Abstract
The role of Rho kinase in Ca2+ sensitization of the contractile apparatus in smooth muscle was investigated in the bovine middle cerebral artery.
U46619, a thromboxane A2 analog, induced a greater sustained contraction with a smaller [Ca2+]i elevation than that seen with 118 mM K+. The level of myosin light chain (MLC) phosphorylation obtained in the initial phase of the contraction was higher than that seen with 118 mM K+; thereafter, it gradually declined to a comparable level in the late phase.
During the steady state of the U46619-induced contraction, Y27632 (10 μM), a Rho-kinase inhibitor, partially inhibited [Ca2+]i, although it substantially inhibited tension and MLC phosphorylation. Wortmannin (10 μM), an MLC kinase inhibitor, had no significant effect on [Ca2+]i, but it completely inhibited MLC phosphorylation and partially inhibited tension. The wortmannin-resistant tension development was thus not associated with MLC phosphorylation, and this component was completely inhibited by Y27632.
In conclusion, U46619 enhanced Ca2+ sensitivity in a manner both dependent and independent of MLC phosphorylation in the bovine middle cerebral artery. Both mechanisms of Ca2+ sensitization can be inhibited by the Rho-kinase inhibitor.
Keywords: Ca2+ sensitization, cerebral artery, thromboxane A2, myosin light chain, myosin light chain kinase, Rho kinase
Introduction
Cerebral vasospasm is one of the critical determinants in the prognosis of subarachnoid hemorrhage (Proust et al., 1995). It is thus essential to elucidate the molecular mechanism of post-hemorrhagic vasospasm to improve the outcome of subarachnoid hemorrhage. The expression of Rho kinase, a kinase regulated by a small GTP-binding protein RhoA (Ishizaki et al., 1996), was upregulated in the basilar artery in the rat model of subarachnoid hemorrhage (Miyagi et al., 2000). Rho-kinase inhibitor fasudil, which was originally developed as an inhibitor of myosin light chain (MLC) kinase (MLCK), and hydroxyfasudil, the active metabolite of fasudil, have been successfully used to treat posthemorrhagic vasospasm (Tachibana et al., 1999; Nagumo et al., 2000). Rho kinase is thus suggested to play an important role in the pathogenesis of posthemorrhagic vasospasm (Sato et al., 2000). However, the mechanism for the therapeutic effect of Rho-kinase inhibition remains to be determined.
The contraction of the vascular smooth muscle is primarily regulated by the intracellular Ca2+ signal (Somlyo & Somlyo, 1994). An elevation of cytosolic Ca2+ concentration ([Ca2+]i) activates a Ca2+-calmodulin-dependent kinase MLCK, and thereby increases MLC phosphorylation and induces contraction (Hartshorne, 1987). On the other hand, MLC phosphatase (MLCP) dephosphorylates MLC and causes relaxation (Hartshorne et al., 1998). However, the extent of the tension developed for a given increase in [Ca2+]i varies with the type of contractile stimulation (Hirano et al., 1991; Somlyo & Somlyo, 1994). The alteration of the Ca2+ sensitivity of contractile apparatus as well as Ca2+ signal is thus suggested to play a critical role in the regulation of smooth muscle contraction. The molecular mechanism for regulating the myofilament Ca2+ sensitivity is still under investigation. Mechanisms which are both dependent on and independent of change in MLC phosphorylation have been proposed (Moreland et al., 1992; Somlyo & Somlyo, 1994). Their relative contribution to the smooth muscle contraction still remains to be determined. In the MLC phosphorylation-dependent regulation of the Ca2+ sensitivity, the Ca2+-independent phosphorylation of MLC and inhibition of MLCP are suggested to play a key role (Hartshorne et al., 1998). Rho kinase has been shown to phosphorylate MLC in a Ca2+-independent manner (Amano et al., 1996). It was also shown to phosphorylate the regulatory subunit of MLCP and inhibit its activity (Kimura et al., 1996). Furthermore, Rho kinase was shown to phosphorylate CPI-17, an inhibitor protein of MLCP originally isolated as a substrate for protein kinase C, which in turn inhibits the activity of MLCP (Koyama et al., 2000). It is thus possible that the inhibition of Rho kinase decreases the Ca2+ sensitivity by lowering MLC phosphorylation, and thus causing vasorelaxation. On the other hand, the precise mechanism for MLC phosphorylation-independent regulation of myofilament Ca2+ sensitivity remains to be elucidated. The actin-binding proteins such as calponin and caldesmon, or intermediate filaments, have been proposed to play a critical role in the regulation of the Ca2+ sensitivity (Rasmussen et al., 1987). Rho kinase has recently been reported to cause Ca2+ sensitization, with no increase in MLC phosphorylation in the fibroblast fibers reconstituted in collagen gel (Nobe et al., 2003).
In the present study, we first determined the mechanisms of the contraction induced by a stable analog of thromboxane A2, U46619, in the bovine middle cerebral artery. U46619 was used as a representative contractile stimulation in the cerebral artery, because thromboxane A2 is a potent vasoconstrictor agonist produced by platelets, and it was shown to be involved in posthemorrhagic vasospasm (Sasaki et al., 1982). We simultaneously monitored [Ca2+]i and tension by using front-surface fura-2 fluorometry, and evaluated the Ca2+ sensitivity in intact smooth muscle. We also determined MLC phosphorylation to assess the relative contribution of MLC phosphorylation-dependent and -independent mechanisms in the regulation of the Ca2+ sensitivity during U46619-induced contraction. We next elucidated the effect of a Rho-kinase inhibitor, Y27632, on [Ca2+]i, tension and MLC phosphorylation, and compared it to that of an MLCK inhibitor, wortmannin.
Methods
Tissue preparation
Bovine middle cerebral arteries were obtained from a local slaughterhouse and brought to the laboratory in ice-cold normal physiological salt solution (normal PSS). Segments of the middle cerebral artery 1–3 cm from the bifurcation of the internal carotid artery were excised. Under a binocular microscope, the arachnoid membrane was carefully dissected away, and the arteries were cut into circular strips (1.0 wide, 4.0 mm long). To remove the endothelium, the inner surfaces of the arteries were rubbed with a cotton swab.
Fura-2 loading
Vascular strips were loaded with the Ca2+ indicator dye fura-2 by incubating in Dulbecco's modified Eagle's medium containing 25 μM fura-2/AM (an acetoxymethyl ester form of fura-2) and 5% fetal bovine serum for 4 h at 37°C under aeration with 95% O2–5% CO2. The fura-2-loaded strips were washed with normal PSS to remove the dye in the extracellular space, and then were incubated further in normal PSS at 25°C for at least 1 h before the initiation of measurements. Each strip was mounted vertically in a quartz organ bath filled with normal PSS.
Simultaneous measurement of cytosolic Ca2+ concentration and tension development with front-surface fluorometry
The strips were mounted vertically in a quartz organ bath, and connected to a force transducer (TB-612T, Nihon Kohden, Tokyo, Japan). The resting load was adjusted to 200 mg to obtain the maximal developed tension with 118 mM K+ depolarization. During the 1 h equilibration period after the fura-2 loading, the strips were stimulated with 118 mM K+ every 15 min. Changes in [Ca2+]i were monitored using a front-surface fluorometer CAM-OF-3 (Hirano et al., 1990; Kanaide, 1999). The fluorescence intensities at 500 nm obtained with 340 nm (F340) and 380 nm excitation (F380), and their ratio (F340/F380), were monitored as an indication of [Ca2+]i. The tension and the fluorescence ratio were expressed as a percentage, assigning the values in normal PSS (5.9 mM K+) and 118 mM K+-PSS to be 0 and 100%, respectively.
Measurement of MLC phosphorylation
The extent of MLC phosphorylation was determined using the urea–glycerol gel electrophoresis technique (Kamm & Stull, 1986; Persechini et al., 1986), followed by immunoblot detection with a specific mouse monoclonal anti-MLC antibody (Zhou et al., 1999). The strips were pulled to 1.2-fold the resting length and pinned onto a rubber block to keep the resting load similar to that given in the force measurement. At the indicated time after stimulation, arterial strips were transferred into 90% acetone, 10% trichloroacetic acid and 10 mM dithiothreitol (DTT) prechilled at −80°C to stop the reaction. Tissues were then extensively washed and stored in acetone containing 10 mM DTT at −80°C. After the tissue was air-dried to remove acetone, the cellular protein was extracted in the sample buffer (8 M urea, 20 mM Tris (hydroxymethyl) aminomethane, 23 mM glycine, 0.004% bromophenol blue and 10 mM DTT) at room temperature for 2 h. The supernatant was subjected to electrophoresis on 10% polyacrylamide gel containing 40% glycerol, followed by transfer onto polyvinylidene difluoride membrane (BioRad, Hercules, CA, U.S.A.) in 10 mM Na2HPO4 (pH 7.6). Since the amount of extracted protein was not determined because of interference due to the high concentration of urea in the extract, the same volume of the extract was loaded onto the gel. The amount of protein loaded could vary to some extent due to variation in the extraction efficiency. The 20 kDa MLC, both unphosphorylated and phosphorylated, was detected by the specific antibody (200 × dilution) and a secondary antibody (1000 × dilution). The immune complex was detected using the enhanced chemiluminescence technique (ECL plus kit; Amersham, Buckinghamshire, U.K.). X-OMAT AR Film (Kodak, Rochester, NY, U.S.A.) was used to detect light emission. After obtaining the image of the X-ray film with a gel documentation system equipped with a CCD camera, Printgraph AE-6911CX (Atto, Tokyo, Japan), the density of unphosphorylated and phosphorylated MLCs was determined by Gel Plotting Macros of the NIH image ver. 1.61 (National Institute of Health, U.S.A.). The percentage of the phosphorylated form in total MLC (sum of unphosphorylated and phosphorylated forms) was calculated to indicate the extent of MLC phosphorylation.
α-Toxin permeabilization of the bovine middle cerebral artery
For the study with permeabilized fibers, strips (approximately 200 μm wide, 1.0 mm long) smaller than those used in the study with intact preparation were prepared. The strips were permeabilized by treatment with 5000 U ml−1 staphylococcal α-toxin in Ca2+-free cytosolic substitution solution (CSS) for 1 h at 25°C, as previously described (Nishimura et al., 1988). The arterial strips were then mounted onto two tungsten wires bathed in wells filled with Ca2+-free CSS on a plate, by passing the tungsten wires through the longitudinal slit made in the center of the strip. One of the wires was fixed and the other was connected to a force transducer (U Gauge; Minebea, Nagano, Japan). The strip was then stretched to twice its resting length, and was allowed to relax completely in Ca2+-free CSS for 30 min. The extent of tension development was expressed as a percentage, assigning values of the tension obtained in Ca2+-free CSS (resting state) and 10 μM Ca2+-containing CSS (maximum contraction) to be 0 and 100%, respectively.
Drugs and solution
The composition of normal PSS was as follows (in mM): NaCl 123, KCl 4.7, NaHCO3 15.5, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.25 and D-glucose 11.5. High K+ PSS was prepared by replacing NaCl with equimolar KCl. PSS was bubbled with a mixture of 95% O2 and 5% CO2, with the resulting pH being 7.4. The composition of CSS was as follows (in mM): EGTA 10, K-methanesulfonate 100, MgCl2 3.38, Na2ATP 2.2, creatine phosphate 10, Tris maleate 20 (pH 6.8) and the indicated concentration of free Ca2+. The Ca2+ CSS containing the indicated concentration of free Ca2+ was prepared by adding an appropriate amount of CaCl2, using the EGTA-Ca2+-binding constant of 106 mol l (Saida & Nonomura, 1978). Ca2+, ATP and creatine phosphate were omitted in the rigor solution (Zhou et al., 1999). Fura-2/AM was purchased from Dojindo Laboratories (Kumamoto, Japan); wortmannin was from Kyowa Hakko (Tokyo, Japan); U46619 (9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α) was from Funakoshi (Tokyo, Japan); ATPγS (adenosine 5′-O-(3-thiotriphosphate)) was from Boehringer-Mannheim (Mannheim, Germany); Staphylococcus aureus α-toxin and a monoclonal anti-MLC antibody (clone MY-21) were purchased from Sigma (St Louis, MO, U.S.A.); Y27632 was from Calbiochem (San Diego, CA, U.S.A.).
Data analysis
The data are expressed as the mean±s.e.m. Student's t-test was used to determine the statistical significance between the two groups, and an analysis of variance was used to determine the concentration-dependent effect of Y27632 and wortmannin. P-values of less than 0.05 were considered to have statistical significance. All data were collected using a computerized data-acquisition system (MacLab; Analog Digital Instruments, Australia, and Macintosh; Apple Computer, U.S.A.).
Results
Time course of changes in [Ca2+]i, tension and MLC phosphorylation induced by 118 mM K+ and U46619 contraction
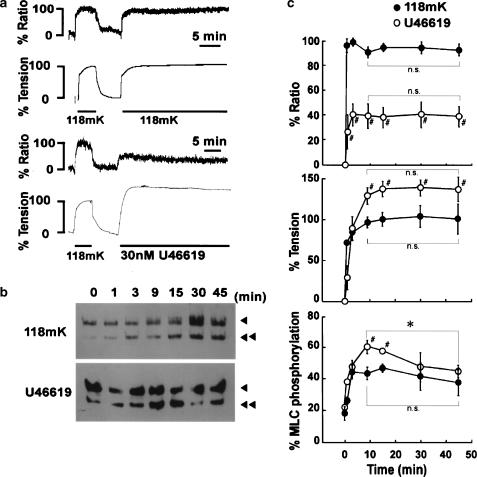
High K+ depolarization induced a sustained increase in [Ca2+]i and tension in the bovine middle cerebral artery (Figure 1a). Upon stimulation with 118 mM K+, [Ca2+]i and tension increased and reached a sustained level within 3 and 10 min, respectively. The levels of [Ca2+]i and tension obtained with 118 mM K+ were 93.8±2.9 and 101.6±8.5% at 15 min, and were 91.3±5.6 and 101.9±18.5% at 45 min (Figure 1c). There was no significant decrease in [Ca2+]i or tension during the sustained phase of the 118 mM K+-induced contraction. Similarly, 118 mM K+ induced a sustained increase in MLC phosphorylation (Figure 1b), reaching a sustained level within 5 min (Figure 1c). The levels of MLC phosphorylation at 0 (resting), 15 and 45 min were 19.2±4.4, 48.8±2.9 and 39.4±8.2% (n=4–6), respectively. There was no significant decrease in MLC phosphorylation level during the sustained phase of the 118 mM K+-induced contraction. As a result, the changes in [Ca2+]i, MLC phosphorylation and tension correlated well with each other during the 118 mM K+-induced sustained contraction.
Figure 1.
Changes in [Ca2+]i, tension and MLC phosphorylation induced by 118 mM K+ and U46619 in the bovine middle cerebral artery. (a) Representative traces showing changes in [Ca2+]i (% ratio) and % tension, and (b) a representative Western blot showing the changes in MLC phosphorylation induced by 118 mM K+ and 30 nM U46619 in bovine middle cerebral arterial strips. The levels of [Ca2+]i and tension at rest (5.9 mM K+) and those at steady state of the first contraction induced by 118 mM K+ depolarization were assigned to be 0 and 100%, respectively. In panel (b), arrowheads and double arrowheads indicate unphosphorylated and phosphorylated MLC, respectively. (c) Summary of changes in [Ca2+]i (% ratio), tension and MLC phosphorylation induced by 118 mM K+ and 30 nM U46619. The data are the mean±s.e.m. (n=4–7). #P<0.05 compared to the values obtained during the 118 mM K+-induced contraction; *P<0.05; n.s., not significantly different.
On the other hand, U46619 induced a sustained elevation of [Ca2+]i and tension (Figure 1a, c), while it induced a transient and gradually declining increase in MLC phosphorylation (Figure 1b, c). Upon stimulation with 30 nM U46619, [Ca2+]i and tension rapidly increased and reached a sustained level within 3 and 15 min, respectively (Figure 1a). The levels of [Ca2+]i and tension obtained with 30 nM U46619 were 41.6±8.0 and 138.4±9.5% at 15 min, and 41.0±7.0 and 140.0±9.5% at 45 min (Figure 1c). There was no significant decrease in [Ca2+]i and tension during the sustained phase of the U46619-induced contraction. On the other hand, MLC phosphorylation increased and reached its peak within 9 min, and, thereafter, gradually declined (Figure 1b). The levels of MLC phosphorylation at 0 (resting), 9 (peak) and 45 min were 22.4±1.0, 60.9±4.2 and 45.1±4.0% (n=5–6), respectively. The level of MLC phosphorylation at 45 min was significantly (P<0.05) lower than the peak level, but still significantly (P<0.05) higher than the resting level.
As a consequence, 30 nM U46619 induced a greater sustained contraction with a lower sustained increase in [Ca2+]i than that seen with 118 mM K+ (Figure 1c). Furthermore, the peak elevation of MLC phosphorylation obtained with 30 nM U46619 was significantly greater than that obtained with 118 mM K+, and the level of MLC phosphorylation at 45 min was comparable to that obtained with 118 mM K+, although the level of [Ca2+]i seen during the U46619-induced sustained contraction was significantly lower than that obtained with 118 mM K+ (Figure 1c). Importantly, the enhanced contraction seen in the late sustained phase of the U46619-induced contraction (30–40 min) was thus not associated with the enhanced elevation of MLC phosphorylation.
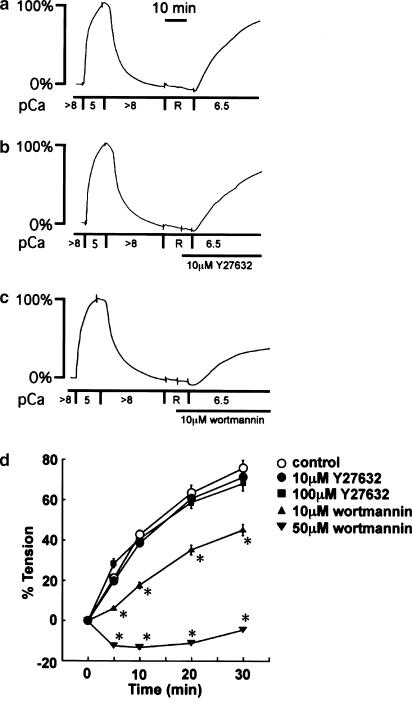
Effect of Y27632 and wortmannin on the contraction induced by ATPγS in the α-toxin-permeabilized strips of the bovine middle cerebral artery
To elucidate the mechanism of U46619-induced enhancement of MLC phosphorylation and contraction in comparison to the 118 mM K+-induced contraction, the effects of Y27632 and wortmannin on the U46619-induced contraction were examined. Y27632 and wortmannin have been wildly used as inhibitors of Rho kinase and MLCK, respectively (Nakanishi et al., 1992; Uehata et al., 1997). We first evaluated their specificity in the bovine middle cerebral artery, by examining the effects on the contraction induced by 2 mM ATPγS and 300 nM Ca2+ (pCa=6.5) in the α-toxin-permeabilized strips (Figure 2), as we previously described (Nakamura et al., 2001). After recording the reference contraction induced by 10 μM Ca2+, the strips were exposed to the rigor solution for 15 min (Figure 2a). The subsequent application of 2 mM ATPγS in the presence of 300 nM Ca2+ induced a progressive contraction (Figure 2a). This contraction is considered to be due to Ca2+-dependent thiophosphorylation of MLC, thus indicating that the rate of this contraction mostly reflects the activity of MLCK. The ATPγS-induced contraction was inhibited strongly by 10 μM wortmannin and completely by 50 μM wortmannin (Figure 2d). However, Y27632 had no significant effect on this contraction even at 100 μM Y27632 (Figure 2d).
Figure 2.
Effect of Y27632 and wortmannin on the contractions induced by ATPγS and Ca2+ in the α-toxin-permeabilized strips of the bovine middle cerebral artery. (a) Representative recordings showing the contraction induced by 2 mM ATPγS and 300 nM (pCa=6.5) Ca2+ in the α-toxin-permeabilized strips, and the effects of (b) 10 μM Y27632 and (c) 10 μM wortmannin. The strips were first contracted by 10 μM (pCa=5) Ca2+, and this level was assigned to be the 100% level. After complete relaxation in the relaxing solution, the strips were once treated with a rigor buffer (R), and then, the contraction was initiated by 2 mM ATPγS and 300 nM (pCa=6.5) Ca2+-containing buffer, in the absence and presence of Y27632 or wortmannin. Y27632 and wortmannin were applied 5 min before initiation of the contraction. (d) Summary of the effects of Y27632 (10, 100 μM) and wortmannin (10, 50 μM) on the time course of the contraction induced by 2 mM ATPγS and 300 nM Ca2+ in the α-toxin-permeabilized strips. The levels of tension obtained in the Ca2+-free solution and 10 μM Ca2+-containing solution were assigned to be 0 and 100%, respectively. Data are the mean±s.e.m. (n=4). *Significantly (P<0.05) different from the control value.
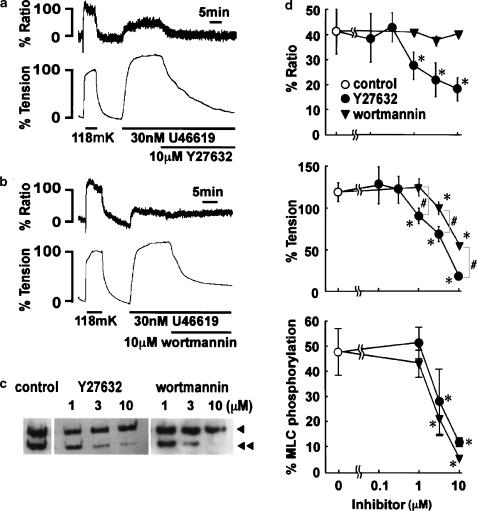
Effects of Y27632 and wortmannin on [Ca2+]i and tension during U46619-induced sustained contraction in the middle cerebral arterial strips
Both Y27632 and wortmannin induced a marked decrease in tension when applied during the sustained phase of the U46619-induced contraction (Figure 3a, b). The application of 10 μM Y27632 induced a significant decrease in both [Ca2+]i and tension within 30 min (Figure 3a), while 10 μM wortmannin had little effect on [Ca2+]i but induced a partial decrease in tension (Figure 3b). The concentration-dependent effects of these inhibitors were thus evaluated at 30 min after their application (Figure 3d). The significant decrease in [Ca2+]i and tension was obtained with 1 μM and higher concentrations of Y27632. The levels of [Ca2+]i and tension obtained with 10 μM Y27632 were 18.5±4.6 and 19.5±4.4% (n=6), respectively. The decreases in [Ca2+]i and tension were associated with a decrease in MLC phosphorylation. The significant decrease in MLC phosphorylation was obtained at 3 μM and higher concentration (Figure 3c, d). On the other hand, wortmannin decreased tension at the concentration of 3 μM and higher concentrations, while having no significant effect on the [Ca2+]i (Figure 3d). Moreover, the extent of inhibition in tension obtained by wortmannin was significantly smaller than that obtained by Y27632, when compared at the same concentrations (Figure 3d). However, wortmannin decreased MLC phosphorylation in a way similar (P>0.05) to that seen with Y27632 (Figure 3c, d).
Figure 3.
Changes in [Ca2+]i, tension and MLC phosphorylation induced by Y27632 and wortmannin during the U46619-induced sustained contraction in the bovine middle cerebral artery. (a, b) Representative recordings showing the effect of 10 μM Y27632 (a) and 10 μM wortmannin (b) on the U46619-induced sustained elevation of [Ca2+]i and tension. Y27632 and wortmannin were applied 15 min after the application of 30 nM U46619. The levels of [Ca2+]i and tension at rest (5.9 mM K+) and those at steady state of contraction induced by 118 mM K+ depolarization were assigned to be 0 and 100%, respectively. (c) Representative Western blots showing the extent of MLC phosphorylation obtained 45 min after the initiation of contraction by 30 nM U46619, that is, 30 min after the application of Y27632 and wortmannin. The arrowheads and double arrowheads indicate unphosphorylated and phosphorylated MLC, respectively. (d) Concentration-dependent effect of Y27632 and wortmannin on the U46619-induced elevations of [Ca2+]i (% ratio), tension and MLC phosphorylation obtained 45 min after the initiation of the contraction, that is, 30 min after the application of Y27632 and wortmannin. The data are the mean±s.e.m. (n=4–6). *Significantly (P<0.05) different from the control value; #P<0.05.
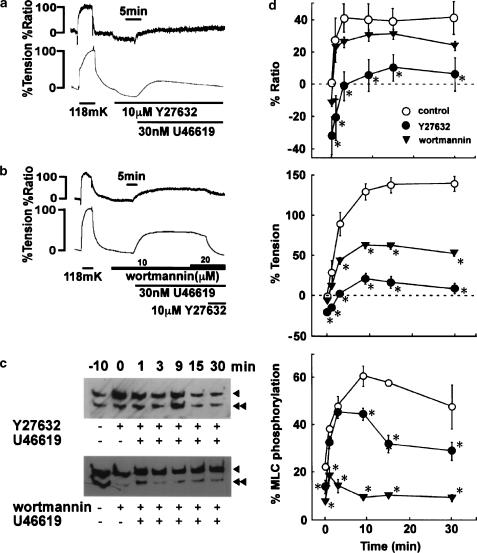
Effects of pretreatment with Y27632 and wortmannin on the contraction induced by U46619 in the bovine middle cerebral artery
When 10 μM Y27632 was applied during the resting state, the resting levels of [Ca2+]i and tension were significantly decreased to −31.5±10.5 and −18.9±1.0% (n=5), respectively (Figure 4a, d). In the presence of 10 μM Y27632, 30 nM U46619 induced elevation of [Ca2+]i and tension significantly smaller than those seen in control (Figure 1). [Ca2+]i increased only slightly above the resting level (Figure 4a, d). The tension development was also substantially inhibited and the sustained level was slightly higher than the resting level (Figure 4a, d). The treatment with 10 μM Y27632 slightly, but significantly, decreased the resting level of MLC phosphorylation. However, U46619 increased the MLC phosphorylation to a similar extent to that obtained in the control within 6 min after the initiation of contraction. Thereafter, the level of MLC phosphorylation during the sustained phase of contraction was significantly suppressed in the presence of Y27632. The MLC phosphorylation was 28.4±3.8% (n=4) at 30 min after the initiation of contraction.
Figure 4.
Effects of pretreatment with Y27632 and wortmannin on the contraction induced by U46619 in the bovine middle cerebral artery. (a, b) Representative recordings showing the effects of pretreatment with 10 μM Y27632 (a) and 10 μM wortmannin (b) on the U46619-induced elevation of [Ca2+]i and tension. Y27632 and wortmannin were applied 10 min before the application of U46619. (c) Representative Western blots showing the effects of pretreatment with Y27632 and wortmannin on MLC phosphorylation at the indicated time after stimulation with 30 nM U46619. MLC phosphorylation seen just before adding Y27632 and wortmannin was shown as −10 (min). Arrowheads, unphosphorylated MLC; double arrowheads, phosphorylated MLC. (d) Summary of the time course of changes in [Ca2+]i (% ratio), tension and MLC phosphorylation induced by 30 nM U46619 in the absence (control) and the presence of 10 μM Y27632 and 10 μM wortmannin. The data are the mean±s.e.m. (n=4–6). Control data were the same as in Figure 1c. *Significantly (P<0.05) different from the control value.
On the other hand, wortmannin slightly, but significantly, decreased the resting level of [Ca2+]i, and this decrease was much smaller than that observed with Y27632 (Figure 4d). MLC phosphorylation also significantly decreased after the application of wortmannin, and this decrease was greater than that seen with Y27632 (Figure 4d). However, wortmannin had little effect on the resting tension in contrast to the case with Y27632 (Figure 4d). In the presence of 10 μM wortmannin, U46619 induced a [Ca2+]i elevation similar (P>0.05) to that observed in the control (Figure 4d). However, wortmannin greatly inhibited the elevation of MLC phosphorylation in both the initial and sustained phases of contraction (Figure 4c, d). The level of MLC phosphorylation obtained at 9, 15 and 30 min after the application of U46619 did not significantly differ from that obtained just before application. Despite the strong suppression of MLC phosphorylation, U46619 induced a significant amount of sustained contraction (53.8±3.6% at 30 min) in the presence of 10 μM wortmannin (Figure 4b). During this sustained contraction, the increment of wortmannin to 20 μM induced no further decrease in [Ca2+]i and tension. However, 10 μM Y27632 induced a further decrease in both the [Ca2+]i and tension level (Figure 4b).
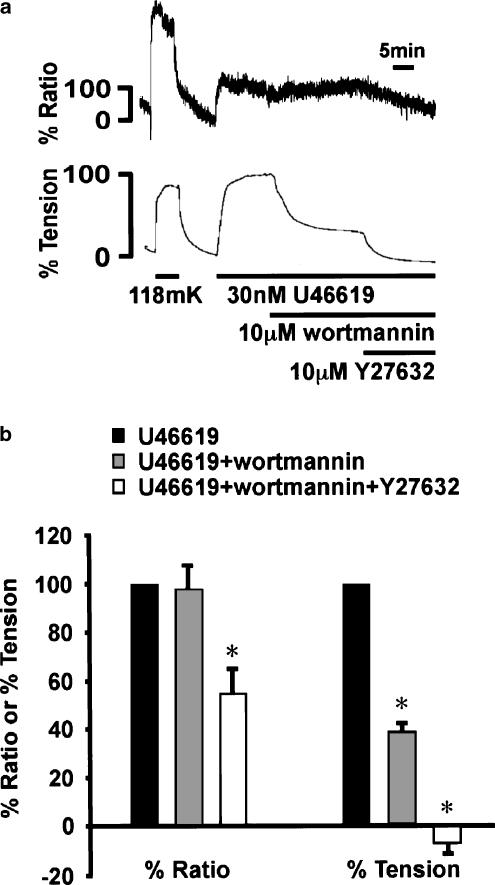
Effect of Y27632 on wortmannin-resistant component of U46619-induced sustained contraction in the middle cerebral arterial strips
Finally, we quantitatively examined the effect of Y27632 on the wortmannin-resistant component of the U46619-induced contraction (Figure 5). When applied during the sustained phase of U46619-induced contraction, 10 μM wortmannin had no significant effect on the level of [Ca2+]i, but significantly decreased tension (Figure 5). The tension reached a new steady-state level, and remained at this level for more than 20 min (data not shown). The addition of 10 μM Y27632 decreased significantly, but partly, [Ca2+]i while it decreased tension to a level similar to the resting level (Figure 5b).
Figure 5.
Changes in [Ca2+]i and tension induced by Y27632 after the application of wortmannin during the U46619-induced sustained contraction in the bovine middle cerebral artery. (a) Representative recording showing the effect of additive applications of 10 μM wortmannin and 10 μM Y27632 on [Ca2+]i and tension during the U46619-induced sustained contraction. The response to 118 mM K+ was recorded prior to the experimental protocol. (b) Summary of the effect of additive application of 10 μM wortmannin and 10 μM Y27632 on [Ca2+]i and tension during U46619-induced sustained contraction. The levels of [Ca2+]i and tension at rest (5.9 mM K+) and those obtained just prior to the application of wortmannin during the U46619-induced contraction were assigned to be 0 and 100%, respectively. The data are the mean±s.e.m. (n=4). *Significantly (P<0.05) different from the control value.
Discussion
Herein, we demonstrated that both MLC phosphorylation-dependent and -independent mechanisms contributed to the enhancement of myofilament Ca2+ sensitivity during the U46619-induced contraction in the bovine middle cerebral artery. Importantly, their relative contribution varied with time. The MLC phosphorylation-independent mechanism plays a more important role in the late phase than in the initial phase of the sustained contraction. This conclusion was supported by the following observations: U46619 produced a greater contraction with a lower increase in [Ca2+]i than high K+ depolarization. The level of MLC phosphorylation in the initial phase of the sustained contraction was higher than that seen with 118 mM K+-induced contraction, while that in the late phase of the sustained contraction was similar to that obtained with 118 mM K+. However, it should be noted that U46619 induced a greater MLC phosphorylation for a given level of [Ca2+]i in both initial and late phases of contraction than that seen during 118 mM K+. Namely, the [Ca2+]i-MLC phosphorylation relationship was enhanced throughout the U46619-induced contraction.
The enhancement of MLC phosphorylation for a given change in [Ca2+]i and its resultant potentiation of the myofilament Ca2+ sensitivity could be achieved by either activating Ca2+-independent phosphorylation or inhibiting dephosphorylation of MLC (Somlyo & Somlyo, 1998). Many kinases including Rho kinase have been reported to phosphorylate MLC in a Ca2+-independent manner, while MLC kinase is the only Ca2+-dependent kinase that phosphorylates MLC (Amano et al., 1996; Van Eyk et al., 1998; Deng et al., 2001; Niiro & Ikebe, 2001). On the other hand, the activity of myosin phosphatase can be inhibited by the phosphorylation of MYPT1, by inhibitor proteins such as CPI-17 or by a perturbation of the subunit structure (Hirano et al., 2003). The phosphorylation of MYPT1 at the inhibitory site was reported to be possibly catalyzed by various kinases including Rho kinase (Ichikawa et al., 1996; Kimura et al., 1996; MacDonald et al., 2001). In the present study, we thus examined two kinase inhibitors, Y27632 and wortmannin, to elucidate the mechanism of U46619-induced Ca2+ sensitization.
We first confirmed the specificity of wortmannin and Y27632 by examining their effect on the ATPγS- and Ca2+-induced contraction in the permeabilized strips (Figure 2). The rate of contraction induced by Ca2+ in the presence of ATPγS is considered to reflect the MLCK activity (Nakamura et al., 2001). Based on the observation that wortmannin but not Y27632 inhibited this contraction, we concluded that Y27632 had no inhibitory effect on MLCK even at 100 μM in the bovine middle cerebral artery, and that wortmannin is effective in inhibiting MLCK. Wortmannin (10 μM) almost completely inhibited the MLC phosphorylation induced by U46619 with no effect on [Ca2+]i, regardless of whether it was applied during the U46619-induced sustained contraction (Figure 3) or before the stimulation with U46619 (Figure 4). This observation suggested that MLCK plays a primary role in the MLC phosphorylation induced by U46619. However, there is a possibility that wortmannin may inhibit other kinases that are involved in the sustained contraction induced by U46619. On the other hand, pretreatment with Y27632 had no significant effect on the MLC phosphorylation in the developing phase of contraction (<3 min), while it significantly, but only partly, inhibited the MLC phosphorylation in the sustained phase. This observation suggested that the MLC phosphorylation is primarily determined by MLCK activity in the developing phase of the U46619-induced contraction, and that the enhancement of MLC phosphorylation seen during the sustained contraction was mediated by Y27632-sensitive kinases other than MLCK. In line with this finding, when applied during the sustained phase, Y27632 completely inhibited MLC phosphorylation, and this phenomenon was associated with a decrease in [Ca2+]i. Rho kinase has been reported to phosphorylate MYPT1 and inhibit the myosin phosphatase activity (Kimura et al., 1996). It also reported to phosphorylate CPI-17 at the site responsive for inhibiting myosin phosphatase (Koyama et al., 2000). Moreover, Rho kinase was shown to directly phosphorylate MLC independently of Ca2+ (Amano et al., 1996). It is conceivable that all of these effects of Rho kinase lead to an enhancement of MLC phosphorylation independent of MLCK. Therefore, we suggest that Y27632 antagonized these effects of Rho kinase and decreased MLC phosphorylation level during the sustained phase of the U46619-induced contraction. However, it is possible that the inhibition of MLC phosphorylation by Y27632 was partially due to a decrease in [Ca2+]i and the resultant inhibition of MLCK.
A fraction of MLC (∼20%) was phosphorylated under the resting conditions. This level of resting phosphorylation is consistent with many other reports in the literature (Persechini et al., 1986; Hartshorne, 1987). The addition of wortmannin under resting conditions significantly and substantially decreased this MLC phosphorylation, suggesting that MLCK plays a major role in phosphorylation under the resting condition. Moreover, Y27632 also slightly, but significantly, decreased the resting level of phosphorylation. MLC has been shown to be phosphorylated not only by MLCK in a Ca2+-dependent manner, but also by other kinases, including Rho kinase, integrin-linked kinase, Zip kinase and p21-activated protein kinase, in a Ca2+-independent manner (Amano et al., 1996; Van Eyk et al., 1998; Deng et al., 2001; Niiro & Ikebe, 2001). The Y27632-sensitive kinase, such as Rho kinase, is thus suggested to play some roles in the maintenance of the MLC phosphorylation under resting conditions.
Although wortmannin completely inhibited MLC phosphorylation induced by U46619, it inhibited the contraction only partly and significantly less than that seen with Y27632. The residual contraction observed in the presence of wortmannin was thus suggested to be independent of MLC phosphorylation, and may represent the component of the U46619-induced contraction enhanced by a mechanism independent of MLC phosphorylation. In the present study, we demonstrated that Y27632 inhibited the wortmannin-resistant component of the U46619-induced contraction. As a result, Y27632 was suggested to induce relaxation during the U46619-induced contraction by inhibiting not only MLC phosphorylation-dependent but also -independent mechanism of contraction. This study is thus the first report demonstrating that Y27632 induces relaxation in a manner independent of MLC phosphorylation in smooth muscle contraction. Our observation is thus consistent with the recent report demonstrating that Rho kinase mediated serum-induced contraction in fibroblast fibers independent of MLC phosphorylation (Nobe et al., 2003). However, the biochemical basis of the MLC phosphorylation-independent contractile mechanism and the role of Rho kinase remain to be investigated.
The MLC phosphorylation-independent sustained contraction was previously described as a latch contraction, and this contraction was considered to be dependent on Ca2+, but to require lower Ca2+ than that required for MLC phosphorylation-dependent contraction (Murphy et al., 1990). This contractile mechanism could thus contribute to the enhancement of Ca2+ sensitivity. Y27632 completely inhibited the MLC phosphorylation-independent contraction with concomitant decrease in [Ca2+]i. It is thus possible that decrease in [Ca2+]i is linked to the inhibition of contraction. However, the complete relaxation was associated with partial decrease in [Ca2+]i, thus suggesting that Y27632 decreased Ca2+ sensitivity. In other words, the Y27632-sensitive, MLC phosphorylation-independent component could contribute to the enhancement of Ca2+ sensitivity of the U46619-induced sustained contraction. On the other hand, it is also possible that Y27632-induced decrease in [Ca2+]i was not linked to the relaxation, and that the Y27632-sensitive, MLC phosphorylation-independent component of contraction is also Ca2+-independent. The actin-binding proteins such as calponin and caldesmon have been proposed to contribute to MLC phosphorylation-independent contraction. The phosphorylation of calponin and caldesmon was reported to regulate their effect on contraction (Winder & Walsh, 1993; Gerthoffer & Pohl, 1994). Furthermore, calponin has been shown to be a substrate of Rho kinase (Kaneko et al., 2000), while it was also shown to be dephosphorylated by PP1c (Ichikawa et al., 1993). It is thus possible that Y27632 disinhibited myosin phosphatase and promoted the dephosphorylation of calponin and the calponin-mediated inhibition of contraction, thereby causing MLC phosphorylation-independent relaxation. However, these possibilities remain to be elucidated.
The mechanism of the Y27632-induced [Ca2+]i decrease remains to be determined. The [Ca2+]i elevation during the sustained phase of the U46619-induced contraction mainly depends on the influx of the extracellular Ca2+, because the removal of extracellular Ca2+ abolished the [Ca2+]i elevation in the sustained phase (data not shown). Y27632 is thus suggested to inhibit the Ca2+ influx in the bovine middle cerebral artery. Our observation is thus consistent with previous reports demonstrating that Y27632 inhibited [Ca2+]i elevation due to Ca2+ influx induced by either agonist stimulation or depolarization (Takizawa et al., 1993; Ito et al., 2001; 2002). Hydroxyfasudil, another Rho-kinase inhibitor chemically different from Y27632 (Nagumo et al., 2000), also inhibited the [Ca2+]i elevation due to Ca2+ influx induced by both high K+ depolarization and norepinephrine in the rat aortic smooth muscle (Takizawa et al., 1993). The Rho kinase is thus suggested to be involved in the activation of Ca2+ influx by agonist stimulations or depolarization in some, if not all, smooth muscle tissues. It is thus possible that Y27632 inhibited the Rho-kinase activity and thereby decreased [Ca2+]i elevation due to the Ca2+ influx. However, it is also possible that Y27632 directly blocked Ca2+ entry channels activated by U46619. These two possibilities remain to be evaluated.
In conclusion, U46619 enhanced Ca2+ sensitivity of the contractile apparatus and induced a greater contraction for a given [Ca2+]i level than high K+ depolarization in the bovine middle cerebral artery. Ca2+ sensitization was found to be partly due to an enhancement of MLC phosphorylation and partly due to the activation of the contractile mechanism independent of MLC phosphorylation. The inhibition of MLCK activity by wortmannin caused a marked but not complete relaxation, while it had no effect on [Ca2+]i. Y27632 inhibited this MLC phosphorylation-independent contraction in addition to inhibiting MLC phosphorylation. Rho kinase may therefore be a potently effective therapeutic target in the management of the cerebral vasospasm.
Acknowledgments
We thank Mr Brian Quinn for comments and help with the manuscript. This study was supported in part by Grants-in-Aid for Scientific Research (Nos. 13470149, 14657174, 14570675, 15590758) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, by the Research Grant for Cardiovascular Diseases (13C-4) from the Ministry of Health, Labour and Welfare, Japan and by grants from the Japan Space Forum and the Naito Foundation.
Abbreviations
- ATPγS
adenosine 5′-O-(3-thiotriphosphate)
- [Ca2+]i
cytosolic Ca2+ concentrations
- CPI-17
17 kDa protein kinase C-potentiated inhibitory protein of type 1 protein phosphatase
- CSS
cytosolic substitution solution
- DTT
dithiothreitol
- fura-2/AM
an acetoxymethyl ester form of fura-2
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- MYPT1
myosin phosphatase target subunit 1
- PSS
physiological salt solution
References
- AMANO M., ITO M., KIMURA K., FUKATA Y., CHIHARA K., NAKANO T., MATSUURA Y., KAIBUCHI K. Phospho-rylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- DENG J.T., VAN LIEROP J.E., SUTHERLAND C., WALSH M.P. Ca2+-independent smooth muscle contraction. A novel function for integrin-linked kinase. J. Biol. Chem. 2001;276:16365–16373. doi: 10.1074/jbc.M011634200. [DOI] [PubMed] [Google Scholar]
- GERTHOFFER W.T., POHL J. Caldesmon and calponin phosphorylation in regulation of smooth muscle contraction. Canad. J. Physiol. Pharmacol. 1994;72:1410–1414. doi: 10.1139/y94-203. [DOI] [PubMed] [Google Scholar]
- HARTSHORNE D.J.Biochemistry of the contractile process in smooth muscle Physiology of the Gastro-intestinal Tract 1987New York: Raven Press; 423–482.ed. Johnson, L.R. pp [Google Scholar]
- HARTSHORNE D.J., ITO M., ERDODI F. Myosin light chain phosphatase: subunit composition, interactions and regulation. J. Muscle Res. Cell Motil. 1998;19:325–341. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- HIRANO K., DMITRY D.N., HIRANO M., NISHIMURA J., KANAIDE H. Protein kinase network in the regulation of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Mol. Cell. Biochem. 2003;248:105–114. doi: 10.1023/a:1024180101032. [DOI] [PubMed] [Google Scholar]
- HIRANO K., HIRANO M., ABE S., KANAIDE H.Cytosolic calcium transients in vascular smooth muscle Ion Channels of Vascular Smooth Muscle Cells and Endothelial Cells 1991New York: Elsevier; 93–105.ed. Sperelakis, N. & Kuriyama, H. pp [Google Scholar]
- HIRANO K., KANAIDE H., ABE S., NAKAMURA M. Effects of diltiazem on calcium concentrations in the cytosol and on force of contractions in porcine coronary arterial strips. Br. J. Pharmacol. 1990;101:273–280. doi: 10.1111/j.1476-5381.1990.tb12700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICHIKAWA K., ITO M., HARTSHORNE D.J. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J. Biol. Chem. 1996;271:4733–4740. doi: 10.1074/jbc.271.9.4733. [DOI] [PubMed] [Google Scholar]
- ICHIKAWA K., ITO M., OKUBO S., KONISHI T., NAKANO T., MINO T., NAKAMURA F., NAKA M., TANAKA T. Calponin phosphatase from smooth muscle: a possible role of type 1 protein phosphatase in smooth muscle relaxation. Biochem. Biophys. Res. Commun. 1993;193:827–833. doi: 10.1006/bbrc.1993.1700. [DOI] [PubMed] [Google Scholar]
- ISHIZAKI T., MAEKAWA M., FUJISAWA K., OKAWA K., IWAMATSU A., FUJITA A., WATANABE N., SAITO Y., KAKIZUKA A., MORII N., NARUMIYA S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- ITO S., KUME H., HONJO H., KATOH H., KODAMA I., YAMAKI K., HAYASHI H. Possible involvement of Rho kinase in Ca2+ sensitization and mobilization by MCh in tracheal smooth muscle. Am. J. Physiol. 2001;280:L1218–L1224. doi: 10.1152/ajplung.2001.280.6.L1218. [DOI] [PubMed] [Google Scholar]
- ITO S., KUME H., YAMAKI K., KATOH H., HONJO H., KODAMA I., HAYASHI H. Regulation of capacitative and noncapacitative receptor-operated Ca2+ entry by rho-kinase in tracheal smooth muscle. Am. J. Respir. Cell Mol. Biol. 2002;26:491–498. doi: 10.1165/ajrcmb.26.4.4701. [DOI] [PubMed] [Google Scholar]
- KAMM K.E., STULL J.T. Activation of smooth muscle contraction: relation between myosin phosphorylation and stiffness. Science. 1986;232:80–82. doi: 10.1126/science.3754063. [DOI] [PubMed] [Google Scholar]
- KANAIDE H.Measurement of [Ca2+]i in smooth muscle strips using front surface fluorometry Methods in Molecular Biology 1999Totowa, NJ: Humana Press Inc.269–277.ed. Lambert, D.G. pp [DOI] [PubMed] [Google Scholar]
- KANEKO T., AMANO M., MAEDA A., GOTO H., TAKAHASHI K., ITO M., KAIBUCHI K. Identification of calponin as a novel substrate of Rho-kinase. Biochem. Biophys. Res. Commun. 2000;273:110–116. doi: 10.1006/bbrc.2000.2901. [DOI] [PubMed] [Google Scholar]
- KIMURA K., ITO M., AMANO M., CHIHARA K., FUKATA Y., NAKAFUKU M., YAMAMORI B., FENG J., NAKANO T., OKAWA K., IWAMATSU A., KAIBUCHI K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- KOYAMA M., ITO M., FENG J., SEKO T., SHIRAKI K., TAKASE K., HARTSHORNE D.J., NAKANO T. Phosphorylation of CPI-17, an inhibitory phosphoprotein of smooth muscle myosin phosphatase, by Rho-kinase. FEBS Lett. 2000;475:197–200. doi: 10.1016/s0014-5793(00)01654-9. [DOI] [PubMed] [Google Scholar]
- MACDONALD J.A., BORMAN M.A., MURANYI A., SOMLYO A.V., HARTSHORNE D.J., HAYSTEAD T.A. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2419–2424. doi: 10.1073/pnas.041331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAGI Y., CARPENTER R.C., MEGURO T., PARENT A.D., ZHANG J.H. Upregulation of rho A and rho kinase messenger RNAs in the basilar artery of a rat model of subarachnoid hemorrhage. J. Neurosurg. 2000;93:471–476. doi: 10.3171/jns.2000.93.3.0471. [DOI] [PubMed] [Google Scholar]
- MORELAND S., NISHIMURA J., VAN BREEMEN C., AHN H.Y., MORELAND R.S. Transient myosin phosphorylation at constant Ca2+ during agonist activation of permeabilized arteries. Am. J. Physiol. 1992;263:C540–C544. doi: 10.1152/ajpcell.1992.263.2.C540. [DOI] [PubMed] [Google Scholar]
- MURPHY R.A., REMBOLD C.M., HAI C.M. Contraction in smooth muscle: what is latch. Prog. Clin. Biol. Res. 1990;327:39–50. [PubMed] [Google Scholar]
- NAGUMO H., SASAKI Y., ONO Y., OKAMOTO H., SETO M., TAKUWA Y. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am. J. Physiol. 2000;278:C57–C65. doi: 10.1152/ajpcell.2000.278.1.C57. [DOI] [PubMed] [Google Scholar]
- NAKAMURA K., NISHIMURA J., HIRANO K., IBAYASHI S., FUJISHIMA M., KANAIDE H. Hydroxyfasudil, an active metabolite of fasudil hydrochloride, relaxes the rabbit basilar artery by disinhibition of myosin light chain phosphatase. J. Cereb. Blood Flow Metab. 2001;21:876–885. doi: 10.1097/00004647-200107000-00013. [DOI] [PubMed] [Google Scholar]
- NAKANISHI S., KAKITA S., TAKAHASHI I., KAWAHARA K., TSUKUDA E., SANO T., YAMADA K., YOSHIDA M., KASE H., MATSUDA Y. Wortmannin, a microbial product inhibitor of myosin light chain kinase. J. Biol. Chem. 1992;267:2157–2163. [PubMed] [Google Scholar]
- NIIRO N., IKEBE M. Zipper-interacting protein kinase induces Ca2+-free smooth muscle contraction via myosin light chain phosphorylation. J. Biol. Chem. 2001;276:29567–29574. doi: 10.1074/jbc.M102753200. [DOI] [PubMed] [Google Scholar]
- NISHIMURA J., KOLBER M., VAN BREEMEN C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem. Biophys. Res. Commun. 1988;157:677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- NOBE H., NOBE K., FAZAL F., DE LANEROLLE P., PAUL R.J. Rho kinase mediates serum-induced contraction in fibroblast fibers independent of myosin LC20 phosphorylation. Am. J. Physiol. 2003;284:C599–C606. doi: 10.1152/ajpcell.00188.2002. [DOI] [PubMed] [Google Scholar]
- PERSECHINI A., KAMM K.E., STULL J.T. Different phosphorylated forms of myosin in contracting tracheal smooth muscle. J. Biol. Chem. 1986;261:6293–6299. [PubMed] [Google Scholar]
- PROUST F., HANNEQUIN D., LANGLOIS O., FREGER P., CREISSARD P. Causes of morbidity and mortality after ruptured aneurysm surgery in a series of 230 patients. The importance of control angiography. Stroke. 1995;26:1553–1557. doi: 10.1161/01.str.26.9.1553. [DOI] [PubMed] [Google Scholar]
- RASMUSSEN H., TAKUWA Y., PARK S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987;1:177–185. [PubMed] [Google Scholar]
- SAIDA K., NONOMURA Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J. Gen. Physiol. 1978;72:1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASAKI T., WAKAI S., ASANO T., TAKAKURA K., SANO K. Prevention of cerebral vasospasm after SAH with a thromboxane synthetase inhibitor, OKY-1581. J. Neurosurg. 1982;57:74–82. doi: 10.3171/jns.1982.57.1.0074. [DOI] [PubMed] [Google Scholar]
- SATO M., TANI E., FUJIKAWA H., KAIBUCHI K. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ. Res. 2000;87:195–200. doi: 10.1161/01.res.87.3.195. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. From pharmacomechanical coupling to G-proteins and myosin phosphatase. Acta Physiol. Scand. 1998;164:437–448. doi: 10.1046/j.1365-201X.1998.00454.x. [DOI] [PubMed] [Google Scholar]
- TACHIBANA E., HARADA T., SHIBUYA M., SAITO K., TAKAYASU M., SUZUKI Y., YOSHIDA J. Intra-arterial infusion of fasudil hydrochloride for treating vasospasm following subarachnoid haemorrhage. Acta Neurochir. (Wien) 1999;141:13–19. doi: 10.1007/s007010050260. [DOI] [PubMed] [Google Scholar]
- TAKIZAWA S., HORI M., OZAKI H., KARAKI H. Effects of isoquinoline derivatives, HA1077 and H-7, on cytosolic Ca2+ level and contraction in vascular smooth muscle. Eur. J. Pharmacol. 1993;250:431–437. doi: 10.1016/0014-2999(93)90030-l. [DOI] [PubMed] [Google Scholar]
- UEHATA M., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- VAN EYK J.E., ARRELL D.K., FOSTER D.B., STRAUSS J.D., HEINONEN T.Y., FURMANIAK-KAZMIERCZAK E., COTE G.P., MAK A.S. Different molecular mechanisms for Rho family GTPase-dependent, Ca2+-independent contraction of smooth muscle. J. Biol. Chem. 1998;273:23433–23439. doi: 10.1074/jbc.273.36.23433. [DOI] [PubMed] [Google Scholar]
- WINDER S.J., WALSH M.P. Calponin: thin filament-linked regulation of smooth muscle contraction. Cell Signal. 1993;5:677–686. doi: 10.1016/0898-6568(93)90029-l. [DOI] [PubMed] [Google Scholar]
- ZHOU Y., HIRANO K., SAKIHARA C., NISHIMURA J., KANAIDE H. NH2-terminal fragments of the 130 kDa subunit of myosin phosphatase increase the Ca2+ sensitivity of porcine renal artery. J. Physiol. (Lond.) 1999;516:55–65. doi: 10.1111/j.1469-7793.1999.055aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]