Abstract
Accumulating data have been published emphasizing the important role of 5-hydroxytryptamine (5-HT) receptors in proximal stomach relaxation. However, a proper in vivo characterization of 5-HT receptors mediating gastric relaxation is still missing. In the current study, we focus on the in vivo characterization of 5-HT1A receptors mediating relaxation of the proximal stomach in conscious dogs.
Beagle dogs were equipped with a gastric fistula. In the conscious state, volume changes within an intragastric bag were measured at constant pressure by means of a barostat. Results are presented as the maximum volume increase after treatment (mean±s.e.m.). All drugs were injected intravenously.
The 5-HT1A receptor agonist flesinoxan (10, 50, 100 and 150 μg kg−1) induced a dose-dependent relaxation of the canine proximal stomach (50±10, 230±51, 290±38 and 275±33 ml, respectively; n=9–11). The selective 5-HT1A receptor antagonist WAY-100635 dose-dependently inhibited the flesinoxan-induced relaxation. NG-nitro-L-arginine methyl ester did not affect this relaxation, suggesting that nitrergic nerves are not involved.
After supradiaphragmatic vagotomy, the baseline of the intragastric volume was larger compared to that before vagotomy (317±50 vs 142±28 ml, respectively; n=5). Compensation for this by either reduction of the intraballoon pressure or infusion of a contractile dose of bethanechol did not establish a condition in which flesinoxan was able to relax the stomach. In contrast, nitroprusside induced a significant gastric relaxation when tone was increased by bethanechol.
It is concluded that flesinoxan induces proximal gastric relaxation in conscious dogs via 5-HT1A receptors. The response is mediated through a vagal pathway without involvement of nitrergic nerves.
Keywords: 5-HT1A, barostat, conscious dog, gastric relaxation, flesinoxan, proximal stomach, vagotomy, WAY-100635
Introduction
The proximal stomach plays an important role in the accommodation of food. In order to do this the proximal stomach is able to relax in response to different stimuli. Distension of the pharynx, esophagus and stomach, for example, lead to and maintain pronounced relaxation of the proximal stomach (Undeland et al., 1995; Shafik, 1999). Regulation of gastric tone is mainly mediated through vago-vagal pathways by both cholinergic and nitrergic efferent nerves. Gastric tone is based on a balance between the cholinergic contractile and the nitrergic relaxant drive. Interruption of the constant cholinergic drive maintaining gastric tone, by vagal cooling (Azpiroz & Malagelada, 1987) or vagotomy (Abrahamsson & Thoren, 1973), markedly decreases gastric tone. Similarly, administration of atropine causes a relaxation of the fundus and an increase in proximal gastric compliance (Lidums et al., 2000). Nitric oxide (NO) has been shown to mediate gastric relaxation in different species (Meulemans et al., 1995; Esplugues, 2002). Administration of the NO-synthase inhibitor NG-nitro-L-arginine (L-NNA) increases gastric tone, corresponding to a gastric contraction in dogs (Paterson et al., 2000). This illustrates the importance of a continuous NO release in the maintenance of gastric tone. NG-monomethyl-L-arginine (L-NMMA) impairs gastric accommodation and enhances meal-induced satiety in humans (Tack & Demedts, 2002).
In general, 5-hydroxytryptamine (5-HT; Hoyer et al., 2002) has a stimulatory influence on gastric smooth muscle activity. Stimulation of 5-HT2A, 5-HT2B, 5-HT3 and 5-HT4 receptors was shown to induce gastric contraction or to enhance contractility in different species (Baxter et al., 1994; Buchheit & Buhl, 1994; Prins et al., 2001; Janssen et al., 2002). Bulbring & Gershon (1967) first reported gastric relaxation to exogenous administration of 5-HT. They described relaxations to 5-HT in the guinea-pig stomach as part of the vagal inhibitory innervation. More recently, the antimigraine drug sumatriptan, which has affinity at different 5-HT1 receptor subtypes, was shown to induce feline and human gastric fundus relaxation (Tack et al., 1995; 2000; Coulie et al., 1999). In dogs, studied with a barostat, sumatriptan shifted gastric pressure–volume curves towards higher volumes and enhanced gastric accommodation, an effect mediated by 5-HT1B receptors (De Ponti et al., 2003). In isolated guinea-pig whole stomach preparation, the sumatriptan-induced relaxation was antagonized by the 5-HT1A receptor antagonist NAN-190 (Meulemans et al., 1996). Buspirone, a 5-HT1 receptor agonist with high affinity at 5-HT1A receptors, confirmed the possible involvement of 5-HT1A receptor involvement in the regulation of gastric tone in humans (Tack et al., 1999). In rats, on the other hand, as determined by isobaric distension of the stomach, 5-HT1A receptor agonists 8-OH-DPAT and flesinoxan reduced the gastric volume in comparison with vehicle (Rouzade et al., 1998).
The aim of this study was to investigate in vivo properly whether gastric relaxation can be induced by activation of 5-HT1A receptors. A selective 5-HT1A receptor antagonist was used to characterize the receptor involved and the contribution of the vagal pathway was assessed by use of vagotomized dogs.
Methods
Preparation of animals
Experiments were performed on 11 adult female Beagle dogs (9–14 kg body weight), trained to stand quietly in Pavlov frames. The local ethics committee approved the experiments.
Implantation of gastric cannula
After a fasting period (water was available ad libitum) of 24 h, the dogs were premedicated (SC) with an association of 0.315 mg kg−1 fentanyl and 10 mg kg−1 fluanisone (Hypnorm®, Janssen Animal Health, Beerse, Belgium) and anesthetized (i.v.) with 15 mg kg−1 pentobarbital sodium. The anesthesia was maintained with a mixture of halothane 2% (Fluothane®, AstraZeneca, Destelbergen, Belgium), nitrous oxide 43% and oxygen 55% through an endotracheal tube. The respiratory rate was 20 breaths per minute with a volume of 15 ml kg−1. A heating element placed under the animal maintained the body temperature. The level of anesthesia was continuously assessed via heart rate and ECG measurements. After a midline laparotomy, an incision was made through the ventral gastric wall in the longitudinal direction between the greater and the lesser curvature, 2 cm above the nerves of Latarjet. A gastric cannula (∅ 18 mm) was secured to the gastric wall by means of a double purse string suture and brought out via a stab wound at the left quadrant of the hypochondrium. Dogs were allowed a recovery period of 2 weeks. For the first 10 days after the operation, the dogs were treated (PO) with an antibiotic, enrofloxacin, at a dose of 5 mg kg−1 day−1 (Baytril®, Bayer, Leverkusen, Germany).
Supradiaphragmatic vagotomy
Via randomization five out of the 11 dogs used were assigned to study the influence of flesinoxan before and after vagotomy.
Anesthesia was induced as described above. The dogs were positioned on the right side, and a thoracotomy was performed between the left seventh and eighth rib. After skin and conjunctive tissue incision, the different layers of the striated muscles and the pleura were incised. The ribs and muscle tissue were protected with moistened sponges and a rib retractor was inserted to expose the lung and other tissues. The left diaphragmatic lobe of the lung was reclined in the cranial direction to access the esophagus, 2 cm of the dorsal and ventral ramifications of the left and right vagus nerves were excised. Hereafter, the different muscles layers and the skin were closed with proper suturing. The skin was stitched using an intra-dermis suture. A drain tube was positioned in the thorax until a complete recovery of the dog (1–4 h) to avoid any postoperation complications. Dogs were allowed a recovery period of 2 weeks, with the antibiotic therapy as described above.
Measuring insulin-induced gastric secretion assessed completeness of vagotomy. The test was performed approximately 2 weeks before and after vagotomy. Dogs were fasted for 24 h prior to the experiment (water was available ad libitum). At the beginning of the experiment the gastric cannula was opened to remove any gastric juice or food remnants and cleansed with lukewarm water. After a 30 min stabilization period with open cannula, the gastric secretion was collected over a period of 75 min. Then insulin (0.2 IU kg−1) was administered intravenously and again gastric secretion was collected over a period of 75 min. The volume and acidity of the collections were measured. The acidity was determined by titration of a 0.25 ml sample of the collection with NaOH (0.1 N) to a pH of 7. The amount of added NaOH (0.1 N) was then multiplied by 4 and by the volume of the collected sample; the secretion results are expressed as 10−4 mol H+. Before vagotomy, insulin increased the secretion significantly from 3.5±1.0 to 30.3±5.0 (n=5; P<0.005). After vagotomy, insulin was not able to stimulate gastric secretion (1.5±1.1 to 1.1±0.6, n=5) demonstrating the complete vagus denervation of the stomach.
Recording of gastric volume
Variations in gastric tone were measured with an electronic barostat (model JS 10987, Janssen Scientific Instruments, Janssen Pharmaceutica, Beerse, Belgium) maintained at constant pressure. LabVIEW 2-software, version 5.1 was used for process-control and data storage. The barostat had a maximum displacement of approximately 800 ml and maintained a constant pressure in a polyethylene bag that had a capacity of approximately 1100 ml.
Dogs were fasted 24 h prior to the experiment (water was available ad libitum). At the beginning of the experiment, the gastric cannula was opened to remove any gastric juice or food remnants and cleansed with lukewarm water. After calibration of the barostat, the bag was positioned into the proximal stomach through the gastric cannula. A rubber stopper was used to close the space between the tubing and the wall of the cannula. In order to ensure easy unfolding of the intragastric bag during the experiment, the following protocol, taking approximately 5 min, was followed before the beginning of the experiment: the intragastric pressure was raised to 20 mmHg in one step, leading to a progressive increase of the volume in the bag; when a volume of 400 ml was reached pressure was returned to 2 mmHg; after a short stabilization period at 2 mmHg, the intragastric pressure was raised to 10 mmHg until a volume of 300 ml was reached. Hereafter, the pressure was reduced to 2 mmHg awaiting the beginning of the experimental protocol.
Experiments were performed at constant intragastric pressure (6 mmHg), while the gastric volume changes were monitored (condition 1). Owing to the loss of vagal control on stomach motility, the baseline gastric volume at an intragastric pressure of 6 mmHg was significantly higher after vagotomy than before (317±50 vs 142±28 ml, respectively; P<0.05; n=5). Therefore, after vagotomy, the drugs were tested in two additional experimental conditions. In one set of experiments, the pressure in the bag was decreased in order to obtain a mean baseline volume approximately equal to the mean baseline volume at 6 mmHg before vagotomy (condition 2). In another set of experiments, the intrabag pressure was set at 6 mmHg, but after a 15 min stabilization period bethanechol was administered as a bolus i.v. (0.015 mg kg−1) followed by an i.v. infusion (0.05 mg kg−1 h−1) to increase gastric tone (condition 3).
Drug administration
Drugs were administered after a baseline period of at least 15 min at the constant pressure used. Per experiment, a single dose of flesinoxan was administered. Saline, NG-nitro-L-arginine methyl ester (L-NAME) and the 5-HT1A receptor antagonist N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-2-pyridinyl-cyclohexanecarboxamide (WAY-100635) were tested vs a single dose of flesinoxan, and administered 15 min prior to flesinoxan. For each experiment, dogs were randomly assigned to a dose in such a manner that each dog had received all treatments after completion of the set of experiments. After administration of the test compound, intragastric volume was measured during 60 min. A minimum washout period of 48 h was allowed between two consecutive experiments in the same animal. In the vagotomized dogs, the influence of the NO-donor nitroprusside, inducing smooth muscle relaxation, was compared to the effect induced by flesinoxan. All drugs, except atropine, were administered via injection in the cephalic vein (maximum injected volume: 1 ml 5 kg−1); agonists and antagonists were administered in opposite paws. Atropine was injected subcutaneously.
Analysis of data
A regression curve (Lowess regression) was calculated from the original gastric volume data to correct for extreme outliers. The Lowess regression method obtains a smoothed Y value at a given X by fitting a linear regression to the data in the neighborhood of the X value (Cleveland, 1979; Neter et al., 1996). SigmaPlot 2000 for Windows Version 6 was used for the calculation of the Lowess regression curve. Gastric volume changes induced by a given treatment were calculated as the difference between the highest (upon volume increase) or lowest (upon volume decrease) volume after treatment, and the mean baseline volume in the last 5 min before treatment (=mean baseline volume, MBV), as determined from the Lowess regression curve. Results were expressed as mean±s.e.m.
All comparisons of two or more treatment sessions within a group of dogs were analyzed using PROC MIXED (SAS System for Windows V8; SAS code available on request). A level of P<0.05 was taken as significant and the number of dogs used is denoted by n.
Drugs
The following drugs were used (abbreviations and respective suppliers in parentheses): atropine sulfate (Janssen Chimica, Belgium), bethanechol (Merck, Germany), flesinoxan HC1 (Solvay Pharma, Belgium), insulin (Actrapid® HM (100 UI ml−1); Novo Nordisk Pharma, Brussels, Belgium), L-NAME, WAY-100635 (Sigma-Aldrich, Belgium), sodium nitroprusside (nitroprusside; Johnson & Johnson Pharmaceutical Research & Development, Beerse, Belgium).
All compounds were dissolved in 0.9% NaCl, except for WAY-100635 that was dissolved in three equivalents 2,3-dihydroxybutanedioic acid +0.9% NaCl. Administration of solvents alone did not change the intragastric volume in comparison with the baseline volume. All drugs used were dissolved isotonically and the pH was between 4 and 7.
Results
Influence of flesinoxan
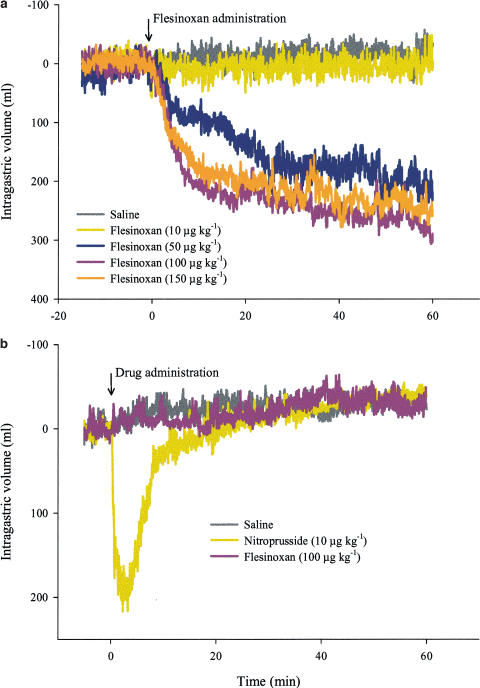
The dose–response curve of flesinoxan was established in 11 conscious dogs. The mean intragastric baseline volume (MBV) in dogs treated with saline was 180±23 ml (n=11); this did not significantly differ from the MBV before addition of 10, 50, 100 and 150 μg kg−1 flesinoxan (190±29, 219±27, 191±28 and 192±38 ml, respectively). At 100 and 150 μg kg−1, the administration of flesinoxan induced a moderate degree of restlessness and salivation in the dogs that lasted for the entire duration of the experiment. In order to prevent adverse effects, the dose of 150 μg kg−1 was not exceeded. Flesinoxan (10, 50, 100 and 150 μg kg−1) progressively induced a dose-dependent gastric relaxation vs saline (50±10, 230±51, 290±38 and 275±33 vs 26±8 ml maximum gastric volume increase, respectively; Figure 1a). The maximal intragastric volume increase was reached at 41±8, 55±2 and 46±5 min for 50, 100 and 150 μg kg−1 flesinoxan, respectively.
Figure 1.
Mean barostat recordings of canine intragastric volume (at 6 mmHg intrabag pressure). All curves are shown after subtraction of the mean baseline volume. (a) Dose–response curves of flesinoxan (n=9–11; i.v.). (b) Influence of flesinoxan, nitroprusside and saline after vagotomy (n=5; i.v.). In order to obtain a comparable baseline volume as before vagotomy, a bethanechol infusion was applied (condition 3).
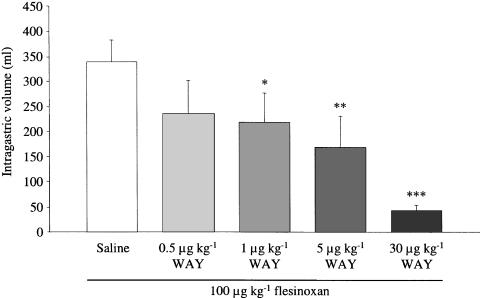
A dose of 100 μg kg−1 flesinoxan was selected to study the influence of inhibitors (n=6). The MBV before administration of flesinoxan in the control session was 195±30 ml and did not significantly differ from the MBV after addition of 0.5, 1, 5 and 30 μg kg−1 WAY-100635 (257±37, 287±51, 244±31 and 189±41 ml, respectively). WAY-100635 dose-dependently antagonized the effect of flesinoxan (Figure 2). After pretreatment with 30 μg kg−1 WAY-100635, the maximum flesinoxan-induced intragastric volume increase (43±11 ml) did not significantly differ from the maximum intragastric volume increase after saline addition in the same dogs (37±12 ml). Pretreatment with 30 μg kg−1 WAY-100635 decreased markedly the salivation and restlessness adverse effects normally induced by flesinoxan. Addition of L-NAME (10 mg kg−1) caused a significant (P<0.05) gastric contraction compared to saline addition (intragastric volume decrease of 72±18 vs 13±5 ml, respectively). The maximum effect of L-NAME was reached after 10±2 min. Nevertheless, the MBV after L-NAME addition did not significantly differ from that in the same dogs after treatment with saline (140±26 vs 195±30 ml, respectively). After addition of L-NAME, the flesinoxan-induced maximal intragastric volume increase was 379±44 ml; this was not significantly different from the maximal intragastric volume increase in the control session (339±44 ml).
Figure 2.
Maximal intragastric volume increase induced by 100 μg kg−1 flesinoxan (i.v.) after pretreatment with saline or different doses of WAY-100635 (WAY; i.v.). Intragastric volumes shown as mean±s.e.m.; n=6 *P<0.05, **P<0.01, ***P<0.0001 compared to saline addition.
Influence of vagotomy on the effect of flesinoxan
Five dogs were tested under three experimental conditions after vagotomy (Table 1).
Table 1.
Maximal intragastric volume increase (ml) after administration of different drugs before vagotomy and in three different conditions after vagotomy
| Treatment | Dose (μg kg−1) | Prevagotomy | Condition 1 | Condition 2 | Condition 3 |
|---|---|---|---|---|---|
| Saline | 7±6 | 72±21 | 50±34 | 0±3 | |
| Nitroprusside | 10 | ND | 107±16 | 106±14 | 178±30** |
| Flesinoxan | 100 | 238±69* | 46±16 | 34±19 | 18±5 |
| Atropine | 160 | 396±40*** | 168±31** | ND | ND |
Intragastric volumes are shown as mean±s.e.m. (n=5). Condition 1 (6 mmHg IGP), Condition 2 (decreased IGP), Condition 3 (6 mmHg IGP, bethanechol infusion); IGP: intragastric pressure.
P<0.01
**P<0.001 and ***P<0.0001 vs saline addition in the same Condition; ND: not determined.
Condition 1: The dogs were first studied at a constant intragastric bag pressure of 6 mmHg to mimic the experimental condition as was used before vagotomy. At this pressure after vagotomy, the MBV in the saline treatment session was significantly greater than before vagotomy (317±50 vs 142±28 ml, respectively; P<0.05). In condition 1, the MBV in the saline session did not significantly differ from that before addition of nitroprusside, flesinoxan and atropine (363±43, 339±64 and 301±61 ml, respectively). Nitroprusside and flesinoxan were not able to induce a significant relaxation vs saline administration. Injection of 160 μg kg−1 atropine on the other hand induced a significant gastric relaxation vs saline. The maximal intragastric volume increase by atropine after vagotomy was significantly decreased compared to before vagotomy (P<0.05).
In order to compare effects of drugs on a similar MBV as before vagotomy (142±28 ml), two additional experimental conditions were used.
Condition 2: The intragastric pressure was decreased (intragastric pressure ranging from 3 to 5 mmHg) in order to obtain an MBV approximately equal to that at 6 mmHg before vagotomy. The MBV of the saline session in condition 2 was 169±12 ml and did not significantly differ from the MBV before vagotomy. The MBV of the saline session was not different from that in the nitroprusside and flesinoxan treatment sessions in condition 2 (171±14 and 186±47 ml, respectively). Nitroprusside and flesinoxan were not able to induce a significant relaxation vs saline in this condition.
Condition 3: The intrabag pressure was set at 6 mmHg but the cholinergic agonist bethanechol was administered (bolus (0.015 mg kg−1) followed by infusion (0.05 mg kg−1 h−1)). Bethanechol infusion induced an intragastric volume decrease of 214±43 ml. Profuse salivation was noted in all bethanechol experiments. The MBV after bethanechol administration was 177±31 ml. This was not significantly different from the MBV before vagotomy and in condition 2. Within condition 3, the MBV of the saline session did not significantly differ from the nitroprusside and flesinoxan treatment sessions (163±11 and 184±12 ml, respectively). Flesinoxan was not able to induce an intragastric volume increase compared to saline, but administration of nitroprusside induced a significant stomach relaxation (Figure 1b). As nitroprusside had not been studied in the same dogs before vagotomy, it was tested in the six non-vagotomized dogs to investigate its influence in basal conditions; it induced a significant stomach relaxation vs saline addition (218±33 vs 25±10 ml; P<0.001; n=6). This nitroprusside-induced volume increase was not significantly different from that induced in the five vagotomized dogs under condition 3.
Discussion
In this study, we investigated the effect of the 5-HT1A receptor agonist flesinoxan on the gastric tone of conscious dogs by means of a barostat. The selective 5-HT1A receptor antagonist WAY-100635 was used to characterize the receptor involved. The flesinoxan-induced effect after vagotomy was compared to the effect before vagotomy in order to determine the importance of vagal pathways in the response.
Flesinoxan induced a dose-dependent intragastric volume increase reflecting gastric relaxation from 50 μg kg−1 onwards. The maximal effect was reached after administration of 100 μg kg−1 flesinoxan. Similar doses of flesinoxan were used to study 5-HT1A receptor-mediated blood pressure and heart rate effects in cats and dogs (Wouters et al., 1988) and are associated with 5-HT1A receptor involvement. A slowly developing response profile was observed as has earlier been described for the flesinoxan-induced change in body temperature (Hadrava et al., 1995). The estimated half-life for terminal elimination of flesinoxan upon intravenous administration is 136 min in rats (Zuideveld et al., 2002b) and this might contribute to the long duration of the gastric relaxant response upon administration of flesinoxan. In our experiments, injection of 100 and 150 μg kg−1 flesinoxan induced a moderate degree of restlessness and salivation that lasted for the entire experiment. Salivation and restlessness are well-described adverse effects of flesinoxan in dogs and may reflect a canine type of ‘5-HT syndrome' (Di Francesco, 1994).
The interaction of flesinoxan with 5-HT1A receptors was confirmed by the use of WAY-100635. WAY-100635 is a highly selective and high-affinity antagonist at 5-HT1A receptors in vivo (Forster et al., 1995; Zuideveld et al., 2002a). In the presence of WAY-100635, the flesinoxan-induced restlessness and salivation adverse effects were blocked, indicating that these symptoms were 5-HT1A receptor-mediated effects. In our experiments, WAY-100635 dose-dependently inhibited the flesinoxan-induced gastric relaxation from 1 μg kg−1 onwards. In the presence of 30 μg kg−1 WAY-100635, the flesinoxan-induced relaxation was completely blocked. At this dose, WAY-100635 is known to have antagonistic properties towards 5-HT1A receptor-mediated effects in rat (Assie & Koek, 2000; Zuideveld et al., 2002a).
As it was earlier shown that flesinoxan-induced effects can be centrally mediated by alteration of the vagal tone (Wouters et al., 1988), we assessed the effect of flesinoxan after vagotomy. During fasting, the proximal stomach is in a continuous state of tonic contraction that is maintained by a vagally mediated input. This is illustrated by vagotomy or vagal cooling experiments performed on conscious dogs; vagotomy or vagal cooling markedly decreased fundic tone, indicating a significant contribution of vagal pathways to the maintenance of canine gastric tone (Abrahamsson & Thoren, 1973; Azpiroz & Malagelada, 1987). It is well known that the main component of this vagal drive is cholinergic (Lidums et al., 2000). This was confirmed in our experiments where administration of atropine induced a significant stomach relaxation, indicative of the constant cholinergic input. Although significantly reduced compared to the situation before vagotomy, atropine still induced a gastric volume increase after vagotomy. As no vagal cholinergic pathways are present after vagotomy, this might be explained by a local cholinergic drive. Different studies described local compensatory mechanisms after chronic vagotomy. These adaptive changes are explained as alternative control mechanisms that have responded to the permanent removal of a more dominant control mechanism that is normally present (Hall et al., 1982). These compensatory mechanisms are most likely mediated by the intrinsic neural pathways of the stomach (Hakanson et al., 1984; Grundy et al., 1993; Takahashi & Owyang, 1997). Although these local compensatory mechanisms can contribute to a partial recovery of the gastric tone, they cannot fully compensate the loss of vagal cholinergic input. This is illustrated in our experiments by the less pronounced effect of atropine and by the fact that the intragastric MBV at an intragastric balloon pressure of 6 mmHg after vagotomy is significantly higher compared to the MBV before vagotomy. The difference in MBV before and after vagotomy makes it difficult to interpret drug effects before and after vagotomy. Therefore, after vagotomy, drugs were not only tested at a constant intragastric pressure of 6 mmHg (condition 1) but also in two additional conditions. In condition 2, drugs were tested at a decreased intraballoon pressure and in condition 3 they were tested together with a bethanechol infusion. Intravenous infusion of bethanechol is a classical method to maintain the gastric tone after vagal cooling or vagotomy (De Ponti et al., 1987). The intraballoon pressure in condition 2 and the bethanechol dose in condition 3 were chosen so that the MBV in these conditions was not different from that before vagotomy. In the three experimental conditions after vagotomy, the influence of flesinoxan and the NO-donor nitroprusside was compared to that of saline administration. Nitroprusside significantly relaxed the stomach of non-vagotomized dogs. After vagotomy, nitroprusside was only able to induce a stomach relaxation that was significantly more pronounced compared to saline administration in condition 3. This indicates that the infusion of bethanechol is a better means to compensate for the loss in cholinergic input and the increased basal stomach volume, than decreasing the intraballoon pressure during registration. The effect of drugs after vagotomy can thus best be studied in condition 3. Flesinoxan was not able to induce a stomach relaxation after vagotomy in all the three conditions, indicating that the vagus plays a crucial role in the flesinoxan-induced stomach relaxation.
In different studies describing flesinoxan-mediated effects, the mechanism of action is centrally mediated. In cats, for example, flesinoxan acts centrally to cause brachycardia and blood pressure decrease by sympathoinhibition and an increase in vagal tone (Ramage et al., 1988). Also, the antiemetic effect of flesinoxan in cats is most likely to be mediated centrally (Lucot, 1994). Flesinoxan in dogs seems to act central in order to mediate blood pressure changes (Huber et al., 1991). A first possibility is thus that flesinoxan activates centrally localized 5-HT1A receptors that mediate stomach relaxation through the vagus. 5-HT1A receptors have been found throughout the whole central nervous system and also in the dorsal motor nucleus of the vagus (Browning & Travagli, 2001). As the dorsal motor nucleus mediates many excitatory and inhibitory responses to the gastrointestinal tract (Roman & Gonella, 1981), it is plausible that the site of action of flesinoxan to induce gastric relaxation would be located there. A possible vagal pathway by which flesinoxan might induce gastric relaxation comprises vagal fibers ending on intrinsic nitrergic neurones in the stomach (Paterson et al., 2000). To assess this possibility, the influence of the NO synthase inhibitor L-NAME was investigated. A concentration of L-NAME was chosen, previously shown to affect intestinal motility in dogs (Alemayehu et al., 1994). The administration of L-NAME caused a decrease in gastric volume, reflecting a stomach contraction. This illustrates that there is tonic nitrergic input towards the stomach as shown before (Paterson et al., 2000). L-NAME did not influence the relaxant effect of flesinoxan illustrating that this is not mediated via nitrergic nerves. Flesinoxan might also act centrally by activating other vagal pathways that in turn stimulate purinergic or peptidergic neurotransmitter release in the enteric nervous system and thus relax the stomach (Azpiroz & Malagelada, 1986). Another possible mechanism for centrally located 5-HT1A receptors to relax the stomach is to inhibit the constant cholinergic vagal drive that maintains the gastric tone (Chang et al., 2003); this cholinergic drive is indeed impaired after vagotomy (this study; Azpiroz & Malagelada, 1987).
In contrast, activation of centrally located 5-HT1A receptors in rats by flesinoxan and 8-OH-DPAT was associated with smaller gastric volumes in response to a distension pressure (10 mmHg) compared to vehicle, corresponding with an increase in gastric tone (Rouzade et al., 1998). Although it was not shown that activation of these receptors actually contracts the stomach and species differences might hinder comparison to our results, these experiments in rats do not correlate with centrally located 5-HT1A receptors mediating gastric relaxation. Bulbring & Gershon (1967) showed that activation of 5-HT receptors located in the myenteric plexus of the guinea-pig isolated stomach inhibits acetylcholine release and subsequently relaxes the stomach. Tack et al. (1992) demonstrated 5-HT1A receptors inhibiting the release of acetylcholine at presynaptic nerve endings in the myenteric plexus of guinea-pig stomach tissue. Activation of these presynaptic 5-HT1A receptors on cholinergic motor neurones will result in decreased acetylcholine release and thus in stomach relaxation. 5-HT1A receptors have also been identified on myenteric interneurons (Kirchgessner et al., 1996). Flesinoxan might thus also act on the peripheral end of vagal motor fibers or on interneurons in the myenteric plexus in series with the vagal motor nerves. The latter possibility implies that the local cholinergic compensatory mechanism after vagotomy does not involve the interneurons in series with the vagal nerve.
In conclusion, as demonstrated by the antagonistic properties of WAY-100635, flesinoxan acts via the 5-HT1A receptor to relax the canine stomach in vivo. After vagotomy, flesinoxan was not able to induce stomach relaxation demonstrating that the effect is mediated through the vagal pathway; the actual results do not allow concluding as to whether flesinoxan interacts with the vagal nerves at a central or peripheral level. Nitrergic nerves are not involved, as L-NAME did not influence the effect of flesinoxan.
Abbreviations
- Flesinoxan
(+)-N-[2-[4-(2,3-Dihydro-2-hydroxymethyl-1,4-benzodioxin-5-yl)-1-piperazinyl]ethyl]-4-fluorobenzamide hydrochloride
- 5-HT
5-hydroxytryptamine
- IGP
intragastric pressure
- L-NAME
NG-nitro-L-arginine methyl ester
- MBV
mean baseline volume
- WAY-100635
N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-2-pyridinyl-cyclohexanecarboxamide
References
- ABRAHAMSSON H., THOREN P. Vomiting and reflex vagal relaxation of the stomach elicited from heart receptors in the cat. Acta Physiol. Scand. 1973;88:433–439. doi: 10.1111/j.1748-1716.1973.tb05472.x. [DOI] [PubMed] [Google Scholar]
- ALEMAYEHU A., LOCK K.R., COATNEY R.W., CHOU C.C. L-NAME, nitric oxide and jejunal motility, blood flow and oxygen uptake in dogs. Br. J. Pharmacol. 1994;111:205–212. doi: 10.1111/j.1476-5381.1994.tb14045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASSIE M.B., KOEK W. Estimation of apparent pA2 values for WAY 100635 at 5-HT1A receptors regulating 5-hydroxytryptamine release in anaesthetised rats. Eur. J. Pharmacol. 2000;409:173–177. doi: 10.1016/s0014-2999(00)00839-6. [DOI] [PubMed] [Google Scholar]
- AZPIROZ F., MALAGELADA J.R. Vagally mediated gastric relaxation induced by intestinal nutrients in the dog. Am. J. Physiol. 1986;251:G727–G735. doi: 10.1152/ajpgi.1986.251.6.G727. [DOI] [PubMed] [Google Scholar]
- AZPIROZ F., MALAGELADA J.R. Importance of vagal input in maintaining gastric tone in the dog. J. Physiol. 1987;384:511–524. doi: 10.1113/jphysiol.1987.sp016467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAXTER G.S., MURPHY O.E., BLACKBURN T.P. Further characterization of 5-hydroxytryptamine receptors (putative 5-HT2B) in rat stomach fundus longitudinal muscle. Br. J. Pharmacol. 1994;112:323–331. doi: 10.1111/j.1476-5381.1994.tb13072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWNING K.N., TRAVAGLI R.A. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J. Physiol. 2001;531:425–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCHHEIT K.H., BUHL T. Stimulant effects of 5-hydroxytryptamine on guinea pig stomach preparations in vitro. Eur. J. Pharmacol. 1994;262:91–97. doi: 10.1016/0014-2999(94)90031-0. [DOI] [PubMed] [Google Scholar]
- BULBRING E., GERSHON M.D. 5-Hydroxytryptamine participation in the vagal inhibitory innervation of the stomach. J. Physiol. 1967;192:823–846. doi: 10.1113/jphysiol.1967.sp008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG H.Y., MASHIMO H., GOYAL R.K. Musings on the wanderer: what's new in our understanding of vago-vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G357–G366. doi: 10.1152/ajpgi.00478.2002. [DOI] [PubMed] [Google Scholar]
- CLEVELAND W.S. Robust locally weighted regression and smoothing scatterplots. J. Am. Stat. Assoc. 1979;74:386–829. [Google Scholar]
- COULIE B., TACK J., SIFRIM D., ANDRIOLI A., JANSSENS J. Role of nitric oxide in fasting gastric fundus tone and in 5-HT1 receptor-mediated relaxation of gastric fundus. Am. J. Physiol. 1999;276:G373–G377. doi: 10.1152/ajpgi.1999.276.2.G373. [DOI] [PubMed] [Google Scholar]
- DE PONTI F., AZPIROZ F., MALAGELADA J.R. Reflex gastric relaxation in response to distention of the duodenum. Am. J. Physiol. 1987;252:G595–G601. doi: 10.1152/ajpgi.1987.252.5.G595. [DOI] [PubMed] [Google Scholar]
- DE PONTI F., CREMA F., MORO E., NARDELLI G., FRIGO G., CREMA A. Role of 5-HT1B/D receptors in canine gastric accommodation: effect of sumatriptan and 5-HT1B/D receptor antagonists. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:G96–G104. doi: 10.1152/ajpgi.00280.2002. [DOI] [PubMed] [Google Scholar]
- DI FRANCESCO G.F. Are the cardiovascular effects and ‘5-HT syndrome' induced by MDL 73,975 and flesinoxan in the dog mediated by 5-HT1A receptors. Eur. J. Pharmacol. 1994;262:205–215. doi: 10.1016/0014-2999(94)90734-x. [DOI] [PubMed] [Google Scholar]
- ESPLUGUES J.V. NO as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002;135:1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORSTER E.A., CLIFFE I.A., BILL D.J., DOVER G.M., JONES D., REILLY Y., FLETCHER A. A pharmacological profile of the selective silent 5-HT1A receptor antagonist. WAY-100635. Eur. J. Pharmacol. 1995;281:81–88. doi: 10.1016/0014-2999(95)00234-c. [DOI] [PubMed] [Google Scholar]
- GRUNDY D., GHARIB-NASERI M.K., HUTSON D. Plasticity in the gastric inhibitory innervation after immunization against VIP and vagotomy in the ferret. Am. J. Physiol. 1993;265:G432–G439. doi: 10.1152/ajpgi.1993.265.3.G432. [DOI] [PubMed] [Google Scholar]
- HADRAVA V., BLIER P., DENNIS T., ORTEMANN C., DE MONTIGNY C. Characterization of 5-hydroxytryptamine 1A properties of flesinoxan: in vivo electrophysiology and hypothermia study. Neuropharmacology. 1995;34:1311–1326. doi: 10.1016/0028-3908(95)00098-q. [DOI] [PubMed] [Google Scholar]
- HAKANSON R., VALLGREN S., EKELUND M., REHFELD J.F., SUNDLER F. The vagus exerts trophic control of the stomach in the rat. Gastroenterology. 1984;86:28–32. [PubMed] [Google Scholar]
- HALL K.E., EL SHARKAWY T.Y., DIAMANT N.E. Vagal control of migrating motor complex in the dog. Am. J. Physiol. 1982;243:G276–G284. doi: 10.1152/ajpgi.1982.243.4.G276. [DOI] [PubMed] [Google Scholar]
- HOYER D., HANNON J.P., MARTIN G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- HUBER S., GROHS J.G., RABERGER G. Cardiovascular and side effects of flesinoxan in conscious hypertensive dogs. Modulation by prazosin. Eur. J. Pharmacol. 1991;202:1–7. doi: 10.1016/0014-2999(91)90246-m. [DOI] [PubMed] [Google Scholar]
- JANSSEN P., PRINS N.H., MEULEMANS A.L., LEFEBVRE R.A. Smooth muscle 5-HT(2A) receptors mediating contraction of porcine isolated proximal stomach strips. Br. J. Pharmacol. 2002;137:1217–1224. doi: 10.1038/sj.bjp.0704992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRCHGESSNER A.L., LIU M.T., RAYMOND J.R., GERSHON M.D. Identification of cells that express 5-hydroxytryptamine1A receptors in the nervous systems of the bowel and pancreas. J. Comp. Neurol. 1996;364:439–455. doi: 10.1002/(SICI)1096-9861(19960115)364:3<439::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- LIDUMS I., HEBBARD G.S., HOLLOWAY R.H. Effect of atropine on proximal gastric motor and sensory function in normal subjects. Gut. 2000;47:30–36. doi: 10.1136/gut.47.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCOT J.B. Antiemetic effects of flesinoxan in cats: comparisons with 8-hydroxy-2-(di-n-propylamino)tetralin. Eur. J. Pharmacol. 1994;253:53–60. doi: 10.1016/0014-2999(94)90756-0. [DOI] [PubMed] [Google Scholar]
- MEULEMANS A.L., EELEN J.G., SCHUURKES JA. NO mediates gastric relaxation after brief vagal stimulation in anesthetized dogs. Am. J. Physiol. 1995;269:G255–G261. doi: 10.1152/ajpgi.1995.269.2.G255. [DOI] [PubMed] [Google Scholar]
- MEULEMANS A.L., HELSEN L.F., DE RIDDER W.J.E., SCHUURKES J.A. Effects of sumatriptan on the guinea-pig isolated stomach. Neurogastroenterol. Motil. 1996;8:A183. [Google Scholar]
- NETER J., KUTNER M.H., NACHTSHEIM C.J., WASSERMAN W. Applied Linear Statistical Models 1996Chicago: Irwin; 4th edn [Google Scholar]
- PATERSON C.A., ANVARI M., TOUGAS G., HUIZINGA J.D. Nitrergic and cholinergic vagal pathways involved in the regulation of canine proximal gastric tone: an in vivo study. Neurogastroenterol. Motil. 2000;12:301–306. doi: 10.1046/j.1365-2982.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., VAN DER GRIJN A., LEFEBVRE R.A., AKKERMANS L.M., SCHUURKES J.A. 5-HT(4) receptors mediating enhancement of contractility in canine stomach; an in vitro and in vivo study. Br. J. Pharmacol. 2001;132:1941–1947. doi: 10.1038/sj.bjp.0703985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAGE A.G., WOUTERS W., BEVAN P. Evidence that the novel antihypertensive agent, flesinoxan, causes differential sympathoinhibition and also increases vagal tone by a central action. Eur. J. Pharmacol. 1988;151:373–379. doi: 10.1016/0014-2999(88)90533-x. [DOI] [PubMed] [Google Scholar]
- ROMAN C., GONELLA J. Extrinsic Control of Digestive Tract Motility 1981New York: Raven Press; 289–333.ed. Johnson, L.R. pp [Google Scholar]
- ROUZADE M.L., FIORAMONTI J., BUENO L. Decrease in gastric sensitivity to distension by 5-HT1A receptor agonists in rats. Dig. Dis. Sci. 1998;43:2048–2054. doi: 10.1023/a:1018859214758. [DOI] [PubMed] [Google Scholar]
- SHAFIK A. Effect of distension of the pharynx and esophagus on the stomach in dogs: experimental evidence for a pharyngoesophagogastric reflex. Digestion. 1999;60:17–21. doi: 10.1159/000007584. [DOI] [PubMed] [Google Scholar]
- TACK J., JANSSENS J., VANTRAPPEN G., WOOD J.D. Actions of 5-hydroxytryptamine on myenteric neurons in guinea pig gastric antrum. Am. J. Physiol. 1992;263:G838–G846. doi: 10.1152/ajpgi.1992.263.6.G838. [DOI] [PubMed] [Google Scholar]
- TACK J.F., COULIE B., WILMER A., JANSSENS J. Sumatriptan, a 5-HT1-receptor agonist, causes a significant relaxation of the gastric fundus in man. Gastroenterology. 1995;108:A696. [Google Scholar]
- TACK J., PIESSEVAUX H., COULIE B., FISCHLER B., DE GUCHT V., JANSSENS J. A placebo-controlled trial of buspirone, a fundus-relaxing drug, in functional dyspepsia: effect on symptoms and gastric sensory and motor function. Gastroenterology. 1999;166:A325. [Google Scholar]
- TACK J., COULIE B., WILMER A., ANDRIOLI A., JANSSENS J. Influence of sumatriptan on gastric fundus tone and on the perception of gastric distension in man. Gut. 2000;46:468–473. doi: 10.1136/gut.46.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACK J., DEMEDTS I. Role of nitric oxide in the gastric accommodation reflex and in meal induced satiety in humans. Gut. 2002;51:219–224. doi: 10.1136/gut.51.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI T., OWYANG C. Characterization of vagal pathways mediating gastric accommodation reflex in rats. J. Physiol. 1997;504:479–488. doi: 10.1111/j.1469-7793.1997.479be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDELAND K.A., HAUSKEN T., GILJA O.H., ROPERT R., GALMICHE J.P., BERSTAD A. Gastric relaxation in response to a soup meal in healthy subjects. A study using a barostat in the proximal stomach. Scand. J. Gastroenterol. 1995;30:1069–1076. doi: 10.3109/00365529509101609. [DOI] [PubMed] [Google Scholar]
- WOUTERS W., TULP M.T., BEVAN P. Flesinoxan lowers blood pressure and heart rate in cats via 5-HT1A receptors. Eur. J. Pharmacol. 1988;149:213–223. doi: 10.1016/0014-2999(88)90651-6. [DOI] [PubMed] [Google Scholar]
- ZUIDEVELD K.P., TREIJTEL N., MAAS H.J., GUBBENS-STIBBE J.M., PELETIER L.A., DER GRAAF P.H., DANHOF M. A competitive interaction model predicts the effect of WAY-100,635 on the time course of R-(+)-8-hydroxy-2-(di-n-propylamino)tetralin-induced hypothermia. J. Pharmacol. Exp. Ther. 2002a;300:330–338. doi: 10.1124/jpet.300.1.330. [DOI] [PubMed] [Google Scholar]
- ZUIDEVELD K.P., VAN GESTEL A., PELETIER L.A., VAN DER GRAAF P.H., DANHOF M. Pharmacokinetic–pharmacodynamic modelling of the hypothermic and corticosterone effects of the 5-HT1A receptor agonist flesinoxan. Eur. J. Pharmacol. 2002b;445:43–54. doi: 10.1016/s0014-2999(02)01665-5. [DOI] [PubMed] [Google Scholar]