KIM J.H., CHO C.K., YOO S.Y. Enhancement of radiation-inducible hepatic glutathione S-trans-ferase Ya1, Yb1, Yb2, Yc1, and Yc2 expression by oltipraz: possible role in radioprotection. Mol. Pharmacol. 1997;51:225–233. doi: 10.1124/mol.51.2.225. [DOI] [PubMed] [Google Scholar]
- KIM W.Y., KIM J.M., HAN S.B., LEE S.K., KIM N.D., PARK M.K., KIM C.K., PARK J.H. Steaming of ginseng at high temperature enhances biological activity. J. Nat. Prod. 2000;63:1702–1704. doi: 10.1021/np990152b. [DOI] [PubMed] [Google Scholar]
- KUBES P. Inducible nitric oxide synthase: a little bit of good in all of us. Gut. 2000;47:6–9. doi: 10.1136/gut.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWON S.W., HAN S.B., PARK I.H., KIM J.M., PARK M.K., PARK J.H. Liquid chromatographic determination of less polar ginsenosides in processed ginseng. J. Chromatogr. A. 2001;921:335–339. doi: 10.1016/s0021-9673(01)00869-x. [DOI] [PubMed] [Google Scholar]
- LEE A.K., SUNG S.H., KIM Y.C., KIM S.G. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-alpha and COX-2 expression by sauchinone effects on I-kappa B alpha phosphorylation, C/EBP and AP-1 activation. Br. J. Pharmacol. 2003;139:11–20. doi: 10.1038/sj.bjp.0705231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Z., NIWA Y., SAKAMOTO S., SHONO M., CHEN X., NAKAYA Y. Induction of inducible nitric oxide synthase by ginsenosides in cultured porcine endothelial cells. Life Sci. 2000;67:2983–2989. doi: 10.1016/s0024-3205(00)00880-8. [DOI] [PubMed] [Google Scholar]
- LIU W., KATO M., ITOIGAWA M., MURAKAMI H., YAJIMA M., WU J., ISHIKAWA N., NAKASHIMA I. Distinct involvement of NF-κB and p38 mitogen-activated protein kinase pathways in serum deprivation-mediated stimulation of inducible nitric oxide synthase and its inhibition by 4-hydroxynonenal. J. Cell. Biochem. 2001;83:271–280. doi: 10.1002/jcb.1234. [DOI] [PubMed] [Google Scholar]
- MARCZIN N., PAPAPETROPOULOS A., CATRAVAS J.D. Tyrosine kinase inhibitors suppress endotoxin- and IL-1β-induced NO synthesis in aortic smooth muscle cells. Am. J. Physiol. 1993;265:H1014–H1018. doi: 10.1152/ajpheart.1993.265.3.H1014. [DOI] [PubMed] [Google Scholar]
- MOMOSE I., TERASHIMA M., NAKASHIMA Y., SAKAMOTO M., ISHINO H., NABIKA T., HOSOKAWA Y., TANIGAWA Y. Phorbol ester synergistically increases interferon regulatory factor-1 and inducible nitric oxide synthase induction in interferon-γ-treated Raw264.7 cells. Biochim. Biophys. Acta. 2000;1498:19–31. doi: 10.1016/s0167-4889(00)00072-0. [DOI] [PubMed] [Google Scholar]
- NISHIZUKA Y. The molecular heterogeneity of protein kinase C and its implication for cellular regulation. Nature. 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- PALMER R.M., ASHTON D.S., MONCADA S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988;333:664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- PANG H., WANG H.L., FU L., SU C.Y. Pharmacokinetic studies of 20(R)-ginsenoside Rg3 in human volunteers. Yao Xue Xue Bao. 2001;36:170–173. [PubMed] [Google Scholar]
- RAHMAN A., TRUE A.L., ANWAR K.N., YE R.D., VOYNO-YASENETSKAYA T.A., MALIK A.B. Gα(q) and Gβγ regulate PAR-1 signaling of thrombin-induced NF-κB activation and ICAM-1 transcription in endothelial cells. Circ. Res. 2002;91:398–405. doi: 10.1161/01.res.0000033520.95242.a2. [DOI] [PubMed] [Google Scholar]
- SALH B., WAGEY R., MAROTTA A., TAO J.S., PELECH S. Activation of phosphatidylinositol 3-kinase, protein kinase B, and p70 S6 kinases in lipopolysaccharide-stimulated Raw 264.7 cells: differential effects of rapamycin, LY294002, and wortmannin on nitric oxide production. J. Immunol. 1998;161:6947–6954. [PubMed] [Google Scholar]
- SCHREIBER E., HARSHMAN K., KEMLER I., MALIPIERO U., SCHAFFNER W., FONTANA A. Astrocytes and glioblastoma cells express novel octamer-DNA binding proteins distinct from the ubiquitous Oct-1 and B cell type Oct-2 proteins. Nucleic Acids Res. 1990;18:5495–5503. doi: 10.1093/nar/18.18.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA S., TANAKA O., ANDO T., SADO M., TSUSHIMA S., OHSAWA T. Chemical studies on oriental plant drugs. XIV. Protopanaxadiol, a genuine sapogenin of ginseng saponins. Chem. Pharm. Bull. 1966;14:595–600. doi: 10.1248/cpb.14.595. [DOI] [PubMed] [Google Scholar]
- SHIRAKAWA F., MIZEL S.B. In vitro activation and nuclear translocation of NF-κB catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol. Cell. Biol. 1989;9:2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRSJO A., SODERKVIST P., SUNDQVIST T., CARLSSON M., OST M., GIDLOF A. Different induction mechanisms of mRNA for inducible nitric oxide synthase in rat smooth muscle cells in culture and in aortic strips. FEBS Lett. 1994;338:191–196. doi: 10.1016/0014-5793(94)80363-3. [DOI] [PubMed] [Google Scholar]
- SUNG J., HAN K.H., ZO J.H., PARK H.J., KIM C.H., OH B.H. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am. J. Chin. Med. 2000;28:205–216. doi: 10.1142/S0192415X00000258. [DOI] [PubMed] [Google Scholar]
- SZABO C., OHSHIMA H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide. 1997;1:373–385. doi: 10.1006/niox.1997.0143. [DOI] [PubMed] [Google Scholar]
- TODA N., AYAJIKI K., FUJIOKA H., OKAMURA T. Ginsenoside potentiates NO-mediated neurogenic vasodilatation of monkey cerebral arteries. J. Ethnopharmacol. 2001;76:109–113. doi: 10.1016/s0378-8741(01)00217-3. [DOI] [PubMed] [Google Scholar]
- TREISMAN R. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- WONG J.M., BILLIAR T.R. Regulation and function of inducible nitric oxide synthase during sepsis and acute inflammation. Adv. Pharmacol. 1995;34:155–170. doi: 10.1016/s1054-3589(08)61084-4. [DOI] [PubMed] [Google Scholar]
- WRIGHT S.D., RAMOS R.A., TOBIAS P.S., ULEVITCH R.J., MATHISON J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- XIE Q.W., KASHIWABARA Y., NATHAN C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- XIE Q.W., WHISNANT R., NATHAN C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon-γ and bacterial lipopolysaccha-ride. J. Exp. Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU X., POTOKA D.A., BOYLE P., NADLER E.P., MCGINNIS K., FORD H.R. Aminoguanidine renders inducible nitric oxide synthase knockout mice more susceptible to Salmonella typhimurium infection. FEMS Microbiol. Lett. 2002;206:93–97. doi: 10.1111/j.1574-6968.2002.tb10992.x. [DOI] [PubMed] [Google Scholar]