Abstract
The objective of this study was to determine the concentration-dependent effects of nisoldipine, a dihydropyridine Ca2+ channel blocker, on K+ currents in guinea-pig ventricular myocytes.
Myocytes in the conventional whole-cell configuration were bathed in normal Tyrode's solution or K+-free Tyrode's solution for the measurement of the effects of 0.01–100 μM nisoldipine on rapidly activating delayed-rectifier K+ current (IKr), slowly activating delayed-rectifier K+ current (IKs), inwardly rectifying K+ current (IK1), and reference L-type Ca2+ current (ICa,L).
Nisoldipine inhibited IKr with an IC50 of 23 μM, and IKs with an IC50 of 40 μM. The drug also had weak inhibitory effects on inward- and outward-directed IK1; the IC50 determined for outward-directed current was 80 μM.
Investigation of nisoldipine action on IKs showed that inhibition occurred in the absence of previous pulsing, and with little change in the time courses of activation and deactivation. However, the drug-induced inhibition was significantly weaker at ⩾+30 mV than at +10 mV.
We estimate that nisoldipine is about 30 times less selective for delayed-rectifier K+ channels than for L-type Ca2+ channels in fully polarised guinea-pig ventricular myocytes, and several orders less selective in partially depolarised myocytes.
Keywords: Guinea-pig ventricular myocytes, nisoldipine, L-type Ca2+ current, rapidly activating delayed-rectifier K+ current, slowly activating delayed-rectifier K+ current, inward-rectifying K+ current
Introduction
Members of the 1,4-dihydropyridine class of compounds have important clinical application in the management of cardiovascular disorders, and the basis of this use is the binding of drug molecules to high-affinity inhibitory sites on L-type Ca2+ channels (Kohlhardt & Fleckenstein, 1977; Singh et al., 1983; Sanguinetti & Kass, 1984; Schwartz & Triggle, 1984; McDonald et al., 1994; Roden, 1996; Kamp et al., 1999). Dihydropyridines can also block other ion channels, including voltage-dependent Na+ channels (Yatani & Brown, 1985) and delayed-rectifier K+ channels, in a variety of excitable and inexcitable cells (Nishi et al., 1983; Hume, 1985; Terada et al., 1987; Richard et al., 1988; Jacobs & DeCoursey, 1990; Tatsuta et al., 1994).
Delayed-rectifier K+ channels in cardiac cells are also affected by dihydropyridines. In particular, ca. 10 μM concentrations of nifedipine and/or nisoldipine have been shown to have marked inhibitory effects on the fast-activating K+ channels that carry transient outward current (Ito) (Kass, 1982; Gotoh et al., 1991; Jahnel et al., 1994) and ultrarapid K+ current (IKur) (Zhang et al., 1997). However, the susceptibility of these channels to nifedipine/nisoldipine action is not representative of all cardiac delayed-rectifier K+ channels. For example, we have recently reported that 10 μM nifedipine has no significant effect on the two components of delayed-rectifier K+ current (IK) in guinea-pig ventricular myocytes, rapidly activating IKr and slowly activating IKs; however, higher concentrations produced a concentration-dependent block with IC50 values near 300 μM (Zhabyeyev et al., 2000). Whether nisoldipine (which is a much more potent inhibitor of L-type Ca2+ channel blocker than nifedipine) is an equally weak inhibitor of these K+ currents has not been established.
The primary objective of the present study was to determine the concentration-dependent effects of nisoldipine on delayed-rectifier IKr and IKs in guinea-pig ventricular myocytes. For comparison with these data, we also measured the effects of the drug on inwardly rectifying K+ current (IK1) and on ICa,L. Nisoldipine had concentration-dependent inhibitory actions on all the three K+ currents, and these are discussed and compared with pertinent findings in earlier studies.
Methods
All the procedures were carried out in accordance with the national and university regulations on the care and treatment of laboratory animals.
Ventricular myocytes
Male guinea-pigs (250–300 g) were killed by cervical dislocation, and single ventricular myocytes were enzymatically isolated as described previously (Ogura et al., 1995). The excised hearts were mounted on a Langendorff column, and retrogradely perfused through the aorta with Ca2+-free Tyrode's solution (37°C) containing collagenase (0.08–0.12 mg ml−1; Yakult Pharmaceutical Co., Tokyo, Japan) for 10–15 min. The cells were dispersed and stored at 22°C in a high-K+, low-Na+ solution supplemented with 50 mM glutamic acid and 20 mM taurine. A few drops of the cell suspension were placed in a 0.3-ml perfusion chamber mounted on an inverted microscope stage. After the cells had settled to the bottom, the chamber was perfused (2 ml min−1) with Tyrode's solution at 36°C. The Tyrode's solution contained (in mM) NaCl 140, KCl 5.4, CaCl2 1.8, MgCl2 1, glucose 10, and N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) 5 (pH 7.4 with NaOH). In some experiments, the Tyrode's solution was replaced by a Na+-free solution (equimolar substitution with trimethylammonium ion), or by a K+-, Ca2+-free Tyrode's solution (KCl and CaCl2 omitted) that also contained 0.2 mM Cd2+ to suppress Ca2+ channel current.
Whole-cell membrane currents were recorded using an EPC-7 amplifier (List Electronic, Darmstadt, Germany). Recording pipettes were fabricated from thick-walled borosilicate glass capillaries (H15/10/137, Jencons Scientific Ltd, Bedfordshire, U.K.) and filled with a solution that contained (in mM) KCl 40, potassium aspartate 106, MgCl2 1, K2-ATP 4, ethylene glycol-bis(b-aminoethyl ether)-N,N,N,N-tetraacetic acid (EGTA) 5, and HEPES 5 (pH 7.2 with KOH). The pipettes had resistances of 1.5–2.5 MΩ when filled with pipette solution, and liquid junction potentials between external and pipette-filling solution were nulled prior to patch formation. Series resistance ranged between 3 and 7 MΩ, and was compensated by 60–80%. Membrane current signals were filtered at 3 kHz, and digitised with an A/D converter (Digidata 1200A, Axon Instruments) and pCLAMP software (Axon Instruments) at a sampling rate of 8 kHz prior to analysis.
Drugs
Nisoldipine was kindly provided by Bayer Inc. (Etobicoke, ON, Canada). The drug was dissolved in dimethyl sulphoxide (DMSO) (Sigma Chemical Co., St Louis, MO, U.S.A.) (0.1 M stock solution) and stored in the dark at −20°C. Appropriate amounts of stock solution were freshly added to bathing solutions, and these were protected from the light during all experiments. The highest final concentration of DMSO was 0.1%, a concentration that has no significant effect on membrane currents in guinea-pig ventricular cells (Ogura et al., 1995). Nevertheless, the appropriate concentrations of DMSO were included in the control solutions used in experiments with high concentrations of nisoldipine. E4031 was obtained from Eisai (Tokyo, Japan) and dissolved in the bathing solution.
Statistics
Results are expressed as means±s.e.m., and Student's t-test or one-way ANOVA followed by Dunnett's multiple comparison test was used to determine the significance of drug effects. Differences were considered to be significant when P<0.05.
Results
Inhibition of total IK
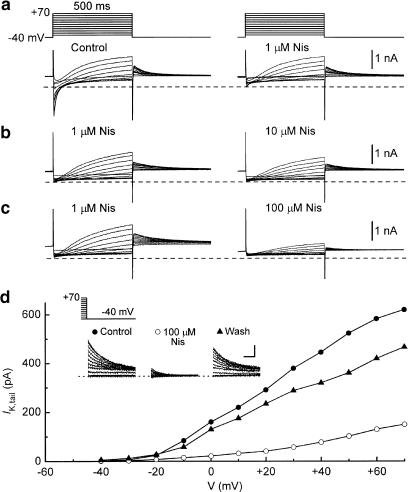
Figure 1a shows records of membrane currents obtained from a representative myocyte bathed in normal Tyrode's solution and depolarised for 500 ms from −40 mV to potentials up to +70 mV. The depolarisations elicited inward ICa,L that reached a maximal amplitude near 0 mV, and activated time-dependent outward K+ current that increased with positive potential and deactivated when the membrane was repolarised to −40 mV (IK,tail). Exposure to 1 μM nisoldipine for 10 min resulted in near-complete inhibition of ICa,L, but had little effect on the amplitudes of IK elicited by depolarisations and repolarisations. However, concentrations of nisoldipine higher than 1 μM had marked concentration-dependent inhibitory effects on IK. In the examples shown in Figure 1b and c, 10 μM drug depressed the time-dependent current by about 20% and 100 μM depressed it by about 80%. These inhibitions were registered in myocytes pretreated with 1 μM nisoldipine, that is, they were essentially independent of changes in ICa,L.
Figure 1.
Effects of nisoldipine on membrane currents in guinea-pig ventricular myocytes. The myocytes were bathed in normal Tyrode's solution, held at −80 mV, and depolarised after 200-ms prepulses (−40 mV) to more positive potentials for 500 ms at 0.1 Hz; tail currents were recorded on repolarisations to −40 mV. (a) Selective inhibition of inward ICa,L by 1 μM nisoldipine (Nis). (b,c) Inhibition of outward K+ currents at positive potentials, and K+ tail currents at −40 mV, by 8-min exposures to 10 and 100 μM nisoldipine. The myocytes were pretreated with 1 μM nisoldipine for 10 min. The dashed lines on the records here and in other figures indicate zero-current levels. (d) IK,tail–V relationships determined before, during, and 10 min after removal of 100 μM nisoldipine. The calibration bars on the IK,tail records (top) indicate 100 ms and 200 pA.
The pronounced inhibition of IK by high concentrations of nisoldipine was not a manifestation of toxicity because ca. 10-min washouts generally restored the current to at least 50% of predrug amplitude (e.g., Figure 1d) (also see below). By comparison, washout of 1 μM nisoldipine for 13±2 min only restored ICa,L to 29±5% of control (n=4), and washout of 50 μM nisoldipine for 14±2 min only restored the current to 9±4% of control (n=5).
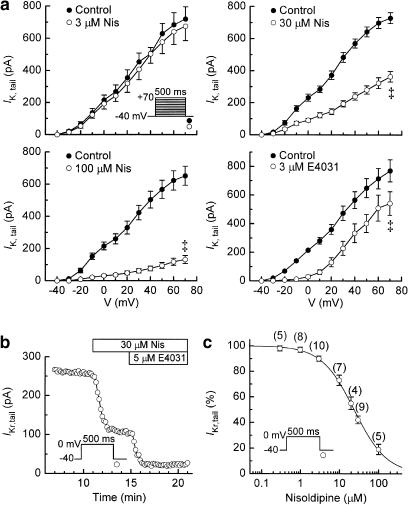
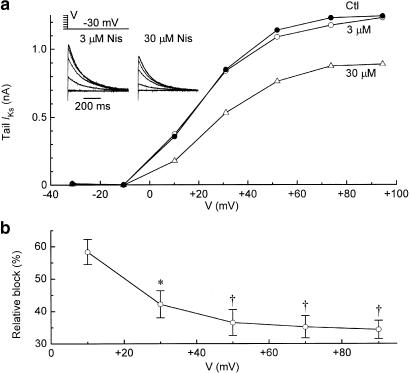
Myocytes were repolarised to −40 mV for measurement of IK,tail elicited after 500-ms depolarisations to test potentials (V) between −30 and +70 mV. Average IK,tail–V relationships recorded before and 7–10 min after additions of 3, 30, and 100 μM nisoldipine are shown in Figure 2a. The low concentration had little effect on the IK,tail–V relationship, whereas the 30 and 100 μM concentrations depressed its amplitude by about 50 and 80%, respectively. For reference in the interpretation of these data, Figure 2a also shows the effects of 3 μM E4031 on the IK,tail–V relationship. The selective IKr inhibitor (Sanguinetti & Jurkiewicz, 1990) almost completely abolished IK,tail elicited after depolarisations from −40 mV up to test potential 0 mV, but had little effect on the increment in tail amplitude induced by depolarisations to more positive potentials.
Figure 2.
Inhibition of IKr by nisoldipine. Myocytes were bathed in normal Tyrode's solution and depolarised from prepulse −40 mV to more positive potentials for 500 ms at 0.1 Hz for the measurement of IK,tail on repolarisations to −40 mV. (a) Average IK,tail–V relationships determined from myocytes treated with single concentrations of nisoldipine (3 μM (n=4), 30 μM (n=5), 100 μM (n=6)). Also shown (lower right) are the effects of the IKr-selective inhibitor E4031 (3 μM) (n=7); the results of the E4031 series (near complete inhibition of IK,tail at ⩽0 mV) validate the method (IK,tail amplitude after pulses to 0 mV) used for measurement of IKr in (b, c) below. ‡P<0.001 (paired t-test at +70 mV). (b) Time course of inhibition of IKr,tail (0 mV) by 30 μM nisoldipine. (c) Dependence of IKr inhibition on nisoldipine concentration. Myocytes were treated for 7–10 min with one or two concentrations of the drug. The Hill equation fitting the data has an IC50 of 23±2 μM and a coefficient of 1.05. Numbers of observations are shown in parentheses.
Inhibition of IKr
The result with E4031 in Figure 2a indicated that the amplitudes of tail currents after 500-ms depolarisations to 0 mV could be used to evaluate the inhibition of IKr by nisoldipine. In practice, the amplitude of IKr,tail was measured following 500-ms depolarisations to 0 mV at 0.1 Hz. The data in Figure 2b indicate that the inhibition by nisoldipine reached a steady state within 5 min. As illustrated by the significant recovery of current at 0 mV following 10-min washout of 100 μM drug in Figure 1d, the inhibition of IKr by high concentrations of nisoldipine was slowly reversible. For example, in four myocytes treated with 30 μM nisoldipine for 5–8 min, IKr recovered to 89±6% of predrug amplitude after a 10–15 min washout period.
The dependence of IKr,tail inhibition on the concentration of nisoldipine is shown in Figure 2c. The lowest concentration that produced a significant inhibition was 3 μM (reduction to 90±2% of predrug control (n=10), P<0.05, paired t-test). The overall data are well described by the Hill equation with an IC50 of 23±2 μM and a coefficient of 1.05.
Inhibition of IKs
Dependence on drug concentration
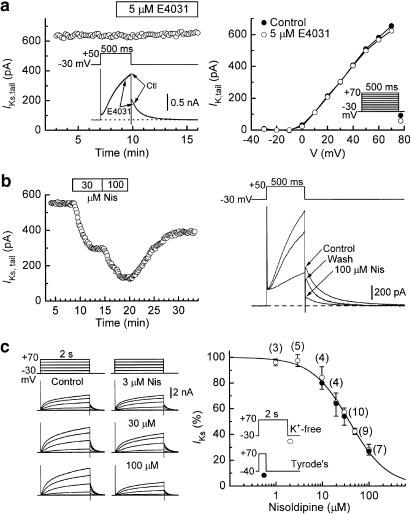
Inhibition of IKs by nisoldipine was evaluated in myocytes that were superfused with a solution (K+-, Ca2+-free plus 0.2 mM Cd2+) that enhances IKs and suppresses IKr and Ca2+-dependent currents (Sanguinetti & Jurkiewicz, 1990; Jones et al., 1998). Under these conditions, both the time-dependent currents elicited by 500-ms depolarisations and the subsequent tail currents at −30 mV were insensitive to 5 μM E4031 (Figure 3a). However, IKs isolated in this manner was rapidly inhibited by high concentrations of nisoldipine, and the inhibition was only slowly and partially reversible upon removal of the drug (Figure 3b). In four myocytes treated with 100 μM nisoldipine for 5 min, a 10-min washout period restored IKs to 73±5% of predrug control amplitude.
Figure 3.
Inhibition of IKs by nisoldipine. Myocytes were bathed in K+-, Ca2+-free Cd2+ solution to suppress IKr and Ca2+-dependent currents, and IKs was activated by 500-ms or 2-s depolarisations from −30 mV to more positive potentials at 0.1 Hz. (a) Results from two myocytes demonstrating the lack of effect of IKr inhibitor E4031 on the IK activated under the foregoing experimental conditions. (b) Time course of inhibition of IKs,tail by 30 and 100 μM nisoldipine. (c) Dependence of inhibition on nisoldipine concentration. Left: examples of records obtained from myocytes depolarised with 2-s pulses. Right: data (open symbols) obtained from myocytes exposed to one or two concentrations of the drug for 6–10 min each. Inhibition was measured from changes in the amplitudes of tail currents after 2-s depolarisations to +70 mV, and the Hill equation fitting the data has an IC50 of 40±1 μM and a coefficient of 1.07. Numbers of observations are shown in parentheses. For comparison, the plot also shows data (filled symbols) obtained when the inhibition of IKs was evaluated as the drug-induced change in the amplitude of time-dependent current elicited by 500-ms pulses to +70 mV in myocytes bathed in normal Tyrode's solution (n=4–10).
The dependence of IKs inhibition on nisoldipine concentration was evaluated from measurements of the amplitudes of tail currents after 2-s depolarisations to +70 mV before and during steady-state drug action (Figure 3c). The data from these determinations are well described by the Hill equation with an IC50 of 40±1 μM and a coefficient of 1.06 (Figure 3c, open circles).
The dependence of IKs inhibition on ⩾10 μM nisoldipine was also assessed from changes in the amplitude of time-dependent outward currents elicited by 500-ms depolarisations to +70 mV in myocytes superfused with normal Tyrode's solution and pretreated with 1 μM nisoldipine (see Figure 1 for representative traces). The filled circles on the plot in Figure 3c indicate that the nisoldipine–IKs relationship determined in this manner was similar to that determined from measurements of tail IKs under K+-free conditions.
Time- and voltage-dependent features
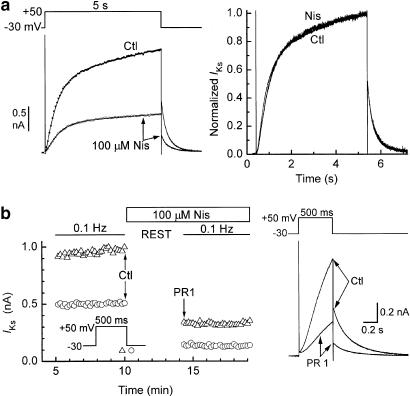
Inhibition of fast-activating types of delayed-rectifier K+ current by dihydropyridines generally increases with time during a depolarising pulse to a positive potential (Gotoh et al., 1991; Jahnel et al., 1994; Zhang et al., 1997). In the present study, we examined the effect of 100 μM nisoldipine on the time course of development of IKs during long (5 s) depolarisations to +50 mV. With the exception of the first 100–200 ms, the current records were satisfactorily fitted with single exponential functions (Figure 4a, left). Although the time constants of these functions were longer in the presence of the drug (587±37 ms) than in its absence (predrug, 424±31 ms; after washout, 518±23 ms) (n=4 myocytes), the differences were not significant at the 0.05 level (paired t-tests). When records obtained before and after addition of nisoldipine were scaled and superimposed, it was evident that drug treatment did not modify the ratio of the amplitudes of activating IKs and IKs,tail (Figure 4a, right). The same figure indicates that the time course of IKs,tail was also unchanged.
Figure 4.
Characteristics of nisoldipine block of IKs in myocytes bathed in K+-, Ca2+-free Cd2+ solution. (a) Lack of significant effect of 100 μM nisoldipine on the time courses of IKs activation and deactivation. Left: original records whose activation time courses have been fitted with single exponentials (small dots), having time constants of 488 ms (control) and 576 ms (nisoldipine). Right: superimposition after scaling of the nisoldipine record to the control record. (b) Effect of rest on the development of block. Left: measurements of IKs amplitudes. Right: records obtained on the last pulse before rest (Ctl) and the first postrest pulse (PR 1).
To determine whether block of IKs by nisoldipine has classical use-dependent features, myocytes were stimulated at 0.1 Hz (500-ms pulses from −30 to +50 mV), rested at −80 mV, exposed to 100 μM drug, and restimulated 4 min later. The records and time plot in Figure 4b indicate that near-maximal inhibition was reached on the first postrest depolarisation. In four experiments of this type, tail IKs on the first postrest pulse was 32±6% of predrug control, and tail IKs on the 30th postrest pulse was a similar 33±4%.
The dependence of block on IKs-activating voltage was determined by measuring the amplitudes of tail currents elicited after 2-s depolarisations to different test potentials. Data from a representative myocyte illustrate that, in contrast to low concentrations of the drug, 30 μM nisoldipine decreased the amplitude of tail currents after depolarisations to all test voltages between +10 and +90 mV (Figure 5a). When tail IKs–V data from six experiments with 30 μM nisoldipine were normalised, it was evident that tails after small depolarisations were inhibited to a greater degree than tails after large depolarisations. As depicted in Figure 5b, the relative block declined from 0.58±0.04 at +10 mV to 0.34±0.03 at +90 mV, with most of the voltage dependence arising between +10 and +50 mV. Analysis of the data (one-way ANOVA followed by Dunnett's multiple comparison test) indicates that block at +10 mV was significantly larger than block at +30, +50, +70, and +90 mV (P<0.05–0.01).
Figure 5.
Dependence of nisoldipine block of IKs on the voltage of the activating pulse. Myocytes were bathed in K+-, Ca2+-free Cd2+ solution and depolarised for 2 s to potentials V for evaluation of IKs,tail amplitude on repolarisation. (a) Records and IKs,tail analysis from a myocyte exposed to 3 μM and then 30 μM nisoldipine. (b) Dependence of the degree of block on voltage. The data are from six myocytes that were exposed to 30 μM nisoldipine. *P<0.05, ‡P<0.01 (versus +10 mV data).
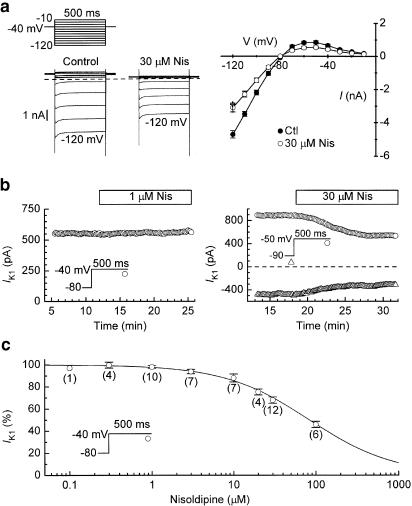
Inhibition of IK1
When myocytes were bathed in Tyrode's solution that contained 0.2 mM Cd2+ (to block ICa,L), 500-ms pulses from −40 mV to potentials between −120 and −10 mV elicited currents that were large and inward up to −80 mV, and smaller, outward, and quasi-time-independent at more positive potentials (Figure 6a and b); these features, and the negative slope in the I–V relationship at <−50 mV, are typical for IK1 over this voltage region (Jones et al., 1999). High concentrations of nisoldipine lowered the end-of-pulse amplitudes of both inward and outward IK1; in four myocytes, 30 μM nisoldipine inhibited the inward current at −120 mV by 34±4%, and the outward current at −40 mV by 35±5% (both P<0.01, multiple comparison test). Inhibition caused by ⩽30 μM nisoldipine was only partially reversed by washout of the drug for ca. 10 min (not shown).
Figure 6.
Inhibition of IK1 by nisoldipine. Myocytes were bathed in Tyrode's solution that contained 0.2 mM Cd2+, and held at −80 or −90 mV. (a) Effects of 30 μM nisoldipine on IK1 measured as the end-of-pulse current amplitude on 500-ms pulses from prepulse −40 mV to potentials between −120 and −10 mV. Left: records from a representative experiment; right: summary of data from four experiments. †P<0.01 (multiple comparison test at −120 mV). (b) Time courses of changes in two representative experiments. Left: lack of effect of 1 μM nisoldipine on outward-directed IK1 at −40 mV; right: inhibition of inward (−90 mV) and outward (−50 mV) IK1 by 30 μM nisoldipine. (c) Dependence of inhibition of outward IK1 on the concentration of nisoldipine. The Hill equation fitting the data has an IC50 of 80±3 μM and a coefficient of 0.83. Noncumulative drug exposures lasted for 8–10 min. Numbers of myocytes are shown in parentheses.
The time course and concentration dependence of nisoldipine-induced inhibitions of outward-directed IK1 were evaluated by measuring the outward current amplitude at −40 mV (Jones et al., 1999). The time plots in Figure 6b indicate that outward IK1 at −40 mV was generally stable over tens of minutes, and unaffected by 1 μM nisoldipine, but quickly inhibited by 30 μM nisoldipine. The steady-state effects of the drug are well described by the Hill equation with an IC50 of 80±3 μM and a coefficient of 0.83 (Figure 6c).
Inhibition of ICa,L
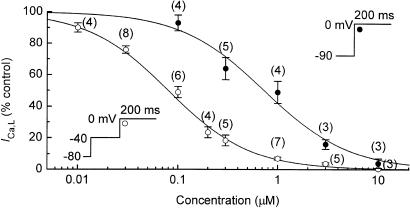
For comparison with the foregoing data on the inhibition of K+ currents by nisoldipine, we determined concentration–response relations for inhibition of ICa,L by the drug. To obtain data that might be applicable to inhibition during an action potential in fully polarised ventricular myocytes, a group of myocytes was bathed in Na+-free Tyrode's solution to eliminate Na+ current, held at –90 mV, and depolarised to 0 mV for 200 ms at 0.1 Hz. The myocytes were pretreated with 3 μM E4031 to suppress IKr, and exposed to 0.4 mM Cd2+ after 5–7 min nisoldipine to establish the background current level. A second group of myocytes was bathed in normal Tyrode's solution, and subjected to a voltage protocol similar to that used in many of the experiments on K+ currents. These myocytes were depolarised by a prepulse to –40 mV for 200 ms to inactivate Na+ current, and then pulsed to 0 mV at 0.1 Hz; they were also treated with 3 μM E4031 and exposed to Cs2+ after the effect of nisoldipine had reached a steady state.
The results obtained from the two groups of myocytes are summarised in Figure 7. The data from the group held at –90 mV are described by the Hill equation with IC50 of 0.73±0.13 μM and a coefficient of 1.03, whereas the data from the other group have an IC50 of 0.08±0.01 μM and a coefficient of 1.07. The difference in IC50 is an indication of the voltage dependence of nisoldipine action on L-type Ca2+ channels (Kass, 1982; McDonald et al., 1994).
Figure 7.
Inhibition of ICa,L by nisoldipine. Myocytes were either bathed in Na+-free Tyrode's held at –90 mV, and depolarised to 0 mV for 200 ms (0.1 Hz), or bathed in normal Tyrode's solution, held at –80 mV and depolarised by a prepulse to −40 to 0 mV for 200 ms (0.1 Hz). At 5 min postpatch, the myocytes were pretreated with 3 μM E4031 for 3 min and then exposed to single concentrations of nisoldipine for 5–7 min prior to application of 0.4 mM Cd2+ to establish the background current level at 0 mV. Peak ICa,L was measured as the background current level minus inward peak level. The Hill equation fitting the data obtained from myocytes held at –90 mV has an IC50 of 0.73±0.13 μM and a coefficient of 1.03. The fit of the data from the other group of myocytes has an IC50 of 0.08±0.01 μM and a coefficient of 1.07.
Discussion
The present study provides new information on the sensitivity of cardiac inward-rectifier and delayed-rectifier K+ channels to inhibition by nisoldipine. The results are discussed and compared with findings from previous studies on interactions between nisoldipine and K+ channels.
Effects on inwardly rectifying K+ current
Nisoldipine inhibited guinea-pig ventricular IK1 with an IC50 of 80 μM. The inhibition appeared to be independent of voltage and direction of the current, and was at least partially reversible with prolonged washout of the drug. In an earlier study on nisoldipine, Kass (1982) observed that concentrations up to 100 μM had little effect on outward IK1 in cardiac Purkinje fibres. A possible reason for the discrepancy with the present findings is that the Purkinje fibres had been injected with tetrabutylammonium bromide to block Ito prior to the trials with nisoldipine. The pretreatment may have interfered with the measurements because the quaternary compound is itself a blocker of inwardly rectifying K+ current (Barros et al., 1992).
Inhibition of delayed-rectifier K+ currents
Fast-activating currents
Dihydropyridine Ca2+-channel blockers have previously been shown to inhibit fast-activating types of delayed-rectifier K+ currents in a variety of noncardiac cells. In most cases, nifedipine was the drug under investigation (e.g., Nishi et al., 1983; Gola & Ducreux, 1985; Jacobs & DeCoursey, 1990; Tatsuta et al., 1994). However, Nerbonne & Gurney (1987) included nisoldipine in their study on fast-activating inactivating K+ current in Aplysia bag cell neurons, and reported that the IC50 of the drug was ca. 4 μM.
The fast-activating inactivating Ito in rabbit atrial myocytes has also been found to be quite sensitive to nisoldipine (IC50 4.7 μM) (Gotoh et al., 1991).
Slower-activating cardiac K+ currents
There have been two previous studies on the effects of nisoldipine on slower-activating global IK in cardiac cells. In the first of these, Kass (1982) found that 10 μM nisoldipine had no effect on IK (Ix) elicited by 1-s depolarisations of cardiac Purkinje fibres. Subsequently, Hume (1985) reported that 10 μM nisoldipine reduced IK in frog atrial myocytes by about 40%. In relation to the present findings on IKr and IKs, it is important to note that frog atrial IK has been characterised as a single K+ conductance (Hume & Giles, 1983) that resembles IKs in some respects (slowly activating, noninactivating), but not in others (half-activation near 0 mV, saturation at ca. +40 mV) (see Hume, 1985). Thus, direct comparison of the findings on guinea-pig ventricular IKr (IC50 23 μM) and IKs (IC50 40 μM) with those on frog atrial IK (IC50 16 μM) may not be justified. Nevertheless, these determinations taken together indicate that nisoldipine has a weaker action on slower-activating types of cardiac delayed-rectifier K+ channels than has been reported for fast-activating Ito-carrying channels by Gotoh et al. (1991).
Characteristics of IKs block
Block of IKs by nisoldipine had the following features: (i) the time course of nonblocked current in the presence of the drug was similar to that of current in the absence of the drug, (ii) block was fully established on the first pulse after a rest in the presence of the drug, and (iii) the degree of block was dependent on the voltage of the activating depolarisation. The first two features suggest (but do not prove) that, in contrast to dihydropyridine block of cardiac Ito (Gotoh et al., 1991; Jahnel et al., 1994) and hKv1.5 current (Zhang et al., 1997), block of IKs by nisoldipine occurs via binding of drug molecules to closed rather than open channels.
A dependence of dihydropyridine block on voltage has been ruled out in some studies on noncardiac delayed-rectifier K+ channels (e.g., Nerbonne & Gurney, 1987; Barros et al., 1992; Pappone & Ortiz-Miranda, 1993), but has been detected in two previous studies on cardiac delayed-rectifier channels. Hume (1985) observed that inhibition of frog atrial IK by 10 μM nisoldipine was weaker on depolarisations to positive potentials than to negative ones, and Zhang et al. (1997) reported that block of hKv1.5 channels by 30 μM nifedipine was weaker at potentials above +20 mV. In the present case, the block of IKs by 30 μM nisoldipine was approximately 40% weaker at ⩾+50 mV than at +10 mV. Since nisoldipine has a very low pKa and is almost completely in the neutral form at pH 7.4, it is difficult to view the voltage dependency in terms of charged drug molecules under the influence of the electrical field. However, Hume (1985) noted that this type of voltage-dependent block could be due to a drug-induced positive shift of the voltage dependence of current activation. It seems unlikely that a shift is the major cause of the voltage dependence of the IKs block studied here, because the time course of IKs at +50 mV was little affected by 100 μM nisoldipine. Rather, the voltage dependence may arise from a coupling of the binding site to a voltage-sensitive process such that unbinding is enhanced at more positive potentials. As proposed by Zhang et al. (1997) for nifedipine block of cardiac hKv1.5 channels, the dihydropyridine site on Ks channels may be near the external mouth of the pore, and relief of block may be linked to increased K+ permeation at more positive voltages.
Selectivity of nisoldipine action on cardiac K+ and Ca2+ channels
The IC50 values for nisoldipine block of IKr, IKs, and IK1 were 23, 40, and 80 μM, respectively. Thus, nisoldipine is far from being a potent blocker of these K+ pathways. This is particularly the case with regard to IKr because there are numerous clinical and experimental drugs that inhibit this current with an IC50<1 μM (e.g., Sanguinetti & Jurkiewicz, 1990; Carmeliet, 1992; Salata et al., 1995; Roden, 1996; Yang et al., 1997; Jones et al., 1998; Zhang et al., 1999). Nisoldipine is a potent blocker of L-type Ca2+ channels in cardiac cells, and is commonly used to abolish ICa,L in electrophysiological studies on these cells. The present results indicate that any spillover effects on K+ currents are likely to be negligible when the drug is used to abolish ICa,L in studies on guinea-pig ventricular myocytes. The placing of a numerical value on the relative selectivity of nisoldipine for its binding site in L-type Ca2+ channels over those in delayed-rectifier K+ channels is complicated by the fact that binding to the Ca2+ channels is strongly dependent on the holding potential (Sanguinetti & Kass, 1984; McDonald et al., 1994). For this reason, the IC50 for ICa,L in cardiac Purkinje fibres declined from 1.34 μM at a holding potential −80 mV, to ca. 0.2 μM at −50 mV, and a calculated 0.001 μM at ca. −20 mV (Sanguinetti & Kass, 1984).
In the present study, the IC50 for block of ICa,L at 0 mV was 0.73±0.13 μM when myocytes were held at –90 mV, and a much lower 0.08±0.1 μM when myocytes were held at –80 mV and depolarised by a prepulse to –40 mV for 200 ms prior to the test pulses to 0 mV. Since the block of K+ currents was not influenced by holding potential, the relative selectivity of nisoldipine for block of Ca2+ channels over delayed-rectifier channels is estimated to be about 30 when well-polarised myocytes undergo rapid depolarisation to a plateau potential (for example, during an action potential). The selectivity increases to near 400 when depolarisation to a plateau potential occurs after a prepulse from –80 to –40 mV (a protocol frequently used in electrophysiological studies). The latter estimate of relative selectivity is close to the 1000-fold value estimated by Hume (1985), whose reference IC50 for frog atrial ICa,L was 0.016 μM (holding potential –60 mV).
Acknowledgments
We thank Ms Gina Dickie for excellent technical assistance. This study was supported by the Heart and Stroke Foundation of New Brunswick, and the Canadian Institutes of Health Research.
Abbreviations
- DMSO
dimethyl sulphoxide
- EGTA
ethylene glycol-bis(b-aminoethyl)-N,N,N,N-tetraacetic acid
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- I–V
current–voltage
- IC50
concentration that produces 50% of maximal inhibition
- ICa,L
L-type Ca2+ current
- IK
delayed-rectifier K+ current
- IKr
rapidly activating component of IK
- IKs
slowly activating component of IK
- IKur
ultrarapid K+ current
- IK1
inward-rectifying K+ current
- Ito
transient outward current
References
- BARROS F., DELGADO L.M., DEL CAMINO D., DE LA PENA P. Characteristics and modulation by thyrotropin-releasing hormone of an inwardly rectifying K+ current in patch-perforated GH3 anterior pituitary cells. Pflügers Arch. 1992;422:31–39. doi: 10.1007/BF00381510. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J. Pharmacol. Exp. Ther. 1992;262:809–817. [PubMed] [Google Scholar]
- GOLA M., DUCREUX C. D600 as a direct blocker of Ca-dependent K currents in Helix neurons. Eur. J. Pharmacol. 1985;117:311–322. doi: 10.1016/0014-2999(85)90004-4. [DOI] [PubMed] [Google Scholar]
- GOTOH Y., IMAIZUMI Y., WATANABE M., SHIBATA E.F., CLARK R.B., GILES W.R. Inhibition of transient outward K+ current by DHP Ca2+ antagonists and agonists in rabbit cardiac myocytes. Am. J. Physiol. 1991;260:H1737–H1742. doi: 10.1152/ajpheart.1991.260.5.H1737. [DOI] [PubMed] [Google Scholar]
- HUME J.R. Comparative interactions of organic Ca++ channel antagonists with myocardial Ca++ and K+ channels. J. Pharmacol. Exp. Ther. 1985;234:134–140. [PubMed] [Google Scholar]
- HUME J.R., GILES W. Ionic currents in single isolated bullfrog atrial cells. J. Gen. Physiol. 1983;81:153–194. doi: 10.1085/jgp.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E.R., DECOURSEY T.E. Mechanisms of potassium channel block in rat alveolar epithelial cells. J. Pharmacol. Exp. Ther. 1990;255:459–472. [PubMed] [Google Scholar]
- JAHNEL U., KLEMM P., NAWRATH H. Different mechanisms of the inhibition of the transient outward current in rat ventricular myocytes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;349:87–94. doi: 10.1007/BF00178211. [DOI] [PubMed] [Google Scholar]
- JONES S.E., KASAMAKI Y., OGURA T., SHUBA L.M., MCCULLOUGH J.R., MCDONALD T.F. Inhibition of cardiac inward-rectifier K+ current by terodiline. Eur. J. Pharmacol. 1999;370:319–327. doi: 10.1016/s0014-2999(99)00130-2. [DOI] [PubMed] [Google Scholar]
- JONES S.E., OGURA T., SHUBA L.M., MCDONALD T.F. Inhibition of the rapid component of the delayed-rectifier K+ current by therapeutic concentrations of the antispasmodic agent terodiline. Br. J. Pharmacol. 1998;125:1138–1143. doi: 10.1038/sj.bjp.0702173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMP T.J., ZHOU Z., ZHANG S., MAKIELSKI J.C., JANUARY C.T.The pharmacology of T- and L-type calcium channels in the heart Cardiac Electrophysiology: From Cell to Bedside 1999Philadelphia, PA: W.B. Saunders Co. Inc.ed. Jalife, J. & Zipes, D. [Google Scholar]
- KASS R.S. Nisoldipine: a new, more selective calcium current blocker in cardiac Purkinje fibres. J. Pharmacol. Exp. Ther. 1982;223:446–456. [PubMed] [Google Scholar]
- KOHLHARDT M., FLECKENSTEIN A. Inhibition of the slow inward current by nifedipine in mammalian ventricular myocardium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1977;298:267–272. doi: 10.1007/BF00500899. [DOI] [PubMed] [Google Scholar]
- MCDONALD T.F., PELZER S., TRAUTWEIN W., PELZER D.J. Regulation and modulation of calcium channels incardiac, skeletal, and smooth muscle cells. Physiol. Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- NERBONNE J.M., GURNEY A.M. Blockade of Ca2+ and K+ currents in bag cell neurons of Aplysia californica by dihydropyridine Ca2+ antagonists. J. Neurosci. 1987;7:882–893. doi: 10.1523/JNEUROSCI.07-03-00882.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHI K., AKAIKE N., OYAMA Y., ITO H. Actions of calcium antagonists on calcium currents in Helix neurons. Specificity and potency. Circ. Res. 1983;52:I53–I59. [PubMed] [Google Scholar]
- OGURA T., SHUBA L.M., MCDONALD T.F. Action potentials, ionic currents and cell water in guinea pig ventricular preparations exposed to dimethyl sulfoxide. J. Pharmacol. Exp. Ther. 1995;273:1273–1286. [PubMed] [Google Scholar]
- PAPPONE P.A., ORTIZ-MIRANDA S.I. Blockers of voltage-gated K channels inhibit proliferation of cultured brown fat cells. Am. J. Physiol. 1993;264:C1014–C1019. doi: 10.1152/ajpcell.1993.264.4.C1014. [DOI] [PubMed] [Google Scholar]
- RICHARD S., CHARNET P., OUADID H., TIAHO F., NARGEOT J. Effects of the Ca-antagonist nicardipine on K+ currents and Na+–Ca2+ exchange in frog atrial fibres. J. Mol. Cell. Cardiol. 1988;20:1133–1140. doi: 10.1016/0022-2828(88)90593-7. [DOI] [PubMed] [Google Scholar]
- RODEN D.M.Antiarrhythmic drugs Goodman & Gilman's The Pharmacological Basis of Therapeutics 1996New York: McGraw-Hill; 839–874.ed. Hardman, J.G. & Limbird, L.E. pp [Google Scholar]
- SALATA J.J., JURKIEWICZ N.K., WALLACE A.A., STUPIENSKI R.F., GUINOSSO P.J., LYNCH J.J. Cardiac electrophysiological actions of the histamine H1-receptor antagonists astemizole and terfenadine compared with chlorpheniramine. Circ. Res. 1995;76:110–119. doi: 10.1161/01.res.76.1.110. [DOI] [PubMed] [Google Scholar]
- SANGUINETTI M.C., JURKIEWICZ N.K. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J. Gen. Physiol. 1990;96:195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., KASS R.S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fibre by dihydropyridine calcium channel antagonists. Circ. Res. 1984;55:336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ A., TRIGGLE D.J. Cellular action of calcium channel blocking drugs. Annu. Rev. Med. 1984;35:325–339. doi: 10.1146/annurev.me.35.020184.001545. [DOI] [PubMed] [Google Scholar]
- SINGH B.N., NADEMANEE K., BAKY S.H. Calcium antagonists. Clinical use in the treatment of arrhythmias. Drugs. 1983;25:125–153. doi: 10.2165/00003495-198325020-00003. [DOI] [PubMed] [Google Scholar]
- TATSUTA H., UEDA S., MORISHIMA S., OKADA Y. Voltage- and time-dependent K+ channel currents in the basolateral membrane of villus enterocytes isolated from guinea pig small intestine. J. Gen. Physiol. 1994;103:429–446. doi: 10.1085/jgp.103.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERADA K., KITAMURA K., KURIYAMA H. Different inhibitions of the voltage-dependent K+ current by Ca2+ antagonists in the smooth muscle cell membrane of rabbit small intestine. Pflügers Arch. 1987;408:558–564. doi: 10.1007/BF00581156. [DOI] [PubMed] [Google Scholar]
- YANG T., SNYDERS D.J., RODEN D.M. Inhibition of cardiac potassium currents by the vesnarinone analog OPC-18790: comparison with quinidine and dofetilide. J. Pharmacol. Exp. Ther. 1997;280:1170–1175. [PubMed] [Google Scholar]
- YATANI A., BROWN A.M. The calcium channel blocker nitrendipine blocks sodium channels in neonatal rat cardiac myocytes. Circ. Res. 1985;56:868–875. doi: 10.1161/01.res.56.6.868. [DOI] [PubMed] [Google Scholar]
- ZHABYEYEV P., MISSAN S., JONES S.E., MCDONALD T.F. Low-affinity block of cardiac K+ currents by nifedipine. Eur. J. Pharmacol. 2000;401:137–143. doi: 10.1016/s0014-2999(00)00413-1. [DOI] [PubMed] [Google Scholar]
- ZHANG S., ZHOU Z., GONG Q., MAKIELSKI J.C., JANUARY C.T. Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ. Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]
- ZHANG X., ANDERSON J.W., FEDIDA D. Characterization of nifedipine block of the human heart delayed rectifier, hKv1.5. J. Pharmacol. Exp. Ther. 1997;281:1247–1256. [PubMed] [Google Scholar]