Abstract
In addition to its central role in blood coagulation and hemostasis, human α-thrombin is a powerful regulator of inflammatory responses and is known to affect cell-mediated immunity. Interleukin (IL)-12 is a strong promoter of the development of Th1-type lymphocytes and its downregulation implies a positive feedback mechanism for development of Th2 responses. We have previously shown that thrombin enhances the release of IL-6, a Th2-related cytokine, in human peripheral blood mononuclear cells (PBMC).
Here we show that thrombin downregulates IL-12 production at both protein and mRNA levels in human PBMC. The inhibition of IL-12 production was accompanied by an enhanced release of IL-10, which inhibits Th1-related processes and promotes Th2-type responses.
The use of proteolytically inactive thrombin and of the specific thrombin receptor agonist peptide, SFLLRN, reveals that this downregulation is thrombin-specific and requires thrombin proteolytic activity.
In addition, activation of coagulation inhibits IL-12 production in whole blood cultures, confirming the tight relationship between the coagulation pathway, where thrombin is a key enzyme, and inflammation.
Decreased IL-12 production appears to be related also to IL-10 production, since the addition of an anti-IL-10 monoclonal antibody to thrombin-treated PBMC resulted in a partial restoration of IL-12 production.
In conclusion, the observation that thrombin significantly affects the production of IL-12, as well as of IL-10, implies a concerted role orchestrated by thrombin in PBMC that could be crucial to effective immunity and inflammation.
Keywords: Thrombin, protease-activated receptor, cytokine, mononuclear cells, inflammation
Introduction
Activation of the coagulation cascade culminates in the production of the serine protease thrombin. Besides its fundamental role for hemostasis thrombin is an important inflammatory mediator, which exerts direct effects on different cell types (Grand et al., 1996). Thrombin enhances T lymphocyte and monocyte activation and induces the release of proinflammatory cytokines, such as interleukin (IL)-6 and IL-8 (Naldini et al., 1993; Johnson et al., 1998). Recent reports indicate that thrombin promotes T lymphocyte recruitment through mechanisms involving NF-κB (Kaur et al., 2001).
Many of the cellular events induced by thrombin have been shown to require proteolytic activation of one or more of the seven transmembrane domain receptors for thrombin (protease-activated receptors (PARs)), including PAR-1 (Vu et al., 1991), PAR-3 (Ishihara et al., 1997) or PAR-4 (Kahn et al., 1998); PAR-2 receptor is selectively activated by trypsin (Schmidlin & Bunnett, 2001). The synthetic peptide SFLLRN, which mimics the first six amino acids of the new amino terminus unmasked by receptor cleavage, functions as an agonist for PAR-1 and activates the receptor independent of thrombin and proteolysis. PAR-1 expression has been associated with the inflammatory activities exerted by thrombin (Cirino et al., 2000). PAR-1 activation mediates thrombin-dependent, cell-mediated renal inflammation (Cunningham et al., 2000) and enhances the release of cytokines such as monocyte chemotactic protein-1 (Colotta et al., 1994) and IL-1 (Naldini et al., 2002).
IL-12 is an heterodimeric cytokine produced primarily by macrophage, monocytes and dendritic cells. Only IL-12 p70, consisting of a p40 and p35 chain, encoded by two separate genes, is biologically active (Trinchieri, 1998). IL-12 is instrumental for development of a Th1 response and promotes IFN-γ production and enhancement of cytolytic activity in T cells and NK cells (Trinchieri, 1993). By contrast, in the absence of IL-12, a Th2 response can be developed (Romagnani, 1997). One of the most important endogenous inhibitor of IL-12 is IL-10, which is considered an anti-inflammatory cytokine and promotes Th2 responses (D'Andrea et al., 1993). Aberrant regulation of Th1 or Th2 responses leads to immunopathology; thus, modulation of IL-12 during an immune response and specific physio-pathological processes is crucial for the outcome of disease (Romagnani et al., 2000).
It has been recently reported that several inflammation mediators can control the release and the expression of specific cytokines, such as IL-12 (van der Pouw Kraan et al., 1998). Indeed, histamine, prostaglandin E2 (PGE2) and cyclooxygenase-2 (COX-2) have been shown to inhibit the release of IL-12 suggesting that the immunosuppression observed after injury may be the result of the downregulation of IL-12 expression (Schwacha et al., 2002). For these reasons, the role of IL-12 has been considered crucial for the onset of infectious diseases and for an increased susceptibility to sepsis and multiple organ failure (Lauw et al., 1999).
Coagulation and inflammation are intimately related, but the complex mechanisms involved are not fully elucidated yet. Thrombin and IL-12 play pivotal roles in both processes, however, to our knowledge, the relationship between thrombin and IL-12 expression has not been analyzed until now. We have previously reported that thrombin promotes the production of Th2-type cytokines, such as IL-6 (Naldini et al., 1993), and inhibits lymphokine-activated killer cell cytotoxicity by a PAR-1 mediated mechanism (Naldini & Carney, 1996). These studies suggest that thrombin could be involved in a negative feedback regulation of Th1-type responses.
We show here and for the first time that thrombin inhibits the release and the expression of IL-12 in human peripheral blood mononuclear cells (PBMC). Such inhibition was accompanied by enhancement of IL-10 release. IL-12 downregulation is thrombin-specific and requires thrombin proteolytic activity, since SFLLRN peptide mimics thrombin effects. Downregulation of IL-12 was dependent upon IL-10, since IL-12 production was restored after the addition a monoclonal antibody to IL-10. Activation of the extrinsic pathway of coagulation cascade resulted in a downregulation of IL-12 production, similar to that mediated by thrombin, confirming the pivotal role of IL-12 in the coagulative response. These studies suggest that thrombin may play a more central role during immunity/inflammation then previously recognized, by modulating the expression of both Th1- and Th2-type cytokines. Our findings may be relevant for trying to understand how coagulation and inflammation may control the development of several disorders.
Methods
Cell cultures
Human PBMC were isolated from heparinized venous blood of healthy volunteers by a gradient of Lympholite/M (Cedarlane Labs, Ontario, Canada). The gradients were spun at 400 × g and the PBMC at the interface were removed, washed twice and resuspended in serum-free medium (HYQ-CCM1, HyClone, UT, U.S.A.). The PBMC preparations essentially contain only lymphocytes (∼90%) and monocytes (∼10%), as determined by flow cytometric analysis. PBMC were incubated in serum-free medium (105 cells per 100 μl) in 96-well microtiter plates (Costar, MA, U.S.A.) at 37°C in the presence of indicated concentrations of thrombin, diisopropylfluorophosphate-treated thrombin (DIP-thrombin) or synthetic agonist peptide (SFLLRN). After 1 h, 100 μl medium supplemented with 1% heat-inactivated FCS was added and incubation continued in the presence or absence of either 5 μg ml−1 of phytohemagglutinin (PHA) or 0.1 μg ml−1 of lipopolysaccharide (LPS). Following indicated time points, cell-free supernatants were obtained after centrifugation and aliquots from supernatants were frozen at −20°C pending assay.
The isolation of whole blood and the subsequent culturing of whole blood were performed as previously described (Johnson et al., 1996).
Cytokine measurements
IL-12 (p70 and p40) and IL-10 concentrations were assessed from PBMC supernatants by ELISA using flat-bottomed microtiter plates and commercially available monoclonal antibodies and high-performance ELISA reagents, according to the manufacturer's instructions. Thereafter, plates were read at 450 nm in a Titertek Multiskan reader. Background absorbance at 620 nm was substracted. Whole blood culture supernatants were analyzed by ELISA, carried out as described before (van der Pouw Kraan et al., 1995). None of the assays showed cross-reactivity with other cytokines. The minimum detectable doses were: IL-12 <3 pg ml−1; IL-10 <5 pg ml−1.
RNase protection assay
Freshly isolated PBMC, at a concentration of 106 cells ml−1, were incubated in 25-cm2 tissue culture flasks and exposed to thrombin in the presence of PHA, as described above. At the appropriate time of culture, cells were harvested by centrifugation and lysed, and total RNA was isolated according to the manufacturer's instructions using RNAWIZ™.
IL-12 p40 mRNA was evaluated by RNase protection assay. The RNA probes were synthesized using T7 phage polymerase (MAXIscript) and a biotin RNA labeling mixture. The DNA template was a multiprobe template set (hCK-2, Riboquant RNase protection assay System). Total RNA (1 μg) and the appropriate labeled probe were hybridized and then digested by RNase A/T1 and treated with proteinase K. The products of RNase protection assay were then separated for analysis on a denaturing polyacrylamide gel (5% acrylamide/8 M urea) by running the gel at 200 V for 1 h. Gels were then transferred to a positively charged nylon membrane, using a semi-dry transfer unit (Hoefer Pharmacia Biotech, San Francisco, CA, U.S.A.). The nucleic acids were then immobilized by UV cross-linking. Nonisotopic detection was performed with the BrightStar BioDetect kit, following the manufacturer's instructions. The identity and quantity of each mRNA species in the original sample was then determined from the signal intensities given by the appropriately sized protected probe fragment bands (protected nucleotide size: IL-12 p40: 321 nt). Antisense L32 (housekeeping gene) probe transcripts were synthesized and used in a series of parallel reactions, to normalize the amounts of RNA present in the samples (protected nucleotide size for L32: 112 nt). Gel electrophoretic autoradiographs were then quantitated by Sigma Gel analysis software (Jandel Scientific, U.S.A.).
Materials and reagents
Highly purified human α-thrombin (99% α-form; specific activity 3683 NIH U mg−1 protein) and DIP-thrombin were kindly provided by Dr J.W. Fenton (Albany, NY, U.S.A.) and human PAR-1 agonist peptide (SFLLRN) was a gift of Dr D.H. Carney (University of Texas Medical Branch Peptide Synthesis Laboratory, Galveston, TX, U.S.A.). Anti-CD3 (CLB-T3.4/E), anti-CD28 (CLB-CD28/1) were obtained from CLB (Amsterdam, NL, U.S.A). Anti-IL-10 (BT-10) was a gift of Dr J. Wijdenes (Diaclone, Besancon, France) and recombinant human tissue factor pathway inhibitor (TFPI) was a gift of Dr A. Creasy (Chiron Co., Emeryville, CA, U.S.A.). PHA and LPS were purchased from Biochrom KG, Berlin, D. Kits for cytokine assays (ELISA) were from Euroclone (Devon, U.K.). Reagents for RNAse protection assay (RNAWIZ, MAXIscript, BrightStar BioDetect kit) were obtained from Ambion (Austin, TX, U.S.A.) and the biotin RNA labeling mixture from Boehringer (Mannheim, Germany). The DNA template was purchased from Pharmingen (San Diego, CA, U.S.A.) as multiprobe template set (hCK-2, Riboquant RNase protection assay System).
Statistical analysis
Values presented are the means±s.e.m. of the results obtained from three to five independent experiments, unless specifically described. Statistical significance between means was determined using ANOVA or Student's t-test. Bonferroni's correction was used for multiple t-test comparisons.
Results
Thrombin inhibits the release of IL-12 in PBMC
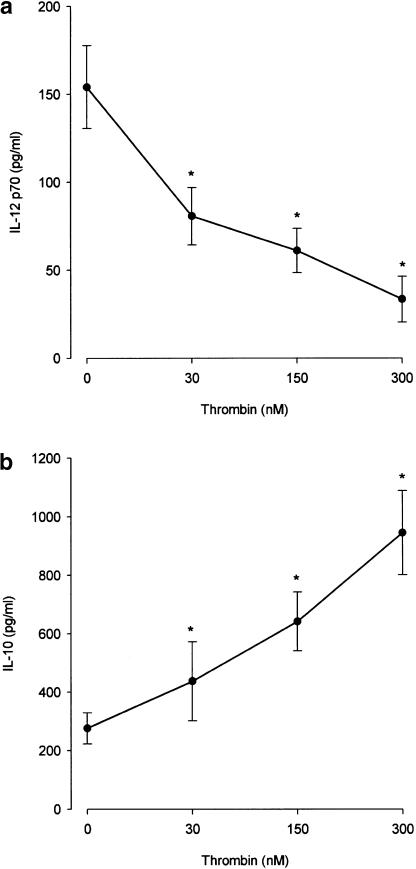
A previous study of thrombin generation, under conditions in which all procoagulant factors and inhibitors are at their mean plasma concentrations, indicates that, after an initiation phase, thrombin reaches a maximum concentration of approximately 300 nM (Butenas et al., 1999). Figure 1a shows the effect of 30–300 nM thrombin on the release of IL-12 p70 from PBMC activated with PHA, a specific polyclonal activator of T lymphocytes. It is evident that 30 nM thrombin was sufficient to lead to a mean inhibition of IL-12 release of 50%, while 300 nM thrombin reduced IL-12 production to 22%. As IL-12 directs the development of naïve CD4+ T cells into Th1-type cells, downregulation of IL-12 suggests that thrombin is a strong inhibitor of the Th1-type response. Downregulation of Th1 responses is usually associated with upregulation of Th-2 type responses and IL-10 is considered a Th2-related cytokine. In the same cultures, IL-10 production was already significantly enhanced in the presence of 30 nM thrombin (by approximately 60%), whereas 300 nM thrombin led to a four-fold increase of IL-10 production (Figure 1b).
Figure 1.
Thrombin modulates cytokine production. PBMC were cultured at a concentration of 106 cells ml−1 in the presence of different concentrations of thrombin for 1 h at 37°C, then supplemented with FCS (1%) in the presence of PHA (5 μg ml−1). After 16 h of culture, cell-free supernatants were obtained and IL-12 p70 (panel a) and IL-10 (panel b) present in the supernatants were determined by ELISA. The means±s.e.m. of five independent experiments are presented. Asterisks indicate statistically significant (P<0.05) differences between cytokine release in cultures treated with thrombin versus untreated, as determined using ANOVA.
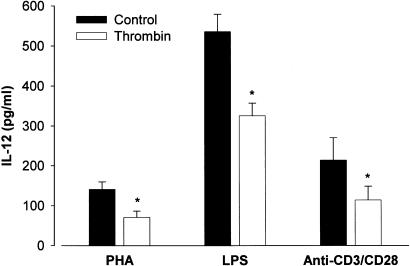
IL-12 inhibition by thrombin was not unique to PHA-stimulated PBMC. Indeed, different stimuli induce IL-12 production in PBMC. Both the bacterial product LPS and the combination of monoclonal antibodies anti-CD3 and anti-CD28 induce significant IL-12 p70 production. As shown in Figure 2, 150 nM thrombin led to a mean inhibition of IL-12 production of more than 40% in the presence of LPS. A similar inhibition of IL-12 production was achieved in PBMC activated with anti-CD3/anti-CD28.
Figure 2.
Thrombin inhibits IL-12 production. PBMC were cultured at a concentration of 106 cells ml−1 in the absence (filled bars) and in the presence of 150 nM thrombin (open bars) for 1 h at 37°C, then supplemented with FCS (1%) in the presence of different activators: PHA (5 μg ml−1), LPS (0.1 μg ml−1) and anti-CD3 (0.1 μg ml−1)/anti-CD28 (1 μg ml−1). After 16 h of incubation, cell-free supernatants were obtained and IL-12 present in the supernatants were determined by ELISA. The means±s.e.m. of three independent experiments are presented. Asterisks indicate statistically significant (P<0.05) differences between IL-12 release in cultures treated with thrombin versus untreated, as determined using Student's t-test. In the absence of the activators, levels of IL-12 were undetectable.
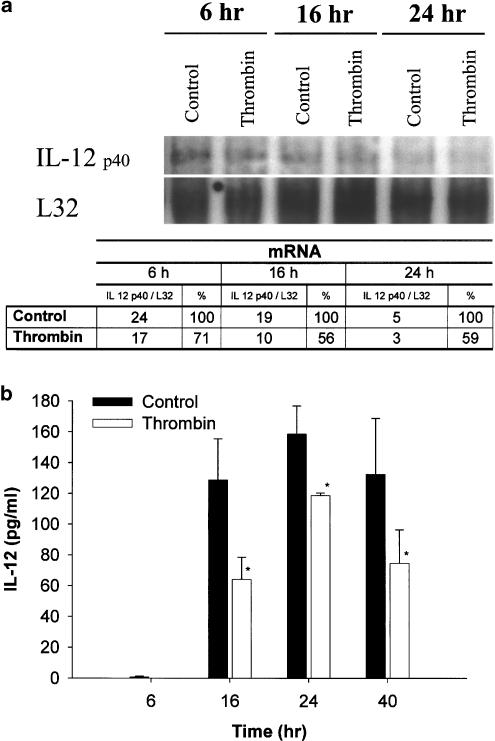
Thrombin inhibits IL-12 production at mRNA level
IL-12 p40 mRNA expression was assessed by RNase protection assays. First, we examined the kinetics of IL-12 p40 mRNA expression. The amount of IL-12 p40 mRNA is expressed relative to the amount of L-32 transcripts as a measure for the total RNA isolated. Figure 3a shows that at 6 h PHA induces high levels of IL-12 p40 mRNA. A 6-h stimulation appeared to be optimal for IL-12 p40 mRNA production, as a prolonged stimulation (16–24 h) with PHA led to a decrease of mRNA level. The addition of 150 nM thrombin to PHA-activated PBMC reduced IL-12 p40 mRNA expression to about 70 and 50%, after a stimulation of 6 and 16 h, respectively. Thrombin-induced inhibition was still evident after prolonged stimulations, such as 24 h. The reduction of IL-12 p40 mRNA was accompanied by an inhibition of IL-12 p70 protein production. Such inhibition was significant after 16 h of incubation and was still evident at 40 h (Figure 3b).
Figure 3.
Kinetics of thrombin inhibition of IL-12 production. PBMC were cultured at a concentration of 106 cells ml−1 in the presence of 150 nM thrombin for 1 h at 37°C, then supplemented with FCS (1%) in the presence of PHA (5 μg ml−1). (a) At the appropriate time, cell lysates were obtained and analyzed by RNase protection assay. L32 was used as a housekeeping gene. Quantification of IL-12 p40 expression was achieved with Sigma Gel analysis software and the results, representing the percentage of area of each band, calibrated against the L32 housekeeping gene band, are presented in the table. (b) In a parallel culture, cell-free supernatants were obtained after 6, 16, 24 and 40 h of incubation and IL-12 p70 present in the supernatant was determined by ELISA. The means±s.e.m. of triplicate cultures are presented. Asterisks indicate statistically significant (P<0.05) differences between IL-12 p70 release in cultures treated with thrombin (open bars) versus untreated (filled bars), as judged by Student's t-test. At zero time the levels of IL-12 p70 were undetectable.
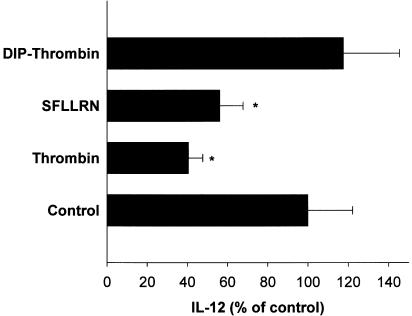
Thrombin inhibition requires proteolytic activity and involves activation of PAR-1
Thrombin proteolytic activity and PAR-1 receptor activation are often involved in the biological effects exerted by thrombin. First, we analyzed whether proteolytically inactive thrombin (DIP-thrombin) was able to mimic thrombin-induced IL-12 downregulation. As shown in Figure 4, 300 nM DIP-thrombin failed to inhibit downregulation of IL-12 in PHA-stimulated PBMC. We next investigated whether PAR-1 activation was involved in thrombin-induced IL-12 downregulation. Figure 4 shows that at a concentration of 40 μM, the PAR-1 agonist peptide SFLLRN led to an inhibition of IL-12 production of more than 50%, similar, although to a lesser extent, to the inhibition exerted by 150 nM thrombin. These results suggest that thrombin inhibition of IL-12 production requires thrombin proteolytic activity and may be mediated, at least in part, by PAR-1 activation.
Figure 4.
Thrombin inhibition of IL-12 production requires thrombin proteolytic activity and is thrombin-specific. PBMC were cultured at a concentration of 106 cells ml−1 in the presence of 300 nM DIP-thrombin, 40 μM SFLLRN peptide or 150 nM thrombin for 1 h at 37°C, then supplemented with FCS (1%) in the presence of PHA (5 μg ml−1). After 16 h of culture, cell-free supernatants were obtained and IL-12 p70 present in the supernatants were determined by ELISA. The means±s.e.m. of five independent experiments are presented. Asterisks indicate statistically significant (P<0.05) differences between IL-12 p70 release in cultures treated with thrombin or thrombin derivatives versus controls, as determined using Student's t-test and Bonferroni's correction.
Thrombin affects IL-12 production via IL-10
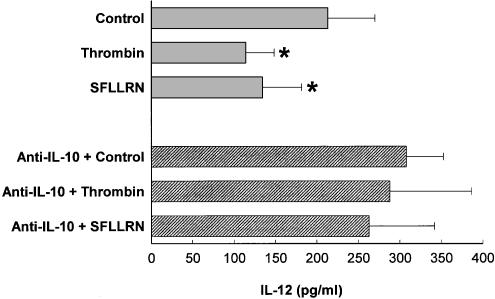
It is well established that IL-10 inhibits IL-12 production in monocytes (Moore et al., 2001). Since thrombin induces high amounts of IL-10 (Figure 1), we asked whether IL-10 is involved in the suppression of IL-12 in thrombin-treated PBMC. PBMC were therefore treated with thrombin in the presence of anti-CD3/anti-CD28 and/or anti-IL10, and cell-free supernatants were assayed for IL-12 release. Figure 5 shows that 150 nM thrombin and 40 μM SFLLRN peptide inhibits IL-12 production by 50 and 45%, respectively. The same figure shows that neutralization of IL-10 in cultures of anti-CD3/anti-CD28-treated PBMC in the presence of either thrombin or SFLLRN peptide partially abolished IL-12 downregulation. Similar results were obtained in PBMC activated by other stimuli, such as PHA (data not shown). Therefore, IL-12 downregulation by thrombin may be mediated by IL-10.
Figure 5.
Thrombin inhibition of IL-12 production is related to IL-10. PBMC (106 cells ml−1) were cultured in the presence of 150 nM thrombin and 40 μM SFLLRN peptide for 1 h at 37°C, then supplemented with FCS (1%) and anti-CD3 (0.1 μg ml−1)/anti-CD28 (1 μg ml−1), in the presence or absence of 10 μg ml−1 of anti-IL-10 (BT-10). After 16 h of culture, cell-free supernatants were obtained and IL-12 p70 present in the supernatants was determined by ELISA. The mean±s.e.m. of three independent experiments are presented. Asterisks indicate statistically significant (P<0.05) differences between IL-12 release in cultures treated with thrombin or thrombin derivatives versus controls, as determined using Student's t-test and Bonferroni's correction.
Both thrombin and PAR-1 have been demonstrated to induce the release of PGE2 from various cells types (Asokananthan et al., 2002) and PGE2 is known to inhibit the production of IL-12 (van der Pouw Kraan et al., 1995). To determine whether thrombin-induced IL-12 donregulation was dependent on PGE2, we performed additional experiments where PHA-activated PBMC were incubated in the presence of thrombin and indomethacin. However, in three independent experiments, preincubation of PHA-activated PBMC with 10 μM indomethacin before 300 nM thrombin exposure did not significantly alter the release of IL-12 (12±2% of control) when compared with that obtained with PHA-activated cells exposed to thrombin alone (17±1% of control).
Coagulation activation inhibits IL-12 production in whole blood cultures via IL-10
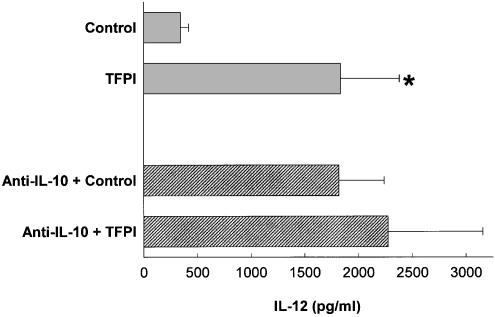
Since thrombin inhibits IL-12 production and both thrombin and activation of the coagulation cascade induced proinflammatory vascular responses, we next investigated whether coagulation activation inhibits the release of IL-12. To test this hypothesis, nonheparinized whole blood cultures were challenged with LPS in the presence of the inhibitor of the extrinsic pathway of coagulation, TFPI. Figure 6 shows that whole blood cultures challenged with LPS and in the presence of TFPI enhanced IL-12 production by four-fold. As expected, neutralization of IL-10 increased the levels of IL-12 in the cultures where TFPI was absent, since it was abolished the negative feedback exerted by IL-10 on IL-12 production by the monocytes present in the whole blood cultures. However, when TFPI was added to the same cultures, we could not observe a further and significant IL-12 upregulation. Similar results were obtained in whole blood cultures challenged with LPS and other inhibitors of the coagulation cascade, such as hirudin and heparin (data not shown). Therefore, coagulation activation resulted in IL-12 downregulation by an a mechanism mediated, at least in part, by IL-10. Thus, these results support the finding that thrombin may inhibit IL-12 release via IL-10.
Figure 6.
Activation of coagulation inhibits IL-12 production in LPS-activated whole blood cultures. Whole blood (diluted 1 : 10 in IMDM) was cultured with TFPI (50 μg ml−1) and LPS (0.1 ng ml−1) in the presence or in the absence of 10 μg ml−1 of anti-IL-10 (B-T10). After 16 h of culture, cell-free supernatants were obtained and IL-12 p40 present in the supernatants was determined by ELISA. The means±s.e.m. of five independent experiments are presented. Asterisks indicate statistically significant (P<0.05) differences between IL-12 release in cultures treated with TFPI versus controls, as judged by Student's t-test.
Discussion
The present data indicate that thrombin is a strong inhibitor of IL-12 production in activated PBMC. Further, thrombin enhances IL-10 production. IL-12 downregulation is dependent upon IL-10, since treatment of PBMC with anti-IL10 resulted in a restoration of IL-12 release. Treatment of PBMC with the PAR-1 agonist peptide SFLLRN also resulted in a decreased IL-12 production, whereas DIP-inactivated thrombin was unable to inhibit IL-12 release. Thus, it appears that thrombin downregulates Th1-related responses and that this downregulation is thrombin-specific and requires thrombin proteolytic activity. Coagulation activation also resulted in downregulation of IL-12 release, since treatment of whole blood with TFPI, the main inhibitor of the extrinsic coagulation cascade, elicited an enhancement of IL-12 production. This is in agreement with the hypothesis, supported by several reports, that deficient IL-12 production has been implicated in immunosuppression and increased susceptibility to sepsis after injury. Indeed, IL-12 acts as both proinflammatory cytokine and an immunomodulator which contributes to a Th1-type response (Trinchieri, 1998).
IL-12 may play a role in the hemostatic disorders frequently observed during sepsis (Jansen et al., 1996), as it causes a late fibrinolytic responses after initial activation of coagulation (Lauw et al., 1999). Interestingly, thrombin is increased during sepsis and plays a role in dysregulated coagulation (Woodman et al., 2000). Acquired antithrombin deficiencies are commonly found in sepsis and the level is predictive of outcome (White & Perry, 2001). Indeed, recombinant human antithrombin III improves survival and attenuates inflammatory responses in baboons lethally challenged with Escherichia coli (Minnema et al., 2000). Thus, it is evident that thrombin and IL-12 may interact in the onset of sepsis and in the outcome of such disorders.
PGE2 and COX-2 are associated with downregulation of IL-12 production after injury (Schwacha et al., 2002). However, IL-12 downregulation by thrombin appeared to be independent of PGE2, since pretreatment of PHA-activated PBMC with indomethacin did not restore IL-12 production. This is in agreement with other reports, showing that indomethacin treatment does not ablate PAR-1-induced IL-6 and IL-8 release from human respiratory epithelial cells (Asokananthan et al., 2002). Thus, thrombin affects IL-12 expression directly and, along with other proinflammatory mediators, may play a concerted role in immunosuppression and increased susceptibility to sepsis, after injury. At the same time, thrombin, along with other proinflammatory mediators, by reducing the release of IL-12, may control the inflammatory response by a negative feedback mechanism. This will avoid a prolonged inflammatory response and IL-12 downregulation may be especially important to modulate the effects of thrombin during inflammation.
It is well recognized that thrombin elicits proinflammatory activities by activation of PAR-1 receptor (Cirino et al., 2000; Vergnolle et al., 2001). In previous studies, we showed that PAR-1 expression and responsiveness of cells to thrombin were linked to IFNγ-induced differentiation (Naldini et al., 1998). Other laboratories have also shown that leukocytes express PAR-1 (Howells et al., 1993) and that PARs are associated with inflammation (Cirino et al., 1996). Therefore, it is not surprising that thrombin may control IL-12 production through a mechanism that requires thrombin proteolytic activity and our results suggest that IL-12 downregulation by thrombin may be an additional regulatory mechanism in blocking the proinflammatory effects generated by thrombin itself. Thus, thrombin ability to inhibit IL-12 may be extremely valuable in a feedback mechanism between PBMC and thrombin during an inflammatory response.
The ability of thrombin to inhibit IL-12 and to enhance IL-10 release in human PBMC could have additional important physiological consequences in the orchestration of the immune response. In whole blood and in PBMC cultures, IL-12 is predominantly released by monocytes and the IL-12 produced is responsible for driving the development of a Th1 response (Trinchieri, 1993). While Th1 cytokines are mainly involved in cell-mediated immunity, Th2 cytokines are responsible for strong humoral immune response and inhibit macrophage functions (Del Prete et al., 1994). In addition, different factors have been shown to be responsible for the polarization of T lymphocytes into a Th1 or Th2 profile and all these factors influence Th1/Th2 type polarization by the presence of specific cytokines in the microenvironment, where the cross-talk between monocytes and lymphocytes is crucial (O'Garra & Arai, 2000). It should be pointed out that our experiments were conducted using PBMC, where both monocytes and lymphocytes were present. Moreover, T cell clones with a Th1 profile are capable of promoting procoagulant activity and tissue factor induction, while Th2-polarized clones are not (Del Prete et al., 1995). Thus, thrombin, by downregulating IL-12 and enhancing IL-10 release, may antagonize the development of a Th1 profile, promote a Th2-type response and control a further promotion of procoagulant activity and tissue factor induction.
IL-12 downregulation by thrombin may be relevant also in another patho-physiological processes associated with inflammation, such as angiogenesis. Thrombin is a strong proangiogenic mediator (Tsopanoglou et al., 1993) and induces the release of proangiogenic cytokines, such as IL-8 (Johnson et al., 1998). Our results show that thrombin inhibits the release of IL-12, well known for its antiangiogenic effect (Voest et al., 1995; Cavallo et al., 2001; Wigginton et al., 2001). Thus, downregulation of IL-12 may be an additional mechanism by which thrombin promotes angiogenesis. The above effects mediated by thrombin in inflammation and angiogenesis require the involvement of PAR-1 receptors (Tsopanoglou & Maragoudakis, 1999; Cunningham et al., 2000; Griffin et al., 2001; Naldini et al., 2002). Thus, it may be possible to use specific agonists or antagonists of PAR-1, or agents which regulate expression of these receptors, to abolish the nonbeneficial effects induced by thrombin itself especially in inflammation. These results strongly support that thrombin is an important modulator of the cytokine cascade and a key mediator in inflammation/immunity, and propose a novel role for thrombin as a regulator of Th1/Th2 responses.
Acknowledgments
We thank Mr S. Focardi for the excellent technical assistance. This work was supported in part by MIUR (Cofin, 2001; 2002) and by PAR (Progetti, 2000, Università di Siena), to A.N.
Abbreviations
- IL
interleukin
- LPS
lipopolysaccharide
- PAR
protease-activated receptor
- PBMC
peripheral blood mononuclear cells
- PHA
phytohemagglutinin
- Th
T helper
- TFPI
tissue factor pathway inhibitor
References
- ASOKANANTHAN N., GRAHAM P.T., FINK J., KNIGHT D.A., BAKKER A.J., MCWILLIAM A.S., THOMPSON P.J., STEWART G.A. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E(2) release from human respiratory epithelial cells. J. Immunol. 2002;168:3577–3585. doi: 10.4049/jimmunol.168.7.3577. [DOI] [PubMed] [Google Scholar]
- BUTENAS S., VAN'T VEER C., MANN K.G. ‘Normal' thrombin generation. Blood. 1999;94:2169–2178. [PubMed] [Google Scholar]
- CAVALLO F., QUAGLINO E., CIFALDI L., DI CARLO E., ANDRE A., BERNABEI P., MUSIANI P., FORNI G., CALOGERO R.A. Interleukin 12-activated lymphocytes influence tumor genetic programs. Cancer Res. 2001;61:3518–3523. [PubMed] [Google Scholar]
- CIRINO G., BUCCI M., CICALA C., NAPOLI C. Inflammation-coagulation network: are serine protease receptors the knot. Trends Pharmacol. Sci. 2000;21:170–172. doi: 10.1016/s0165-6147(00)01469-3. [DOI] [PubMed] [Google Scholar]
- CIRINO G., CICALA C., BUCCI M.R., SORRENTINO L., MARAGANORE J.M., STONE S.R. Thrombin functions as an inflammatory mediator through activation of its receptor. J. Exp. Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLOTTA F., SCIACCA F.L., SIRONI M., LUINI W., RABIET M.J., MANTOVANI A. Expression of monocyte chemotactic protein-1 by monocytes and endothelial cells exposed to thrombin. Am. J. Pathol. 1994;144:975–985. [PMC free article] [PubMed] [Google Scholar]
- CUNNINGHAM M.A., RONDEAU E., CHEN X., COUGHLIN S.R., HOLDSWORTH S.R., TIPPING P.G. Protease-activated receptor 1 mediates thrombin-dependent, cell-mediated renal inflammation in crescentic glomerulonephritis. J. Exp. Med. 2000;191:455–462. doi: 10.1084/jem.191.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ANDREA A., ASTE-AMEZAGA M., VALIANTE N.M., MA X., KUBIN M., TRINCHIERI G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL PRETE G., DE CARLI M., LAMMEL R.M., D'ELIOS M.M., DANIEL K.C., GIUSTI B., ABBATE R., ROMAGNANI S. Th1 and Th2 T-helper cells exert opposite regulatory effects on procoagulant activity and tissue factor production by human monocytes. Blood. 1995;86:250–257. [PubMed] [Google Scholar]
- DEL PRETE G., MAGGI E., ROMAGNANI S. Human Th1 and Th2 cells: functional properties, mechanisms of regulation, and role in disease. Lab. Invest. 1994;70:299–306. [PubMed] [Google Scholar]
- GRAND R.J.A., TURNELL A.S., GRABHAM P.W. Cellular consequences of thrombin-receptor activation. Biochem. J. 1996;313:353–368. doi: 10.1042/bj3130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN C.T., SRINIVASAN Y., ZHENG Y.W., HUANG W., COUGHLIN S.R. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science. 2001;293:1666–1670. doi: 10.1126/science.1061259. [DOI] [PubMed] [Google Scholar]
- HOWELLS G.L., MACEY M., CURTIS M.A., STONE S.R. Peripheral blood lymphocytes express the platelet-type thrombin receptor. Br. J. Haematol. 1993;84:156–160. doi: 10.1111/j.1365-2141.1993.tb03039.x. [DOI] [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.-W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- JANSEN P.M., VAN DER POUW KRAAN T.C., DE JONG I.W., VAN MIERLO G., WIJDENES J., AARDEN L., CHANG A.A., TAYLOR F.B., JR, HACK C.E. The release of interleukin-12 in Escherichia coli-induced lethal and sublethal primate sepsis. Ann. N.Y. Acad. Sci. 1996;795:351–353. doi: 10.1111/j.1749-6632.1996.tb52690.x. [DOI] [PubMed] [Google Scholar]
- JOHNSON K., AARDEN L., CHOI Y., DE GROOT E., CREASEY A. The proinflammatory cytokine response to coagulation and endotoxin in whole blood. Blood. 1996;87:5051–5060. [PubMed] [Google Scholar]
- JOHNSON K., CHOI Y., DEGROOT E., SAMUELS I., CREASEY A., AARDEN L. Potential mechanisms for a proinflammatory vascular cytokine response to coagulation activation. J. Immunol. 1998;160:5130–5135. [PubMed] [Google Scholar]
- KAHN M.L., ZHENG Y.-W., HUANG W., BIGORNIA V., ZENG D., MOFF S., FARESE R.V., JR, TAM C., COUGHLIN S.R. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- KAUR J., WOODMAN R.C., OSTROVSKY L., KUBES P. Selective recruitment of neutrophils and lymphocytes by thrombin: a role for NF-kappaB. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H784–H795. doi: 10.1152/ajpheart.2001.281.2.H784. [DOI] [PubMed] [Google Scholar]
- LAUW F.N., DEKKERS P.E., TE VELDE A.A., SPEELMAN P., LEVI M., KURIMOTO M., HACK C.E., VAN DEVENTER S.J., VAN DER P.T. Interleukin-12 induces sustained activation of multiple host inflammatory mediator systems in chimpanzees. J. Infect. Dis. 1999;179:646–652. doi: 10.1086/314636. [DOI] [PubMed] [Google Scholar]
- MINNEMA M.C., CHANG A.C., JANSEN P.M., LUBBERS Y.T., PRATT B.M., WHITTAKER B.G., TAYLOR F.B., HACK C.E., FRIEDMAN B. Recombinant human antithrombin III improves survival and attenuates inflammatory responses in baboons lethally challenged with Escherichia coli. Blood. 2000;95:1117–1123. [PubMed] [Google Scholar]
- MOORE K.W., DE WAAL M.R., COFFMAN R.L., O'GARRA A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- NALDINI A., CARNEY D.H. Thrombin modulation of natural killer activity in human peripheral lymphocytes. Cell Immunol. 1996;172:35–42. doi: 10.1006/cimm.1996.0212. [DOI] [PubMed] [Google Scholar]
- NALDINI A., CARNEY D.H., BOCCI V., KLIMPEL K.D., ASUNCION M., SOARES L.E., KLIMPEL G.R. Thrombin enhances T cell proliferative responses and cytokine production. Cell. Immunol. 1993;147:367–377. doi: 10.1006/cimm.1993.1076. [DOI] [PubMed] [Google Scholar]
- NALDINI A., CARNEY D.H., PUCCI A., PASQUALI A., CARRARO F. Thrombin regulates the expression of proangiogenic cytokines via proteolytic activation of protease-activated receptor-1. Gen. Pharmacol. 2002;35:255–259. doi: 10.1016/s0306-3623(01)00113-6. [DOI] [PubMed] [Google Scholar]
- NALDINI A., SOWER L.E., BOCCI V., MEYERS B., CARNEY D.H. Thrombin receptor expression and responsiveness of human monocytic cells to thrombin is linked to interferon-induced cellular differentiation. J. Cell. Physiol. 1998;177:76–84. doi: 10.1002/(SICI)1097-4652(199810)177:1<76::AID-JCP8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- O'GARRA A., ARAI N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542–550. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- ROMAGNANI P., ANNUNZIATO F., PICCINNI M.P., MAGGI E., ROMAGNANI S. Th1/Th2 cells, their associated molecules and role in pathophysiology. Eur. Cytokine Network. 2000;11:510–511. [PubMed] [Google Scholar]
- ROMAGNANI S. The Th1/Th2 paradigm. Immunol. Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- SCHMIDLIN F., BUNNETT N.W. Protease-activated receptors: how proteases signal to cells. Curr. Opin. Pharmacol. 2001;1:575–582. doi: 10.1016/s1471-4892(01)00099-6. [DOI] [PubMed] [Google Scholar]
- SCHWACHA M.G., CHUNG C.S., AYALA A., BLAND K.I., CHAUDRY I.H. Cyclooxygenase 2-mediated suppression of macrophage interleukin-12 production after thermal injury. Am. J. Physiol. Cell Physiol. 2002;282:C263–C270. doi: 10.1152/ajpcell.00357.2001. [DOI] [PubMed] [Google Scholar]
- TRINCHIERI G. Interleukin-12 and its role in the generation of Th1 cells. Immunol. Today. 1993;14:335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- TRINCHIERI G. Proinflammatory and immunoregulatory functions of interleukin-12. Int. Rev. Immunol. 1998;16:365–396. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- TSOPANOGLOU N.E., MARAGOUDAKIS M.E. On the mechanism of thrombin-induced angiogenesis: potentiation of vascular endothelial gowth factor activity on endothelial cells by upregulation of its receptor. J. Biochem. 1999;274:23969–23976. doi: 10.1074/jbc.274.34.23969. [DOI] [PubMed] [Google Scholar]
- TSOPANOGLOU N.E., PIPILI-SYNETOS E., MARAGOUDAKIS M.E. Thrombin promotes angiogenesis by a mechanism independent of fibrin formation. Am. J. Physiol. 1993;264:C1302–C1307. doi: 10.1152/ajpcell.1993.264.5.C1302. [DOI] [PubMed] [Google Scholar]
- VAN DER POUW KRAAN T.C., BOEIJE L.C., SMEENK R.J., WIJDENES J., AARDEN L.A. Prostaglandin-E2 is a potent inhibitor of human interleukin 12 production. J. Exp. Med. 1995;181:775–779. doi: 10.1084/jem.181.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER POUW KRAAN T.C., SNIJDERS A., BOEIJE L.C., DE GROOT E.R., ALEWIJNSE A.E., LEURS R., AARDEN L.A. Histamine inhibits the production of interleukin-12 through interaction with H2 receptors. J. Clin. Invest. 1998;102:1866–1873. doi: 10.1172/JCI3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., WALLACE J.L., BUNNETT N.W., HOLLENBERG M.D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 2001;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- VOEST E.E., KENYON B.M., O'REILLY M.S., TRUITT G., D'AMATO R.J., FOLKMAN J. Inhibition of angiogenesis in vivo by interleukin 12. J. Natl. Cancer Inst. 1995;87:581–586. doi: 10.1093/jnci/87.8.581. [DOI] [PubMed] [Google Scholar]
- VU T.-K.H., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- WHITE B., PERRY D. Acquired antithrombin deficiency in sepsis. Br. J. Haematol. 2001;112:26–31. doi: 10.1046/j.1365-2141.2001.02396.x. [DOI] [PubMed] [Google Scholar]
- WIGGINTON J.M., GRUYS E., GEISELHART L., SUBLESKI J., KOMSCHLIES K.L., PARK J.W., WILTROUT T.A., NAGASHIMA K., BACK T.C., WILTROUT R.H. IFN-gamma and Fas/FasL are required for the antitumor and antiangiogenic effects of IL-12/pulse IL-2 therapy. J. Clin. Invest. 2001;108:51–62. doi: 10.1172/JCI10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODMAN R.C., TEOH D., PAYNE D., KUBES P. Thrombin and leukocyte recruitment in endotoxemia. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1338–H1345. doi: 10.1152/ajpheart.2000.279.3.H1338. [DOI] [PubMed] [Google Scholar]