Abstract
Effects of ergosterol peroxide (C28H44O3; Cpd 6A) from Cordyceps cicadae on phytohemagglutinin (PHA)-stimulated cell proliferation were studied in primary human T cells.
The results showed that Cpd 6A suppressed T-cell proliferation for about 24 h after stimulation with PHA. Cell cycle analysis indicated that Cpd 6A arrested the cell cycle progression of activated T cells from the G1 transition to the S phase.
To localize the point in the cell cycle where arrest occurred, a set of key regulatory events leading to the G1/S boundary, including the expression of cyclins D2, E, A1, and B1, interleukin (IL)-2, IL-4, interferon-γ (IFN-γ), and activating protein-1 (AP-1), was examined.
Cpd 6A suppressed, in activated T lymphocytes, the production and mRNA expression of cyclin E, IL-2, IL-4, IL-10, and IFN-γ in a dose-dependent manner.
Expression of AP-1 proteins, consisting of c-Fos and c-Jun, in activated T lymphocytes was decreased by Cpd 6A. The kinetic study indicated that the inhibitory effects of Cpd 6A on IL-2 mRNA expressed in T cells might be related to blocking c-Fos protein synthesis. T-cell proliferation after Cpd 6A treatment was partially restored by addition of IL-2, IL-4, and IFN-γ.
These suppressant effects of Cpd 6A on T-cell proliferation, activated by PHA, appeared to be mediated, at least in part, through the inhibition of early gene transcripts, especially those of cyclin E, IFN-γ, IL-2, and IL-4, and by arresting cell cycle progression in the cells.
Keywords: Ergosterol peroxide, T cell, cyclin E, IL-2, IL-4, IL-10, IFN-γ, c-Fos, c-Jun
Introduction
Cordyceps cicadae Shing (Ascomycetes) is a fungus parasitic on the larvae of Cicada flammata Dist. It is commonly used in traditional Chinese medicine for the treatment of cancer and asthma (Ukai et al., 1982). Many fungi belonging to the genus Cordyceps have been demonstrated to yield natural products possessing various biological activities (Kuo et al., 1994; 1996; 2001). Several polysaccharides isolated from C. cicadae have been demonstrated to possess antitumor activity (Ukai et al., 1983; Kiho et al., 1989). Although this fungus has been utilized in Chinese herbal medicinal prescriptions for the relief of asthma for a long time, there is a relative scarcity of definitive evidence to establish its immunopharmacological activity. In order to identify the active ingredients in this fungus that are responsible for its putative clinical effects, pure compounds from C. cicadae were evaluated in immune response assays.
Hypersensitivity reactions are harmful inflammatory responses that produce tissue injury and may cause serious disorders such as asthma and contact dermatitis (Charles et al., 1997). There is now convincing evidence that cytokines such as interleukin-10 (IL-10), interferon-γ (IFN-γ), interleukin-2 (IL-2), and interleukin-4 (IL-4), secreted by T lymphocytes in response to antigen stimulation, play a role in lung inflammation and asthma (Goodman et al., 1996). In patients with asthma, the levels of T cells and cytokines have been shown to be significantly elevated in bronchoalveolar lavage fluids, suggesting a possible pathological role for T cells and cytokines (Bradely et al., 1991; Corrigan & Kay, 1992). One of the therapeutic objectives in asthma and bronchial inflammation is to reduce the local inflammatory response. Blockade of T lymphocyte activation, proliferation, and cytokine production is one such anti-inflammatory means (Arai et al., 1990).
T-lymphocyte activation and proliferation is a highly regulated process involving ordered expression of a series of control genes such as c-fos, c-jun, as well as the cytokines IL-2 and IFN-γ (Cantrell, 1996). Activating protein-1 (AP-1) is a transcription factor formed by the dimerization of c-Jun and c-Fos (Angel & Herrlich, 1994). AP-1 cooperates with other transcription factors to regulate the expression of inducible genes in T cells (Karin, 1995). Proliferation of eucaryotic cells is strictly regulated and governed by checkpoints located at distinct points in the cell cycle (Norbury & Nurse, 1992). Transition through G2/M is controlled by cyclins A and B associated with p34cdc2. A distinct class of G1 cyclins including cyclins C, D, and E regulates the progression of the cell cycle at the G1/S transition (Parde, 1989; Lew et al., 1991). Growth modulators or other external events that affect the T-cell proliferation are ultimately likely to act by controlling the expression or functions of the products of these genes (Ajchenbaum et al., 1993).
In a previous study, we found that the ethanolic extract of C. cicadae inhibited proliferation and IL-2 production in human peripheral blood mononuclear cells activated by phytohemagglutinin (PHA) (Weng et al., 2002). Based on a bioassay-guided fractionation procedure, ergosterol peroxide (Cpd 6A) was identified in extracts from C. cicadae (Kuo et al., 2002). In the present study, primary human T lymphocytes were isolated from peripheral blood and used as target cells. In order to elucidate the mechanisms of action of Cpd 6A on T-cell proliferation, tritiated thymidine was used for the detection of total cellular DNA synthesis in the cultures. Furthermore, we determined the effects of Cpd 6A on cell cycle progression, production, and gene expression of cytokines, cyclins, c-fos, and c-jun in T cells stimulated with PHA, and analyzed their roles in the regulation of T-cell proliferation.
Methods
Isolation of ergosterol peroxide from C. cicadae
C. cicadae was separated into the rod-like ascocarps and insect-body portions. The insect-body portion (9.7 kg) was ground into powder and dried at 45–50°C, and then extracted with methanol (MeOH) (1L, x3) at room temperature. The methanolic extracts were fractionated by the method as described previously (Kuo et al., 2002). The solvent was removed under reduced pressure and the residue (1.5 kg) was partitioned between H2O and ethyl acetate (EtOAc). The organic layer was combined and concentrated to yield the crude extract (535 g). The sample, absorbed on silica gel (sample/adsorbent (v/v)=1/8), was subjected to dry flash column chromatography. Sufficient hexane was passed through the column to expel all of the air. Extensive gradient elutions were then employed using hexane and EtOAc to yield 14 fractions. These fractions were combined to give 10 main fractions with monitoring by thin-layer chromatography (TLC) and the solvent was removed under reduced pressure. Each combined fraction was further purified by rechromatography and recrystallization. Ergosterol peroxide was identified from the sixth fraction. The purity of all compounds isolated from C. cicadae was verified by a high-performance liquid chromatography (HPLC). They were dissolved in dimethylsulfoxide (DMSO) and then stored at 4°C until use.
General methods for compound purification
Melting points were measured on a micro melting point hot-stage apparatus and were uncorrected. 1H-, 13C-, and 2D-nuclear magnetic resonance (NMR) spectra were taken on a Bruker ACP-300 spectrophotometer with deuterated solvents as internal standards. Dry flash column chromatography was performed on silica gel (230–400 mesh, Merck). TLC was carried out on precoated kiesel gel 60 F254 plates (silica gel plated, 0.25 mm thick, Merck). Spots were visualized under UV light (254 and 365 nm) irradiation and by spraying with 10% molybdatophosphoric acid solution, followed by heating at 120°C.
Human subjects
In all, 20 healthy male subjects (28–37 years, mean age 32 years) were chosen for this investigation. The experimental protocol had been reviewed and approved by the institutional human experimentation committee. Written informed consent was obtained from each and every subject.
Preparation of primary human T lymphocytes
Heparinized human peripheral blood (80 ml) was obtained from normal healthy volunteers. Human peripheral blood mononuclear cells were isolated by the Ficoll–Hypaque gradient density method as described previously (Kuo et al., 2000). After the depletion of adherent cells on plastic dishes, T lymphocytes were isolated by erythrocyte rosetting. The erythrocyte rosette positive fraction contained <5% monocytes or B lymphocytes, as assessed by flow-cytometric analysis. T cells were resuspended to a concentration of 2 × 106 cells ml−1 in RPMI-1640 medium (GIBCO), supplemented with 2% heat-inactivated fetal calf serum (FCS; Hyclone), 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin.
Lymphoproliferation test
The lymphoproliferation test was modified from that previously described (Kuo et al., 2000). The concentration of T lymphocytes was adjusted to 2 × 106 cells ml−1 before use. A volume of 100 μl of cell suspension was applied onto each well of a 96-well flat-bottomed plate (Nunc 167008, Nunclon, Raskilde, Denmark) with or without 5 μg ml−1 PHA. Cyclosporin A was used as a positive control (Schreiber & Crabtree, 1992). Cpd 6A, cyclosporin A, IL-2, IL-4, or IFN-γ was added to the cells at varying concentrations or at different times. The plates were incubated in 5% CO2-air-humidified atmosphere at 37°C for 3 days. Subsequently, tritiated thymidine (1 μCi well−1, NEN) was added to each well. After a 16 h incubation, the cells were harvested on glass fiber filters by an automatic harvester (Dynatech, Multimash 2000, Billingshurst, U.K.). Radioactivity in the filters was measured by scintillation counting. The inhibitory activity of Cpd 6A on T-lymphocyte proliferation was calculated by the formula:
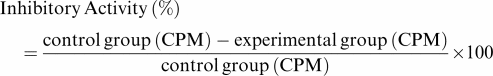 |
Determination of cytokine production in T lymphocytes
T lymphoctyes (2 × 105 cells well−1) were cultured with PHA alone or in combination with varying concentrations of Cpd 6A for 3 days. The cell supernatants were then collected and assayed for IL-2, IL-4, IL-10, and IFN-γ concentrations by EIA.
Extraction of total cellular RNA
Cells (5 × 106) were treated with or without PHA and cocultured with varying concentrations of Cpd 6A isolated from C. cicadae for different time periods. T cells were collected and total cellular RNA was extracted by TRIzol reagent according to the manufacturer's protocol. The isolated RNA was precipitated with 100% cold ethanol, pelleted by centrifugation and redissolved in diethyl pyrocarbonate (DEPC)-treated H2O. The concentration of the extracted RNA was calculated by measuring the optical density at 260 nm. The ratio of the optical density at 260 nm to that at 280 nm was always higher than 1.8. The quality of RNA was assessed by the integrity of 28S and 18S bands and lack of degradation on agarose-gel electrophoresis.
Synthesis of first-strand complementary deoxyribonucleic acid
Aliquots of 1 μg of RNA were reverse-transcribed using the Advantage™ reverse transcription (RT)-for-polymerase chain reaction (PCR) kit according to the manufacturer's instructions. Briefly, 1 μg RNA in 12.5 μl of DEPC-treated H2O was mixed with 20 μM of oligodeoxythymidine (oligo dT)18, and heated at 70°C for 10 min, then quick-chilled on ice. The following reagents were added to the tube: 6.5 μl of concentrated synthesis buffer (50 mM Tris-HCl, pH 8.3; 75 mM KCl; 3 mM MgCl2; 0.5 mM dNTPs; and 0.5 U RNase inhibitor), and 200 U of the Moloney murine leukemia virus (MMLV) reverse transcriptase. The reaction was initially incubated at 42°C for 1 h, and then at 94°C for 5 min to terminate the reaction. A volume of 80 μl of DEPC-treated H2O was added to the tube and the contents stored at −20°C for use in the PCR.
PCR
The PCR was performed in an air thermocycler according to the manufacturer's instructions, as described previously (Kuo et al., 2000). Briefly, 10 μl of the first-strand complementary deoxyribonucleic acid (cDNA) was mixed with 0.75 μM primers, 4 U of Taq polymerase, 10 μl of reaction buffer (2 mM Tris-HCl, pH 8.0; 0.01 mM ethylenediaminetetraacetate, EDTA; 0.1 mM dithiothreitol, DDT; 0.1% Triton X-100; 5% glycerol; and 1.5 mM MgCl2), and 25 μl of water, in a total volume of 50 μl. All primer pairs for the cytokines and cyclins were designed based on published human cDNA sequence data (Gray et al., 1982; Taniguchi et al., 1983; Hirano, 1986; Kim et al., 1992; Ajchenbaum et al., 1993; Marone et al., 1998). The c-fos and c-jun specific primers used were: forward 5′-CTTCTGCTCTAAAAGCTGCG-3′ and reverse 5′-CGACCTGCAGCTCGAGCACA-3′, and forward 5′-GCTCGATGAGTGACCGCGA-3′ and reverse 5′-CGCACTCTTACTTGTCGACTCG-3′, respectively. The PCR was carried out under the following conditions: denaturing temperature of 94°C for 1 min, annealing temperature of 60°C for 1 min, and elongation temperature of 72°C for 80 s for the first 30 cycles, and, finally, an elongation temperature of 72°C for 10 min. Following the reaction, the amplified product was taken out of the tubes and run on 2% agarose gel.
Cell cycle analysis
Procedures for cell cycle analysis have been described previously (Kuo et al., 2000). The concentration of T lymphocytes was adjusted to 2 × 106 cells ml−1 before use. A volume of 1 ml of cell suspension was placed into each well of a six-well flat-bottomed plate (Greiner, Germany) with or without 5 μg ml−1 PHA. Cpd 6A (25 μM) was added to the cells and the plates were incubated in 5% CO2-air-humidified atmosphere at 37°C for different time periods. The cells were harvested by centrifugation, washed in phosphate-buffered saline (PBS; pH 7.2), and then fixed in 70% ethanol for 30 min at −20°C. After washing, the cells were stained once with PBS and DNA was stained with propidium iodide (4 μg ml−1) containing 100 μg ml−1 of ribonuclease A. Flow cytometry analysis was conducted using a Becton-Dickinson FASCan.
Western blot analysis
Total cellular proteins was extracted from T lymphocytes by a method described previously (Kuo et al., 2000). Cells (1 × 107) were placed into each well of a six-well flat-bottomed plate (Greiner, Germany) with or without 5 μg ml−1 PHA, and treated with 25 μM of Cpd 6A for different times. The extracted cellular proteins (50 μg) were dissolved in dissociation buffer (2% SDS, 5% β-mercaptoethanol, 0.05 M Tris-HCl, and 20% glycerol, pH 7.6) and resolved by 10% SDS—PAGE, and then transferred to nitrocellulose filters. After blocking the filters, the filters were incubated with mouse monoclonal antibodies. Specific reactive proteins were detected by an enhanced chemiluminescence method, employing a rabbit anti-mouse Ig Ab linked to horseradish peroxidase.
Determination of cell viability
Approximately 2 × 105 primary human T lymphocytes were with or without PHA and cocultured with 0.1% DMSO or 100 μM of Cpd 6A for 4 days. The total, viable, and nonviable cell numbers were counted under the microscope with the help of a hemocytometer following trypan blue staining. The viability was calculated as
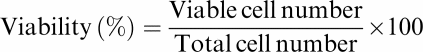 |
Materials
Reagents were obtained from Sigma or Merck. Antibodies were purchased from BD PharMingen (San Diego, CA, U.S.A.). Rabbit anti-mouse Ig Ab linked to horseradish peroxidase for detection in the Western blots was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, U.S.A.). The kits for enzyme immunoassay of cytokines (EIA) were from R&D Systems (Minneapolis, U.S.A.). TRIzol reagent for RNA extraction was obtained from Life Technologies Inc., Grand Island, NY, U.S.A. and the Advantage RT-for-PCR kit from CLONTECH, Palo Alto, CA, U.S.A. C. cicadae was purchased from Chinese medicine shops in Taipei and identified by Professors Cheng-Jen Chou and Tun-Tschu Chang. A voucher specimen (No. TFRIA46) has been deposited in the herbarium of Taiwan Forestry Research Institute, Taipei, Taiwan.
Statistical analysis
Data are presented as mean±s.d., and the differences between means were assessed by Student's t-test.
Results
Identification of ergosterol peroxide from C. cicadae
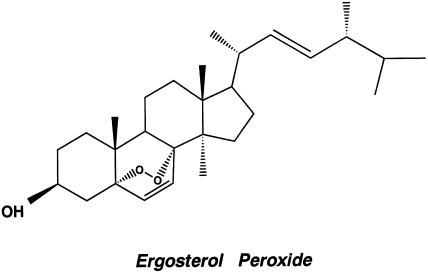
To extract pure active compounds from the C. cicadae, we used the complete isolation process in each chromatographic cycle and finally by HPLC. The most active compound (15 mg) was obtained from the sixth combined fraction as colorless needles with m.p. 180–182°C. NMR and mass spectrum analyses indicated a structure as shown in Figure 1. The chemical name of this bioactive component is 3β-hydroxy-5,8-epidioxyergosta-6,22-diene (C28H44O3; MW 428; Cpd 6A). The mass and NMR spectra data for the compound were compatible with those previously reported for ergosterol peroxide by Bok et al. (1999). The purity of ergosterol peroxide isolated from C. cicadae was assessed by HPLC, using a 5C18-ARII column. The column was eluted with MeOH and eluates analyzed by a UV detector at 210 nm. Ergosterol peroxide appeared as a single peak at 6.69 min retention time and its purity was 93.98%.
Figure 1.
The structure of Cpd 6A purified from C. cicadae.
Effects of Cpd 6A on T-cell proliferation
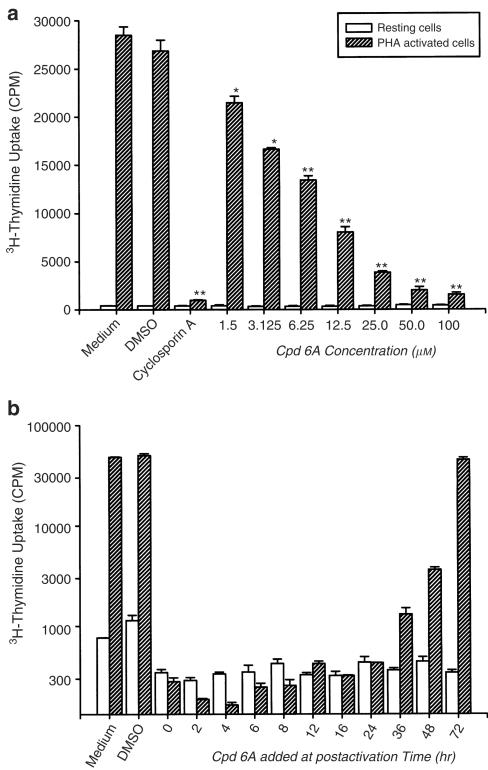
As shown in Figure 2a, treatment with PHA for 3 days stimulated cell proliferation, as indicated by about 67-fold increase in tritiated thymidine uptake (402±16 vs 26846±1124 c.p.m., P<0.001). Treatment with the vehicle DMSO affected neither the tritiated thymidine uptake in the resting state nor that in the stimulated state. While being similar to cyclosporin A in having little effects on tritiated thymidine uptake in resting T cells, both Cpd 6A and cyclosporin A significantly suppressed the enhanced uptake observable in activated cells. Furthermore, the inhibitory effects of Cpd 6A on activated T cells were concentration dependent. At 1.5 μM, Cpd 6A suppressed PHA-treated T-cell proliferation to the extent of 20.0±4.0%. The corresponding degrees of inhibition for 6.25, 25, and 100 μM were 50.0±6.2, 85.6±8.9, and 94.2±8.1%, respectively, with an IC50 of 6.25±2.8 μM. The inhibitory effects of Cpd 6A on T cells were not related to direct cytotoxicity, since the viability of resting (90.4±7.6 vs 88.4±2.7%) or activated T cells (82.3±13.2% vs 78.6±15.8%) was not significantly decreased following treatment with 100 μM of Cpd 6A for 4 days.
Figure 2.
Inhibitory effects of Cpd 6A on T-lymphocyte proliferation. T cells (2 × 105 well−1) were treated with cyclosporin A (6.25 μM) or the indicated concentration of Cpd 6A with or without PHA (5 μg ml−1) at the same time (a). In the kinetic study, the cells were activated with or without PHA and then Cpd 6A (25 μM) was added at the indicated time (b). Cells were pulsed on day 3 for 16 h with 1 μCi well−1 tritiated thymidine. Proliferation assay was performed as described in Methods. Each bar represents the mean of three independent experiments. *P<0.01 vs the cells treated with DMSO. **P<0.001 vs the cells treated with DMSO.
Time course of the effect of Cpd 6A on PHA-stimulated T-cell proliferation
Time-course experiments were performed to determine at what point in the activation process Cpd 6A inhibited T-cell proliferation. Cpd 6A was added to cultures at 0, 2, 4, 6, 8, 12, 16, 24, 36, 48, and 72 h, and the proliferation assay was performed at 88 h. The results indicated that, after stimulation, addition of Cpd 6A between 0 and 24 h appeared to exert the highest suppressory effect on T-cell proliferation (Figure 2b). The addition of Cpd 6A to the cultures at 36, 48, and 72 h postactivation only attenuated the cell proliferation. The fact that Cpd 6A was inhibitory when added during the first 24 h suggested that the inhibitory effects of Cpd 6A might be related to the blocking of biochemical events or gene expression necessary for T-cell proliferation activated with PHA during this time.
Effects of Cpd 6A on the cell cycle
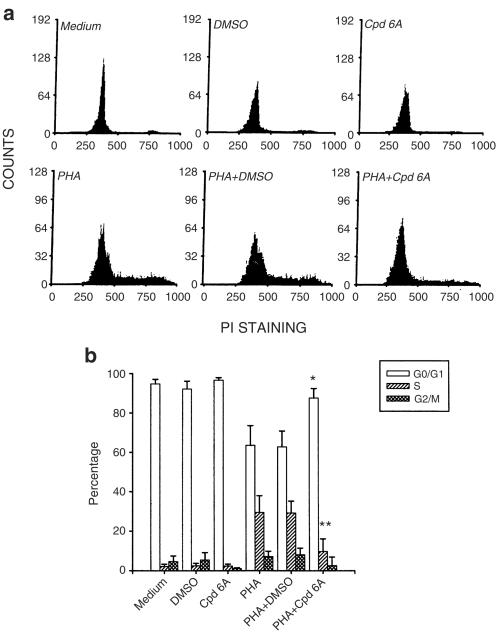
After stimulation with PHA, T lymphocytes enter the G1 phase in 2–4 h, the S phase after approximately 18–24 h, and reach the G2/M phase by 36–48 h (Morice et al., 1993). As the above data suggested, Cpd 6A inhibited T-cell proliferation during the first 24 h after stimulation with PHA. We further examined where in the cell cycle of T cells the arrest took place. After incubation with or without Cpd 6A for 3 days, cell cycle analyses were performed using a commercially prepared propidium iodide reagent for staining nuclear DNA prior to flow cytometry analysis. As shown in Figure 3a, resting T lymphocytes existed almost exclusively in the G0/G1 phase, which was not affected by DMSO or Cpd 6A treatment. When the cells were stimulated with PHA to initiate the cell cycle, fluorescence intensity increased from that of the G0/G1 phase to the S phase, and G2/M phase and DMSO did not affect this fluorescence change. By contrast, after adding Cpd 6A to PHA-activated T lymphoctyes, almost all cells were still blocked at the G0/G1 stage in comparison to the control groups. A computer program was then used to determine the percentage of T cells in the G0/G1, S, and G2/M phases (Figure 3b). Results indicated that addition of Cpd 6A at 2 h after PHA stimulation inhibited 95.6% of the cells from entering the S phase (Table 1 ). Addition of Cpd 6A between 36 and 72 h gradually increased the percentage of cells in the S and G2/M phases. These data are in agreement with the DNA synthesis data, which showed that the addition of Cpd 6A at 0–24 h after PHA stimulation blocks T-cell proliferation.
Figure 3.
Ability of Cpd 6A to block T-cell progression into the S phase of the cell cycle. T cells (2 × 106) were incubated in 25 μM of Cpd 6A with or without PHA (5 μg ml−1) for 3 days. For determining cells entering into the different parts of the cell cycle, cells from a representative subject were stained with propidium iodide, and the DNA content of the cells was analyzed by flow cytometry (a). A computer program was then used to determine the percentage of T cells in the G0/G1, S, and G2/M phases. *P<0.01 vs the cells treated with PHA and DMSO. **P<0.001 vs the cells treated with PHA and DMSO. Each bar is the mean of three independent experiments (b).
Table 1.
Cpd 6A inhibits T cell cycle progression into S phase of the cell cycle
| % Cell | |||
|---|---|---|---|
| G0/G1 | S | G2/M | |
| Condition | |||
| Unstimulated | 95.3±5.5 | 1.6±4.2 | 3.1±2.5 |
| Time after PHA stimulation (h) | |||
| 0 | 92.7±4.7 | 1.4±2.6 | 5.9±3.0 |
| 2 | 91.8±3.8 | 4.3±4.0 | 3.9±2.7 |
| 4 | 93.0±3.2 | 3.9±2.9 | 3.1±1.0 |
| 6 | 91.8±2.7 | 2.8±1.2 | 5.4±4.9 |
| 8 | 91.3±1.6 | 5.9±0.4 | 2.8±3.8 |
| 12 | 86.4±2.1 | 8.9±2.4 | 4.7±1.6 |
| 16 | 85.1±3.0 | 11.2±2.5 | 3.7±2.9 |
| 24 | 77.8±2.6 | 16.8±3.1 | 5.4±1.8 |
| 36 | 66.8±3.9 | 24.7±2.6 | 8.5±0.8 |
| 48 | 63.3±5.5 | 27.9±3.9 | 8.8±4.3 |
| 72 | 61.9±3.8 | 29.5±4.2 | 8.6±5.3 |
| Time of addition of 25 μM Cpd 6A after PHA stimulation (h) | |||
| 0 | 95.5±4.8 | 0.9±0.5 | 3.6±3.0 |
| 2 | 95.6±3.2 | 2.2±2.0 | 2.2±1.8 |
| 4 | 97.9±3.9 | 1.4±0.8 | 0.7±0.3 |
| 6 | 93.4±4.5 | 1.9±1.8 | 4.7±3.9 |
| 8 | 94.7±5.1 | 2.6±1.9 | 2.7±1.7 |
| 12 | 89.7±2.6 | 3.7±2.1 | 6.6±5.0 |
| 16 | 91.3±7.0 | 3.3±3.0 | 5.4±1.2 |
| 24 | 88.9±6.9 | 5.0±2.9 | 6.1±3.3 |
| 36 | 80.0±4.2 | 14.3±3.5 | 5.7±4.4 |
| 48 | 74.3±1.5 | 23.3±2.2 | 6.4±1.3 |
| 72 | 61.9±1.0 | 30.0±3.2 | 8.1±5.6 |
In PHA controls, T cells (2 × 106) were stimulated with PHA (5 μg ml−1) and collected for cell cycle analysis at the indicated time. In experiment groups, after PHA stimulation, 25 μM of Cpd 6A was added at the indicated time and progression of cell cycle was analyzed at the third day. Analysis of cell cycle was performed as described in Methods. These data are representative of three separate experiments.
Effects of Cpd 6A on cyclin production in T lymphocytes
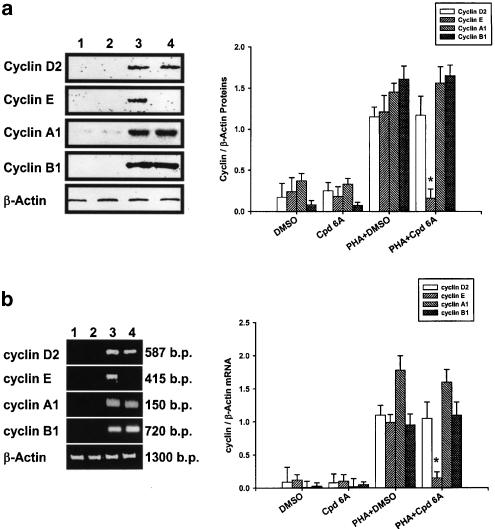
To assess if the blockade of PHA-induced T cell cycle progression at the G0/G1 stage by Cpd 6A was related to expression of G1 cyclins, the cells were incubated with or without Cpd 6A for 24 h. Total cellular proteins were then extracted, and the production of Cyclins D2, E, A1, and B1 in T lymphocytes was assayed by Western blot analyses. As shown in Figure 4a, Cpd 6A did not affect the level of 35 kDa Cyclin D2, 65 kDa Cyclin A1, and 62 kDa Cyclin B1 expressed in T lymphocytes activated with PHA. By contrast, the stimulated production of 50 kDa Cyclin E in activated T cells was significantly suppressed by Cpd 6A. Furthermore, laser densitometry analysis demonstrated that the ratio of Cyclin E to β-actin proteins in PHA-activated T cells was significantly decreased by Cpd 6A.
Figure 4.
Inhibition of cyclin E production and gene expression in T lymphocytes by Cpd 6A. T cells (1 × 107) were stimulated with PHA (5 μg ml−1) in the presence or absence of 25 μM Cpd 6A for 24 h. Lysates (50 μg of protein) were run on a 10% SDS–PAGE gel and analyzed by immunoblotting with anti-Cyclin D2, E, A1 or B1 antibody (a). The total cellular RNA was isolated from T cells (5 × 106) treated with Cpd 6A for 18 h and the RT–PCR was done as described in Methods. Following the reaction, the amplified product was taken out of the tubes and run on 2% agarose gel (b). Unstimulated cells (lane 1), unstimulated cells treated with Cpd 6A (lane 2), stimulated cells (lane 3), and stimulated cells in the presence of Cpd 6A (lane 4). The results are representative of three experiments. Bar graphs represent the ratio of each cyclin to β-actin signal. *P<0.001 vs the cells treated with PHA and DMSO.
Cyclin mRNA expression in Cpd 6A-treated T cells detected by RT–PCR
As Cyclin E proteins in activated T lymphocytes were decreased by Cpd 6A, we examined whether the cyclin E mRNA expression in activated T cells was affected by Cpd 6A. Total cellular RNA was extracted from activated T cells in the presence or absence of 25 μM Cpd 6A for 18 h and used for RT–PCR. Initially, we examined the dose–response relationship of the PCR amplification of cDNA. The exponential phase of amplification was determined by performing for 20, 30, 40, and 50 cycles. We determined that 30 cycles of PCR were optimal for amplication of cDNA for cyclins D2, E, A1, and B1 (data not shown). The results of RT–PCR analyses are shown in Figure 4b. The mRNA for β-actin was detectable in the samples treated with DMSO (lane 1), Cpd 6A (lane 2), PHA and DMSO (lane 3), and PHA and Cpd 6A (lane 4), respectively. The results indicated that neither Cpd 6A nor DMSO affected β-actin mRNA expression in T lymphocytes. While resting T cells expressed low levels of cyclin D2, E, A1, and B1 mRNAs, after PHA stimulation, levels of these cyclin transcriptions were significantly increased in the cells. The data indicated that Cpd 6A did not affect the transcriptions of cyclins D2, A1, and B1 in T cells induced by PHA. RT–PCR products for cyclin E amplified from RNA preparations of PHA-treated T cells were attenuated by Cpd 6A. Analysis of laser densitometry demonstrated that the ratio of cyclin E to β-actin mRNAs in PHA-activated T cells was significantly impaired by Cpd 6A.
Effects of Cpd 6A on cytokine production in T lymphocytes
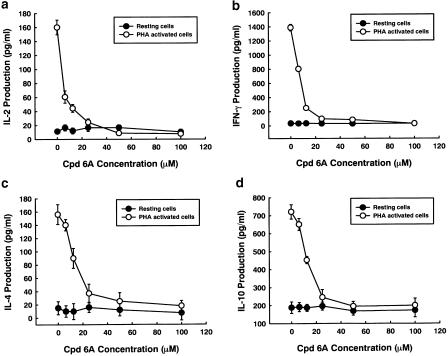
To evaluate the relevance of cytokine production to the inhibitory effects of Cpd 6A on T-cell proliferation after stimulation with PHA, T cells were incubated with or without varying concentrations of Cpd 6A for 3 days. Supernatants were then collected, and the production of IL-2, IL-4, IL-10, and IFN-γ was assayed by EIA. As shown in Figure 5a–d, the stimulated production of these cytokines in activated T cells was significantly suppressed by Cpd 6A. Furthermore, the inhibitory activities of Cpd 6A were concentration dependent. At 100 μM, the stimulated production of cytokines in activated T cells was completely blocked by Cpd 6A, with their concentrations returning to almost the same levels as those in resting cells.
Figure 5.
Cytokine production in T lymphoctyes treated with Cpd 6A. T cells (2 × 105 well−1) were treated with 0, 6.25, 12.5, 25, 50, or 100 μM of Cpd 6A with or without PHA (5 μg ml−1) for 3 days. Then, the cell supernatants were collected and IL-2 (a), IFN-γ (b), IL-4 (c), and IL-10 (d) concentration was determined by EIA, respectively. Each point is the mean of three independent experiments.
Effects of Cpd 6A on cytokine gene expression in T lymphocytes
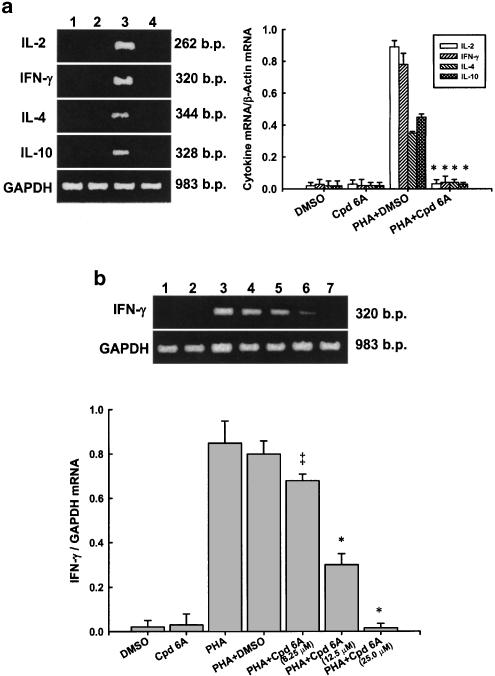
As the production of several cytokines in activated T lymphocytes was decreased by Cpd 6A, we examined whether the expression of cytokine mRNA in activated T cells was affected by Cpd 6A. Total cellular RNA was extracted from activated T cells in the presence or absence of 25 μM of Cpd 6A for 18 h and subjected to RT–PCR. The results of RT–PCR analyses are shown in Figure 6a. The mRNA for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detectable in the samples treated with DMSO (lane 1), Cpd 6A (lane 2), DMSO and PHA (lane 3), and Cpd 6A and PHA (lane 4), respectively. The results indicated that neither Cpd 6A nor DMSO affected GAPDH mRNA expression in T lymphocytes, and that resting T cells expressed low levels of IL-2, IL-4, IL-10, and IFN-γ mRNAs. After PHA stimulation, the levels of each cytokine mRNA were significantly increased in the cells. By contrast, Cpd 6A treatment attenuated mRNAs of IL-2, IL-4, IL-10, and IFN-γ in activated T cells, as indicated by PCR amplification. Laser densitometry analysis demonstrated that the mRNAs of those cytokines were suppressed by Cpd 6A. RT–PCR analysis confirmed that IFN-γ mRNA expression in PHA-activated T cells was blocked by Cpd 6A in a dose-dependent manner (Figure 6b). Similar results were also obtained from other cytokines (data not shown). Thus, the decreased production of IL-2, IL-4, IL-10, and IFN-γ was related to blocking of their mRNA expression in T cells treated with Cpd 6A.
Figure 6.
Effects of Cpd 6A on cytokine mRNA expression in T cells, detected by RT–PCR analysis. T cells (5 × 106) were activated with or without PHA (5 μg ml−1) in the presence or absence of 25 μM Cpd 6A for 18 h. The total cellular RNA was isolated from T cells treated with DMSO (Lane 1), Cpd 6A (lane 2), PHA and DMSO (lane 3), or PHA and Cpd 6A (lane 4), respectively. Aliquots of 1 μg of RNA were reverse-transcribed for synthesis of first-strand cDNA. Briefly, 10 μl of the first-strand cDNA was applied for PCR, as described in Methods. Following the reaction, the amplified product was taken out of the tubes and run on 2% agarose gel (a). The inhibitory activity of Cpd 6A on IFN-γ mRNA production in T cells was concentration dependent (b). Graphical representation of laser densitometry of cytokine expression in resting or PHA-stimulated T cells in the presence or absence of Cpd 6A. The ratio of each cytokine mRNA to GAPDH mRNA was calculated. P<0.05 vs the cells treated with PHA and DMSO. *P<0.001 vs the cells treated with PHA and DMSO. Each bar is the mean of three independent experiments.
Expression of c-Fos and c-Jun proteins in Cpd 6A-treated T cells
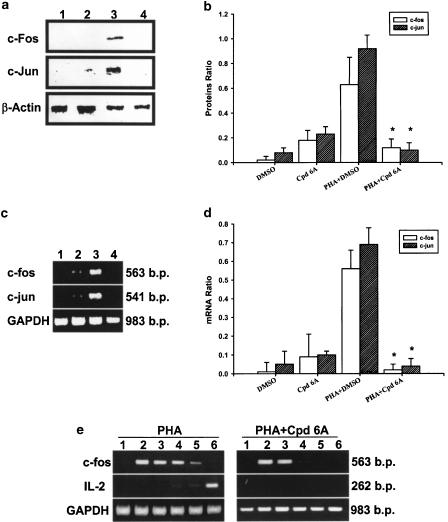
Next, we assessed the synthesis of c-Fos and c-Jun proteins during the suppression of IL-2 mRNA by Cpd 6A in T cells stimulated with PHA. The cells were pretreated with or without Cpd 6A for 30 min and stimulated with PHA. Cellular proteins were then extracted, and c-Fos and c-Jun protein levels detected by Western blot analysis. The results indicated that T cells expressed 55 kDa c-Fos and 39 kDa c-Jun proteins after PHA stimulation for 4 h (Figure 7a). The cells incubated with PHA in the presence of 25 μM Cpd 6A showed a significant decrease in c-Fos and c-Jun protein levels in comparison with the control group. As shown in Figure 7b, analysis of laser densitometry demonstrated that the values for c-Fos and c-Jun proteins in Cpd 6A-treated cells were significantly lower than those in untreated cells.
Figure 7.
c-Fos and c-Jun protein and mRNA levels are decreased by Cpd 6A and kinetic studies of c-fos and IL-2 mRNA expression in activated T cells. T cells (1 × 107) were pretreated with 25 μM of Cpd 6A for 30 min and then PHA (5 μg ml−1) for 4 h. Lysates (50 μg of protein) were run on a 10% SDS–PAGE gel and analyzed by immunoblotting with anti-c-Fos or c-Jun antibodies (a). Bar graphs represent the ratio of the c-Fos or c-Jun to β-actin proteins. These results are representative of three separate experiments. *P<0.001 vs the cells treated with PHA and DMSO (b). The total cellular RNA was isolated from T cells (5 × 106) pretreated with Cpd 6A for 30 min and then activated with PHA for 2 h. Following RT–PCR, the amplified product was taken out of the tubes and run on 2% agarose gel. The results are representative of three experiments (c). Bar graphs represent the ratio of c-fos or c-jun to the GAPDH mRNA signal. *P<0.001 vs the cells treated with PHA and DMSO (d). Unstimulated cells (lane 1), unstimulated cells treated with Cpd 6A (lane 2), stimulated cells (lane 3), and stimulated cells in the presence of Cpd 6A (lane 4). In kinetic studies of c-fos mRNA and IL-2 mRNA expression, T cells were activated with PHA (5 μg ml−1) or 25 μM Cpd 6A was added at 2 h postactivation. Then, total cellular RNA was isolated from T cells at 2 min (lane 1), 30 min (lane 2), 2 h (lane 3), 4 h (lane 4), 6 h (lane 5), and 8 h (lane 6) postactivation. Following RT–PCR, the amplified product was taken out of the tubes and run on 2% agarose gel (e).
Effects of Cpd 6A on c-fos and c-jun gene expression in T lymphocytes
As the production of c-Fos and c-Jun proteins in activated T lymphocytes was decreased by Cpd 6A, we examined whether the mRNAs for these proteins were affected by Cpd 6A. T cells were pretreated with or without Cpd 6A for 30 min. Then, the total cellular RNA was extracted from T cells in the presence or absence of PHA for 2 h and analyzed by RT–PCR. These results are shown in Figure 7c. The mRNA for GAPDH was detectable in the samples treated with DMSO (lane 1), Cpd 6A (lane 2), DMSO and PHA (lane 3), and Cpd 6A and PHA (lane 4), respectively. While resting T cells expressed the basal levels of c-fos and c-jun mRNAs, the levels of each mRNA were significantly increased in the cells treated with PHA. However, PCR products for c-fos and c-jun amplified from PHA-treated T-cell RNA preparations were attenuated by Cpd 6A. The laser densitometry analysis demonstrated that the ratios of c-fos and c-jun mRNAs to GAPDH mRNA in PHA-activated T cells were significantly decreased by Cpd 6A (Figure 7d). Furthermore, similar results were obtained from the cells treated with PHA and Cpd 6A at the same time (data not shown).
To prove that decreased expression of c-fos preceded a change in cytokine production, the c-fos and IL-2 mRNA expressed in T cells treated with PHA for different time periods was analyzed by RT–PCR. The data showed that c-fos mRNA was expressed in the cells 30 min after activation, and decreased 6 h after activation (Figure 7e). The IL-2 mRNA could be detected in T cells 8 h after activation. When the cells were treated with Cpd 6A 2 h after activation, c-fos mRNA was decreased 4 h after activation and IL-2 mRNA expression was blocked 8 h after activation.
The roles of IL-2, IL-4, and IFN-γ in restoring T-cell proliferation and cell cycle progression after Cpd 6A treatment
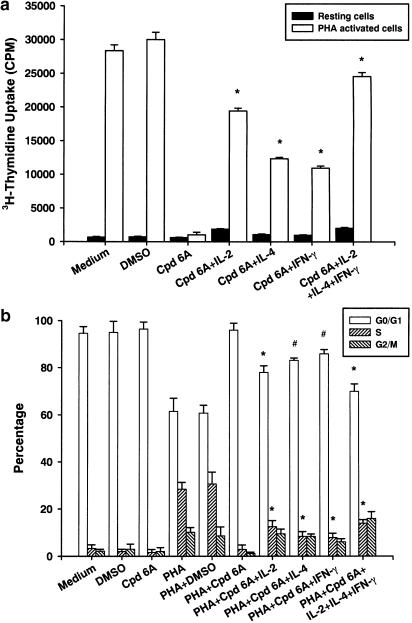
In order to assess the roles played by IL-2, IL-4, and IFN-γ in T-cell proliferation when treated with Cpd 6A, the cells were incubated with Cpd 6A, IL-2, IL-4, or IFN-γ with or without PHA for 3 days. Then, cell proliferation was determined on the basis of uptake of tritiated thymidine. Flow cytometry was used to analyze the percentage of the cell population that could enter S phase in the presence of these agents. As shown in Figure 8a, Cpd 6A significantly suppressed the cell proliferation in T cells stimulated with PHA (1045±373 vs 30012±1094 c.p.m., P<0.001). After addition of exogenous IL-2, IL-4, IFN-γ or a combination of IL-2, IL-4, and IFN-γ to T cells, Cpd 6A began to lose its inhibitory effect. Cell cycle analysis demonstrated that when T lymphocytes were incubated with Cpd 6A and PHA, approximately 2.9% of the cells were in the S phase. When IL-2, IL-4, IFN-γ, or a combination of these agents was added, 12.5±2.6, 8.4±2.0, 7.9±1.8, and 14.0±1.5% of the cells were in the S phase, respectively. Taken together, these results indicate that Cpd 6A inhibited T-cell proliferation by blocking cells in the G0/G1 phase of the cell cycle, and these inhibitory effects could be decreased through the addition of exogenous IL-2, IL-4, and IFN-γ.
Figure 8.
Inhibitory activities of Cpd 6A on T-lymphocyte proliferation and cell cycle progression were decreased by addition of exogenous IL-2, IL-4, and IFN-γ. T cells (2 × 105 well−1) were treated with Cpd 6A (25 μM), IL-2 (1.0 ng ml−1), IL-4 (0.5 ng ml−1), IFN-γ (3.0 ng ml−1) or a combination of IL-2, IL-4, and IFN-γ with or without PHA (5 μg ml−1). Cells were pulsed on day 3 for 16 h with 1 μCi well−1 tritiated thymidine. Proliferation assays were performed as described. Each bar represents the mean of three independent experiments. *P<0.001 vs the cells treated with Cpd 6A alone (a). In cell cycle analysis, T cells (2 × 106) were treated with Cpd 6A (25 μM), IL-2 (1.0 ng ml−1), IL-4 (0.5 ng ml−1), IFN-γ (3.0 ng ml−1) or a combination of IL-2, IL-4, and IFN-γ with or without PHA (5 μg ml−1) for 3 days. The DNA content of the cells was analyzed by flow cytometry and a computer program was then used to determine the percentage of T cells in the G0/G1, S, and G2/M phases. #P<0.01 vs the cells treated with PHA and Cpd 6A. *P<0.001 vs the cells treated with PHA and Cpd 6A. Each bar is the mean of three independent experiments (b).
Discussion
In the present study, the action mechanisms of Cpd 6A identified from C. cicadae on human T lymphocyte proliferation activated with PHA were defined. The results demonstrated that the growth-suppressive actions of Cpd 6A were not explained by a drug-induced reduction in cell viability and that delayed addition of Cpd 6A reduced its antiproliferative activity. Cell cycle analysis revealed that Cpd 6A inhibited the entry of PHA-stimulated T cells into the S phase of the cell cycle, observations that were consistent with the data that Cpd 6A suppressed PHA-driven T-cell proliferation. In addition, we observed that Cpd 6A decreased the gene expression of cyclin E, IL-2, IL-4, IL-10, and IFN-γ in activated T cells. Cpd 6A impaired c-fos and c-jun protein synthesis and mRNA transcription in T cells stimulated with PHA. After addition of exogenous IL-2, IL-4, and IFN-γ to the cultures, the inhibitory functions of Cpd 6A on cell cycle progression and proliferation in T cells stimulated with PHA were attenuated. We suggest that Cpd 6A interferes with some regulatory events required for the entry of PHA-activated T cells into the S phase and then the cell proliferation is suppressed.
Cpd 6A is a sterol and has been isolated from many fungi such as Ganoderma carnosum, C. sinensis, and Aspergillus species (Keller et al., 1997; Bok et al., 1999; Rösecke & König, 2000). There are several biological functions described for ergosterol peroxide. It possesses an antiviral action (Lindequist et al., 1989) and antitumor activity (Mizushina et al., 1998). Ergosterol peroxide is identified as an immunosuppressive component (Fujimoto et al., 1994). The present results showed that Cpd 6A suppressed the activation and proliferation signals in primary human T lymphocytes induced by PHA. The possible inhibitory effect of DMSO on T cells was studied in these experiments. T-cell proliferation and viability were not changed by DMSO. Therefore, the inhibitory function of Cpd 6A was unlikely to be related to DMSO. The morphology and characteristics of T cells treated with or without Cpd 6A were similar, suggesting that the inhibitory effects of Cpd 6A were not related to the pH, osmolarity, or other physiological variables in different preparations (data not shown). Moreover, the effects of Cpd 6A on T lymphocytes were determined at the third day or early after PHA activation, and, in almost all experiments, the concentration of Cpd 6A used was 25 μM. The results of cell viability indicated that there was no significant cell death in T-cell cultures after treatment with 100 μM Cpd 6A for 4 days. We suggest that, at concentrations below 100 μM and during this time frame, the inhibitory effects of Cpd 6A on T cells were not through cytotoxic effects. We cannot exclude the possibility that Cpd 6A may have toxic effects on T cells following chronic treatment or at higher concentrations.
The central event in the generation of immune responses is the activation and clonal expansion of T cells (Charles et al., 1997). During the process of differentiation, T cells spontaneously arrest in G0 and may remain quiescent for long periods of time, until exposed to specific antigen or mitogens. Interaction of T cells with antigens or PHA initiates a cascade of biochemical events, which induces the resting T cells to enter the cell cycle and then to proliferate and differentiate (Charles, 1993). In our studies, the results indicated that almost all unstimulated primary T lymphocytes existed at the G0/G1 phase and, after stimulation with PHA, entry into cell cycle was induced. It has been demonstrated in many previous studies with T cells that a series of genes such as c-fos, cyclins, IFN-γ, and IL-2 are included in a carefully controlled order as the cells pass through G0, G1, and S phases (Ajchenbaum et al., 1993). For example, the transition from G0 to G1 is marked by transcriptional activation of the IL-2 receptor and IL-2 genes (and in some cases IL-4). Data from primary human T lymphocytes indicate that cyclin E is likely to play a regulatory role in the cell cycle from G1 transition to the S phase (Charles, 1993). Inhibition of the function of cyclin E and its related cyclin-dependent kinase cdk2 prevents mammalian cells from entering the S phase (Pagano et al., 1993). Subsequent G1 events and initiation of DNA synthesis are dependent on the induction of IL-2 receptor and on a supply of IL-2 from autocrine or external sources. Although the molecular mechanisms involved in regulating the passage through cell cycle in T cells stimulated with PHA remain largely unknown, growth modulators or other external events that affect the T-cell proliferation are ultimately likely to act by controlling the expression or function of the products of these genes (Ajchenbaum et al., 1993). The present results indicate that Cpd 6A suppressed the proliferation of PHA-activated T cells and blocked PHA-induced progression of the T cell cycle from G1 transition to S phase. We also found that the levels of cyclin E mRNA and proteins in PHA-activated T cells were decreased by Cpd 6A. The deficiencies in cyclin E proteins were caused by defects in cyclin E mRNA production in activated T cells. Thus, we suggest that the inhibitory effects of Cpd 6A on T-cell proliferation may be related to the reduction of cyclin E gene expression and arrest of cell cycle progression in the cells.
On the other hand, the production of cytokines such as IL-2, IL-10, IFN-γ, and IL-4 in PHA-activated T-cell cultures was decreased by Cpd 6A. These decreases in cytokine production were related to Cpd 6A suppressing the mRNA transcripts for these cytokines. In the present study, exogenous IL-2, IL-4, and IFN-γ were added to T-cell cultures in the presence of Cpd 6A, and the cell cycle progression and proliferation were determined. Although approximately 50% of the effects of Cpd 6A on T cells could be reversed by IL-2, other cytokines such as IL-4 and IFN-γ were also effective. Gelfand et al. (1987) showed that decreased intracellular Ca2+ concentration, [Ca2+]i, would lead to decreased IL-2 production in T lymphocytes. The increase in [Ca2+]i. induced by PHA in T cells was not reduced by Cpd 6A, even at high concentrations (data not shown). Thus, suppression of the expression of cyclin E, IL-2, IL-4, IL-10, and IFN-γ in activated T lymphocytes by Cpd 6A was not related to the effects on [Ca2+]i. Although we could not exclude the possibility that other mechanisms such as interference with signal transduction downstream of PHA might also act cooperatively to inhibit T-cell activation and proliferation, we believe that one of the factors contributing to the arrest of cell cycle progression is a deficiency in IL-2, IL-4, and IFN-γ production in T cells. These actions are similar to those of cyclosporin A, which induces arrest early in the G1 phase of T-cell cycle by inhibiting IL-2 transcription (Schreiber & Crabtree, 1992).
In addition, many previous studies showed that c-Fos combined with c-Jun proteins as the AP-1 complex is an important regulatory factor for IL-2 mRNA transcription in T lymphocytes (Charles et al., 1997). The c-fos and c-jun genes are required for cell cycle progression and cell proliferation in PHA-stimulated lymphocytes (Tay et al., 1996). During the G1 phase of the cell cycle, activation of AP-1 can be detected within hours following T-cell stimulation, and involves the transcription of stimulus-induced genes (Angel & Herrlich, 1994). Whisler et al. (1993) also report that the age-related impairments in G1 progression of PHA-stimulated T cells are associated with decreased expression of c-jun and IL-2 genes. In our studies, although, in some cases, the production and gene expressions of c-fos and c-jun could be detected in unstimulated T cells, both gene products could be significantly increased in the cells after PHA stimulation. Western blot analysis indicated that Cpd 6A decreased c-Fos and c-Jun proteins in T cells activated with PHA. The data demonstrated that Cpd 6A reduced the levels of c-Fos and c-Jun proteins through inhibition of c-fos and c-jun mRNA expressions in activated T cells. The kinetic studies indicated that the decreased expression of c-fos mRNA preceded a change in IL-2 mRNA. We therefore suggest that defects in IL-2 mRNA transcription in activated T cells are related to the inhibitory effect of Cpd 6A on c-Fos protein synthesis. Attenuation of c-fos and c-jun gene expression in T cells may be one of the mechanisms by which Cpd 6A suppressed T-cell proliferation induced by PHA.
In summary, we hypothesize that the inhibitory mechanisms of Cpd 6A on T-cell proliferation activated by PHA are, at least in part, related to: (1) Cpd 6A changing the c-fos and c-jun mRNA levels and protein levels in the cells, (2) Cpd 6A affecting cyclin E and cytokine mRNA levels in the cells, (3) Cpd 6A reducing the production of cytokine and Cyclin E in T cells activated with PHA, (4) Cyclin E and cytokine production decrease as the entry into S phase of the cell cycle induced by PHA is interrupted, and (5) inhibition of the entry into the S phase of the cell cycle, causing the antiproliferative effect of Cpd 6A on T cells. It is believed that asthma is a chronic disorder in which T-cell-mediated inflammation causes smooth muscle contraction and narrowing of the airways (Corrigan & Kay, 1992; Goodman et al., 1996). Cytokines such as IFN-γ, IL-4, IL-10, and IL-5 play an important role in supporting chronic airway inflammation. Thus, results of the present study indicate that Cpd 6A contained in extracts of C. cicadae may also have acted to reduce tissue inflammation – in part by inhibiting T-lymphocyte proliferation and IL-4, IL-10, and IFN-γ gene expression. Our observations correlate with the putative pharmacological activities attributed to C. cicadae. Although in vitro studies do not necessarily predict in vivo outcomes, such studies have provided insights into molecular targets, as illustrated by the effects of Cpd 6A on cytokine, cyclins, and AP-1 genes. The relative contributions of these activities to the potent immunosuppression by Cpd 6A in vivo remain to be elucidated. Its detailed mechanisms of actions on cell cycle progression and cyclin E and cytokine gene transcriptions in PHA-activated T lymphocytes are subjects for further study.
Acknowledgments
This study was partially supported by grants-in aid from The National Science Council, Republic of China (NSC90-2320-B-077-006 and NSC91-2320-B-077-019).
Abbreviations
- Cpd 6A
ergosterol peroxide
- DEPC
diethyl pyrocarbonate
- DMSO
dimethyl sulfoxide
- IFN-γ
interferon-γ
- IL
interleukin
- PCR
polymerase chain reaction
- PHA
phytohemagglutinin
References
- AJCHENBAUM F., ANDO K., DECAPRIO J.A., GRIFFIN J.D. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J. Biol. Chem. 1993;268:4113–4119. [PubMed] [Google Scholar]
- ANGEL P.E., HERRLICH P.A. The FOS and JUN Families of Transcription Factors. Boca Raton, FL: CRC Press; 1994. [Google Scholar]
- ARAI K., LEE F., MIYAJIMA A. Cytokines: coordinators of immune and inflammatory response. Annu. Rev. Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- BOK J.W., LERMER L., CHILTON J., KLINGEMAN H.G., TOWERS G.H. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry. 1999;51:891–898. doi: 10.1016/s0031-9422(99)00128-4. [DOI] [PubMed] [Google Scholar]
- BRADELY B., AZZAWI L., JACOBSON M., ASSOUFI B., COLLINS J.V., IRANI A.A., SCHWARTZ L.B., DURHAM S.R., JEFFERY P.K., KAY A.B. Eosinophils, T lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J. Allergy Clin. Immunol. 1991;88:661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- CANTRELL D. T cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- CHARLES A.J., JR, PAUL T., HUNT S., WALPORT M. Immunobiology. New York: Current Biology Ltd; 1997. [Google Scholar]
- CHARLES J.S. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- CORRIGAN C.J., KAY A.B. T cells and eosinophils in the pathogenesis of asthma. Immunol. Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO H., NAKAYAMA M., NAKAYAMA Y., YAMAZAKI M. Isolation and characterization of immunosuppressive components of three mushrooms, Pisolithus tinctorius, Microporus flabelliformis and Lenzites betulina. Chem. Pharm. Bull. 1994;42:694–697. doi: 10.1248/cpb.42.694. [DOI] [PubMed] [Google Scholar]
- GELFAND E.W., MILLS G.B., CHEUNG R.K., LEE J.W., GRINSTEIN S. Transmembrane ion influxes during activation of human T lymphocytes. Role of Ca2+, Na+/H+ exchange and phospholipid turnover. Immunol. Rev. 1987;95:59–87. doi: 10.1111/j.1600-065x.1987.tb00500.x. [DOI] [PubMed] [Google Scholar]
- GOODMAN R.B., STRIETER R.M., MARTIN D.P., STEINBERG K.P., MILBERG J.A., MAUNDER R.J., KUNKEL S.L., WALZ A., HUNDSON L.D., MARTIN T.R. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1996;154:602–611. doi: 10.1164/ajrccm.154.3.8810593. [DOI] [PubMed] [Google Scholar]
- GRAY P.W., AGGRAWAL B.B., BENTON C.V., BRINGMAN T.S., HENZEL W.J., JARRETT J.A., LEUNG D.W., MOFFAT B. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982;295:503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- HIRANO T. Complementary DNA for a novel interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- KARIN M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995;28:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- KELLER A.C., KELLER J., MAILARD M.P., HOSTETTMANN K. A lanostane-type steroid from the fungus Ganoderma carnosum. Phytochemistry. 1997;46:963–965. [Google Scholar]
- KIHO T., ITO M., YOSHIDA I., NAGAI K., HARA C., UKAI S. Polysaccharides in fungi. XXIV. A (1 → 3)-β-D-glucan from the alkaline extract of the insect-body portion of Chan Hua (fungus: Cordyceps cicadae) Chem. Pharm. Bull. 1989;37:2770–2772. [Google Scholar]
- KIM J.M., BRANNAN C.I., COPELAND N.G., JENKINS N.A., KHAM T.A., MOORE K.W. Structure of the mouse interleukin-10 gene and chromosomal localization of the mouse and human genes. J. Immunol. 1992;148:3618–3623. [PubMed] [Google Scholar]
- KUO Y.C., LIN C.Y., TSAI W.J., WU C.L., CHEN C.F., SHIAO M.S. Growth inhibitors against tumor cells in Cordyceps sinensis other than cordycepin and polysaccharides. Cancer Invest. 1994;12:611–615. doi: 10.3109/07357909409023046. [DOI] [PubMed] [Google Scholar]
- KUO Y.C., LIN L.C., DON M.J., LIAO H.F., TSAI Y.P., LEE G.H., CHOU C.J. Cyclodesipeptide and dioxomorpholine derivatives isolated from the insect-body portion of the fungus Cordyceps cicadae. J. Chin. Med. 2002;13:209–219. [Google Scholar]
- KUO Y.C., TSAI W.J., SHIAO M.S., CHEN C.F., LIN C.Y. Cordyceps sinensis as an immunomodulatory agent. Am. J. Chin. Med. 1996;XXIV:111–125. doi: 10.1142/S0192415X96000165. [DOI] [PubMed] [Google Scholar]
- KUO Y.C., TSAI W.J., WANG J.Y., CHANG S.C., LIN C.Y., SHIAO M.S. Regulation of bronchoalveolar lavage fluids cell function by the immunomodulatory agents from Cordyceps sinensis. Life Sci. 2001;68:1067–1082. doi: 10.1016/s0024-3205(00)01011-0. [DOI] [PubMed] [Google Scholar]
- KUO Y.C., YANG N.S., CHOU C.J., LIN L.C., TSAI W.J. Regulation of cell proliferation, gene expression, production of cytokines, and cell cycle progression in primary human T lymphocytes by piperlactam S isolated from Piper kadsura. Mol. Pharmacol. 2000;58:1057–1066. doi: 10.1124/mol.58.5.1057. [DOI] [PubMed] [Google Scholar]
- LEW D.J., DULIC V., REED S.I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- LINDEQUIST U., LESNAU A., TEUSCHER E., PILGRIM H. The antiviral action of ergosterol peroxide. Pharmazie. 1989;44:579–580. [PubMed] [Google Scholar]
- MARONE M., SCAMBIA G., GIANNITELLI C., FERRANDINA G., MASCIULLO V., BELLACOSA A., BENEDETTI-PANICI P., MANCUSO S. Analysis of cyclin E and CDK2 in ovarian cancer: gene amplification and RNA overexpression. Int. J. Cancer. 1998;75:34–39. doi: 10.1002/(sici)1097-0215(19980105)75:1<34::aid-ijc6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- MIZUSHINA Y., WATANABE I., TOGASHI H., HANASHIMA L., TAKEMURA M., OHTA K., SUGAWARA F., KOSHINO H., ESUMI Y., UZAWA J., MATSUKAGE A., YOSHIDA S., SAKAGUCHI K. An ergosterol peroxide, a natural product that selectively enhances the inhibitory effect of linoleic acid on DNA polymerase beta. Biol. Pharm. Bull. 1998;21:444–448. doi: 10.1248/bpb.21.444. [DOI] [PubMed] [Google Scholar]
- MORICE W.G., BRUNN G.J., WIEDERRECHT G., SIEKIERKA J.J., ABRAHAM R.T. Rapamycin-induced inhibition of p34cdc2 kinase activation is associated with G1/S-phase growth arrest in T lymphocytes. J. Biol. Chem. 1993;268:3734–3738. [PubMed] [Google Scholar]
- NORBURY C., NURSE P. Animal cell cycles and their control. Annu. Rev. Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- PAGANO M., PEPPERKOK R., VEERDE F., BALDIN V., ANSORGE W., BARTEK J. Regulation of the cell cycle by the cdk2 protein kinase in cultured human fibroblasts. J. Cell Biol. 1993;121:101–111. doi: 10.1083/jcb.121.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDE A.B. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- RÖSECKE J., KÖNIG W.A. Constituents of various wood-rotting basidiomycetes. Phytochemistry. 2000;54:603–610. doi: 10.1016/s0031-9422(00)00165-5. [DOI] [PubMed] [Google Scholar]
- SCHREIBER S.L., CRABTREE G.R. The mechanism of action of cyclosporin A and FK 506. Immunol. Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI T., MATSUI H., FUJITA T., TAKAOKA C., KASHIMA N., YOSHIMOTO R., HAMURO J. Structure and expression of a cloned cDNA for interleukin-2. Nature. 1983;302:305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- TAY D.L., HOFFBRAND A.V., WICKREMASINGHE G.R. Expression of c-fos and c-jun proteins and AP-1 binding activity during cell cycle progression of HL60 cells and phytohemagglutinin-stimulated lymphocytes. Exp. Hematol. 1996;24:277–284. [PubMed] [Google Scholar]
- UKAI S., KIHO T., HARA C., MORITA M., GOTP A., IMAIZUMI N., HASEGAWA Y. Polysaccharides in fungi. XIII. Antitumor activity of various polysaccharides isolated from Dictyophora indusiata, Ganoderma japonicum, Cordyceps cicadae, Auricularia auricuiajudae and Auricularia species. Chem. Pharm. Bull. 1983;31:741–744. doi: 10.1248/cpb.31.741. [DOI] [PubMed] [Google Scholar]
- UKAI S., MATSUURA S., HARA C., KIHO T., HIROSE K. Structure of a new galactomannan from the ascocarps of Cordyceps cicadae Shing. Carbohydrate Res. 1982;101:109–116. [Google Scholar]
- WENG S.C., CHOU C.J., LIN L.C., TSAI W.J., KUO Y.C. Immunomodulatory functions of extracts from Chinese medicinal fungus Cordyceps cicadae. J. Ethnopharmacol. 2002;83:79–85. doi: 10.1016/s0378-8741(02)00212-x. [DOI] [PubMed] [Google Scholar]
- WHISLER R.L., LIU B., WU L.C., CHEN M. Reduced activation of transcriptional factor AP-1 among peripheral blood T cells from elderly humans after PHA stimulation: restroactive effect of phorbol diesters. Cell. Immunol. 1993;152:96–109. doi: 10.1006/cimm.1993.1270. [DOI] [PubMed] [Google Scholar]