Abstract
Little is known about the cellular effects induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy), although changes in gene expression have been observed following treatments with other psychostimulants. Thus, the aim of this study was to investigate in mice, the relationships between the ras-dependent protein kinase ERK and MDMA-induced reinforcement using the conditioned place preference (CPP) and locomotor activity measurements. This was completed using real-time quantitative PCR method by a study of immediate early-genes (IEGs) transcription known to be involved in neuronal plasticity.
A significant CPP was observed after repeated MDMA treatment in CD-1 mice at a dose of 9 mg kg−1 i.p. but not at 3 and 6 mg kg−1. This rewarding effect was abolished by the selective inhibitor of ERK activation, SL327 (50 mg kg−1; i.p.). Similar results were obtained on MDMA-induced locomotor activity, clearly suggesting a role of ERK pathway in these behavioral responses.
Following acute i.p. injection, MDMA induced a strong c-fos transcription in brain structures, such as caudate putamen, nucleus accumbens and hippocampus, whereas egr-1 and egr-3 transcripts were only increased in the caudate putamen. MDMA-induced IEGs transcription was selectively suppressed by SL327 in the caudate putamen, suggesting a role for other signaling pathways in regulation of IEGs transcription in the other brain structures. In agreement with these results, MDMA-induced c-fos protein expression was blocked by SL327 in the caudate putamen.
This study confirms and extends to mice the reported role of ERK pathway in the development of addiction-like properties of MDMA. This could facilitate studies about the molecular mechanism of this process by using mutant mice.
Keywords: MDMA, locomotor activity, ERK, place preference, immediate-early genes
Introduction
The rewarding properties of cocaine and various psycho-stimulants including 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) are related to their ability to enhance the release of dopamine (DA) in the nucleus accumbens (see review in Di Chiara, 1995). Moreover, MDMA was also shown to induce an increase in 5-hydroxytryptamine (5-HT) in the same structure. DA and serotonin (5-HT) interact with G protein-coupled receptors (GPCRs) expressed in different brain structures, including the mesolimbic pathway and hippocampus, which are involved in drug reinforcement and learning, respectively. Addiction is generally believed to result from molecular and cellular adaptations in specific brain neurons caused by repeated exposure to a drug of abuse (see review in Nestler, 2001). One of the present challenges is to identify and characterize the cellular targets accounting for the persistent aspects of addiction. This occurs by drug-induced stimulation of the receptors triggering alterations in intracellular biochemical processes and changes in gene expression. Thus, stimulation of D1 DA receptor by psychostimulants led to an increase in cAMP production through activation of the adenylyl cyclase by the αs subunit of the G protein, with subsequent activation of the protein kinase A (Lovenberg et al., 1991). This latter enzyme induces a phosphorylation of the transcription factor CREB, which under this activated form was shown to trigger the expression of early genes such as c-fos and egr family.
On the other hand, several studies have shown the existence of a link between the signaling pathway involving GPCR and the MAPK cascade, through βγ subunits of the G-protein, leading to a ras-dependent cascade of phosphorylation (Lowes et al., 2002). A key component of this cascade is the protein kinase ERK, which is activated by another protein kinase MEK. In the case of cocaine, inhibition of this phosphorylation process in mice reversed both the rewarding effects and the enhanced c-fos expression evoked by the drug (Valjent et al., 2000). The level of c-fos expression was also shown to be increased following acute MDMA administration in rats (Dragunow et al., 1991; Erdtmann-Vourliotis et al., 1999, 2000; Stephenson et al., 1999), but no data are available in mice. Thus, owing to the usefulness of mice for gene manipulations and the recent description of the entire mouse genome (Blake et al., 2003), it was of interest to investigate whether MDMA is able to induce a conditioned place preference in mice, and if the behavioral effects of the drug are modified following ERK inhibition, using a specific inhibitor of ERK kinase (MEK), SL327 (Selcher et al., 1999). Moreover, we analyzed the levels of c-fos, egr-1 and egr-3 transcription in discrete brain structures involved in drug dependence after injection of MDMA in mice, and we evaluated the role of ERK pathway in these effects.
The inhibition of ERK phosphorylation by SL327 completely abolished the place preference induced by MDMA showing the involvement of the ras-dependent signaling cascade in the observed reinforcement process. SL327 was also able to inhibit the acute effect of MDMA, by reversing the hyperlocomotor activity induced by this compound. Several early genes such as c-fos, egr-1 and egr-3 were found overexpressed by MDMA in particular structures, not necessarily identical. This overexpression was selectively inhibited by SL327 in the caudate putamen, suggesting that the neuronal plasticity induced by MDMA in this structure is probably controlled by ERK signaling pathway.
Methods
Animals and drugs
Male CD-1 mice (Iffa Credo, France) weighing 22–24 g were housed in a room with 12 h alternating ligth/dark cycle and controlled temperature (21±1°C). Food and water were available ad libitum. All drugs were injected intraperitoneally (i.p.). D,L-MDMA (Lipomed, Switzerland) was dissolved in saline 0.9%. The MEK inhibitor SL327, a generous gift of Bristol-Myers Squibb (Wilmington), was dissolved in 100% DMSO, as previously described in behavioral studies (Selcher et al., 1999), and injected 1 h before MDMA treatment. Volumes of injection were 0.1 and 0.02 ml per 10 g of body weight for MDMA and SL327, respectively. In preliminary studies, it has been verified that injection of DMSO 1 h before MDMA did not modify any effect as compared to MDMA alone in behavioral and biochemical studies.
The animals were treated in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals (1985) and in agreement with the local ethical committee.
Place preference paradigm
A previously described unbiased place conditioning method was used (Valverde et al., 2001). The place preference apparatus consisted of two conditioning compartments (15 × 15 × 15 cm3) that were distinguished by different patterns on floors and walls, separated from each other by a neutral area. The movement and location of mice were recorded by computerized monitoring software (Videotrack, Viewpoint, Lyon, France). Briefly, the protocol consisted of three phases.
Preconditioning phase. Drug-naive mice had free access to both compartments for 20 min, and the time spent in each compartment was recorded.
Conditioning phase. This phase consisted of 6 days where each conditioning chamber was closed. In experiment A (MDMA induced place-preference), on the first conditioning day, mice (n=9–10/group) were treated with MDMA (1, 3 or 9 mg kg−1; i.p.), and were placed 20 min after injection in one of the conditioning environments individually for 20 min. The following conditioning day, mice were given saline in the opposite compartment and this sequence alternated during the next 6 days. Control animals received saline every day. In experiment B (effect of SL327 pretreatment on MDMA induced place-preference), on days 1, 3 and 5, according to their group, animals (n=7–8/group) received SL327 (50 mg kg−1; i.p.) or DMSO 1 h before MDMA (9 mg kg−1; i.p.) or saline injection, and 20 min after, were placed in one of the conditioning environments individually for 20 min. All mice received DMSO/saline on days 2, 4 and 6.
Postconditioning phase. This phase took place 24 h after the final conditioning session and was carried out exactly as the preconditioning phase.
Results are expressed in scores (mean±s.e.m.) calculated as the difference between postconditioning and preconditioning time spent in the drug-paired compartment.
Locomotor activity
Locomotion was evaluated in transparent activity boxes (10 × 18 × 14 cm3; Imetronic, Bordeaux, France). Displacements were measured by photocell beams located across the long axis, 20 mm above the floor (horizontal activity). Naive mice, not prehabituated to the activity boxes (n=10–12/group), received MDMA (9 mg kg−1; i.p.) or saline injection 1 h after SL327 (50 mg kg−1; i.p.) or DMSO, and their locomotor activity was immediately recorded for 120 min. Locomotor activity was expressed in scores (mean±s.e.m.) as the total number of interruption of the photocell beams, recorded every 15 min.
Drug treatment for RT–PCR analysis
Mice were injected with SL327 (50 mg kg−1; i.p.) or DMSO, followed 1 h later by MDMA treatment (9 mg kg−1; i.p.) or saline. At 1 h after the last injection, mice were killed by cervical dislocation, brain was removed and the following structures were dissected: caudate putamen, nucleus accumbens and hippocampus. Structures were frozen in isopentane at −50°C, and stored at −80°C until RNA extraction.
RNA extraction and real-time quantitative RT–PCR
Total RNA were extracted from the different brain structures by a modified acid-phenol guanidinum method (RNABle®, Eurobio). Each RNA sample was prepared with tissues pooled from five mice, which received the same treatment. The quality of the RNA samples was determined by electrophoresis through agarose gels and staining with ethidium bromide. Reverse transcription of RNA was performed in first-strand buffer (Invitrogen, France), 500 μM of each dNTP, 20 U of Rnasin ribonuclease inhibitor (Promega, France), 10 mM dithiothreitol, 100 U Superscript II Rnase H− reverse transcriptase (Invitrogen, France), 0.15 μg μl−1 random hexa-nucleotide primers (Amersham Biosciences, France) and 1 μg of total RNA. The samples were incubated at 25°C for 10 min, 42°C for 30 min and 99°C for 5 min. PCR reactions were performed on a Light-Cycler® instrument (Roche Diagnostics) using the LC-FastStart DNA Master SYBR Green I kit (Roche Diagnostics). The PCR reactions were performed with 3–4 samples/drug treatment, in duplicate for each sample. Quantification was made on the basis of a calibration curve using total RNA from mouse brain. In addition to c-fos, egr-1 and egr-3 transcripts, the TBP mRNA (a component of the DNA binding complex TFIID) was quantified and each sample was normalized on the basis of its TBP content. The results are expressed as gene of interest transcript/TBP transcript.
Immunohistochemistry
Mice (n=5–6/group) were injected with SL327 (50 mg kg−1; i.p.) or DMSO, followed 1 h later by MDMA treatment (9 mg kg−1; i.p.) or saline. At 2 h after the last injection, mice were deeply anesthetized by intraperitoneal injection of pentobarbital (Sanofi, France) prior to intracardiac perfusion of 4% paraformaldehyde in 0.1 M Na2HPO4/NaH2PO4 buffer, pH 7.5 (PB), delivered with a peristaltic pump at 20 ml min−1 over 5 min. The brains were then removed, postfixed for 4 h in the same fixative solution, and cryoprotected overnight in 30% sucrose in PB. Sections (60 μm) were cut with a microtome and kept in a solution containing 30% ethylene glycol, 30% glycerol in PBS, pH 7.4 at −20°C until they were processed for immunochemistry. Free-floating sections were rinsed in PB, incubated for 30 min at room temperature in a blocking solution of 3% normal goat serum in PB with 0.3% Triton X-100, and were then incubated overnight at 4°C in the primary antiserum directed against the c-fos protein (Tebu, sc-52, diluted at 1 : 6000). The sections were washed three times in 1% normal goat serum in PB with 0.3% Triton X-100 (NGST) and incubated in biotinylated goat anti-rabbit immunoglobulin G for 1 h at room temperature, then washed twice in NGST and incubated for 1 h in avidin–biotin–peroxidase complex (Vectastain, Vector Laboratories, Burlingame). Finally, the sections were washed three times in PB and developed using VIP chromogen (Vector Laboratories, Burlingame). After processing, the tissue sections were mounted on gelatin-coated slides, air-dried, dehydrated through a graded alcohol series, xylene treated and cover-slipped for light-microscopic examination. c-fos-positive neurons were counted at × 63 magnification using a computerized image analyser (NIH image software). Cell counts were performed for each mouse in the caudate putamen from bregma +0.26 to +1.54 mm (four to five sections per mouse), according to The Mouse Brain Paxinos and Franklin Atlas (Academic Press, 2nd edn, 2001). These counts were used to calculate an average number of c-fos-positive cells per section in the caudate putamen region.
Statistical analysis
Data were analyzed using one-way ANOVA between subjects for score values of conditioned place preference, locomotor activity, quantitative RT–PCR and immunohistochemistry. Post hoc comparisons were made using the Fisher–PLSD test. Effects of treatments on locomotor activity counts were analyzed by two-way ANOVA for repeated measures. The level of significance was set at P<0.05.
Results
Effect of MDMA in the place preference paradigm in mice
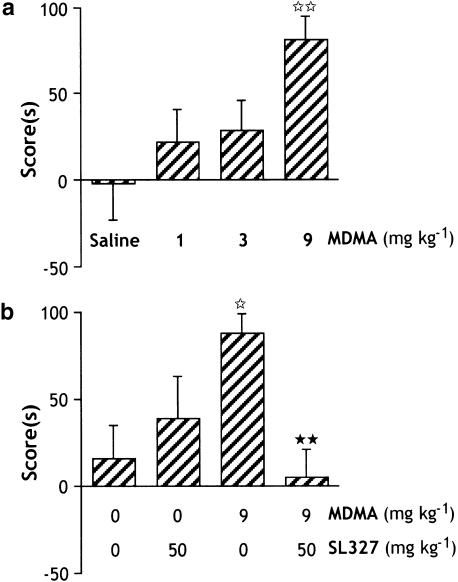
Comparison of preconditioning times spent in the drug-paired compartment did not show any significant difference between the groups (F3,33=1.301; P=0.2905), indicating the unbiased characteristics of the experimental design (Table 1a). One-way ANOVA (F3,33=2.921; P=0.0485) revealed that repeated administration of MDMA induced a conditioned place preference (CPP). Post hoc comparisons (P=0.0072) showed a significant effect of MDMA at the highest dose used (9 mg kg−1), while no significant effect was observed with the doses of 1 and 3 mg kg−1 (Figure 1a).
Table 1.
Time spent in drug-associated compartment during the preconditioning and the testing phase
| Preconditioning (s) | Testing (s) | |
|---|---|---|
| (a) MDMA-induced place preferencea | ||
| Saline | 369±16 | 367±32 |
| MDMA 1 mg kg−1 | 365±10 | 386±21 |
| MDMA 3 mg kg−1 | 360±15 | 388±20 |
| MDMA 9 mg kg−1 | 335±5 | 416±17## |
| (b) Effect of SL327 pretreatment on MDMA-induced place preferenceb | ||
| DMSO/saline | 331±21 | 347±25 |
| SL327/saline | 330±14 | 369±28 |
| DMSO/MDMA | 331±13 | 418±20# |
| SL327/MDMA | 327±12 | 331±14** |
Values are mean±s.e.m. from nine or 10 independent mice per group.
P<0.01 as compared to saline-treated group (Fisher–PLSD).
Values are mean±s.e.m. from seven or eight independent mice per group.
P<0.05 as compared to control group;
P<0.01 as compared to MDMA-treated group (Fisher–PLSD). Statistical analysis was performed on the scores data, calculated as the difference between testing and preconditioning times spent in drug-associated compartment.
Figure 1.
(a) Effects of MDMA in the place preference paradigm. The mice received MDMA (1, 3 and 9 mg kg−1; i.p.) or saline (i.p.) 20 min before being confined in the associated compartment (see methods for details). Data are expressed in scores calculated as the difference between postconditioning and preconditioning time spent in the compartment associated with the drug (means±s.e.m.). ⋆⋆P<0.01 as compared to saline-treated group (Fisher–PLSD). (b) Effect of pretreatment with the MEK inhibitor, SL 327 (50 mg kg−1; i.p.) on MDMA (9 mg kg−1; i.p.)-induced CPP. Data are expressed in scores calculated as the difference between postconditioning and preconditioning time spent in the compartment associated with the drug (means±s.e.m.). ⋆P<0.05 as compared to control group; **P<0.01 as compared to MDMA-treated group (Fisher–PLSD).
Role of ERK activity on rewarding properties of MDMA
The selective MEK inhibitor SL327 (50 mg kg−1; i.p.) was administered 1 h before MDMA (9 mg kg−1; i.p.). Comparison of preconditioning times spent in the drug-paired compartment did not show any significant difference between the groups (F3,26=0.009; P=0.9989), indicating the unbiased nature of the protocol (Table 1b) One-way ANOVA (F3,26=3.188; P=0.0403) revealed a significant treatment effect between the four groups of animals treated during the conditioning phase with DMSO+Sal, DMSO+MDMA, SL327+Sal or SL327+MDMA. Post hoc comparisons showed a significant effect of MDMA vs control group; and interestingly, pretreatment with SL327 during the conditioning phase completely abolished the place preference induced by MDMA (P=0.0089). No effect was observed after administration of SL327 alone (Figure 1b).
Role of ERK activity on MDMA-induced locomotor activity
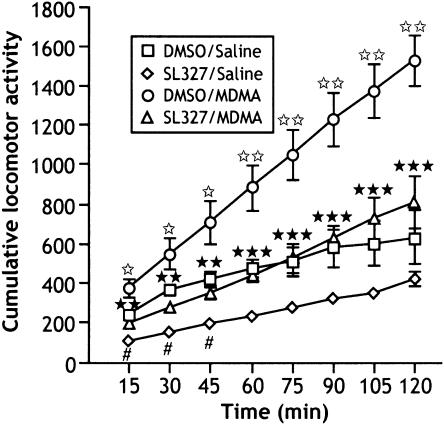
Two-way ANOVA for repeated measures showed a significant treatment effect (F3,39=11.901; P<0.0001), time effect (F7,273=132.78; P<0.0001) and time × treatment interaction (F2,273=13.030; P<0.0001). Administration of MDMA (9 mg kg−1) increased locomotor activity compared to saline-treated animals. This hyperactivity was antagonized by preinjection of SL327 (50 mg kg−1) 1 h before MDMA treatment (after 120 min: P=0.0002). Interestingly, SL327 pretreatment at 50 mg kg−1 significantly decreased spontaneous locomotor activity of mice at the beginning of the test (Figure 2).
Figure 2.
Effects of SL327 on spontaneous motor activity and MDMA-induced hyperlocomotion. Mice were i.p. injected with SL 327 (50 mg kg−1; 0,02 ml 10 g−1 of body weight) or vehicle (DMSO, 0,02 ml 10 g−1 of body weight) followed 1 h later by MDMA (9 mg kg−1; i.p.) or saline. The motor activity was monitored each 15 min for 120 min and started immediately after MDMA administration. Data represent means±s.e.m. of cumulative photobeam disruptions (n=10–12 mice). ⋆P<0.05 and ⋆⋆P<0.01 vs DMSO/saline group. **P<0.01 and ***P<0.001 vs DMSO/MDMA group. #P<0.05 vs DMSO/saline group (Fisher–PLSD).
Role of ERK activation on MDMA-induced IEG transcription
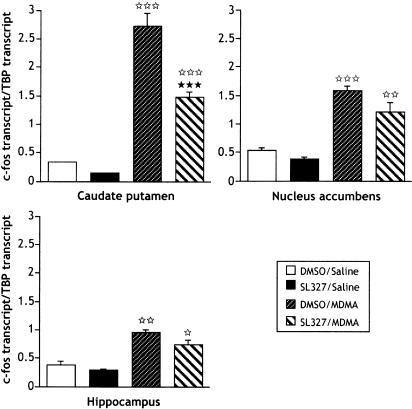
The effects of SL327 pretreatment on MDMA-induced c-fos transcription were analyzed by real-time quantitative RT–PCR (Figure 3). One-way ANOVA revealed significant differences of c-fos transcript level in the brain structures selected: caudate putamen (F3,12=60.534; P<0.0001), nucleus accumbens (F3,11=16.521; P=0.0002) and hippocampus (F3,12=9.807; P=0.0015). Post hoc comparisons (Fisher–PLSD) showed a significant increase of c-fos transcription 1 h after MDMA administration (9 mg kg−1) in these different regions: eight-fold (P<0.0001), three-fold (P=0.0004) and 2.5-fold (P=0.0013). Moreover, as shown in Figure 3, SL327 (50 mg kg−1) injected 1 h before MDMA partially decreased (P<0.0001), and did not modify MDMA-induced c-fos transcription in caudate putamen and hippocampus, respectively . Although not significant, the P-value (0.0761) indicates a trend to the decrease of MDMA-induced c-fos transcription by SL327 administration in the nucleus accumbens. SL327 alone did not modify the levels of c-fos transcription as compared to control group in all the structures studied.
Figure 3.
Real-time quantitative RT–PCR of c-fos transcript after acute treatment with MDMA (9 mg kg−1; i.p.)±SL327 (50 mg kg−1; i.p.). SL327 was administered 1 h before MDMA, and brains were rapidly removed and the caudate putamen, nucleus accumbens and hippocampus were dissected 1 h after administration of MDMA (see Methods for details). Data represent means±s.e.m. of c-fos/TBP fluorescence ratio (n=3–4 samples/group; each sample is composed of structures pooled from five mice). ⋆P<0.05, ⋆⋆P<0.01 and ⋆⋆⋆P<0.001 vs DMSO/saline group. ***P<0.001 vs DMSO/MDMA group (Fisher–PLSD).
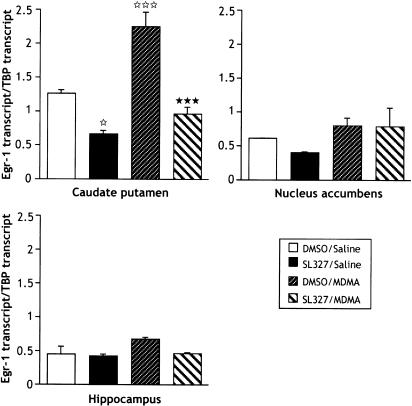
The effect of MDMA on egr-1 transcription was also investigated by real-time quantitative RT–PCR (Figure 4). A significant increase (1.8-fold) was only observed in the caudate putamen, as revealed by one-way ANOVA (F3,12=20.780; P<0.0001) and post hoc comparison (P=0.0006), while no significant differences in egr-1 transcription after MDMA injection were determined in the nucleus accumbens or hippocampus. In the caudate putamen, administration of SL327 1 h before MDMA completely abolished MDMA-induced egr-1 transcription (P<0.0001). However, SL327 pretreatment also reduced basal level of egr-1 transcript (P<0.05).
Figure 4.
Real-time quantitative RT–PCR of egr-1 transcript after acute treatment with MDMA (9 mg kg−1; i.p.)±SL327 (50 mg kg−1; i.p.). SL 327 was administered 1 h before MDMA, and brains were rapidly removed and the caudate putamen, nucleus accumbens and hippocampus were dissected 1 h after administration of MDMA (see Methods for details). Data represent means ±s.e.m. of egr-1/TBP fluorescence ratio (n=3–4 samples/group; each sample is composed of structures pooled from five mice). ⋆P<0.05 and ⋆⋆⋆P<0.001 vs DMSO/saline group. ***P<0.001 vs DMSO/MDMA group (Fisher–PLSD).
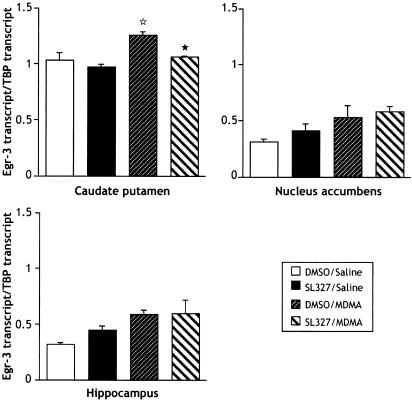
The profile of MDMA-induced egr-3 transcription was very similar to egr-1, as shown in Figure 5. A slight but significant increase (1.2-fold) was only observed in the caudate putamen (F3,12=4.386; P=0.0265 as revealed by one-way ANOVA; post hoc comparison: P=0.0221), which was abolished by SL327 pretreatment (P=0.039). MDMA did not modify the basal level of egr-3 transcription in the nucleus accumbens and hippocampus.
Figure 5.
Real-time quantitative RT–PCR of egr-3 transcript after acute treatment with MDMA (9 mg kg−1; i.p.)±SL327 (50 mg kg−1; i.p.). SL 327 was administered 1 h before MDMA, and brains were rapidly removed and the caudate putamen, nucleus accumbens and hippocampus were dissected 1 h after administration of MDMA (see Methods for details). Data represent means±s.e.m. of egr-3/TBP fluorescence ratio (n=3–4 samples/group; each sample is composed of structures pooled from five mice). ⋆P<0.05 vs DMSO/saline group. *P<0.05 vs DMSO/MDMA group (Fisher–PLSD).
Effect of SL327 on MDMA-induced c-fos protein expression in the caudate putamen
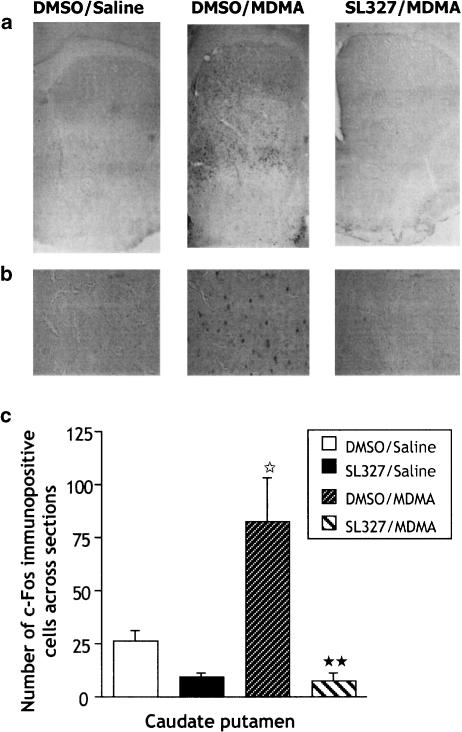
MDMA (9 mg kg−1) produced a three-fold induction of c-fos-positive neurons in the caudate putamen 2 h after injection, as revealed by immunohistochemistry (Figure 6; P=0.0232). This c-fos induction followed a specific pattern, with immunopositive cells predominantly in the dorso-medial region of the caudal caudate-putamen and in the rostral caudate putamen. Pretreatment with SL327 (50 mg kg−1) 1 h before MDMA injection completely abolished this increase (P=0.0054).
Figure 6.
Immunohistochemistry of c-fos 2 h after acute treatment with MDMA (9 mg kg−1; i.p.)±SL327 (50 mg kg−1; i.p.). SL327 was administered 1 h before MDMA and mice were perfused 2 h after the last injection (see Methods for details). (a) Representative example showing c-fos immunoreactivity in the whole striatum. (b) High magnification of caudate putamen region ( × 400) showing c-fos-positive nuclei. (c) Data represent means±s.e.m. of c-fos immunopositive neurons (n=5–6/group). ⋆P<0.05 vs DMSO/saline group. **P<0.01 vs DMSO/MDMA group (Fisher–PLSD).
Discussion
MDMA has been studied in rats, but to date no data are available on rewarding effects of this substituted amphetamine in mice and on its mechanims of action. However, studies in these animals appear very interesting as spontaneous mutants or mice with genetic modifications, including null mutant mice (knockout) represent very useful animal models to investigate the molecular mechanisms involved in MDMA effects.
This work reports for the first time that MDMA is able to generate a CPP in mice at a dose of 9 mg kg−1. This is consistent with the dose used in rats to produce a reliable CPP (Bilsky & Reid, 1991). Moreover, the effect observed following repeated administration of MDMA in the CPP reflects the reinforcing properties of MDMA, which was reported to induce feeling of euphoria and well-being in humans (Teter & Guthrie, 2001). This effect is very likely due to the effects of MDMA on the release and reuptake of different monoamines in the mesolimbic pathway. Thus, administration of MDMA results in increased synaptic levels of both 5-HT and DA in the rat nucleus accumbens (White et al., 1996; Kankaanpaa et al., 1998). DA increase, mainly in the core of the nucleus accumbens, has been shown to play a critical role in reward and drug dependence and is a common response generated by all drugs of abuse (see reviews in Koob, 1992; Di Chiara, 1995).
The initial event leading to addiction involves the action of the drug on its target(s) protein(s) and on neurons that express these proteins, followed by a repercussion on the intracellular signaling. The best established molecular adaptation to chronic drug exposure is an upregulation of the cAMP pathway (Terwilliger et al., 1991; Ortiz et al., 1995; Schoffelmeer et al., 1996; Unterwald et al., 1996). However, this phenomenon is just one of a large number of changes at the molecular and cellular levels that have been documented after chronic drug administration, involving, for instance, activation of MAP kinases or changes in protein kinase C activity (Narita et al., 1994). We have focused our attention on the ERK pathway, as once activated ERK can phosphorylate and activate other protein kinases as well as an array of effector proteins, which include proteins and transcription factors (Seger & Krebs, 1995). Thus, ERK is an important regulator of neuronal functions, and has been shown to be implicated in various neurobiological events such as synaptic plasticity and memory (see review in Sweatt, 2001). In this study, we used the selective MEK inhibitor SL327, which has no significant effect on a variety of other kinases such as PKC, CAMKII or PKA (Atkins et al., 1998; Selcher et al., 1999). This inhibitor totally abolished the expression of MDMA-induced CPP, demonstrating that the ERK pathway is involved in MDMA-induced rewarding effects. This suggests that ERK may be a common pathway to different drugs of abuse, leading to addictive state, as ERK has also been directly related to the development of THC- and cocaine-rewarding properties in vivo (Valjent et al., 2000; 2001).
To evaluate the role of ERK pathway in MDMA-induced acute behavioral effect, we have also investigated the effect of SL327 on MDMA-induced hyperlocomotion. SL327 alone was able to induce a short-lasting significant decrease of locomotor activity in mice. At the moment, we have no clear explanation for this effect occurring more specifically during the exploration of the activity boxes. This behavior might be expected to engage dopaminergic systems, known to activate the ERK signaling pathway. In rats, a trend toward a decrease in locomotor activity measured in an open-field has already been observed following SL327 injection (Atkins et al., 1998). The most important result obtained in this experiment is the demonstration that ERK pathway seems to be involved in the acute behavioral effect of MDMA, as SL327 completely reversed the hyperlocomotion induced by a single administration of this amphetamine derivative. This suggests that psychostimulant drugs, belonging to different chemical series, may have several common mechanisms of action as illustrated by the partial inhibition of cocaine-induced hyperlocomotor effect by SL327 (Valjent et al., 2000). MDMA-induced hyperactivity is very likely mediated by both DA, acting on D1 receptor, and by 5-HT, as this substituted amphetamine is known to act by increasing the extracellular levels of these neurotransmitters (see above). Moreover, it has been demonstrated that 5-HT1B and 5-HT2A receptors play a role in the MDMA-induced locomotor activity (Kehne et al., 1996; Scearce-Levie et al., 1999). Thus, activation of these receptors by MDMA-induced endogenous 5-HT release may lead to ERK activation, as this was associated to a coupling to different second messengers, including ERK pathway (Pullarkat et al., 1998; Watts et al., 2001). Other systems may also play a role in the effects observed following MDMA administration, as this compound can directly interact with relatively high affinities to α2-adrenoreceptors, M-1 muscarinic and H-1 histamine receptors (Battaglia et al., 1988), all possibly activating ERK pathways (Berkeley et al., 2001).
This leads to the phosphorylation of important proteins, effectors and transcription factors, and results in a variety of responses. Thus, for instance, both Elk-1 and CREB transcription factors, critically involved in immediate-early genes (IEG) mRNA induction, may be phosphorylated by ERK (Seger & Krebs, 1995). IEG have certainly a key role for persistent drug-induced plasticity, as regulation of gene expression is one mechanism that should lead to relatively stable changes within neurons. As previously mentioned, a prime nuclear target of activated ERK is the ternary complex factor Elk-1, which acts as a transcriptional activator via the serum-response element (SRE) of various IEGs, including c-fos. In mice, using for the first time, the real-time quantitative PCR method, we observed a strong induction by MDMA of c-fos transcription in three brain structures: nucleus accumbens, caudate putamen and hippocampus. This is in agreement with previous studies performed in rats, showing, by in situ hybridization or immunohistochemistry, an increase in c-fos transcription and expression in the medial prefrontal cortex, caudate putamen and nucleus accumbens following acute MDMA treatment (Erdtmann-Vourliotis et al., 1999; Stephenson et al., 1999). One of the most important result was the inhibition of MDMA-induced c-fos transcription when mice were pretreated with SL327, which clearly appears to be tissue-specific. Thus, the MEK inhibitor partially blocked MDMA-induced c-fos expression in the caudate putamen, while no inhibition was observed in the nucleus accumbens or the hippocampus. This suggests that MDMA-induced c-fos transcription can be mediated by different signaling pathways, one ERK-dependent and the other one -independent. Two DNA regulatory elements, at least, are crucial for the transcriptional regulation of c-fos: the cAMP/calcium-responsive element (CRE) and the SRE. This latter site is essentially controlled by phosphorylation of Elk-1 by ERK proteins, while activation of the CRE site is dependent on CREB phosphorylation, which may occur following activation of different kinases, PKA, CAMKs or ERK (Herdegen & Leah, 1998). However, as revealed by immunohistochemistry, c-fos increase was totally blocked by SL327 pretreatment, indicating that ERK inhibition is suffisant to suppress its induction at the protein level.
Other IEGs may be regulated by CRE and/or SRE sites, such as egr-1 or egr-3, which code for transcription factors that have become popular neurobiological tools for imaging functional activity, and Egr-1 or Egr-3 proteins may be involved in neuronal plasticity (O'Donovan et al., 1999). The present study showed that MDMA is also able to induce egr-1 and egr-3 transcription in the caudate putamen of mice, consistent with the data obtained in rat following acute administration of MDMA or cocaine, respectively (Shirayama et al., 2000; Jouvert et al., 2002). However, the changes noted in egr-3 transcripts were small and may not be biologically significant at the protein level. In the caudate putamen, pretreatment with the MEK inhibitor abolished MDMA-induced egr-1 or egr-3 transcription. It is interesting to observe that ERK inhibition also reduced basal egr-1 transcription. This could result from the fact that egr-1 mRNA is constitutively expressed at high level in several brain structures (Herdegen et al., 1995). However, the absence of significant difference between SL327/MDMA and SL327/saline groups clearly shows that MDMA-mediated egr-1 transcription is dependent on ERK activation.
All these findings demonstrate that the abused drug MDMA induces a rapid and transient expression of several IEGs in mice. However, these genes can be activated by a wide range of stimuli (Ali et al., 1999; Thiriet et al., 2001; Nakao et al., 2002). Thus, the challenge in the next few years will be to identify downstream effectors, which have their expression levels modulated by these IEGs, and are specifically related to MDMA's action. However, it is interesting to observe that activation of ERK through phosphorylation, and Egr-1 protein are implicated in numerous forms of neuronal plasticity, including long-term synaptic changes, such as those occurring during long-term potentiation (Atkins et al., 1998; Davis et al., 2000; Williams et al., 2000), which appears to play a key role in the development of drug addiction (Davis et al., 2000; Ungless et al., 2001). Thus, the identification of the molecular events leading to ERK activation by MDMA will be of great interest.
In summary, these data show for the first time the presence of a rewarding effect and the induction of IEGs in specific brain structures by MDMA in mice. Moreover, this study clearly reveals the major role of ERK in the behavioral responses induced by this substituted amphetamine. Finally, the ERK pathway appears differentially implicated in MDMA-induced IEG transcription depending on the brain structure and the IEG studied, showing therefore the complex MDMA action at the intracellular level.
Acknowledgments
The SL327 was a generous gift from Dr Trazkos (Bristol-Myers Squibb, Wilmington). We thank C. Canestrelli for the invaluable help in animals caring. J. Salzmann was supported by a fellowship from the ‘Ministère de la Recherche' (MERT).
Abbreviations
- CPP
conditioned place preference
- IEG
immediate-early genes
- MDMA
3,4-methylenedioxymethamphetamine
References
- ALI S.F., THIRIET N., ZWILLER J. Acute ibogaine injection induces expression of the immediate early genes, egr-1 and c-fos, in mouse brain. Mol. Brain Res. 1999;74:237–241. doi: 10.1016/s0169-328x(99)00283-1. [DOI] [PubMed] [Google Scholar]
- ATKINS C.M., SELCHER J.C., PETRAITIS J.J., TRZASKOS J.M., SWEATT J.D. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- BATTAGLIA G., BROOKS B.P., KULSAKDINUN C., DE SOUZA E.B. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur. J. Pharmacol. 1988;149:159–163. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- BERKELEY J.L., GOMEZA J., WESS J., HAMILTON S.E., NATHANSON N.M., LEVEY A.I. M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol. Cell Neurosci. 2001;18:512–524. doi: 10.1006/mcne.2001.1042. [DOI] [PubMed] [Google Scholar]
- BILSKY E.J., REID L.D. MDL72222, a serotonin 5-HT3 receptor antagonist, blocks MDMA's ability to establish a conditioned place preference. Pharmacol. Biochem. Behav. 1991;39:509–512. doi: 10.1016/0091-3057(91)90217-p. [DOI] [PubMed] [Google Scholar]
- BLAKE J.A., RICHARDSON J.E., BULT C.J., KADIN J.A., EPPIG J.T. MGD: the Mouse Genome Database. Nucleic Acids Res. 2003;31:193–195. doi: 10.1093/nar/gkg047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS S., VANHOUTTE P., PAGES C., CABOCHE J., LAROCHE S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J. Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI CHIARA G. The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend. 1995;38:95–137. doi: 10.1016/0376-8716(95)01118-i. [DOI] [PubMed] [Google Scholar]
- DRAGUNOW M., LOGAN B., LAVERTY R. 3,4-Methylene-dioxymethamphetamine induces Fos-like proteins in rat basal ganglia: reversal with MK 801. Eur. J. Pharmacol. 1991;206:255–258. doi: 10.1016/s0922-4106(05)80027-6. [DOI] [PubMed] [Google Scholar]
- ERDTMANN-VOURLIOTIS M., MAYER P., RIECHERT U., HOLLT V. Acute injection of drugs with low addictive potential (Δ(9)-tetrahydrocannabinol, 3,4-methylenedioxy-methamphetamine, lysergic acid diamide) causes a much higher c-fos expression in limbic brain areas than highly addicting drugs (cocaine and morphine) Mol. Brain Res. 1999;71:313–324. doi: 10.1016/s0169-328x(99)00207-7. [DOI] [PubMed] [Google Scholar]
- ERDTMANN-VOURLIOTIS M., MAYER P., RIECHERT U., HOLLT V. Prior experience of morphine application alters the c-fos response to MDMA (‘ecstasy') and cocaine in the rat striatum. Mol. Brain Res. 2000;77:55–64. doi: 10.1016/s0169-328x(00)00040-1. [DOI] [PubMed] [Google Scholar]
- HERDEGEN T., LEAH J.D. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res. Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- HERDEGEN T., KOVARY K., BUHL A., BRAVO R., ZIMMERMANN M., GASS P. Basal expression of the inducible transcription factors c-Jun, JunB, JunD, c-Fos, FosB, and Krox-24 in the adult rat brain. J. Comp. Neurol. 1995;354:39–56. doi: 10.1002/cne.903540105. [DOI] [PubMed] [Google Scholar]
- JOUVERT P., DIETRICH J.B., AUNIS D., ZWILLER J. Differential rat brain expression of EGR proteins and of the transcriptional corepressor NAB in response to acute or chronic cocaine administration. Neuromol. Med. 2002;1:137–151. doi: 10.1385/NMM:1:2:137. [DOI] [PubMed] [Google Scholar]
- KANKAANPAA A., MERIRINNE E., LILLSUNDE P., SEPPALA T. The acute effects of amphetamine derivatives on extracellular serotonin and dopamine levels in rat nucleus accumbens. Pharmacol. Biochem. Behav. 1998;59:1003–1009. doi: 10.1016/s0091-3057(97)00527-3. [DOI] [PubMed] [Google Scholar]
- KEHNE J.H., KETTELER H.J., MCCLOSKEY T.C., SULLIVAN C.K., DUDLEY M.W., SCHMIDT C.J. Effects of the selective 5-HT2A receptor antagonist MDL 100,907 on MDMA-induced locomotor stimulation in rats. Neuropsychopharmacology. 1996;15:116–124. doi: 10.1016/0893-133X(95)00160-F. [DOI] [PubMed] [Google Scholar]
- KOOB G.F. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- LOVENBERG T.W., NICHOLS D.E., NESTLER E.J., ROTH R.H., MAILMAN R.B. Guanine nucleotide binding proteins and the regulation of cyclic AMP synthesis in NS20Y neuroblastoma cells: role of D1 dopamine and muscarinic receptors. Brain Res. 1991;556:101–107. doi: 10.1016/0006-8993(91)90552-7. [DOI] [PubMed] [Google Scholar]
- LOWES V.L., IP N.Y., WONG Y.H. Integration of signals from receptor tyrosine kinases and γ protein-coupled receptors. Neurosignals. 2002;11:5–19. doi: 10.1159/000057317. [DOI] [PubMed] [Google Scholar]
- NAKAO S., MIYAMOTO E., MASUZAWA M., KAMBARA T., SHINGU K. Ketamine-induced c-Fos expression in the mouse posterior cingulate and retrosplenial cortices is mediated not only via NMDA receptors but also via sigma receptors. Brain Res. 2002;926:191–196. doi: 10.1016/s0006-8993(01)03338-8. [DOI] [PubMed] [Google Scholar]
- NARITA M., MAKIMURA M., FENG Y., HOSKINS B., HO I.K. Influence of chronic morphine treatment on protein kinase C activity: comparison with butorphanol and implication for opioid tolerance. Brain Res. 1994;650:175–179. doi: 10.1016/0006-8993(94)90224-0. [DOI] [PubMed] [Google Scholar]
- NESTLER E.J. Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- O'DONOVAN K.J., TOURTELLOTTE W.G., MILLBRANDT J., BARABAN J.M. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22:167–173. doi: 10.1016/s0166-2236(98)01343-5. [DOI] [PubMed] [Google Scholar]
- ORTIZ J., FITZGERALD L.W., CHARLTON M., LANE S., TREVISAN L., GUITART X., SHOEMAKER W., DUMAN R.S., NESTLER E.J. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- PULLARKAT S.R., MYSELS D.J., TAN M., COWEN D.S. Coupling of serotonin 5-HT1B receptors to activation of mitogen-activated protein kinase (ERK-2) and p70 S6 kinase signaling systems. J. Neurochem. 1998;71:1059–1067. doi: 10.1046/j.1471-4159.1998.71031059.x. [DOI] [PubMed] [Google Scholar]
- SCEARCE-LEVIE K., VISWANATHAN S.S., HEN R. Locomotor response to MDMA is attenuated in knockout mice lacking the 5-HT1B receptor. Psychopharmacology (Berl) 1999;141:154–161. doi: 10.1007/s002130050819. [DOI] [PubMed] [Google Scholar]
- SCHOFFELMEER A.N., VOORN P., JONKER A.J., WARDEH G., NESTBY P., VANDERSCHUREN L.J., DE VRIES T.J., MULDER A.H., TJON G.H. Morphine-induced increase in D1 receptor regulated signal transduction in rat striatal neurons and its facilitation by glucocorticoid receptor activation: possible role in behavioral sensitization. Neurochem. Res. 1996;21:1417–1423. doi: 10.1007/BF02532383. [DOI] [PubMed] [Google Scholar]
- SEGER R., KREBS E.G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- SELCHER J.C., ATKINS C.M., TRZASKOS J.M., PAYLOR R., SWEATT J.D. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIRAYAMA Y., HASHIMOTO K., IYO M., WATANABE K., HIGUCHI T., MINABE Y. 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy)-induced egr-1 mRNA in rat brain: pharmacological manipulation. Eur. J. Pharmacol. 2000;402:215–222. doi: 10.1016/s0014-2999(00)00521-5. [DOI] [PubMed] [Google Scholar]
- STEPHENSON C.P., HUNT G.E., TOPPLE A.N., MCGREGOR I.S. The distribution of 3,4-methylenedioxymethamphetamine “Ecstasy”-induced c-fos expression in rat brain. Neuroscience. 1999;92:1011–1023. doi: 10.1016/s0306-4522(99)00049-4. [DOI] [PubMed] [Google Scholar]
- SWEATT J.D. The neuronal MAP kinase cascade: a biochemical signal integration system subserving synaptic plasticity and memory. J. Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- TERWILLIGER R.Z., BEITNER-JOHNSON D., SEVARINO K.A., CRAIN S.M., NESTLER E.J. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- TETER C.J., GUTHRIE S.K. A comprehensive review of MDMA and GHB: two common club drugs. Pharmacotherapy. 2001;21:1486–1513. doi: 10.1592/phco.21.20.1486.34472. [DOI] [PubMed] [Google Scholar]
- THIRIET N., ZWILLER J., ALI S.F. Induction of the immediate early genes egr-1 and c-fos by methamphetamine in mouse brain. Brain Res. 2001;919:31–40. doi: 10.1016/s0006-8993(01)02991-2. [DOI] [PubMed] [Google Scholar]
- UNGLESS M.A., WHISTLER J.L., MALENKA R.C., BONCI A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- UNTERWALD E.M., FILLMORE J., KREEK M.J. Chronic repeated cocaine administration increases dopamine D1 receptor-mediated signal transduction. Eur. J. Pharmacol. 1996;318:31–35. doi: 10.1016/s0014-2999(96)00841-2. [DOI] [PubMed] [Google Scholar]
- VALJENT E., CORVOL J.C., PAGES C., BESSON M.J., MALDONADO R., CABOCHE J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J. Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALJENT E., PAGES C., ROGARD M., BESSON M.J., MALDONADO R., CABOCHE J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur. J. Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- VALVERDE O., NOBLE F., BESLOT F., DAUGE V., FOURNIE-ZALUSKI M.C., ROQUES B.P. Δ9-tetra-hydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur. J. Neurosci. 2001;13:1816–1824. doi: 10.1046/j.0953-816x.2001.01558.x. [DOI] [PubMed] [Google Scholar]
- WATTS S.W., YANG P., BANES A.K., BAEZ M. Activation of Erk mitogen-activated protein kinase proteins by vascular serotonin receptors. J. Cardiovasc. Pharmacol. 2001;38:539–551. doi: 10.1097/00005344-200110000-00006. [DOI] [PubMed] [Google Scholar]
- WHITE S.R., OBRADOVIC T., IMEL K.M., WHEATON M.J. The effects of methylenedioxymethamphetamine (MDMA, “Ecstasy”) on monoaminergic neurotransmission in the central nervous system. Prog. Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.M., BECKMANN A.M., MASON-PARKER S.E., ABRAHAM W.C., WILCE P.A., TATE W.P. Sequential increase in Egr-1 and AP-1 DNA binding activity in the dentate gyms following the induction of long-tenn potentiation. Mol. Brain Res. 2000;77:258–266. doi: 10.1016/s0169-328x(00)00061-9. [DOI] [PubMed] [Google Scholar]