Abstract
Theophylline, a nonselective phosphodiesterase inhibitor, has long been regarded as a major bronchodilator in the treatment of human asthma. Using front-surface fluorometry with fura-2 and α-toxin permeabilization, the effects of theophylline on intracellular Ca2+ concentration ([Ca2+]i), tension development and Ca2+ sensitivity of the contractile apparatus were investigated in porcine tracheal smooth muscle strips.
Application of theophylline induced a relaxation without a significant decrease in [Ca2+]i when strips were precontracted by 40 mM K+ depolarization, while theophylline significantly decreased both [Ca2+]i and tension induced by carbachol.
The effects of theophylline on the increases in [Ca2+]i and tension induced by carbachol were significantly inhibited by iberiotoxin, an inhibitor of large-conductance Ca2+-activated K+ channels.
In the absence of extracellular Ca2+, theophylline significantly attenuated carbachol-induced transient increases in tension development, while it did not affect carbachol-induced transient increase in [Ca2+]i.
The [Ca2+]i–force relationship, which was determined by cumulative applications of extracellular Ca2+ (0–5 mM) during 40 mM K+ depolarization, was significantly shifted to the right by theophylline.
In α-toxin permeabilized strips, theophylline significantly increased the EC50 value of [Ca2+]i for contraction and enhanced the effect of cAMP, but not of cGMP.
These results indicate that theophylline induces relaxation of the porcine tracheal smooth muscle through an activation of BK channels, and a resultant decrease in [Ca2+]i and an attenuation of Ca2+ sensitivity, presumably through the action of cAMP.
Keywords: Theophylline, porcine tracheal smooth muscle, intracellular Ca2+ concentration, Ca2+ sensitivity, large-conductance Ca2+-activated K+ channel, iberiotoxin, cAMP, cGMP
Introduction
Theophylline has been used worldwide for the treatment of asthma and chronic obstructive pulmonary diseases for several decades, due in part to its low cost and its ease of administration. Theophylline is a bronchodilator that may also be used to enhance respiratory muscle function and mucociliary clearance; it also acts in the central nervous system to enhance ventilation. Theophylline has been shown to relax an isolated airway smooth muscle preparation. The mechanisms whereby theophylline causes tracheal smooth muscle relaxation are not well understood.
Theophylline-induced airway smooth muscle relaxation has been attributed to the increased cellular cAMP and cGMP levels, because theophylline is known to be a nonselective phosphodiesterase (PDE) inhibitor. It is thought that cyclic nucleotides such as cAMP and cGMP cause smooth muscle relaxation mainly by decreasing intracellular Ca2+ concentrations ([Ca2+]i), as well by attenuating Ca2+ sensitivity of the contractile apparatus (Nishimura & van Breemen, 1989; Ushio-Fukai et al., 1993). To explain the cAMP- and/or cGMP-induced decrease in [Ca2+]i, several mechanisms have been proposed, including inhibition of Ca2+ influx due to hyperpolarization via stimulation of Ca2+-activated K+ channels (Sadoshima et al., 1988), a stimulation of Ca2+ uptake into the intracellular stores (Mueller & van Breemen, 1979) and an increase in Ca2+ extrusion from cells through the salcolemmal Ca2+ pump (Bülbring & Tomita, 1987). However, the relative importance of these various mechanisms in theophylline-induced relaxation of the airway smooth muscle remains unclear. Moreover, it is not known whether theophylline decreases the Ca2+ sensitivity of the contractile apparatus in airway smooth muscle.
In this study using simultaneous measurements of [Ca2+]i and tension in fura-2-loaded intact muscle strips, as well as the receptor-coupled permeabilization by the α-toxin technique, we examined the mechanisms underlying theophylline-induced relaxation of the porcine tracheal smooth muscle. We determined that theophylline decreases the Ca2+ sensitivity of the contractile apparatus and also reduces [Ca2+]i through opening of iberiotoxin (IbTX)-sensitive channels, presumably large-conductance Ca2+-activated K+ channels (BK channels). The combined effects of reduction in Ca2+ sensitivity and inhibition of Ca2+ entry would promote smooth muscle relaxation.
Methods
Tissue preparation
The tracheas were dissected from adult pigs at a local slaughterhouse, using a protocol approved by the Animal Research Committee of the Research Institute of Angiocardiology, Graduate School of Medical Sciences, Kyushu University. The tracheas were placed in ice-cold physiological salt solution (PSS). The lower end of the trachea (just above the first branching of the bronchus branching), comprising three tracheal rings in length, was used for the experiments. The posterior portion of the trachea was excised longitudinally, and all cartilage was detached. Both the mucosal and adventitial tissues were carefully removed under microscopic observation. The muscle sheets were transversely cut into rectangular strips measuring approximately 3 mm in length and 1 mm in width (Kai et al., 1993).
Fura-2 loading
Tracheal strips were loaded with the Ca2+ indicator dye fura-2 in the form of acetoxymethyl ester (fura-2/AM), as previously described (Kai et al., 1993). The strips were incubated in 1 ml aerated (95% O2 : 5% CO2) Dulbecco's modified Eagle's medium (DMEM) containing 50 μM fura-2/AM and 5% fetal bovine serum for 3 h at 37°C. After loading with fura-2, the strips were washed with normal PSS to remove excess dye from the extracellular space, and were then equilibrated in normal PSS for at least 1 h.
Tension recordings
Each strip was mounted vertically in a 6 ml quartz organ bath containing PSS maintained at 37°C and bubbled with 95% O2 and 5% CO2. The lower end of the strip was fixed, and the upper end of the strip was connected to a force transducer (TB-612T, Nihon Koden, Japan) to record isometric tension. During the 1 h post-fura-2 loading equilibration period, strips were stimulated with 40 mM K+ PSS at 5–10 min intervals, and the muscle length increased in a stepwise manner after each stimulation until the developed tension reached a maximum. When exposed to 40 mM K+ PSS, most strips produced a stable tension within 10 min, with or without an initial transient force response (Kai et al., 1993; 1996; Yoshimura et al., 1995; Setoguchi et al., 2001). Any strips showing instability in tension, as induced by 40 mM K+ PSS, were excluded from the study. The responsiveness of each strip to 40 mM K+ PSS was then recorded before starting the experimental protocol, since maximal contractile responses to high K+ depolarization were obtained at this concentration of K+. In experiments to determine the effects of IbTX on the theophylline-induced decreases in tension, the developed tension was expressed as a percentage, assigning the values in normal (5.9 mM K+) PSS and the steady state of the contraction induced by 100 nM carbachol (CCh) as 0 and 100%, respectively. For the rest of the experiments, the developed tension was expressed as a percentage, assigning the values in normal PSS and the steady-state contraction induced by 40 mM K+ PSS to be 0 and 100%, respectively.
Measurements of fura-2 fluorescence
Changes in the fluorescence intensity of the fura-2–Ca2+ complex were monitored using a front-surface fura-2 fluorometer (model CAM-OF). The details of our front-surface fluorometry system have been described elsewhere (Hirano et al., 1990; Kai et al., 1993; Kanaide, 1999). In brief, two wavelengths of excitation light (340 and 380 nm) were obtained spectroscopically from a Xenon light source. The strips were illuminated by guiding the two alternating (400 Hz) wavelengths of excitation light through quartz optic fibers. The surface fluorescence of the strip was collected by glass optic fibers and introduced through a 500 nm band pass filter into a photomultiplier. We thus measured the fura-2 fluorescence intensity emission, which was induced by alternating two wavelengths of excitation light (340 and 380 nm), at 500 nm emitting light.
The ratio of the fluorescence intensities (fluorescence ratio) at 340 nm excitation to that at 380 nm excitation was monitored to estimate the changes in [Ca2+]i. In experiments to determine the effects of IbTX on theophylline-induced decreases in [Ca2+]i, the ratio was expressed as a percentage, assigning the values in normal PSS and a steady state of the [Ca2+]i induced by 100 nM CCh to be 0 and 100%, respectively. For the rest of experiments, the ratio was expressed as a percentage, assigning the values in normal PSS and a steady state of the [Ca2+]i induced by 40 mM K+ PSS to be 0 and 100%, respectively.
Tension measurement in α-toxin permeabilized tracheal strips
Permeabilization of tracheal strips by α-toxin was performed according to previously described methods (Nishimura et al., 1988), with minor modifications. In brief, thin strips (about 0.5 mm in width and 2 mm in length) of the porcine tracheal smooth muscle were mounted between two tungsten wires, one of which was fixed and the other one was attached to a force transducer (UL2; Minebea Co., Japan). Permeabilization was carried out in a Ca2+-free cytosolic substitution solution (CSS; in mM: potassium methanesulfonate 100, Na2ATP 2.2, MgCl2 3.38, EGTA 10, creatine phosphate 10, Tris-maleate 20 (pH 6.8)) with 5000 U ml−1 Staphylococcus aureus α-toxin for 30 min. The composition of Ca2+ solution (activating solution) was the same as the CSS described above, except that it contained the indicated concentration of Ca2+ buffered by 10 mM EGTA. All experiments using permeabilized tissue were performed at room temperature. In experiment to determine the Ca2+–force relationship in the absence or presence of 300 μM theophylline, the resting tension in the relaxing solution and the maximal tension induced by 10 μM Ca2+ were taken as 0 and 100%, respectively. In experiments to determine the relaxant effect of cAMP and cGMP on tension induced by 500 nM Ca2+ in the absence or presence of 100 μM theophylline, the resting tension in relaxing solution and the tension induced by 500 nM Ca2+ just before the application of theophylline and at the corresponding time point of the time-matched control were taken as 0 and 100%, respectively.
Solutions and drugs
Normal PSS was of the following composition (in mM): NaCl 123, KCl 4.7, NaHCO3 15.5, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.25 and D-glucose 11.5. High K+ PSS was identical to normal PSS, except for an equimolar substitution of KCl for NaCl. Ca2+-free PSS was produced by exclusion of CaCl2 from the composition of normal PSS. PSS was bubbled with 95% O2 and 5% CO2, with a resulting pH of 7.4 at 37°C.
DMEM was purchased from Gibco (Grand Island, NY, U.S.A.). CCh, theophylline, α-toxin, IbTX, cAMP and cGMP were from Sigma (St Louis, MO, U.S.A.). EGTA and fura-2/AM were obtained from Dojindo (Kumamoto, Japan).
Data analysis
The measured values were expressed as mean±s.e.m. (n=number of experiments using a number of different animals). Analysis of covariance was used to determine the statistical significance of the shift of the [Ca2+]i–tension relationship (Figure 5). Paired Student's t-test was used to determine the statistical significance of the effect of IbTX on the CCh-induced tension and [Ca2+]i (Figure 3c), and the relaxant effect of theophylline on the CCh-induced tension and [Ca2+]i in the absence or presence of IbTX (Figure 3d) and the EC50 values of [Ca2+]i in the absence or presence of 300 μM theophylline (Figure 6). Repeated measures analysis of variance and contrast analysis were used to determine the statistical significance of the effect of cAMP and cGMP in the absence or presence of 100 μM theophylline (Figure 7). For the rest of the measurements, an unpaired t-test was used. P-values less than 0.05 were considered to be significant.
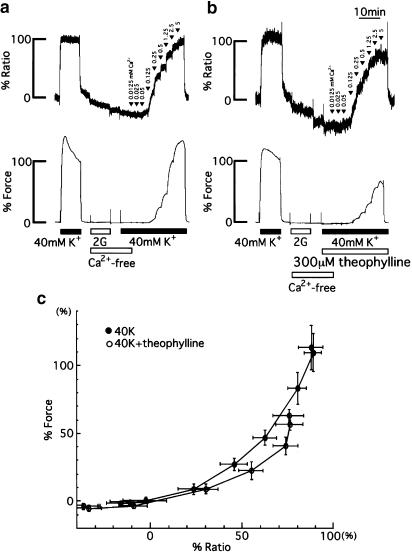
Figure 5.
Representative recordings of the changes in [Ca2+]i and force induced by cumulative applications of extracellular Ca2+ (0–5 mM) during 40 mM K+ depolarization in the absence (a) and the presence of theophylline (b). The numbers noted by the arrowheads indicate the final concentration of extracellular Ca2+ (mM) at each step. The steady-state [Ca2+]i–force curves (c) in the absence (closed circle) or presence of theophylline (open circle) were constructed with the data obtained from four independent experiments made in a manner similar to that in (a) and five independent experiments done as in (b). The data were obtained at the time when force reached the maximal level at each application of extracellular Ca2+. The vertical and horizontal bars represent s.e.m.
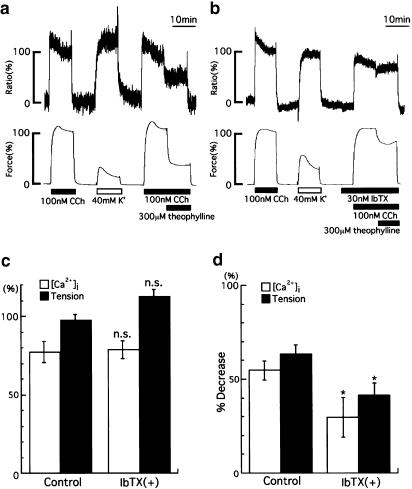
Figure 3.
Effect of theophylline on [Ca2+]i and tension induced by CCh in the absence or presence of IbTX. After the responses (100%) to 100 nM CCh had been recorded, strips were incubated in normal PSS for 10 min, and then in 40 mM K+ PSS for 10 min to fill intracellular Ca2+ stores. (a) The strips were incubated with normal PSS again, and then with 100 nM CCh. At 10 min, theophylline (300 μM) was then applied during the CCh-induced contraction. (b) The recordings were obtained under similar conditions as above (a), except that 30 nM IbTX was applied 5 min before the second application of CCh. (c) A summary of the effect of IbTX on the increases in [Ca2+]i and tension development induced by CCh. IbTX has no direct effect on the [Ca2+]i or tension levels. (d) A summary of the percentage decreases induced by theophylline during the CCh-induced contraction in the absence or presence of IbTX. The data are expressed as the means±s.e.m. (shown as vertical bars) (n=5). *P<0.05; n.s., difference not significant (statistical significance was determined by using paired t-test).
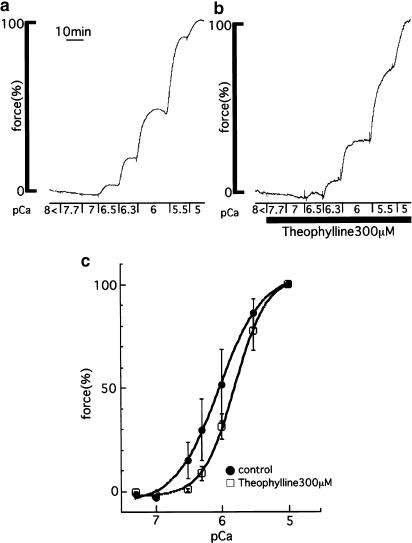
Figure 6.
Effects of theophylline on the contraction of the α-toxin permeabilized strips. (a, b) Representative recordings show the Ca2+-induced contraction in the absence (a) or presence (b) of 300 μM theophylline. The resting tension in the relaxing solution and the maximal tension induced by 10 μM Ca2+ were taken as 0 and 100%, respectively. Theophylline (300 μM) did not affect the contraction induced by 10 μM Ca2+ (data not shown). (c) A summary of the results obtained from experiments performed in a similar manner as in (a, b). The data are expressed as the means±s.e.m. (shown as vertical bars) (n=5).
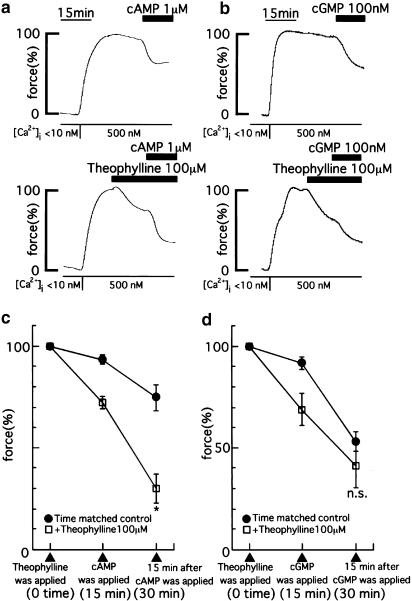
Figure 7.
Effects of theophylline on the relaxation of cyclic nucleotides on the contraction of the α-toxin permeabilized strips. (a, b) Representative recordings show the relaxant effect of 1 μM cAMP (a) and 100 nM cGMP (b) on the contraction induced by 500 nM Ca2+ with or without 100 μM theophylline, respectively. The resting tension in the relaxing solution and the tension induced by 500 nM Ca2+ just before the application of theophylline and at the corresponding time point of the time-matched control were taken as 0 and 100%, respectively. (c) A summary of the relaxant effect of 1 μM cAMP with or without 100 μM theophylline (n=5). (d) A summary of the relaxant effect of 100 nM cGMP with or without 100 μM theophylline (n=7). The data are expressed as the means±s.e.m. (shown as vertical bars). *P<0.05; n.s., difference not significant (statistical significance was determined by using repeated measures analysis of variance and contrast analysis).
Results
Effects of theophylline on increases in [Ca2+]i and tension induced by 40 mM K+ PSS and CCh
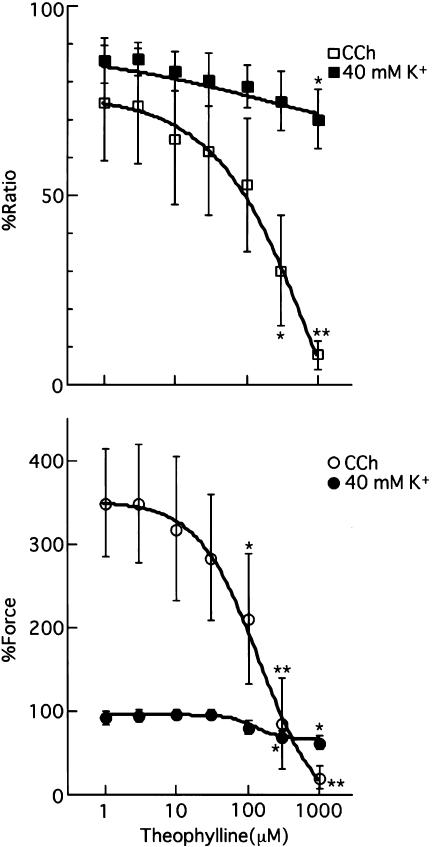
After recording the 100% response levels by the depolarization with 40 mM K+ PSS, CCh or 40 mM K+ was applied. Both CCh and 40 mM K+ depolarization increased [Ca2+]i and tension levels, which reached steady-state levels within 10 min. The cumulative application of theophylline (1 μM–1 mM) during the maintained phase of the contraction induced by CCh or 40 mM K+ depolarization caused concentration-dependent reductions in [Ca2+]i and tension (Figure 1). Comparisons were made with tension and [Ca2+]i values prior to the application of theophylline. Theophylline significantly attenuated both the increases in [Ca2+]i (>300 μM theophylline) and tension (>100 μM theophylline) induced by CCh. The increases in tension and [Ca2+]i produced by 40 mM K+ were both inhibited by theophylline at concentrations above 300 μM and 1 mM, respectively. Application of 1 mM theophylline decreased the [Ca2+]i level and tension induced by CCh to 7.89±3.78 (P<0.01) and 20.7±13.9% (P<0.01), respectively (n=4). On the other hand, application of 1 mM theophylline decreased the [Ca2+]i level and tension induced by 40 mM K+ depolarization to 70.2±7.95 (P<0.05) and 62.6±8.18% (P<0.05), respectively (n=4). A maximal concentration of theophylline (1 mM) did not alter the basal tension or [Ca2+]i (data not shown).
Figure 1.
The concentration-dependent effect of theophylline on the increases in [Ca2+]i level and tension development induced by 40 mM K+ depolarization (closed squares and closed circles, n=4) and 100 nM CCh (open squares and open circles, n=4). The fluorescence ratio and tension were expressed as a percentage by assuming the values in normal PSS (5.9 mM K+ PSS) and the steady state during 40 mM K+ depolarization to be 0 and 100%, respectively. The data are expressed as the means±s.e.m. (shown as vertical bars). *P<0.05, compared with values just before the application of theophylline; **P<0.01, compared with values just before the application of theophylline (statistical significance was determined by using unpaired t-test).
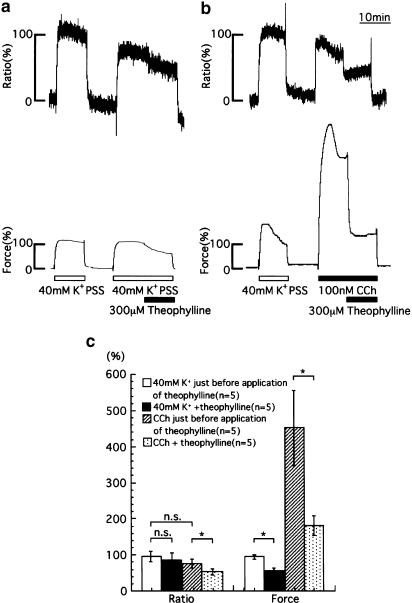
A representative time course of the effect of theophylline (300 μM) on the increases in [Ca2+]i and tension of porcine tracheal smooth muscle strips during contraction induced by 40 mM K+ PSS is shown in Figure 2a. When theophylline (300 μM) was added 10 min after the application of 40 mM K+ PSS, there was a significant reduction of tension (from 94.9±5.94 to 57.3±5.54%, n=5, P<0.05) with an insignificant decrease in [Ca2+]i (from 96.3±14.7 to 86.0±18.3%, n=5, P>0.05). Figure 2b shows a representative time course of the effect of theophylline (300 μM) on the increases in [Ca2+]i and tension induced by 100 nM CCh. The application of theophylline (300 μM) 10 min after the addition of 100 nM CCh caused a significant reduction of tension (from 452±104 to 181±27.1%, n=5, P<0.05), accompanied by a significant decrease in [Ca2+]i (from 76.7±12.8 to 53.0±9.6%, n=5, P<0.05). Figure 2c summarizes the changes in [Ca2+]i and tension induced by the applications of theophylline (300 μM) to porcine tracheal smooth muscle strips precontracted with 40 mM K+ PSS or 100 nM CCh. Theophylline significantly decreased both [Ca2+]i and tension when strips were precontracted by CCh, while it decreased tension without reducing [Ca2+]i when strips were precontracted with 40 mM K+ PSS.
Figure 2.
Effects of 300 μM theophylline on [Ca2+]i and tension of the porcine tracheal smooth muscle. Representative recordings of the effects of 300 μM theophylline on the increases in [Ca2+]i and tension induced by 40 mM K+ PSS (a) and 100 nM CCh (b). Theophylline was applied 10 min after the application of 40 mM K+ PSS (n=5) or 100 nM CCh (n=5). (c) A summary of the results obtained from the indicated number of experiments (using a number of different animals) performed in a manner similar to those in (a, b). The data are expressed as the means±s.e.m. (shown as vertical bars). *P<0.05; n.s., difference not significant (statistical significance was determined by using the unpaired t-test).
Effects of IbTX on the theophylline-induced decreases in [Ca2+]i and tension induced by CCh
Figure 3a and b shows representative recordings of the effects of theophylline on the increases in [Ca2+]i and tension induced by 100 nM CCh in the absence (a) or presence (b) of 30 nM IbTX, a BK channel blocker. When 100 nM CCh was applied, both the [Ca2+]i and tension responses rapidly increased and reached plateau phases within 10 min. The values at resting and plateau phases were designated as 0 and 100%, respectively. IbTX did not directly affect the increases in [Ca2+]i (77.3±6.9% in the absence and 78.7±5.8% in the presence of IbTX) and tension (97.5±3.5% in the absence and 113±4.4% in the presence of IbTX) development induced by CCh (Figure 3b and c). The application of theophylline induced sustained decreases in [Ca2+]i and tension within 1 min (Figure 3a and b). In the absence of IbTX, the percent decreases in [Ca2+]i and tension, calculated from the values just before and 10 min after the application of theophylline, were 54.8±5.0 and 63.4±4.7%, respectively (n=5). In the presence of 30 nM IbTX, these values were inhibited significantly (29.8±10.5% for [Ca2+]i, 41.3±6.6% for tension, n=5, P<0.05). Figure 3d summarizes the effects of IbTX on the theophylline-induced decreases in [Ca2+]i and tension during contraction induced by CCh. IbTX significantly inhibited the theophylline-induced decreases in [Ca2+]i and tension.
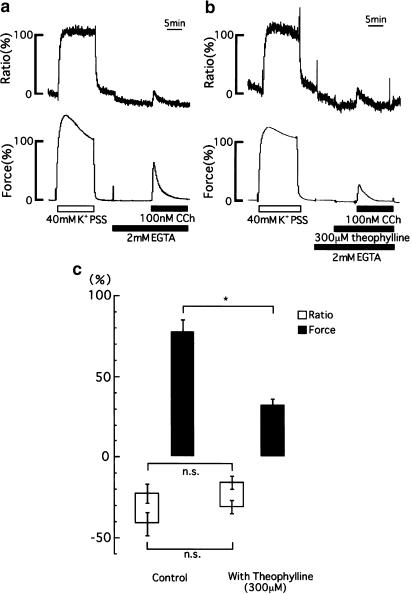
Effects of theophylline on the increases in [Ca2+]i and tension development induced by CCh in the absence of extracellular Ca2+
Figure 4a shows the representative time courses of the changes in [Ca2+]i and tension development induced by 100 nM CCh in the Ca2+-free PSS containing 2 mM EGTA. Under these conditions, basal [Ca2+]i gradually declined to reach a steady state, whereas the tension remained unchanged. The application of 100 nM CCh after a 10 min incubation in Ca2+-free PSS caused transient increases in [Ca2+]i (−22.6±5.7%, n=8) and tension (77.5±7.4%, n=8). As shown in Figure 4b, pretreatment of strips with theophylline for 5 min significantly attenuated the transient increases in tension development (32.1±3.8%, n=8, P<0.01) induced by CCh, while it did not affect the transient increases in [Ca2+]i (−15.9±4.2%, n=8, P>0.05). Figure 4c summarizes the changes in [Ca2+]i and tension development induced by CCh in Ca2+-free PSS containing 2 mM EGTA with or without theophylline.
Figure 4.
Effect of theophylline on increases in [Ca2+]i level and tension development induced by CCh in Ca2+-free PSS containing 2 mM EGTA. Representative recordings show changes in [Ca2+]i and tension development induced by 100 nM CCh in Ca2+-free PSS containing 2 mM EGTA, in the absence (a) or presence of 300 μM theophylline (b). The developed tension and [Ca2+]i were expressed as a percentage, assigning the values in normal PSS and the steady-state contraction induced by 40 mM K+ PSS to be 0 and 100%, respectively. (c) A summary of the results obtained from experiments performed in a similar manner as in (a, b). The bottom and top of each column indicate [Ca2+]i and tension levels just before and at the peak of CCh-induced contraction. The data are expressed as the means±s.e.m. (shown as vertical bars) (n=8). *P<0.01; n.s., difference not significant (statistical significance was determined by using unpaired t-test).
Effects of theophylline on the [Ca2+]i–force relationships
Figure 5a shows representative recordings of the control (without theophylline) [Ca2+]i–force relationship of contractions during membrane depolarization with 40 mM K+ PSS. After the responses (100%) to 40 mM K+ depolarization had been recorded, the strips were incubated in Ca2+-free PSS containing 2 mM EGTA for 10 min, followed by a 5 min exposure to Ca2+-free PSS without EGTA. The solution was then changed to a Ca2+-free 40 mM K+-depolarization solution, and extracellular Ca2+ (0–5 mM) was cumulatively applied. Both [Ca2+]i and force increased stepwise with elevations of extracellular Ca2+ concentration. Figure 5b shows the representative recordings of responses to the cumulative application of external Ca2+ during 40 mM K+ depolarization in the presence of 300 μM theophylline. In Figure 5c, the [Ca2+]i–force relationships were plotted with data points obtained from the experiments carried out in a similar manner as described in Figure 5a and b. Theophylline significantly shifted the [Ca2+]i–force relationships to the right (P<0.05), indicating that theophylline induces weaker tension development for given levels of [Ca2+]i.
Effects of theophylline on the contraction of the α-toxin permeabilized strips
To confirm the inhibitory effects of theophylline on Ca2+ responsiveness, we applied theophylline to the α-toxin permeabilized strips of porcine tracheal smooth muscle. Figures 6a and b show representative recordings of the Ca2+-induced contraction without (a) or with (b) 300 μM theophylline. The resting tension in the relaxing solution and the maximal tension induced by 10 μM Ca2+ were taken as 0 and 100%, respectively. In primary experiments, we determined that 300 μM theophylline did not affect the contraction induced by 10 μM Ca2+ (data not shown). Figure 6c summarizes the Ca2+–force relationship obtained from experiments carried out in a similar manner as in Figure 6a and b. In the presence of 300 μM theophylline, the EC50 value of [Ca2+]i for contraction was significantly increased from 0.97±0.22 (n=5) to 1.43±0.28 μM (n=5, P<0.05).
Effects of theophylline on cyclic nucleotide-induced relaxation of α-toxin permeabilized strips
Figures 7a and b show representative recordings of the effects of cAMP and cGMP on 500 nM Ca2+-induced contraction of α-toxin permeabilized strips with or without 100 μM theophylline, respectively. In the presence of 100 μM theophylline, 1 μM cAMP significantly accelerated relaxation compared with the application of 1 μM cAMP alone. On the other hand, 100 μM theophylline did not accelerate the relaxation caused by 100 nM cGMP. The concentrations of cAMP (1 μM) and cGMP (100 nM) were chosen to induce similar extents of relaxation of the 500 nM Ca2+-induced contraction. Figures 7c and d summarize the effect of 1 μM cAMP and 100 nM cGMP on the 500 nM Ca2+-induced contraction of α-toxin permeabilized strips with or without 100 μM theophylline, respectively.
Discussion
In this study, using simultaneous measurements of [Ca2+]i and tension development as well as α-toxin permeabilized preparations, we determined that the major mechanisms for theophylline-induced relaxation of the porcine tracheal smooth muscle are: (1) activation of BK channels and (2) attenuation of Ca2+ sensitivity, presumably through the action of cAMP. In addition, the data also demonstrate that theophylline minimally effects the intracellular Ca2+ release or cGMP-mediated pathways.
Smooth muscle tone is regulated not only by [Ca2+]i levels but also by the Ca2+ sensitivity of the contractile apparatus (Somlyo et al., 1999). In order to evaluate the effects of a drug on Ca2+ mobilization and Ca2+ sensitivity, it is thus necessary to measure both [Ca2+]i and the tension of intact smooth muscle strips in addition to measuring the tension of receptor-coupled permeabilized strips. In this study, we demonstrate that theophylline induces a concentration-dependent reduction in the [Ca2+]i and tension induced by CCh or 40 mM K+ depolarization (Figure 1). Importantly, the theophylline-induced reduction in [Ca2+]i and tension induced by 40 mM K+ were much weaker than those induced by CCh. To investigate this difference, we applied 300 μM theophylline to the contraction induced by CCh or 40 mM K+ depolarization, since this concentration of theophylline had submaximal effects on the increases in [Ca2+]i and tension induced by CCh. Theophylline (300 μM) induced significant relaxation without changing [Ca2+]i during the contraction induced by 40 mM K+ depolarization (Figure 2a). On the other hand, 300 μM theophylline significantly decreased both [Ca2+]i level and tension induced by CCh (Figure 2b). As we reported previously, CCh induced a greater contraction than K+ depolarization at similar levels of [Ca2+]i, persuasive evidence for CCh-induced Ca2+ sensitization (Yoshimura et al., 1995). We thus speculated that theophylline decreases [Ca2+]i during CCh-induced contraction through its action on K+ channels, because the reduction of [Ca2+]i could be observed only in the presence of a physiological concentration of K+. We also directly examined the possibility that theophylline decreases Ca2+ sensitivity, since it induced relaxation without decreasing [Ca2+]i levels during 40 mM K+ depolarization-induced contraction. We designed experiments to confirm these speculations.
Experiments were made to identify the K+ channels responsible for theophylline-induced reductions of [Ca2+]i. Application of IbTX, a selective inhibitor of BK channels, significantly inhibited the effects of theophylline on the increases in [Ca2+]i and tension induced by CCh (Figure 3). In agreement with this finding, BK channel blockade has also been associated with the inhibitory effects of aminophylline, a complex of theophylline and ethylenediamine (Jones et al., 1990). Our results suggest that theophylline induces hyperpolarization and relaxation of the porcine tracheal smooth muscle as a result of opening BK channels. Since theophylline is a known nonselective PDE inhibitor, it also likely to elevate intracellular levels of cAMP and/or cGMP. Others have already reported that BK channels are activated via cAMP- (Kume et al., 1989; 1994) and cGMP- (Robertson et al., 1993; Stockand & Sansom, 1996) dependent protein kinases (PKA and PKG, respectively) pathways. Ca2+-activated K+ channels are subdivided into three families: small-, intermediate- and large-conductance Ca2+-activated K+ channels. Methylxanthines, including theophylline, activate the human, intermediate-conductance and Ca2+-activated K+ channels, and directly interact with the protein of the channel (Schröder et al., 2000). However, methylxanthines did not directly effect BK or small-conductance Ca2+-activated K+ channels, demonstrating that the effects were not secondary to a rise in intracellular Ca2+. Our study does not determine whether theophylline directly activates BK channels. It has been reported that BK channel blockers attenuated the relaxant effects of salbutamol, isoprenaline, dibutyryl cAMP and sodium nitroprusside on CCh-contracted tracheal smooth muscle (Jones et al., 1990; 1993). These results support the notion that the elevation of cAMP and/or cGMP induced by theophylline activated PKA and/or PKG pathways, which subsequently open BK channels and lead to relaxation of the CCh-contracted tracheal smooth muscle.
Caffeine, a methylxanthine, induces intracellular Ca2+ release in smooth muscle. However, theophylline, which is also methylxanthine, did not induce intracellular Ca2+ release (Figure 4b). We also examined whether theophylline affects CCh-induced Ca2+ release from the sarcoplasmic reticulum, since isoprenaline is known to attenuate agonist-induced intracellular Ca2+ release in vascular smooth muscle (Ushio-Fukai et al., 1992). As shown in Figure 4, theophylline had no significant effect on Ca2+ release by CCh. The theophylline-induced inhibition of contraction induced by CCh in the absence of extracellular Ca2+ could be explained by theophylline-induced decreases in Ca2+ sensitivity, as discussed below. It was thus concluded that theophylline has little effect on the intracellular Ca2+ release mechanism.
We next examined the effects of theophylline on Ca2+ sensitivity in both intact and permeabilized preparations. As shown in Figure 5, theophylline significantly shifted the [Ca2+]i–force curve to the right in intact strips. This observation was further confirmed in α-toxin permeabilized strips. Theophylline significantly increased the Ca2+ EC50 concentration and shifted the pCa–tension curve to the right (Figure 6). These results were consistent with previous findings that cAMP and/or cGMP attenuates Ca2+ sensitivity in smooth muscle (Nishimura & van Breemen, 1989; Jones et al., 1999). From these results, we concluded that theophylline decreased the Ca2+ sensitivity of the contractile apparatus of the porcine tracheal smooth muscle, presumably through the action of cAMP and/or cGMP.
The effects of theophylline on tracheal smooth muscles are probably due to its inhibitory effect on PDE, leading to increase in intracellular cAMP and/or cGMP levels. We explored the relative importance of cAMP and cGMP in theophylline-induced decreases in Ca2+ sensitivity. As shown in Figure 7, theophylline preferentially enhanced the relaxant effect of cAMP, while it did not enhance the relaxant effect of cGMP. This finding suggests that the theophylline-induced decrease in Ca2+ sensitivity is mainly mediated by the cAMP-PKA pathway in tracheal smooth muscle. PDE is now acknowledged to represent the activity of a large superfamily of enzymes comprised of at least 11 distinct isoenzymes. In airways, PDE4 appears to be the most important isoenzyme, based on its distribution in the airway smooth muscle and inflammatory cells (Schmidt et al., 1999). This isoform (PDE4) degrades cAMP and thus represents an attractive rationale for the observation that theophylline accelerates relaxation by cAMP and not cGMP.
Theophylline is frequently used as a bronchodilator, and its ability to control chronic asthma is disproportionately greater than is explainable by its relatively small degree of bronchodilator activity (Schmidt et al., 1999). One explanation for this discrepancy is that theophylline has any other beneficial effects on asthmatics: anti-inflammatory action and improvement in function of respiratory muscle tone (Jenne, 1995; Weinberger & Hendeled, 1996; Danialou et al., 1998).
In summary, this study describes some of the mechanisms that underlie the direct effects of theophylline on tracheal smooth muscle relaxation. We determine that the major mechanisms for theophylline-induced relaxation of the porcine tracheal smooth muscle include the activation of BK channels as well as the attenuation of Ca2+ sensitivity presumably through the actions of cAMP.
Acknowledgments
We thank Dr I. Laher (UBC, Vancouver) for comments and help with the manuscript. This study was supported in part by Grants-in-Aid for Scientific Research (Nos. 13470149, 14657174, 14570675, 15590758) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, by the Research Grant for Cardiovascular Diseases (13C-4) from the Ministry of Health, Labour and Welfare, Japan, and by grants from the Japan Space Forum and the Naito Foundation.
Abbreviations
- BK channel
large-conductance Ca2+-activated K+ channel
- [Ca2+]i
intracellular Ca2+ concentration
- CCh
carbachol
- CSS
cytosolic substitution solution
- DMEM
Dulbecco's modified Eagle's medium
- EGTA
ethylene glycol-bis(beta-aminoethyl ether) N,N,N′,N-tetraacetic acid
- fura-2/AM
fura-2 acetoxymethyl ester
- IbTX
iberiotoxin
- PDE
phosphodiesterase
- PKA
cAMP-dependent protein kinase
- PKG
cGMP-dependent protein kinase
- PSS
physiological salt solution
References
- BÜLBRING E., TOMITA T. Catecholamine action on smooth muscle. Pharmacol. Rev. 1987;39:49–96. [PubMed] [Google Scholar]
- DANIALOU G., VICAUT E., AUBIER M., BOCZKOWSKI J. Theophylline dilates rat diaphragm arterioles via the prostaglandins pathway. Br. J. Pharmacol. 1998;124:1355–1362. doi: 10.1038/sj.bjp.0701962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRANO K., KANAIDE H., ABE S., NAKAMURA M. Effects of diltiazem on calcium concentrations in the cytosol and on force of contractions in porcine coronary arterial strips. Br. J. Pharmacol. 1990;101:273–280. doi: 10.1111/j.1476-5381.1990.tb12700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENNE J.W. Two new roles for theophylline in the asthmatic. J. Asthma. 1995;32:89–95. doi: 10.3109/02770909509083229. [DOI] [PubMed] [Google Scholar]
- JONES K.A., WONG G.Y., JANKOWSKI C.J., AKAO M., WARNER D.O. cGMP modulation of Ca2+ sensitivity in airway smooth muscle. Am. J. Physiol. 1999;276:L35–L40. doi: 10.1152/ajplung.1999.276.1.L35. [DOI] [PubMed] [Google Scholar]
- JONES T.R., CHARETTE L., GARCIA M.L., KACZOROWSKI G.J. Selective inhibition of relaxation of guinea-pig trachea by charybdotoxin, a potent Ca++-activated K+ channel inhibitor. J. Pharmacol. Exp. Ther. 1990;255:697–706. [PubMed] [Google Scholar]
- JONES T.R., CHARETTE L., GARCIA M.L., KACZOROWSKI G.J. Interaction of iberiotoxin witadrenoreceptor agonists and sodium nitroprusside on guinea pig trachea. J. Appl. Physiol. 1993;74:1879–1884. doi: 10.1152/jappl.1993.74.4.1879. [DOI] [PubMed] [Google Scholar]
- KAI T., NISHIMURA J., KOBAYASHI S., TAKAHASHI S., YOSHITAKE J., KANAIDE H. Effects of lidocaine on intracellular Ca2+ and tension in airway smooth muscle. Anesthesiology. 1993;78:954–965. doi: 10.1097/00000542-199305000-00021. [DOI] [PubMed] [Google Scholar]
- KAI T., TAKAHASHI S., KANAIDE H. Halothane counteracts acetylcholine-induced increase in Ca2+ sensitivity of the contractile apparatus in airway smooth muscle. Eur. J. Pharmacol. 1996;315:313–318. doi: 10.1016/s0014-2999(96)00617-6. [DOI] [PubMed] [Google Scholar]
- KANAIDE H.Measurement of [Ca2+]i in smooth muscle strips using front-surface fluorimetry Methods in Molecular Biology 1999Totowa, NJ: Humana Press Inc.269–277.ed. Lambert, D.G. pp [DOI] [PubMed] [Google Scholar]
- KUME H., HALL I.P., WASHABAU R.J., TAKAGI K., KOTLIKOFF M.I. β-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J. Clin. Investig. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUME H., TAKAI A., TOKUNO H., TOMITA T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989;341:152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- MUELLER E., VAN BREEMEN C. Role of intracellular Ca2+ sequestration in beta-adrenergic relaxation of a smooth muscle. Nature. 1979;281:682–683. doi: 10.1038/281682a0. [DOI] [PubMed] [Google Scholar]
- NISHIMURA J., KOLBER M., VAN BREEMEN C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem. Biophys. Res. Commun. 1988;157:677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- NISHIMURA J., VAN BREEMEN C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem. Biophys. Res. Commun. 1989;163:929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- ROBERTSON B.E., SCHUBERT R., HESCHELER J., NELSON M.T. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am. J. Physiol. 1993;265:C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- SADOSHIMA J., AKAIKE N., KANAIDE H., NAKAMURA N. Cyclic AMP modulates Ca-activated K channel in cultured smooth muscle cells of rat aortas. Am. J. Physiol. 1988;255:H754–H759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- SCHMIDT D., DENT G., REBE K.F. Selective phosphodiesterase inhibitors for the treatment of bronchial asthma and chronic obstructive pulmonary disease. Clin. Exp. Allergy. 1999;29 Suppl 2:99–109. doi: 10.1046/j.1365-2222.1999.00018.x. [DOI] [PubMed] [Google Scholar]
- SCHRÖDER R.L., JENSEN B.S., STROBAEK D., OLESEN S.P., CHRISTOPHERSEN P. Activation of the human, intermediate-conductance, Ca2+-activated K+-channel by methylxanthines. Pflugers Arch. 2000;440:809–818. doi: 10.1007/s004240000364. [DOI] [PubMed] [Google Scholar]
- SETOGUCHI H., NISHIMURA J., HIRANO K., TAKAHASHI S., KANAIDE H. Leukotrien C4 enhances the contraction of porcine tracheal smooth muscle through the activation of Y-27632, a rho kinase inhibitor, sensitive pathway. Br. J. Pharmacol. 2001;132:111–118. doi: 10.1038/sj.bjp.0703780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMLYO A.P., WU X., WALKER L.A., SOMLYO A.V. Pharmacomechanichal coupling: the role of calcium, G-proteins, kinases and phosphatases. Rev. Physiol. Biochem. Pharmacol. 1999;134:201–234. doi: 10.1007/3-540-64753-8_5. [DOI] [PubMed] [Google Scholar]
- STOCKAND J.D., SANSOM S.C. Mechanism of activation by cGMP-dependent protein kinase of large Ca2+-activated K+ channels in mesangial cells. Am. J. Physiol. 1996;271:C1669–C1677. doi: 10.1152/ajpcell.1996.271.5.C1669. [DOI] [PubMed] [Google Scholar]
- USHIO-FUKAI M., ABE S., KOBAYASHI S., NISHIMURA J., KANAIDE H. Effect of isoprenaline on cytosolic calcium concentrations and on tension in porcine coronary artery. J. Physiol. (Lond.) 1993;462:679–696. doi: 10.1113/jphysiol.1993.sp019576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USHIO-FUKAI M., NISHIMURA J., AOKI H., KOBAYASHI S., KANAIDE H. Endothelin-1 inhibits and enhances contraction of porcine coronary arterial strips with an intact endothelium. Biochem. Biophys. Res. Commun. 1992;184:518–524. doi: 10.1016/0006-291x(92)91225-f. [DOI] [PubMed] [Google Scholar]
- WEINBERGER M., HENDELED L. Theophylline in asthma. N. Engl. J. Med. 1996;334:1380–1388. doi: 10.1056/NEJM199605233342107. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA H., KAI T., NISHIMURA J., KOBAYASHI S., TAKAHASHI S., KANAIDE H. Effects of midazolam on intracellular Ca2+ and tension in airway smooth muscles. Anesthesiology. 1995;83:1009–1020. doi: 10.1097/00000542-199511000-00015. [DOI] [PubMed] [Google Scholar]