Abstract
A large body of evidence now exists to substantiate that the endocannabinoid, anandamide, activates TRPV1 receptors. It is a low intrinsic efficacy TRPV1 agonist that behaves as a partial agonist in tissues with a low receptor reserve, while in tissues with high receptor reserve and in circumstances associated with certain disease states, it behaves as a full agonist. The efficacy of anandamide as a TRPV1 agonist is influenced by a succession of factors including receptor reserve, phosphorylation, metabolism and uptake, CB1 receptor activation, voltage, temperature, pH and bovine serum albumin. There are indications that the endocannabinoid system may play a role in the modulation of TRPV1 receptor activation. The activation of TRPV1 receptors by anandamide has potential implications in the treatment of inflammatory, respiratory and cardiovascular disorders. The relative importance of anandamide as a physiological and/or pathophysiological TRPV1 receptor agonist in comparison to other potential candidates has yet to be revealed.
Keywords: Anandamide, vanilloid, TRPV1, cannabinoid, endocannabinoid, endovanilloid, lipoxygenase
Introduction
The vanilloid VR1 or TRPV1 receptor is part of a family of transient receptor potential (TRP) channels (see Benham et al., 2002), whose expression is largely associated with small diameter primary afferent fibres. This receptor is a nonselective cation channel that integrates multiple noxious stimuli and is associated with the pathophysiology of various major diseases (Szallasi, 2002). It is activated by the naturally occurring vanilloids, capsaicin and resiniferatoxin (RTX), noxious heat and acid (see Szallasi, 2002). Anandamide (N-arachidonoyl-ethanolamide) is an ‘endocannabinoid', as defined by its ability to bind to and activate cannabinoid CB1 and CB2 receptors (see Pertwee & Ross, 2002); however, the pharmacology of this compound is complex (see Di Marzo, 2002; Di Marzo et al., 2002b). The search for endogenous TRPV1 receptor activators or ‘endovanilloids' is ongoing and recent advances suggest that anandamide may be one such compound (Di Marzo et al., 2001). Since the first revelations of the structural similarity of anandamide to capsaicin and of its ability to activate TRPV1 receptors (Melck et al., 1999; Zygmunt et al., 1999; Smart et al., 2000), this topic has been the focus of exciting and sometimes controversial debate.
Various reviews cover the interaction of anandamide with TRPV1 receptors in pain pathways (see Di Marzo et al., 2002a; Iversen & Chapman, 2002; Rice et al., 2002; Walker & Huang, 2002), the cardiovascular system (see Högestätt & Zygmunt, 2002; Ralevic et al., 2002; Randall et al., 2002) and immune cells (see Parolaro et al., 2002). This article will focus on the most recent findings and, in particular, those aspects relating to the pharmacology of anandamide.
The basics: affinity, potency and efficacy
There is ample evidence that the interaction of anandamide with TRPV1 receptors is specific: TRPV1 actions are blocked by receptor-specific antagonists and not by antagonists of CB1 and CB2 receptors; desensitisation of TRPV1 via capsaicin pretreatment abrogates the effects of anandamide; neonatal capsaicin treatment prevents anandamide activation of TRPV1; anandamide-mediated TRPV1 effects are absent from untransfected cells that do not express TRPV1 receptors; anandamide displaces radiolabelled RTX from specific binding sites (Zygmunt et al., 1999; Smart et al., 2000; Ross et al., 2001b).
It seems appropriate to begin this article by reviewing the literature relating to the pharmacology of anandamide and TRPV1. Traditionally, the foundations of the pharmacological analysis of any compound are the three basic parameters of affinity, potency and efficacy. Numerous studies confirm that anandamide activates TRPV1 receptors; those that specifically measure the affinity, efficacy or potency at native and recombinant TRPV1 receptors are shown in Tables 1 and 2 .
Table 1.
Studies measuring the affinity, potency and efficacy of anandamide at recombinant TRPV1 receptors
| Cell line | Affinity (Ki) | Measured response | pH | Potency (pEC50) | Efficacy (Emax) (%)a | Reference |
|---|---|---|---|---|---|---|
| HEK 293(rat) | Inward current | 7.4 | 5.31±0.06 | ∼30–50 | Zygmunt et al. (1999) | |
| [Ca2+]i (FLIPR) | 7.4 | 5.73±0.04 | 100 | Ralevic et al. (2001) | ||
| 6.4 | 5.76±0.04 | — | Ralevic et al. (2001) | |||
| [Ca2+]i (FLIPR) | — | 5.85±0.01 | 100 | Jerman et al. (2002) | ||
| [Ca2+]i (Fura-2) | — | 5.85±0.12 (22°C) | 63 | Sprague et al. (2001) | ||
| 5.15±0.04 (37°C) | 72 | Sprague et al. (2001) | ||||
| <4 (50°C) | 120 | Sprague et al. (2001) | ||||
| HEK 293 (human) | [Ca2+]i (FLIPR) | 7.4 | 5.94±0.06 | 100 | Smart et al. (2000) | |
| 6.4 | 5.76±0.04 | 100 | Smart et al. (2000) | |||
| 7.4 | 5.60±0.10 | 100 | Ralevic et al. (2001) | |||
| 7.4 | 6.66 (with LEA)b | — | Smart et al. (2000) | |||
| 7.4 | 5.74 (no LEA) | — | Smart et al. (2002) | |||
| — | [Ca2+]i (Fluro-3 imaging) | 7.4 | 6.20 | 100 | De Petrocellis et al. (2001a) | |
| 5.40 (with PEA) | 7.4 | 6.66 (with PEA)c | — | De Petrocellis et al. (2001b) | ||
| 4.72 (no PEA) | 6.36 (no PEA) | — | De Petrocellis et al. (2001b) | |||
| CHO (rat) | 5.78±0.06 (with PMSF) | [Ca2+]i ([45Ca2+] uptake) | 7.4 | 5.80±0.04 (with PMSF)d | 100 | Ross et al. (2001b) |
| <5 (no PMSF) | 7.4 | <5 (no PMSF) | — | Ross et al. (2001b) | ||
| CHO (guinea-pig) | [Ca2+]i | 7.5 | 5.01 | ∼22 | Savidge et al. (2002) | |
| NIH 3T3 (rat) | [Ca2+]i ([45Ca2+] uptake) | 5.5 | 4.85 | — | Olah et al. (2001) | |
| 6.0 | 4.60 | — | Olah et al. (2001) | |||
| 6.5 | 4.20 | — | Olah et al. (2001) |
Relative to that of capsaicin (100%).
Lauroylethanolamide (LEA) is an N-acylethanolamide.
Palmitoylethanolamide (PEA) is an N-acylethanolamide.
Phenylmethylsulphonyl fluoride (PMSF) is an inhibitor of FAAH.
Table 2.
Studies measuring the affinity, potency and efficacy of anandamide at native TRPVI receptors
| Tissue | Measured response | Potency (pEC50) | Efficacy (Emax) (%)a | Reference |
|---|---|---|---|---|
| Guinea-pig basilar artery | Relaxation | 6.02±0.11 | 100±1 | Zygmunt et al. (1999) |
| Rat hepatic artery | Relaxation | 6.45±0.11 | 92±3 | Zygmunt et al. (1999) |
| Rat mesenteric artery | Relaxation | 6.1±0.1 | 98±1 | Andersson et al. (2002) |
| Rat mesenteric bed | Relaxation | 6.32±0.04 | ∼100% | Ralevic et al. (2001) |
| Guinea-pig bronchi | Contraction | 5.23±0.1 (with PMSF)b | 46.5±6.0 | Tucker et al. (2001) |
| 5.26±0.05 (no PMSF) | 41.6±5.8 | Tucker et al. (2001) | ||
| 5.18±0.11 (with PMSF) | 49.5±3.9 | Craib et al. (2001) | ||
| 4.15±0.64 (no PMSF) | — | Craib et al. (2001) | ||
| <5.4±0.1 | >28±6 | Andersson et al. (2002) | ||
| Guinea-pig ileum | [3H]Ach release | 6.3 | — | Mang et al. (2001) |
| Increased muscle tone | 6.3 | — | ||
| Mouse trigeminal neurones | Outward current | 5.3±0.1 | 38±2 | Roberts et al. (2001) |
| Rat DRG | Inward current | — | 10–50 | Smart et al. (2001) |
| Single channel currents (inside-out patches) | 4.93 | — | Hwang et al. (2001) | |
| [Ca2+]i ([5Ca2+ ] uptake) | 5.15 (pH 5.5) | — | Olah et al. (2001) | |
| 5 (pH 6) | ∼23% | Olah et al. (2001) | ||
| Inactive (pH 6.6) | — | Olah et al. (2001) | ||
| [Ca2+]i (FLIPR) | — | ∼63% | Jerman et al. (2002) |
Relative to that of capsaicin (100%).
Phenylmethylsulphonyl fluoride (PMSF) is an inhibitor of FAAH.
Affinity
Radioligand displacement assays in recombinant cell lines using the high-affinity TRPV1 agonist [3H]RTX demonstrate that anandamide has a low affinity for the receptor. While the Ki value of ∼2 μM for anandamide is high, it is similar to that of the established receptor agonist capsaicin (De Petrocellis et al., 2001b; Ross et al., 2001b). This assay is, of course, reliant on RTX binding to the same site on TRPV1 as other agonists. It has been observed that RTX has a significantly lower Ki/EC50 ratio than capsaicin as measured in radioligand binding and functional Ca2+ uptake assays (see Di Marzo et al., 2002a) in both native and recombinant TRPV1 expression systems. Such findings can be explained on the basis that RTX has a lower intrinsic efficacy than capsaicin. Indeed, there is direct evidence that RTX is a lower efficacy agonist at TRPV1 than capsaicin (Wardle et al., 1997; Ross et al., 2001b; Andersson et al., 2002). However, it is possible that RTX and capsaicin bind to different domains on the receptor. There is now evidence that RTX behaves differently from capsaicin: the activation of TRPV1 by RTX leads to a Ca2+-independent Ca2+ mobilisation that is not observed with capsaicin (Marshall et al., 2003). Such data question the usefulness of [3H]RTX as a radioligand for the direct measurement of the TRPV1 receptor ‘affinity' of anandamide and capsaicin: the inhibition of binding of [3H]RTX does not necessarily imply a direct competition and may be explained by allosteric binding sites on the TRPV1 receptor.
There is good evidence that the structural determinants for capsaicin binding and sensitivity are also essential for the interaction of anandamide with the receptor (Jordt & Julius, 2002). Consequently, in mutant receptors rendered insensitive to capsaicin, anandamide is also inactive, even at low pH. While the wild-type VRL-1 receptor does not normally bind either capsaicin or anandamide; VRL-1 receptor mutants, in which capsaicin displaces the specific binding of [3H]RTX, also have an affinity for anandamide (Jordt & Julius, 2002).
Potency
While anandamide has a similar affinity for TRPV1 to that of capsaicin, it has a significantly lower potency. In high expression recombinant cell lines using various methods, the potency (EC50) of anandamide has been measured in the range of 0.7 –5 μM (Table 1). In native systems, the potency of anandamide ranges from 0.3 to 0.8 μM in blood vessels (relaxation) compared with 6 –10 μM in bronchus (contraction) and DRG neurones ([Ca2+]i and inward current) (Table 2).
Efficacy: full or partial agonist?
While it is clear that anandamide can activate TRPV1 receptors, significant differences emerge when comparisons are made of the efficacy of anandamide in native and recombinant receptor systems. There are also significant differences between tissues. Using the measurement of [Ca2+]i in high expression recombinant cell lines expressing either rat or human TRPV1, anandamide appears to be a full agonist as defined by an Emax value that is not significantly different from that of capsaicin (De Petrocellis et al., 2001a; Smart et al., 2000; Ralevic et al., 2001; Ross et al., 2001b; Sprague et al., 2001; Jerman et al., 2002). Some authors find the Emax value of anandamide to be less than 100% in recombinant expression systems. Measurements of inward current using whole-cell patch-clamp electrophysiology in HEK 293 cells Zygmunt et al. (1999) estimate the efficacy of anandamide to be 30 – 50% that of capsaicin. In the same cells, Sprague et al. (2001) find that, at 22 and 37°C, the Emax value for anandamide is 63 and 72% of the response to capsaicin. In CHO cells expressing guinea-pig TRPV1, anandamide has a significantly lower efficacy (22%) than that of capsaicin (Savidge et al., 2002). Similar differences are found in tissues expressing native TRPV1 receptors and the efficacy of anandamide appears to be tissue dependent (Table 2). Thus, it is a full agonist in blood vessels and mesenteric arterial bed; it is a partial agonist in the bronchus, dorsal root ganglion (DRG) and trigeminal neurones (Table 2). In the substantia gelatinosa neurones of the dorsal horn, anandamide activates TRPV1 receptors to increase the frequency of mEPSPs and stimulate neuropeptide release, but only at high concentrations of 10 and 50 μM (see Morisset et al., 2001).
The differences in the efficacy of anandamide between tissues and as measured using different pharmacological end points can be accounted for by a low intrinsic efficacy. Agonist potency is a function of both affinity and efficacy. The ratio of Ki (inhibition constant) to EC50 value is indicative of the relative intrinsic efficacy of a compound. In CHO cells expressing rTRPV1, Ross et al. (2001b) found that, although anandamide and AM404 have Emax values of 100%, they display a low Ki/EC50 ratio as determined from radioligand binding and [45Ca2+] uptake data. This is indicative of a low intrinsic efficacy. As a consequence of the low intrinsic efficacy of anandamide at TRPV1, the Emax value will vary with the pharmacological end point that is measured. Thus, while the elevation of [Ca2+]i has a high degree of signal amplification, measurement of the TRPV1 current has no downstream amplification. Accordingly, analyses of TRPV1 currents in sensory neurones reveal that anandamide is a partial agonist (Zygmunt et al., 1999; Roberts et al., 2002). When the response in the elevation in [Ca2+]i is measured, anandamide is found to be either a partial or a full agonist depending on the tissue used (see Tables 1 and 2).
The low intrinsic efficacy of anandamide at the TRPV1 receptor has important physiological implications. A low intrinsic efficacy agonist will attenuate the effects of a full agonist. Indeed, in trigeminal neurones, coapplication of anandamide with capsaicin significantly reduces the currents produced by capsaicin (Roberts et al., 2002). If endogenous activators of TRPV1 exist that are full agonists, then compounds such as anandamide may have an inhibitory role and serve as anti-inflammatory and analgesic compounds. Indeed, a high efficacy TRPV1 agonist, N-arachidonoyl-dopamine (NADA), has recently been isolated from the central nervous system (Huang et al., 2002).
Factors influencing the efficacy and potency of anandamide at TRPV1
The intrinsic efficacy of anandamide at TRPV1 is relatively low in comparison to that observed for this compound at the CB1 receptor (see Pertwee & Ross, 2002) and as a result, the physiological relevance of anandamide as an endogenous TRPV1 receptor agonist is controversial. Recently, however, a succession of factors have been shown to influence the efficacy and potency of anandamide at TRPV1 significantly.
Receptor reserve
The receptor reserve in a given tissue or cell line will influence the efficacy of a low intrinsic efficacy agonist, whereby such a compound may behave as a full agonist or a partial agonist/antagonist in situations of high or low receptor reserve, respectively. Thus, although anandamide and AM404 may behave as full agonists in certain systems, one would predict that they may behave as partial agonists in tissues with a low receptor reserve. Andersson et al. (2002) performed computer simulations of the impact of the receptor reserve on the concentration – response curves for various TRPV1 agonists, their hypothesis being that the bronchus has a significantly lower receptor reserve than mesenteric vessels. The simulations accurately modelled the experimental data from these tissues. Thus, while capsaicin is equally potent in both tissues, RTX, olvanil and anandamide are less potent in the bronchi.
The low intrinsic efficacy of anandamide indicates that its efficacy is significantly affected by changes in the receptor reserve in a given tissue. Circumstances that increase TRPV1 expression levels will conspire to increase the efficacy of anandamide such that it behaves as a full agonist. In vitro, the nerve growth factor (NGF) and glial-derived neurotrophic factor regulate DRG neurone responses to capsaicin (Winter et al., 1988) and the treatment of DRG neurones with NGF increases the levels of TRPV1 mRNA (Winston et al., 2001). This has two important implications for the activation of TRPV1 by anandamide. Firstly, the levels of NGF used in cultures of DRG neurones may vary between labs and this may affect the efficacy and potency of anandamide measured by different research groups. Secondly, alterations in the levels of these growth factors may be associated with the development of chronic pain (for review see Di Marzo et al., 2002a) and any associated changes in the TRPV1 receptor expression will alter the efficacy of anandamide.
The TRPV1 receptor is associated with the pathophysiology of various major diseases (Szallasi, 2002). Alterations in the expression and sensitivity of TRPV1 receptors is concurrent with the development of chronic pain (see Di Marzo et al., 2002a) and inflammation (Amaya et al., 2003). The expression of TRPV1 is dramatically increased in colonic nerve fibres of patients with active inflammatory bowel disease (Yiangou et al., 2001). Patients with asthma show a significantly enhanced sensitivity to capsaicin as compared to normal subjects (see Spina & Page, 2002), and TRPV1 may be a key mediator in the pathophysiology of asthma (see Hwang & Oh, 2002). Hudson et al. (2001) demonstrate that in an animal model of neuropathic pain, VR1 immunoreactivity increases significantly in both C and A fibres in undamaged DRG neurones.
Anandamide uptake and metabolism
Anandamide membrane transporter
The agonist binding site on TRPV1 is thought to be intracellular (Jung et al., 1999) and consequently the potency of exogenously added anandamide is affected by its ability to enter the cell. The anandamide membrane transporter (AMT) is responsible for the uptake of extracellular anandamide and compounds that alter the function of the AMT modulate the potency of anandamide in HEK cells expressing TRPV1 (De Petrocellis et al., 2001a; and see Di Marzo et al., 2002a). The AMT is activated by nitric oxide (Maccarrone et al., 2000a) and the TRPV1 receptor-mediated component of anandamide-induced vasorelaxation in the mesenteric arterial bed is attenuated by the nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester (L-NAME) (Harris et al., 2002). A recent study by Andersson et al. (2002) provides evidence that primary afferent fibres express the AMT and that variability in the expression levels of the transporter affects the potency of anandamide. Thus the AMT inhibitor, VDM13, causes a 2.3-fold rightward shift in the log concentration – response curve for vasodilatation of mesenteric arteries by anandamide, but does not affect the contractile response to anandamide in the bronchus. However, even in the presence of the uptake inhibitor, anandamide is still a full agonist at TRPV1 in mesenteric arteries as compared to a partial agonist in the bronchi. As discussed earlier, the authors demonstrate that the remaining tissue differences are due to a lower receptor reserve in the bronchus. It should be noted that the existence of the AMT is controversial (see Glaser et al., 2003). A number of AMT inhibitors are also inhibitors of fatty acid amide hydrolase (FAAH) and as such, may increase the intracellular anandamide levels by inhibiting hydrolysis.
Fatty acid amide hydrolase (FAAH)
Inside the cell, anandamide is rapidly metabolised by FAAH to yield arachidonic acid and ethanolamide. There is evidence that FAAH inhibitors enhance, attenuate or have no effect on TRPV1 activation by anandamide. Thus, as has been demonstrated for CB1 receptors (Cravatt et al., 2001), the affinity and potency of anandamide at TRPV1 is enhanced by the inhibition of FAAH in certain tissues including CHO cells (Ross et al., 2001b), HEK 293 (De Petrocellis et al., 2001a), rat ileum (McVey et al., 2002) and neuroblastoma cells (Maccarrone et al., 2000c). On the other hand, in the mesenteric bed, FAAH inhibitors attenuate the TRPV1 receptor-mediated relaxation by anandamide (Orliac et al., 2003). In the bronchus, however, the potency of anandamide as a TRPV1 agonist is unaffected by FAAH inhibitors (Tucker et al., 2001; Andersson et al., 2002).
The intraluminal administration of anandamide causes inflammation of the rat ileum resulting in enteritis. The effect involves stimulation of substance P release from sensory fibres and is antagonised by capsazepine (McVey et al., 2002). It is more potent in the presence of FAAH inhibitors. Inflammation of the ileum by Clostridium diffificle toxin A is known to involve the TRPV1 receptor activation and McVey et al. (2002) have also demonstrated that toxin A stimulates the release of anandamide. Moreover, FAAH inhibitors significantly increase the inflammatory effects of this toxin, indicating that endocannabinoids may mediate its inflammatory actions. In endotoxaemic rats in which LPS has been used to mimic Gram-negative sepsis, anandamide causes a reduction in the contractile responses of mesenteric beds to noradrenaline (Orliac et al., 2003). In this case, the effect of anandamide is inhibited by capsazepine and an FAAH inhibitor and thus appears to involve a metabolite of anandamide that is as a TRPV1 receptor agonist.
Lipoxygenase
The lipoxygenase metabolites of arachidonic acid, particularly 12-(S)-hydroperoxyeicosatetraenoyl acid (12-(S)-HPETE), 5-(S)HETE and leukotriene B4 (LTB4), are agonists of the TRPV1 receptor (Hwang et al., 2001; Piomelli, 2001). Recent studies of the action of the potent inflammatory mediator bradykinin, provide more compelling evidence for the role of lipoxygenase metabolites in the activation of TRPV1. Thus, bradykinin activation of TRPV1 receptors in both cultured DRG neurones and the skin is significantly attenuated by lipoxygenase inhibitors (Shin et al., 2002). Of the neurones that respond electrophysiologically to bradykinin, 75% coexpress mRNAs encoding the TRPV1, B2 receptor and 12-lipoxy-genase. Furthermore, extracellular recording from C fibre receptive fields in guinea-pig isolated airways reveals that lipoxygenase inhibitors dramatically inhibit bradykinin-induced action potentials that are TRPV1 receptor mediated (Carr et al., 2002): the nonselective lipoxygenase inhibitor, ETYA, and the 12-lipoxygenase inhibitor, baicalein, reduce the effect of bradykinin by 76 and 60%, respectively. It is known that 15-HPETE is synthesised by bronchial epithelial cells and induces persistent airway hyper-responsiveness that is sensitive to pretreatment with capsaicin (see Spina & Page, 2002). In addition, protease-activated receptor 2 causes TRPV1 receptor-mediated coronary vasodilation that involves a lipoxygenase-derived product (McLean et al., 2002).
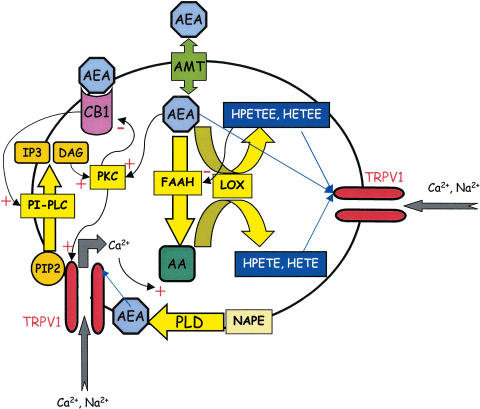
In addition to hydrolysis by FAAH, anandamide can also be metabolised by a range of oxygenase enzymes that are already known to convert arachidonic acid into potent biologically active compounds (see Kozak & Marnett, 2002; Ross et al., 2002). These include cyclooxygenase-2, lipoxygenase and P450 enzymes. In vitro, 12- and 15-lipoxygenase convert anandamide into 12- and 15-HPETE ethanolamide (HPETEE), respectively, the reaction rates being similar to those for arachidonic acid (see Kozak & Marnett, 2002). Thus, the pharmacology of anandamide may be directed by tissue-specific differences in the balance of metabolic enzymes. For example in platelets, which lack significant expression of COX-2 or FAAH, the primary metabolism of anandamide is by lipoxygenase (Edgemond et al., 1998). As an alternative substrate for lipoxygenase enzymes, anandamide may attenuate the production of metabolites of arachidonic acid that are TRPV1 receptor agonists. Alternatively, anandamide may activate TRPV1 via lipoxygenase metabolites of arachidonic acid formed subsequent to its metabolism by FAAH. A further possibility is that anandamide lipoxygenase metabolites may themselves activate TRPV1 receptors (Figure 1). In the bronchus, the TRPV1 receptor-mediated contractile action of anandamide is little affected by FAAH inhibitors, but is significantly attenuated by lipoxygenase inhibitors (Craib et al., 2001). These data suggest that, in this tissue, anandamide may be metabolised to hydroperoxyeicosatetraenoyl ethanolamides (HPETEEs) and LTB4 ethanolamides that, like the hydroperoxy derivatives of arachidonic acid (HPETEs) and LTB4, may be vanilloid receptor agonists. In hTRPV1-HEK cells, the anandamide lipoxygenase metabolites, 11(S)-HPETEE and 5(S)-HPETEE, do not induce an increase in [Ca2+]i and 15(S)-HPETEE has only a modest effect at concentrations above 10 μM (De Petrocellis et al., 2001a). However, it is feasible that these compounds and related molecules are more potent as TRPV1 agonists if produced intracellularly via the metabolism of arachidonic acid and/or anandamide close to the intracellular TRPV1 agonist binding site.
Figure 1.
Metabolic pathways of anandamide and arachidonic acid related to the TRPV1 receptor. Anandamide (AEA) is rapidly hydrolysed by FAAH to yield arachidonic acid and ethanolamide. Arachidonic acid (AA) is oxygenated by lipoxygenase enzymes: the products include 12-(S)- and 15-(S)-HPETE, 5-(S)HETE and LTB4 that are TRPV1 agonists (Hwang et al., 2001). Anandamide is also a substrate for lipoxygenase (see Kozak & Marnett, 2002), yielding equivalent HPETE ethanolamides (HPETEE) and HETE ethanolamides (HETEE) that may also be TRPV1 agonists (Craib et al., 2001). The lipoxygenase products of anandamide are potent inhibitors of FAAH (Maccarrone et al., 2000d). PKC can activate the TRPV1 receptor directly (Premkumar & Ahern, 2000; Olah et al., 2002), and it will also sensitise the receptor to other agonists (Vellani et al., 2001). Anandamide can activate PKC directly (De Petrocellis et al., 1995; Premkumar & Ahern, 2000). CB1 receptor activation is coupled to the stimulation of PLC, hydrolysis of PIP2 and release of the TRPV1 receptor from the tonic inhibitory effect of this compound (see Hermann et al., 2003). Anandamide formation occurs through phosphodiesterase-mediated cleavage of a phospholipid precursor, N-arachidonoyl-phosphatidylethanolamine (NAPE), and its production in and release from neurones is calcium dependent (Di Marzo et al., 1994). In cultured neurones, anandamide is synthesised in response to TRPV1 receptor activation (Ahluwalia et al., 2003).
In the bronchus, lipoxygenase inhibitors modestly attenuate the contractile action of capsaicin (Craib et al., 2001), raising the possibility that the increase in intracellular calcium elicited by TRPV1 receptor activation leads to the release of arachidonic acid and/or anandamide, whose hydroxylation by lipoxygenase may lead to the formation of compounds that are themselves vanilloid agonists. Indeed, mass spectrometric analysis shows that capsaicin and depolarisation (KCl) induce a significant release of anandamide in DRG cultures (Ahluwalia et al., 2003). It is perhaps notable that the capsaicin-evoked release of anandamide is significantly attenuated when the FAAH inhibitor, MAFP, is excluded from the buffer, demonstrating that anandamide is rapidly metabolised in DRG neurones. Metabolic products of anandamide have also been implicated in anandamide-induced depolarisation of the guinea-pig isolated vagus nerve that is TRPV1 receptor mediated (Kagaya et al., 2002). In this preparation, depolarisation by anandamide, but not capsaicin, is inhibited by lipoxygenase inhibitors, but only in the presence of calcium. It is not clear, however, whether these active metabolites are produced via direct lipoxygenase metabolism of anandamide or via the metabolism of arachidonic acid, because these experiments did not include FAAH inhibitors.
Potent FAAH inhibitors have recently been synthesised, which enhance the levels of anandamide significantly, and these compounds may be of considerable therapeutic benefit (Boger et al., 2000). In the event of inhibition of FAAH metabolism of anandamide, increased levels of endogenous anandamide may lead to the production of significant levels of the HPETE ethanolamides. It is also notable that specific inhibitors of 5-lipoxygenase markedly enhance the level of hydrolysis of anandamide by FAAH in human mast cells (Maccarrone et al., 2000b). Thus, Maccarrone et al. hypothesise that 5-lipoxygenase metabolites of anandamide may be acting as endogenous inhibitors of FAAH. To complement the hypothesis, they have demonstrated that various HPETE and HETE ethanolamides are potent endogenous inhibitors of FAAH (Maccarrone et al., 2000d; van der Stelt et al., 2002). Consequently, lipoxygenase metabolism of anandamide may enhance TRPV1 receptor activation by increasing the levels of available anandamide.
Entourage effects
Anandamide is one of a group of N-acylethanolamides that include palmitoylethanolamide (PEA). PEA is cosynthesised with anandamide and acts as an ‘entourage' compound to inhibit the degradation of anandamide and thereby increase the available levels. PEA enhances both the affinity and the potency of anandamide at TRPV1 receptors (De Petrocellis et al., 2001b), an effect shared by other N-acylethanolamides (e.g. lauroylethanolamide) (Smart et al., 2002) (see Table 1). The enhancement of anandamide-induced TRPV1 receptor activation by these compounds does not correlate with their ability to inhibit anandamide metabolism by FAAH (Smart et al., 2002). This suggests that these compounds enhance the action of anandamide by a mechanism other than the inhibition of enzymatic degradation. Therefore, in circumstances that increase the synthesis of N-acylethanolamides, the actions of anandamide at TRPV1 may be enhanced.
Phosphorylation
Phosphorylation of TRPV1 by protein kinase C (PKC) and protein kinase A (PKA) sensitises TRPV1 receptors to vanilloids, anandamide, heat and protons (see Di Marzo et al., 2002a). Premkumar & Ahern (2000) report that the repeated application of anandamide evokes progressively larger TRPV1 currents in oocytes and that anandamide treatment enhances proton-evoked currents. The potentiation by anandamide is significantly attenuated by compounds that inhibit PKC, and the authors suggest that anandamide stimulates PKC to enhance agonist-evoked responses (Figure 1). These experiments were performed in an oocyte expression system injected with TRPV1, thus anandamide actions are presumably cannabinoid receptor independent. Indeed, there is evidence that by binding directly to the diacylglycerol site, anandamide has a dual modulatory effect on PKC in the rat brain in vitro: while anandamide increases phosphatidylserine-induced PKC activation, it also inhibits dioleylglycerol-induced potentiation of Ca2+-induced PKC activation (De Petrocellis et al., 1995).
PKC is a series of isozymes that are specific for different substrates and there is controversy as to which isozyme is responsible for PKC-mediated enhancement of TRPV1 responses (see Cesare et al., 1999; Numazaki et al., 2002; Olah et al., 2002). In cultured DRG neurons, the PKC activator, phorbol 12,13 dibutyrate (PBDu), induces TRPV1 receptor activation; in cultures treated chronically with PBDu, there is a significant downregulation of the PKCα isoform (and only a partial downregulation of PKC β, δ, ɛ isoforms) with concomitant loss of TRPV1 receptor activation (Olah et al., 2002). While anandamide activates TRPV1 receptors in cultures in which PKCα is downregulated, it is four-fold less potent and this may implicate PKCα in modulating the potency of anandamide (Olah et al., 2002).
Phosphorylation of TRPV1 is likely to be associated with inflammation (Cesare et al., 1999). A comparative study of PKCγ immunoreactivity in the dorsal horn before and after the induction of inflammation in the rat hind paw reveals that there are persistent alterations that parallel the time course of allodynia (Martin et al., 1999). Kamei et al. (2001) have produced evidence that allodynia and hyperalgesia in diabetic mice may be due to the sensitisation of VR1 receptors in primary sensory neurones and there is evidence of an upregulation of certain isoforms of PKC in diabetes (Borghini et al., 1994; Koya & King, 1998). Furthermore, PKC inhibitors have been shown to have beneficial effects on diabetic neuropathy (Cameron et al., 1999).
As discussed above, PKC and PKA facilitate the activation of TRPV1 by anandamide. In contrast, PKC-mediated phosphorylation of the CB1 receptor prevents cannabinoid-mediated activation of Kir currents and the inhibition of P/Q-type Ca2+ channels (Garcia et al., 1998). The CB1 receptor inhibits adenylyl cyclase via the α-subunit of Gi resulting in the inhibition of PKA activation. Thus, one may anticipate that PKC and PKA are pivotal in regulating the balance of CB1 and TRPV1 activation by anandamide (see Di Marzo et al., 2002a; Hermann et al., 2003) (Figure 1).
Phosphatidyl-inositol-bis phosphate
The TRPV1 receptor is under the inhibitory control of phosphatidyl-inositol-bis phosphate (PIP2); both antibody sequestration of plasma membrane PIP2 and phospholipase C (PLC) hydrolysis of PIP2 potentiate the TPRV1 channel activation (Chuang et al., 2001). The potent inflammatory mediators, bradykinin and NGF, activate PLC signalling pathways in DRG neurones leading to the hydrolysis of PIP2 to inositol-tris-phosphate and diacylglycerols (DAGs) (Chuang et al., 2001). The authors suggest that, in addition to sensitisation via a PKC-dependent mechanism, TRPV1 is also sensitised by a PKC-independent mechanism consequent to the hydrolysis of PIP2.
In HEK-293 cells overexpressing both the CB1 and TRPV1 receptors, pretreatment with a cannabinoid receptor agonist significantly enhances capsaicin-evoked increases in [Ca2+]i (Hermann et al., 2003). This effect is inhibited by SR141716A and is absent from cells that express TRPV1 only, indicating that CB1 receptor activation leads to an enhanced activation of TRPV1. Furthermore, anandamide produces a significantly greater increase in intracellular calcium in CB1-TRPV1-HEK as compared to TRPV1-HEK cells. The CB1 receptor-mediated enhancement of TRPV1 activation is attenuated by the inhibitors of phosphoinositide-3-kinase (PI-3-K) and PLC. PI-3-K is responsible for the formation of PIP2, while PI-PLC catalyses PIP2 hydrolysis and the result of inhibition of these enzymes will be the attenuation of the turnover and hydrolysis of PIP2. CB1 receptor activation is coupled to the stimulation of PLC and PI-3-K (Ho et al., 1999; Netzeband et al., 1999; Gomez Del Pulgar et al., 2002) and hence may release TRPV1 from tonic inhibition by PIP2. PKC is also activated as a consequence of the hydrolysis of PIP2 to DAG. Hermann et al. (2003) suggest that these mechanisms may underlie the CB1 receptor-mediated enhancement of TRPV1 receptor activation (Figure 1).
Hermann et al. (2003) also found that if cAMP-mediated signalling is activated, CB1 agonists mediate the inhibition and not the enhancement of TRPV1 receptor activation: when PKA is activated via forskolin stimulation of adenylyl cyclase, CB1 receptor agonists inhibit TRPV1 receptor activation by capsaicin. In cells in which there is coexpression of these receptors, the findings of Hermann et al. (2003) raise two possibilities for CB1 receptor influence on the activation of TRPV1. Firstly, CB1 receptor activation may lead to an enhanced TRPV1 receptor activation. One may predict such an outcome in cells in which the CB1 receptor couples to pathways that facilitate that gating of TRPV1. Alternatively, CB1 receptor activation may lead to an inhibition of TRPV1 receptor activation. Such an outcome may be observed in cells in which CB1 receptors are not coupled to PI-PLC or PI-3-K or in which the TRPV1 receptor is phosphorylated by cyclic AMP-dependent PKA activation.
CB1 receptor activation
Within the DRG, there is a heterogeneous population of cells containing small, intermediate and large diameter neurones that give rise to C, Aδ or Aβ fibre axons. Both in culture and in situ, DRG neurones express both CB1 and TRPV1 receptors. The localisation of CB1 and TRPV1 receptor is controversial (see Rice et al., 2002); some studies suggest a high degree of colocalisation (Ahluwalia et al., 2000; 2002) and others suggest little colocalisation (Farquhar-Smith et al., 2000; Khasabova et al., 2002). Anandamide has a dual effect on neuropeptide release from cultured DRG neurones: at low concentrations, anandamide induces a CB1 receptor-mediated inhibition of electrically stimulated neuropeptide release, presumably by the inhibition of voltage-gated Ca2+ channels (Ross et al., 2001a), while at higher concentrations anandamide evokes a TRPV1 receptor-mediated release (Tognetto et al., 2001). Anandamide also attenuates capsaicin-evoked neuropeptide release from DRG neurones and isolated paw skin (Richardson et al., 1998; Nagy et al., 2002). Furthermore, in the presence of a CB1 receptor antagonist, anandamide becomes equipotent with capsaicin as a TRPV1 receptor agonist (Nagy et al., 2002). Similarly, in the guinea-pig ileum, anandamide increases acetylcholine release in a capsazepine-sensitive manner and this effect is enhanced in the presence of the CB1 receptor antagonist, SR141716A (Mang et al., 2001). Finally, in human neuroblastoma cells, cannabinoid receptor antagonists potentiate the TRPV1 receptor-mediated cell death induced by anandamide (Maccarrone et al., 2000c). The implication of such data is that the activation of the CB1 receptor by anandamide may attenuate its TRVP1 receptor-mediated action. In the spinal cord, superfusion of the CB1 receptor antagonist significantly enhances the release of neuropeptide evoked by capsaicin (Lever & Malcangio, 2002). The activation of the TRPV1 receptor may evoke the release of endocannabinoids that subsequently activate CB1 receptors to attenuate the TRPV1 receptor-mediated release of neuropeptide. This hypothesis is supported by the finding that capsaicin induces a significant release of anandamide in DRG cultures (Ahluwalia et al., 2003). Cannabinoid receptors are constitutively active and there are numerous examples of the CB1 receptor antagonist, SR141716A, producing inverse cannabimimetic effects (see Pertwee, 2003; Hurst et al., 2002). In DRG neurons, a cannabinoid agonist inhibits and antagonist enhances voltage-gated Ca2+ currents (Ross et al., 2001a). It is possible that in DRG neurones and the spinal cord, constitutively active CB1 receptors may exert a tonic inhibitory effect on the TRPV1 receptor by attenuating cAMP-dependent PKA activation. In this scenario, inverse agonists may enhance TRPV1 receptor activation.
Ellington et al. (2002) find that the CB1 receptor agonist, CP55940, inhibits capsaicin-evoked calcitonin gene-related peptide (CGRP) release from the paw skin of both the control and diabetic animals. While anandamide inhibits CGRP release from the paw skin of control animals, it produces a small stimulation of CGRP release in diabetic animals. This demonstrates that there is an alteration in the pharmacology of anandamide in the disease state that may be associated with changes in TRPV1 receptor expression and/or sensitivity in sensory fibres of diabetic animals.
Anandamide also has bidirectional effects on cough in conscious guinea-pigs (Jia et al., 2002). When given by aerosol, anandamide induces cough, an effect that is prevented by pretreatment with capsazepine and not CB1 or CB2 receptor antagonists. When airways are pretreated with the ligand for a longer time in experimental conditions, anandamide inhibits cough through CB1 receptors.
Voltage
Voltage-dependent priming of TRPV1 receptors is yet another mechanism by which the activation by anandamide may be enhanced. Ahern & Premkumar (2002) demonstrate that depolarisation acts synergistically with agonists to enhance the TRPV1 current in both oocytes expressing TRPV1 and cultured DRG neurones. Hence, voltage modulates the TRPV1 currents activated by anandamide, protons, heat and PKC (PBDu treatment). Intriguingly, voltage evokes a significantly larger current rise with anandamide and PKC as compared to that observed with capsaicin. Furthermore, the extent of the activation of TRPV1 receptors by voltage is inversely related to the agonist concentration. Consequently, this mechanism is significantly more pronounced for low efficacy agonists such as anandamide.
Temperature, pH, BSA, ethanol
Heat is known to enhance the TRPV1 receptor-mediated responses. Accordingly, the pEC50 values for the elevation of [Ca2+]i in HEK293 cells by anandamide are significantly lower at 37°C compared to 22°C (Sprague et al., 2001). Hence, temperature may contribute to the low efficacy of anandamide in experiments that are conducted at room temperature, including patch-clamp electrophysiology. Olah et al. (2001) found that in both cultured DRG neurones and an NIH3T3 cell line stably expressing TRPV1 receptors, low acidic pH (6.0 and 5.5) markedly enhances the stimulation of [45Ca2+] uptake by anandamide. However, in TRPV1-transfected HEK 293 cells, lowering the pH of the buffer from 7.4 to 6.4 does not affect the potency of anandamide (Ralevic et al., 2001; Smart et al., 2001). De Petrocellis et al. (2001c) demonstrate that the potency of anandamide at TRPV1 is markedly reduced in the presence of bovine serum albumin (BSA): 1 μM elevates [Ca2+]i by ∼65, ∼10 and ∼2% in the presence of 0, 0.1 and 0.2% BSA, respectively. The authors suggest that BSA prevents the uptake of anandamide, interfering with the carrier-mediated internalisation of this compound, and the subsequent activation of intracellular TRPV1. Ethanol (0.1–3%) enhances TRPV1 receptor activation by capsaicin, heat, protons and anandamide as measured using either FLIPR or electrophysiology (Trevisani et al., 2002). It is conceivable that TRPV1 receptor activation by anandamide may be greater if ethanol is used as the vehicle as opposed to DMSO.
Central nervous system
TRPV1 receptors have been thought to be primarily associated with the peripheral nervous system and in particular, primary afferent fibres, yet PCR studies show that TRPV1 mRNA is present in brain tissue and is especially high in the CA1 and CA3 regions of the hippocampus (Mezey et al., 2000). When paired-pulse stimulation is applied to hippocampal slices, there is a depression of the second population spike evoked at interpulse intervals of 5 – 200 ms. The inhibitory feedback is due to the release of GABA following the first stimulation. Al-Hayani et al. (2001) demonstrate that the TRPV1 receptor agonists RTX, capsaicin and anandamide increase paired-pulse depression in the CA1 region of the rat hippocampal slice. The effect is blocked by the TRPV1 receptor antagonist, capsazepine. In contrast, the CB1 receptor agonist, WIN55212, and the endocannabinoid 2-arachidonoyl glycerol, reduce paired-pulse depression, an effect that is blocked by the CB1 receptor antagonist AM251 and is consistent with the CB1 receptor-mediated inhibition of GABA release (see Davies et al., 2002). In view of the low intrinsic efficacy of anandamide at TRPV1 as compared to CB1, it is unexpected that anandamide should preferentially activate the TRPV1 receptor in this preparation. An interesting possibility is that anandamide activation of the CB1 receptor leads to an enhancement of TRPV1 activation by liberating TRPV1 from tonic inhibition by PIP2 (see Hermann et al., 2003). The experiments were performed in the absence of an FAAH inhibitor and another explanation may be the rapid conversion of anandamide to an active metabolite. Other possibilities include those discussed earlier, in particular receptor reserve and the phosphorylation states of TRPV1 and CB1 in the CA1 region of the hippocampus. A further possibility is that, despite displaying the characteristics of TRPV1, this may be a novel site at which anandamide has a higher potency. Additional effects of vanilloids have been noted in the hippocampus. Hájos & Freund (2002a) found that the CB1/CB2 receptor agonists (WIN55212 and CP55940) and capsaicin modulate excitatory transmission, an effect that is antagonised by capsazepine. The results suggest the existence of a novel site on the hippocampal excitatory axons at which both cannabinoids and vanilloids act to suppress glutamatergic transmission (see Davies et al., 2002; Hájos & Freund, 2002b).
Vanilloid effects have also been reported in the brain stem: microinjection of capsaicin into the sensory nerve terminals of the commissural nucleus of the solitary tract (cNTS), significantly reduces the respiration rate (Geraghty & Mazzone, 2002). The finding that RTX is significantly more potent than capsaicin and that the effects are blocked by capsazepine suggest that this is TRPV1 receptor-mediated. While anandamide and olvanil do not inhibit respiration in this preparation, these compounds significantly attenuate the effect of RTX in a concentration-dependent manner. TRPV1 receptor activation in the cNTS is desensitised by very low concentrations of RTX and the authors suggest that anandamide may be desensitising the receptor. Alternatively, anandamide may be behaving as a partial agonist to antagonise RTX in this preparation.
Marinelli et al. (2002) demonstrate that capsaicin causes an increase in miniature excitatory postsynaptic currents in the rat locus coeruleus, an effect that is mediated by presynaptic TRPV1 receptors that potentiate glutamate and adrenaline/noradrenaline release. Similarly, there is evidence that the substantia nigra pars compacta (SNc) contains presynaptic TRPV1 receptors on glutamatergic synapses to dopaminergic neurones. Spontaneous EPSCs (sEPSCs) are increased and inhibited by a TRPV1 agonist and antagonist respectively (Marinelli et al., 2003). The tissue contains high levels of endogenous anandamide and the authors suggest that the stimulation of TPRV1 in the SNc by endocannabinoids may effect tonic facilitation of glutamate release. In the presence of the CB1 receptor antagonist AM281, anandamide increases the frequency of sEPSCs, albeit at the high concentration of 30 μM. At the lower concentration of 10 μM, anandamide is only active when PKA is stimulated by the adenylyl cyclase activator, forskolin.
Conclusions
A large body of evidence now exists to substantiate that anandamide activates TRPV1 receptors. It is a low intrinsic efficacy TRPV1 agonist behaving as a partial agonist in tissues with a low receptor reserve, while in tissues with high receptor reserve and in circumstances associated with certain disease states, it behaves as a full agonist. Furthermore, there are indications that the endocannabinoid system may play a role in the modulation of TRPV1 receptor activation. The activation of TRPV1 receptors by anandamide has potential implications in the treatment of inflammatory, respiratory and cardiovascular disorders. The relative importance of anandamide as a physiological and/or pathophysiological TRPV1 receptor agonist in comparison to other potential candidates, such as 15-HPETE and NADA, has yet to be revealed.
Abbreviations
- Anandamide
(arachidonyl ethanolamide)
- capsaicin
(3-methoxy-4-hydroxy)benzyl-8-methyl-6-nonenamide
- CB1
cannabinoid receptor
- DRG
dorsal root ganglion
- HETE
hydroxyeicoatetraenoic acids
- HPETE
hydroperoxy-eicosatetraenoic acids
- PDBu
phorbol 12,13 dibutyrate
- PKC
protein kinase C
- RTX
resiniferatoxin
- TRP
transient receptor potential
References
- AHERN G.P., PREMKUMAR L.S. Voltage-dependent priming of rat vanilloid receptor: effects of agonist and protein kinase C activation. J. Physiol.-London. 2002;545:441–451. doi: 10.1113/jphysiol.2002.029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AHLUWALIA J., URBAN L., BEVAN S., CAPOGNA M., NAGY I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurones. Neuroscience. 2002;110:747–753. doi: 10.1016/s0306-4522(01)00601-7. [DOI] [PubMed] [Google Scholar]
- AHLUWALIA J., URBAN L., CAPOGNA M., BEVAN S., NAGY I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- AHLUWALIA J., YAQOOB M., URBAN L., BEVAN S., NAGY I. Activation of capsaicin-sensitive primary sensory neurones induces anandamide production and release. J. Neurochem. 2003;84:585–591. doi: 10.1046/j.1471-4159.2003.01550.x. [DOI] [PubMed] [Google Scholar]
- AL-HAYANI A., WEASE K.N., ROSS R.A., PERTWEE R.G., DAVIES S.N. The endogenous cannabinoid anandamide activates vanilloid receptors in the rat hippocampal slice. Neuropharmacology. 2001;41:1000–1005. doi: 10.1016/s0028-3908(01)00145-9. [DOI] [PubMed] [Google Scholar]
- AMAYA F., OH-HASHI K., NARUSE Y., IIJIMA N., UEDA M., SHIMOSATO G., TOMINAGA M., TANAKA Y., TANAKA M. Local inflammation increases vanilloid receptor-1 expression within distinct subgroups of DRG neurones. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- ANDERSSON D.A., ADNER M., HOGESTATT E.D., ZYGMUNT P.M. Mechanisms underlying tissue selectivity of anandamide and other vanilloid receptor agonists. Mol. Pharmacol. 2002;62:705–713. doi: 10.1124/mol.62.3.705. [DOI] [PubMed] [Google Scholar]
- BENHAM C.D., DAVIS J.B., RANDALL A.D. Vanilloid and TRP channels: a family of lipid-gated cation channels. Neuropharmacology. 2002;42:873–888. doi: 10.1016/s0028-3908(02)00047-3. [DOI] [PubMed] [Google Scholar]
- BOGER D.L., SATO H., LERNER A.E., HEDRICK M.P., FECIK R.A., MIYAUCHI H., WILKIE G.D., AUSTIN B.J., PATRICELLI M.P., CRAVATT B.F. Exceptionally potent inhibitors of fatty acid amide hydrolase: the enzyme responsible for degradation of endogenous oleamide and anandamide. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5044–5049. doi: 10.1073/pnas.97.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORGHINI I., ANIA-LAHUERTA A., REGAZZI R. α, βI, βII, δ and ɛ protein kinase C isoforms and compound activity in sciatic nerve of normal and diabetic rats. J. Neurochem. 1994;62:686–696. [PubMed] [Google Scholar]
- CAMERON N.E., COTTER M.A., JACK A.M., BASSO M.D., HOHMANN T.C. Protein kinase C effects on nerve function, perfusion, Na+, K-ATPase activity and glutathione content in diabetic animals. Diabetologia. 1999;42:1120–1130. doi: 10.1007/s001250051280. [DOI] [PubMed] [Google Scholar]
- CARR M.J., KOLLARIK M., MEEKER S.N., UNDEM B.J. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J. Pharmacol. Exp. Ther. 2002;304:1275–1279. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- CESARE P., DEKKER L.V., SARDINI A., PARKER P.J., MCNAUGHTON P.A. Specific involvement of PKC-ɛ in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- CHUANG H.H., PRESCOTT E.D., KONG H.Y., SHIELDS S., JORDT S.E., BASBAUM A.I., CHAO M.V., JULIUS D. Brady-kinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P-2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- CRAIB S.J., ELLINGTON H.C., PERTWEE R.G., ROSS R.A. A possible role of lipoxygenase in the activation of vanilloid receptors by anandamide in the guinea-pig isolated bronchus. Br. J. Pharmacol. 2001;134:30–37. doi: 10.1038/sj.bjp.0704223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAVATT B.F., DEMAREST K., PATRICELLI M.P., BRACEY M.H., GIANG D.K., MARTIN B.R., LICHTMAN A.H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES S.N., PERTWEE R.G., RIEDEL G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology. 2002;42:993–1007. doi: 10.1016/s0028-3908(02)00060-6. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., BISOGNO T., MACCARRONE M., DAVIS J.B., FINAZZI-AGRO A., DI MARZO V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J. Biol. Chem. 2001a;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., DAVIS J.B., DI MARZO V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001b;506:253–256. doi: 10.1016/s0014-5793(01)02934-9. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., HARRISON S., BISOGNO T., TOGNETTO M., BRANDI I., SMITH G.D., CREMINON C., DAVIS J.B., GEPPETTI P., DI MARZO V. The vanilloid receptor (VR1)-mediated effects of anandamide are potently enhanced by the cAMP-dependent protein kinase. J. Neurochem. 2001c;77:1660–1663. doi: 10.1046/j.1471-4159.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., ORLANDO P., DI MARZO V. Anandamide, an endogenous cannabinomimetic substance, modulates rat brain protein kinase C in vitro. Biochem. Mol. Biol. Int. 1995;36:1127–1133. [PubMed] [Google Scholar]
- DI MARZO V. Foreword-endocannabinoids in the new millennium. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:91–92. doi: 10.1054/plef.2001.0339. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BISOGNO T., DE PETROCELLIS L. Anandamide: some like it hot. Trends Pharmacol. Sci. 2001;22:346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BLUMBERG P.M., SZALLASI A. Endovanilloid signaling in pain. Curr. Opin. Neurobiol. 2002a;12:372–379. doi: 10.1016/s0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., DE PETROCELLIS L., FEZZA F., LIGRESTI A., BISOGNO T. Anandamide receptors. Prostaglandins Leukot. Essent. Fatty Acids. 2002b;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., FONTANA A., CADAS H., SCHINELLI S., CIMINO G., SCHWARTZ J.C., PIOMELLI D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- EDGEMOND W.S., HILLARD C.J., FALCK J.R., KEARN C.S., CAMPBELL W.B. Human platelets and polymorphonuclear leukocytes synthesize oxygenated derivatives of arachidonylethanolamide (anandamide): their affinities for cannabinoid receptors and pathways of inactivation. Mol. Pharmacol. 1998;54:180–188. doi: 10.1124/mol.54.1.180. [DOI] [PubMed] [Google Scholar]
- ELLINGTON H.C., COTTER M.A., CAMERON N.E., ROSS R.A. The effect of cannabinoids on capsaicin-evoked calcitonin gene-related peptide (CGRP) release from the isolated paw skin of diabetic and non-diabetic rats. Neuropharmacology. 2002;42:966–975. doi: 10.1016/s0028-3908(02)00040-0. [DOI] [PubMed] [Google Scholar]
- FARQUHAR-SMITH W.P., EGERTOVA M., BRADBURY E.J., MCMAHON S.B., RICE A.S.C., ELPHICK M.R. Cannabinoid CB1 receptor expression in rat spinal cord. Mol. Cell. Neurosci. 2000;15:510–521. doi: 10.1006/mcne.2000.0844. [DOI] [PubMed] [Google Scholar]
- GARCIA D.E., BROWN S., HILLE B., MACKIE K. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J. Neurosci. 1998;18:2834–2841. doi: 10.1523/JNEUROSCI.18-08-02834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERAGHTY D.P., MAZZONE S.B. Respiratory actions of vanilloid receptor agonists in the nucleus of the solitary tract: comparison of resiniferatoxin with non-pungent agents and anandamide. Br. J. Pharmacol. 2002;137:919–927. doi: 10.1038/sj.bjp.0704931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER S., ABUMRAD N.A., FATADE F., KACZOCHA M., STUDHOLME K.M., DEUTSCH D. Evidence against the presence of an anandamide transporter. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMEZ DEL PULGAR T., DE CEBALLOS M.L., GUZMAN M., VELASCO G. Cannabinoids protect astrocytes from ceramide-induced apoptosis through the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 2002;277:36527. doi: 10.1074/jbc.M205797200. [DOI] [PubMed] [Google Scholar]
- HÁJOS N., FREUND T.F. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacolgy. 2002a;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- HÁJOS N., FREUND T.F. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem. Phys. Lipids. 2002b;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- HARRIS D., MCCULLOCH A.I., KENDALL D.A., RANDALL M.D. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J. Physiol. 2002;539:893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMANN H., DE PETROCELLIS L., BISOGNO T., SCHIANO MORIELLO A., LUTZ B., DI MARZO V. Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor-mediated response. Cell. Mol. Life Sci. 2003;60:607–616. doi: 10.1007/s000180300052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO B.Y., UEZONO Y., TAKADA S., TAKASE I., IZUMI F. Coupling of the expressed cannabinoid CB1 and CB2 receptors to phospholipase C and G-protein-coupled inwardly rectifying K+ channels. Receptors Channels. 1999;6:363–374. [PubMed] [Google Scholar]
- HÖGESTÄTT E.D., ZYGMUNT P.M. Cardiovascular pharmacology of anandamide. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:343–351. doi: 10.1054/plef.2001.0346. [DOI] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., TREVISANI M., AL-HAYANI A., DE PETROCELLIS L., FEZZA F., TOGNETTO M., PETROS T.J., KREY J.F., CHU C.J., MILLER J.D., DAVIES S.N., GEPPETTI P., WALKER J.M., DI MARZO V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUDSON L.J., BEVAN S., WOTHERSPOON G., GENTRY C., FOX A.M., WINTER J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur. J. Neurosci. 2001;12:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- HURST D.P., LYNCH D.L., BARNETT-NORRIS J., HYATT S.M., SELTZMAN H.H., ZHONG M., SONG Z.H., NIE J.J., LEWIS D., REGGIO P.H. N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Mol. Pharmacol. 2002;62:1274–1287. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- HWANG S.W., HAWOON C., KWAK J., LEE S.-Y., KANG C.-J., JUNG J., CHO S., MIN K.H., SUH Y.-G., KIM G., OH U. Direct activation of capsaicin receptors by products of lipoxygenase: endogenous capsaicin-like substances. Proc. Nat. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG S.W., OH U. Hot channels in airways: pharmacology of the vanilloid receptor. Curr. Opin. Pharmacol. 2002;2:235–242. doi: 10.1016/s1471-4892(02)00149-2. [DOI] [PubMed] [Google Scholar]
- IVERSEN L., CHAPMAN V. Cannabinoids: a real prospect for pain relief. Curr. Opin. Pharmacol. 2002;2:50–55. doi: 10.1016/s1471-4892(01)00120-5. [DOI] [PubMed] [Google Scholar]
- JERMAN J.C., GRAY J., BROUGH S.J., OOI L., OWEN D., DAVIS J.B., SMART D. Comparison of effects of anandamide at recombinant and endogenous rat vanilloid receptors. Br. J. Anesthesia. 2002;89:882–887. doi: 10.1093/bja/aef281. [DOI] [PubMed] [Google Scholar]
- JIA Y.L., MCLEOD R.L., WANG X., PARRA L.E., EGAN R.W., HEY J.A. Anandamide induces cough in conscious guinea-pigs through VR1 receptors. Br. J. Pharmacol. 2002;137:831–836. doi: 10.1038/sj.bjp.0704950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDT S.E., JULIUS D. Molecular basis for species specificity to ‘hot' chili peppers. Cell. 2002;108:421–430. doi: 10.1016/s0092-8674(02)00637-2. [DOI] [PubMed] [Google Scholar]
- JUNG J., HWANG S.W., KWAK J., LEE S.Y., KANG C.J., KIM W.B., KIM D., OH U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J. Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGAYA M., LAMB J., ROBBINS J., PAGE C.P., SPINA D. Characterization of the anandamide induced depolarization of guinea-pig isolated vagus nerve. Br. J. Pharmacol. 2002;137:39–48. doi: 10.1038/sj.bjp.0704840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMEI J., ZUSHIDA K., MORITA K., SASAKI M., TANAKA S.I. Role of vanilloid VR1 receptor in thermal allodynia and hyperalgesia in diabetic mice. Eur. J. Pharmacol. 2001;422:83–86. doi: 10.1016/s0014-2999(01)01059-7. [DOI] [PubMed] [Google Scholar]
- KHASABOVA I.A., SIMONE D.A., SEYBOLD V.S. Cannabinoids attenuate depolarization-dependent Ca2+ influx in intermediate-size primary afferent neurons of adult rats. Neuroscience. 2002;115:613–625. doi: 10.1016/s0306-4522(02)00449-9. [DOI] [PubMed] [Google Scholar]
- KOYA D., KING G.L. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–866. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- KOZAK K.R., MARNETT L.J. Oxidative metabolism of endocannabinoids. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:211–220. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- LEVER I.J., MALCANGIO M. CB1 receptor antagonist SR141716A increases capsaicin-evoked release of substance P from the adult mouse spinal cord. Br. J. Pharmacol. 2002;135:21–24. doi: 10.1038/sj.bjp.0704506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACCARRONE M., BARI M., LORENZON T., BISOGNO T., DI MARZO V., FINAZZI-AGRO A. Anandamide uptake by human endothelial cells and its regulation by nitric oxide. J. Biol. Chem. 2000a;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- MACCARRONE M., FIORUCCI L., ERBA F., BARI M., FINAZZIAGRO A., ASCOLI F. Human mast cells take up and hydrolyze anandamide under the control of 5-lipoxygenase and do not express cannabinoid receptors. FEBS Lett. 2000b;468:176–180. doi: 10.1016/s0014-5793(00)01223-0. [DOI] [PubMed] [Google Scholar]
- MACCARRONE M., LORENZON T., BARI M., MELINO G., FINAZZI-AGRO A. Anandamide induces apoptosis in human cells via vanilloid receptors – evidence for a protective role of cannabinoid receptors. J. Biol. Chem. 2000c;275:31938–31935. doi: 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- MACCARRONE M., SALVATI S., BARI M., FINAZZI-AGRO A. Anandamide and 2-arachidonoylglycerol inhibit fatty acid amide hydrolase by activating the lipoxygenase pathway of the arachidonate cascade. Biophys. Res. Commun. 2000d;278:576–583. doi: 10.1006/bbrc.2000.3869. [DOI] [PubMed] [Google Scholar]
- MANG C.F., ERBELDING D., KILBINGER H. Differential effects of anandamide on acetylcholine release in the guinea-pig ileum mediated via vanilloid and non-CB1 cannabinoid receptors. Br. J. Pharmacol. 2001;134:161–167. doi: 10.1038/sj.bjp.0704220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINELLI S., DI MARZO V., BERRETTA N., MATIAS I., MACCARRONE M., BERNARDI G., MERCURI N.B. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurones of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J. Neurosci. 2003;23:3136–3144. doi: 10.1523/JNEUROSCI.23-08-03136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINELLI S., VAUGHAN C.W., CHRISTIE M.J., CONNOR M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J. Physiol. 2002;543:531–540. doi: 10.1113/jphysiol.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARSHALL I.C.B., OWEN D.E., CRIPPS T.V., DAVIS J.B., MCNULTY S., SMART D. Activation of vanilloid receptor 1 by resiniferatoxin mobilizes calcium from inositol 1,4,5-triphosphate-sensitive stores. Br. J. Pharmacol. 2003;138:172–176. doi: 10.1038/sj.bjp.0705003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN W.J., LIU H., WANG H., MALMBERG A.B., BASBAUM A.I. Inflammation-induced up-regulation of protein kinase Cγ immunoreactivity in rat spinal cord correlates with enhanced nociceptive processing. Neuroscience. 1999;88:1267–1274. doi: 10.1016/s0306-4522(98)00314-5. [DOI] [PubMed] [Google Scholar]
- MCCLEAN P.G., ASTON D., SARKAR D., AHLUWALIA A. Protease-activated receptor-2 activation causes EDHF-like coro-nary vasodilation. Circ. Res. 2002;90:465–472. doi: 10.1161/hh0402.105372. [DOI] [PubMed] [Google Scholar]
- MCVEY D.C., SCHMID P.C., SCHMID H.H.O., VIGNA S.R. Endocannabinoids induce ileitis in rats via the capsaicin receptor (VR1) J. Pharmacol. Exp. Ther. 2002;304:713–722. doi: 10.1124/jpet.102.043893. [DOI] [PubMed] [Google Scholar]
- MELCK D., BISOGNO T., DE PETROCELLIS L., CHUANG H.H., JULIUS D., BIFULCO M., DI MARZO V. Unsaturated long-chain N-acyl-vanillyl-amides (N-AVAMs): vanilloid receptor ligands that inhibit anandamide-facilitated transport and bind to CB1 cannabinoid receptors. Biochem. Biophys. Res. Commun. 1999;262:275–284. doi: 10.1006/bbrc.1999.1105. [DOI] [PubMed] [Google Scholar]
- MEZEY E., TOTH Z.E., CORTRIGHT D.N., ARZUBI M.K., KRAUSE J.E., ELDE R., GOU A., BLUMBERG P.M., SZALLASI A. Distribution of mRNA for vanilloid subtype 1 (VR1), and VR1-like immunoreactivity in the central nervous system of the rat and human. Proc. Nat. Acad. U.S.A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORISSET V., AHLUWALIA J., NAGY I., URBAN L. Possible mechanisms of cannabinoid-induced antinociception in the spinal cord. Eur. J. Pharmacol. 2001;429:93–100. doi: 10.1016/s0014-2999(01)01309-7. [DOI] [PubMed] [Google Scholar]
- NAGY I., AHLUWALIA J., URBAN L. Dual effect of anandamide on nociceptive primary sensory neurones. Soc. Neurosci. Abstr. 2002;2002:65. [Google Scholar]
- NETZEBAND J.G., CONROY S.M., PARSONS K.L., GRUOL D.L. Cannabinoids enhance NMDA-elicited Ca2+ signals in cerebellar granule neurons in culture. J. Neurosci. 1999;19:8765–8777. doi: 10.1523/JNEUROSCI.19-20-08765.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUMAZAKI M., TOMINAGA T., TOYOOKA H., TOMINAGA M. Phosphorylation of capsaicin receptor VR1 by protein kinase C and identification of two target serine residues. J. Biol. Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- OLAH Z., KARAI L., IADAROLA M.J. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J. Biol. Chem. 2001;276:31163–31170. doi: 10.1074/jbc.M101607200. [DOI] [PubMed] [Google Scholar]
- OLAH Z., KARAI L., IADAROLA M.J. Protein kinase C alpha is required for vanilloid receptor 1 activation – evidence for multiple signaling pathways. J. Biol. Chem. 2002;277:35752–35759. doi: 10.1074/jbc.M201551200. [DOI] [PubMed] [Google Scholar]
- ORLIAC M.L., PERONI R., CELUCH S.M., ADLER-GRACHINSKY E. Potentiation of anandamide effects in mesenteric beds isolated from endotoxemic rats. J. Pharmacol. Exp. Ther. 2003;304:179–184. doi: 10.1124/jpet.102.041095. [DOI] [PubMed] [Google Scholar]
- PAROLARO D., MASSI P., RUBINO T., MONTI E. Endocannabinoids in the immune system and cancer. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:319–332. doi: 10.1054/plef.2001.0355. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G.Inverse agonism at cannabinoid receptors Esteve Foundation Symposium X. Inverse Agonism 2003Amsterdam: Elsevier; (in press) [Google Scholar]
- PERTWEE R.G., ROSS R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- PIOMELLI D. The ligand that came from within. Trends Pharmacol. Sci. 2001;22:17–19. doi: 10.1016/s0165-6147(00)01602-3. [DOI] [PubMed] [Google Scholar]
- PREMKUMAR L.S., AHERN G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., JERMAN J.C., MIDDLEMISS D.N., SMART D. Cannabinoid activation of recombinant and endogenous vanilloid receptors. Eur. J. Pharmacol. 2001;424:211–219. doi: 10.1016/s0014-2999(01)01153-0. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., KENDALL D.A., RANDALL M.D., SMART D. Cannabinoid modulation of sensory neurotransmission via cannabinoid and vanilloid receptors: roles in regulation of cardiovascular function. Life Sci. 2002;71:2577–2594. doi: 10.1016/s0024-3205(02)02086-6. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., HARRIS D., KENDALL D.A., RALEVIC V. Cardiovascular effects of cannabinoids. Pharmacol. Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- RICE A.S.C., FARQUHAR-SMITH W.P., NAGY I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- RICHARDSON J.D., KILO S., HARGREAVES K.M. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- ROBERTS L.A., CHRISTIE M.J., CONNOR M. Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons. Br. J. Pharmacol. 2002;137:421–428. doi: 10.1038/sj.bjp.0704904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R.A., COUTTS A.A., MCFARLANE S., IRVING A.J., PERTWEE R.G., MACEWAN D.J., SCOTT R.H. Actions of cannabinoid receptor ligands on rat cultured sensory neurones: implications for antinociception. Neuropharmacology. 2001a;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., CRAIB S.J., STEVENSON L.A., PERTWEE R.G., HENDERSON A., TOOLE J., ELLINGTON H.C. Pharmacological characterization of the anandamide cyclooxygenase metabolite: prostaglandin E2 ethanolamide. J. Pharmacol. Exp. Ther. 2002;301:900–907. doi: 10.1124/jpet.301.3.900. [DOI] [PubMed] [Google Scholar]
- ROSS R.A., GIBSON T.M., BROCKIE H.C., LESLIE M., PASHMI G., CRAIB S.J., DI MARZO V., PERTWEE R.G. Structure – activity relationship for the endogenous cannabinoid, anandamide, and certain of its analogues at vanilloid receptors in transfected cells and vas deferens. Br. J. Pharmacol. 2001b;132:631–640. doi: 10.1038/sj.bjp.0703850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVIDGE J., DAVIS C., SHAH K., COLLEY S., PHILLIPS E., RANASINGHE S., WINTER J., KOTSONIS P., RANG H., MCINTYRE P. Cloning and functional characterization of the guinea pig vanilloid receptor 1. Neuropharmacology. 2002;43:450–456. doi: 10.1016/s0028-3908(02)00122-3. [DOI] [PubMed] [Google Scholar]
- SHIN J., CHO H., HWANG S.W., JUNG J., SHIN C.Y., LEE S.-Y., KIM S.H., LEE M.G., CHOI Y.H., KIM J., HABER N.A., REICHLING D.B., KHASAR S., LEVINE J.D., OH U. Bradykinin-12-lipoxygenase-VR1 signalling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. U.S.A. 2002;99:10150–10155. doi: 10.1073/pnas.152002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., JERMAN J.C., GUNTHORPE M.J., BROUGH S.J., RANSON J., CAIRNS W., HAYES P.D., RANDALL A.D., DAVIS J.B. Characterisation using FLIPR of human vanilloid VR1 receptor pharmacology. Eur. J. Pharmacol. 2001;417:51–58. doi: 10.1016/s0014-2999(01)00901-3. [DOI] [PubMed] [Google Scholar]
- SMART D., JONSSON K.O., VANDEVOORDE S., LAMBERT D.M., FOWLER C.J. ‘Entourage' effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br. J. Pharmacol. 2002;136:452–458. doi: 10.1038/sj.bjp.0704732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPRAGUE J., HARRISON C., ROWBOTHAM D.J., SMART D., LAMBERT D.G. Temperature-dependent activation of recombinant rat vanilloid VR1 receptors expressed in HEK293 cells by capsaicin and anandamide. Eur. J. Pharmacol. 2001;423:121–125. doi: 10.1016/s0014-2999(01)01123-2. [DOI] [PubMed] [Google Scholar]
- SPINA D., PAGE C.P. Asthma – a need for a re-think. TIPS. 2002;23:311–315. doi: 10.1016/s0165-6147(02)02022-9. [DOI] [PubMed] [Google Scholar]
- SZALLASI A. Vanilloid (capsaicin) receptors in health and disease. Am. J. Clin. Pathol. 2002;118:110–121. doi: 10.1309/7AYY-VVH1-GQT5-J4R2. [DOI] [PubMed] [Google Scholar]
- TREVISANI M., SMART D., GUNTHORPE M.J., TOGNETTO M., BARBIERI M., CAMPI B., AMADESI S., GRAY J., JERMAN J.C., BROUGH S.J., OWEN D., SMITH G.D., RANDALL A.D., HARRISON S., BIANCHI A., DAVIS J.B., GEPPETTI P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat. Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- TOGNETTO M., AMADESI S., HARRISON S., CREMINON C., TREVISANI M., CARRERAS M., MATERA M., GEPPETTI P., BIANCHI A. Anandamide excites central terminals of dorsal root ganglion neurons via vanilloid receptor-1 activation. J. Neurosci. 2001;21:1104–1109. doi: 10.1523/JNEUROSCI.21-04-01104.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUCKER R.C., KAGAYA M., PAGE C.P., SPINA D. The endogenous cannabinoid agonist, anandamide stimulates sensory nerves in guinea-pig airways. Br. J. Pharmacol. 2001;132:1127–1135. doi: 10.1038/sj.bjp.0703906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER STELT M., VAN KUIK J.A., BARI M., VAN ZADELHOFF G., LEEFLANG B.R., VELDINK G.A., FINAZZI-AGRO A., VLIEGENTHART J.F.G., MACCARRONE M. Oxygenated metabolites of anandamide and 2-arachidonoylglycerol: conformational analysis and interaction with cannabinoid receptors, membrane transporter, and fatty acid amide hydrolase. J. Med. Chem. 2002;45:3709–3720. doi: 10.1021/jm020818q. [DOI] [PubMed] [Google Scholar]
- VELLANI V., MAPPLEBECK S., MORIONDO A., DAVIS J.B., MCNAUGHTON P.A. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J. Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER J.M., HUANG S.M. Endocannabinoids in pain modulation. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:235–242. doi: 10.1054/plef.2001.0361. [DOI] [PubMed] [Google Scholar]
- WARDLE K.A., RANSON J., SANGER G.J. Pharmacological characterisation of the vanilloid receptor in the rat dorsal spinal cord. Br. J. Pharmacol. 1997;121:1012–1016. doi: 10.1038/sj.bjp.0701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINSTON J., TOMA H., SHENOY M., PASRICHA P.J. Nerve growth factor regulates VR-1 mRNA levels in cultures of adult dorsal root ganglion neurones. Pain. 2001;89:181–186. doi: 10.1016/s0304-3959(00)00370-5. [DOI] [PubMed] [Google Scholar]
- WINTER J., FORBES C.A., CAMPBELL E.A. Nerve growth factor (NGF) regulates adult rat cultured dorsal root ganglion neuron responses to excitotoxin capsaicin. Neuron. 1988;1:972–981. doi: 10.1016/0896-6273(88)90154-7. [DOI] [PubMed] [Google Scholar]
- YIANGOU Y., FACER P., DYER N.H., CHAN C.L., KNOWLES C., WILLIAMS N.S., ANAD P. Vanilloid receptor 1 immunoreactivity in inflamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SΦRGÅRD M., DI MARZO V., JULIUS D., HÖGESTÄTT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]