Abstract
The neuroprotective drug riluzole has multiple effects on cellular signaling. We found that riluzole rapidly and reversibly inhibited spontaneous Ca2+ oscillations in both immortalized GnRH-secreting hypothalamic neurons (GT1 cells) and in the prolactin and growth-hormone-secreting GH3 cell line. At lower concentrations (100 nM–5 μM), riluzole reduced the amplitude and frequency of spontaneous Ca2+ oscillations, whereas at higher concentrations it abolished spontaneous Ca2+ signaling.
Whole-cell current clamp recordings in GH3 cells revealed that riluzole decreased the action potential frequency, amplitude, and duration.
Riluzole inhibited voltage-gated Na+ currents, increased iberiotoxin-sensitive voltage-gated K+ currents, and had no effect on voltage-gated Ca2+ currents in GH3 cells. Riluzole also inhibited voltage-gated Na+ currents and increased voltage-gated K+ channels in GT1 cells.
The inhibitory effects of riluzole on Ca2+ signaling were blocked by pretreatment with iberiotoxin in GH3 cells, but only partially reduced by iberiotoxin in GT1 cells.
These results indicate that riluzole inhibits Ca2+ signaling primarily by activation of K+ channels in GH3 cells, and also by inhibition of Na+ channels in GT1 cells. Riluzole's inhibition of spontaneous excitability and Ca2+ signaling may be involved in its multiple effects on cellular function in the nervous system.
Keywords: Riluzole, Ca2+ oscillation, GT1, GH3, K+ channels, Ca2+ channels, Na+ channels, iberiotoxin, GnRH
Introduction
Riluzole (2-amino-6(trifuromethoxy) benzothiazole) is a compound that has been reported to have neuroprotective effects in multiple in vitro and in vivo models (Jehle et al., 2000; Obinu et al., 2002). It is used clinically in the treatment of motor neuron disease (Bensimon et al., 1994; McKee et al., 1994). It has anesthetic properties at high concentrations (Mantz et al., 1992), and has also been reported to have anticonvulsant effects (De Sarro et al., 2000). The mechanisms of action by which riluzole exerts its effects are complex. Riluzole blocks the presynaptic release of glutamate (Martin et al., 1993; Mantz et al., 1994; Rothstein & Kuncl, 1995; De Sarro et al., 2000). This mechanism has been proposed as the one responsible for the drug's neuroprotective effects. However, riluzole has also been shown to have other effects on cell physiology. Multiple studies have shown that riluzole inhibits Na+ channels (Benoit & Escande, 1991; Hebert et al., 1994; Siniscalchi et al., 1997; Song et al., 1997; Stefani et al., 1997; Yokoo et al., 1998; Zona et al., 1998). Some of these studies found that riluzole inhibits the fast transient Na+ current by shifting the steady-state inactivation curve towards more negative potentials. Riluzole has also been reported to activate multiple types of K+ channels, including SK channels (Grunnet et al., 2001) and two pore-domain channels (Lesage, 2003).
In this study, we have used Ca2+ imaging and patch-clamp techniques to investigate the effects of riluzole on the spontaneous activity of two neuroendocrine cell lines, the prolactin and growth-hormone-secreting pituitary cell line (GH3 cells), and GnRH-secreting immortalized hypothalamic neurons (GT1 cells). Each of these cell lines shows spontaneous activity characterized by bursts of action potentials and corresponding oscillations in intracellular Ca2+ concentration (Charles & Hales, 1995; Charles et al., 1999). We have found that riluzole reversibly inhibits this spontaneous activity via multiple mechanisms of ion channel modulation.
Methods
Cell cultures
Both GH3 and GT1 cells were maintained in Dulbecco's modified minimum essential medium (DMEM) supplemented with 5% fetal calf serum, 5% fetal horse serum, penicillin (100 U ml−1), and streptomycin (100 μg ml−1) (Cellgro) at 37°C in a humidified atmosphere with 5% CO2. Cells were transferred from 25 cm2 flasks onto poly-L-lysine-coated glass coverslips, on which they were grown for 2–3 days to a confluence of ∼50–70% before experimentation.
Measurement of [Ca2+]i
Changes in [Ca2+]i were measured using a video imaging system as previously described (Costantin & Charles, 2001) GH3 or GT1 cells were incubated at room temperature for 30 min–1 h in Hanks' balanced salt solution (HBSS) containing fluo-3-acetoxymethyl ester as fluorescent Ca2+ indicator at a final concentration of 5 μM, and 0.1% anhydrous dimethyl sulfoxide and Pluronic F-127 (0.01% w v−1) as the dispersing agent (Molecular Probes, Eugene, OR, U.S.A.). The cells were then washed and maintained in fresh medium for 10–30 min to allow complete de-esterification of fluo-3. Cells were then placed in an open slide flow chamber on the stage of a Nikon inverted microscope. Cells were superfused with extracellular recording solutions for 5 min to remove any extracellular fluo-3 before the beginning of each experiment. Changes in [Ca2+]i in fields of cells (typically 80–100 cells per field) were measured using a 488-nm excitation via a Nikon Plan Apo × 20 epifluorescence objective. Fluorescence at 510 nm was recorded with a silicon-intensified tube camera (Hamamatsu), and digitized at a resolution of 640 × 480 pixels using an Axon Image Lightning board and Image WorkBench software. The fluorescence (F) of fluo-3 was displayed as a continuous record showing the time course of changes of ΔF from an individual region of interest. During the experiment, the cells were continuously superfused with the control solution at a flow rate of 3–2 ml min−1. The drugs were applied using a superfusion system, by switching from drug-free control solution to drugs-containing solution.
Electrophysiological recording
The spontaneous electrical activity in individual cells was measured in current-clamp configuration using Nystatin perforated-patch technique (Zimber & Simasko, 2000), and voltage clamp was used for recording the whole-cell Na+, Ca2+, and K+ ion channel currents, as described previously (Hamill et al., 1981; Costantin & Charles, 2001). The patch electrodes were fabricated from a borosilicate glass capillary (1.5 mm OD, 1.16 mm ID, Warner Instrument) using a microprocessor-controlled puller (P-87; Sutter, U.S.A.) to a resistance of 4–6 MΩ. Voltage and current signals were collected at 10 kHz and analog-filtered at 5 kHz, amplified by an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, U.S.A.). All signals were digitized with a 12-bit A/D converter (Digidata 1200) and stored on computer hard disk for off-line analysis. Junction potentials ranged from −8 to −10 mV in all experiments, and were not corrected for.
Solutions
Current clamp
The bath solution consisted of (in mM): 140 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, and 10 N-(2-hydroxyethyl)piperazine-N-2-ethanesulfonic acid (HEPES). Nystatin was dissolved in dimethyl sulfoxide (DMSO) and added to the internal solution to yield a final concentration of 250 μg ml−1. The tip of the pipette was filled with an internal solution containing (in mM): 140 K-gluconate, 10 HEPES, and 2 MgCl2, with Tris-base pH 7.4. The remainder of the pipette was filled with the same internal solution supplemented with Nystatin. Cell characteristics, that is, the resting membrane potential, after hyperpolarization (AHP), half-decay time of the AHP, input resistance, spike amplitude, spike half-width, and membrane time constant (τ), were obtained only after the cell's membrane potential stabilized with no holding current.
Na+ currents
The bathing solution had the following composition (in mM): 150 NaCl, 2 CaCl2, 0.5 CdCl2, 10 HEPES, and 10 glucose, pH 7.3 with NaOH. The pipette solution in these experiments was filled with (in mM): 100 CsCl, 30 NaCl, 10 EGTA, 1 CaCl2, 2 MgCl2, 2 Na2ATP, and 10 HEPES, pH 7.3 with CsOH.
Ca2+ currents
Ca2+ channel activity was recorded with Ba2+ as the charge carrier. The bathing solution contained (in mM): 130 NaCl, 1.2 MgCl2, 10 BaCl2, 10 glucose, and 10 HEPES, with tetrodotoxin (TTX, 500 nM) added to block Na+ channels. The pH was adjusted to 7.3 with NaOH. The pipette solution in these experiments contained (in mM): 135 CsCl, 1 MgCl2, 10 HEPES, and 4 Na2-ATP, pH adjusted to 7.3 with CsOH.
K+ currents
The bathing solution contained (in mM): 135 NaCl, 5 KCl, 4 MgCl2, 1 CaCl2, 10 glucose, 10 HEPES, and 500 nM of TTX. The pH was adjusted to 7.3 with NaOH. The electrode solution contained (in mM): 120 K-gluconate, 20 KCl, 10 HEPES, 0.2 EGTA, 2 Mg2Cl, and 4 Na2ATP. pH was adjusted to 7.3 with KOH added to extracellular solutions.
Drugs
All drugs were bath-applied by gravity. TTX, iberiotoxin, and riluzole (Sigma, St Louis, MO, U.S.A) were made up as concentrated stocks in water or DMSO, and stored at −20°C. Stocks were thawed and diluted immediately prior to use. Riluzole was first prepared as a 50 mM stock solution in ethanol. The final concentration of the vehicle (0.05%) did not affect the properties of the cells.
Data analysis
Fluorescence and electrophysiological data were analyzed off-line with custom software written in LabVIEW and with the Mini Analysis Program (Synaptosoft). In voltage-clamp recordings, a ‘leak' current was estimated off-line by fitting the linear portion of the current during a depolarizing ramp to a straight line using pClamp8. It was then subtracted off the trace digitally. All curve fitting was performed with the SigmaPlot 8 software (SPSS, Chicago, IL, U.S.A.). Data were presented as means±s.e. Student's t-test, as appropriate, was applied to the data to determine statistical significance. P<0.05 was considered significant.
Results
Riluzole inhibits Ca2+ oscillations in GH3 cells
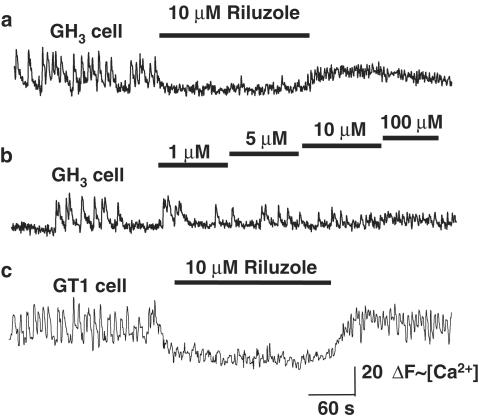
Under resting conditions, 80% of GH3 cells (n=353 in 12 experiments) displayed spontaneous [Ca2+]i oscillations. In spontaneously active GH3 cells, riluzole (1–10 μM) inhibited Ca2+ oscillations in a concentration-dependent manner (Figure 1). Lower concentrations of riluzole (1–5 μM) reduced both the amplitude and frequency of Ca2+ oscillations, whereas higher concentrations of riluzole (10 μM) completely abolished Ca2+ oscillations (95% of spontaneously active cells). Ca2+ oscillations were rapidly restored upon washout of riluzole, and there was a sustained ‘rebound' increase in the frequency of Ca2+ oscillations compared with baseline, following the washout of riluzole.
Figure 1.
Riluzole inhibits spontaneous Ca2+ oscillations in GH3 cells and GT1 cells. (a) Representative trace of fluo-3 ΔF (∼[Ca2+]i) vs time for a single GH3 cell. Bath application of riluzole (10 μM) abolishes spontaneous Ca2+ oscillations. Ca2+ oscillations resume immediately upon washout of riluzole. There is a sustained increase in the frequency of Ca2+ oscillations compared with the baseline frequency following washout of riluzole. (b) Representative trace of ΔF (∼[Ca2+]i) vs time for a single GH3 cell exposed to increasing concentrations of riluzole. Lower concentrations of riluzole (100 nM–5 μM) reduce the frequency and amplitude of Ca2+ oscillations, whereas higher concentrations abolish Ca2+ oscillations. (c) Representative trace of ΔF (∼[Ca2+]i) vs time for a single GT1 cell, showing the effect of bath application of riluzole. The response is similar to that observed with GH3 cells.
Riluzole inhibits Ca2+ oscillations in GT1 cells
Riluzole had a similar effect on spontaneous Ca2+ oscillations in GT1 cells. Riluzole (1 μM) reduced the frequency and amplitude of Ca2+ oscillations, whereas 10 μM riluzole abolished Ca2+ oscillations (98% of spontaneously active cells, Figure 1c). As with GH3 cells, washout of riluzole resulted in a sustained increase in the frequency of Ca2+ oscillations compared with the baseline frequency.
Effect of riluzole on spontaneous action potentials in GH3 cells
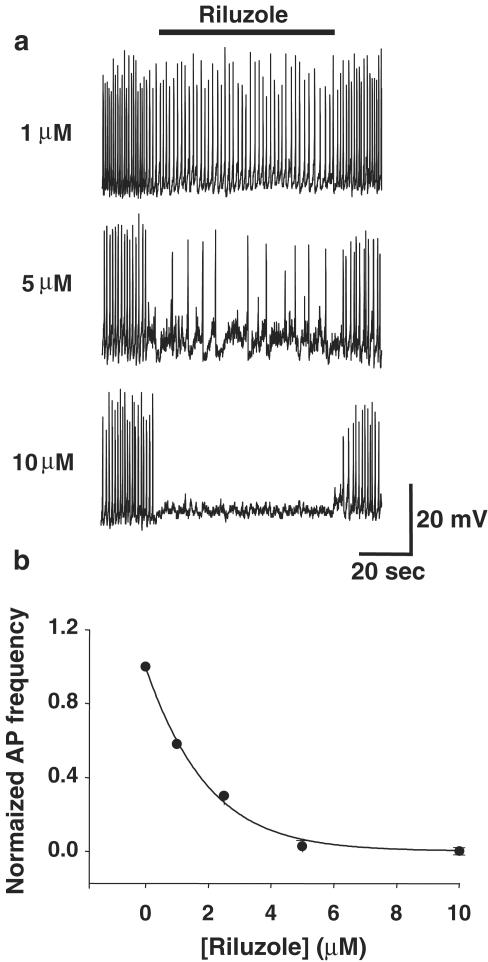
The current-clamp configuration was used to examine the effect of riluzole on spontaneous action potentials in GH3 cells. Action potentials had an average peak potential of −22±2.8 mV, a threshold potential of −36.1±1.1 mV, and an AHP potential of −43.6±2.2 mV (n=20 cells). The mean action potential duration measured at the threshold potential was 135±76 ms, and the mean firing frequency was 0.728±0.05 Hz (at zero holding current). These parameters are similar to those reported previously (Sankaranarayanan & Simasko, 1998). Bath application of riluzole (1 μM) exerted inhibitory effects on action potentials in 15 out of 20 cells (Figure 2). The action potential frequency was significantly decreased from 0.782±0.5 Hz in control medium to 0.423±0.1 Hz after application of riluzole (P<0.0002; n=5). Riluzole (1 μM) also significantly decreased the average amplitude of these action potentials to 15.8±0.6 mV from 20.0±0.7 mV in controls (P<0.005; n=5). The amplitude of 20 action potentials measured 30 s before and after the beginning of riluzole application was used for the calculation. Cells did not show a more hyperpolarized resting membrane potential in the presence of riluzole (average resting potential −42.3±1.2 mV) as compared with control conditions (average resting potential −41.2±1.4 mV) or changes in membrane resistance (Rm).
Figure 2.
Effect of riluzole on spontaneous action potentials in GH3 cells. (a) Traces show perforated patch recordings of spontaneous action potentials in the whole-cell current-clamp configuration. Spontaneous action potentials were inhibited in a dose-dependent fashion by riluzole. At 1 and 5 μM, riluzole reduced the frequency and amplitude of action potentials, whereas 10 μM riluzole abolished action potentials. See also Figure 5. (b) Plot of average action potential frequency (normalized to control frequency) vs riluzole concentration showing dose-dependent inhibition of action potentials.
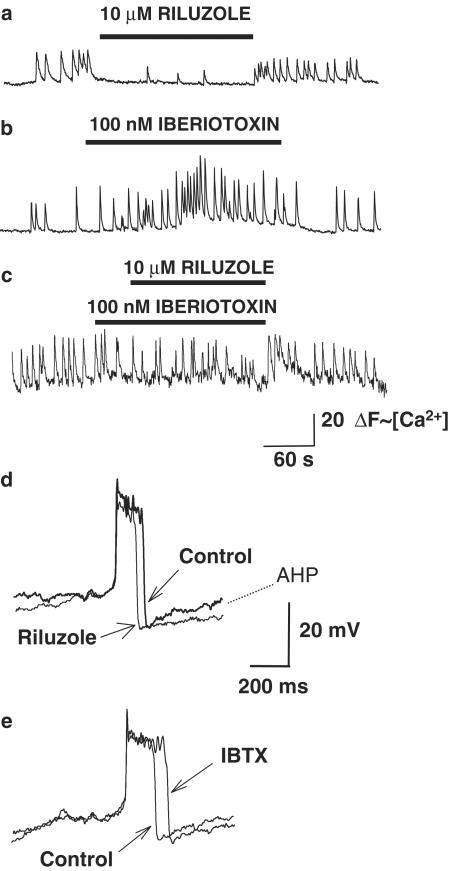
Although riluzole (1 μM) had no effect on the peak amplitude of the AHP following action potentials, it significantly increased the time of recovery to resting membrane potential following the AHP in all cells tested (Figure 6). The average τ for recovery of membrane potential following the AHP was 1460±102 ms in the presence of riluzole compared with 380±80 ms under control conditions (n=6; P<0.05). The specific BK channel blocker iberiotoxin (100 nM) blocked the action of riluzole on the AHP (n=4, data not shown, see below).
Figure 6.
Iberiotoxin blocks the inhibitory effect of riluzole on Ca2+ signaling and action potentials in GH3 cells. (a) Representative trace showing ΔF(∼[Ca2+]i) vs time for a single cell. Bath application of riluzole (10 μM) markedly inhibits spontaneous Ca2+ oscillations. (b) Bath application of iberiotoxin (100 nM) increases the frequency and amplitude of Ca2+ oscillations in this representative cell. (c) Prior application of iberiotoxin blocks the inhibitory action of riluzole on spontaneous Ca2+ oscillations. (d) Traces show representative whole-cell current-clamp recordings of action potentials from a GH3 cell before and after application of 1 μM riluzole. The traces are superimposed to highlight the differences in the action potentials. Riluzole significantly decreased the amplitude and duration of action potentials, and increased the recovery time of the AHP. (e) Superimposed recordings of action potentials from a GH3 cell before and after the application of iberiotoxin (100 nM). Iberiotoxin significantly increased the amplitude and duration of the action potential.
Effect of riluzole on Ca2+ channels in GH3 cells
To investigate the possibility that the inhibition of Ca2+ oscillations by riluzole was mediated by inhibition of Ca2+ channels, we evaluated the effect of riluzole on voltage-gated Ca2+ currents using Ba2+ as the charge carrier. We found that riluzole had no significant effect on Ca2+ currents. Under control conditions, IBa was activated at approximately −30 mV and the current was maximal at 0 mV with a reversal potential of approximately +50 mV. In the presence of 10 μM riluzole, the I–V relationship exhibited similar voltage dependence and peak IBa amplitude (data not shown, n=10). Ca2+ channel currents were also unaffected by riluzole when Ca2+ was the charge carrier (data not shown). Bath application of 200 μM Cd2+ completely blocked the depolarization-activated Ba2+ current, both in the absence and the presence of riluzole (n=10).
Riluzole inhibits Na+ currents
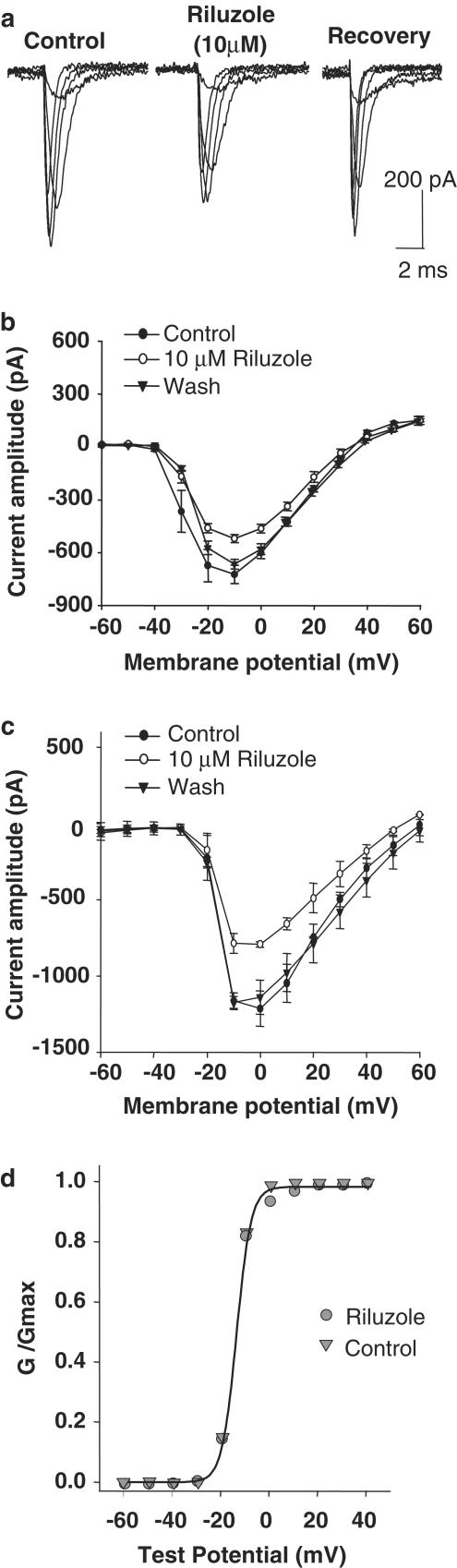
We then examined the effects of riluzole on whole-cell voltage-gated Na+ currents in GH3 cells. Inward Na+ currents were maximally activated at −20 mV with a peak amplitude of 650±29 pA, n=10. Riluzole (10 μM) diminished the amplitude of the Na+ current in all cells (n=10, Figure 3). This inhibitory effect was evident along the entire voltage range of activation for the Na+ current (between −40 and +30 mV). Riluzole did not modify the voltage threshold for the current activation (i.e. −40 to 30 mV) or the potential at which the inward current reached maximal values. The action of riluzole was dose-dependent and full recovery of the current occurred within 30–60 s following washout. The Na+ current that was inhibited by riluzole was also completely inhibited by 500 nM TTX. However, the same concentration of TTX had no effect on spontaneous Ca2+ oscillations. This result indicates that the effect of riluzole on Na+ currents was not responsible for its inhibitory effect on Ca2+ oscillations.
Figure 3.
Riluzole inhibits Na+ currents in GH3 and GT1 cells. (a) Traces show whole-cell Na+ currents in a GH3 cell activated by depolarizing voltage steps at 10 mV intervals from a holding potential of −80 mV up to −30 mV. Riluzole (10 μM) significantly reduced the amplitude of Na+ currents (center trace). The effect of riluzole on Na+ currents was rapidly reversible (right trace). (b) Current–voltage plot showing the effects of riluzole on Na+ currents in GH3 cells (average values±standard error of means from 10 experiments). Riluzole inhibited Na+ currents in GH3 cells throughout the voltage range in which they were activated. (c) Current–voltage plot showing the effect of riluzole on Na+ currents in GT1 cells (average values±standard error of means from five experiments). GT1 cells show significantly greater Na+ currents than GH3 cells, and these currents were also reversibly inhibited by riluzole. (d) Conductance–voltage relationship for sodium channels in the absence and presence of 10 μM riluzole. The conductance (gNa) was calculated according to equation, gNa=INa/(Vg–Vr), where INa is the peak amplitude of sodium current, Vg is the test potential, and Vr is the reversal potential. Curves were drawn according to the equation gNa/maxgNa=1/(1+exp[Vg0.5−Vg]/kg), where maxgNa is the maximum value for gNa, Vg0.5 is the potential at which gNa is 0.5 maxgNa and kg is the slope factor (potential required for an e-fold change (n=10)). These curves are indistinguishable because riluzole did not change the voltage dependence of Na+ conductance.
Riluzole also inhibited voltage-gated Na+ currents in GT1 cells (Figure 3). Na+ currents in GT1 cells were activated at −30 mV, and had an average peak potential of 1.2 pA. In the presence of riluzole (10 μM), the average peak amplitude of Na+ currents was reduced by 35% (n=5 cells). In contrast to previous reports with other cell types, we found that, in GT1 cells, riluzole did not affect the steady-state inactivation of Na+ currents (Figure 4). Voltage-activated inward Na+ currents were inhibited by 30% by 50 nM TTX (Figure 7), and were abolished by 500 nM TTX. In contrast to GH3 cells, TTX abolished spontaneous Ca2+ oscillations in GT1 cells (Figures 7, 8), indicating that Na+ channels play a primary role in their spontaneous activity. Ca2+ oscillations were abolished by a concentration of TTX (10 nM) that caused a level of inhibition of Na+ currents that was similar to that observed with riluzole. Thus, the inhibitory effects of riluzole on Ca2+ signaling in GT1 cells may involve its inhibition of Na+ channels.
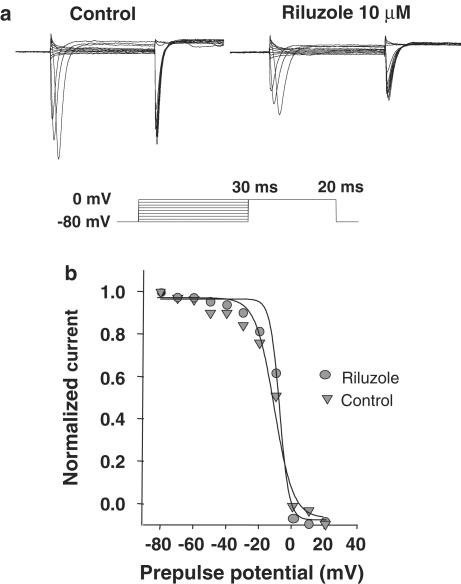
Figure 4.
Riluzole does not affect slow steady-state inactivation curves to sodium channels in GT1-1 cells. (a) Na+ current traces in a representative GT1 cell before (left trace) and during (right trace) exposure to 10 μM riluzole. A 30 ms prepulse from a holding potential of −80 mV to voltages between −80 and −40 mV was immediately followed by a 20 ms step depolarization to 0 mV. The peak amplitudes of the sodium current normalized to their respective maximum values are plotted according to the same equation as described for (d).
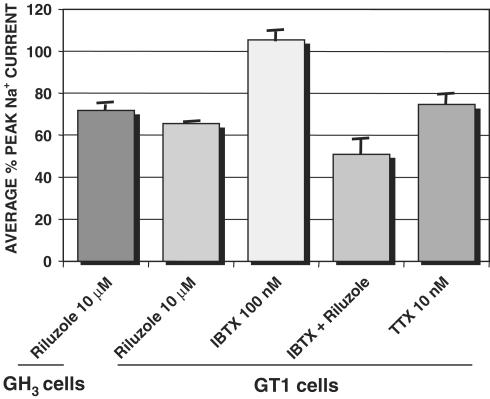
Figure 7.
Inhibition of Na+ currents. The graph shows the average percentage of peak voltage-gated Na+ current amplitude under each condition as compared with control conditions (n=5 for each condition). Similar inhibition of Na+ currents by riluzole was observed in GH3 and GT1 cells, and this inhibition was comparable to that caused by a low concentration of TTX. Iberiotoxin did not have a significant effect on Na+ currents in GT1 cells, nor did it alter the inhibition caused by riluzole.
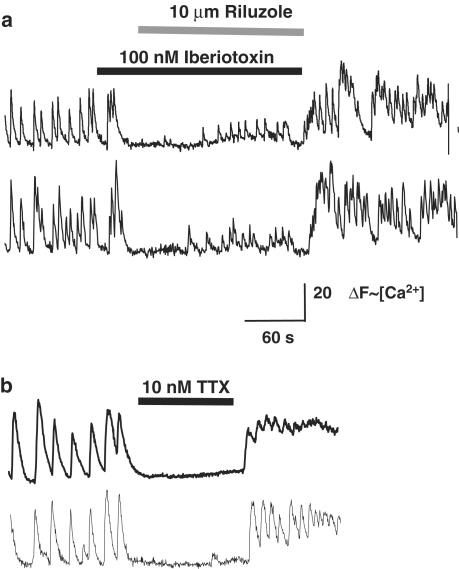
Figure 8.
Iberiotoxin does not block the inhibitory effect of riluzole on Ca2+ signaling in GT1 cells, which is similar to that caused by a low concentration of TTX. Traces show ΔF (∼[Ca2+]i) vs time for two different GT1 cells for each condition. (a) Prior addition of iberiotoxin does not block the inhibitory action of riluzole on spontaneous Ca2+ oscillations. Only a few Ca2+ oscillations with markedly reduced amplitude and frequency are observed when riluzole is applied in the presence of iberiotoxin. (b) Bath application of a TTX at a concentration (10 nM) that causes a similar inhibition of Na+ currents as riluzole also reversibly inhibits spontaneous Ca2+ oscillations.
Riluzole increases K+ currents
Whole-cell perforated patch recordings from GH3 cells revealed both transient and sustained outward K+ currents. Bath application of 10 μM riluzole increased the sustained outward current at +20 to +80 mV (Figure 5). The effect of riluzole was reversed by iberiotoxin (100 nM), a highly specific blocker of BKCa channels. On average, iberiotoxin inhibited sustained outward K+ current (at +80 mV) by 30±0.9% (n=5; P<0.03). Riluzole also increased K+ currents in GT1 cells. In the presence of 10 μM riluzole, sustained outward K+ currents in GT1 cells (at +80 mV) were increased by 18±0.5% (n=5).
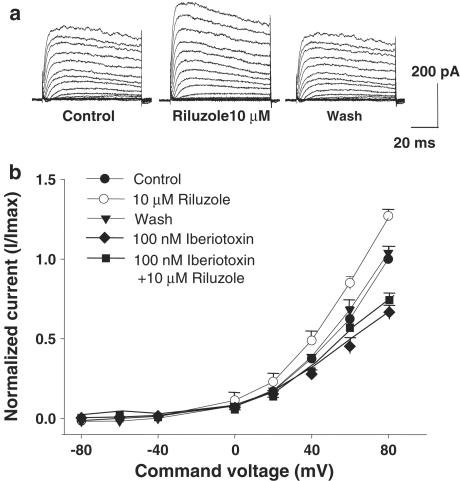
Figure 5.
(a) Riluzole potentiates K+ currents in GH3 cells. (a) Effect of riluzole on outward K+ currents in GH3 cells. Depolarizing voltage steps (10 mV intervals up to +80 mV from a holding potential of −80 mV) activated outward K+ currents in GH3 cells (left trace). The amplitude of outward K+ currents was significantly increased in the presence of 10 μM riluzole (center trace). K+ currents returned to baseline levels upon washout of riluzole (right trace). (b) Effect of riluzole and iberiotoxin on current–voltage relationships of K+ currents in GH3 cells. I–V curves show average normalized values from experiments. K+ current amplitude is increased by riluzole, but decreased by iberiotoxin.
Iberiotoxin blocks the inhibitory effects of riluzole on Ca2+ oscillations in GH3 cells but not in GT1 cells
Bath application of iberiotoxin (10–100 nM) evoked an increase in the frequency and amplitude of Ca2+ oscillations in GH3 cells (Figure 6). Iberiotoxin also increased the duration of action potentials in GH3 cells (Figure 6). In the presence of iberiotoxin (100 nM), riluzole (10 μM) resulted in only a slight inhibition of Ca2+ oscillations in GH3 cells. By contrast, in GT1 cells, the presence of iberiotoxin did not significantly change the inhibition of Ca2+ oscillations by riluzole (Figures 7, 8). Ca2+ oscillations were abolished in most cells, and only a few low-amplitude oscillations persisted in some cells.
Discussion
Both GH3 cells and GT1 cells have spontaneous oscillations of membrane potential leading to bursts of action potentials and influx of Ca2+ resulting in oscillations in intracellular Ca2+. The spontaneous activity of GH3 and GT1 cells involves the coordinated activity of multiple ion channels, including Na+ channels, Ca2+ channels, and K+ channels. In GT1 cells, spontaneous Ca2+ oscillations are abolished by TTX, indicating a critical role of voltage-gated Na+ channels (Hales et al., 1994; Costantin & Charles, 1999). By contrast, TTX has no effect on the spontaneous activity of GH3 cells (Dubinsky & Oxford, 1984). Ca2+ oscillations are abolished by nimodipine in both cell types, indicating that Ca2+ oscillations are generated by influx of Ca2+ through voltage-gated channels (Charles & Hales, 1995; Charles et al., 1999). In both cell types, the inward rectifier K+ channel specifically modulates the frequency of Ca2+ oscillations, whereas sustained voltage and Ca2+-activated outward K+ currents modulate the amplitude of Ca2+ oscillations (Charles et al., 1999; Costantin & Charles, 2001).
Riluzole reversibly inhibited spontaneous Ca2+ signaling in both GH3 and GT1 cells. At lower concentrations, riluzole reduced the frequency and amplitude of Ca2+ oscillations, whereas at higher concentrations it completely abolished spontaneous activity. Riluzole had a corresponding effect on spontaneous action potentials in GH3 cells. At lower concentrations, it reduced their amplitude, duration, and frequency, whereas at higher concentrations they were abolished completely. In our studies, the IC50 for riluzole's inhibition of action potentials was 5 μM. Siniscalchi et al. (1997) reported a higher IC50 of >29 μM for inhibition of action potentials by current injection in cortical neurons in a slice preparation. The differences in IC50 values could be accounted for by the fact that different cell types were studied in a slice preparation as compared with a monolayer culture. We consistently observed that washout of riluzole resulted in a sustained increase in the frequency of Ca2+ oscillations. The mechanism for this sustained ‘rebound' effect is not known.
The absence of any effect on Ca2+ currents indicates that riluzole's effects were not mediated by inhibition of Ca2+ influx through Ca2+ channels. Riluzole inhibited Na+ currents in both GH3 and GT1 cells, and activated iberiotoxin-sensitive K+ currents in both cell types. In GH3 cells, but not in GT1 cells, iberiotoxin reversed the inhibitory effect of riluzole on spontaneous Ca2+ signaling, indicating that the activation of iberiotoxin-sensitive K+ channels is a primary mechanism by which riluzole inhibits spontaneous activity in GH3 cells. In GT1 cells, where Na+ channels play a more significant role in spontaneous Ca2+ oscillations, the inhibition of K+ channels by iberiotoxin was not sufficient to overcome the inhibitory effects of riluzole. In these cells, riluzole's inhibition of Na+ channels is also likely an important mechanism by which it inhibits spontaneous Ca2+ signaling. We found that a concentration of TTX (10 nM) that only partially inhibited voltage-gated Na+ channels in GT1 cells also inhibited Ca2+ oscillations, confirming that even partial inhibition of Na+ currents can abolish spontaneous Ca2+ signaling in these cells. In contrast to previous reports (Benoit & Escande, 1991), we found that riluzole did not change the steady-state inactivation of Na+ currents in GT1 cells. This difference could be explained by our use of a lower concentration of riluzole than was used in the previous reports, or by a different effect of riluzole on Na+ channels in different cell types.
The effects of iberiotoxin, a specific BK channel blocker, indicate that riluzole's effects in GH3 cells and GT1 cells are primarily on this type of K+ channel. Rilzuole's effects of reducing action potential duration and increasing the duration of AHP are consistent with a primary effect on Ca2+-activated K+ channels. Previous studies have reported that riluzole activates multiple types of K+ channels, including SK channels (Grunnet et al., 2001) and two pore channels (Lesage, 2003). However, the SK channel blocker apamin has little effect on spontaneous Ca2+ signaling or K+ currents in either GT1 cells (Costantin & Charles, 2001) or GH3 (Sankaranarayanan & Simasko, 1998) cells, indicating a minor role for this channel in these cell types. We also found that riluzole had no effect on resting membrane potential or input resistance, suggesting that it did not independently open K+ channels or activate K+ channels (such as two pore channels) that are open near the resting membrane potential.
Riluzole has been reported to inhibit ‘low-probability' synaptic transmitter release, while not affecting ‘high-probability' release (Prakriya & Mennerick, 2000). This effect could be due to changes in action potential characteristics and the resultant inhibition of Ca2+ influx. At lower concentrations (100 nM–1 μM), riluzole reduced the amplitude, duration, and frequency of action potentials. This corresponded with a reduced amplitude and frequency of Ca2+ transients. In some cells, very low amplitude Ca2+ oscillations persisted in the presence of riluzole. This effect on Ca2+ transients could explain the ability of riluzole to inhibit exocytosis requiring higher Ca2+ transients, while maintaining exocytosis triggered at a lower Ca2+ threshold.
Riluzole has been reported to reduce glutamate release, and its neuroprotective effects have been attributed to this mechanism (Martin et al., 1993; Doble, 1996). While riluzole inhibits the release of specific neurotransmitters differently, it is not selective for glutamate (Jehle et al., 2000). Neither GH3 nor GT1 cells release glutamate, nor do they show significant glutamate-mediated Ca2+ signaling responses. Thus, the effects of riluzole on these neuroendocrine cell lines clearly do not involve glutamate. However, in glutamatergic neurons, inhibition of Ca2+ signaling by similar mechanisms could underlie riluzole's inhibition of glutamate release. Alternatively, inhibition of Na+ channels, activation of K+ channels, and the resultant inhibition of Ca2+ signaling may have significant functional consequences that are independent of glutamate release. The specific mechanisms by which riluzole exerts its neuroprotective, anesthetic, and anticonvulsant actions in vitro and in vivo remain to be determined.
Acknowledgments
This work was supported by NSF IBN-9982585 and NIDA DA05010 (AC).
Abbreviations
- GnRH
gonadotropin-releasing hormone
- TTX
tetrodotoxin
References
- BENOIT E., ESCANDE D. Riluzole specifically blocks inactivated Na channels in myelinated nerve fibre. Pflug. Arch. 1991;419:603–609. doi: 10.1007/BF00370302. [DOI] [PubMed] [Google Scholar]
- BENSIMON G., LACOMBLEZ L., MEININGER V. A controlled trial of riluzole in amyotrophic lateral sclerosis ALS/Riluzole Study Group. N. Engl. J. Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- CHARLES A.C., HALES T.G. Mechanisms of spontaneous calcium oscillations and action potentials in immortalized hypothalamic (GT1-7) neurons. J. Neurophysiol. 1995;73:56–64. doi: 10.1152/jn.1995.73.1.56. [DOI] [PubMed] [Google Scholar]
- CHARLES A.C., PIROS E.T., EVANS C.J., HALES T.G. L-type Ca2+ channels and K+ channels specifically modulate the frequency and amplitude of spontaneous Ca2+ oscillations and have distinct roles in prolactin release in GH3 cells. J. Biol. Chem. 1999;274:7508–7515. doi: 10.1074/jbc.274.11.7508. [DOI] [PubMed] [Google Scholar]
- COSTANTIN J.L., CHARLES A.C. Spontaneous action potentials initiate rhythmic intercellular calcium waves in immortalized hypothalamic (GT1-1) neurons. J. Neurophysiol. 1999;82:429–435. doi: 10.1152/jn.1999.82.1.429. [DOI] [PubMed] [Google Scholar]
- COSTANTIN J.L., CHARLES A.C. Modulation of Ca(2+) signaling by K(+) channels in a hypothalamic neuronal cell line (GT1-1) J. Neurophysiol. 2001;85:295–304. doi: 10.1152/jn.2001.85.1.295. [DOI] [PubMed] [Google Scholar]
- DE SARRO G., SINISCALCHI A., FERRERI G., GALLELLI L., DE SARRO A. NMDA and AMPA/kainate receptors are involved in the anticonvulsant activity of riluzole in DBA/2 mice. Eur. J. Pharmacol. 2000;408:25–34. doi: 10.1016/s0014-2999(00)00709-3. [DOI] [PubMed] [Google Scholar]
- DOBLE A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- DUBINSKY J.M., OXFORD G.S. Ionic currents in two strains of rat anterior pituitary tumor cells. J. Gen. Physiol. 1984;83:309–339. doi: 10.1085/jgp.83.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNNET M., JESPERSEN T., ANGELO K., FROKJAER-JENSEN C., KLAERKE D.A., OLESEN S.P., JENSEN B.S. Pharmacological modulation of SK3 channels. Neuropharmacology. 2001;40:879–887. doi: 10.1016/s0028-3908(01)00028-4. [DOI] [PubMed] [Google Scholar]
- HALES T.G., SANDERSON M.J., CHARLES A.C. GABA has excitatory actions on GnRH-secreting immortalized hypothalamic (GT1-7) neurons. Neuroendocrinology. 1994;59:297–308. doi: 10.1159/000126671. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patchclamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflug. Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HEBERT T., DRAPEAU P., PRADIER L., DUNN R.J. Block of the rat brain IIA sodium channel alpha subunit by the neuroprotective drug riluzole. Mol. Pharmacol. 1994;45:1055–1060. [PubMed] [Google Scholar]
- JEHLE T., BAUER J., BLAUTH E., HUMMEL A., DARSTEIN M., FREIMAN T.M., FEUERSTEIN T.J. Effects of riluzole on electrically evoked neurotransmitter release. Br. J. Pharmacol. 2000;130:1227–1234. doi: 10.1038/sj.bjp.0703424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESAGE F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003;44:1–7. doi: 10.1016/s0028-3908(02)00339-8. [DOI] [PubMed] [Google Scholar]
- MANTZ J., CHERAMY A., THIERRY A.M., GLOWINSKI J., DESMONTS J.M. Anesthetic properties of riluzole (54274 RP), a new inhibitor of glutamate neurotransmission. Anesthesiology. 1992;76:844–848. doi: 10.1097/00000542-199205000-00023. [DOI] [PubMed] [Google Scholar]
- MANTZ J., LAUDENBACH V., LECHARNY J.B., HENZEL D., DESMONTS J.M. Riluzole, a novel antiglutamate, blocks GABA uptake by striatal synaptosomes. Eur. J. Pharmacol. 1994;257:R7–R8. doi: 10.1016/0014-2999(94)90716-1. [DOI] [PubMed] [Google Scholar]
- MARTIN D., THOMPSON M.A., NADLER J.V. The neuroprotective agent riluzole inhibits release of glutamate and aspartate from slices of hippocampal area CA1. Eur. J. Pharmacol. 1993;250:473–476. doi: 10.1016/0014-2999(93)90037-i. [DOI] [PubMed] [Google Scholar]
- MCKEE P., FULLER G.N., STEVENS D.L.Riluzole in amyotrophic lateral sclerosis N. Engl. J. Med. 1994331272, 273–274.author reply [PubMed] [Google Scholar]
- OBINU M.C., REIBAUD M., BLANCHARD V., MOUSSAOUI S., IMPERATO A. Neuroprotective effect of riluzole in a primate model of Parkinson's disease: behavioral and histological evidence. Mov. Disord. 2002;17:13–19. doi: 10.1002/mds.1272. [DOI] [PubMed] [Google Scholar]
- PRAKRIYA M., MENNERICK S. Selective depression of low-release probability excitatory synapses by sodium channel blockers. Neuron. 2000;26:671–682. doi: 10.1016/s0896-6273(00)81203-9. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN J.D., KUNCL R.W. Neuroprotective strategies in a model of chronic glutamate-mediated motor neuron toxicity. J. Neurochem. 1995;65:643–651. doi: 10.1046/j.1471-4159.1995.65020643.x. [DOI] [PubMed] [Google Scholar]
- SANKARANARAYANAN S., SIMASKO S.M. Potassium channel blockers have minimal effect on repolarization of spontaneous action potentials in rat pituitary lactotropes. Neuroendocrinology. 1998;68:297–311. doi: 10.1159/000054378. [DOI] [PubMed] [Google Scholar]
- SINISCALCHI A., BONCI A., MERCURI N.B., BERNARDI G. Effects of riluzole on rat cortical neurones: an in vitro electrophysiological study. Br. J. Pharmacol. 1997;120:225–230. doi: 10.1038/sj.bjp.0700905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG J.H., HUANG C.S., NAGATA K., YEH J.Z., NARAHASHI T. Differential action of riluzole on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp. Ther. 1997;282:707–714. [PubMed] [Google Scholar]
- STEFANI A., SPADONI F., BERNARDI G. Differential inhibition by riluzole, lamotrigine, and phenytoin of sodium and calcium currents in cortical neurons: implications for neuroprotective strategies. Exp. Neurol. 1997;147:115–122. doi: 10.1006/exnr.1997.6554. [DOI] [PubMed] [Google Scholar]
- YOKOO H., SHIRAISHI S., KOBAYASHI H., YANAGITA T., YAMAMOTO R., WADA A. Selective inhibition by riluzole of voltage-dependent sodium channels and catecholamine secretion in adrenal chromaffin cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;357:526–531. doi: 10.1007/pl00005203. [DOI] [PubMed] [Google Scholar]
- ZIMBER M.P., SIMASKO S.M. Recruitment of calcium from intracellular stores does not occur during the expression of large spontaneous calcium oscillations in GH(3) cells and lactotropic cells in primary culture. Neuroendocrinology. 2000;72:242–251. doi: 10.1159/000054593. [DOI] [PubMed] [Google Scholar]
- ZONA C., SINISCALCHI A., MERCURI N.B., BERNARDI G. Riluzole interacts with voltage-activated sodium and potassium currents in cultured rat cortical neurons. Neuroscience. 1998;85:931–938. doi: 10.1016/s0306-4522(97)00604-0. [DOI] [PubMed] [Google Scholar]