Abstract
The pharmacological profile of a novel glutamate transport inhibitor, WAY-855 (3-amino-tricyclo[2.2.1.02.6]heptane-1,3-dicarboxylic acid), on the activity of the human forebrain glutamate transporters EAAT1, EAAT2 and EAAT3 expressed in stable mammalian cell lines and in Xenopus laevis oocytes is presented.
WAY-855 inhibited glutamate uptake mediated by all three subtypes in a concentration-dependent manner, with preferential inhibition of the CNS-predominant EAAT2 subtype in both cells and oocytes. IC50 values for EAAT2 and EAAT3 inhibition in cells were 2.2 and 24.5 μM, respectively, while EAAT1 activity was inhibited by 50% at 100 μM (IC50 values determined in oocytes were 1.3 μM (EAAT2), 52.5 μM (EAAT3) and 125.9 μM (EAAT1)).
Application of WAY-855 to EAAT-expressing oocytes failed to induce a transporter current, and the compound failed to exchange with accumulated [3H]D-aspartate in synaptosomes consistent with a nonsubstrate inhibitor. WAY-855 inhibited D-aspartate uptake into cortical synaptosomes by a competitive mechanism, and with similar potency to that observed for the cloned EAAT2.
WAY-855 failed to agonise or antagonise ionotropic glutamate receptors in cultured hippocampal neurones, or the human metabotropic glutamate receptor subtype 4 expressed in a stable cell line.
WAY-855 represents a novel structure in glutamate transporter pharmacology, and exploration of this structure might provide insights into the discrimination between EAAT2 and other EAAT subtypes.
Keywords: Excitatory aminoacid transporter, EAAT, WAY-855, glutamate transporter, inhibitor
Introduction
Glutamate is the predominant excitatory neurotransmitter in the mammalian central nervous system. Targets for synaptically released glutamate are the ligand-gated ion channel receptors, termed the ionotropic receptors (Barnard, 1997; Ziff, 1999), and the seven transmembrane domain G-protein-coupled receptors, termed metabotropic glutamate receptors (Conn & Pin, 1997; Schoepp et al., 1999). Activation of these receptors is responsible for the physiological actions of glutamate; however, overstimulation of the ionotropic receptors contributes to much of the excitotoxic actions attributed to glutamate. Consequently, synaptic glutamate levels are regulated in order to maintain the integrity of synaptic transmission and to limit or prevent the pathophysiological activity of this excitatory neurotransmitter.
A family of high-affinity Na+-dependent glutamate transporters expressed in the plasma membranes of both neurones and astroglia is believed to be responsible for the clearance of extracellular glutamate by mediating the cellular uptake of glutamate in a process driven largely by the energy of the transmembrane Na+ gradient (reviewed in Danbolt, 2001). Five members of this transporter family have been identified by molecular cloning and are designated EAAT1/GLAST, EAAT2/GLT-1, EAAT3/EAAC1, EAAT4 and EAAT5 (EAAT: excitatory aminoacid transporter; GLAST: glutamate/aspartate transporter; GLT-1: glutamate transporter 1; EAAC1: excitatory aminoacid carrier 1) (Kanai & Hediger, 1992; Pines et al., 1992; Storck et al., 1992; Arriza et al., 1994; 1997; Fairman et al., 1995). The designation EAAT adopted for the human clones reflects the fact that, in addition to glutamate, a number of other endogenous excitatory amino acids serve as substrates for these plasma membrane carriers. GLAST, GLT-1 and EAAC1 represent nonhuman homologs, which were cloned prior to the isolation of their human counterparts.
Although five transporter subtypes have been identified, it has been recognised that the EAAT2 subtype is the predominant transporter expressed in forebrain and accounts for the bulk of the transport activity measured in forebrain preparations, a proposal supported by several independent lines of evidence. Transgenic mice deficient in EAAT2 lose over 90% of the glutamate uptake measured in forebrain preparations, and exhibit increased vulnerability to an experimental head trauma insult (Tanaka et al., 1997). Consistent with these observations, chronic administration of antisense oligonucleotides, to knock down levels of EAAT2, results in greatly diminished glutamate transport capacity, elevated extracellular glutamate levels and increased neurodegeneration in the treated animals (Rothstein et al., 1996). Finally, the pharmacological profile of glutamate transport measured in forebrain preparations is well correlated with the pharmacology that has been described for both the rat GLT-1 and human EAAT2 subtypes expressed in mammalian cell lines (Dunlop et al., 1999a; Tan et al., 1999), in particular with respect to inhibition by the selective EAAT2 blockers dihydrokainate and kainate.
The availability of pharmacological tools for the discrimination of EAAT subtypes is important for a number of reasons including the determination of the physiological roles of individual subtypes, the characterisation of biological systems in which EAAT activity is known to be expressed and the generation of pharmacaphore models of transporter binding sites. However, there are few agents known that can be used to discriminate between different EAAT subtypes (reviewed in Bridges et al., 1999), with dihydrokainate, a relatively selective inhibitor of EAAT2, being a notable exception. We have been primarily focused on the identification of molecules that can enhance the transport activity of the EAAT2 subtype, on the basis that such molecules may have neuroprotective activity in both acute and chronic neurodegenerative diseases (Dunlop et al., 1999b). During the course of these efforts we have identified several EAAT2 uptake inhibitors and in this study we present a characterisation of WAY-855 (3-amino-tricyclo[2.2.1.02.6]heptane-1,3-dicarboxylic acid), a novel nonsubstrate transport inhibitor exhibiting preferential inhibition of the EAAT2 subtype.
Materials and methods
Chemicals and reagents
Madin – Darby canine kidney (MDCK) cells expressing EAAT2 were from Dr Susan Amara (Vollum Institute, Portland, OR, U.S.A.) as were the cDNAs for the human EAAT1 and EAAT3 subtypes which were used for the generation of stable MDCK cell lines. L-[3H]glutamate and D-[3H]aspartate were obtained from Amersham (Arlington Heights, IL, U.S.A.) and cell culture reagents were from Life Technologies (Rockville, MD, U.S.A.). Amino acids L-glutamate and D-aspartate were from Sigma (St Louis, MO, U.S.A.) and threo-3-methylglutamate was from Tocris Cookson (Ballwin, MO, U.S.A.).
Uptake in stable cell lines
Stable MDCK cell lines expressing each of the glutamate transporter subtypes EAAT1 – 3 were maintained in T175 flasks at 37°C in a humidified atmosphere with 5% CO2. Cells were detached by trypsinisation and replated at 20,000 cells well−1 in 96-well culture plates the day prior to measurements of glutamate uptake. Uptake assays were performed in Dulbecco's phosphate-buffered saline (D-PBS) in the presence of 1 μM glutamate and 0.2 μCi ml−1 L-[3H]glutamate (specific activity, 50 Ci mmol−1) in a final volume of 100 μl. Such an assay format has been previously validated (Dunlop et al., 1999a; Tan et al., 1999) and the conditions used allowed for the evaluation of compounds in the 96-well format achieving medium throughput screening capacity. Cells were washed with D-PBS before incubation with substrate in the absence or presence of compounds for 20 min at room temperature. Assays were stopped by aspiration followed by two ice-cold D-PBS washes. Cells were solubilised with 0.5 N NaOH before addition of Microscint 20 for the determination of [3H] accumulation in the wells using a Packard TopCount. The uptake was linear for incubation times up to 30 min (data not shown); thus, data were analysed as true rates. Nonspecific uptake was corrected for by performing all experiments in the absence and presence of sodium (replacement of sodium chloride with choline chloride). Sodium-independent uptake accounted for <5% of total uptake and was subtracted prior to any further data calculation. IC50 determinations for inhibition of uptake in cell lines and synaptosomes (below) and inhibition of glutamate-induced currents in oocytes (below) were performed by nonlinear regression analysis using KaleidaGraph.
Glutamate-induced currents in oocytes
Electrophysiological assessment of glutamate uptake was performed by measuring glutamate-induced currents in Xenopus oocytes injected with 50 nl capped cRNA encoding each of the individual subtypes 2 – 5 days prior to assay. Assays were performed in ND-96 buffer (in mM: 96 NaCl, 5 NaHEPES, 2 KCl, 1.8 CaCl2, 1 MgCl2, pH 7.4) in the presence of 30 μM glutamate added alone or in the presence of varying concentrations of WAY-855. Recordings were made using standard two-electrode voltage clamp. Oocytes were voltage clamped to −70 mV and stepped to a range of potentials from −100 to +20 mV for 200 ms. Currents elicited by substrate were calculated as the difference between currents measured in the presence of glutamate and currents measured in its absence.
Uptake in rat cortical synaptosomes
P2 synaptosomal fractions isolated from rat cerebral cortex were prepared by homogenisation of tissue in ice cold isolation medium (310 mM sucrose, 10 mM HEPES, pH 7.4) followed by centrifugation at 1000 × g for 5 min. The resulting supernatant was collected and centrifuged at 20,000 × g for 20 min to obtain the crude synaptosomal P2 pellet, which was used for uptake studies at a protein concentration of 1 mg ml−1 in HEPES-buffered saline (HBS). D-[3H]aspartate uptake was assayed in a final volume of 300 μl HBS containing 50 μg synaptosomal protein, 1 μM D-aspartate and 0.25 μCi assay−1 D-[3H]aspartate (50 nM) in the absence and presence of drug for the determination of IC50 values. Kinetic experiments were performed in the presence of 0.25 μCi assay−1 D-[3H]aspartate (specific activity, 15 Ci mmol−1) over a range of cold D-aspartate concentrations in both the absence and presence of WAY-855. Uptake assays were incubated for 4 min at room temperature and were terminated by filtration on a Packard 96-well Unifilter (Whatman GF/B) plate followed by two washes with ice-cold buffer. Microscint 20 was added directly to filter plates for the determination of [3H] retained on the filters using a Packard TopCount. Uptake was determined to be linear up to 5 min incubation period and data were corrected to represent sodium-dependent uptake.
Synaptosomal exchange
Rat cortical P2 synaptosome fractions were resuspended in HBS at a protein concentration of 1 mg ml−1 and incubated in the presence of 1 μCi ml−1 D-[3H]aspartate for 1 h at room temperature with constant shaking. Synaptosome suspension (100 μl) equilibrated with [3H] label was diluted into 100 μl HBS containing compound at 2 × final concentration immediately after the 1 h loading period. Synaptosomes were incubated with drug for 5 min at room temperature followed by centrifugation to separate pellet and supernatant. An aliquot of the supernatant was subsequently removed for the determination of [3H] efflux, a direct index of drug-mediated exchange.
Selectivity studies
Primary cultures of hippocampal neurones were used for the evaluation of ionotropic glutamate receptor effects of WAY-855. Briefly, rapid application (200 ms) of drug, achieved by picospritzer, was used to examine the activation of a mixed ionotropic glutamate receptor current. L-Glutamate (1 mM) application was utilised as a positive control. In order to examine the effect of WAY-855 on a representative of the metabotropic glutamate receptor (mGluR) family, a [35S]GTPγS binding assay with the cloned human mGluR4 subtype expressed in Chinese hamster ovary (CHO) cells was used (Kowal et al., 1998). Membranes prepared from CHO/hmGluR4 cells were prepared in assay buffer (20 mM HEPES, 100 mM NaCl, 10 mM MgCl2, pH 7.4) at a final protein concentration of 250 μg ml−1. WAY-855 (1 – 100 μM) in the presence or absence of 40 μM L-glutamate was preincubated with 10 μM GDP and 25 μg membrane preparation for 20 min at room temperature followed by 15 min on ice. Subsequently, [35S]GTPγS (1250 Ci mmol−1) was added to each assay tube (0.1 nM final) and incubated for a further 30 min at 30°C, before assay termination by addition of 3 ml ice cold buffer followed by filtration using Whatman GF/B filter paper. Filters were washed × 3 with ice cold buffer and radioactivity retained by the filters was determined by liquid scintillation counting.
Data analysis
For graphical display, data in the uptake inhibition studies were converted to % control defined as the uptake of [3H]-transmitter observed in the absence of drug. Similarly, in the oocyte experiments, data were converted to % control defined as the glutamate-induced current measured in the absence of added drug. The pIC50 values were defined as the midpoint between the maximum and minimum of each individual concentration – response curve of a nonlinear regression analysis (Kaleidagraph).
Statistical analysis
Analysis of variance (ANOVA) was performed on overall data followed by individual comparisons with control according to the method of Dunnett.
Results
WAY-855 (Figure 1) was initially identified from a screening effort focused on the identification of positive modulators of the EAAT2 subtype, and was observed to have dramatic inhibitory activity on this subtype. Subsequently, the inhibitory activity of WAY-855 on the forebrain glutamate transporters EAAT1, EAAT2 and EAAT3 was examined in addition to its interaction with the uptake system in rat cortical synaptosomes.
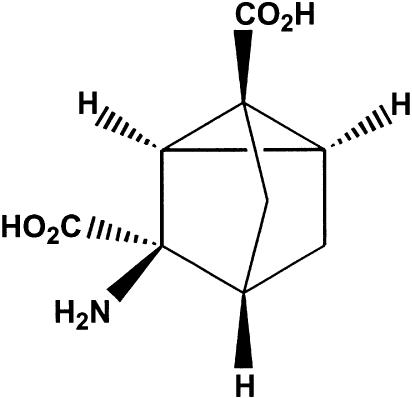
Figure 1.
Chemical structure of WAY-855 (3-amino-tricyclo[2.2.1.02.6] heptane-1,3-dicarboxylic acid).
Inhibition of cloned transporter activity
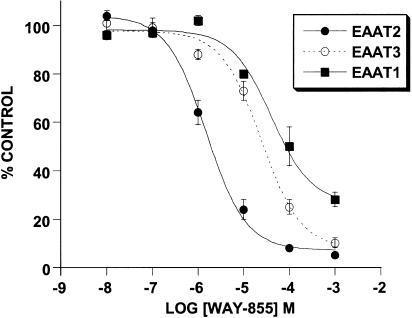
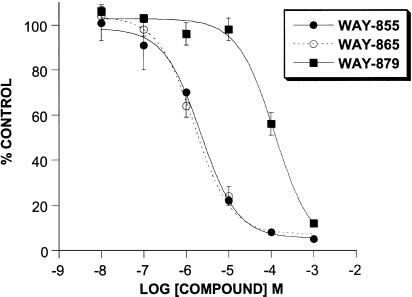
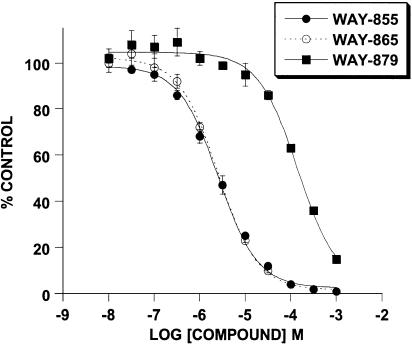
The inhibitory effect of WAY-855 on glutamate uptake mediated by the cloned transporters expressed in MDCK cells was examined using a six-point concentration response ranging from 0.01 to 1000 μM. WAY-855 produced a concentration-dependent inhibition of all three subtypes (Figure 2), and pIC50 values estimated from the log-concentration – response curves for EAAT2 and EAAT3 were 5.66±0.06 (2.2 μM) and 4.61±0.11 (24.5 μM), respectively, indicating an 11-fold selectivity for the EAAT2 subtype over EAAT3. Maximal inhibition of the EAAT1 subtype was not obtained at the highest concentration tested; however, 50% inhibition of EAAT1 was observed at 100 μM WAY-855 allowing for the estimation of selectivity for EAAT2 over EAAT1 as 45-fold. Preparation of the optical isomers of WAY-855 was achieved by subjecting suitably derivatised spiro-hydantoin precursors to chiral chromatography (Whelk-O column; 2 × 25 cm, ethanol : hexane 1 : 1, flow rate 20 ml min−1) (Greenfield, unpublished). Absolute stereochemistry for the resolved enantiomers could not be assigned due to the lack of a chiral synthesis or crystal structure. Evaluation of the HPLC-resolved enantiomers for EAAT2 inhibitory activity is illustrated in Figure 3, indicating that the inhibitory activity was strongly stereoselective for one of the enantiomers (designated WAY-865). Estimated pIC50 values for WAY-865 and WAY-879 were 5.81±0.06 (1.5 μM) and 3.92±0.11 (120 μM) indicating that the active enantiomer was 80-fold more potent than its distomer.
Figure 2.
Concentration-dependent inhibition of glutamate uptake mediated by the cloned human glutamate transporters EAAT1 – 3 expressed in MDCK cells. Glutamate uptake into cells expressing each of the transporter subtypes was examined in the absence and presence of increasing concentrations of WAY-855. Data are expressed as % control uptake observed in the absence of drug and represent mean values±s.e.m. from three independent experiments.
Figure 3.
Inhibition of EAAT2-mediated glutamate uptake is stereoselective in favour of the WAY-865 enantiomer. Glutamate uptake into EAAT2-expressing cells was evaluated in the absence and presence of WAY-855 and its resolved enantiomers. Data are expressed as % control uptake observed in the absence of drug and represent mean values±s.e.m. from three independent experiments.
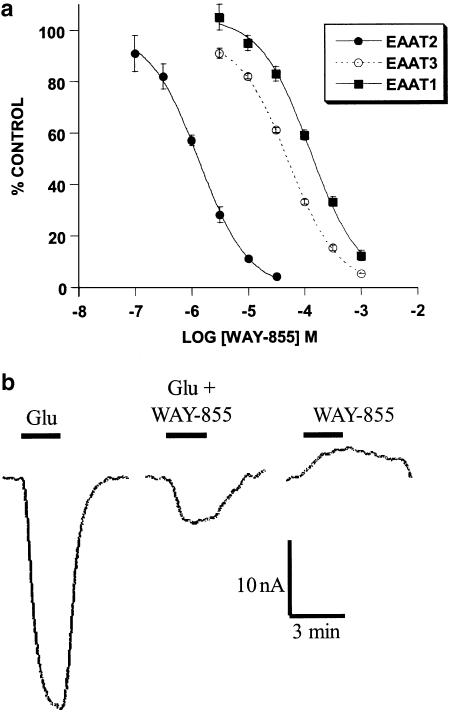
Further characterisation of the effect of WAY-855 on glutamate-induced currents in oocytes expressing each of the subtypes EAAT1 – 3 produced essentially similar results as obtained for the inhibition of glutamate uptake in stable cell lines. Inward currents generated by the application of 30 μM glutamate were measured in the absence and presence of WAY-855 tested over the concentration range 0.1 – 1000 μM. Log-concentration – response curves for the inhibition of glutamate-induced currents are depicted in Figure 4a and, the pIC50 values were estimated as 5.87±0.04 (1.3 μM) for EAAT2, 4.28±0.02 (52.5 μM) for EAAT3 and 3.90±0.06 (125.9 μM) for EAAT1. Moreover, the application of WAY-855 to EAAT2-injected oocytes failed to produce an inward current although the compound blocked the inward current generated by glutamate (Figure 4b), an observation consistent with WAY-855 acting as a nonsubstrate EAAT2 inhibitor. In contrast, application of the substrate inhibitor L-trans-2,4-pyrollidine dicarboxylate (L-trans-2,4-PDC) generated an inward current (not shown) comparable to results described by others (Arriza et al., 1994). The small outward current observed following application of WAY-855 is attributable to the block of a leak conductance observed in the absence of substrate. Similar outward currents elicited by the nonsubstrate EAAT2 inhibitor dihydrokainate have been described by others (Bergles & Jahr, 1997; Otis & Jahr, 1998). WAY-855 also failed to generate currents in EAAT1- and EAAT3-injected oocytes (data not shown).
Figure 4.
WAY-855 inhibits glutamate-induced currents in oocytes expressing each of the cloned human glutamate transporters EAAT1 – 3 in a concentration-dependent manner (a) and acts as a nonsubstrate inhibitor of EAAT2 (b). (a) Currents induced by application of 30 μM glutamate to EAAT-expressing oocytes were measured in the absence and presence of increasing concentrations of WAY-855. Data are expressed as % control current observed in the absence of drug and represent mean values±s.e.m. from three independent experiments. (b) A 2 min application of 30 μM glutamate to EAAT2-injected oocytes induces an inward current. WAY-855 (10 μM) blocks the glutamate-induced current but fails to generate an inward current when applied on its own.
Inhibition of transport into rat cortical synaptosomes
Excitatory amino acid (EAA) uptake into synaptosomes prepared from rat cortex exhibits a pharmacological profile resembling that of the cloned EAAT2 subtype and fits with a one-site interaction consistent with the predominant contribution of EAAT2 to the uptake signal. Using D-[3H]aspartate as a substrate, the inhibitory activity of WAY-855 and the enantiomers was evaluated (Figure 5). Consistent with the correlation between synaptosomal and EAAT2-mediated uptake, there was excellent agreement between the inhibitory potencies determined using rat cortical synaptosomes and those described above for the cloned EAAT2 subtype. The estimated pIC50 values for the inhibition of D-[3H]aspartate uptake into cortical synaptosomes were 5.59±0.04 (2.6 μM) for WAY-855, 5.59±0.02 (2.6 μM) for WAY-865 and 3.85±0.06 (141.3 μM) for WAY-879.
Figure 5.
Concentration-dependent inhibition of D-aspartate uptake into P2 synaptosome fractions prepared from rat cortex by WAY-855 and the enantiomers WAY-865 and WAY-879. Synaptosomal D-aspartate uptake was measured in the presence and absence of increasing concentrations of compounds, and data are expressed as % control uptake. Mean values±s.e.m. from three independent experiments are presented.
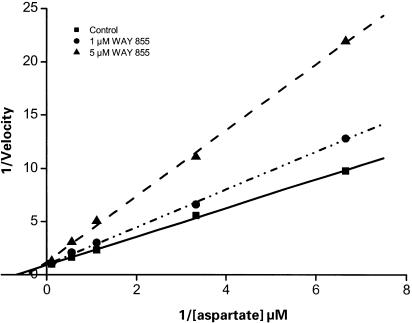
The synaptosomal preparation was also used to examine the effect of WAY-855 on uptake kinetics. Lineweaver – Burk transformations of saturable D-[3H]aspartate uptake data obtained in the absence and presence of WAY-855 (Figure 6) indicate that the compound interacts with the EAA uptake system in a competitive manner, producing a decrease in the apparent affinity for glutamate (Km) with no change in maximal transport capacity (Vmax). The calculated Ki values of 0.2 and 0.5 μM determined in the presence of 1 and 5 μM WAY-855, respectively, are in good agreement with its inhibitory IC50 reported above. Km and Vmax determinations were derived from the Lineweaver – Burke transformations using linear regression analysis.
Figure 6.
WAY-855 interacts with the excitatory amino-acid uptake system in rat cortical synaptosomes in a competitive manner. Synaptosomal D-aspartate uptake was measured in the presence of increasing concentrations of D-aspartate substrate with or without the addition of WAY-855. Lineweaver – Burk transformations of the saturable uptake data obtained in the absence (solid line) and presence of 1 μM (dotted line) or 5 μM WAY-855 (dashed line) obtained in a representative experiment are depicted. The profile is consistent with a competitive mode of inhibition, and the Ki values estimated in the presence of 1 and 5 μM WAY-855 were 0.2 and 0.5 μM, respectively.
Synaptosomal exchange
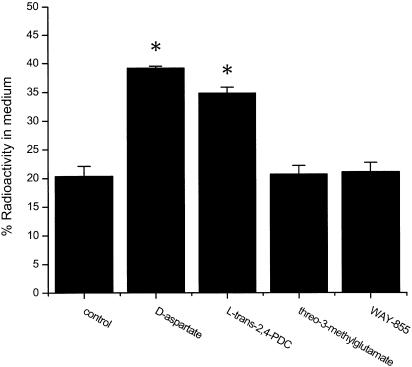
Our electrophysiological studies indicate that WAY-855 acts as a nonsubstrate transport blocker. A biochemical index that can be used for the determination of substrate versus nonsubstrate activity is the capacity for drug-mediated heteroexchange of accumulated D-[3H]aspartate substrate (Griffiths et al., 1994; Koch et al., 1999; Dunlop, 2001). Cortical synaptosomes were equilibrated with D-[3H]aspartate prior to the addition of exogenous unlabelled D-aspartate, WAY-855 or threo-3-methylglutamate (T3MG), a nonsubstrate inhibitor of EAAT2 (Vandenberg et al., 1997), to assess drug-mediated [3H] efflux. As illustrated, addition of exogenous D-aspartate to D-[3H]aspartate-loaded synaptosomes stimulated the efflux of [3H] label (Figure 7); however, both WAY-855 and T3MG failed to increase [3H] efflux over control levels consistent with these compounds behaving as nonsubstrate inhibitors.
Figure 7.
WAY-855 fails to promote exchange of previously accumulated D-[3H]aspartate from rat brain synaptosome fractions. Synaptosomes were pre-equilibrated with D-[3H]aspartate for 1 h prior to dilution into buffer with or without compounds at 2 × concentration for 5 min followed by centrifugation to separate pellet and supernatant. An aliquot of the supernatant was removed for the determination of [3H] efflux and the [3H] remaining in the pellet was determined. Data are expressed as % of radioactivity: radioactivity in supernatant/(radioactivity in supernatant+radioactivity in pellet) × 100%, and represent mean values±s.e.m. from three independent experiments. WAY-855 (100 μM) was compared with the substrates D-aspartate (30 μM) and L-trans-2,4-PDC (100 μM) and the nonsubstrate EAAT2 inhibitor threo-3-methylglutamate (300 μM). *P<0.01 compared to baseline control.
Selectivity
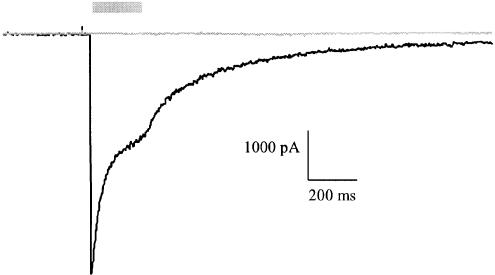
Rapid application by picospritzer (200 ms) of 1 mM WAY-855 to cultures of primary hippocampal neurones failed to activate a mixed glutamate receptor current or affect input resistance in four of four neurones tested (Figure 8). In contrast, in the same neurones, rapid application of 1 mM L-glutamate activated a large desensitising inward current. Similarly, bath application of WAY-855 (1 – 100 μM) did not activate a mixed glutamate receptor current, and also failed to block the current activated by bath application of 100 μM L-glutamate in three of three neurones tested (not shown). These observations are consistent with WAY-855 having no agonist or antagonist activity at ionotropic glutamate receptors.
Figure 8.
WAY-855 does not activate ionotropic glutamate receptors. Rapid application (200 ms) of 1 mM WAY-855 did not activate a mixed glutamate receptor current in cultured hippocampal neurones (grey trace). In contrast, 1 mM L-glutamate activated a large desensitising inward current (black trace). Representative profiles are presented. Grey bar indicates period of drug application.
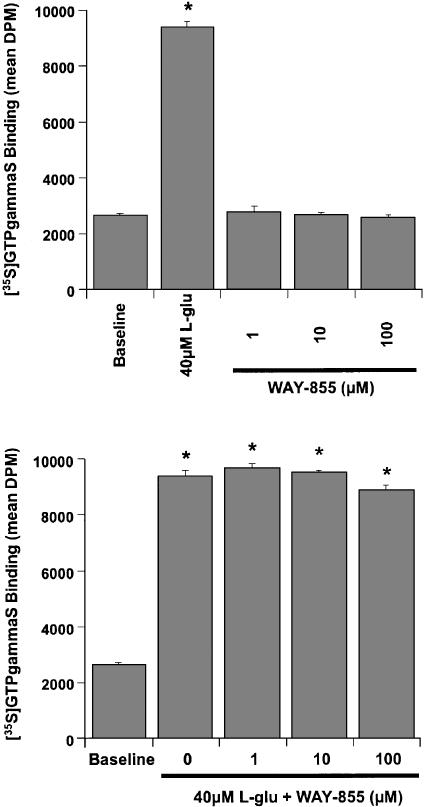
Similarly, WAY-855 failed to agonise or antagonise the cloned human mGluR4 receptor expressed in CHO cells. L-Glutamate (40 μM) stimulated [35S]GTPγS binding to the mGluR4 receptor subtype, while WAY-855 (1 – 100 μM) did not (Figure 9a). In addition, WAY-855 (1 – 100 μM) failed to antagonise the mGluR4 [35S]GTPγS binding stimulated by 40 μM L-glutamate (Figure 9b).
Figure 9.
WAY-855 is not an mGluR4 receptor agonist or antagonist. Membranes from CHO cells expressing the human mGluR4 receptor subtype were incubated in the presence of L-glutamate or WAY-855 (a). L-Glutamate stimulated [35S]GTPγS binding to the mGluR4 receptor whereas WAY-855 (1 – 100 μM) did not. (b) Membranes were incubated with 40 μM L-glutamate in the absence (0) and presence of WAY-855 (1 – 100 μM). WAY-855 failed to antagonise [35S]GTPγS binding to mGluR4 stimulated by 40 μM L-glutamate. Data represent mean±s.e.m (n=3). *P<0.001 compared to baseline control. No difference was observed in the magnitude of the L-glutamate-stimulated response in the presence of WAY-855.
Discussion
WAY-855, a conformationally restricted glutamate analogue, was identified during a screening effort aimed at identifying novel modulators of the predominant forebrain glutamate transporter EAAT2. The results presented in this study present a characterisation of WAY-855 as a novel nonsubstrate inhibitor of high-affinity Na+-dependent glutamate uptake exhibiting preferential inhibition of the predominant EAAT2 subtype. Additionally, although acting as a nonsubstrate EAAT2 inhibitor, WAY-855 interacts with the glutamate uptake system in a competitive manner as determined using cortical synaptosomes as a native preparation in which the EAAT2 subtype is predominantly expressed. This latter observation suggests that WAY-855 overlaps with the glutamate recognition site of the transporter and presumably, given the conformationally restrained nature of the molecule, presents a rigid glutamate-like conformation to the substrate-binding site and is unable to be translocated.
Characterisation of the inhibitory activity of WAY-855 was undertaken with the cloned human forebrain transporters EAAT1 – 3 and rat cortical synaptosomes, a native preparation that expresses predominantly the EAAT2 subtype. By using either glutamate uptake mediated by the cloned transporters in MDCK cells or glutamate-induced currents in EAAT-injected oocytes, it was determined that the transporter selectivity of WAY-855 was EAAT2>EAAT3>EAAT1. Results from the cell line uptake studies indicate selectivity for EAAT2 inhibition over EAAT3 and EAAT1 of 11- and 45-fold, respectively. A greater separation was observed in the measurement of inhibition of glutamate-induced currents in oocytes where the EAAT2 selectivity was 40- and 90-fold, respectively, over EAAT3 and EAAT1. This discrepancy between the measures of glutamate uptake and currents induced by glutamate transport might arise from differential effects of WAY-855 on transporter current components that are coupled or uncoupled to the inward translocation of substrate. Nevertheless, the results are in agreement that WAY-855 interacts preferentially with the EAAT2 subtype and represents a novel tool for studying transporter function.
The resolution of WAY-855 into the enantiomers WAY-865 and WAY-879 allowed for the determination that EAAT2 inhibition was strongly stereoselective in favour of the enantiomer designated as WAY-865. This separation was observed both for glutamate uptake mediated by the cloned EAAT2 subtype and in rat cortical synaptosomes with either an 80- or 54-fold separation, respectively, in the potency of WAY-865 compared with WAY-879 for the inhibition of uptake. Interestingly, the essentially superimposable nature of the inhibition curves observed for WAY-855 and enantiomers on the cloned EAAT2 transport activity and the uptake system in rat cortical synaptosomes provides further data in support of the idea that the transport observed in the synaptosomal preparation is attributable to EAAT2 (Tan et al., 1999).
Glutamate transporter pharmacology has recently been reviewed in some detail (Bridges et al., 1999) and it is important to note that WAY-855 represents a completely novel structure in the arena of glutamate transporter inhibitors. Compounds known to interact with glutamate transporters include simple glutamate and aspartate analogues incorporating sulphinic and sulphonic acid or methyl and phenyl group substitutions in a linear carbon backbone. Cyclic derivatives include the carboxycyclopropyl glycines (Nakamura et al., 1993), aminocyclobutane dicarboxylates (Fletcher et al., 1991) and the pyrrolidine dicarboxylates (Bridges et al., 1991) representing three-, four- and five-member ring structures, respectively. Generally, the simple linear analogues have been shown to discriminate poorly between transporter subtypes and have no particular value as pharmacological tools, with the exception of α-amino adipate, which inhibits both the EAAT4 subtype (Fairman et al., 1995) and the transport activity in cerebellar synaptosomes (Robinson et al., 1991) while having negligible activity on other EAAT targets. A more recent addition is the threo-hydroxyaspartate derivative threo-β-benzyloxyaspartate (TBOA) described originally as an EAAT2 inhibitor with some selectivity over EAAT1 – Ki values for the inhibition of EAAT1 and EAAT2 in COS-1 cells were 42 and 6 μM (Shimamoto et al., 1998) – and subsequently as a nonselective EAAT inhibitor (Shigeri et al., 2001).
A limited number of the cyclic analogues have been found to exhibit greater pharmacological specificity between EAAT subtypes. This is best illustrated by the pyrollidine dicarboxylates, dihydrokainate and kainate, which have been shown to act as selective inhibitors of the EAAT2 subtype (Arriza et al., 1994), and are essentially inactive at other transporter subtypes. Consequently, dihydrokainate has become the best marker available for the EAAT2 subtype, while kainate has been used to a lesser extent by virtue of its interaction with glutamate receptors. However, even dihydrokainate may interact to some extent with ionotropic receptors (Wang et al., 1998). Similarly, the analogue L-trans-2,3-pyrrolidine dicarboxylate (L-trans-2,3-PDC) has been shown to be selective for EAAT2 (Ki∼10 μM) over EAAT1 and EAAT3, which were largely unaffected by the compound (Koch et al., 1999). However, as with kainate (and dihydrokainate), L-trans-2,3-PDC interacts with glutamate receptors, which may confound its use in native preparations.
WAY-855 incorporates the glutamate backbone in a six-member heptane dicarboxylate ring structure resulting in the presentation of a restricted conformation of the amino acid, as is the case for the pyrrolidine dicarboxylate inhibitors. Pharmacologically, potent EAAT2 inhibition is observed with WAY-855 and in this respect the compound is as potent as other known EAAT2 inhibitors. The nature of inhibition was found to be competitive although the compound was not translocated, an observation analogous to the pyrrolidine dicarboxylate inhibitor L-trans-2,3-PDC. The activity of such compounds, in particular WAY-855, as nonsubstrate inhibitors presumably is a consequence of the bulk conferred by the ring structure and presented to the substrate-binding site. In the case of the pyrollidine dicarboxylates, the picture is more complex since L-trans-2,4-pyrollidine dicarboxylate and L-anti-endo-2,4-methanopyrollidine dicarboxylate exhibit partial substrate activity while L-trans-2,3-PDC is not transported indicating that ring substitution also impacts the discrimination between substrate and nonsubstrate inhibitors. Although good selectivity was observed for WAY-855 with respect to the EAAT1 subtype, a smaller separation was observed for the EAAT3 subtype, although the compound did exhibit some selectivity for EAAT2: 11- and 40-fold for glutamate uptake and glutamate-induced currents, respectively. Exploration of WAY-855 analogues will be required to determine if greater separation between the EAAT2 and EAAT3 targets can be achieved. A significant property associated with WAY-855, presented in this study, is its lack of activity on the ionotropic and metabotropic glutamate receptor targets examined. As discussed above, overlapping pharmacology with several glutamate uptake inhibitors has frequently been observed with glutamate receptor targets. Consequently, WAY-855 represents a selective tool for the exploration of EAAT pharmacology in the absence of nonselective effects on other glutamate targets.
In summary, this study describes the pharmacological profile of the novel glutamate transport inhibitor WAY-855. Data presented reveal that WAY-855 exhibits preferential inhibition of the predominant EAAT2 subtype, behaves as a competitive nonsubstrate transport inhibitor and exhibits good selectivity over nontransporter glutamate targets.
Acknowledgments
We thank Steve Kurtz, Renee Sykes and Liz Fiddler for construction of stable cell lines and John Butera for helpful discussions during preparation of the manuscript.
Abbreviations
- EAAC1
excitatory aminoacid carrier 1
- EAAT
excitatory aminoacid transporter
- GLAST
glutamate/aspartate transporter
- GLT-1
glutamate transporter-1
- HBS
HEPES-buffered saline
- L-trans-2,3-PDC
L-trans-2,3-pyrrolidine dicarboxylate
- L-trans-2,4-PDC
L-trans-2,4-pyrollidine dicarboxylate
- MDCK
Madin – Darby canine kidney
- T3MG
threo-3-methylglutamate
- TBOA
threo-β-benzyloxy-aspartate
- WAY-855
3-amino-tricyclo[2.2.1.02.6]heptane-1,3-dicarboxylic acid
References
- ARRIZA J.L., ELIASOF S., KAVANAUGH M.P., AMARA S.G. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARRIZA J.L., FAIRMAN W.A., WADICHE J.L., MURDOCH G.H., KAVANAUGH M.P., AMARA S.G. Functional comparison of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNARD E.A. Ionotropic glutamate receptors: new types and new concepts. Trends Pharmacol. Sci. 1997;18:141–148. doi: 10.1016/s0165-6147(97)01053-5. [DOI] [PubMed] [Google Scholar]
- BERGLES D.E., JAHR C.E. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- BRIDGES R.J., KAVANAUGH M.P., CHAMBERLIN A.R. A pharmacological review of competitive inhibitors and substrates of high-affinity, sodium-dependent glutamate transport in the central nervous system. Curr. Pharmaceutical Des. 1999;5:363–379. [PubMed] [Google Scholar]
- BRIDGES R.J., STANLEY M.S., ANDERSON M.W., COTMAN C.W., CHAMBERLIN A.R. Conformationally defined neurotransmitter analogues. Selective inhibition of glutamate uptake by one pyrrolidine-2,4-dicarboxylate diastereomer. J. Med. Chem. 1991;34:717–723. doi: 10.1021/jm00106a037. [DOI] [PubMed] [Google Scholar]
- CONN P.J., PIN P.J. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- DANBOLT N.C. Glutamate uptake. Prog. Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- DUNLOP J. Substrate exchange properties of the high-affinity glutamate transporter EAAT2. J. Neurosci. Res. 2001;66:482–486. doi: 10.1002/jnr.1239. [DOI] [PubMed] [Google Scholar]
- DUNLOP J., LOU Z., ZHANG Y., MCILVAIN H.B. Inducible expression and pharmacology of the human excitatory amino acid transporter 2 subtype of L-glutamate transporter. Br. J. Pharmacol. 1999a;128:1485–1490. doi: 10.1038/sj.bjp.0702945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNLOP J., ZALESKA M.M., ELIASOF S., MOYER J.A. Excitatory amino acid transporters as emerging targets for central nervous system therapeutics. Emerging Ther. Targets. 1999b;3:543–570. [Google Scholar]
- FAIRMAN W.A., VANDENBERG R.J., ARIZZA J.L., KAVANAUGH M.P., AMARA S.G. An excitatory amino acid transporter with properties of a ligand gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- FLETCHER E.J., MEWETT K.N., DREW C.A., ALLAN R.D., JOHNSTON G.A. Inhibition of L-glutamate uptake into rat cortical synaptosomes by the conformationally restricted analogue of glutamic acid, cis-1-aminocyclobutane-1,3-dicarboxylic acid. Neurosci. Lett. 1991;121:133–135. doi: 10.1016/0304-3940(91)90667-i. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS R., DUNLOP J., GORMAN A., SENIOR J., GRIEVE A. L-trans-pyrrolidine-2,4-dicarboxylate and cis-1-aminocyclobutane-1,3-dicarboxylate behave as transportable competitive inhibitors of the high-affinity glutamate transporters. Biochem. Pharmacol. 1994;47:267–274. doi: 10.1016/0006-2952(94)90016-7. [DOI] [PubMed] [Google Scholar]
- KANAI Y., HEDIGER M.A. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- KOCH H.P., KAVANAUGH M.P., ESSLINGER C.S., ZERANGUE N., HUMPHREY J.M., AMARA S.G., CHAMBERLIN A.R., BRIDGES R.J. Differentiation of substrate and non substrate inhibitors of the high-affinity, sodium-dependent glutamate transporters. Mol. Pharmacol. 1999;56:1095–1104. doi: 10.1124/mol.56.6.1095. [DOI] [PubMed] [Google Scholar]
- KOWAL D., HSIAO C.-L., GE A., WARDWELL J., GHOSH K., TASSE R. A [35S]GTPγS binding assessment of metabotropic glutamate receptor standards in Chinese hamster ovary cell lines expressing the human metabotropic receptor subtypes 2 and 4. Neuropharmacology. 1998;37:179–187. doi: 10.1016/s0028-3908(98)00011-2. [DOI] [PubMed] [Google Scholar]
- NAKAMURA Y., KATAOKA K., ISHIDA M., SHINOZAKI H. (2S,3S,4R)-2-(carboxycyclopropyl) glycine, a potent and competitive inhibitor of both glial and neuronal uptake of glutamate. Neuropharmacology. 1993;32:833–837. doi: 10.1016/0028-3908(93)90137-r. [DOI] [PubMed] [Google Scholar]
- OTIS T.S., JAHR C.E. Anion currents and predicted glutamate flux through a neuronal glutamate transporter. J. Neurosci. 1998;18:7099–7110. doi: 10.1523/JNEUROSCI.18-18-07099.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINES G., DANBOLT N.C., BJORAS M., ZHANG Y., BENDAHAN A., EIDE L., KOEPSELL H., STORM-MATHISEN J., SEEBERG E., KANNER B.I. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- ROBINSON M.B., HUNTER-ENSOR M., SINOR J. Pharmacologically distinct sodium-dependent L-[3H]glutamate transport processes in rat brain. Brain Res. 1991;544:196–202. doi: 10.1016/0006-8993(91)90054-y. [DOI] [PubMed] [Google Scholar]
- ROTHSTEIN J.D., DYKES-HOBERG M., PARDO C.A., BRISTOL L.A., JIN L., KUNCL R.W., KANAI Y., Hediger M.A., WANG Y., SCHIELKE J.P., WELTY D.F. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- SCHOEPP D.D., JANE D.E., MONN J.A. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- SHIGERI Y., SHIMAMOTO K., YASUDA-KAMATANI Y., SEAL R.P., YUMOTO N., NAKAJIMA T., AMARA S.G. Effects of threo-beta-hydroxyaspartate derivatives on excitatory amino acid transporters (EAAT4 and EAAT5) J. Neurochem. 2001;79:297–302. doi: 10.1046/j.1471-4159.2001.00588.x. [DOI] [PubMed] [Google Scholar]
- SHIMAMOTO K., LEBRUN B., YASUDA-KAMATANI Y., SAKAITANI M., SHIGERI Y., YUMOTO N., NAKAJIMA T. DL-threo-β-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol. Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- STORCK T., SCHULTE S., HOFMANN K., STOFFEL W. Structure, expression and functional analysis of a Na+-dependent glutamate/aspartate transporter from rat brain. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAN J., ZELENAIA O., CORREALE D., ROTHSTEIN J.D., ROBINSON M.B. Expression of the GLT-1 subtype of Na+-dependent glutamate transporter: pharmacological characterization and lack of regulation by protein kinase C. J. Pharm. Exp. Ther. 1999;289:1600–1610. [PubMed] [Google Scholar]
- TANAKA K., WATASE K., MANABE T., YAMADA K., WATANABE M., TAKAHASHI K., IWAMA H., NISHIKAWA T., ICHIHARA N., KIKUCHI T., OKUYAMA S., KAWASHIMA N., HORI S., TAKIMOTO M., WADA K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- VANDENBERG R.J., MITROVIC A.D., CHEBIB M., BALCAR V.J., JOHNSTON G.A. Contrasting modes of action of methylglutamate derivatives on the excitatory amino acid transporters, EAAT1 and EAAT2. Mol. Pharmacol. 1997;51:809–815. doi: 10.1124/mol.51.5.809. [DOI] [PubMed] [Google Scholar]
- WANG G.J., CHUNG H.J., SCHNUER J., LEA E., ROBINSON M.B., POTTHOFF W.K., AIZENMAN E., ROSENBERG P.A. Dihydrokainate-sensitive neuronal glutamate transport is required for protection of rat cortical neurons in culture against synaptically released glutamate. Eur. J. Neurosci. 1998;10:2523–2531. doi: 10.1046/j.1460-9568.1998.00256.x. [DOI] [PubMed] [Google Scholar]
- ZIFF E.B. Recent excitement in the ionotropic glutamate receptor field. Ann. NY Acad. Sci. 1999;868:465–473. doi: 10.1111/j.1749-6632.1999.tb11315.x. [DOI] [PubMed] [Google Scholar]