Abstract
Dronedarone is a noniodinated benzofuran derivative that has been synthesized to overcome the limiting iodine-associated adverse effects of the potent antiarrhythmic drug amiodarone. In this study, the acute electrophysiological effects of dronedarone on repolarizing potassium channels were investigated to determine the class III antiarrhythmic action of this compound. HERG and KvLQT1/minK potassium channels conduct the delayed rectifier potassium current IK in human heart, being a primary target for class III antiarrhythmic therapy.
HERG and KvLQT1/minK were expressed heterologously in Xenopus laevis oocytes, and the respective potassium currents were recorded using the two-microelectrode voltage-clamp technique.
Dronedarone blocked HERG channels with an IC50 value of 9.2 μM and a maximum tail current reduction of 85.2%.
HERG channels were blocked in the closed, open, and inactivated states. The half-maximal activation voltage was shifted by −6.1 mV, and HERG current block by dronedarone was voltage-dependent, but not use-dependent.
Dronedarone exhibited a weaker block of KvLQT1/minK currents (33.2% at 100 μM drug concentration), without causing significant changes in the corresponding current–voltage relationships.
In conclusion, these data demonstrate that dronedarone is an antagonist of cloned HERG potassium channels, with additional inhibitory effects on KvLQT1/minK currents at higher drug concentrations, providing a molecular mechanism for the class III antiarrhythmic action of the drug.
Keywords: Amiodarone, arrhythmia, antiarrhythmic drug, dronedarone, electrophysiology, ion channels, K+ channel, torsade de pointes, Xenopus oocytes
Introduction
Amiodarone has been widely used as an effective drug in the treatment of supraventricular and ventricular arrhythmias (Singh, 1995). Concerns have been raised about noncardiac adverse effects of amiodarone, especially ocular and pulmonary toxicity or thyroid dysfunction, aggravated by its long biologic half-life. In search for a potential alternative to amiodarone, the noniodinated benzofuran derivative dronedarone (SR33589B), which is structurally related to amiodarone, has been developed. Its short-term electrophysiological effects in rabbit hearts have been shown to be similar to those of amiodarone (Sun et al., 1999,2002). Moreover, a study by Chatelain et al. (1995) revealed that dronedarone exhibits antiadrenergic properties. Recent electrophysiological studies confirmed that this agent displays a wide pharmacological spectrum, since dronedarone inhibits the rapid component of the delayed rectifier potassium current IKr and L-type calcium currents, ICa(L), in isolated canine cardiomyocytes (Varro et al., 2001). In addition, dronedarone blocks both components of the delayed rectifier potassium current, IKr and IKs, as well as ICa(L) and inward rectifier potassium currents in guinea-pig cardiomyocytes (Gautier et al., 2003).
In human cardiomyocytes, repolarization is mainly carried out by the rapidly and slowly activating components IKr and IKs of the delayed rectifier potassium current (Sanguinetti & Jurkiewicz, 1990). The underlying potassium channel genes encoding IKr and IKs are HERG (Sanguinetti et al., 1995) and KvLQT1/minK (Barhanin et al., 1996; Sanguinetti et al., 1996), respectively.
The aim of the present study was to investigate the acute electrophysiological effects of dronedarone on the human molecular counterparts of the crucial repolarizing current IK, HERG, and KvLQT1/minK, expressed heterologously in Xenopus oocytes.
Methods
Molecular biology
The cDNA clones encoding for HERG and KvLQT1 were generously donated by Dr M.T. Keating, and the minK clone was kindly provided by Dr A.M. Brown. Procedures for in vitro transcription and oocyte injection have been published previously (Kiehn et al., 1999). Briefly, HERG (Warmke & Ganetzky, 1994), KvLQT1 (Sanguinetti et al., 1996), and minK (Sanguinetti et al., 1996) cRNAs were prepared with the mMESSAGE mMACHINE kit (Ambion, Austin, U.S.A.), using SP6 RNA polymerase after linearization with EcoRI (Roche Diagnostics, Mannheim, Germany). Stage V–VI defolliculated Xenopus oocytes were injected with 46 nl of cRNA per oocyte. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institute of Health (NIH Publication No. 85-23, revised 1996). All experiments followed the European Community guidelines for the use of experimental animals.
Electrophysiology and statistics
Two-microelectrode voltage-clamp recordings from Xenopus laevis oocytes were carried out as published previously (Thomas et al., 1999). In brief, recordings were performed using a Warner OC-725A amplifier (Warner Instruments, Hamden, U.S.A.) and pClamp software (Axon Instruments, Foster City, U.S.A.) for data acquisition and analysis. Microelectrodes had tip resistances ranging from 1 to 5 MΩ. The recording chamber was continually perfused. All experiments were carried out at room temperature (20–22°C), and no leak subtraction was done during the experiments.
Concentration–response relationships for dronedarone block were fit with a Hill equation of the form Idrug/Icontrol=1/[1+(D/IC50)n], where I indicates the current, D is the drug concentration, n is the Hill coefficient, and IC50 is the concentration necessary for 50% block. Activation curves were fit with a Boltzmann distribution: G(V)=Gmax/(1−exp[(V1/2−V)/k]), where V is the test pulse potential, V1/2 is the half-maximal activation potential, and k is the slope of the activation curve. All data are expressed as mean±s.d., unless otherwise specified. We used paired and unpaired Student's t-tests (two-tailed tests) to compare the statistical significance of the results; a P-value <0.05 was considered statistically significant. The effects of dronedarone and ethanol on HERG and KvLQT1/minK currents, respectively, were compared using one-way ANOVA. If the hypothesis of equal means could be rejected at the 0.05-level, pairwise comparisons of groups were made and the probability values were adjusted for multiple comparisons using the Bonferroni correction.
Solutions and drug administration
Voltage clamp measurements of Xenopus oocytes were performed in a solution containing (in mM): 5 KCl, 100 NaCl, 1.5 CaCl2, 2 MgCl2, and 10 HEPES (pH 7.4 with NaOH). Current and voltage electrodes were filled with 3 M KCl solution. N-[2-butyl-3-(4-[3-dibutylaminopropoxy]-benzoyl)-benzofuran-5-yl]-methanesulfonamide (dronedarone) hydrochloride (kindly provided by Sanofi-Synthelabo, Montpellier, France) was prepared as 10 mM stock solution in ethanol and stored at 4°C. On the day of the experiments, aliquots of the stock solution were diluted to the desired concentration with the bath solution.
Results
Inhibition of HERG currents by dronedarone
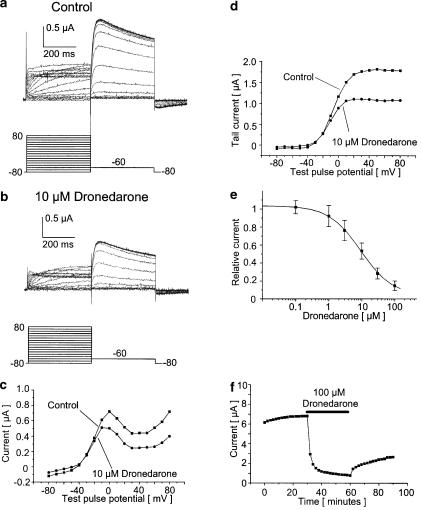
Injection of oocytes with cRNA encoding HERG channels resulted in potassium currents showing inward rectification at positive membrane potentials (Figure 1a). HERG currents were measured with a two-step protocol. From the holding potential (−80 mV), currents were activated by a variable test pulse from −80 to 80 mV (400 ms), followed by a return pulse to −60 mV (400 ms) to evoke outward tail currents. Pulses were applied at a frequency of 0.2 Hz. The amplitude of the outward tail currents exceeded the amplitude of the activating currents during the test pulse, due to slow activation, fast inactivation, rapid recovery from inactivation, and slow deactivation, which is characteristic of HERG currents (Sanguinetti et al., 1995; Kiehn et al., 1996,1999; Smith et al., 1996). After application of 10 μM dronedarone for 30 min, HERG-activating currents (measured at the end of the 10 mV step) and HERG peak tail currents were significantly reduced by 33.5±10.9 and 48.6±6.8%, respectively (Figure 1) (n=4). The half-maximal activation voltage V1/2 (Figure 1d) was shifted by −6.1±1.6 from −4.1±3.6 mV under control conditions to −10.2±2.5 mV after dronedarone incubation (n=4). This difference was statistically significant. The IC50 value for dronedarone block of HERG tail currents yielded 9.2 μM with a Hill coefficient of 0.91 (n=4–5; Figure 1e), with a maximum block of 85.2% at 100 μM dronedarone. The time course of HERG channel inhibition by dronedarone is shown in Figure 1f, displaying relatively slow onset and very slow unblock kinetics. Neither HERG step currents (n=12) nor the half-maximal activation voltage (n=8) were significantly altered during a control period of 30 min without drug application. The control HERG peak tail current amplitude, however, was increased by 12.3±4.0% (n=7). In addition, 1% (v v−1) ethanol (the maximum bath concentration) had no significant effects on HERG outward tail currents (n=7) or the half-maximal activation voltage (n=4), whereas HERG step currents were reduced by 9.2±2.3% (n=6).
Figure 1.
Inhibition of HERG currents by dronedarone. Original current traces obtained before (a) and after incubation with 10 μM dronedarone (b) in a representative oocyte. Outward currents (measured at 10 mV) and peak tail currents were significantly reduced by 33.8 and 39.1%, respectively. This is illustrated by the I–V relationships for HERG outward currents (c) and peak tail currents (d), where current amplitudes were plotted against the respective test pulse potentials (data obtained from the experiments shown in (a) and (b)). (e) Concentration–response curve for block of HERG tail currents by dronedarone after a test pulse to 30 mV. The IC50 yielded 9.2 μM with a Hill coefficient of 0.91 (n=4–5). (f) Time course of HERG tail current inhibition by 100 μM dronedarone in one representative cell. For simplicity, not all current measurements are displayed. After a control period of 30 min, currents decreased upon perfusion with the drug solution within 10 min, reaching the maximum block after 30 min. Upon washout of dronedarone, the blocking effects on HERG were partially reversible within 30 min.
The biophysical mechanism of HERG current inhibition
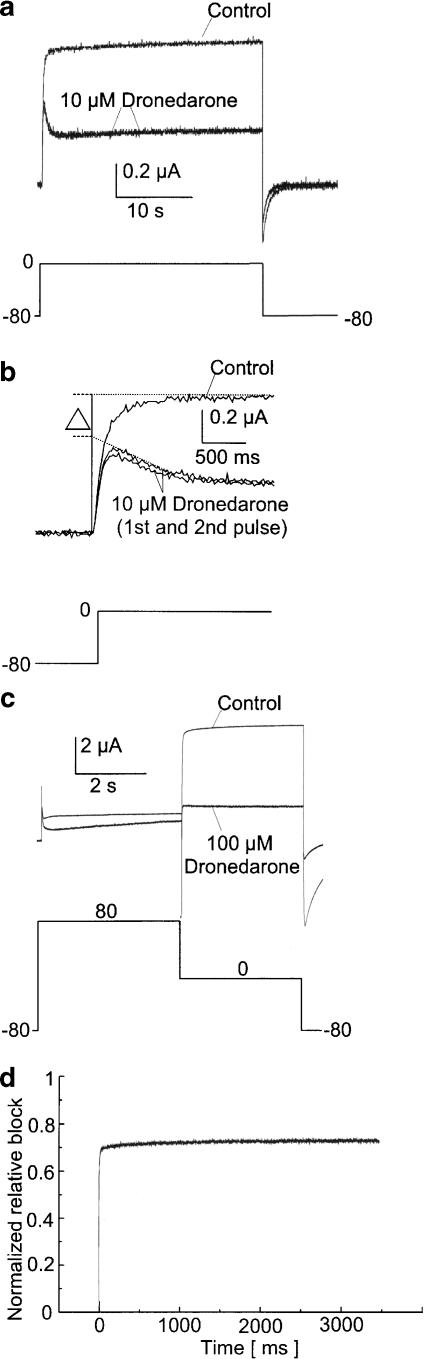
To determine whether dronedarone blocks HERG channels in the closed or open state, we recorded activating currents during a long depolarizing voltage step to 0 mV (30 s) to resolve the slow kinetics of dronedarone block. Figure 2a displays the graphical overlay of the control measurement and two consecutive recordings (2 min apart) after bath application of 10 μM dronedarone for 30 min, revealing two components of the block. The reduced initial peak current after dronedarone application reflects the blockade of closed channels, whereas the additional time-dependent reduction that developed during the test pulse is caused by inhibition of open HERG channels. In a series of four cells, the peak current was reduced by 41.5±11.0%, and maximum block yielded 53.4±11.5%. The open-channel block component was reversible within 2 min, since the second current trace in the presence of dronedarone recorded 2 min after the first recording displayed equal peak current amplitudes compared to the first pulse (Figure 2a). The inhibition of closed and open channels is further illustrated in an enlargement of the first 2 s of the blocking and control curves (Figure 2b). The control curve and the first recording after dronedarone application are continued with dotted lines, revealing a current amplitude difference (Δ) when extrapolated to the beginning of the measurement. This difference, Δ, reflects the degree of closed-channel block that occurred before the first test pulse during dronedarone incubation. The activation time course during the first second does not interfere with this idea, since the lower availability during activation of the control current would have produced an even smaller Δ value (if there was no closed state block, Δ would be even negative, above the extrapolated control current in Figure 2b). The amount of block in addition to the closed-state block Δ can be interpreted as the block of open channels.
Figure 2.
Biophysical mechanism of HERG channel inhibition by dronedarone. (a) Dronedarone block of HERG currents consists of two components, as illustrated by a graphical overlay of the control recording and the first two measurements after incubation with 10 μM dronedarone. Inhibition of closed channels is reflected by reduced initial peak currents, whereas the additional time-dependent component can be interpreted as block of open channels. (b) Enlargement of the first 2 s of (a). The current traces extrapolated to the beginning of the recordings demonstrate an amplitude difference Δ, which is the result of the closed-state block. (c) Inhibition of inactivated channels by 100 μM dronedarone is displayed in this representative single experiment. HERG channels were inactivated by a first voltage step to +80 mV, followed by channel opening at 0 mV. The corresponding normalized relative block during the 0 mV step is displayed in (d). Maximum inhibition was already achieved in the inactivated state, and weak further time-dependent development of block occurred upon channel opening after the inactivating voltage step (see text for voltage protocols).
To address the question whether HERG channels are blocked by dronedarone in the inactivated state, a long test pulse to +80 mV (4 s) was applied to inactivate the channels, followed by a second voltage step (0 mV, 3.5 s) to open HERG channels (n=9). Typical current traces under control conditions and after application of 100 μM dronedarone for 30 min while holding the cell at −80 mV are displayed in Figure 2c. Figure 2d depicts the normalized relative block upon channel opening during the second voltage pulse (0 mV), illustrating that pronounced inhibition of HERG channels had already been obtained during the preceding inactivating +80 mV pulse, and virtually no additional time-dependent block of open channels was observed during the 0 mV pulse.
It is concluded from these experiments that dronedarone inhibits HERG channels in the closed, open, and inactivated states.
Voltage dependence of HERG channel block by dronedarone
The voltage dependence of HERG blockade by dronedarone was analyzed by using long test pulses (21 s) ranging from −60 to 80 mV to reach steady-state block conditions, followed by a second voltage protocol to determine the amount of block during the preceding long pulse. The second protocol consisted of a first step to −120 mV (120 ms) to close HERG channels, a second step to 40 mV (50 ms) to activate channels, and a third step to −120 mV (110 ms) to record tail currents, representing the degree of current inhibition during the variable long 21-s pulse. Under control conditions, the peak tail currents displayed equivalent amplitudes, independent of the preceding test pulse voltage. In Figure 3a, the block values after incubation with 10 μM dronedarone (30 min) are plotted versus the test pulse potentials. HERG current block increased at voltages between −60 and 20 mV where maximum inhibition was reached, followed by a slight decrease at potentials above 20 mV (n=6).
Figure 3.
Voltage and frequency dependence of HERG current block. (a) Relative inhibition of peak tail currents as a function of the respective preceding test pulse potential, illustrating the voltage dependence of the block (n=6). (b) Mean relative tail current amplitudes obtained from six oocytes are plotted versus time. Trains of pulses were applied at different intervals (see the inlet), revealing that HERG current inhibition by dronedarone was not use-dependent. (c) The values for steady-state block after 80 s did not differ significantly (see the text for voltage protocols).
Lack of frequency dependence of HERG current block by dronedarone
The frequency dependence was investigated after dronedarone block (bath concentration 10 μM) had reached steady-state conditions (30 min incubation time). HERG potassium channels were rapidly activated by depolarizing steps to 100 mV for 400 ms, and outward tail currents were recorded at −60 mV (400 ms), before returning to the holding potential of −80 mV. Pulses were applied at intervals of 2, 4, 6, or 10 s. The time course of blockade is displayed in Figure 3b (n=6). The level of blockade after 80 s is a measure for the frequency dependence of the block. Figure 3c depicts the mean relative tail current block obtained from six cells under steady-state conditions (after 80 s), illustrating that dronedarone block was not frequency-dependent.
Effects of dronedarone on KvLQT1/minK currents
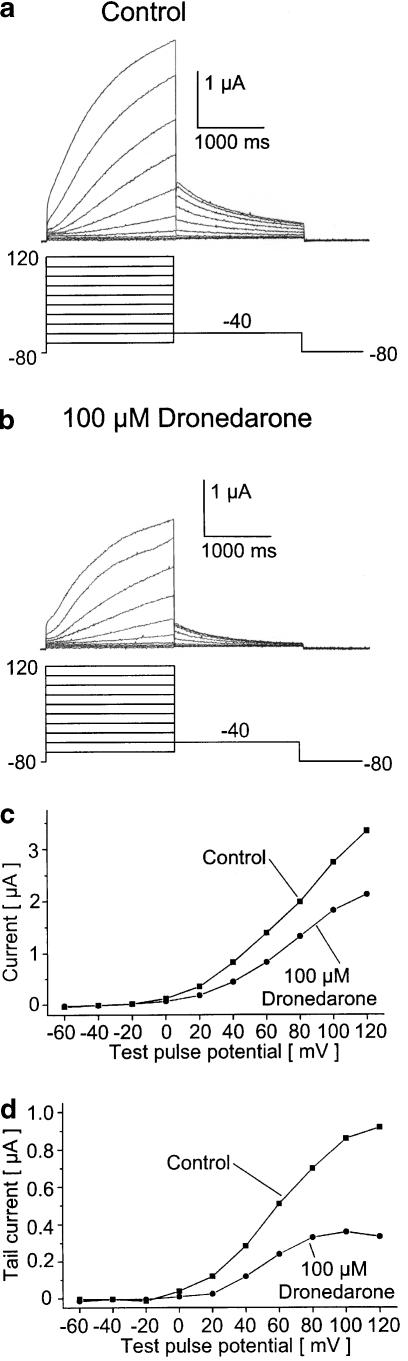
Human IKs current was resembled by injection of oocytes with cRNA encoding KvLQT1 and minK, resulting in outward potassium currents characterized by a linear current–voltage (I–V) relationship (Barhanin et al., 1996; Sanguinetti et al., 1996). Currents were activated during depolarizing steps to potentials ranging from −60 to 120 mV (2 s), and tail currents were recorded at −40 mV (2 s). The holding potential was −80 mV, and pulses were applied at a frequency of 0.2 Hz. Addition of 100 μM dronedarone to the bath for 30 min significantly reduced KvLQT1/minK outward currents (measured at 80 mV membrane potential) by 27.3±11.1% (Figure 4a and c), and peak tail currents were blocked by 50.3±18.8% (Figure 4b and d; n=6). The corresponding I–V relationships for KvLQT1/minK currents were not significantly altered (control: V1/2=53.1±3.0 mV; drug: V1/2=50.3±5.9 mV; n=5) (Figure 4d). A control period of 30 min (n=6) had no significant effects on KvLQT1/minK step currents, outward tail currents, or the half-maximal activation voltage. In contrast, incubation with 1% (v v−1) ethanol (the maximum bath concentration) for 30 min also reduced KvLQT1/minK tail currents by 17.1±10.3%, without significantly affecting the outward step currents or the half-maximal activation voltage V1/2 (n=5). However, the inhibition of KvLQT1/minK currents by 100 μM dronedarone was significantly more pronounced.
Figure 4.
Acute effects of dronedarone on KvLQT1/minK currents. (a, b) Representative current traces recorded from a cell expressing KvLQT1 and minK proteins before and after application of 100 μM dronedarone (30 min). (c) I–V relationship for currents measured at the depolarizing test pulse. The maximum current amplitude at 80 mV was reduced by 32.5% in the measurement with dronedarone. (d) I–V relationship for peak tail currents, illustrating a reduction by 58.8% in this oocyte (see the text for voltage protocols).
Discussion
In the present study, the heterologous Xenopus oocyte expression system was used to determine the molecular electrophysiological basis of the class III antiarrhythmic action of dronedarone, focusing on the acute effects of dronedarone on delayed rectifier potassium currents. Since inhibition of these currents is most effective in prolonging refractoriness, they constitute a primary target for class III antiarrhythmic agents. HERG and KvLQT1/minK genes were expressed to resemble the respective human potassium currents IKr and IKs. This approach allowed detailed biophysical studies, without the necessity to discriminate between the different native ion currents on the basis of kinetics or pharmacology.
Acute effects of dronedarone on HERG and KvLQT1/minK currents
Application of dronedarone resulted in a concentration-dependent decrease in HERG currents at moderate drug concentrations (IC50=9.2 μM). These results provide a molecular basis for the inhibition of native IKr by dronedarone, observed in canine and guinea-pig cardiomyocytes (Varro et al., 2001; Gautier et al., 2003). In canine cells, 10 μM dronedarone blocked IKr almost completely. In our experiments, the concentration required for maximum inhibition (85.2%) reached 100 μM. This difference can be explained by specific properties of Xenopus oocytes (e.g. the vitelline membrane and the yolk), reducing the actual concentration of drugs at the cell membrane. Similar observations have been made when HERG channel block by dofetilide (Kiehn et al., 1996), BRL-32872 (Thomas et al., 2001) or chlorpromazine (Thomas et al., 2003) was investigated. Inhibition of KvLQT1/minK currents required a drug concentration of 100 μM. The degree of blockade reached 50.3%, but the solvent ethanol also reduced KvLQT1/minK tail currents by 17.1%, leaving approximately 33.2% block attributable to dronedarone. The IC50 value could not be determined, since application of drug concentrations higher than 100 μM was prevented by increasing ethanol bath concentrations (>1% v v−1). In summary, taking into consideration the specific properties of the expression system used, it is reasonable to assume that inhibition of KvLQT1/minK channels underlies the reduction of native IKs in guinea-pig heart observed with dronedarone, where the IC50 yielded approximately 10 μM (Gautier et al., 2003).
The biophysical mechanism of potassium current reduction by dronedarone: comparison with amiodarone and clinical implications
Dronedarone blocks HERG channels in the closed, open, and inactivated states, as demonstrated using specially designed voltage protocols to discriminate between the different states. Compared with the results obtained in a previous study (Kiehn et al., 1999), the acute actions of dronedarone and amiodarone on HERG channels are similar (Table 1 ), with the exception that dronedarone displayed no frequency dependence and stronger maximum inhibition. Thus, despite the deletion of iodine from the drug molecule, the molecular electrophysiological effects on HERG as a major target of the class III antiarrhythmic drug action are equally or even more pronounced compared with amiodarone. In contrast to amiodarone, frequency dependence could not be observed with dronedarone. Furthermore, dronedarone application caused a −6.1 mV shift in the HERG activation curve, which may cause an increase in the current amplitude if other biophysical parameters remained unchanged. However, due to pronounced pharmacological HERG channel block, current increase could not be detected in our study.
Table 1.
Comparison of the electrophysiological parameters of HERG channel block by dronedarone and amiodarone
| Amiodarone (Kiehn et al., 1999) | Dronedarone (this study) | |
|---|---|---|
| IC50 | 9.8 μM | 9.2 μM |
| Maximum inhibition | 62.8% | 85.2% |
| Blocked states | Closed, open, inactivated | Closed, open, inactivated |
| Voltage dependence | + | + |
| Frequency dependence | + | − |
Dronedarone blocked KvLQT1/minK potassium currents at relatively high drug concentrations without causing significant changes in the half-maximal activation voltage, suggesting that activation kinetics were not markedly affected by the drug. Additional data obtained from guinea-pig ventricular cardiomyocytes revealed that IKs is blocked in a voltage-dependent, but time-, frequency-, and use-independent manner (Gautier et al., 2003).
Despite its inhibitory effects on delayed rectifier potassium currents, acute application of dronedarone did not lengthen the action potential duration (APD) of guinea-pig and rabbit papillary muscle preparations at various stimulation rates (Sun et al., 1999; Gautier et al., 2003). In fact, Sun et al. (1999) reported APD shortening after acute dronedarone treatment. The discrepancy between potassium current inhibition and the lack of APD lengthening might be explained by multichannel blocking properties of dronedarone. In particular, APD-shortening inhibition of inward currents (L-type calcium currents and sodium currents; Varro et al., 2001; Gautier et al., 2003) by dronedarone might oppose its APD-lengthening blockade of outward potassium currents, which could lead to the homogenization of repolarization.
Furthermore, the inhibition of HERG currents in combination with the blockade of L-type calcium currents by dronedarone (Varro et al., 2001; Gautier et al., 2003) might result in a relatively low proarrhythmic potential, as suggested by earlier studies for several other drugs (Bril et al., 1996; Chouabe et al., 1998; Zhang et al., 1999; Thomas et al., 2001,2002,2003). Due to the similarity between the molecular structure of dronedarone and thyroid hormone, the potentially beneficial electrophysiological effect of dronedarone might also be caused by cardioselective inhibition of T3 receptors in cardiomyocytes, or by a combination of both mechanisms.
Conclusions
Our results demonstrate that dronedarone is an antagonist of cloned HERG potassium channels with additional inhibitory effects on KvLQT1/minK currents at high drug concentrations, providing a molecular mechanism for the class III antiarrhythmic action of the drug. In comparison with amiodarone, dronedarone displays large similarities in the biophysical blocking properties of HERG currents. Further in vitro and particularly in vivo studies need to be conducted carefully with respect to possible proarrhythmic risk to evaluate whether dronedarone is an effective tool in the treatment of atrial and ventricular tachyarrhythmias.
Acknowledgments
The excellent technical assistance of K. Güth, S. Lück, and R. Bloehs is gratefully acknowledged. We thank Dr P. Gautier (Sanofi-Synthelabo, France) for helpful comments on the manuscript. This work was supported by grants from the Deutsche Forschungsgemeinschaft (project KI 663/1-1 to J.K.; project KA 1714/1-1 to C.A.K.), from the University of Heidelberg (AiP+F to D.T.), from the Novartis-Foundation (to D.T.), and from the Foundation Cardiology 2000 (Forssmann-Scholarship to D.T.).
Abbreviations
- APD
action potential duration
- HERG
human ether-a-go-go-related gene
- IK
delayed rectifier potassium current
- IKr
rapidly activating component of the delayed rectifier potassium current
- IKs
slowly activating component of the delayed rectifier potassium current
References
- BARHANIN J., LESAGE F., GUILLEMARE E., FINK M., LAZDUNSKI M., ROMEX G. KvLQT1 and IsK (minK) proteins associate to form the IKs potassium channel. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- BRIL A., GOUT B., BONHOMME M., LANDAIS L., FAIVRE J.F., LINEE P., POYSER R.H., RUFFALO R.R. Combined potassium and calcium channel blocking activities as a basis for antiarrhythmic efficacy with low proarrhythmic risk: experimental profile of BRL-32872. J. Pharmacol. Exp. Ther. 1996;276:637–646. [PubMed] [Google Scholar]
- CHATELAIN P., MEYSMANS L., MATTEAZZI J.R., BEAUFORT P., CLINET M. Interaction of the antiarrhythmic agents SR 33589 and amiodarone with the beta-adrenoceptor and adenylate cyclase in rat heart. Br. J. Pharmacol. 1995;116:1949–1956. doi: 10.1111/j.1476-5381.1995.tb16397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOUABE C., DRICI M.D., ROMEY G., BARHANIN J., LAZDUNSKI M. HERG and KvLQT1/IsK, the cardiac K+ channels involved in long QT syndromes, are targets for calcium channel blockers. Mol. Pharmacol. 1998;54:695–703. [PubMed] [Google Scholar]
- GAUTIER P., GUILLEMARE E., MARION A., BERTRAND J.P., TOURNEUR Y., NISATO D. Electrophysiologic characterization of dronedarone in guinea pig ventricular cells. J. Cardiovasc. Pharmacol. 2003;41:191–202. doi: 10.1097/00005344-200302000-00007. [DOI] [PubMed] [Google Scholar]
- KIEHN J., LACERDA A.E., WIBLE B.A., BROWN A.M. Molecular physiology and pharmacology of HERG. Circulation. 1996;94:2572–2579. doi: 10.1161/01.cir.94.10.2572. [DOI] [PubMed] [Google Scholar]
- KIEHN J., THOMAS D., KARLE C.A., SCHÖLS W., KÜBLER W. Inhibitory effects of the class III antiarrhythmic drug amiodarone on cloned HERG potassium channels. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:212–219. doi: 10.1007/pl00005344. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., CHAGAN J., CURRAN M.E., KEATING M.T. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:1–20. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., CURRAN M.E., ZOU A., SHEN J., SPECTOR P.S., ATKINSON D.L., KEATING M.T. Coassembly of KvLQT1 and minK (IsK) proteins to form IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of the delayed rectifier K+ current: differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH B.N. Expanding indications for the use of class III agents in patients at high risk for sudden death. J. Cardiovasc. Electrophysiol. 1995;6:887–900. doi: 10.1111/j.1540-8167.1995.tb00365.x. [DOI] [PubMed] [Google Scholar]
- SMITH P., BAUKROWITZ T., YELLEN G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- SUN W., SARMA J.S.M., SINGH B.N. Electrophysiological effects of dronedarone (SR33589), a noniodinated benzofuran derivative, in the rabbit heart. Comparison with amiodarone. Circulation. 1999;100:2276–2281. doi: 10.1161/01.cir.100.22.2276. [DOI] [PubMed] [Google Scholar]
- SUN W., SARMA J.S.M., SINGH B.N. Chronic and acute effects of dronedarone on the action potential of rabbit atrial muscle preparations: comparison with amiodarone. J. Cardiovasc. Pharmacol. 2002;39:677–684. doi: 10.1097/00005344-200205000-00008. [DOI] [PubMed] [Google Scholar]
- THOMAS D., GUT B., WENDT-NORDAHL G., KIEHN J. The antidepressant drug fluoxetine is an inhibitor of human ether-a-go-go-related gene (HERG) potassium channels. J. Pharmacol. Exp. Ther. 2002;300:543–548. doi: 10.1124/jpet.300.2.543. [DOI] [PubMed] [Google Scholar]
- THOMAS D., WENDT-NORDAHL G., RÖCKL K., FICKER E., BROWN A.M., KIEHN J. High-affinity blockade of HERG human cardiac potassium channels by the novel antiarrhythmic drug BRL-32872. J. Pharmacol. Exp. Ther. 2001;297:753–761. [PubMed] [Google Scholar]
- THOMAS D., WU K., KATHÖFER S., KATUS H.A., SCHOELS W., KIEHN J., KARLE C.A. The antipsychotic drug chlorpromazine inhibits HERG potassium channels. Br. J. Pharmacol. 2003;139:567–574. doi: 10.1038/sj.bjp.0705283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS D., ZHANG W., KARLE C.A., KATHÖFER S., SCHÖLS W., KÜBLER W., KIEHN J. Deletion of protein kinase A phosphorylation sites in the HERG potassium channel inhibits activation shift by protein kinase A. J. Biol. Chem. 1999;274:27457–27462. doi: 10.1074/jbc.274.39.27457. [DOI] [PubMed] [Google Scholar]
- VARRO A., TAKACS J., NEMETH M., HALA O., VIRAG L., IOST N., BALATI B., AGOSTON M., VERECKEI A., PASTOR G., DELBRUYERE M., GAUTIER P., NISATO D., PAPP J.G. Electrophysiological effects of dronedarone (SR 33589), a noniodinated amiodarone derivative in the canine heart: comparison with amiodarone. Br. J. Pharmacol. 2001;133:625–634. doi: 10.1038/sj.bjp.0704106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARMKE J.W., GANETZKY B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG S., ZHOU Z., GONG Q., MAKIELSKI J.C., JANUARY C.T. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ. Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]