Abstract
The present study investigated the mechanisms involved in the increased 5-hydroxytryptamine (5-HT) vasoconstriction observed in rat middle cerebral arteries exposed in vitro to lipopolysaccharide (LPS, 10 μg ml−1) for 1–5 h. Functional, immunohistochemical and Western blot analysis and superoxide anion measurements by ethidium fluorescence were performed.
LPS exposure increased 5-HT (10 μM) vasoconstriction only during the first 4 h. In contrast to control tissue, indomethacin (10 μM), the COX-2 inhibitor NS 398 (10 μM), the TXA2/PGH2 receptor antagonist SQ 29,548 (1 μM) and the TXA2 synthase inhibitor furegrelate (1 μM) reduced 5-HT contraction of LPS-treated arteries from hour one. The iNOS inhibitor aminoguanidine (0.1 mM) increased 5-HT contraction from hour three of LPS incubation.
The superoxide anion scavenger superoxide dismutase (SOD, 100 U ml−1) and the H2O2 scavenger catalase (1000 U ml−1), as well as the respective inhibitors of NAD(P)H oxidase and xanthine oxidase, apocynin (0.3 mM) and allopurinol (0.3 mM), reduced 5-HT contraction after LPS incubation. LPS induced an increase in superoxide anion levels that was abolished by PEG-SOD.
Subthreshold concentrations of the TXA2 analogue U 46619, xanthine/xanthine oxidase and H2O2 potentiated, whereas those of sodium nitroprusside inhibited, the 5-HT contraction.
COX-2 expression was increased at 1 and 5 h of LPS incubation, while that of iNOS, Cu/Zn-SOD and Mn-SOD was only increased after 5 h. All the three vascular layers expressed COX-2 and Cu/Zn-SOD. iNOS expression was detected in the endothelium and adventitia after LPS.
In conclusion, increased production of TXA2 from COX-2, superoxide anion and H2O2 enhanced vasoconstriction to 5-HT during the first few hours of LPS exposure; iNOS and SOD expression counteracted that increase at 5 h. These changes can contribute to the disturbance of cerebral blood flow in endotoxic shock.
Keywords: Lipopolysaccharide, middle cerebral artery, nitric oxide, thromboxane, oxygen radical
Introduction
Endotoxic shock is associated to an early decrease in cerebral blood flow and perfusion pressure, and an increase in cerebrovascular resistance (Parker & Emerson, 1977; Ekström-Jodal et al., 1982), in spite of systemic hypotension. These cerebrovascular alterations would be involved in the impaired neurological function found in sepsis. Topical (Brian et al., 1995; 1998) and intracerebroventricular (Okamoto et al., 1998) administration of lipopolysaccharide (LPS), a component of the outer membrane of Gram-negative bacteria, produces vasodilatation of cerebral arterioles. However, the results obtained in cerebral arterioles cannot be extrapolated to large arteries, because different mechanisms are involved in the regulation of its vascular tone (Faraci & Heistad, 1990). On the other hand, large cerebral arteries contribute importantly to total cerebrovascular resistance, and are the main determinants of local microvascular pressure (Faraci & Heistad, 1990).
Expression of inducible isoforms of nitric oxide synthase (iNOS) and cyclooxygenase (COX-2) has been generally associated with several pathological events that occur in sepsis (Kirkeboen & Strand, 1999; Ermert et al., 2000; Yamagata et al., 2001) and underlie the vasodilatation of cerebral arterioles after LPS exposure (Brian et al., 1995; 1998; Okamoto et al., 1998). Furthermore, the increased production of superoxide anion and other strongly vasoactive reactive oxygen species is also responsible for vascular alterations in sepsis (Brandes et al., 1999). However, it is unclear whether iNOS- and COX-2-derived metabolites and reactive oxygen species are involved in the cerebral vasoconstriction observed during the early phases of endotoxic shock.
5-hydroxytryptamine (5-HT) induces potent vasoconstrictor responses in large cerebral vessels mainly through 5-HT2A receptor activation (Roon et al., 1999), and is involved in the control of cerebrovascular tone (Lincoln, 1995). Moreover, endotoxin administration was found to increase cerebral vascular resistance in dogs through a 5-HT receptor-mediated mechanism (Ekström-Jodal et al., 1982). In contrast to previous studies where PGF2α contraction of rat cerebral arteries was decreased (Briones et al., 2002a), our preliminary experiments also revealed that LPS treatment enhanced 5-HT contraction in rat cerebral arteries.
Therefore, the purpose of the present study was to investigate the mechanisms involved in the enhanced 5-HT contractions induced by LPS in rat middle cerebral arteries (MCAs). The role of iNOS- and COX-2-derived products and reactive oxygen species in the effects of LPS on 5-HT responses in isolated rat MCAs was analysed. To our knowledge, this is the first study in which the participation of all these mediators in the LPS effect has been studied in large cerebral arteries.
Methods
Male Wistar Kyoto rats, 6-month old, were obtained from the Animal Quarters of the Facultad de Medicina of the Universidad Autónoma de Madrid. Rats were euthanized by decapitation. MCAs were dissected for functional studies, immunohistochemistry, superoxide anion measurement and cytosolic Cu/Zn-superoxide dismutase (SOD) and Mn-SOD protein expression. All arteries from the circle of Willis were used for iNOS and COX-2 protein expression analysis to obtain a sufficient amount of protein.
All experiments comply with the current Spanish and European laws (RD 223/88 MAPA and 609/86).
Reactivity experiments
MCA segments (2 mm in length, 234±2 μm diameter, n=67) were fitted with two 40 μm wires and mounted in a small-vessel dual-chamber myograph for measurement of isometric tension, according to the method described by Mulvany & Halpern (1977). After a 30 min equilibration period in Krebs–Henseleit solution (KHS, see composition below) bubbled with a 95% O2–5% CO2 mixture at 37°C and pH=7.4, the segments were stretched to their optimal lumen diameter for active tension development. This was determined based on the internal circumference–wall tension ratio of the segments by setting their internal circumference, L0, to 90% of the circumference the vessels would have if they were exposed to a passive tension that was equivalent to that produced by a transmural pressure of 100 mmHg (Mulvany & Halpern, 1977). The effective lumen diameter was calculated as L0/π. Afterwards, the segments were washed and left to equilibrate for 30 min. Then, segment contractility was tested by an initial exposure to a high K+ solution (120 mM K+-KHS).
Once the presence of endothelium was determined by the ability of 1 μM bradykinin to relax the segments precontracted with 10 μM 5-HT, five consecutive additions of 5-HT were performed at 1 h intervals in the presence or absence of 10 μg ml−1 LPS. To analyse the participation of NO from iNOS on the effect of LPS, aminoguanidine was used. The participation of COX-derived prostanoids was analysed using indomethacin, NS 398, SQ 29,548 and furegrelate; the involvement of reactive oxygen species was studied using allopurinol, apocynin, SOD and catalase. All drugs were added 30 min before the corresponding 5-HT administration.
In some experiments, concentration–response curves for U 46619 and H2O2 were performed. The effect of superoxide anion exogenously generated by xanthine plus xanthine oxidase was studied; catalase was added to scavenge the generated H2O2. Paired experiments were designed to analyse the effects of U 46619, exogenously generated superoxide anion, H2O2 and the NO donor, sodium nitroprusside (SNP), on 5-HT vasoconstrictor responses. For this, two successive administrations of 5-HT at 1 h intervals were made and subthreshold concentrations of those drugs were added 3 min before the second 5-HT administration.
At the end of the experiment, KHS was replaced by 120 mM K+-KHS; once the contraction was stable, 0.1 mM papaverine was added.
Immunohistochemistry
At the end of the functional studies, arteries were fixed with cold (4°C) 4% paraformaldehyde, pH 7.0, for 1 h and embedded in paraffin. Longitudinal sections 5 μm thick were obtained using a rotation microtome and placed on glass slides. The sections were processed following the avidin–biotin–peroxidase method (Hsu & Raine, 1981). Thus, the sections were incubated overnight at 4°C in a humidified chamber with either mouse monoclonal antibody anti-iNOS (1 : 200, Transduction Laboratories, Lexington, U.K.), rabbit polyclonal antibody anti-COX-2 (1 : 700, Cayman Chemical, Ann Arbor, MI, U.S.A.), anti-Cu/Zn-SOD (1 : 1000, StressGen, Victoria, Canada) or anti-Mn-SOD (1 : 4000, StressGen) diluted in 1% bovine serum albumin. Then, the sections were reacted with biotinylated anti-mouse or anti-rabbit immunoglobulins for 20 min at room temperature and incubated with streptavidin, which was conjugated to horseradish peroxidase for 20 min at room temperature (LSAB 2 kit for rat tissue, DAKO, Denmark); finally, the immunocomplex was visualized as a brown product after incubating with 0.05% 3,3-diamino-benzidine and 0.02% H2O2. Controls were obtained using MCA sections that had been incubated without the corresponding primary antibody.
Western blot
Cerebral arteries were incubated for 1 or 5 h in oxygenated KHS (37°C) with or without LPS. Afterwards, the arteries were quick-frozen in liquid nitrogen and kept at −70°C until protein expression analysis.
Proteins from homogenized cerebral arteries (15 μg for iNOS and COX-2 and 5 μg for Cu/Zn- and Mn-SOD) were separated by 7.5% (iNOS and COX-2) or 12% (Cu/Zn- and Mn-SOD) sodium dodecylsulphate (SDS)–polyacrylamide gel electrophoresis. Proteins were transferred to polyvinyl difluoride membranes overnight and then the membranes were incubated with mouse monoclonal antibody for iNOS (1 : 10,000), rabbit polyclonal antibody for COX-2 (1 : 250), Cu/Zn-SOD (0.05 μg ml−1) or Mn-SOD (0.02 μg ml−1). After washing, membranes were incubated with antimouse (1 : 2000) or anti-rabbit (1 : 2000) IgG antibody conjugated to horseradish peroxidase. The immunocomplexes were detected using an enhanced horseradish peroxidase/luminol chemiluminiscence system (ECL Plus, Amersham International plc, Little Chalfont, U.K.) and subjected to autoradiography (Hyperfilm ECL, Amersham International plc). Signals on the immunoblot were quantified using a computer program (NIH Image V1.56). The same membrane was used to determine α-actin expression using a mouse monoclonal antibody (1 : 3,000,000, Boehringer Mannheim, Mannheim, Germany).
Stimulated mouse macrophage homogenates were used as a positive control for iNOS and COX-2, and rat brain homogenates were used as a positive control for Cu/Zn- and Mn-SOD.
Superoxide anion measurement
The oxidative fluorescent dye hydroethidine was used to evaluate the in situ production of superoxide anion, as described previously (Miller et al., 1998). Hydroethidine is freely permeable to cells and, in the presence of superoxide anion, is oxidized to ethidium bromide which is trapped by intercalation with DNA. Ethidium bromide is excited at 488 nm and has an emission spectrum of 610 nm.
At the end of the functional studies, segments removed from the myographs were immersed in 30% saccharose for 30 min; after that, segments were immersed in the embedding medium Tissue Tek OCT (Bayer Química Farmacéutica, Barcelona, Spain), frozen in liquid nitrogen and kept at −70°C until superoxide anion measurement. Frozen ring segments were cut into 14 μm-thick sections and placed on a glass slide. Serial sections were equilibrated under identical conditions for 30 min at 37°C in Krebs-HEPES buffer. Fresh buffer containing hydroethidine (2 μM) was topically applied to each tissue section and coverslipped. Slides were incubated in a light-protected humidified chamber at 37°C for 30 min. Images were obtained with a Leica TCS SP2 laser-scanning confocal microscope equipped with a krypton/argon laser. Control- and LPS-incubated tissues were processed and imaged in parallel. Laser settings were identical for acquisition of images from all specimens. Fluorescence was detected with a 543 nm long-pass filter. Autofluorescence of the internal elastic lamina was detected with the 488 nm line of the microscope. In each experiment, some of the sequentially cut sections from LPS-treated arteries were incubated for 2 h with polyethylene glycol-conjugated SOD (PEG-SOD, 500 U ml−1).
Data analysis and statistics
Vasoconstrictor responses induced by the successive additions of 5-HT in the absence and presence of LPS were calculated as active wall tension (Mulvany & Halpern, 1977), and expressed as a percentage of the contraction elicited by the first 5-HT administration, which was considered the contraction at 0 h. Some results were expressed as a percentage of maximum response determined as the difference between the tone generated by 120 mM K+-KHS and that produced by 0.1 mM papaverine (2.1±0.1 N m−1, n=67).
Data for protein expression are expressed as the ratio between the immunoblot signals for the studied protein and the α-actin signals.
Results are expressed as mean±s.e.m. for the number of rats indicated in each case. Statistically significant differences for paired or unpaired experiments were calculated by means of Student's t-test with Bonferroni corrections for multiple comparisons. The effect of the incubation time was analysed by two-way analysis of variance (ANOVA). A P-value <0.05 was considered statistically significant.
Drugs and solutions
KHS contained (mM): 115 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4·7H2O, 2.5 CaCl2, 1.2 KH2PO4, 11.1 glucose and 0.01 Na2EDTA. The drugs used were 5-HT creatinine sulphate, indomethacin, aminoguanidine hemisulphate, furegrelate, SOD, PEG-SOD, allopurinol, apocynin, catalase, bradykinin acetate, LPS (E. coli, serotype 055:B5), xanthine sodium salt, xanthine oxidase, hydroethidine, H2O2, bovine serum albumin and SNP dihydrate (Sigma Chemical Co., St Louis, MO, U.S.A.); NS 398 and U 46619 (Calbiochem-Novabiochem GmbH, Bad Soden, Germany); SQ 29,548 (ICN Iberica, Barcelona, Spain); SDS and acrylamide (Bio-Rad Laboratories, Hercules, CA, U.S.A.); xylene (Merck Eurolab, Albertslund, Denmark); paraformaldehyde (Kebo Lab, Albertslund, Denmark); ethanol (De Danske Sprit, Aalborg, Denmark); Mayer's acid haematoxylin (Bie&Amp, Berntsen, Hojbjerg, Denmark) and 3,3-diamino-benzidine (Dako, Glostrup, Denmark).
Drug solutions were made in bidistilled water except for indomethacin, SQ 29,548 and NS 398, which were, respectively, dissolved in NaHCO3 (0.5% w v−1), absolute ethanol and DMSO. Stock solutions were kept at −20°C, and appropriate dilutions were made in bidistilled water.
Results
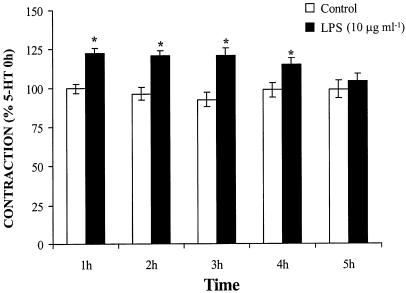
5-HT (10 μM) induced a stable contraction (1.23±0.03 N m−1; n=67), which was maintained through the five administrations of 5-HT at intervals of 1 h (Figure 1). The response to 5-HT increased from the first to the fourth hour of LPS (10 μg ml−1) incubation and returned to control levels after 5 h (Figure 1). The incubation with LPS did not modify the basal tone (results not shown) or the contraction elicited by K+-KHS (n=25, results not shown).
Figure 1.
LPS transiently enhances the contraction elicited by five successive administrations of 5-HT (10 μM) in rat MCAs (n=23–25). *P<0.05.
Participation of NO and prostanoids on the effect of LPS
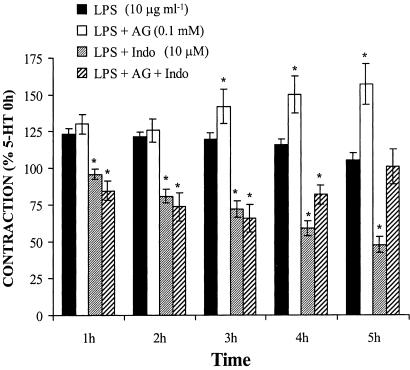
The specific inhibitor of iNOS, aminoguanidine (0.1 mM), increased the 5-HT contraction in the presence of LPS in a time-dependent way (P<0.05) from the third hour of incubation. However, the COX-inhibitor indomethacin (10 μM) induced an LPS-incubation time-dependent inhibition (P<0.05) of the contraction to 5-HT from the first hour of LPS incubation (Figure 2). When aminoguanidine and indomethacin were used together, a reduction in the contraction to 5-HT was still observed between 1 and 3 h of LPS incubation, but they increased this contraction at the following incubation times (P<0.05); after 5 h of LPS incubation, the contraction induced by 5-HT was similar in the absence and presence of these drugs (Figure 2).
Figure 2.
COX- and iNOS-derived products participate in the enhanced 5-HT vasoconstriction after LPS incubation. Effect of aminoguanidine (AG, n=11), indomethacin (Indo, n=11) and AG plus Indo (n=9) on the contraction to 5-HT (10 μM) after LPS incubation in rat MCAs. *P<0.05 vs LPS.
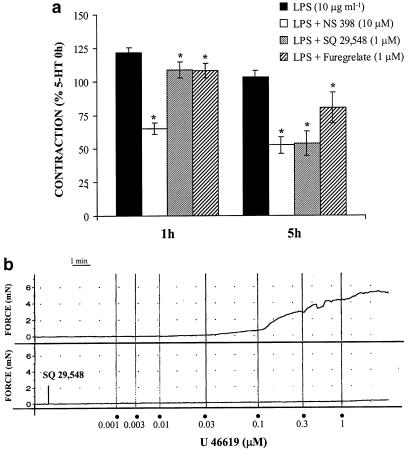
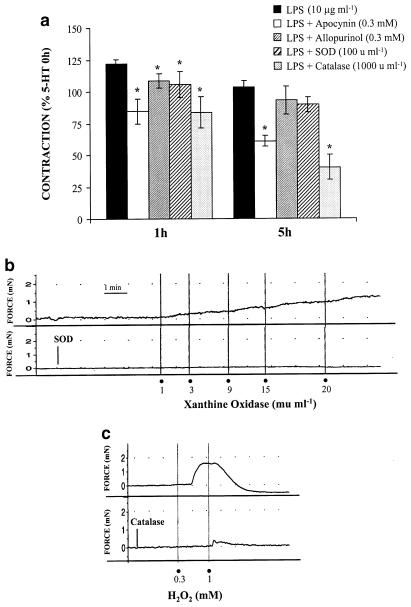
The specific COX-2 inhibitor NS 398 (10 μM) the thromboxane A2/prostaglandin H2 (TXA2/PGH2) receptor antagonist SQ 29,548 (1 μM), and the TXA2 synthase inhibitor, furegrelate (1 μM), reduced the vasoconstrictor response induced by 5-HT after 1–5 h of LPS incubation; Figure 3a only shows the effect at 1 and 5 h. The effect of SQ 29,548 was incubation time-dependent (P<0.05).
Figure 3.
COX-2-derived contractile prostanoids participate in the enhanced 5-HT vasoconstriction after LPS incubation. (a) Effect of NS 398 (n=11), SQ 29,548 (n=7) and furegrelate (n=9) on the contraction to 5-HT (10 μM) after LPS incubation in rat MCAs. *P<0.05 vs LPS. (b) Representative tracings of the contraction to U 46619 in the absence or presence of SQ 29,548 (n=9).
Aminoguanidine, indomethacin, NS 398, SQ 29,548 and furegrelate did not modify the 5-HT response in the absence of LPS (results not shown).
In the absence of LPS, the basal tone was not modified by the above drugs (results not shown). In the presence of LPS, aminoguanidine induced a slight increase in basal tone at the longest incubation times studied (9.4±2.4% of maximum response), while a small decrease, from the first hour of LPS incubation, was observed with indomethacin (−4.0±0.3 and −5.9±1.9% of maximum response after 1 and 5 h LPS incubation, respectively) and NS 398 (−3.6±0.7 and −6.4±1.8% of maximum response after 1 and 5 h LPS incubation, respectively).
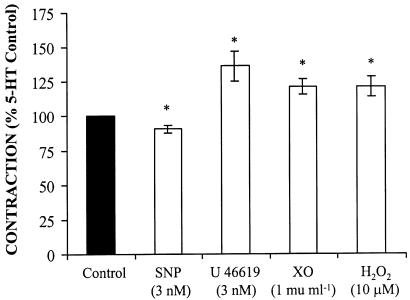
In control arteries, the TXA2 analogue U 46619 (0.001–1 μM) induced concentration-dependent contractions that were completely abolished by 1 μM SQ 29,548 (Figure 3b). In addition, the incubation of arteries with low concentrations of U 46619 (3 nM) potentiated the 5-HT-induced contraction (Figure 4); this concentration of U 46619 did not induce any vasoactive response. On the other hand, a subthreshold concentration of SNP (3 nM) inhibited 5-HT-induced contraction (Figure 4).
Figure 4.
SNP inhibits the contraction to 5-HT (10 μM) in rat MCAs, while U 46619, xanthine oxidase (XO, in the presence of 100 μM xanthine and 1000 U ml−1 catalase) and H2O2 potentiate it (n=6). *P<0.05 vs control.
Participation of reactive oxygen species on the effect of LPS
The inhibitor of NAD(P)H oxidase, apocynin (0.3 mM), inhibited the vasoconstrictor response elicited by 5-HT from the first hour of LPS incubation. Both the xanthine oxidase inhibitor allopurinol (0.3 mM) and the superoxide anion scavenger SOD (100 U ml−1) reduced the 5-HT contraction from 1 to 4 h of LPS incubation, but not at 5 h. The H2O2 scavenger catalase (1000 U ml−1) reduced the vasoconstrictor response elicited by 5-HT during 1–5 h of LPS incubation. Figure 5a only shows the effect of these drugs at 1 and 5 h.
Figure 5.
Reactive oxygen species participate in the enhanced 5-HT vasoconstriction after LPS incubation. (a) Effect of apocynin (n=7), allopurinol (n=9), SOD (n=7) and catalase (n=6) on the contraction to 5-HT (10 μM) after LPS incubation in rat MCAs. *P<0.05 vs LPS. (b) Representative tracings of the contraction induced by xanthine oxidase (in the presence of 100 μM xanthine and 1000 U ml−1 catalase) and H2O2 (n=5–9). The effect of SOD and catalase on the contraction to xanthine oxidase and H2O2, respectively, is also shown.
Allopurinol, SOD and catalase did not modify the vasoconstrictor response to 5-HT in the absence of LPS (results not shown). Apocynin reduced the 5-HT contraction in the absence of LPS; after 2–5 h of incubation, this reduction was lower (P<0.05) than that observed in the presence of LPS (% of reduction at 5 h: control, 27.1±5.4; LPS, 44.8±3.0, n=7, P<0.05); however, at 1 h of incubation, the effect of apocynin was similar in both the absence and presence of LPS (control: 22.8±4.9, LPS: 35.7±7.6% of reduction, n=7, P>0.05). Apocynin also decreased the vasoconstrictor response to K+-KHS by 15.7±5.6% (n=5) in control segments.
These drugs did not modify the basal tone in the absence of LPS (results not shown). However, in the presence of LPS, the incubation with SOD induced a slight decrease in basal tone after 1 h (−6.1±2.1% of maximum response), but not after 5 h of LPS incubation.
H2O2 (0.3 mM) as well as xanthine oxidase (1–20 mU ml−1), in the presence of xanthine (100 μM) plus catalase (1000 U ml−1), induced concentration-dependent contractions in control arteries; catalase abolished the H2O2 contraction, while SOD abolished the contraction to xanthine oxidase (Figure 5b). In addition, the incubation with both 1 mU ml−1 xanthine oxidase (in the presence of xanthine plus catalase) and 10 μM H2O2 potentiated the contraction induced by 5-HT (Figure 4). At these concentrations, these drugs did not increase basal tone (results not shown).
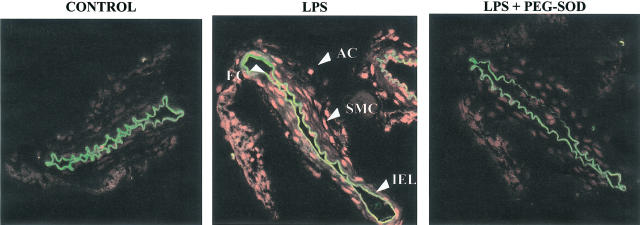
In LPS-treated arteries, an important increase in ethidium bromide fluorescence was observed, reflecting an increase in superoxide anion. Incubation with PEG-SOD abolished ethidium bromide fluorescence, confirming the specificity of the fluorescent signal for superoxide anion. Ethidium bromide fluorescence was prominent in all the three layers of the cerebral arterial segments (Figure 6).
Figure 6.
LPS increases the superoxide anion level. Representative fluorescent photomicrographs (n=3) of confocal microscopic sections of MCAs from Wistar Kyoto rats incubated without (control) or with LPS (10 μg ml−1). Vessels were labelled with the oxidative dye hydroethidine, which produces a red fluorescence when oxidized to ethidium bromide by superoxide anion. Sequentially cut sections were incubated in the presence of polyethylene glycol-conjugated SOD (LPS+PEG-SOD). AC, adventitial cells; EC, endothelial cells and SMC, smooth muscle cells. IEL indicates internal elastic lamina and its autofluorescence provides a means way to separate endothelial cells from smooth muscle cells.
Expression and cellular localization of iNOS, COX-2 and SOD
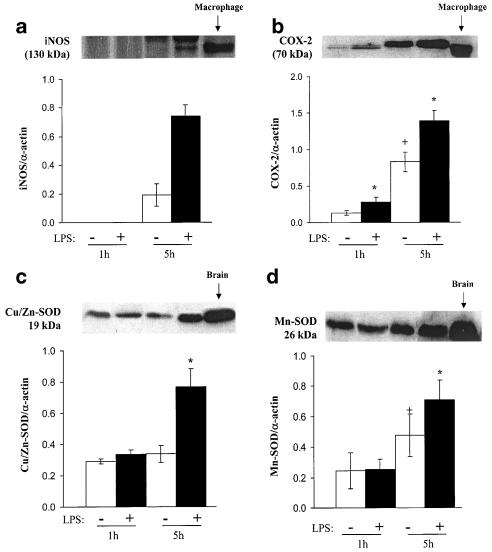
iNOS expression was not detected in homogenates from cerebral arteries incubated for 1 h with or without LPS. After 5 h of KHS incubation, iNOS expression was detected and was markedly increased after 5 h of LPS incubation (Figure 7a). iNOS was rarely detected in control sections. However, after 5 h of LPS incubation, iNOS immunoreactivity was observed in the endothelial cell layer, the outer media and adventitia (Figure 8).
Figure 7.
LPS increases iNOS, COX-2, cytosolic Cu/Zn- and Mn-SOD expressions. Representative Western blots and quantitative analysis for (a) iNOS, (b) COX-2, (c) Cu/Zn-SOD and (d) Mn-SOD expressions in rat cerebral arteries incubated for 1 and 5 h in the absence or presence of LPS (10 μg ml−1). *P<0.05 vs absence of LPS, +P<0.05 vs 1 h, n=4–7.
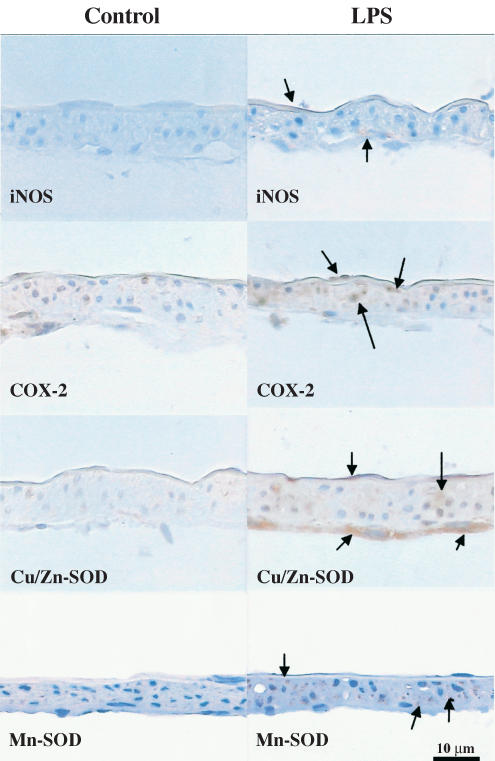
Figure 8.
Immunohistochemical localization of iNOS, COX-2, cytosolic Cu/Zn- and Mn-SOD in rat MCAs. Sections of arterial segments here show the endothelial cell layer upwards. The arteries were treated for 5 h in the absence (control) or presence of LPS (10 μgml−1) (n=5). Positive immunoreaction is observed as a brown precipitate, while cell nuclei are stained blue with Mayer's haemotoxylin. Magnification × 40.
COX-2 expression was detected after 1 h of KHS incubation and was increased after 5 h in control tissue (Figure 7b). Incubation for 1 or 5 h with LPS increased COX-2 expression (Figure 7b). This isoform was found in the three layers of control sections; in LPS-treated tissues, immunoreactivity increased markedly in the endothelium and media and less markedly in adventitia (Figure 8).
Cu/Zn- and Mn-SOD expressions were detected in MCA after 1 and 5 h of KHS incubation; the expression of Mn-SOD was greater after 5 h than after 1 h. Incubation with LPS for 5 h increased the expression of both isoforms (Figure 7c, d). Cu/Zn-SOD was sparsely located in all the three layers of control sections and was marked in the endothelium and adventitia of LPS-treated segments (Figure 8). Immunoreactivity for Mn-SOD was detected mainly in the media of control sections, but the reaction was more pronounced in this layer of LPS-treated sections (Figure 8).
Discussion
Hypotension and hyporesponsiveness to vasoconstrictors are described in human septic shock (Parrillo et al., 1990) and in experimental models (Szabó et al., 1993). However, an early decrease of cerebral blood flow and perfusion pressure and increased cerebrovascular resistance (Parker & Emerson, 1977) have been described in experimental septic shock; the vasoconstrictor 5-HT has been proposed to be involved in these alterations (Ekström-Jodal et al., 1982). The main finding of the present study is that LPS treatment transiently enhances vasoconstrictor responses to 5-HT in rat MCA, and that activation of TXA2/PGH2 receptors, formation of superoxide anions and H2O2 seem to play a role in this effect. These events are parallel to the induction of COX-2. In addition, iNOS is induced at a later time point and iNOS-derived NO as well as increased expression of SOD seem to counteract vasoconstriction.
LPS increases vasoconstrictor responses to 5-HT in rat MCA from the first until the fourth hour of incubation; after 5 h of LPS-incubation, the contraction to 5-HT returns to the control. These findings suggest that formation of both vasoconstrictors and vasodilators is involved in the effect of LPS. In contrast, LPS did not modify the potassium contraction. In a previous study in these arteries, we found that LPS reduced the PGF2α contraction (Briones et al., 2002a) and a reduction of the U 46619 contraction after LPS exposure has also been described in cerebral arteries by Ueno & Lee (1993). The fact that both PGF2α and U 46619 activate the TXA2/PGH2 receptor (Dorn et al., 1992) suggests that the effect of possible vasoconstrictor prostanoids released following LPS treatment (see below) would be masked. In addition, differences in the LPS effect depending on the vasoconstrictor agent used have been described. Thus, in rat aorta, Wylam et al. (2001) found that LPS increased the 5-HT response, in agreement with our results, while contraction to other vasoconstrictors was reduced.
LPS administration leads to NO overproduction after iNOS induction (Szabó et al., 1993). A role of NO from iNOS in the LPS effects on cerebral vessels has been reported by our group (Briones et al., 2002a) and others (Ueno & Lee, 1993; Brian et al., 1995). In MCA, 5 h LPS treatment increased iNOS expression in the endothelium, outer media and adventitia. In agreement, Zhang et al. (1999) described iNOS expression in the endothelium and adventitia of rat aorta. The iNOS inhibitor aminoguanidine increased both basal tone and 5-HT response after 3 h of LPS incubation. These results suggest the involvement of NO generated after iNOS induction in the LPS effect on 5-HT response during the longest incubation time period studied. The fact that SNP, at a concentration that did not modify basal tone, reduced 5-HT contraction supports this proposal.
In addition to iNOS, LPS induces other enzymes, including COX-2 (Cirino et al., 1996; Brian et al., 1998; Okamoto et al., 1998; Briones et al., 2002b). In the present study, indomethacin decreased 5-HT contraction and basal tone from the first hour of LPS incubation. These results suggest that vasoconstrictors from COX are involved in the LPS effects at the earliest incubation times, and these effects are counteracted by the NO from iNOS that appears later on, as mentioned above. This is also supported by the finding that aminoguanidine plus indomethacin reduced the 5-HT contraction from hour 1 to 3 h, and increased the contraction from the fourth hour of LPS incubation.
The COX-2 selective inhibitor NS 398 also decreased the 5-HT contraction as well as basal tone from the first hour of LPS incubation, suggesting the involvement of COX-2-derived contractile products in the LPS effect on MCA after short incubation times. This hypothesis was reinforced by the fact that after 1 h of LPS incubation COX-2 expression was increased. Other authors have also found that COX-2 expression increases after short incubation times with LPS (Hori et al., 2001; Briones et al., 2002b). In addition, it has been described that COX-2 is induced more quickly than iNOS and that the early production of prostanoids stimulates iNOS expression (Hori et al., 2001). Immunohistochemical studies revealed positive staining for COX-2 after LPS administration in the three layers of the vessels. COX-2 expression after LPS treatment has been reported in the vascular smooth muscle and endothelial cells (Vagnoni et al., 1999; Yamagata et al., 2001). Although COX-2 expression has not previously been described in adventitia, COX-2 is also induced in macrophages (Paul et al., 1999) and fibroblasts (Noguchi et al., 1996) activated by LPS, and may explain the finding of COX-2 expression in the adventitia of MCA.
The inhibitor of the thromboxane synthase, furegrelate, and the TXA2/PGH2 receptor antagonist SQ 29,548 had the same effect as indomethacin and NS 398, since they reduced the enhanced 5-HT contraction in LPS-treated arteries. These results suggest that TXA2 is involved in the potentiation of the 5-HT contraction after LPS incubation. These results agree with studies in peripheral arteries where participation of TXA2 in LPS effects was also described (Cirino et al, 1996; Ermert et al., 2000; Briones et al., 2002b). A synergistic effect between 5-HT and TXA2 has been described in several vessels such as human cerebral arteries (Hempelmann et al., 1997) and rat aorta (Lee et al., 1998). In the human coronary artery, endogenously produced TXA2 enhances contractions induced by 5-HT1-agonists, such as sumatriptan (MaassenVanDenBrink et al., 1996). On the other hand, organ culture was found to preferentially increase the 5-HT1B/D receptor subtype in rat cerebral arteries (Hoel et al., 2001). The present study does not determine whether any particular subtype of 5-HT receptor is upregulated. However, the augmented 5-HT contraction observed after incubation with a subthreshold concentration of the TXA2 analogue U 46619, together with the inhibitory effect of SQ 29,548 and furegrelate observed in LPS-treated arteries, provide evidence that low concentrations of TXA2, generated after COX-2 induction by LPS, could amplify the 5-HT contraction.
Several clinical studies have shown increased oxidative stress and decreased antioxidant defences in septic patients (Goode et al., 1995). In agreement, our results show that superoxide anion levels were increased in all the three layers of MCAs treated with LPS. Superoxide anion production has been associated to an increase in xanthine oxidase and NAD(P)H oxidase expressions after LPS (Brandes et al., 1999). In MCA, both the NAD(P)H oxidase inhibitor apocynin and the xanthine oxidase inhibitor allopurinol reduced 5-HT contraction after LPS incubation, suggesting that both enzymatic systems could be sources of superoxide anion after LPS treatment and could therefore participate in the enhanced 5-HT response. However, the fact that apocynin reduced 5-HT contraction compared to control at 2–5 h but not at 1 h, while allopurinol reduced this contraction at 1–4 h but not at 5 h, suggests that the time course of superoxide anion production from each source is different. In addition, other sources of superoxide anion, such as iNOS and COX-2, cannot be excluded (Xia et al., 1998; Kulkarni & Armstead, 2002). The participation of superoxide anion in LPS-evoked potentiation of NA contraction has also recently been described in mesenteric resistance arteries (Briones et al., 2002b).
The following results further support the participation of superoxide anions in the LPS effect on 5-HT contraction: (1) although only extracellular superoxide anion could be affected by exogenous SOD, this drug reduced the basal tone and the vasoconstrictor response to 5-HT after LPS incubation. (2) A concentration of exogenously generated superoxide anion that did not alter the basal tone enhanced 5-HT contraction. Other authors have found that endothelial superoxide anion production is responsible for the hypercontraction to 5-HT induced by regenerated endothelium after intimal injury (Lin et al., 1991). (3) Higher concentrations of superoxide anion induced contractions that were inhibited by SOD, as was also found in rabbit basilar arteries (Didion et al., 2001), although vasodilator responses mediated by this anion have also been reported (Didion & Faraci, 2002).
In the present work, low levels of superoxide anion were observed in the control tissue. Recent studies in cerebral vessels have suggested that superoxide anion is mainly generated by NAD(P)H oxidase but not by xanthine oxidase (Didion & Faraci, 2002). In agreement with these observations, we also found that, in the absence of LPS, the inhibitor of NAD(P)H oxidase, apocynin, but not the inhibitor of xanthine oxidase, allopurinol, reduced 5-HT contraction. Nonspecific effects were observed at high concentrations of apocynin (Grimminger et al., 1995), and it did cause some inhibition also of high potassium contraction in the present study. However, depolarization of endothelial cells in the absence of LPS was also demonstrated to enhance NAD(P)H-derived superoxide formation (Sohn et al., 2000). Therefore, NAD(P)H oxidase-derived superoxide anion could contribute to the regulation of 5-HT contraction in normal arteries, and is markedly enhanced in the presence of LPS.
The Mn- and Cu/Zn-SOD isoforms were found in control sections and at 5 h of LPS incubation. In addition, increased expression of both isoforms was observed after 5 h of LPS incubation. Other authors have also demonstrated induction of Mn-SOD after LPS in several cell types (Leach et al., 1998; Brandes et al., 1999; Yu et al., 1999). However, results concerning the effect of LPS on the Cu/Zn-SOD isoform are still contradictory; in fact, both reduction (Leach et al., 1998) and no alteration (Yu et al., 1999) of this isoform have been described. In spite of the increased expression of Mn- and Cu/Zn-SOD after 5 h LPS, this was not enough to compensate the superoxide anion production, which was elevated at this incubation time.
When superoxide anion is dismutated by SOD, H2O2 is generated, and it could also participate in the LPS effects (Brandes et al., 1999). The following findings confirm the participation of H2O2 in the observed LPS effect in MCA: (1) the H2O2 scavenger catalase reduced the contraction to 5-HT from the first hour of incubation with LPS; (2) a subthreshold concentration of H2O2 potentiated the 5-HT vasoconstrictor response in the absence of LPS, as has been described by Watanabe et al. (1996) in human umbilical arteries and (3) higher concentrations of H2O2 induced catalase-sensitive vasoconstrictor responses, as also reported in canine cerebral arteries (Yang et al., 1999). Vasorelaxation mediated by H2O2 was also reported in some vessels, including cerebral arteries (Wei et al., 1996; Matoba et al., 2002). Since H2O2 induces vasoconstriction and enhances the 5-HT response, as it does the superoxide anion, it is difficult to identify the contribution of each mediator in the final effect of exogenous SOD. Thus, the production of hydrogen peroxide by SOD could explain why 5 h LPS incubation with SOD does not modify the contraction to 5-HT, while catalase reduces this response.
The drugs used in this study, except apocynin, did not modify 5-HT response in control arteries. However, some of the drugs (indomethacin, NS 398, SQ 29,548, catalase and indomethacin plus aminoguanidine) not only reduced the additional vasoconstriction induced after LPS, but also reduced the 5-HT contraction below the control response in the absence of the endotoxin. This effect is observed from the first hours of incubation, when iNOS is not yet induced. These results suggest the participation of vasodilators other than iNOS-derived NO, which would counteract the effect of vasoconstrictor mediators. Thus, synthesis of NO from eNOS (Szabó et al., 1995), release of CGRP (Brian et al., 1995) and an increase of carbon monoxide (Gaine et al., 1999), among others, have been described after LPS exposure. Thus, the LPS effect on vasoconstrictor response induced by 5-HT would be the result of the time-dependent formation of different vasodilator and vasoconstrictor mediators.
In conclusion, our results indicate that LPS exposure is followed by an increase of COX-2 expression and enhanced release of thromboxane, superoxide anion and H2O2, which act to enhance the vasoconstriction to 5-HT during the first hours of incubation; an increase of NO from iNOS and the induction of SOD would counteract the enhanced vasoconstriction at a later time point. However, we cannot discard the involvement of other mediators in the LPS effect. Our results would explain the decrease of cerebral blood flow associated to the increase of vasoconstrictor responses in the earlier stages of endotoxic shock (Parker & Emerson, 1977; Ekström-Jodal et al., 1982). Although dilatation of cerebral arterioles after administration of endotoxin has been described (Brian et al., 1998; Okamoto et al., 1998), the largest arteries are the main vessels implicated in the regulation of cerebrovascular tone (Faraci & Heistad, 1990).
Acknowledgments
This study has been supported by grants from DGICYT (BXX2000-0153) and FISS (C0301). Ulf Simonsen was supported by the Danish Heart Foundation. We thank Dr M. Carmen Fernández-Criado for the care of animals, Ms H. Zibrandtsen for her technical assistance and Ms C.F. Warren for her linguistic assistance.
Abbreviations
- COX
cyclooxygenase
- COX-2
inducible cyclooxygenase
- 5-HT
5-hydroxytryptamine
- iNOS
inducible nitric oxide synthase
- KHS
Krebs–Henseleit solution
- LPS
lipopolysaccharide
- MCAs
middle cerebral arteries
- NADPH
β-nicotinamide adenine dinucleotide phosphate, reduced form
- NO
nitric oxide
- NOS
nitric oxide synthase
- SDS
sodium lauryl sulphate (sodium dodecyl sulphate)
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- TXA2
thromboxane A2
References
- BRANDES R.P., KODDENBERG G., GWINNER W., KIM D., KRUSE H.J., BUSSE R., MÜGGE A. Role of increased production of superoxide anions by NAD(P)H oxidase and xanthine oxidase in prolonged endotoxemia. Hypertension. 1999;33:1243–1249. doi: 10.1161/01.hyp.33.5.1243. [DOI] [PubMed] [Google Scholar]
- BRIAN J.E., HEISTAD D.D., FARACI F.M. Dilatation of cerebral arterioles in response to lipopolysaccharide in vivo. Stroke. 1995;26:277–281. doi: 10.1161/01.str.26.2.277. [DOI] [PubMed] [Google Scholar]
- BRIAN J.E., JR, MOORE S.A., FARACI F.M. Expression and vascular effects of cyclooxygenase-2 in brain. Stroke. 1998;29:2600–2606. doi: 10.1161/01.str.29.12.2600. [DOI] [PubMed] [Google Scholar]
- BRIONES A.M., ALONSO M.J., HERNANZ R., MIGUEL M., SALAICES M. Alterations of the nitric oxide pathway in cerebral arteries from spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2002a;39:378–388. doi: 10.1097/00005344-200203000-00009. [DOI] [PubMed] [Google Scholar]
- BRIONES A.M., ALONSO M.J., HERNANZ R., TOVAR S., VILA E., SALAICES M. Hypertension alters the participation of contractile prostanoids and superoxide anions in lipopolysaccharide effects on small mesenteric arteries. Life Sci. 2002b;71:1997–2014. doi: 10.1016/s0024-3205(02)01967-7. [DOI] [PubMed] [Google Scholar]
- CIRINO G., SORRENTINO R., CICALA C., SORRENTINO L., PINTO A. Indomethacin and thromboxane A2/prostaglandin H2 antagonist SQ 29,548 impair in vitro contractions of aortic rings of ex vivo-treated lipopolysaccharide rats. J. Lipid Med. Cell Signal. 1996;13:177–187. doi: 10.1016/0929-7855(95)00051-8. [DOI] [PubMed] [Google Scholar]
- DIDION S.P., FARACI F.M. Effects of NADH and NADPH on superoxide levels and cerebral vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H688–H695. doi: 10.1152/ajpheart.00576.2001. [DOI] [PubMed] [Google Scholar]
- DIDION S.P., HATHAWAY C.A., FARACI F.M. Superoxide levels and function of cerebral blood vessels after inhibition of CuZn-SOD. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1697–H1703. doi: 10.1152/ajpheart.2001.281.4.H1697. [DOI] [PubMed] [Google Scholar]
- DORN G.W., BECKER M.W., DAVIS M. Dissociation of the contractile and hypertrophic effects of vasoconstrictor prostanoids in vascular smooth muscle. J. Biol. Chem. 1992;267:24897–24905. [PubMed] [Google Scholar]
- EKSTRÖM-JODAL B., ELFVERSON J., LARSSON L.E. Early effects of E. coli endotoxin on superior sagittal sinus blood flow. An experimental study in dogs. Acta Anaesth. Scand. 1982;26:171–174. doi: 10.1111/j.1399-6576.1982.tb01747.x. [DOI] [PubMed] [Google Scholar]
- ERMERT M., MERKLE M., MOOTZ R., GRIMMINGER F., SEEGER W., ERMERT L. Endotoxin priming of the cyclooxygenase-2-thromboxane axis in isolated rat lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L1195–L1203. doi: 10.1152/ajplung.2000.278.6.L1195. [DOI] [PubMed] [Google Scholar]
- FARACI F.M., HEISTAD D.D. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ. Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- GAINE S.P., BOOTH G., OTTERBEIN L., FLAVAHAN N.A., CHOI A.M., WIEMER C.M. Induction of heme oxygenase-1 with hemoglobin depresses vasoreactivity in rat aorta. J. Vasc. Res. 1999;36:114–119. doi: 10.1159/000025633. [DOI] [PubMed] [Google Scholar]
- GOODE H.F., COWLEY H.C., WALKER B.E., HOWDLE P.D., WEBSTER N.R. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit. Care Med. 1995;23:646–651. doi: 10.1097/00003246-199504000-00011. [DOI] [PubMed] [Google Scholar]
- GRIMMINGER F., WEISSMANN N., SPRIESTERSBACH R., BECKER E., ROSSEAU S., SEEGER W. Effects of NAD(P)H oxidase inhibitors on hypoxic vasoconstriction in buffer-perfused rabbit lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 1995;268:L747–L752. doi: 10.1152/ajplung.1995.268.5.L747. [DOI] [PubMed] [Google Scholar]
- HEMPELMANN R.G., PRADEL R.H., BARTH H.L., MEDHORN H.M., ZIEGLER A. Interactions between vasoconstrictors in isolated human cerebral arteries. Acta Neurochir. 1997;139:574–582. doi: 10.1007/BF02751003. [DOI] [PubMed] [Google Scholar]
- HOEL N.L., HANSEN-SCHWARTZ J., EDWINSSON L. Selective up-regulation of 5-HT(1B/1D) receptors during organ culture cerebral arteries. Neuroreport. 2001;12:1605–1608. doi: 10.1097/00001756-200106130-00019. [DOI] [PubMed] [Google Scholar]
- HORI M., KITA M., TORIHASHI S., MIYAMOTO S., WON K.J., SATO K., OZAKI H., KARAKI H. Upregulation of iNOS by COX-2 in muscularis resident macrophage of rat intestine stimulated with LPS. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G930–G938. doi: 10.1152/ajpgi.2001.280.5.G930. [DOI] [PubMed] [Google Scholar]
- HSU S.M., RAINE L. Protein A, avidin, and biotin in immunohistochemistry. J. Histochem. Cytochem. 1981;29:1349–1353. doi: 10.1177/29.11.6172466. [DOI] [PubMed] [Google Scholar]
- KIRKEBOEN K.A., STRAND O.A. The role of nitric oxide in sepsis – an overview. Acta Anaesthesiol. Scand. 1999;43:275–288. doi: 10.1034/j.1399-6576.1999.430307.x. [DOI] [PubMed] [Google Scholar]
- KULKARNI M., ARMSTEAD W.M. Relationship between NOC/oFQ, dynorphin, and COX-2 activation in impaired NMDA cerebrovasodilation after brain injury. J. Neurotrauma. 2002;19:965–973. doi: 10.1089/089771502320317113. [DOI] [PubMed] [Google Scholar]
- LEACH M., FRANK S., OLBRICH A., PFEILSCHIFTER J., THIEMERMANN C. Decline in the expression of copper/zinc superoxide dismutase in the kidney of rats with endotoxic shock: effects of the superoxide anion radical scavenger, tempol, on organ injury. Br. J. Pharmacol. 1998;125:817–825. doi: 10.1038/sj.bjp.0702123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE J.-Y., LEE M.-Y., CHUNG S.-M., CHUNG J.-H. Chemically induced platelet lysis causes vasoconstriction by release of serotonin. Toxicol. Appl. Pharmacol. 1998;149:235–242. doi: 10.1006/taap.1998.8387. [DOI] [PubMed] [Google Scholar]
- LIN P.J., PEARSON P.J., CARTIER R., SCHAFF H.V. Superoxide anion mediates the endothelium-dependent contractions to serotonin by regenerated endothelium. J. Thorac. Cardiovasc. Surg. 1991;102:378–385. [PubMed] [Google Scholar]
- LINCOLN J. Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol. Ther. 1995;68:473–501. doi: 10.1016/0163-7258(95)02017-9. [DOI] [PubMed] [Google Scholar]
- MAASSENVANDENBRINK A., BAX W.A., FERRARI M.D., ZIJLSTRA F.J., BOS E., SAXENA P.R. Augmented contraction of the human isolated coronary artery by sumatriptan: a possible role for endogenous thromboxane. Br. J. Pharmacol. 1996;119:855–862. doi: 10.1111/j.1476-5381.1996.tb15751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATOBA T., SHIMOKAWA H., KUBOTA H., MORIKAWA K., FUJIKI T., KUNIHIRO I., MUKAI Y., HIRAKAWA Y., TAKESHITA A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem. Biophys. Res. Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- MILLER F.J., JR, GUTTERMAN D.D., RIOS C.D., HEISTAD D.D., DAVIDSON B.L. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ. Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NOGUCHI K., SHITASHIGE M., YANAI M., MORITA I., NISHIHARA T., MUROTA S., ISHIKAWA I. Prostaglandin production via induction of cyclooxygenase-2 by human gingival fibroblasts stimulated with lipopolysaccharides. Inflammation. 1996;20:555–568. doi: 10.1007/BF01487046. [DOI] [PubMed] [Google Scholar]
- OKAMOTO H., ITO O., ROMAN R.J., HUDETZ A.G. Role of inducible nitric oxide synthase and cyclooxygenase-2 in endotoxin-induced cerebral hyperemia. Stroke. 1998;29:1209–1218. doi: 10.1161/01.str.29.6.1209. [DOI] [PubMed] [Google Scholar]
- PARKER J.L., EMERSON T.E. Cerebral hemodynamics, vascular reactivity, and metabolism during canine endotoxin shock. Circ. Shock. 1977;4:41–53. [PubMed] [Google Scholar]
- PARRILLO J.E., PARKER M.M., NATANSON C., SUFFREDINI A.F., DANNER R.L., CUNNION R.E., OGNIBENE F.P. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- PAUL A., CUENDA A., BRYANT C.E., MURRAY J., CHILVERS E.R., COHEN P., GOULD G.W., PLEVIN R. Involvement of mitogen-activated protein kinase homologues in the regulation of lipopolysaccharide-mediated induction of cyclo-oxygenase-2 but not nitric oxide synthase in RAW 264.7 macrophages. Cell. Signal. 1999;11:491–497. doi: 10.1016/s0898-6568(99)00018-2. [DOI] [PubMed] [Google Scholar]
- ROON K.I., MAASSENVANDENBRINK A., FERRARI M.D., SAXENA P.R. Bovine isolated middle cerebral artery contractions to antimigraine drugs. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:591–596. doi: 10.1007/s002109900095. [DOI] [PubMed] [Google Scholar]
- SOHN H.Y., KELLER M., GLOE T., MORAWIETZ H., RUECKSCHLOSS U., POHL U. The small G-protein Rac mediates depolarization-induced superoxide formation in human endothelial cells. J. Biol. Chem. 2000;275:18745–18750. doi: 10.1074/jbc.M000026200. [DOI] [PubMed] [Google Scholar]
- SZABÓ C., MITCHELL J.A., THIEMERMANN C., VANE J.R. Nitric oxide mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxic shock. Br. J. Pharmacol. 1993;108:786–792. doi: 10.1111/j.1476-5381.1993.tb12879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZABÓ C., SALZMAN A.L., ISCHIROPOULOS H. Peroxynitrite-mediated oxidation of dihydrorhodamine 123 occurs in early stages of endotoxic and hemorrhagic shock and ischemia–reperfusion injury. FEBS Lett. 1995;372:229–232. doi: 10.1016/0014-5793(95)00984-h. [DOI] [PubMed] [Google Scholar]
- UENO M., LEE T.J. Endotoxin decreases the contractile responses of the porcine basilar artery to vasoactive substances. J. Cereb. Blood Flow Metab. 1993;13:712–719. doi: 10.1038/jcbfm.1993.90. [DOI] [PubMed] [Google Scholar]
- VAGNONI K.E., CHRISTIANSEN N.D., HOLYOAK G.R., JANOWIAK M.A., MARTIN P.H. Cellular source in ewes of prostaglandin-endoperoxide synthase-2 in uterine arteries following stimulation with lipopolysaccharide. Biol. Reprod. 1999;61:563–568. doi: 10.1095/biolreprod61.3.563. [DOI] [PubMed] [Google Scholar]
- WATANABE K., OKATANI Y., SAGARA Y. Potentiating effect of hydrogen peroxide on the serotonin-induced vasocontraction in human umbilical artery. Acta Obstet. Gynecol. Scand. 1996;75:783–789. doi: 10.3109/00016349609054704. [DOI] [PubMed] [Google Scholar]
- WEI E.P., KONTOS H.A., BECKMAN J.S. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am. J. Physiol. 1996;271:H1262–H1266. doi: 10.1152/ajpheart.1996.271.3.H1262. [DOI] [PubMed] [Google Scholar]
- WYLAM M.E., METKUS A.P., UMANS J.G. Nitric oxide dependent and independent effects of in vitro incubation or endotoxin on vascular reactivity in rat aorta. Life Sci. 2001;69:455–467. doi: 10.1016/s0024-3205(01)01137-7. [DOI] [PubMed] [Google Scholar]
- XIA Y., ROMAN L.D., MASTERS B.S., ZWEIER J.L. Inducible nitric-oxide synthase generates superoxide from the reductase domain. J. Biol. Chem. 1998;273:22635–22639. doi: 10.1074/jbc.273.35.22635. [DOI] [PubMed] [Google Scholar]
- YAMAGATA K., MATSUMURA K., INOUE W., SHIRAKI T., SUZUKI K., YASUDA S., SUGIURA H., CAO C., WATANABE Y., KOBAYASHI S. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J. Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG Z.W., ZHENG T., WANG J., ZHANG A., ALTURA B.T., ALTURA B.M. H2O2 induces contraction and raises [Ca2+]i in canine cerebral arterial smooth muscle: participation of cellular signaling pathways. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:646–653. doi: 10.1007/s002109900128. [DOI] [PubMed] [Google Scholar]
- YU W.J., LIAU S.S., CHIN W.T., CHENG J.T. Effect of serum in medium on the expression of inducible nitric oxide synthase and superoxide dismutase in cultured C6 glioma cells. Neuroscience. 1999;261:37–40. doi: 10.1016/s0304-3940(98)01007-6. [DOI] [PubMed] [Google Scholar]
- ZHANG H., DU Y., COHEN R.A., CHOBANIAN A.V., BRECHER P. Adventitia as a source of inducible nitric oxide synthase in the rat aorta. Am. J. Hypertens. 1999;12:467–475. doi: 10.1016/s0895-7061(98)00271-4. [DOI] [PubMed] [Google Scholar]