Abstract
The aim of the present study was to explore the mechanisms underlying angiotensin II AT2 receptor modulation of AT1 receptor-mediated vasoconstriction in the rat isolated uterine artery, since previous studies have suggested that AT2 receptors may oppose AT1 receptor-mediated effects.
Segments of uterine artery were obtained from Sprague–Dawley rats and mounted in small vessel myographs. Concentration–response (CR) curves to angiotensin II (0.1 nM–0.1 μM) were constructed in the absence and presence of PD 123319 (AT2 antagonist; 1 μM), HOE 140 (bradykinin B2 antagonist; 0.1 μM), Nω-nitro-L-arginine (NOLA) (NOS inhibitor; 30 μM), as well as combinations of these inhibitors. Contractile responses to angiotensin II were expressed as a percent of the response to a K+ depolarizing solution.
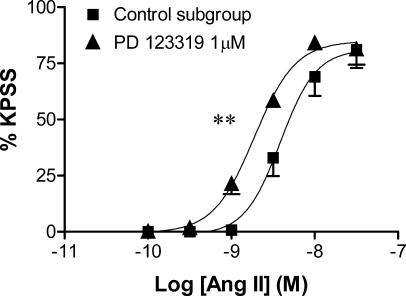
PD 123319 (1 μM) potentiated angiotensin II-induced contractions; reflected by a significant four-fold leftward shift of the angiotensin II CR curve.
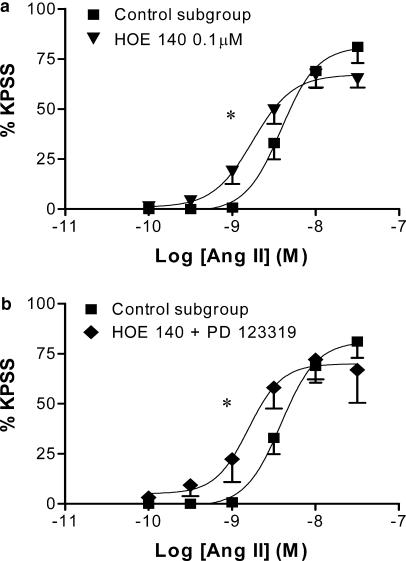
HOE 140 (0.1 μM) significantly increased the pEC50 of the angiotensin II CR curve. The combination of HOE 140 plus PD 123319 did not produce additive potentiation.
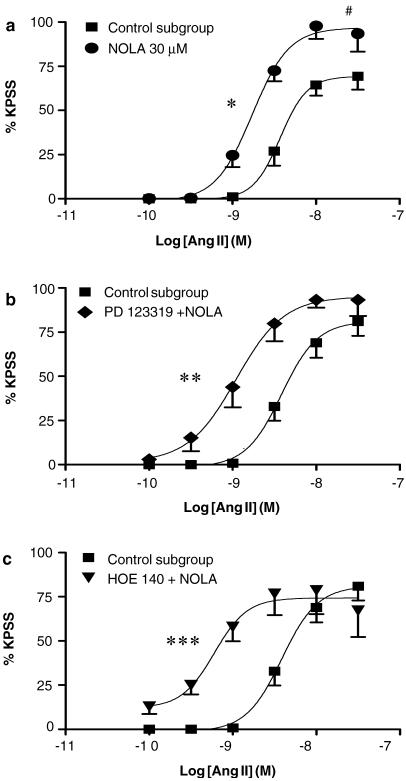
NOLA (30 μM) significantly enhanced sensitivity to angiotensin II, seen as a five-fold leftward shift of the curve, and an augmented maximum contractile response. Combinations of PD 123319 (1 μM) plus NOLA, and of HOE 140 (0.1 μM) plus NOLA, both induced a similar magnitude of potentiation.
Cyclic GMP measurements confirmed angiotensin II-induced activation of the nitric oxide (NO) pathway.
In conclusion, AT2 receptor-mediated inhibition of angiotensin II-induced contraction of the rat uterine artery involves NO production; a component of which occurs through a bradykinin B2 receptor pathway.
Keywords: AT2 receptor, rat uterine artery, angiotensin II, bradykinin, nitric oxide, PD 123319, cyclic GMP, signal transduction
Introduction
The renin–angiotensin system (RAS) plays an integral role in blood pressure regulation and electrolyte balance (Timmermans et al., 1993; Griendling et al., 1996). To date, two pharmacologically distinct angiotensin II receptor subtypes, termed AT1 and AT2, have been characterized (Chiu et al., 1989; Whitebread et al., 1989).
It is through activation of the AT1 receptor that angiotensin II, the main effector peptide of the RAS, exerts many of its well-established cardiovascular effects. By contrast, the physiological role of the AT2 receptor remains incompletely defined. This may be due, in part, to the relatively low levels of AT2 receptor expression in the vasculature (Tsutsumi et al., 1999), and the localization of AT2 receptors in comparatively fewer tissues of cardiovascular relevance than the ubiquitous AT1 receptor (Griendling et al., 1996). However, there has been an increasing body of evidence in the past 10 years, to suggest that AT2 receptors may serve to oppose a range of actions mediated by the AT1 receptor (de Gasparo & Siragy, 1999; Gallinat et al., 2000).
In terms of blood pressure regulation, selective stimulation of unopposed AT2 receptors by circulating angiotensin II has been implicated in the blood pressure-lowering effect of AT1 receptor antagonists in both salt-restricted rats (Siragy et al., 2000) and renal wrap models of hypertension (Siragy & Carey, 1999) Moreover, AT2 receptor stimulation using CGP 42112 has been reported to lower blood pressure in conscious rats (Barber et al., 1999; Carey et al., 2001), and to induce vasodilation in its own right in the perfused rat mesenteric artery (Widdop et al., 2002). It has recently been demonstrated by Widdop et al. (2002) that AT2 receptor-mediated relaxation, in contrast to AT1 receptor-mediated vasoconstriction, is a highly reproducible response, which is maintained even in the presence of chronic AT1 receptor blockade (when circulating angiotensin II levels are elevated). This preservation of AT2 receptor function is critical when considering its potential role in the antihypertensive effect of AT1 receptor antagonists (Siragy & Carey, 1999; Siragy et al., 2000).
A functional interaction between AT1 and AT2 receptors in modulating vascular tone in vitro has been demonstrated previously in our laboratory (Zwart et al., 1998). In isolated uterine arteries from nonpregnant rats, the selective AT2 receptor antagonist PD 123319 potentiated the contractile response to increasing concentrations of angiotensin II, and conversely, the AT2 receptor agonist CGP 42112 inhibited the angiotensin II concentration–response (CR) curve. It is worth noting that the uterine artery is one of few blood vessels reported to contain a predominance of AT2 receptors (Cox et al., 1996); other vascular preparations in which the presence of AT2 receptor mRNA and protein has been established include the rat mesenteric artery (Matrougui et al., 1999) and human renal arteries (Zhuo et al., 1996).
In a number of different experimental models, it has been proposed that the mechanisms underlying AT2 receptor regulation of AT1 receptor-mediated effects may involve the release of the endothelial-derived vasodilators, nitric oxide (NO) and bradykinin (Wiemer et al., 1993; Gohlke et al., 1998; Tsutsumi et al., 1999). Initial studies performed in bovine aortic endothelial cells found that the stimulatory effect of angiotensin II on cellular cyclic GMP content, a measure of NO production, was abolished by both the bradykinin B2 receptor antagonist, HOE 140, and also by the nitric oxide synthase (NOS) inhibitor, L-NNA (Wiemer et al., 1993). In vivo, Siragy and Carey's group have consistently demonstrated, with the use of a renal microdialysis technique, that both endogenous and exogenous angiotensin II-induced increases in cyclic GMP content in renal interstitial fluid of conscious rats is mediated by AT2 receptors (Siragy & Carey, 1996), NO (Siragy & Carey, 1997; 1999) and bradykinin (Siragy & Carey, 1999; Siragy et al., 2000).
The primary aim of the present study was to establish whether an enhanced vasoconstrictor effect of angiotensin II could be unmasked by blocking AT2 receptors, in the rat isolated uterine artery. Given that previous studies have linked AT2 receptor activation with bradykinin and NO release, the second aim of this study was to examine the influence of a bradykinin B2 receptor antagonist and NOS inhibitor on both angiotensin II-induced contractions, and the potentiating effect of PD 123319. We also measured uterine artery cyclic GMP content to determine whether angiotensin II stimulates the NO-cyclic GMP cascade directly in this system. Additionally, we measured phenylephrine-induced, α1-adrenoceptor mediated contractions to determine whether these endothelial-derived vasodilators are associated specifically with angiotensin II responses, or if their modulation of vascular tone extends to other constrictor agents as well.
Methods
General procedures
Female Sprague–Dawley rats (weighing 200–260 g, virgin, nonpregnant) were housed in standard rat cages at 21±3°C, with a 12 h light : dark cycle. Food and water were provided ad libitum.
Animals were killed by stunning and exsanguination. The uterine horns, together with their vasculature, were removed en bloc and placed immediately in ice-cold physiological salt solution (PSS; composition in mM: NaCl 119, KCl 4.7, CaCl2.2H2O 2.5, MgSO4.7H2O 1.17, NaHCO3 25, KH2PO4 1.18, ethylene-diaminetetracetic acid (EDTA) 0.027 and glucose 5.5) gassed with carbogen (95% O2 : 5% CO2).
Small sections of uterine artery (449.0±4.9 μm internal diameter, n=59), with the endothelium intact, were dissected free from surrounding connective tissue under a binocular dissection light microscope (Olympus SZ40) and mounted in small vessel myographs as described previously (McPherson, 1992; Zwart et al., 1998). Briefly, two 40 μm wires were threaded through the lumen of the vessel. One wire was attached to a stationary support connected to a micrometer, while the other was attached to an isometric force (DSC6 strain gauge) transducer. Data was recorded on a printer (Panasonic KX-P1180) and captured by the use of a data acquisition system (CVMS Version 2.0, World Precision Instruments, U.S.A.). The vessel segments were maintained in PSS at 37°C and aerated continuously with carbogen.
Following a 30 min equilibration period at 37°C and under zero force, the diameter of each vessel was normalized to an equivalent transmural pressure of 100 mmHg. Using the diameter of the vessel, calculated from the distance between the two mounting wires, a passive diameter–tension curve was constructed as described previously (Mulvany & Halpern, 1977; see McPherson, 1992 for details). From this curve the effective transmural pressure was calculated. The vessels were then set at tension equivalent to that generated at 0.7 times the diameter of the vessel at 100 mmHg (D100), which has been found previously to be optimal for active tension development in this vessel (Davis, 1994).
After normalization, the tissues were equilibrated for 30 min before being contracted with 124 mM K+ solution (KPSS; composition in mM: KCl 123.7, CaCl2.2H2O 2.5, MgSO4.7H2O 1.17, NaHCO3 25, KH2PO4 1.18, EDTA 0.027 and glucose 5.5). Once the response to KPSS had plateaued, the preparations were washed three to four times and rested for 30 min before an experimental protocol was commenced. In addition, a second KPSS response was performed at the completion of the CR curves to ensure that antagonist treatment did not affect tissue reactivity in a nonspecific manner.
Test for the presence of functional endothelium
Prior to treating the tissues with a given inhibitor or antagonist, phenylephrine was added cumulatively in increasing doses to produce a submaximal response equivalent to approximately 40–50% of the KPSS-induced contraction. Acetylcholine (10 μM) was then added to test for the presence of functional endothelium. Preconstricted tissues in which acetylcholine produced less than 50% relaxation were considered to be endothelium-denuded, and were not included in this report.
Contractile responses to angiotensin II
Cumulative (0.5. log unit) CR curves to angiotensin II (0.1 nM–0.1 μM) were constructed after 30 min equilibration with PD 123319 (1 μM) (n=5). Control curves were obtained similarly but without the addition of PD 123319 (n=7). Only one CR curve was obtained per tissue, since preliminary studies had shown the development of marked tachyphylaxis if a second CR curve to angiotensin II was constructed (data not shown).
Angiotensin II CR curves were also constructed after 30 min equilibration with either HOE 140 (0.1 μM) (n=7) or Nω-nitro-L-arginine (NOLA) (30 μM) (n=5), both alone and in combination with PD 123319 (1 μM) (n=3), to determine whether or not any effects of bradykinin B2 receptor blockade or NOS inhibition, respectively, were enhanced by simultaneous AT2 receptor antagonism.
In addition, a subgroup of tissues were pretreated with NOLA (30 μM) plus HOE 140 (0.1 μM) (n=4) prior to the addition of angiotensin II, to observe whether the combination produced any additive potentiation relative to the effect of each inhibitor given alone.
All concentrations of antagonists were based on previous studies. PD 123319, at a concentration of 1 μM, has previously been reported to selectively inhibit AT2 receptors in vitro without affecting responses to other contractile agents (Zwart et al., 1998). Moreover, 100-fold higher concentrations of PD 123319 are required before the compound develops an AT1 receptor antagonist profile (Macari et al., 1993). The concentration of HOE 140 (0.1 μM) was chosen on the basis that this same dose exhibits effective and selective inhibition of bradykinin-induced relaxation in isolated carotid (Bergaya et al., 2001) and coronary arteries (Mombouli & Vanhoutte, 1992). Concentrations of NOS inhibitors in the 10–100 μM range have been used in numerous isolated vascular preparations to demonstrate the role of NO in modulating arterial tone (Endlich et al., 1999). Furthermore, previous experiments conducted in our laboratory have revealed that NOLA, at 30 μM, is sufficient to abolish endothelium-dependent relaxation to ACh in the rat isolated uterine artery (Hannan et al., 2001). Therefore the same concentration was chosen for the present study.
Effect of ACE inhibition on contractile responses
In a subgroup of experiments, we examined further the involvement of bradykinin in modulating contractile responses to angiotensin II. The ACE inhibitor enalaprilat (10 μM) was added to the PSS, in which tissues were incubated for 30 min prior to the construction of angiotensin II CR curves (n=7–8). In separate tissues, angiotensin I (1 nM–30 μM) CR curves were performed in the absence and presence of enalaprilat-treated PSS; these were used as positive controls (n=6).
Contractile responses to phenylephrine
Cumulative CR curves to phenylephrine (0.1–100 μM) were obtained in the absence of any ligand (n=10), or after 30 min incubation with either PD 123319 (1 μM) (n=5), HOE 140 (0.1 μM) (n=7), NOLA (30 μM) (n=10), or combinations of these three inhibitors.
Uterine artery cyclic GMP content
Segments of uterine artery not used in wire myograph studies were incubated in Eppendorf vials containing carbogenated PSS, at 37°C, for an equilibration period of 30 min. Tissues were left untreated (n=8), or were incubated with a single concentration of either angiotensin II (10 nM) (n=5) or the selective AT2 receptor agonist CGP 42112 (1 μM) (n=7) for 4 min, on the basis of previous functional studies from our laboratory (Zwart et al., 1998). At the conclusion of the treatment period, tissues were snap-frozen in liquid nitrogen and stored at −80°C until cyclic GMP content was determined. Segments of uterine artery from different rats were pooled so as to yield at least 5 mg of tissue frozen weight, the minimum tissue sample weight required for cyclic GMP detection. Thus, the small amount of rat uterine artery harvested precluded full CR analysis.
Frozen samples of pooled tissue (≈5–10 mg) were weighed and homogenized in ice-cold 6% trichloroacetic acid. Following centrifugation, the samples were processed according to published methods (Caputo et al., 1995). Cyclic GMP content was determined using an RIA kit (NEN-Life Science, NEX I-33), and expressed as fmol/mg tissue frozen weight.
Statistical analysis
Responses to contractile agents were measured as increases in force (mN) and expressed as a percent of the initial KPSS-induced contraction. Log CR curves from individual tissues were grouped to generate mean log CR curves to angiotensin II and phenylephrine. CR curves obtained in the absence and presence of the given antagonist and inhibitor combinations were analysed using nonlinear regression (Graphpad Prism 3.0, Graphpad Software, San Diego, CA, U.S.A.), and pEC50 values and maximum responses were obtained. At least three to four preparations were obtained from each rat, but only one vessel from each rat received a particular treatment; n=number of vessels. The pEC50 (−log EC50) was defined as the concentration of agonist required to produce 50% of the maximum response.
When comparing treatment groups, in relation to either EC50 values, maximum contractile responses, or cyclic GMP content, statistical significance was determined using either Student's unpaired t-test, or where more than two groups were being compared, a one-way analysis of variance (ANOVA) with a post hoc Bonferroni's multiple comparison test was employed (Graphpad Software, San Diego, CA, U.S.A.). Values shown are expressed as mean±s.e.m. In all cases, statistical significance was accepted where P<0.05.
Drugs
Drugs and their sources were: acetylcholine HCl (Sigma), angiotensin I, angiotensin II (American Peptide Co., U.S.A.), CGP 42112 (Bachem, Switzerland), enalaprilat (Merck Sharp & Dohme, U.S.A.), HOE 140 (Sigma), NOLA(Sigma), PD 123319 (Parke Davis, U.S.A.) and phenylephrine HCl (Sigma). Stock solutions of all drugs were made up in distilled water, with the exception of NOLA, which was made up in 10% NaHCO3, and phenylephrine, which was made up in catecholamine diluent. All drugs were diluted in distilled water on the day of experimentation.
Results
KPSS responses were obtained before and after the completion of angiotensin II CR curves. None of the treatments used in the study significantly altered the contraction evoked by KPSS (17.1±0.5 mN, n=59), indicating that tissue reactivity had not been affected in a nonspecific manner (data not shown).
Presence of functional endothelium
Acetylcholine (10 μM) relaxed phenylephrine-preconstricted tissues by 79.5±1. 8% (n=59), and these were categorized as endothelium-intact. A similar relaxation response was obtained when acetylcholine was readded at the completion of the angiotensin II CR curves (72.4±1.4%, n=10). There was no significant difference in the extent of acetylcholine-induced relaxation in any of the groups prior to treatment. As expected, treatment of the vessels with NOLA (30 μM) abolished endothelium-dependent relaxation to acetylcholine (2.5±6.0% relaxation) (n=7).
Effect of PD 123319 on contractile responses to angiotensin II
In control tissues, angiotensin II produced concentration-dependent contractions, with a pEC50 value of 8.4±0.1 and a maximum contractile response of 81.0±8.1% of the KPSS response (Figure 1).
Figure 1.
Effect of PD 123319 (1 μM) on the mean log CR curves to angiotensin II in uterine arteries from nonpregnant rats (n=5–7). Responses are expressed as a % of the maximum response to KPSS; values are means and vertical lines represent s.e.m.; **P<0.01 versus control EC50.
PD 123319 (1 μM) did not affect baseline tension, but enhanced the potency of angiotensin II in the uterine artery, which was seen as a significant increase in pEC50 (8.8±0.1, P<0.01; Figure 1). This amounted to an approximate four-fold leftward shift of the angiotensin II CR curve. PD 123319 had no effect on the maximum contractile response to angiotensin II.
Effect of HOE 140, alone and in combination with PD 123319, on contractile responses to angiotensin II
HOE 140 (0.1 μM) did not affect baseline tension, but produced a significant four-fold leftward shift of the angiotensin II CR curve (pEC50=8.8±0.1, P<0.05; Figure 2a), while having no effect on the maximum contractile response. The combination of HOE 140 (0.1 μM) and PD 123319 (1 μM) also potentiated the angiotensin II CR curve (pEC50=8.9±0.2, P<0.05; Figure 2b). However, this degree of potentiation was not significantly greater than that seen in response to either antagonist alone (P>0.05, ANOVA).
Figure 2.
Effect of HOE 140 (0.1 μM) on the mean log CR curves to angiotensin II in uterine arteries from nonpregnant rats: (a) alone (n=7) and (b) in combination with PD 123319 (1 μM) (n=3–7). Responses are expressed as a % of the maximum response to KPSS; values are means and vertical lines represent s.e.m.; *P<0.05 versus control EC50.
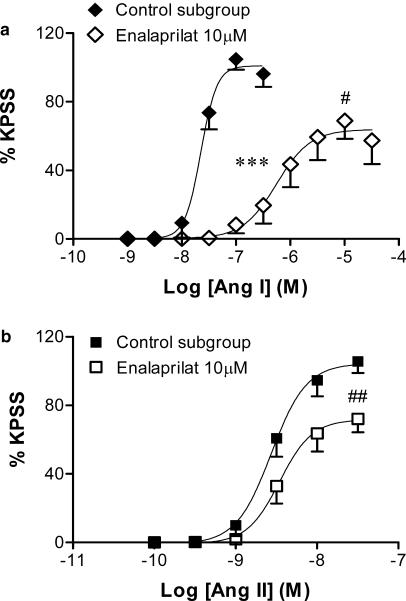
Effect of enalaprilat (10 μM) on contractile responses to angiotensin I and II
In control tissues, angiotensin I produced concentration-dependent contractions, with a pEC50 value of 7.7±0.1 (Figure 3a). Thus, in this subgroup of experiments, angiotensin I was almost 10 times less potent than angiotensin II (pEC50=8.6±0.1, P<0.001, Figure 3b), however, the maximum contractile responses to the two agonists were not significantly different (angiotensin I: 105.8±6.2% KPSS versus angiotensin II: 106.7±6.0% KPSS). Enalaprilat (10 μM) did not affect baseline tension, but significantly decreased the angiotensin I pEC50 (6.1±0.2, P<0.001; Figure 3a), which amounted to an approximate 30-fold rightward shift of the angiotensin I CR curve. However, this shift was not parallel, as the Emax of the angiotensin I CR curve was also significantly reduced by enalaprilat (69.3±10.6% KPSS, P<0.05, Figure 3a).
Figure 3.
Effect of enalaprilat (10 μM) on the mean log CR curves to (a) angiotensin I (n=6) and (b) angiotensin II (n=7–8) in uterine arteries from nonpregnant rats. Responses are expressed as a % of the maximum response to KPSS; values are means and vertical lines represent s.e.m.; ***P<0.001 versus control EC50. #P<0.05, ##P<0.01 versus control maximum response.
Given the potentiating effect of HOE 140 on the angiotensin II CR curve, we examined whether the ACE inhibitor enalaprilat, through increasing endogenous bradykinin availability, could reduce angiotensin II-induced contractions. Enalaprilat (10 μM) tended to cause a rightward shift of the angiotensin II CR curve (pEC50=8.4±0.1, Figure 3b), though this trend was not significant. However, the maximum angiotensin II contractile response was significantly impaired by enalaprilat (74.5±8.5% of the KPSS response, P<0.01, Figure 3b).
Effect of NOLA (30 μM) alone, and in the presence of PD 123319, on contractile responses to angiotensin II
Baseline tension remained unaffected by treatment with NOLA (30 μM), however the NOS inhibitor significantly increased the angiotensin II pEC50 (8.9±0.1, P<0.05; Figure 4a), which resulted in an approximate five-fold leftward shift of the angiotensin II CR curve. In addition, NOLA significantly enhanced angiotensin II-induced maximum contractions (98.8±7.2 versus 69.6±7.5% of the KPSS response, P<0.05, Figure 4a).
Figure 4.
Effect of NOLA (30 μM) on the mean log CR curves to angiotensin II in uterine arteries from nonpregnant rats: (a) alone (n=5), (b) in combination with PD 123319 (1 μM) (n=3–7), and (c) in combination with HOE 140 (0.1 μM) (n=4–7). Responses are expressed as a % of the maximum response to KPSS; values are means and vertical lines represent s.e.m.; *P<0.05, **P<0.01, ***P<0.001 versus control EC50. #P<0.05 versus control maximum response.
The combinations of PD 123319 (1 μM) plus NOLA (30 μM) (pEC50=9.0±0.1, P<0.01; Figure 4b), and of HOE 140 (0.1 μM) plus NOLA (30 μM) (pEC50=9.2±0.1, P<0.001; Figure 4c) significantly potentiated the angiotensin II CR curve, as evidenced by the significant increase in pEC50, without affecting the maximum response. In both cases, the magnitude of potentiation seen was similar to that induced by NOLA (30 μM) alone (Figure 4a). Furthermore, the leftward shift caused by PD 123319 (1 μM) plus NOLA (30 μM) was not significantly different to that seen in response to PD 123319 alone (P>0.05, ANOVA; Figures 1, 4b). However, the degree of potentiation caused by the combination of HOE 140 (0.1 μM) plus NOLA (30 μM) was significantly greater than that induced by HOE 140 alone (P<0.01, ANOVA; Figures 2a, 4c). Maximum angiotensin II contractile responses were not significantly different to control values in the presence of these treatment combinations.
Uterine artery cyclic GMP content
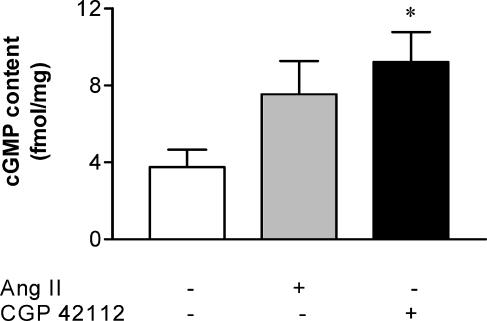
Angiotensin II (10 nM) stimulated an approximate two-fold increase in uterine artery cyclic GMP content relative to basal levels (from 3.8±0.9 to 7.5±1.8 fmol/mg, Figure 5), although this trend was not statistically significant. However, CGP 42112 (1 μM) significantly augmented cyclic GMP content (9.2±1.6 fmol/mg, P<0.05, Figure 5), which amounted to a 2.4-fold increase above basal levels.
Figure 5.
Mean cylic GMP levels in uterine arteries from nonpregnant rats, under control conditions (n=8) and following treatment with either angiotensin II (10 nM) (n=5) or CGP 42112 (1 μM) (n=7). *P<0.05 versus basal levels.
Effect of PD 123319, HOE 140 and NOLA on contractile responses to phenylephrine
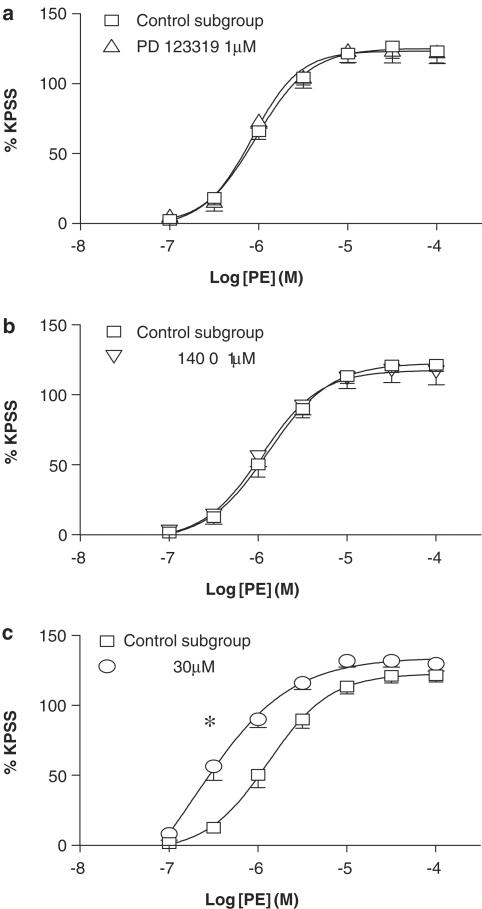
In control tissues, phenylephrine elicited concentration-dependent contractions, with a pEC50 of 5.9±0.1 and a maximum contractile response of 122.3±4.9% of the KPSS response (Figure 6).
Figure 6.
Mean log CR curves to phenylephrine in uterine arteries from nonpregnant rats, under control conditions (n=10), and in the presence of (a) PD 123319 (1 μM) (n=5), (b) HOE 140 (0.1 μM) (n=7), and (c) NOLA (30 μM) (n=10). *P<0.05 versus control EC50.
Neither PD 123319 (1 μM) (Figure 6a) nor HOE 140 (0.1 μM) (Figure 6b) affected CR curves to phenylephrine. In contrast, NOLA (30 μM) significantly augmented the contractile response to phenylephrine, which was reflected by an increased pEC50 (6.5±0.2, P<0.05; Figure 6c), to a similar extent as seen with angiotensin II. This effect was unaltered by cotreatment with either the AT2 receptor or B2 receptor antagonist (data not shown).
Discussion
The main finding of the present study was that, in the uterine artery of nonpregnant rats, AT2 receptor activation selectively opposes angiotensin II-induced contractions. In this preparation, the angiotensin II-mediated responses occur via AT1 receptor stimulation since these are markedly reduced by AT1 receptor blockade (Zwart et al., 1998). Moreover, this modulatory effect involves endogenous NO production; a component of which may occur via a bradykinin B2 receptor pathway.
PD 123319, at a concentration known to selectively antagonize AT2 receptors (Macari et al., 1993), demonstrated the exaggerated vasoconstrictor effect of angiotensin II unmasked by blocking AT2 receptors. Indeed, it was found that PD 123319 potentiated the angiotensin II-induced contractile response by an equivalent magnitude to that reported previously in this vascular preparation under these conditions (Zwart et al., 1998). A similar functional interaction between AT1 and AT2 receptors in modulating tone in the uterine vasculature has also been demonstrated by others in sheep (McMullen et al., 1999; Lambers et al., 2000) and rats (St-Louis et al., 2001).
Previously, we have reported, using this wire myograph system, the lack of direct AT2 receptor-mediated relaxation in precontracted rat uterine arteries (Zwart et al., 1998). Those results contrast with the demonstration of AT2 receptor-mediated relaxation in cannulated, pressurised vessel preparations (Matrougui et al., 1999; Widdop et al., 2002) and point to a lack of sensitivity of the wire myograph to demonstrate modest AT2 receptor-vasodilation directly. In any event, the sensitivity of the wire myograph is obviously sufficient to demonstrate significant counter-regulatory vasodilator effects which were unmasked by AT2 receptor blockade; a phenomenon that has been studied widely, in both in vitro (McMullen et al., 1999; St-Louis et al., 2001) and in vivo (Lambers et al., 2000) settings. Another potential mechanism that may be considered when studying AT1 and AT2 receptor interaction is the fact that these receptors may exist as heterodimers whereby an increased AT2 receptor expression can directly inhibit AT1 receptor signalling (AbdAlla et al., 2001). While this mechanism is unlikely to be involved in the present study, since AbdAlla et al. (2001) found that the AT2 receptor affected AT1 receptors independently of either angiotensin II or PD 123319, it underscores the complex interplay that can occur between angiotensin II receptor subtypes.
To explore the putative link between AT2 receptors, endothelial-derived bradykinin and NO release, we assessed the effects of HOE 140 and NOLA, respectively, on angiotensin II-induced contractions, in the absence and presence of AT2 receptor blockade. HOE 140 alone produced a significant leftward shift of the angiotensin II CR curve, the magnitude of which was equivalent to that seen in the presence of PD 123319. Moreover, the combination of HOE 140 plus PD 123319 did not cause a greater degree of potentiation, relative to that induced by either antagonist alone. This lack of further enhancement suggests that both AT2 receptors and bradykinin B2 receptors activate similar, if not the same intracellular pathways in order to attenuate angiotensin II-induced vasoconstriction.
An involvement of endogenous bradykinin in modulating angiotensin II-induced contractions was supported further by the marked inhibition of angiotensin II-mediated contraction by the ACE inhibitor enalaprilat, although a reduction in angiotensin II-mediated contraction by ACE inhibition was not seen in human vasculature (Chalon et al., 1999; MaassenVanDenBrink et al., 1999) when the effect of bradykinin was potentiated (Chalon et al., 1999). This discrepancy between the present and previous studies may relate to differential counter regulatory influences of AT2 receptors in the vascular preparations studied. While the precise nature of the AT2–bradykinin interaction is not fully understood, it has been proposed that angiotensin II, in reducing intracellular pH levels in endothelial cells, may subsequently activate acid-optimum kininogenases to cleave bradykinin from intracellularly stored kininogens (Wiemer et al., 1993; Tsutsumi et al., 1999). We suggest that a similar interaction may occur in the isolated, endothelium-intact uterine artery preparation.
In agreement with the many studies implicating NO in the regulation of vasomotor tone, NOLA induced a significant leftward shift of the angiotensin II CR curve, together with a potentiated maximum response. In addition, combinations of PD 123319 plus NOLA, and of HOE 140 plus NOLA, both produced a similar degree of enhanced tissue sensitivity to exogenously applied angiotensin II, relative to that caused by NOLA alone. While the HOE 140 plus NOLA combination caused a slightly greater potentiation than NOLA alone (seven- and five-fold, respectively), it would be difficult to argue on this basis alone that bradykinin was activating an additional pathway other than NO. Rather, the relatively similar potentiation seen with the various NOLA combinations would suggest that the counter-regulatory effects of AT2 and bradykinin B2 receptor activation predominantly involve NO production.
Consistent with this interpretation, the leftward shift caused by PD 123319 plus NOLA was not significantly different to that seen in response to PD 123319 alone, which suggests further that, in this preparation, the counter-regulatory vasodilator mechanisms evoked in response to angiotensin II involved AT2 receptor stimulation and NO production. However, the degree of potentiation caused by the combination of HOE 140 plus NOLA was greater than that induced by HOE 140 alone. This could indicate that local kinins, via the activation of B2 receptors, mediate a component of, but not all, the NO produced in response to AT2 receptor stimulation. Similarly, it has been reported that AT2 receptor stimulation is linked to NO production directly, and does not necessarily rely on intermediate bradykinin synthesis in order to activate the NO-cyclic GMP pathway (Siragy et al., 2000).
Our data are consistent with a large body of evidence obtained in a number of diverse experimental settings. In canine coronary arteries, angiotensin II-induced NO release is caused by activation of local kinin production in the vessel wall (Seyedi et al., 1995). Furthermore, the stimulatory effect of angiotensin II on cyclic GMP levels in renal interstitial fluid of conscious rats (Siragy & Carey, 1999; Siragy et al., 2000), in aortic explants from stroke-prone spontaneously hypertensive rats (Gohlke et al., 1998), and in mice overexpressing vascular AT2 receptors (Tsutsumi et al., 1999), is mediated by the AT2 receptor, and occurs via bradykinin- and NO-dependent pathways.
Similarly, in the present study, both angiotensin II and the selective AT2 receptor agonist CGP 42112 increased cyclic GMP production by approximately two-fold in rat isolated uterine artery, and while the effect of angiotensin II did not reach statistical significance, the concentration chosen was submaximal on the basis of functional experiments (Hannan et al., unpublished data). Thus, these results provide evidence that stimulation of AT2 receptors directly activates the cyclic GMP vasodilator cascade in this system, lending support to the results obtained with functional AT2 receptor blockade. However, the possibility that other, non-AT2 receptor mechanisms are involved in activation of the NO-cyclic GMP pathway that serves to modulate angiotensin II-mediated contraction cannot be excluded altogether. Indeed, AT1 receptor activation has itself been associated with endothelial NO synthesis, in rat isolated carotid arteries (Caputo et al., 1995). In any case, the extent of any AT1 receptor-mediated NO release is obviously not sufficient to overcome the overriding contractile response mediated by the same receptor (Zwart et al., 1998).
In a subset of experiments, CR curves to phenylephrine were obtained, to determine on a functional level whether bradykinin and NO are released specifically in response to angiotensin II, or if their modulation of vascular reactivity extends to other constrictor agents. PD 123319 had no effect on the contractile response to the α1-adrenoceptor agonist, which is consistent with previous studies in isolated blood vessels (Maeso et al., 1996; Zwart et al., 1998). Similarly, HOE 140 did not affect phenylephrine CR curves, which suggests that AT2 and B2 receptors are specifically involved in modulating angiotensin II but not other vasoconstrictor responses. By contrast, NOLA caused a significant leftward shift of the phenylephrine CR curve, which was of similar magnitude to its potentiation of angiotensin II-induced vasoconstriction. Thus it appears that endogenous NO plays an important role in modulating uterine arterial responses to various contractile agents, as has also been observed in other vascular preparations (Muller et al., 1997), and is therefore not coupled exclusively to AT2 receptors.
In conclusion, the present study has demonstrated that in the rat isolated uterine artery, AT2 receptor stimulation selectively inhibits AT1 receptor-mediated contraction. This counter-regulatory mechanism appears to involve the activation of the endothelial-derived vasodilator, NO, and subsequent cyclic GMP production; a pathway that may involve, in part, the intermediate synthesis of local kinins and B2 receptor activation. A greater understanding of this AT2 vasodilator cascade, and the signaling mechanisms of its components, under both normal and pathophysiological conditions is of importance. In this context, there are an increasing number of studies implicating AT2 receptor function in some cardioprotective effects of AT1 receptor blockade (Gallinat et al., 2000). Moreover, it has also been suggested that pregnancy is associated with increased AT2 receptor expression (Burrell & Lumbers, 1997) and cyclic GMP production (Magness et al., 1996) in the uterine artery. Whether or not similar counter-regulatory vasodilator mechanisms are operative against angiotensin II in the pregnant state remains to be seen.
Acknowledgments
This work was supported in part by the National Health and Medical Research Council of Australia.
Abbreviations
- ACE
angiotensin-converting enzyme
- CR
concentration–response
- KPSS
high potassium physiological salt solution
- NO
nitric oxide
- NOLA
Nω-nitro-L-arginine
- NOS
nitric oxide synthase
- PSS
physiological salt solution
- RAS
renin–angiotensin system
References
- ABDALLA S., LOTHER H., ABDEL-TAWAB A.M., QUITTERER U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J. Biol. Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- BARBER M.N., SAMPEY D.B., WIDDOP R.E. AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension. 1999;34:1112–1116. doi: 10.1161/01.hyp.34.5.1112. [DOI] [PubMed] [Google Scholar]
- BERGAYA S., MENETON P., BLOCH-FAURE M., MATHIEU E., ALHENC-GELAS F., LEVY B.I., BOULANGER C.M. Decreased flow-dependent dilation in carotid arteries of tissue kallikrein-knockout mice. Circ. Res. 2001;88:593–599. doi: 10.1161/01.res.88.6.593. [DOI] [PubMed] [Google Scholar]
- BURRELL J.H., LUMBERS E.R. Angiotensin receptor subtypes in the uterine artery during ovine pregnancy. Eur. J. Pharmacol. 1997;330:257–267. doi: 10.1016/s0014-2999(97)00167-2. [DOI] [PubMed] [Google Scholar]
- CAPUTO L., BENESSIANO J., BOULANGER C.M., LEVY B.I. Angiotensin II increases cGMP content via endothelial angiotensin II AT1 subtype receptors in the rat carotid artery. Arterioscl. Thromb. Vasc. Biol. 1995;15:1646–1651. doi: 10.1161/01.atv.15.10.1646. [DOI] [PubMed] [Google Scholar]
- CAREY R.M., HOWELL N.L., JIN X.H., SIRAGY H.M. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38:1272–1277. doi: 10.1161/hy1201.096576. [DOI] [PubMed] [Google Scholar]
- CHALON S., BEDARIDA G.V., MORENO H., JR, TEJURA B., URAE A., HOFFMAN B.B., BLASCHKE T.F. Inhibition of angiotensin-converting enzyme in human hand veins. Clin. Pharmacol. Ther. 1999;65:58–65. doi: 10.1016/S0009-9236(99)70122-0. [DOI] [PubMed] [Google Scholar]
- CHIU A.T., HERBLIN W.F., MCCALL D.E., ARDECKY R.J., CARINI D.J., DUNCIA J.V., PEASE L.J., WONG P.C., WEXLER R.R., JOHNSON A.L., TIMMERMANS P.B.M.W.M. Identification of angiotensin II receptor subtypes. Biochem. Biophys. Res. Commun. 1989;165:196–203. doi: 10.1016/0006-291x(89)91054-1. [DOI] [PubMed] [Google Scholar]
- COX B.E., WORD R.A., ROSENFELD C.R. Angiotensin II receptor characteristics and subtype expression in uterine arteries and myometrium during pregnancy. J. Clin. Endocrinol. Metab. 1996;81:49–58. doi: 10.1210/jcem.81.1.8550793. [DOI] [PubMed] [Google Scholar]
- DAVIS E.A. Effects of passive resting tension on reactivity of isolated uterine arteries from pregnant and non-pregnant rats. Can. J. Physiol. Pharmacol. 1994;72:P21.5. [Google Scholar]
- DE GASPARO M., SIRAGY H.M. The AT2 receptor: fact, fancy and fantasy. Regul. Pept. 1999;81:11–24. doi: 10.1016/s0167-0115(99)00023-3. [DOI] [PubMed] [Google Scholar]
- ENDLICH K., MULLER C., BARTHELMEBS M., HELWIG J.J. Role of shear stress in nitric oxide-dependent modulation of renal angiotensin II vasoconstriction. Br. J. Pharmacol. 1999;127:1929–1935. doi: 10.1038/sj.bjp.0702739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLINAT S., BUSCHE S., RAIZADA M.K., SUMNERS C. The angiotensin II type 2 receptor: an enigma with multiple variations. Am. J. Physiol. 2000;278:E357–E374. doi: 10.1152/ajpendo.2000.278.3.E357. [DOI] [PubMed] [Google Scholar]
- GOHLKE P., PEES C., UNGER T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31:349–355. doi: 10.1161/01.hyp.31.1.349. [DOI] [PubMed] [Google Scholar]
- GRIENDLING K.K., LASSEGUE B., ALEXANDER R.W. Angiotensin receptors and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 1996;36:281–306. doi: 10.1146/annurev.pa.36.040196.001433. [DOI] [PubMed] [Google Scholar]
- HANNAN R.E., DAVIS E.A., WIDDOP R.E. The role of nitric oxide in mediating angiotensin II AT2 receptor effects in the rat uterine artery. Proc. Int. Symp. Resist. Art. 2001;7:106. [Google Scholar]
- LAMBERS D.S., GREENBERG S.G., CLARK K.E. Functional role of angiotensin II type 1 and 2 receptors in regulation of uterine blood flow in nonpregnant sheep. Am. J. Physiol. 2000;278:H353–H359. doi: 10.1152/ajpheart.2000.278.2.H353. [DOI] [PubMed] [Google Scholar]
- MAASSENVANDENBRINK A., DE VRIES R., SAXENA P.R., SCHALEKAMP M.A., DANSER A.H. Vasoconstriction by in situ formed angiotensin II: role of ACE and chymase. Cardiovasc. Res. 1999;44:407–415. doi: 10.1016/s0008-6363(99)00249-7. [DOI] [PubMed] [Google Scholar]
- MACARI D., BOTTARI S., WHITEBREAD S., DE GASPARO M., LEVENS N. Renal actions of the selective angiotensin AT2 receptor ligands CGP 42112B and PD 123319 in the sodium-depleted rat. Eur. J. Pharmacol. 1993;249:85–93. doi: 10.1016/0014-2999(93)90665-5. [DOI] [PubMed] [Google Scholar]
- MAESO R., NAVARRO-CID J., MUNOZ-GARCIA R., RODRIGO E., RUILOPE L.M., LAHERA V., CACHOFEIRO V. Losartan reduces phenylephrine constrictor response in aortic rings from spontaneously hypertensive rats. Role of nitric oxide and angiotensin II type 2 receptors. Hypertension. 1996;28:967–972. doi: 10.1161/01.hyp.28.6.967. [DOI] [PubMed] [Google Scholar]
- MAGNESS R.R., ROSENFELD C.R., HASSAN A., SHAUL P.W. Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on PGI2 and NO in pregnancy. Am. J. Physiol. 1996;270:H1914–H1923. doi: 10.1152/ajpheart.1996.270.6.H1914. [DOI] [PubMed] [Google Scholar]
- MATROUGUI K., LOUFRANI L., HEYMES C., LEVY B.I., HENRION D. Activation of AT(2) receptors by endogenous angiotensin II is involved in flow-induced dilation in rat resistance arteries. Hypertension. 1999;34:659–665. doi: 10.1161/01.hyp.34.4.659. [DOI] [PubMed] [Google Scholar]
- MCMULLEN J.R., GIBSON K.J., LUMBERS E.R., BURRELL J.H., WU J. Interactions between AT1 and AT2 receptors in uterine arteries from pregnant ewes. Eur. J. Pharmacol. 1999;378:195–202. doi: 10.1016/s0014-2999(99)00454-9. [DOI] [PubMed] [Google Scholar]
- MCPHERSON G.A. Assessing vascular reactivity of arteries in the small vessel myograph. Clin. Exp. Pharmacol. Physiol. 1992;19:815–825. doi: 10.1111/j.1440-1681.1992.tb00420.x. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.V., VANHOUTTE P.M. Kinins mediate kallikrein-induced endothelium-dependent relaxations in isolated canine coronary arteries. Biochem. Biophys. Res. Commun. 1992;185:693–697. doi: 10.1016/0006-291x(92)91681-f. [DOI] [PubMed] [Google Scholar]
- MULLER C., ENDLICH K., BARTHELMEBS M., HELWIG J.J. AT2-antagonist sensitive potentiation of angiotensin II-induced vasoconstrictions by blockade of nitric oxide synthesis in rat renal vasculature. Br. J. Pharmacol. 1997;122:1495–1501. doi: 10.1038/sj.bjp.0701505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- SEYEDI N., XU X., NASJLETTI A., HINTZE T.H. Coronary kinin generation mediates nitric oxide release after angiotensin receptor stimulation. Hypertension. 1995;26:164–170. doi: 10.1161/01.hyp.26.1.164. [DOI] [PubMed] [Google Scholar]
- SIRAGY H.M., CAREY R.M. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J. Clin. Invest. 1996;97:1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRAGY H.M., CAREY R.M. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J. Clin. Invest. 1997;100:264–269. doi: 10.1172/JCI119531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRAGY H.M., CAREY R.M. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33:1237–1242. doi: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- SIRAGY H.M., DE GASPARO M., CAREY R.M. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension. 2000;35:1074–1077. doi: 10.1161/01.hyp.35.5.1074. [DOI] [PubMed] [Google Scholar]
- ST-LOUIS J., SICOTTE B., BEDARD S., BROCHU M. Blockade of angiotensin receptor subtypes in arcuate uterine artery of pregnant and postpartum rats. Hypertension. 2001;38:1017–1023. doi: 10.1161/hy1101.095008. [DOI] [PubMed] [Google Scholar]
- TIMMERMANS P.B., WONG P.C., CHIU A.T., HERBLIN W.F., BENFIELD P., CARINI D.J., LEE R.J., WEXLER R.R., SAYE J.A., SMITH R.D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol. Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- TSUTSUMI Y., MATSUBARA H., MASAKI H., KURIHARA H., MURASAWA S., TAKAI S., MIYAZAKI M., NOZAWA Y., OZONO R., NAKAGAWA K., MIWA T., KAWADA N., MORI Y., SHIBASAKI Y., TANAKA Y., FUJIYAMA S., KOYAMA Y., FUJIYAMA A., TAKAHASHI H., IWASAKA T. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITEBREAD S., MELE M., KAMBER B., DE GASPARO M. Preliminary biochemical characterization of two angiotensin II receptor subtypes. Biochem. Biophys. Res. Commun. 1989;163:284–291. doi: 10.1016/0006-291x(89)92133-5. [DOI] [PubMed] [Google Scholar]
- WIDDOP R.E., MATROUGUI K., LEVY B.I., HENRION D. AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
- WIEMER G., SCHOLKENS B.A., BUSSE R., WAGNER A., HEITSCH H., LINZ W. The functional role of angiotensin II -subtype AT2- receptors in endothelial cells and isolated ischemic rat hearts. Pharm. Pharmacol. Lett. 1993;3:24–27. [PubMed] [Google Scholar]
- ZHUO J., DEAN R., MACGREGOR D., ALCORN D., MENDELSOHN F.A. Presence of angiotensin II AT2 receptor binding sites in the adventitia of human kidney vasculature. Clin. Exp. Pharmacol. Physiol. 1996;3:S147–S154. [PubMed] [Google Scholar]
- ZWART A.S., DAVIS E.A., WIDDOP R.E. Modulation of AT1 receptor-mediated contraction of rat uterine artery by AT2 receptors. Br. J. Pharmacol. 1998;125:1429–1436. doi: 10.1038/sj.bjp.0702210. [DOI] [PMC free article] [PubMed] [Google Scholar]