Abstract
N-methyl-D-aspartate (NMDA) receptors have been demonstrated to be a pivotal target for ethanol action. The present study examined the actions of acute ethanol exposure on NMDA-induced responses and the acute tolerance to ethanol actions in rat sympathetic preganglionic neurons (SPNs) in vitro and in vivo.
NMDA (50 μM) applied every 5 min induced reproducible membrane depolarizations of SPNs in neonatal spinal cord slice preparations. Ethanol (50 – 100 mM) applied by superfusion for 15 min caused a sustained decrease in NMDA-induced depolarizations in a dose-dependent and reversible manner. When the superfusion time of ethanol (100 mM) was increased to 50 min, NMDA-induced depolarizations were attenuated initially but a gradual recovery was seen in ∼40% of SPNs tested.
Repeated injections of NMDA (2 nmol) intrathecally at 30 min interval caused reproducible increases in mean arterial pressure (MAP) in urethane-anesthetized rats. Intravenous injections of ethanol (0.16 or 0.32 g, 1 ml) inhibited NMDA-induced pressor effects in a blood concentration-dependent manner. The inhibition by ethanol of NMDA-induced pressor effects was reduced over time during continuous infusion of ethanol or on the second injection 3.5 h after prior injection of a higher dose of ethanol.
Ethanol, at concentrations significantly inhibited NMDA-induced responses, had no significant effects on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-induced responses. The study demonstrated the selective inhibition by ethanol of NMDA-induced responses and the development of acute tolerance to the inhibitory effects in SPNs both in vitro and in vivo. These effects may play important roles in the ethanol regulation of cardiovascular function.
Keywords: Ethanol, tolerance, sympathetic preganglionic neurons, NMDA receptors, AMPA receptors, intrathecal, rat
Introduction
Ethanol (alcohol) exerts its neurotoxic effects largely by interacting with neurotransmitter receptors and ion channels in the central nervous system (Faingold et al., 1998; Narahashi et al., 2001). Almost all types of neurotransmitter receptors have been the subjects of investigation. Among theses studies, the NMDA receptors have been demonstrated to be an important target of ethanol action (Wirkner et al., 1999; Woodward, 2000). Electrophysiological studies have shown that acute ethanol exposure at physiologically relevant concentrations inhibited NMDA-mediated responses in various neurons of rats (Lovinger et al., 1990; Nie et al., 1994; Nieber et al., 1998; Popp et al., 1998; Wang et al., 1999; Wirkner et al., 2000). A decrease in the effect of ethanol over a period of exposure, an adaptive phenomenon known as ethanol tolerance, has been reported in many studies. The ratio of motor-impairing effects of ethanol to blood ethanol levels has been used as an index to estimate the development of acute tolerance. Acute tolerance to behavioral impairments of ethanol during a single exposure has been well documented both in humans and in animals (LeBlanc et al., 1975; Portans et al., 1989; Kalant, 1998). The development of acute tolerance to ethanol was evident in rats across ages (Silveri & Spear, 2001). The ability of hippocampal neurons to develop acute tolerance in response to a single ethanol exposure has been shown in free-moving rats; the inhibition by ethanol of neuronal firing rate was reduced during the second exposure within an hour later (Ludvig et al., 2001). Acute tolerance to ethanol inhibition of NMDA-induced responses has been found in several brain areas in vitro. In rat locus coeruleus neurons, the inhibitory action of ethanol on NMDA-induced depolarization disappeared after 50 – 60 min during continuous superfusion of ethanol (100 mM) for 60 min (Poelchen et al., 1997). The development of acute tolerance to ethanol inhibition of NMDA-mediated field excitatory postsynaptic potentials was observed during a 15 min ethanol exposure in CA1 region of most rat hippocampus slices examined (Grover et al., 1994). Similar observations were found in mouse hippocampus in which Fyn tyrosine kinase has been demonstrated to be a determinant of ethanol action and acute tolerance development (Miyakawa et al., 1997).
Sympathetic preganglionic neurons (SPNs), located in thoracolumbar spinal cord, are the only link between the central sympathetic output and the peripheral ganglia. SPNs provide projections to adrenal medulla and sympathetic autonomic ganglia, whose activation may cause changes in cardiovascular function. Glutamate has been shown to be the fast synaptic transmitter mediating the activity of SPNs by acting on both non-NMDA and NMDA receptors (Inokuchi et al., 1992; Deuchars et al., 1995). Thus, changes in cardiovascular function may reflect the responses of non-NMDA and NMDA receptors in SPNs. In SPNs, excitatory postsynaptic currents evoked from dorsal horn are mainly carried by NMDA receptors (Krupp & Feltz, 1995). It is not known, however, whether ethanol affects NMDA receptor activation in SPNs. In addition, ethanol inhibition of responses to NMDA and acute tolerance to ethanol action in neurons were examined mainly in vitro; few studies documented the effects of ethanol on NMDA receptors in vivo. The present study was undertaken to evaluate the action of ethanol on responses of NMDA receptors and the development of acute tolerances in SPNs both in vitro and in vivo. The amplitude of membrane depolarizations induced by superfusion of NMDA and the magnitude of increases in blood pressure induced by intrathecal (i.t.) injection of NMDA were used as indices for responses of NMDA receptors in vitro and in vivo, respectively.
Methods
Animals
A breeding colony of Sprague – Dawley rats purchased from the National Laboratory Animal Breeding and Research Center (Taipei, Taiwan) was established at the Laboratory Animal Center, Tzu Chi University. Animals were housed two per cage in a room maintained at 22±1°C with an alternating 12 h light – dark cycle. Food and water were available ad libitum. Rats at desired age were selected from the colony for use in the present study. All procedures were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee of Tzu Chi University.
Slice preparations and whole-cell recording techniques
Immature (8- to 12-day-old) rats were used in this series of experiments. Under ether anesthesia, animals were decapitated and the spinal segments were rapidly removed. Coronal sections of 500 μm thick were prepared with the use of a Vibratome (1000, Ted Pella, Inc., Redding, CA, U.S.A.). The slices from T7 – T12 segments were incubated in a holding chamber containing Krebs solution at room temperature until use. The Krebs solution consists of (in mM) 117 NaCl, 2.0 KCl, 1.2 KH2PO4, 2.3 CaCl2, 1.3 MgCl2, 26 NaHCO3, and 10 glucose; the solution was saturated with 95% O2 and 5% CO2. To carry out the whole-cell recording, a slice was transferred to a recording chamber (RC-22, Warner Instrument Corp., Hamden, CT, U.S.A.) and was continuously perfused with Krebs solution. The flow rate of Krebs solution was kept constant at 2 – 3 ml min−1. Patch electrodes, filled with the following intracellular solution (in mM): 130 K+ gluconate, 1 MgCl2, 2 CaCl2, 4 ATP, 10 EGTA, and 10 HEPES, had a resistance of 3 – 5 MΩ. The pH of the intracellular solution was adjusted to 7.3 with KOH. Whole-cell recordings were made from neurons located in the area of intermediolateral cell column under current – clamp mode with the use of an Axoclamp 2B. Hyperpolarizing current pulses of constant amplitude with 300 ms duration were injected into the neurons recorded at a frequency of 0.2 Hz. The input resistance of the neurons was calculated from the resulting peak voltage divided by the injected current. SPNs were distinguished from interneurons according to the method described by Deuchars et al. (2001). Interneurons displayed a sag in the voltage responses to hyperpolarizing current pulses and a shorter duration of the action potentials. Signals were displayed on a Tektronix digital oscilloscope (TDS 360) and were also sent to a data acquisition system (DigiPack 1200B and pClamp 7.0, Axon Instruments, Inc., Foster City, CA, U.S.A.) for continuous recording. Recordings were made at room temperature (22±1°C). Reagents were applied by changing superfusion medium by means of a valve controller (VC-6, Warner Instrument Corp.). Tetrodotoxin (0.5 μM) was added in the superfusion medium throughout to inhibit the firing of neurons. NMDA (50 μM) or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) (5 μM) was applied every 5 min by superfusion for 5 – 8 s to induce a 15 – 20 mV depolarization. Ethanol was applied by superfusion when the amplitudes of NMDA-induced depolarization were constant over two consecutive tests. NMDA or AMPA was applied every 5 min throughout the superfusion period of ethanol. The peak amplitude was used to determine the effects of ethanol on NMDA-induced depolarizations.
In vivo experiments
Procedures for i.t. administration to anesthetized rats were similar to those described earlier (Lai et al., 2000; 2002). Adult male rats weighing 250 – 270 g were used in this series of experiments. Under urethane anesthesia (1.2 – 1.5 g kg−1, i.p.), the left femoral artery was cannulated with a polyethylene tubing (PE 50) and connected to a pressure transducer with its output to a Grass pen recorder (Model 7400) for recording of blood pressure. The mean arterial pressure (MAP) was calculated using [(S−D)/3+D], where S indicates systolic blood pressure and D indicates diastolic blood pressure. The right femoral vein was cannulated for intravenous injection of ethanol. Rat was mounted in a stereotaxic header and implanted with a spinal catheter for i.t. injection. A slit was made in the atlanto-occipital membrane and the catheter (PE-10 tubing) was inserted down into the spinal subarachnoid space so that the tip was placed in the vicinity of T7 – T9 segment; the position of the tubing was visually verified at the end of the experiment. NMDA and AMPA at known concentrations were injected i.t. at a volume of 10 μl, which was followed by 10 μl saline to wash the agent. NMDA and AMPA were applied at intervals of 30 and 60 min, respectively. As a negative control, i.t. saline did not elicit any significant changes in blood pressure. After NMDA- or AMPA-induced responses were stable over two consecutive tests, ethanol was administered intravenously 10 min before the next application of NMDA or AMPA; 1 ml of ethanol at known concentrations was injected into femoral vein in 90 s. In order not to disturb the blood pressure recording, blood ethanol concentrations were measured in another group of male rats under the same conditions as the experimental ones. A measure of 0.2 ml of blood each at 10, 40, 70, and 100 min after intravenous injection was withdrawn from femoral artery. The concentration of ethanol was measured by a spectrophotometer (Beckman DU650) according to the instruction of ethanol diagnostic kits available commercially (Sigma Co.).
Chemicals and statistical data analysis
Ethanol was purchased from Riedel-de Haen (Deisenhofen, Germany). Tetrodotoxin citrate was from Alomone Labs (Jerusalem, Israel). NMDA, AMPA, alcohol diagnostic kits, and other chemicals were purchased from Sigma Co. (St Louis, MO, U.S.A.). Stock solutions of reagents were prepared in distilled water. Further dilutions were made with Krebs solution or saline. Ethanol was directly dissolved in Krebs solution or saline. The original data for NMDA-induced depolarizations (mV) or increases in MAP (mmHg) before and after applications of ethanol were analyzed statistically. The time – effect relationship of ethanol on NMDA-mediated responses was analyzed using the repeated measure ANOVA followed by Newman – Keuls post-test. The inhibitory potency of ethanol on NMDA-induced responses between the first and the second administrations was analyzed with two-way ANOVA followed by Bonferroni post-test. P<0.05 is considered statistically significant. The results from ethanol were compared to control and expressed as percentage changes; the peak amplitude of NMDA-induced responses immediately prior to the application of ethanol is taken as control (i.e. 100%). Data were presented as mean±s.e.m. and were plotted and analyzed statistically with Prism version 3.02 for Windows, Graphpad Software (San Diego, CA, U.S.A.).
Results
Effects of ethanol on NMDA-induced depolarizations in vitro
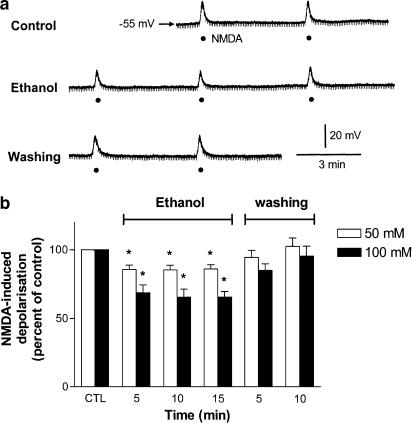
The SPNs recorded were held at resting membrane potentials, being 59±1.0 mV (n=60, range −40 to −70 mV). Superfusion of 100 mM ethanol for 15 min had no significant effects on membrane potentials and input resistances (control, 586±74 MΩ; after ethanol, 597±79 MΩ) of six SPNs. A population of SPNs discharged spontaneously in spinal cord slices harvested from immature rats (Shen et al., 1994). The firing rate (3.9±0.7 Hz, n=4) was not significantly altered by superfusion of ethanol (100 mM) for 15 min. In silent neurons, action potentials were evoked by the injection of positive current pulses sufficient to reach threshold. Ethanol (100 mM) had no effects on the threshold for action potential firing and the amplitude of action potential (n=4). In the following series of experiments, the membrane potential was depolarized by 14 – 20 mV following superfusion of NMDA (50 μM) for 5 – 8 s in the presence of tetrodotoxin (0.5 μM). Repeated applications of NMDA at intervals of 5 min induced similar degree of depolarization. The reproducible depolarizing effects of NMDA lasted for at least 1 h without attenuation of the responses. Superfusion of 10 mM ethanol for 15 min had little effect on NMDA-induced depolarizations (n=4). Higher concentrations of ethanol (50, 100 mM) consistently decreased NMDA-induced depolarizations in a dose-dependent and reversible manner in all neurons tested; the maximal depressions were 18.5±2.6% (n=7) and 40.9±3.6% (n=6) following applications of 50 and 100 mM ethanol for 15 min, respectively. The effect of ethanol was reversed within 5 min upon washout. A representative recording in which ethanol (100 mM) applied by superfusion for 15 min caused sustained decreases in NMDA-induced depolarizations is illustrated in Figure 1a. The time course of the percentage inhibition of NMDA-induced depolarizations by 50 and 100 mM ethanol is shown in Figure 1b.
Figure 1.
Concentration-dependent inhibition by ethanol of NMDA-induced depolarizations in SPNs of spinal cord slices. (a) a Representative continuous recording shows a sustained inhibition of NMDA-induced depolarization by ethanol (100 mM) superfused for 15 min in a sympathetic preganglionic neuron. The neuron was held at a resting membrane potential of −55 mV. The membrane depolarizations were induced by consecutive superfusions of NMDA (50 μM, filled circles) for 7 s at intervals of 5 min in the presence of TTX (0.5 μM). Downward deflections are hyperpolarizing electrotonic potentials induced by constant hyperpolarizing current pulses (not shown). (b) Graph shows percentage change in NMDA-induced depolarization against time in minutes following superfusion of two concentrations of ethanol (50, 100 mM) for 15 min and after removal of ethanol. The magnitude of NMDA-induced depolarization immediately prior to application of ethanol is taken as control (CTL) of 100%. The NMDA-induced depolarizations were 18.2±1.3 mV (n=7) and 17.6±0.9 mV (n=6) before the superfusion of 50 and 100 mM ethanol, respectively. *Significant difference from control.
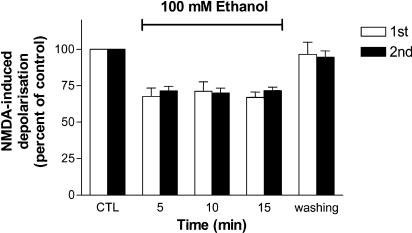
During repeated applications of ethanol at intervals of 15 min, the second application of ethanol exerted a similar degree of decreases in NMDA-induced depolarizations as did the first superfusion. The time courses of percentage inhibition of NMDA-induced depolarizations during the first and the second superfusion of ethanol for the duration of 15 min are shown in Figure 2.
Figure 2.
Graph shows percentage changes in NMDA-induced depolarizations following two consecutive applications of ethanol (100 mM) for 15 min each with 15 min washing between them. NMDA was applied every 5 min to induce membrane depolarizations. The peak amplitude of NMDA-induced depolarizations immediately prior to the application of ethanol is taken as control (CTL) of 100%. There is no statistical difference in percentage inhibition of NMDA-induced depolarizations between the first (1st) and the second (2nd) perfusion of ethanol separated by 15 min. Bars denote mean+s.e.m. from six SPNs.
Tolerance to the inhibition in vitro during continuous superfusion of ethanol
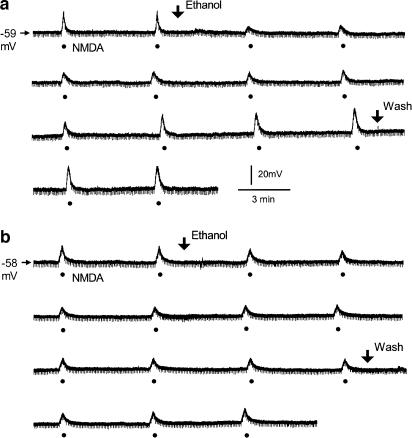
When the superfusion time of ethanol (100 mM) was increased to 50 min, NMDA-induced depolarizations were attenuated initially but a gradual recovery was seen in some of the tested SPNs, suggesting the development of acute tolerance to ethanol. Representative recordings in SPNs with and without the development of tolerance during ethanol superfusion are demonstrated in Figure 3a and b, respectively.
Figure 3.
SPNs with and without the development of tolerance to ethanol inhibition of NMDA-induced depolarizations during the superfusion of ethanol for 50 min. Ethanol (100 mM) was applied by superfusion (between two arrows). (a) A representative continuous recording shows the development of acute tolerance in an SPN. Membrane depolarizations were induced by superfusion of NMDA (50 μM, filled circles) for 7 s at intervals of 5 min. NMDA-induced depolarization was decreased initially by about 60% during the superfusion of ethanol; the inhibition gradually disappeared over time. The neuron was held at a resting membrane potential of −59 mV. (b) A representative continuous recording shows a consistent attenuation of NMDA-induced depolarization during the superfusion of ethanol, and the inhibition gradually recovered after the removal of ethanol from the bath. Membrane depolarizations were induced by superfusion of NMDA (50 μM, filled circles) for 7 s at intervals of 5 min. The neuron was held at resting membrane potential of −58 mV. Recordings in (a) and (b) were taken from two different SPNs. Downward deflections are hyperpolarizing electrotonic potentials induced by constant hyperpolarizing current pulses (not shown).
Since the attenuated action of ethanol following prolonged application did not show in all of the SPNs tested, we characterized the neurons that developed tolerance during the superfusion of ethanol for 50 min according to the following criteria: the maximal inhibition by ethanol of NMDA-induced depolarizations was larger than 25%; it occurred within 20 min during the superfusion; and the inhibition disappeared or decreased by at least 50% of the maximal inhibition after 40 – 50 min. Four of the 40 SPNs recorded from 30 neonatal rats exhibiting less sensitivity (<25%) to the inhibitory action of ethanol were excluded from the consideration of tolerance development. Among the other neurons in which NMDA-induced depolarizations were decreased by more than 25%, 42% (15 of 36) demonstrated tolerance to ethanol inhibition. Augmentation of the sensitivity to NMDA after subsequent removal of ethanol was found in four SPNs that exhibited tolerance. For the neurons (21 of 36) that did not show tolerance, ethanol caused a sustained inhibition during the superfusion and the inhibition was partially or fully reversible upon washing for 10 – 15 min.
Effects of ethanol on AMPA-induced depolarizations in vitro
Membrane potential was depolarized by 14 – 20 mV following superfusion of AMPA (5 μM) for 5 – 8 s in the presence of tetrodotoxin (0.5 μM). Ethanol (100 mM) did not significantly inhibit AMPA-induced depolarization; the AMPA-induced depolarizations were 18±1.0 mV (n=7) and 16±0.9 mV (n=7) before and after 15 min contact with ethanol, respectively.
Effects of ethanol on NMDA-induced pressor effects in vivo
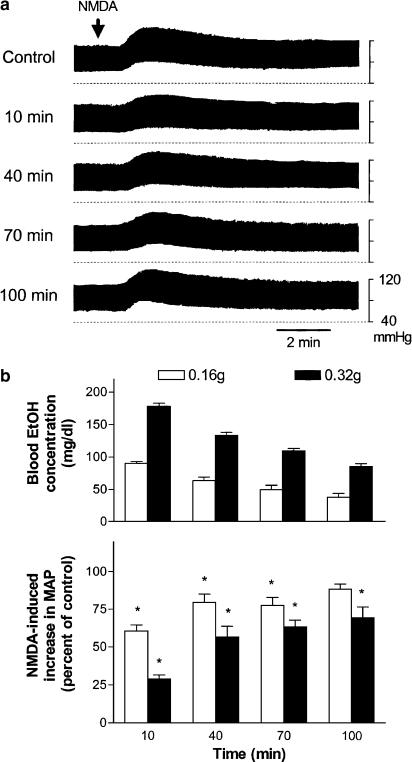
The resting MAP in urethane-anesthetized rats was 81±1.2 mmHg (n=50). In a dose-dependent manner, i.t. NMDA (1, 2, and 4 nmol, 10 μl) increased MAP. The magnitudes of increases in MAP were 13±1.8 (n=3), 22±1.2 (n=40), and 31±2.4 (n=3) mmHg following the applications of 1, 2, and 4 nmol NMDA, respectively. The pressor effects of i.t. NMDA (2 nmol) lasted for 10 – 15 min. Consecutive applications of NMDA (2 nmol) at intervals of 30 min induced similar degree of increases in MAP. The reproducible pressor effects lasted for at least 6 h. Intravenous injection of ethanol (0.16 and 0.32 g, 1 ml) did not cause significant changes in MAP. Around 20% of rats tested following the administration of a high dose of ethanol (0.32 g) showed a greater (>25%) fall in MAP, which reached the maximum within 1 – 3 min, and lasted for less than 10 min. NMDA was applied i.t. 10 min following the administration of ethanol. NMDA-induced pressor effects were attenuated dose dependently by intravenous administration of ethanol. A representative experiment in which an intravenous injection of ethanol (0.16 g) caused significant decreases in NMDA-induced pressor effects at different times after the injection is illustrated in Figure 4a. The time course of percentage inhibition of NMDA-induced pressor effects by and the corresponding blood concentrations of ethanol are illustrated in the bottom and top panels of Figure 4b, respectively. The decline of blood ethanol levels over time was accompanied by a comparable degree of reductions in ethanol inhibition of NMDA-induced pressor effects after a single injection of ethanol. NMDA-induced pressor effects decreased by about 40 and 70% when blood ethanol concentrations were about 90 and 180 mg dl−1 at 10 min after intravenous injections of 0.16 g (1 ml, 20%) and 0.32 g (1 ml, 40%) ethanol, respectively.
Figure 4.
Dose – effect relationship of ethanol (EtOH) given intravenously on NMDA-induced increases in MAP in urethane-anesthetized rats. (a) Representative recordings of changes in blood pressure induced by an i.t. injection of NMDA (2 nmol, indicated by an arrow) and the decreases in NMDA-induced pressor effects at different times after a single intravenous injection of ethanol (0.16 g). NMDA was applied i.t. every 30 min. (b) Bar graphs show the time course of blood ethanol concentrations (top part, n=8 and 9 for 0.16 and 0.32 g ethanol, respectively) and percentage changes in NMDA-induced increases in MAP (bottom part, n=13 and 15 for 0.16 and 0.32 g ethanol, respectively) following intravenous injections of two doses of ethanol (0.16 and 0.32 g). The peak magnitude of NMDA-induced increase in MAP immediately prior to application of ethanol is taken as control (100%). The NMDA-induced increases in MAP were 20.4±1.5 mmHg (n=13) and 22±2 mmHg (n=15) prior to the applications of 0.16 and 0.32 g ethanol, respectively. *Statistically different from control.
Tolerance to the inhibition in vivo following prolonged applications of ethanol
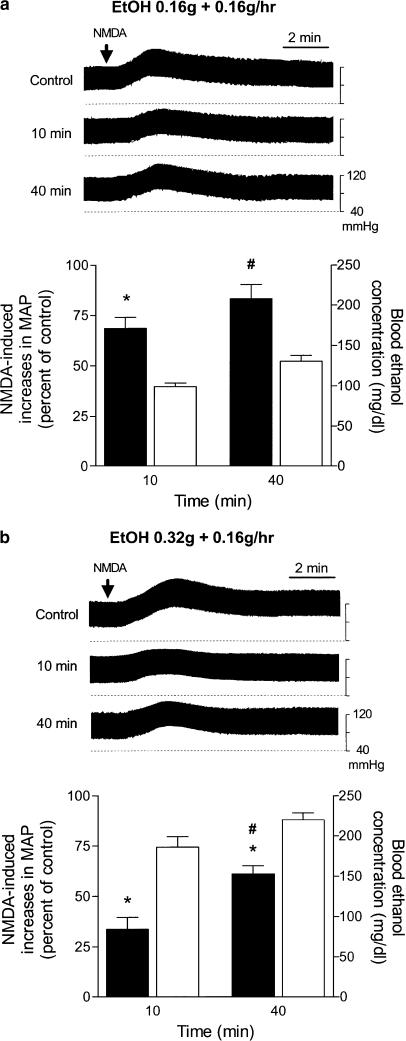
Intravenous injection of a bolus of 0.16 or 0.32 g ethanol (1 ml) followed by a continuous infusion at a constant rate of 0.16 g h−1 caused gradual increases in blood ethanol concentrations. Under this condition, the inhibition by ethanol of NMDA-induced pressor effects was significantly reduced after 40 min. The top parts of Figure 5a and b depict two representative experiments in which NMDA-induced pressor effects at 40 min was inhibited less than that at 10 min following prolonged administrations of two different doses of ethanol, suggesting the development of acute tolerance following continuous infusion of ethanol. The percentage changes in NMDA-induced pressor effects and the corresponding blood ethanol concentrations at 10 and 40 min following prolonged applications of two different doses of ethanol are illustrated in the bottom parts of Figure 5a and b.
Figure 5.
The effects of prolonged applications of ethanol (EtOH) on i.t. NMDA-induced increases in blood pressure in urethane-anesthetized rats. (a) Top panel: Representative recordings show increases in blood pressure induced by an i.t. injection of NMDA (2 nmol, indicated by an arrow) and the decreases in NMDA-induced pressor effects at 10 and 40 min (min) following an intravenous injection of a blous of 0.16 g ethanol (1 ml) followed by a continuous infusion at a constant rate of 0.16 g h−1. NMDA was applied i.t. every 30 min. Bottom panel: Bar graphs show changes in blood ethanol concentrations (right, y-axis; clear bar, n=4) and percentage changes in NMDA-induced increases in MAP (left, y-axis; black bar, n=6) following continuous infusion of ethanol. The peak magnitude of NMDA-induced increase in MAP immediately prior to application of ethanol is taken as control (i.e. 100%); the increase in MAP was 22±3.6 mmHg (n=6). *Statistically different from control; #Statistically different from the changes at 10 min after administration of ethanol. (b) Similar to the legend of (a) except that ethanol was administered with an initial dose of 0.32 g. The blood ethanol concentrations and ethanol effects on NMDA-induced increases in MAP were determined from four and five rats, respectively. NMDA-induced increase in MAP before the application of ethanol was 19±3.2 mmHg (n=5).
Tolerance to the inhibition in vivo after a prior single injection of ethanol
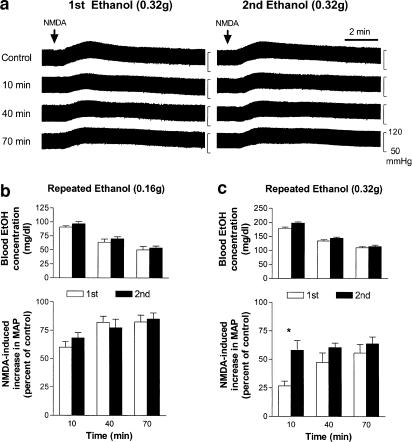
In these series of experiments, the same doses of ethanol (0.16 and 0.32 g) were applied twice at intervals of 3.5 h. As residual blood ethanol (25±8 mg dl−1, n=9) was detected 3.5 h after the first administration of a higher dose of ethanol (0.32 g), the blood ethanol concentrations following the second administration were higher than those after the first administration. Under this condition, however, the inhibitory potency of ethanol (0.32 g) on NMDA-induced pressor effects at 10 min following the second application was significantly reduced compared to that following the first administration. The decreases in NMDA-induced pressor effects were similar between the first and the second applications when a lower dose (0.16 g) of ethanol was given. A representative experiment in which the second injection of a high dose of ethanol (0.32 g) caused a less decrease in NMDA-induced pressor effects at 10 min after the injection is shown in Figure 6a. The time course of inhibition of NMDA-induced pressor effects by and the corresponding blood concentrations of ethanol after the first and second injection of 0.16 and 0.32 g ethanol are illustrated in Figure 6b and c, respectively.
Figure 6.
The effects of repeated applications of ethanol (EtOH) on NMDA-induced increases in blood pressure in urethane-anesthetized rats. (a) Representative recordings show the effects of two intravenous injections of ethanol given at intervals of 3.5 h on NMDA-induced pressor effects at different times after the injection. NMDA (2 nmol, indicated by an arrow) was applied i.t. every 30 min to exert an increase in blood pressure. (b) Bar graphs show blood ethanol concentrations (top part, n=8) and percentage changes in NMDA-induced increases in MAP (bottom part, n=10) following the first (1st, clear bars) and the second (2nd, black bars) injections of 0.16 g ethanol. The peak magnitude of NMDA-induced increase in MAP immediately prior to the application of ethanol is taken as control (i.e. 100%); the NMDA-induced increases in MAP were 20.7±1.7 and 18.9±2.3 mmHg (n=10) before the first and the second applications of ethanol (0.16 g), respectively. (c) Bar graphs show blood ethanol concentrations (top panel, n=9) and percentage changes in NMDA-induced increases in MAP (bottom panel, n=6) following the first (1st, clear bars) and the second (2nd, black bars) injections of 0.32 g ethanol. The NMDA-induced increases in MAP were 20.3±2.7 and 18.9±2.6 mmHg (n=6) before the first and the second applications of ethanol (0.32 g), respectively. *Significant difference between two groups analyzed by two-way ANOVA followed by Bonferroni post-test.
Effects of ethanol on nicotine- or AMPA-induced pressor effects in vivo
Intravenous injection of nicotine (0.1 mg kg−1) increased MAP by 19±1 mmHg (n=5). Ethanol (0.32 g, 1 ml) applied intravenously had no effect on pressor effects of nicotine applied at intervals of 30 min. MAP was increased by i.t. AMPA (1 nmol, 10 μl) by 26±2.5 mmHg (n=5). A gradual reduction in the pressor effects was noticed when AMPA (1 nmol) was applied every 30 min. The application of AMPA at intervals of 60 min induced reproducible increases in MAP; the pressor effects were maintained following upto three consecutive applications. Ethanol (0.32 g) had no significant effects on AMPA-induced pressor effects at 10 min after intravenous administration (n=5).
Discussion
The present study demonstrated the inhibitory actions of ethanol on NMDA-induced responses and the development of acute tolerance to ethanol in SPNs both in vitro and in vivo. Our study shows the first in vitro data of the effects of ethanol on SPNs and the first evidence of acute tolerance to ethanol inhibition of NMDA receptor activation in vivo. Analogues to the studies of NMDA-activated current (potential) in other areas of rat brain and spinal cord in vitro (Lovinger et al., 1990; Nie et al., 1994; Nieber et al., 1998; Wang et al., 1999), ethanol inhibited NMDA-induced depolarization in rat SPNs. In the in vivo model, the pressor effects induced by injection of NMDA into T7 – T9 segments may result largely from the activation of SPNs innervating the adrenal medulla and the subsequent release of catecholamines from the adrenal medulla into the circulation. A single injection of ethanol inhibited NMDA-induced pressor effects in a dose-dependent manner. The in vivo inhibitory action of ethanol on NMDA-induced pressor effects is most likely due to the selective inhibition by ethanol of NMDA receptors based on the following observations: (1) ethanol had no effects on the resting membrane potentials of SPNs; the spontaneous discharge rate, the threshold for firing, and the amplitude of action potential in SPNs were not altered either. These results were similar to those observed in rat locus coeruleus neurons (Nieber et al., 1998). (2) Ethanol, at concentrations significantly inhibited NMDA-induced increases in MAP, had little effects on the intravenous nicotine-induced pressor effects, suggesting the lack of action of ethanol on peripheral ganglionic neurons. (3) The pressor effects induced by i.t. AMPA were much less sensitive to ethanol than those induced by NMDA. However, the possibility that ethanol inhibition of NMDA-induced responses was secondary to the effects of ethanol on other targets instead of NMDA receptors cannot be ruled out. In contrast to NMDA-induced responses, AMPA-induced responses in SPNs were much less sensitive to ethanol both in vitro and in vivo. This result is similar to those found in several neuronal preparations in vitro (Lovinger et al., 1989; 1990; Nie et al., 1994). However, similar inhibitory potency of ethanol on responses of both NMDA and AMPA receptors is also found in a few neuronal preparations in vitro (Nieber et al., 1998; Wirkner et al., 2000).
Based on concentration – effect relationship, a large difference was found in ethanol potency between the inhibitions of NMDA-induced depolarizations in vitro and NMDA-induced pressor effects in vivo. Ethanol at concentration of 100 mM (0.46%, w v−1) caused a mean decrease of less than 40% in NMDA-induced depolarization in vitro. In contrast, the mean decrease of 40% in NMDA-induced pressor effects in vivo was found at blood ethanol concentration of 90 mg dl−1 (0.09%). The differential potency of ethanol between the in vitro and the in vivo studies may be due to the difference in temperature, being room temperature in vitro and body temperature in vivo, and/or the state of receptors in the different experimental conditions. Actually, it has been shown that the conductance of NMDA channels is temperature dependent; the amplitude of channel currents increases with increasing temperature (Chung & Kuyucak, 1995). Temperature may affect the activities of enzymes involved in protein phosphorylation, which have recently been demonstrated to play an important role in ethanol action on NMDA receptors (see below). The inhibitory potency of ethanol in SPNs in the in vitro study was comparable to that in rat locus coeruleus neurons and cultured rat cortical neurons (Poelchen et al., 1997; Wirkner et al., 2000), whose responses were examined at body temperature (35 – 36°C) and room temperature (20 – 22°C), respectively. Thus, the difference in temperature may not completely account for the differences for the two sets of experiments. There is evidence of increased sensitivity to the motor-impairing and hypnotic effects of ethanol during development (Silveri & Spear, 1998), indicating age-related difference as one of the possible contributors for the difference in the potency. The subunit composition of NMDA receptors varies regionally and developmentally (Yamakura & Shimoji, 1999; Cull-Candy et al., 2001) and certain subunit combinations of recombinant NMDA receptors exhibit higher sensitivity to ethanol (Masood et al., 1994; Mirshahi & Woodward, 1995). Thus, differences in the ethanol potency between in vitro neonate and in vivo adult rats may be at least partially due to different subunit composition of NMDA receptors in ontogeny. In addition, the responses induced by the administration of NMDA in vivo may be different from those in vitro, since an i.t. injection of NMDA may be having a multitude of effects via activating not only SPNs but also interneurons, etc. at several levels of the spinal cord.
The acute tolerance to ethanol inhibition of NMDA-induced depolarization in vitro was observed in about 40% instead of all neurons examined. Since both patterns of responses existed in SPNs from the same rats in some of the experiments, the difference in acute tolerance development in ethanol inhibition observed here may reflect variations between individual neurons rather than individual rats. It is interesting to note that the development of acute tolerance was observed following continuous infusion of ethanol in all of the rats examined in vivo. There is evidence for variations in subunit compositions of NMDA receptors within a single cell. For example, the different subunit compositions between synaptic and extrasynaptic NMDA receptors have been found in brain and spinal cord neurons (Rumbaugh & Vicini, 1999; Misra et al., 2000; Momiyama, 2000). Whether different types of NMDA receptors exist in the SPNs remain to be determined. In the present study, responses to NMDA applied by superfusion in vitro or by i.t. administration in vivo may result from the activation of all types of NMDA receptors, if exist, in the SPNs.
Acute tolerance to ethanol inhibition of NMDA-induced pressor effects in vivo developed during prolonged exposure of ethanol or in the second injection 3.5 h after a prior injection of a higher dose of ethanol. It seems that acute tolerance to ethanol inhibition of NMDA-induced responses depends on exposure time and concentration (dose) of ethanol, that is, exposure to a certain level of ethanol for a certain period may be required for the development of the tolerance. The mechanisms for the development of acute tolerance in SPNs remain to be investigated. Fyn tyrosine kinase determines NMDA receptor sensitivity to ethanol in the hippocampus (Miyakawa et al., 1997; Yaka et al., 2003). The regulation by Fyn kinase of the sensitivity of NMDA receptors to ethanol was subunit dependent (Anders et al., 1999). The facilitation of Fyn-mediated phosphorylation of NR2B, resulting from the dissociation of scaffolding protein from NR2B subunit of NMDA receptors during acute exposure to ethanol, may account for acute tolerance development in the hippocampus (Yaka et al., 2003). Other protein kinases such as protein kinase A and C as well as phosphatase have been demonstrated to regulate NMDA receptor activations (Westphal et al., 1999; Lan et al., 2001; Alvestad et al., 2003). The tyrosine phosphatase has been suggested to play an important role in mediating ethanol inhibition of NMDA receptors (Alvestad et al., 2003). Additional experiments will be required to clarify the relationships among protein phosphorylation, the action of ethanol, and acute tolerance development in SPNs.
Consistent with the observations found in behavioral studies (Radlow, 1994; Hiltunen, 1997), sustained exposure or prior single injection of a higher dose of ethanol caused acute tolerance to ethanol actions on NMDA-induced responses. The development of acute tolerance to ethanol inhibition of NMDA-induced pressor effects was not observed following a single intravenous injection of ethanol in vivo probably because blood ethanol concentration does not maintain for a longer duration. In the animal behavioral studies, NMDA antagonists, such as MK-801 and ketamine, have been shown to inhibit acute tolerance to ethanol although these antagonists per se had effects on motor function (Khanna et al., 2002). Thus, acute tolerance to responses mediated by NMDA receptors as shown in the present study may be one of the mechanisms responsible for the acute behavioral tolerance to ethanol seen in animals and humans.
In the in vivo experiments, the involvement of NMDA receptors in the ethanol influence on baroreflex- and chemoreflex-mediated responses has been suggested in rostral ventrolateral medulla (Mao & Abdel-Rahman, 1995; Sun & Reis, 1996). Ethanol inhibition of stress-related tachycardia was also suggested to involve NMDA receptors in the nucleus tractus solitarii (Varga et al., 1996). Taking together with the present study, ethanol intoxication may influence cardiovascular regulation by inhibiting the responses of NMDA receptors in the neurons involved in the cardiovascular control. Consequently, the development of tolerance to ethanol inhibition of NMDA receptors may be a neuroprotective adaptation to avoid interference of NMDA-mediated physiological function. In conclusion, our study demonstrated the selective inhibition by ethanol of NMDA receptor activation and the development of acute tolerance to the inhibition in SPNs both in vitro and in vivo. Such tolerance may play an important role in the ethanol regulation of cardiovascular function.
Acknowledgments
This study was supported by grants from National Science Council (NSC 91-2320-B-320-019 and NSC 91-2745-P-320-001), Taiwan.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- EPSCs
excitatory postsynaptic currents
- fEPSPs
field excitatory postsynaptic potentials
- MAP
mean arterial pressure
- NMDA
N-methyl-D-aspartic acid
- SPN
sympathetic preganglionic neuron
References
- ALVESTAD R.M., GROSSHANS D.R., COULTRAP S.J., NAKAZAWA T., YAMAMOTO T., BROWNING M.D. Tyrosine dephosphorylation and ethanol inhibition of N-methyl-D-aspartate receptor function. J. Biol. Chem. 2003;278:11020–11025. doi: 10.1074/jbc.M210167200. [DOI] [PubMed] [Google Scholar]
- ANDERS D.L., BLEVINS T., SUTTON G., SWOPE S., CHANDLER L.J., WOODWARD J.J. Fyn tyrosine kinase reduces the ethanol inhibition of recombinant NR1/NR2A but not NR1/NR2B NMDA receptors expressed in HEK 293 cells. J. Neurochem. 1999;72:1389–1393. doi: 10.1046/j.1471-4159.1999.721389.x. [DOI] [PubMed] [Google Scholar]
- CHUNG S.H., KUYUCAK S. Changes in the kinetics and conductance of N-methyl-D-aspartate (NMDA)-receptor activated single channels with temperature. Neurosci. Lett. 1995;187:181–184. doi: 10.1016/0304-3940(95)11369-8. [DOI] [PubMed] [Google Scholar]
- CULL-CANDY S., BRICKLEY S., FARRANT M. NMDA receptor subunits: diversity, development and disease. Curr. Opin. Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- DEUCHARS S.A., BROOKE R.E., FRATER B., DEUCHARS J. Properties of interneurones in the intermediolateral cell column of the rat spinal cord: role of the potassium channel subunit Kv3.1. Neuroscience. 2001;106:433–446. doi: 10.1016/s0306-4522(01)00277-9. [DOI] [PubMed] [Google Scholar]
- DEUCHARS S.A., MORRISON S.F., GILBEY M.P. Medullary-evoked EPSPs in neonatal rat sympathetic preganglionic neurones in vitro. J. Physiol. 1995;487:453–463. doi: 10.1113/jphysiol.1995.sp020892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAINGOLD C.L., N'GOUEMO P., RIAZ A. Ethanol and neurotransmitter interactions from molecular to integrative effects. Prog. Neurobiol. 1998;55:509–535. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- GROVER C.A., FRYE G.D., GRIFFITH W.H. Acute tolerance to ethanol inhibition of NMDA-mediated EPSPs in the CA1 region of the rat hippocampus. Brain Res. 1994;642:70–76. doi: 10.1016/0006-8993(94)90906-7. [DOI] [PubMed] [Google Scholar]
- HILTUNEN A.J. Acute alcohol tolerance in social drinkers: changes in subjective effects dependent on the alcohol dose and prior alcohol experience. Alcohol. 1997;14:373–378. doi: 10.1016/s0741-8329(96)00186-3. [DOI] [PubMed] [Google Scholar]
- INOKUCHI H., YOSHIMURA M., YAMADA S., POLOSA C., NISHI S. Fast excitatory postsynaptic potentials and the responses to excitant amino acids of sympathetic preganglionic neurons in the slice of the cat spinal cord. Neuroscience. 1992;46:657–667. doi: 10.1016/0306-4522(92)90152-r. [DOI] [PubMed] [Google Scholar]
- KALANT H. Research on tolerance: what can we learn from history. Alcohol Clin. Exp. Res. 1998;22:67–76. doi: 10.1111/j.1530-0277.1998.tb03618.x. [DOI] [PubMed] [Google Scholar]
- KHANNA J.M., MORATO G.S., KALANT H. Effect of NMDA antagonists, an NMDA agonist, and serotonin depletion on acute tolerance to ethanol. Pharmacol. Biochem. Behav. 2002;72:291–298. doi: 10.1016/s0091-3057(01)00773-0. [DOI] [PubMed] [Google Scholar]
- KRUPP J., FELTZ P. Excitatory postsynaptic currents and glutamate receptors in neonatal rat sympathetic preganglionic neurons in vitro. J. Neurophysiol. 1995;73:1503–1512. doi: 10.1152/jn.1995.73.4.1503. [DOI] [PubMed] [Google Scholar]
- LAI C.-C., LIN H.H., CHEN C.-W., CHEN S.-H., CHIU T.H. Excitatory action of lead on rat sympathetic preganglionic neurons in vitro and in vivo. Life Sci. 2002;71:1035–1045. doi: 10.1016/s0024-3205(02)01789-7. [DOI] [PubMed] [Google Scholar]
- LAI C.-C., WU S.Y., CHEN C.-T., DUN N.J. Nociceptin inhibits rat sympathetic preganglionic neurons in situ and in vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;278:R592–R597. doi: 10.1152/ajpregu.2000.278.3.R592. [DOI] [PubMed] [Google Scholar]
- LAN J.Y., SKEBERDIS V.A., JOVER T., GROOMS S.Y., LIN Y., ARANEDA R.C., ZHENG X., BENNETT M.V., ZUKIN R.S. Protein kinase C modulates NMDA receptor trafficking and gating. Nat. Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- LEBLANC A.E., KALANT H., GIBBINS R.J. Acute tolerance to ethanol in the rat. Psychopharmacology. 1975;41:43–46. doi: 10.1007/BF00421304. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M., WHITE G., WEIGHT F.F. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- LOVINGER D.M., WHITE G., WEIGHT F.F. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J. Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUDVIG N., GEORGE M.A., TANG H.M., GONZALES R.A., BUNGAY P.M. Evidence for the ability of hippocampal neurons to develop acute tolerance to ethanol in behaving rats. Brain Res. 2001;900:252–260. doi: 10.1016/s0006-8993(01)02319-8. [DOI] [PubMed] [Google Scholar]
- MAO L., ABDEL-RAHMAN A.A. Blockade of L-glutamate receptors in the rostral ventrolateral medulla contributes to ethanol-evoked impairment of baroreflexes in conscious rats. Brain Res. Bull. 1995;37:513–521. doi: 10.1016/0361-9230(95)00034-c. [DOI] [PubMed] [Google Scholar]
- MASOOD K., WU C., BRAUNEIS U., WEIGHT F.F. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol. Pharmacol. 1994;45:324–329. [PubMed] [Google Scholar]
- MIRSHAHI T., WOODWARD J.J. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg2+-insensitive mutants. Neuropharmacology. 1995;34:347–355. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- MISRA C., BRICKLEY S.G., FARRANT M., CULL-CANDY S.G.Identification of subunits contributing to synaptic and extrasynaptic NMDA receptors in Golgi cells of the rat cerebellum J. Physiol. 2000524147–162.(Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAKAWA T., YAGI T., KITAZAWA H., YASUDA M., KAWAI N., TSUBOI K., NIKI H. Fyn-kinase as a determinant of ethanol sensitivity: relation to NMDA-receptor function. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- MOMIYAMA A.Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord J. Physiol. 2000523621–628.(Part 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., KURIYAMA K., ILLES P., WIRKNER K., FISCHER W., MUHLBERG K., SCHEIBLER P., ALLGAIER C., MINAMI K., LOVINGER D., LALLEMAND F., WARD R.J., DEWITTE P., ITATSU T., TAKEI Y., OIDE H., HIROSE M., WANG X.E., WATANABE S., TATEYAMA M., OCHI R., SATO N. Neuroreceptors and ion channels as targets of alcohol. Alcohol Clin. Exp. Res. 2001;25:182S–188S. doi: 10.1097/00000374-200105051-00030. [DOI] [PubMed] [Google Scholar]
- NIE Z., MADAMBA S.G., SIGGINS G.R. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J. Pharmacol. Exp. Ther. 1994;271:1566–1573. [PubMed] [Google Scholar]
- NIEBER K., POELCHEN W., SIELER D., ILLES P. Inhibition by ethanol of excitatory amino acid receptors in rat locus coeruleus neurons in vitro. Naunyn Schmiedebergs Arch. Pharmacol. 1998;357:299–308. doi: 10.1007/pl00005171. [DOI] [PubMed] [Google Scholar]
- POELCHEN W., NIEBER K., ILLES P. Tolerance to inhibition by ethanol of N-methyl-D-aspartate-induced depolarisation in rat locus coeruleus neurons in vitro. Eur. J. Pharmacol. 1997;332:267–271. doi: 10.1016/s0014-2999(97)01113-8. [DOI] [PubMed] [Google Scholar]
- POPP R.L., LICKTEIG R., BROWNING M.D., LOVINGER D.M. Ethanol sensitivity and subunit composition of NMDA receptors in cultured striatal neurons. Neuropharmacology. 1998;37:45–56. doi: 10.1016/s0028-3908(97)00186-x. [DOI] [PubMed] [Google Scholar]
- PORTANS I., WHITE J.M., STAIGER P.K. Acute tolerance to alcohol: changes in subjective effects among social drinkers. Psychopharmacology. 1989;97:365–369. doi: 10.1007/BF00439452. [DOI] [PubMed] [Google Scholar]
- RADLOW R. A quantitative theory of acute tolerance to alcohol. Psychopharmacology (Berl) 1994;114:1–8. doi: 10.1007/BF02245438. [DOI] [PubMed] [Google Scholar]
- RUMBAUGH G., VICINI S. Distinct synaptic and extrasynaptic NMDA receptors in developing cerebellar granule neurons. J. Neurosci. 1999;19:10603–10610. doi: 10.1523/JNEUROSCI.19-24-10603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN E., WU S.Y., DUN N.J. Spontaneous and transmitter-induced rhythmic activity in neonatal rat sympathetic preganglionic neurons in vitro. J. Neurophysiol. 1994;71:1197–1205. doi: 10.1152/jn.1994.71.3.1197. [DOI] [PubMed] [Google Scholar]
- SILVERI M.M., SPEAR L.P. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin. Exp. Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- SILVERI M.M., SPEAR L.P. ACUTE, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin. Exp. Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- SUN M.K., REIS D.J. Ethanol inhibits chemoreflex excitation of reticulospinal vasomotor neurons. Brain Res. 1996;730:182–192. doi: 10.1016/0006-8993(96)00445-3. [DOI] [PubMed] [Google Scholar]
- VARGA K., LOVAS G., PALKOVITS M., KUNOS G. Ethanol inhibition of stress-related tachycardia involves medullary NMDA receptors. Eur. J. Pharmacol. 1996;310:145–153. doi: 10.1016/0014-2999(96)00305-6. [DOI] [PubMed] [Google Scholar]
- WANG M.Y., RAMPIL I.J., KENDIG J.J. Ethanol directly depresses AMPA and NMDA glutamate currents in spinal cord motor neurons independent of actions on GABAA or glycine receptors. J. Pharmacol. Exp. Ther. 1999;290:362–367. [PubMed] [Google Scholar]
- WESTPHAL R.S., TAVALIN S.J., LIN J.W., ALTO N.M., FRASER I.D., LANGEBERG L.K., SHENG M., SCOTT J.D. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- WIRKNER K., EBERTS C., POELCHEN W., ALLGAIER C., ILLES P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:568–576. doi: 10.1007/s002100000262. [DOI] [PubMed] [Google Scholar]
- WIRKNER K., POELCHEN W., KOLES L., MUHLBERG K., SCHEIBLER P., ALLGAIER C., ILLES P. Ethanol-induced inhibition of NMDA receptor channels. Neurochem. Int. 1999;35:153–162. doi: 10.1016/s0197-0186(99)00057-1. [DOI] [PubMed] [Google Scholar]
- WOODWARD J.J. Ethanol and NMDA receptor signaling. Crit. Rev. Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- YAKA R., PHAMLUONG K., RON D. Scaffolding of Fyn kinase to the NMDA receptor determines brain region sensitivity to ethanol. J. Neurosci. 2003;23:3623–3632. doi: 10.1523/JNEUROSCI.23-09-03623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAKURA T., SHIMOJI K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog. Neurobiol. 1999;59:279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]