Abstract
The increase in levels of cAMP in leukocytes by selective inhibitors of PDE4 may result in reduction of inflammation, and may be useful in the treatment of pulmonary inflammatory disorders in humans. Here, we have assessed whether oral treatment with the prototype PDE4 inhibitor, rolipram, interfered with the antibacterial host response following pulmonary infection of mice with Klebsiella pneumoniae.
K. pneumoniae infection induced a marked increase in the recruitment of neutrophils to the lungs and the production of proinflammatory cytokines and chemokines, including tumor necrosis factor-α (TNF-α) and keratinocyte-derived chemokine (KC), in bronchoalveolar (BAL) fluid and lung tissue. There were also detectable amounts of interleukin-10 (IL-10) and significant lethality.
Treatment with rolipram (3–30 mg kg−1) was associated with earlier lethality and significant inhibition of the TNF-α production. This was associated with enhanced production of IL-10 in lung tissue of rolipram-treated animals. Rolipram treatment did not affect KC expression and the recruitment of neutrophils in the lung tissue.
Over 70% of neutrophils that migrated into the BAL fluid following K. pneumoniae infection ingested bacteria. Treatment with rolipram inhibited the percentage of neutrophils undergoing phagocytosis of K. pneumoniae in a dose-dependent manner. Maximal inhibition (62%) occurred at doses equal to or greater than 10 mg kg−1.
Thus, treatment of mice with the PDE4 inhibitor rolipram is accompanied by earlier lethality, enhanced bacterial load and decreased capacity of the responding host to produce TNF-α and of neutrophils to phagocytose bacteria. It will be important to investigate whether the shown ability of PDE4 inhibitors to inhibit neutrophil phagocytosis and control experimental bacterial infection will translate into an inhibition of the ability of neutrophils to deal with infectious microorganisms in the clinical setting.
Keywords: Phophodiesterase-4 inhibitors, lung inflammation, neutrophils, chemokines, TNF-α, bacterial infection
Introduction
The host defense against acute pulmonary bacterial infection requires the generation of a vigorous inflammatory response that predominantly involves recruitment and activation of neutrophils (Mehrad & Standiford, 1999). This process is dependent on an intricate balance of the production of lipid mediators, cytokines and chemokines, and may be sufficient to control the replication of invading microorganisms (Mehrad & Standiford, 1999; Teixeira et al., 2001). On the contrary, the inhibition of the production of proinflammatory cytokines, chemokines and failure of neutrophils to migrate into the lung tissue may be associated with uncontrolled bacterial infection and death of the infected host (Mehrad & Standiford, 1999).
The increase in intracellular levels of cAMP and the subsequent activation of cAMP-dependent protein kinases in cells participating in the inflammatory process may result in a reduction of inflammation and immunosupression (Teixeira et al., 1997; Torphy, 1998). The intracellular concentration of cyclic nucleotides is mainly governed on the one hand by the activation of receptor-coupled adenylate cyclase, and on the other by cyclic nucleotide breakdown by phosphodiesterases (PDEs). To date, PDEs are divided into 11 families, of which PDE4 is the most studied and functionally important for the regulation of cAMP in leukocytes (Torphy, 1998; Souness et al., 2000). Due to their potent anti-inflammatory activity, there has been much interest in selective PDE4 inhibitors in the treatment of pulmonary inflammatory disorders (Teixeira et al., 1997; Barnette, 1999; Schimidt et al., 1999; Giembycz, 2002), and at least one study has found a beneficial effect of using PDE4 inhibitors for a chronic inflammatory disease of the lung in humans (Compton et al., 2001). Indeed, the efficacy of PDE4 inhibitors in models of lung injury and shock is very impressive and includes inhibition, very often complete, of the increases in serum levels of tumor necrosis factor-α (TNF-α), tissue injury and mortality (Sekut et al., 1994,1995; Teixeira et al., 1997; De Moraes et al., 1998; Miotla et al., 1998; Souza et al., 2001). If PDE4 inhibitors are to be used for the treatment of acute and/chronic inflammatory diseases of the lungs, it is imperative that we understand whether these drugs may interfere with the antibacterial host response in the lungs of infected animals.
In this study, we have investigated the effects of rolipram, a prototypical and orally active inhibitor of PDE4 in a model of pulmonary infection caused by Gram-negative bacteria. To this end, we have assessed the effects of the treatment with rolipram on the lethality, bacterial counts and inflammatory indices following pulmonary infection of mice with Klebsiella pneumoniae.
Methods
Animals
Balb/C (8–12 weeks old) female mice obtained from the Bioscience unit of our Institution were housed in standard conditions and had free access to commercial chow and water. All procedures described here had prior approval from the animal ethics committee of Instituto de Ciências Biológicas (Belo Horizonte, Brazil).
Bacteria
The bacterium used was K. pneumoniae – ATCC 27 736, which had been kept in the Department of Microbiology, Universidade Federal de Minas Gerais and made pathogenic by 10 passages in Balb/C mice (Soares et al, 2002). Bacteria were frozen when in the log phase of growth and kept in a −70°C freezer at a concentration of 1 × 109 colony-forming units (CFU) ml−1 in tryptic soy broth containing 10% glycerol (v v−1) until use.
Treatment with rolipram
Rolipram (Biomol®) was suspended in 0.1% methylcellulose solution and ground in a homogenizer to ensure a uniform suspension. Control animals received an oral administration of vehicle (0.5 ml), whereas the test group received an oral administration of Rolipram (3–30 mg kg−1). For the experiments measuring infection and inflammatory indices, the drug was administered 24 and 2 h prior to inoculation of bacteria and animals were killed 24 h after inoculation. For lethality experiments, the compound was administered at a dose of 10 mg kg−1 24 and 2 h prior to the inoculation of bacteria and daily thereafter. At 10 mg kg−1, rolipram effectively inhibited some inflammatory parameters, phagocytosis (see below), and has been shown to suppress inflammatory parameters in several models of inflammatory pulmonary disease and shock (Sekut et al., 1994,1995; Kung et al., 2000).
K. pneumoniae inoculation
K. pneumoniae was grown in tryptic soy broth (Difco, Detroit, MI, U.S.A.) for 18 h at 37°C prior to inoculation. The concentration of bacteria in broth was routinely determined by serial 1 : 10 dilutions. A measure of 100 μl of each dilution was plated on McConkey agar plates and incubated for 24 h at 37°C, and then the colonies were counted. Each animal was anesthetized i.p. with 0.2 ml of a solution containing xylazine (0.02 mg ml−1), ketamine (50 mg ml−1) and saline in a proportion of 1 : 0.5 : 3, respectively. The trachea was exposed and 30 μl of a suspension containing 3 × 106 K. pneumoniae or saline was administered with a sterile 26-gauge needle. The skin incision was closed with surgical staples. In further experiments, lethality induced by a lower inoculum (3 × 104) of K. pneumoniae was also investigated.
Bronchoalveolar lavage (BAL)
BAL was performed to obtain leukocytes in the alveolar spaces. The trachea was exposed and a 1.7-mm-outside-diameter polyethylene catheter was inserted. BAL was performed by instilling three 1-ml aliquots of phosphate-buffered saline (PBS), and approximately 2 ml of fluid was retrieved per mouse. The number of total leukocytes was determined by counting them in a modified Neubauer chamber after staining with Turk's solution. Differential counts were obtained from cytospin preparations (Shandon III) by evaluating the percentage of each leukocyte on a slide stained with May–Grunwald–Giemsa. Phagocytosis was assessed by determining the mean percentage of BAL neutrophils that contained at least one bacterium. In total, 200 neutrophils were counted in each condition.
Determination of myeloperoxidase (MPO) activity
The extent of neutrophil accumulation in the lung tissue was measured by assaying MPO activity, as previously described (De Matos et al., 1999). Using the conditions described below, the methodology is very selective for the determination of neutrophils over macrophages (Souza et al., 2002). Briefly, a portion of the left lungs of animals was removed and snap-frozen in liquid nitrogen. Upon thawing, the tissue (0.1 g of tissue per 1.9 ml of buffer) was homogenized in pH 4.7 buffer (0.1 M NaCl, 0.02 M NaPO4, 0.015 M NaEDTA), centrifuged at 3000 × g for 10 min and the pellet subjected to hypotonic lyses (1.5 ml of 0.2% NaCl solution followed 30 s later by the addition of an equal volume of a solution containing NaCl 1.6% and glucose 5%). After a further centrifugation, the pellet was resuspended in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide (HTAB) and rehomogenized. In all, 1 ml aliquots of the suspension were transferred into 1.5 ml-Eppendorf tubes followed by three freeze–thaw cycles using liquid nitrogen. The aliquots were then centrifuged for 15 min at 3000 × g, the pellet was resuspended to 1 ml and samples of lungs were diluted (1 : 20) prior to assay. MPO activity in the resuspended pellet was assayed by measuring the change in optical density (OD) at 450 nm using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM). The results were expressed as ‘myeloperoxidase index' and were calculated by comparing the OD of tissue supernatant with the OD of mouse peritoneal neutrophils processed in the same way. To this end, neutrophils were induced in the peritoneum of mouse by injecting 3 ml of 5% casein. A standard curve of neutrophil (>95% purity) numbers versus OD was obtained by processing purified neutrophils as above and assaying for MPO activity.
Determination of blood and lung K. pneumoniae CFU
At the time of killing, plasma was collected from the brachial plexus, the right ventricle was perfused with 3 ml of sterile saline and the lungs were harvested. Tissues were then homogenized with a homogenizer in a vented hood. The homogenates and blood were placed on ice, and serial 1 : 10 dilutions were made. A measure of 100 μl of each dilution was plated on McConkey agar plates (Difco) and incubated for 24 h at 37°C, and then the number of CFU was counted. The detection limit of the assay was 100 bacteria ml−1 or 100 bacteria per 100 mg of tissue.
Harvesting of the lungs and blood for cytokine analysis
At the designated time point, mice were anesthetized with xylazin/ketamin/saline as above, blood was collected from the brachial plexus and the animals were killed. Prior to the lung removal, the pulmonary vasculature was perfused with 3 ml of PBS via the right ventricle. The right lung was then harvested for assessment of the various cytokine protein levels.
Measurement of cytokine concentrations in serum, BAL and lungs
The cytokine concentrations (TNF-α, KC, MCP-1/JE, IL-10) were measured in serum, BAL and lungs of animals, using ELISA techniques with commercially available antibodies and according to the instructions supplied by the manufacturer (R&D Systems). Serum was obtained from coagulated blood (15 min at 37°C, then 30 min at 4°C) and stored at −20°C until further analysis. Serum and BAL samples were analyzed at a 1 : 3 and 1 : 5 dilution in the assay dilution buffer, respectively. In total, 100 mg of the lung of controls and treated animals were homogenized in 1 ml of PBS (0.4 m NaCl and 10 mM NaPO4) containing antiproteases (0.1 mM PMSF, 0.1 mM benzethonium chloride, 10 mM EDTA and 20 KI aprotinin A) and 0.05% Tween 20. The samples were then centrifuged for 10 min at 3000 × g and the supernatant was immediately used for ELISA assays at a 1 : 5 dilution in the assay dilution buffer. The detection limit of the ELISA assays was 16 pg ml−1.
Statistical analysis
Results are shown as means±s.e.m. Data sets were compared by using analysis of variance (ANOVA) followed by the Student–Newman–Keuls post hoc analysis. The results were considered significant when P<0.05. As the various parameters evaluated in vehicle- and rolipram (10 mg kg−1)-treated noninfected mice were not significantly different (data not shown), the data were pooled and are shown as mean±s.e.m. of noninfected mice.
Results
Effects of Rolipram on tissue inflammation and inflammatory mediator production during K. pneumoniae infection
Our previous experiments have shown that after instillation of K. pneumoniae at an inoculum of 3 × 106 bacteria, there was marked pulmonary (BAL and tissue) neutrophilia, enhanced levels of the chemokines KC and MCP-1 and of the proinflammatory cytokine TNF-α in the lungs of infected mice (Soares et al., 2002). Bacterial loads increased rapidly, but we failed to observe any dissemination of the infection in blood till 48 h after infection (Soares et al., 2002).
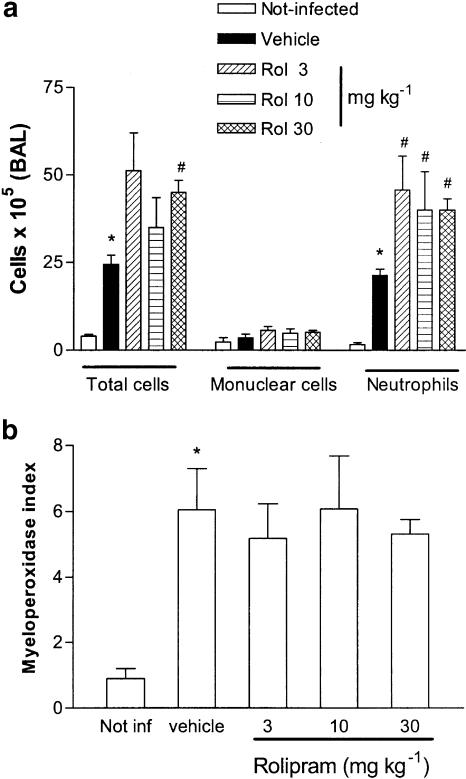
Oral treatment with various doses of rolipram (3–30 mg kg−1) had no significant effect on the number of mononuclear cells that were recovered from the airspaces of infected animals (Figure 1a). Nevertheless, the number of recruited neutrophils in rolipram-treated infected mice was significantly greater than in vehicle-treated infected controls (Figure 1a). This was reflected in the increased number of total cells recovered from BAL fluid, which was significantly greater than controls only at the highest dose of rolipram tested (30 mg kg−1) (Figure 1a). Although rolipram treatment enhanced the number of neutrophils in BAL fluid of infected animals, the drug failed to affect the recruitment of neutrophils in the lung tissue, as assessed by the measurements of tissue MPO (Figure 1b) or histopathological analysis (data not shown).
Figure 1.
Effect of the treatment with rolipram on the influx of leukocytes (a) in the BAL fluid or (b) in the lungs of mice infected with K. pneumoniae. Animals were inoculated with 3 × 106 bacteria or vehicle (30 μl, not infected) and evaluated 24 h after infection. Rolipram (Rol, 3–30 mg kg−1) was administered 24 and 2 h prior to inoculation of bacteria. MPO activity in the lungs was used as an index of neutrophil influx in that tissue. Results are shown as the number of leukocytes or leukocyte index and represent the mean±s.e.m. of six animals in each group. *P<0.01 when compared with noninfected mice and # for P<0.01 when compared to vehicle-treated mice.
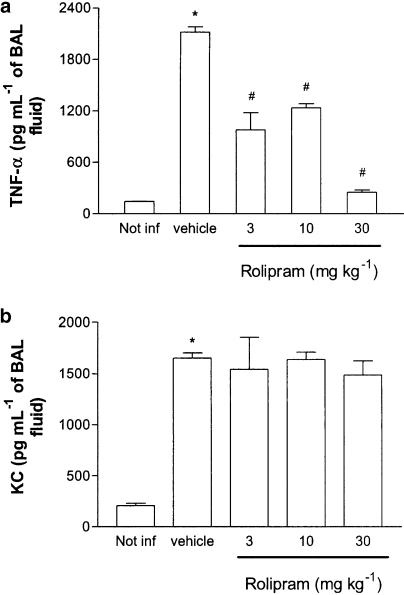
The concentrations of TNF-α, KC, MCP-1 and IL-10 in BAL fluid of infected and noninfected animals were measured 24 h after infection. Treatment with rolipram prevented the increase in the concentrations of TNF-α in BAL fluid of K. pneumoniae-infected mice in a dose-dependent manner (Figure 2a). The maximal inhibition occurred at 30 mg kg−1, but approximately 50% inhibition was attained at 10 mg kg−1. Rolipram failed to affect the elevation in the concentrations of the neutrophil-active chemokine KC observed in infected mice (Figure 2b). The concentrations of MCP-1 and IL-10 were not consistently detectable in the BAL fluid of infected mice, and rolipram treatment had little effect on these parameters (data not shown).
Figure 2.
Effect of the treatment with rolipram on the production of TNF-α (a) and KC (b) in BAL fluid of mice infected with K. pneumoniae. Animals were inoculated with 3 × 106 bacteria or vehicle (30 μl, not infected) and evaluated 24 h after infection. Rolipram (3–30 mg kg−1) was administered 24 and 2 h prior to inoculation of bacteria. Results are shown as the mean±s.e.m. of six animals in each group. *P<0.01 when compared with noninfected mice and # for P<0.01 when compared to vehicle-treated mice.
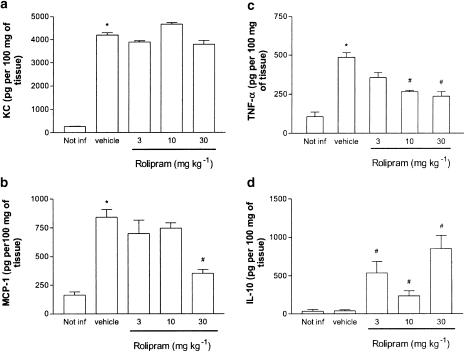
The concentrations of TNF-α, KC, MCP-1 and IL-10 in the pulmonary tissue of rolipram-treated and untreated animals are shown in Figure 3. Rolipram had no effect on the increases in concentrations of KC in the lung tissue of infected animals (Figure 3a). The concentration of MCP-1 in the lung tissue was suppressed significantly (P<0.05) by 42% by 30 mg kg−1 rolipram, but not by the lower doses tested (Figure 3b). Treatment with rolipram was accompanied by a dose-dependent reduction in the concentrations of TNF-α detected in the pulmonary tissue of infected mice (Figure 3c). In the lung tissue, both 10 and 30 mg kg−1 of rolipram had similar inhibitory actions on TNF-α production (around 60% inhibition). Interestingly, treatment with rolipram induced a marked increase in IL-10 production in the lung tissue of K. pneumoniae-infected mice (Figure 3d). The maximal induction of IL-10 production occurred at 30 mg kg−1.
Figure 3.
Effect of the treatment with rolipram on the production of (a) KC, (b) MCP-1, (c) TNF-α and (d) IL-10 in the lung tissue of mice infected with K. pneumoniae. Animals were inoculated with 3 × 106 bacteria or vehicle (30 μl) and evaluated 24 h after infection. Rolipram (3–30 mg kg−1) was administered 24 and 2 h prior to inoculation of bacteria. Results are shown as the mean±s.e.m. of six animals in each group. *P<0.01 when compared with noninfected mice and # for P<0.01 when compared to vehicle-treated mice.
Effects of rolipram on bacterial load and survival during K. pneumoniae infection
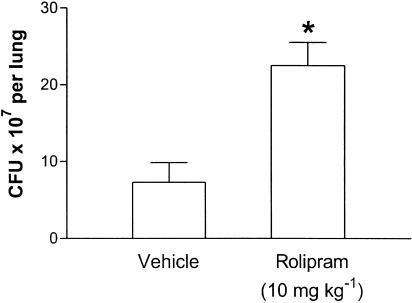
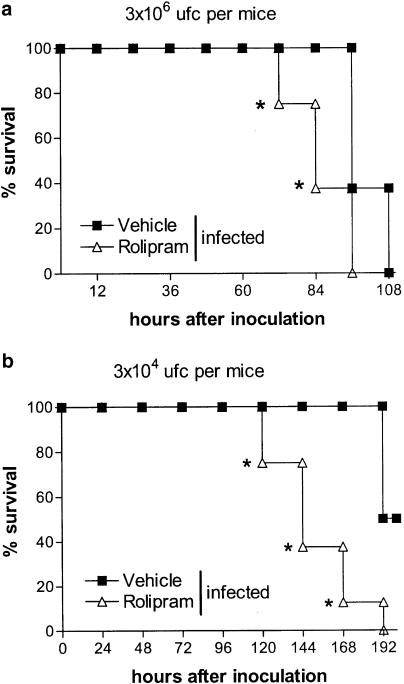
To test the effects of rolipram on bacterial load and survival, an oral dose of rolipram (10 mg kg−1) known to possess anti-inflammatory effects in models of pulmonary inflammation and shock (Sekut et al., 1994,1995; Kung et al., 2000) was used. There were approximately 107 K. pneumoniae in the lungs of mice 24 h after infection with 3 × 106 CFU (Figure 4). Treatment with rolipram was accompanied by an approximately three-fold increase in the number of CFU recovered from the lungs of infected mice (Figure 4). There was no dissemination of the infection at 24 h after infection, as no K. pneumoniae CFU could be determined in blood in either groups at this time point (data not shown). Subsequent experiments were performed to examine the effect of rolipram on the survival of mice infected with K. pneumoniae. Figure 5a shows that all untreated K. pneumoniae-infected animals were alive by 96 h of infection after which time mortality increased substantially, with 100% lethality noted 4.5 days after inoculation. The treatment with rolipram resulted in earlier lethality with 25 and 62.5% of animals dead at 72 and 84 h, respectively (Figure 5a).
Figure 4.
Effect of the treatment with rolipram on the number of colony-forming units (CFU) in the lung tissue of mice infected with K. pneumoniae. Animals were inoculated with 3 × 106 bacteria or vehicle (30 μl) and the number of CFU in lung was homogenates evaluated after 24 h. Rolipram (10 mg kg−1) was administered 24 and 2 h prior to inoculation of bacteria. Results are shown as the mean±s.e.m. of five to six animals in each group. *P<0.01 when compared with vehicle-treated mice.
Figure 5.
Effect of the treatment with rolipram on the survival of mice infected with K. pneumoniae. Animals were inoculated with (a) 3 × 106 or (b) 3 × 104 CFU bacteria or vehicle (30 μl) and the number of dead animals was evaluated (n=8 in each group). Rolipram (10 mg kg−1) was administered 24 and 2 h prior to inoculation of bacteria and daily thereafter. Results are shown as % survival and * for P<0.05 when compared with vehicle-treated mice (n=8–10 in each group).
The results obtained after infection with 3 × 106 CFU of K. pneumoniae show that lethality was precipitated by rolipram treatment. However, the inoculum caused 100% lethality at 108 h even in untreated animals (Figure 5a). To evaluate whether rolipram would also affect survival in mice given a lower inoculum, animals received 3 × 104 CFU and lethality was noted. As seen in Figure 5b, lethality rates were greatly enhanced in rolipram-treated animals. Indeed, at 168 h after infection, 90% rolipram-treated K. pneumoniae-infected mice were dead, whereas none of the vehicle-treated animals had succumbed to the infection (Figure 5b).
Effects of rolipram on the phagocytic activity of neutrophils during K. pneumoniae infection
To examine whether the neutrophils recruited in response to the K. pneumoniae infection were capable of engulfing bacteria present in that organ, the percentage of neutrophils that had ingested bacteria was quantified microscopically. As can be clearly seen in Table 1 , treatment with rolipram dose-dependently inhibited the percentage of neutrophils undergoing phagocytosis of K. pneumoniae. The maximal inhibition had already occurred at 10 mg kg−1 (Table 1).
Table 1.
Percentage of BAL fluid neutrophils containing bacteria in the cytoplasm
| Groups | % neutrophils containing bacteria |
| Vehicle treated | 78.2±6.5 |
| Rolipram treated | |
| 3 mg kg−1 | 63.1±4.8 |
| 10 mg kg−1 | 32.6±8.9* |
| 30 mg kg−1 | 30.0±2.0* |
Animals were inoculated with 3 × 106 bacteria or vehicle (30 μl) and the percentage of neutrophils containing bacteria in the cytoplasm was assessed after 24 h. Rolipram (10 mg kg−1) was administered 24 and 2 h prior to the inoculation of bacteria. Results are shown as the mean±s.e.m. of five to six animals in each group.
P<0.01 when compared with vehicle-treated mice.
Discussion
Cyclic AMP is a ubiquitous second messenger that transduces intracellular signals initiated by many biologically active agents exerting their effects through activation of adenyl cyclase. Inhibition of PDE4 blocks the degradation of cAMP, thereby causing intracellular levels of the nucleotide to increase. cAMP activates protein kinase A with subsequent phosphorylation of protein kinase A-specific substrates (Souness et al., 2000). In vivo, there are several possible mechanisms that appear to be involved in the anti-inflammatory actions of PDE4 inhibitors, including direct inhibition of leukocyte recruitment, inhibition of leukocyte activation, inhibition of proinflammatory cytokine production and enhancement of the production of anti-inflammatory cytokines (Teixeira et al., 1997; Souness et al., 2000). These actions of PDE4 inhibitors are thought to be relevant for any beneficial effects of the drugs on various models of acute and chronic inflammation. However, these actions of PDE4 inhibitors may be deleterious for the ability of a host to control a bacterial infection, as the production of proinflammatory cytokines and recruitment and activation of leukocytes, especially neutrophils, are part of an effective antibacterial host response of an infected animal (Mehrad & Standiford, 1999).
In our experiments, we investigated the effect of rolipram, an inhibitor of PDE4, in a murine model of lung infection with K. pneumoniae. This Gram-negative aerobic organism is an important cause of community-acquired pneumonia in individuals with impaired pulmonary defenses and is a major pathogen for nosocomial pneumonia (Granton & Grossman, 1993; Maloney & Jarvi, 1995). Importantly, studies in our and other laboratories showed that after intratracheal (i.t) inoculation with K. pneumoniae mice developed pneumonia with signs and features resembling the human disease (Mehrad & Standiford, 1999; Soares et al., 2002). Using the above-described model, we now show that treatment of mice with the PDE4 inhibitor rolipram is accompanied by earlier lethality, enhanced bacterial load and decreased capacity of the responding host to produce TNF-α and of neutrophils to phagocytose bacteria. Similarly, a nonspecific inhibitor of PDE, aminophyline, has also been shown to impair pulmonary antibacterial responses (Nelson et al., 1985). One preliminary study evaluated the effects of PDE4 inhibitors in infection models in vivo (DeMarsh et al., 2001). Details of the study are lacking, but it appeared that the administration of the PDE4 inhibitor cilomast concomitantly with a sublethal influenza dose failed to affect viral clearance. Nevertheless, cilomast-treated mice were more resistant to a subsequent challenge with Streptococcus pneumoniae (DeMarsh et al., 2001). Whether PDE4 inhibitors will affect Gram-positive and negative bacterial differentially infection is not known.
The ability of PDE4 inhibitors to block the influx of neutrophils into tissue varies markedly depending on the type of stimulus and model being examined. Thus, neutrophil influx is markedly attenuated in models in which it is TNF-α dependent (De Moraes et al., 1998; Souza et al., 2001) or dependent on the novel expression of cell adhesion molecules, especially E- and P-selectins (Sanz et al., 2002). On the other hand, several studies have failed to demonstrate an effect of rolipram on neutrophil influx in the skin (Teixeira et al., 1994), lungs (Miotla et al., 1998) or into air-pouch models (Klemm et al., 1995). Overall, it appears that the inhibition by rolipram of neutrophil influx relies on the ability of PDE4 inhibitors to prevent mediator and/or cell adhesion molecules expression, rather than a direct effect of the drugs on the neutrophil. Experiments examining the effect of TNF-α blockade in an animal model of K. pneumoniae infection have pinpointed the relevance of TNF-α for infection outcome (Laichalk et al., 1996). In the latter experiments, TNF-α blockade was accompanied by a 50% inhibition of neutrophil influx. Thus, it is clear that TNF-α-independent pathways are relevant for neutrophil influx during pulmonary infection with K. pneumoniae. In our study, treatment with rolipram enhanced neutrophil influx into BAL fluid, but had no significant effect on the influx of neutrophils into pulmonary tissue, as assessed by MPO activity. As previous studies have suggested a role for neutrophil-active CXC chemokines and chemokine receptors for the migration of neutrophils into the lungs of mice infected with Gram-negative bacteria (Moore et al., 2000; Tsai et al., 2000), the levels of the CXC chemokine KC were measured here. In agreement with the lack of effect of the rolipram on neutrophil recruitment into the lung tissue, the tissue concentrations of KC were not different in rolipram-treated and untreated mice. In contrast, there was no direct correlation between KC expression and neutrophil influx in BAL fluid. It is possible that other molecules may be involved in the recruitment of neutrophils to the airspaces following rolipram treatment of K. pneumoniae-infected mice. Thus, because rolipram cannot prevent the generation of the chemokine(s) responsible for neutrophil influx, it cannot prevent the influx of the latter cell type. Moreover, it is clear that an effect on neutrophil influx does not explain the deleterious effect of rolipram treatment on the course of Gram-negative bacteria lung infection.
The CC chemokine MCP-1 appears to play an important role in the pulmonary antifungal response against Aspergillus fumigatus and Cryptococcus neoformans infection in mice (Huffnagle et al., 1995; Blease et al., 2001). Similarly, the administration of MCP-1 prior to a systemic infection with Pseudomonas aeruginosa enhanced survival, an effect associated with enhanced bacterial phagocytosis and killing in vitro (Nakano et al., 1994). In our experiments, the treatment with rolipram had little effect on MCP-1 production (inhibition occurred only at the highest dose tested −30 mg kg–1), suggesting that modulation of the production of this chemokine does appear to account for the immunosuppressive effects observed.
The production and action of TNF-α is part of an effective host response during K. pneumoniae pulmonary infection (Laichalk et al., 1996). On the contrary, the production of IL-10 serves to dampen proinflammatory and antibacterial responses in the same model (Greenberger et al., 1995). In our experiments, treatment with rolipram greatly prevented the enhancement in the release of TNF-α in the lung tissue and BAL fluid of infected animals. The suppression of TNF-α production was associated with an increased production of IL-10, at least in the lung tissue. The ability of PDE4 inhibitors to suppress TNF-α production in in vivo models of inflammation is not novel (e.g. Sekut et al., 1994,1995; De Moraes et al., 1998; Miotla et al., 1998; Souza et al., 2001). Indeed, inhibition of TNF-α production by PDE4 inhibitors may account for several of the anti-inflammatory effects of these drugs in several models of inflammation (Teixeira et al., 1997). Similarly, PDE4 inhibitors have been shown to enhance IL-10 production in several in vitro and in vivo systems (e.g. Kambayashi et al., 1995; Seldon et al., 1998; Procopio et al., 1999). As IL-10 may prevent TNF-α production (e.g. Wang et al., 1995; Procopio et al., 1999), it is possible that the ability of rolipram to induce IL-10 may underlie the observed inhibition of TNF-α release. However, we have previously shown that cAMP-elevating agents may suppress TNF-α production independent of their ability to induce IL-10 (Procopio et al., 1999). Thus, the inhibition of TNF-α production may involve IL-10-dependent and -independent pathways. Regardless of which of these pathways may be more relevant, our studies clearly suggest that both inhibition of TNF-α and enhancement of IL-10 may underlie the detrimental effects of rolipram in our system.
As neutrophils are essential for the host defense against K. pneumoniae infection and rolipram did not appear to prevent neutrophil influx, it was important to evaluate whether the drug interfered with neutrophil activation. To this end, we evaluated the number of bacteria ingested by neutrophils, as an index of neutrophil activation and function. Our results showed that there were fewer neutrophils that had ingested bacteria in the lungs of rolipram-treated mice. The inability of neutrophils to ingest bacteria may underlie the greater number of CFU in the lungs of infected animals and the infection-associated lethality. At least one study in vitro (Au et al., 1998) has shown that treatment of neutrophils with PDE4 inhibitors was associated with inhibition of the phagocytosis of particles and consequent release of proinflammatory mediators. Similarly, the ability of rolipram to prevent neutrophil activation may underlie its protective effects during acute pulmonary injury following LPS and zymosan administration (Miotla et al., 1998). One other study published solely in abstract form failed to demonstrate an effect of the PDE4 inhibitor SB-207499 on bacterial killing (Malus et al., 2001). However, the type of bacteria is not mentioned, neither are the conditions of the study, making it difficult to draw comparisons with our studies. Thus, in addition to inhibiting TNF-α, a cytokine capable of activating neutrophils, rolipram may also act directly on neutrophils to prevent at least one of its functions, the phagocytosis of bacteria.
Previous studies in mice have shown that alveolar macrophages are an important part of the pulmonary response against a challenge with K. pneumoniae infection (Broug-Holub et al., 1997). Of interest, depletion of alveolar macrophages failed to suppress the influx of neutrophils (there was actually a late enhancement), but was accompanied by enhanced lethality and bacterial loads (Broug-Holub et al., 1997). The effects of rolipram treatment on macrophage function were not investigated here, as the great majority of cells recovered from BAL fluid at 24 h were neutrophils. As PDE4 inhibitors can suppress macrophage function (reviewed in Teixeira et al., 1997; Torphy, 1998), an action of orally given rolipram on macrophages could certainly contribute to the inability of the host to deal effectively with a challenge with K. pneumoniae.
There has been much interest in the use of PDE4 inhibitors as anti-inflammatory agents in various pulmonary diseases in which neutrophils are thought to play a major pathophysiological role (Teixeira et al., 1997). In at least one of these diseases, chronic obstructive pulmonary disease, pretreatment with a selective PDE4 inhibitor, cilomilast, improved pulmonary function and quality of life (Compton et al., 2001). Whether the shown ability of PDE4 inhibitors to inhibit neutrophil phagocytosis will translate into an inhibition of the ability of neutrophils to deal with infectious microorganisms in the clinical setting is not known. Investigation of the potential ability of PDE4 inhibitors to prevent effective antibacterial responses in humans is clearly needed.
Acknowledgments
We are grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação De Amparo a Pesquisas do Estado de Minas Gerais (FAPEMIG) for the financial support.
Abbreviations
- BAL
bronchoalveolar lavage
- CFU
colony-forming units
- IL-10
interleukin-10
- KC
keratinocyte-derived chemokine
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- MPO
myeloperoxidase
- PBS
phosphate-buffered saline
- PDE
phosphodiesterase
- PMN
polymorphonuclear leukocyte
- TNF-α
tumor necrosis factor-α
References
- AU B.T., TEIXEIRA M.M., COLLINS P.D., WILLIAMS T.J. Effect of PDE4 inhibitors on zymosan-induced IL-8 release from human neutrophils: synergism with prostanoids and salbutamol. Br. J. Pharmacol. 1998;123:1260–1266. doi: 10.1038/sj.bjp.0701723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNETTE M.S. Phosphodiesterase 4 (PDE)4 inhibitors asthma and chronic obstructive pulmonary disease (COPD) Prog. Drug Res. 1999;63:193–229. doi: 10.1007/978-3-0348-8735-9_5. [DOI] [PubMed] [Google Scholar]
- BLEASE K., MEHRAD B., LUKACS N.W., KUNKEL S.L., STANDIFORD T.J., HOGABOAM C.M. Antifungal and airway remodeling roles for murine monocyte chemoattractant protein-1/CCL2 during pulmonary exposure to Aspergillusfumigatusconidia. J. Immunol. 2001;166:1832–1842. doi: 10.4049/jimmunol.166.3.1832. [DOI] [PubMed] [Google Scholar]
- BROUG-HOLUB E., TOEWS G.B., VAN IWAARDEN J.F., STRIETER R.M., KUNKEL S.L., PAINE R., III, STANDIFORD T.J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMPTON C.H., GUBB J., NIEMAN R., EDELSON J., AMIT O., BAKST A., AYRES J.G., CREEMERS J.P.H.M., SCHULTZE-WERNINGHAUS G., BRAMBILLA C., BARNES N.C. Cilomilast, a selective phosphodiesterase-4 inhibitor for the treatment of patients with chronic obstructive pulmonary disease: a randomised, dose-ranging study. Lancet. 2001;358:265–270. doi: 10.1016/S0140-6736(01)05481-2. [DOI] [PubMed] [Google Scholar]
- DEMARSH P.L., SUCOLOSKI S.K., TAL-SINGER R., WELLS G., DILLON S.B., WOODNUTT G. Effect of Cilomilast, an orally active, selective PDE4 inhibitor in a murine viral/bacterial co-infection model. Am. J. Respir. Crit. Care Med. 2001;163:A381. [Google Scholar]
- DE MATOS I.M., SOUZA D.G., SEABRA D.G., FREIRE-MAIA L., TEIXEIRA M.M. Effects of tachykinin NK1- or PAF-receptor blockade on the lung injury induced by scorpion venom. Eur. J. Pharmacol. 1999;376:293–300. doi: 10.1016/s0014-2999(99)00382-9. [DOI] [PubMed] [Google Scholar]
- DE MORAES V.L.G., SINGER M., VARGAFTIG B.B., CHIGNARD M. Effects of rolipram on cAMP levels in alveolar macrophages and lipopolysaccharide-induced inflammation in mouse lung. Br. J. Pharmacol. 1998;123:631–636. doi: 10.1038/sj.bjp.0701649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEMBYCZ M.A. Development status of second generation PDE4 inhibitors for asthma and COPD: the story so far. Monaldi Arch. Chest Dis. 2002;57:48–64. [PubMed] [Google Scholar]
- GRANTON J.T., GROSSMAN R.F. Community-acquired pneumonia in the elderly patient. Clinical features, epidemiology, and treatment. Clin. Chest Med. 1993;14:537–539. [PubMed] [Google Scholar]
- GREENBERGER M.J., STRIETER R.M., KUNKEL S.L., DANFORTH J.M., GOODMAN R.E., STANDIFORD T.J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J. Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- HUFFNAGLE G.B., STRIETER R.M., STANDIFORD T.J., MCDONALD R.A., BURDICK M.D., KUNKEL S.L., TOEWS G.B. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- KAMBAYASHI T., JACOB C.O., ZHOU D., MAZUREK N., FONG M., STRASSMANN G. Cyclic nucleotide phosphodiesterase type IV participates in the regulation of IL-10 and in the subsequent inhibition of TNF-alpha and IL-6 release by endotoxin-stimulated macrophages. J. Immunol. 1995;155:4909–4916. [PubMed] [Google Scholar]
- KLEMM P., HARRIS H.J., PERRETTI M. Effect of rolipram in a murine model of acute inflammation: comparison with the corticoid dexamethasone. Eur. J. Pharmacol. 1995;281:69–74. doi: 10.1016/0014-2999(95)00232-a. [DOI] [PubMed] [Google Scholar]
- KUNG T.T., CRAWLEY Y., LUO B., YOUNG S., KREUTNER W., CHAPMAN R.W. Inhibition of pulmonary eosinophilia and airway hyperrresponsiviness in allergic mice by rolipram: involvement of endogenously released corticosterone and catecholamines. Br. J. Pharmacol. 2000;130:457–463. doi: 10.1038/sj.bjp.0703308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAICHALK L.L., KUNKEL S.L., STRIETER R.M., DANFORTH J.M., BAILIE M.B., STANDIFORD T.J. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect. Immun. 1996;64:5211–5218. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALONEY S.A., JARVI W.R. Epidemic nosocomial pneumonia in the intensive care unit. Clin. Chest Med. 1995;16:209–223. [PubMed] [Google Scholar]
- MALUS E., CHERAPANOV V., ARORA A., SJOLIN C., DOWNEY G.P. Selective inhibition of neutrophil function by the phosphodiesterase 4 inhibitor SB 207499. Am. J. Respir. Crit. Care Med. 2001;163:A993. [Google Scholar]
- MEHRAD B., STANDIFORD T.J. Role of cytokines in pulmonary antimicrobial host defense. Immunol. Res. 1999;20:15–27. doi: 10.1007/BF02786504. [DOI] [PubMed] [Google Scholar]
- MIOTLA J.M., TEIXEIRA M.M., HELLEWELL P.G. Supression of acute lung injury in mice by an inhibition of phosphodiesterase type 4. Am. J. Respir. Cell Mol. Biol. 1998;18:411–420. doi: 10.1165/ajrcmb.18.3.2913. [DOI] [PubMed] [Google Scholar]
- MOORE T.A., NEWSTEAD M.W., STRIETER R.M., MEHRAD B., BEAMAN B.L., STANDIFORD T.J. Bacterial clearance and survival are dependent on CXC chemokine receptor-2 ligands in a murine model of pulmonary Nocardia asteroides infection. J. Immunol. 2000;164:908–915. doi: 10.4049/jimmunol.164.2.908. [DOI] [PubMed] [Google Scholar]
- NAKANO Y., KASAHARA T., MUKAIDA N., KO Y.C., NAKANO M., MATSUSHIMA K. Protection against lethal bacterial infection in mice by monocyte-chemotactic and -activating factor. Infect. Immunol. 1994;62:377–383. doi: 10.1128/iai.62.2.377-383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON S., SUMMER W.R., KAKAB G.J. Aminophyline-induced suppression of pulmonary antibacterial defenses. Am. Rev. Resp. Dis. 1985;131:923–927. doi: 10.1164/arrd.1985.131.6.923. [DOI] [PubMed] [Google Scholar]
- PROCOPIO D.O., TEIXEIRA M.M., CAMARGO M.M., TRAVASSOS L.R., FEGURSON M.A.J., ALMEIDA I.C., GAZZINELLI R.T. Differential inhibitory mechanism of cAMP on TNF-α and IL-12 synthesis by macrophages exposed to microbial stimuli. Br. J. Pharmacol. 1999;127:1195–1205. doi: 10.1038/sj.bjp.0702624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANZ M.J., ALVAREZ A., PIQUERAS L., CERDÁ M., ISSEKUTZ A.C., LOBB R.R., CORTIJO J., MORCILLO E.J. Rolipram inhibits leukocyte–endothelial cell interaction in vivo through P- and E-selectin down-regulation. Br. J. Pharmacol. 2002;135:1872–1881. doi: 10.1038/sj.bjp.0704644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMIDT D., DENT G., SARGENT C. Selective phosphodiesterase inhibitors in the treatment of bronchial asthma and obstructive pulmonary disease. Clin. Exp. Allergy. 1999;29:99–109. doi: 10.1046/j.1365-2222.1999.00018.x. [DOI] [PubMed] [Google Scholar]
- SEKUT L., MENIUS J.A., BRACKEEN M.F., CONNOLLY K.M. Evaluation of the significance of elevated levels of systemic and localized tumor necrosis factor in different animal models of inflammation. J. Lab. Clin. Med. 1994;124:813–819. [PubMed] [Google Scholar]
- SEKUT L., YARNALL D., STIMPSON S.A., NOEL L.S., BATEMAN-FITE R., CLARK R.L., BRACKEEN M.F., MENIUS J.A., CONNOLY K.M. Anti-inflammatory activity of phosphodiesterases (PDE)-IV inhibitors in acute and chronic models of inflammation. Clin. Exp. Immunol. 1995;100:126–132. doi: 10.1111/j.1365-2249.1995.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELDON P.M., BARNES P.J., GIEMBYCZ M.A. Interleukin-10 does not mediate the inhibitory effect of PDE-4 inhibitors and other cAMP-elevating drugs on lipopolysaccharide-induced tumors necrosis factor-alpha generation from human peripheral blood monocytes. Cell Biochem. Biophys. 1998;29:179–201. doi: 10.1007/BF02737835. [DOI] [PubMed] [Google Scholar]
- SOARES A.C., PINHO V., SOUZA D.G., NICOLI J.R., TEIXEIRA M.M. Role of the platelet-activating factor (PAF) receptor during pulmonary infection with gram negative bacteria. Br. J. Pharmacol. 2002;137:621–628. doi: 10.1038/sj.bjp.0704918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUNESS J.E., ALDOUS D., SARGENT C. Immunosupressive and anti-inflammatory effects of cAMP phosphodiesterase (PDE) type 4 inhibitors. Immunopharmacol. 2000;47:127–162. doi: 10.1016/s0162-3109(00)00185-5. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., CASSALI G.D., POOLE S., TEIXEIRA M.M. Effects of inhibition of PDE4 and TNF-α on local and remote injuries following ischemia and reperfusion injury. Br. J. Pharmacol. 2001;134:985–994. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., SOARES A.C., PINHO V., TORLONI H., REIS L.F.L., TEIXEIRA M.M., DIAS A.A. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am. J. Pathol. 2002;160:1755–1765. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., CUNHA F.Q., FERREIRA S.H.Editorial. Response to an infectious insult Braz. J. Med. Biol. Res. 2001341p (preceding 555) [PubMed] [Google Scholar]
- TEIXEIRA M.M., GRISTWOOD R.W., COOPER N., HELLEWELL P.G. Phosphodiesterase (PDE)4 inhibitors: anti-inflammatory drugs of the future. Trend Pharmacol. Sci. 1997;18:164–170. doi: 10.1016/s0165-6147(97)01049-3. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WILLIAMS T.J., HELLEWELL P.G. Effect of phosphodiesterase isoenzyme inhibitors on cutaneous inflammation in the guinea pig. Br. J. Pharmacol. 1994;112:332–340. doi: 10.1111/j.1476-5381.1994.tb13073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORPHY T.J. Phosphodiesterase isozymes molecular targets for novel antiasthma agents. Am. J. Resp. Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- TSAI W.C., STRIETER R.M., MEHRAD B., NEWSTEAD M.W., ZENG X., STANDIFORD T.J. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect. Immun. 2000;68:4289–4296. doi: 10.1128/iai.68.7.4289-4296.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG P., WU P., SIEGEL M.I., EGAN R.W., BILLAH M.M. Interleukin (IL)-10 inhibits nuclear factor κB (NFκB) activation in human monocytes. IL-10 and IL-4 supress cytokine synthesis by different mechanisms. J. Biol. Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]