Abstract
Although barbiturates, like other general anaesthetics, depress excitatory synaptic transmission in the central nervous system (CNS), the underlying cellular mechanisms remain unresolved. They may increase the likelihood that an action potential will fail to invade every branch of the axonal arbour, thereby decreasing the synaptic drive to the postsynaptic neurons. Alternatively, they may inhibit calcium entry into the presynaptic terminals, thus reducing transmitter release.
To resolve these issues, we have used two-photon microscopy to monitor calcium transients evoked by action potentials in axons, axonal varicosities (synaptic boutons) and fine axon collaterals of hippocampal CA1 neurons.
Pentobarbitone (75–300 μM) did not block the invasion of the axonal arbour or the synaptic boutons, but it did reduce the amplitude of the calcium transients recorded from the axons in a concentration-dependent manner. At 150 μM, pentobarbitone reduced the transients to 78±4% of the control.
Pentobarbitone depressed the calcium transients recorded from the synaptic boutons in a concentration-dependent manner. When 150 μM pentobarbitone was applied, the calcium transients recorded from the boutons were 53±3% of the control. This concentration of pentobarbitone also reduced the amplitude and frequency of the spontaneous excitatory postsynaptic potentials to 54±4 and 42±17% of the control, respectively.
The local anaesthetic procaine (500 μM) had no significant effect on action potential invasion of axon collaterals, even though it reduced the action potential amplitude by 25%.
This data are consistent with the notion that the pentobarbitone-induced depression of presynaptic calcium transients contributes to its depressant effect on excitatory synaptic transmission in the CNS.
Keywords: Axon, bouton, synapse, calcium, anaesthetic, pentobarbitone, procaine, calcium transient, hippocampus
Introduction
General anaesthetics, including the barbiturates, are known to depress excitatory synaptic transmission in the central nervous system (CNS) (Pocock & Richards, 1993 and references therein). The underlying mechanisms have, however, not been fully resolved. The debate has centred on the issue of the relative importance of the presynaptic and postsynaptic effects of these drugs. In model systems such as the adrenal chromaffin cell (Pocock & Richards, 1987,1988) and hippocampal autapses (de Sousa et al., 2000), current evidence suggests that ligand-gated ion channels are more sensitive to modulation by anaesthetics than either voltage-gated channels or the exocytotic cascade. However, several reports based on quantal analysis of excitatory postsynaptic potentials (epsps) suggest that presynaptic effects may be the dominant mechanism by which general anaesthetics act on excitatory synaptic transmission in the CNS (Weakly, 1969; Zorychta & Capek, 1978; Kullmann et al., 1989). Furthermore, a number of workers have suggested that these drugs may prevent action potentials from fully invading the axonal arbour, thereby decreasing the synaptic drive to the postsynaptic neurons (Larrabee & Posternak, 1952; Wall, 1967; Berg-Johnsen & Langmoen, 1986).
While it has not proved feasible to resolve these issues by classical electrophysiological methods, recent advances in confocal (Frenguelli & Malinow, 1996; Emptage et al., 1999) and two-photon microscopy (Cox et al., 2000; Koester & Sakmann, 2000) have shown that it is possible to ascertain whether an action potential has invaded an axon or bouton by recording the calcium transients that accompany the passage of an action potential. We have used this approach to investigate the action of pentobarbitone on the axon collaterals and synaptic boutons of hippocampal CA1 neurons. We have addressed the following questions: does pentobarbitone interfere with the action potential propagation into the axonal arbour or presynaptic nerve terminals? Does it significantly reduce the amplitude of the calcium transients in the axon terminals? Is the action potential invasion of axons more likely to fail during repetitive activation and does exposure to pentobarbitone make such a failure more likely?
We find that action potential propagation into the axonal arbour is reliable and is not affected by concentrations of pentobarbitone that have a significant depressant effect on synaptic transmission. It is also reliable in the presence of concentrations of procaine that significantly reduce the amplitude of the action potential. Pentobarbitone has a greater depressant effect on the calcium transients recorded from the synaptic boutons than on those recorded from axons or their collaterals. This depressant effect occurs at low concentrations and parallels the reduction in the amplitude of the epsps. A preliminary account of our findings has been presented (Baudoux et al., 2001).
Methods
Preparation of hippocampal slice cultures
Transverse slices of hippocampus 250 μm thick were cut from the brains of 8-day-old rat pups. They were subsequently cultured as described by Stoppini et al. (1991) on Millicell CM membranes at a gas/liquid interface for 7–14 days before use.
Electrophysiological methods
The hippocampal slice cultures were mounted in a custom-built recording chamber placed on the stage of a Nikon FN-600 upright microscope equipped with infrared optics and a Bio-Rad 1024 laser confocal scan head. For the majority of experiments, the slices were maintained at 28–30°C and were continuously superfused with oxygenated artificial cerebrospinal fluid (acsf) containing the free radical scavenger Trolox. To control for any confounding effect of low temperature, a further series of experiments was carried out at 34°C. No significant differences were found between the two series.
Electrophysiological recordings were made from the cell bodies of hippocampal CA1 neurons either with conventional intracellular techniques using sharp micropipettes (electrode resistance 100–120 MΩ) or with the whole-cell patch-clamp method (electrode resistance 9–14 MΩ). Sharp electrodes were filled at the tip with 1 mM of the calcium indicator Oregon Green 488 BAPTA-1 (Molecular Probes, Eugene, OR, U.S.A.) in 100 mM potassium acetate and back filled with 3 M potassium acetate. The indicator was loaded by injecting hyperpolarizing current (0.1 nA) for 5–15 min via an Axoclamp 2B amplifier (Axon Instruments, Union City, CA, U.S.A.). For the whole-cell patch-clamp configuration, the electrodes were filled with a standard intracellular recording solution (see below) containing 200 μM Oregon Green BAPTA-1. In a few cases, a 1 : 1 mixture of 100 μM Oregon Green BAPTA-1 and 100 μM Oregon Green BAPTA-2 was used. To allow the indicator to diffuse from the cell body into the axon and its collaterals, we waited 15–20 min before imaging. Action potentials were evoked by depolarizing pulses (0.1–0.2 nA, 10–50 ms) delivered to the cell body via the bridge circuitry of the recording amplifier. Electrophysiological data were collected with a CED 1401 interface linked to an IBM compatible 486 computer. Electrophysiological data were analysed using the Strathclyde Electrophysiology Software WinWCP program version 2.3.
Optical recording
After impalement or break in, cells were visualized by two-photon imaging with a Nikon 60 × NA 1.0 water-immersion objective and pulsed infrared laser light derived from a Millennia V pump laser coupled to a Tsunami mode-locked infrared laser (Spectra Physics). The excitation light had a wavelength of 790 nm and its intensity was regulated with neutral density filters. This provided a fluorescence emission sufficient to give a signal-to-noise ratio greater than 3 : 1 without significant bleaching of the indicator. For most experiments, 5–10% of the available laser power was used. The fluorescence emission was collected with an external detector and was not descanned.
To identify the axon and locate either a branch point or an axon varicosity, the plane of focus was continuously varied during the laser scanning. The axon was identified among the basal dendrites and was traced until a branch point or a bouton was identified. A custom-made scan-rotator circuit (Scientific Systems Design Inc., NJ, U.S.A.) was used to rotate the scan within the plane of focus for optimal alignment with the axon, axon collateral or varicosity.
Since two-dimensional scans are too slow for accurate determination of the time course and amplitude of the calcium transients, line scans were used. These consisted of successive sweeps at 2 ms intervals across a single line in the field of view. Images were collected using Lasersharp Timecourse software (Bio-Rad, U.K.) and analysed using ImageJ (NIH) and Origin 7.0 (Microcal software). At each phase of the experiments (control, drug exposure, recovery), 3–6 line scans were collected and subsequently averaged off-line. This procedure significantly improved the signal-to-noise ratio and avoided any bias due to unconscious selection of individual line scans. After subtracting the background signal, the amplitude of the calcium transients at the recording sites was expressed as the fractional change in basal fluorescence (ΔF/F), which is approximately proportional to the changes in intracellular Ca2+ (Maravall et al., 2000).
Statistical significance was assessed using paired t-tests. All values are expressed as means±s.e.m.
Solutions
The composition of the acsf was (in mM): NaCl 120, KCl 3, MgCl2 1, CaCl2 3, NaHPO4 1.2, NaHCO3 23, glucose 11 and Trolox 1 (a free radical scavenger). It was equilibrated with 95% O2/5% CO2 before use and during the experiment. The anaesthetics (pentobarbitone, 75–300 μM and procaine, 500 μM) were dissolved directly into the bathing solution immediately before use. The patch pipette solution was (in mM): NaCl 5, KCl 10, K+ gluconate 125, Mg-ATP 4, Na+-GTP 0.3, HEPES 10, Oregon Green BAPTA-1 0.2. The pH was adjusted to 7.0 with KOH.
Results
Calcium transients in axons evoked by single action potentials
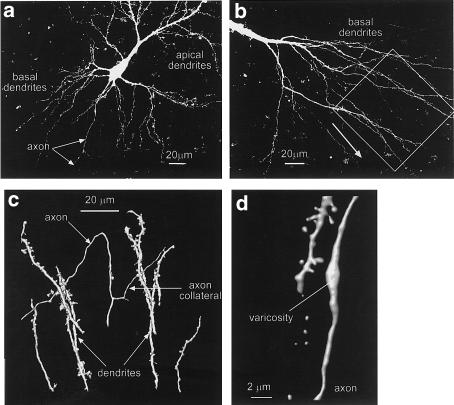
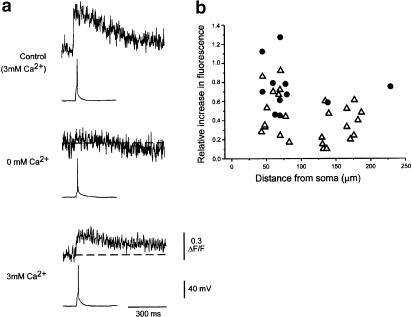
Whole-cell and intracellular recordings were made from 67 neurons in the CA1 region of the hippocampus. Axons were identified among the basal dendrites as smooth processes of small diameter, which often branched to give rise to thin collaterals (see Figure 1a–d). Dendrites were frequently encountered in the same region and were readily distinguished from the axons by their overall morphology and the presence of spines (see Figure 1c–d). The diameter of the axons ranged between 0.4 and 0.8 μm. When an action potential was evoked by a depolarizing pulse, the free calcium concentration [Ca2+]i in the axon rose from baseline to peak within 2–4 ms (see Figure 2a). The amplitude of the rise in [Ca2+]i expressed as the ΔF/F was 0.41±0.04 (n=30) with a range of 0.1–0.9. The calcium transients in the axons declined to the resting level with a time constant of 540±46 ms (means±s.e.m., n=17; range 370–840 ms). Calcium transients in response to single action potentials were recorded both from the main axon and axon collaterals at distances between 45 and 230 μm from the cell body. Despite these differing recording parameters, the amplitude of the calcium transients did not show any significant correlation with the axon diameter or with the distance between the recording site and the cell soma (Figure 2b).
Figure 1.
Examples of hippocampal neurons visualized after labelling with the calcium indicator Oregon green BAPTA-1. All panels show images derived from a series of optical sections. Panel a shows both apical and basal dendrites and the course of the axon (arrows). Panel b shows a view of basal dendrites from another neuron. The area indicated by the box is enlarged and rotated in panel c. The arrowhead in (b) indicates the top of the rotated image. Panel c is a surface rendering of the structures indicated by the box in panel b. The axon is clearly distinguishable from the basal dendrites by its small diameter, lack of spines and branching pattern. Panel d shows a surface rendering of an axon varicosity and a neighbouring dendrite with clearly identifiable spines.
Figure 2.
Characteristics of calcium transients recorded from hippocampal CA1 neurons. Panel a shows that the transients are reversibly suppressed by the removal of extracellular calcium. The voltage and fluorescence calibrations apply to all three panels. The removal of extracellular calcium suppressed the evoked calcium transients, but had no effect on the action potential amplitude. Removal of extracellular calcium increased the excitability of the neuron. Panel b shows the amplitude of the transients recorded from axons (open triangles) and boutons (filled circles) as a function of distance from the soma.
In some cases, the action potential-induced transient was small enough to fall within the noise; in such cases, however, calcium transients could be unmasked by eliciting short trains of action potentials (see below). Depolarizations that were too weak to evoke an action potential did not give rise to calcium responses in the axons.
As the evoked calcium transients could be reversibly suppressed by the removal of extracellular calcium (see Figure 2a), and, as they occur only when an action potential has been evoked, we conclude that these transients reflect a calcium influx into the axons that is triggered by the passage of an action potential. This interpretation is in accord with earlier studies on similar preparations (Cox et al., 2000; Koester & Sakmann, 2000; Emptage et al., 2001).
Calcium transients recorded from axon varicosities
Axonal varicosities were observed both on the main axons and their collateral branches (Figure 1d). Similar structures have been observed along the axons of neocortical neurons (Cox et al., 2000; Koester & Sakmann, 2000). Detailed analysis of electron micrographs (Kincaid et al., 1998; Shepherd & Harris, 1998) and biocytin-labelled cells (Koester & Sakmann, 2000) have shown that virtually all these varicosities are synaptic boutons of the en passage type. Furthermore, a previous study of organotypic hippocampal cultures has shown that these structures contain the presynaptic marker protein synaptophysin (Emptage et al., 2001). Accordingly, we will refer to these structures as synaptic boutons.
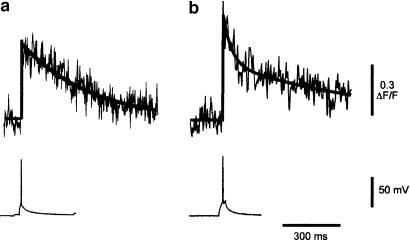
As for the parent axons, the free calcium concentration in the boutons rose rapidly to reach its peak value within 4 ms. The amplitude of the rise in calcium expressed as the ΔF/F was 0.65±0.06 (means±s.e.m., n=13) with a range of 0.3–1.12 (see Figure 2b). This change in fluorescence was significantly greater than that seen in the axons or their branches (P<0.01). In the majority of cases (10/14), the decay of the calcium signal could be fitted with a single exponential with a time constant of 459±75 ms (see Figure 3a). In four cases, the decay was better fitted by the sum of two exponentials with fast and slow time constants of 43±9 and 825±223 ms, respectively (see Figure 3b). The ratio of the amplitudes of the fast and slow components (Af/As) was 0.76±0.18.
Figure 3.
Time course of the calcium transients. Panel a shows an example of a calcium transient fitted with a single exponential (solid line) with a time constant of 367 ms. Panel b shows an example of a calcium transient fitted with a double exponential (solid line). The fast and slow time constants were 41 and 763 ms, respectively. The action potentials responsible for evoking each calcium transient are shown below each record. Calibration bars apply to both panels.
Pentobarbitone does not block action potential propagation into the axonal arbour or synaptic bouton
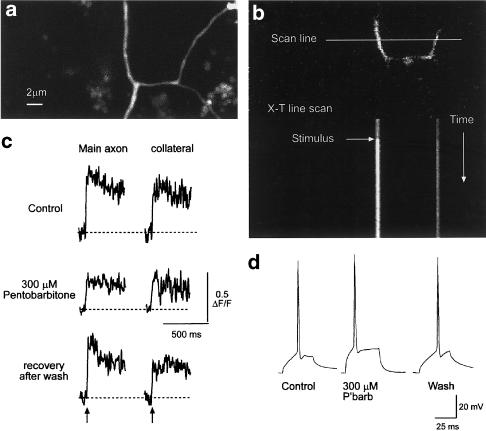
As described earlier, when an action potential was evoked by a depolarizing pulse, the free calcium concentration [Ca2+]i in the axon rapidly rose from baseline to a peak. We have used the appearance of these evoked transients to indicate whether an action potential had invaded an axon, an axon collateral or a synaptic bouton. We recorded evoked calcium transients from 15 main axons and their collaterals distal to clearly identified axon branch points. An example of a branch point is shown in Figure 1c. In each case, the evoked action potential elicited a calcium transient both in the main axon and in its collateral branch (n=77 trials in 15 different neurons). In seven of these preparations, we examined the effects of pentobarbitone at 150 μM (n=5) and 300 μM (n=2) (see Figure 4). In no case did an action potential fail to elicit a calcium transient in both the collateral and its parent axon.
Figure 4.
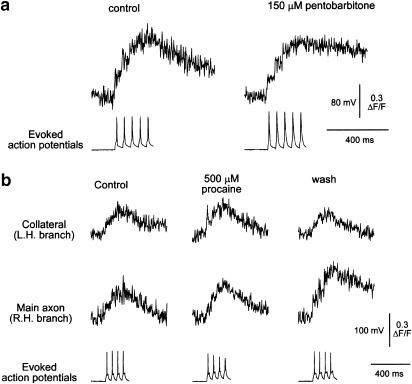
Pentobarbitone does not prevent the propagation of action potentials into collateral branches. Panel a shows a projected image of an axon branch point. After the main axon branches, the collateral branches again. Panel b shows the optical section used for the calcium imaging (top) and the resulting line image (six individual line scans were averaged). In this example, both the main axon and a collateral were captured in the same optical section. Panel c shows the calcium transients recorded from the main axon and the collateral before, during and after exposure to 300 μM pentobarbitone. Arrows indicate the point of stimulation. While this concentration had a substantial depressant effect on the amplitude of the calcium transients, it did not prevent the action potential invading the collateral. The baseline for each trace is indicated by the dotted lines. The calibration bars apply to all three records. Panel d shows examples of the action potentials used to elicit the calcium transients shown in panel c. Note the expanded time base compared to (c).
A further six experiments were carried out at 34°C to check that this security of action potential propagation was not an artefact due to the low temperature used. As for the experiments at 30°C discussed above, the action potential invasion of the axons and their collaterals was reliable (77 trials in six neurons). A further 23 trials were carried out in the presence of pentobarbitone (150–300 μM) and in no case did we observe a failure of the action potential to invade the main axon or the axon collateral. We therefore conclude that action potential invasion of the axonal arbour is reliable even in the presence of concentrations (300 μM) of pentobarbitone that profoundly depress excitatory synaptic transmission.
Similar experiments on synaptic boutons showed no failure of action potential invasion during the application of pentobarbitone (75–300 μM; 69 trials in 14 boutons). This reliable propagation occurred despite the modulatory effects of pentobarbitone on the membrane potential of half the neurons studied: at 150 μM, it reversibly hyperpolarized six (of 12) neurons studied by an average of 4.3±1.5 mV; there was no change in the membrane potential of the remaining six. At 300 μM, the average hyperpolarization of three (of six) neurons was 6.3±2.5 mV. As before, this hyperpolarization was reversible on washing out the drug. As expected, larger depolarizing currents were required to elicit an action potential in those cells that hyperpolarized during exposure to pentobarbitone. These concentrations of pentobarbitone had no significant effect on the amplitude of the evoked action potentials measured from the resting membrane potential to the tip of the overshoot (see Figures 4d and 6).
Figure 6.
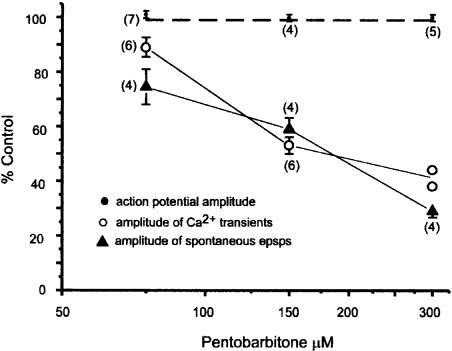
The concentration–response relationship for the effect of pentobarbitone on the mean amplitude of spontaneous epsps in hippocampal CA1 neurons (filled triangles) and the evoked calcium transients recorded from synaptic boutons (open circles). The numbers next to the data points are the number of individual experiments at each concentration. The mean amplitude of the epsps was determined by averaging 30–70 spontaneous synaptic events before, during and after exposure to anaesthetic. Note that these concentrations of pentobarbitone had no significant effect on the amplitude of the evoked action potentials (filled circles, the dotted line indicates 100% of control).
The local anaesthetic procaine (500 μM) reduced the amplitude of the action potential by 25±5% (n=6). It also caused it to broaden: the rise time in the control period was 1.32±0.07 ms (n=11); in procaine it was 2.48±0.3 ms (n=10); after washing out procaine it recovered to 1.36±0.12 (n=9). Nevertheless, this agent did not compromise action potential invasion of the axon (n=102 trials in 10 neurons) or its collaterals (n=35 trials in five neurons), nor did it significantly affect the resting membrane potential.
Pentobarbitone depresses presynaptic calcium transients and spontaneous epsps
The barbiturates and other general anaesthetics inhibit high-threshold voltage-gated calcium channels in adrenal chromaffin cells (Pocock & Richards, 1987; Charlesworth et al., 1994); this effect occurs at concentrations of pentobarbitone likely to be found in the brain during general anaesthesia. Furthermore, as the evoked synaptic potentials in the CNS show a steep dependence on extracellular calcium (Richards & Sercombe, 1970; Dingledine & Somjen, 1981), it is possible that the depressant effects of pentobarbitone on the calcium transients of presynaptic nerve terminals might be greater than those on model systems. To investigate this possibility, we have determined the action of pentobarbitone and procaine on the amplitude of evoked calcium transients recorded from axons and their collaterals.
Pentobarbitone (150 μM) reduced the amplitude of the transients recorded from the axons to 78±4% of control (n=6). At 300 μM, the amplitude was reduced further to 61±8% of the control (n=4). In contrast, the local anaesthetic procaine (500 μM) had no depressant effect on the transients (the amplitude of the transients in the presence of procaine was 107±9% of the control; n=6).
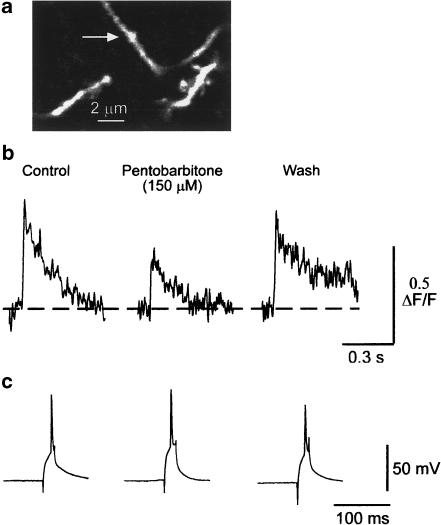
As shown in Figure 5, pentobarbitone also reduced the amplitude of the calcium transients recorded from the synaptic boutons. This effect was concentration dependent and reversible; at a concentration of 75 μM the amplitude of the transients was 89±3.5% (n=6) of the control, at 150 μM they were 53±3.1% (n=7) of the control and at 300 μM (the highest concentration tested) the transients were 41% (n=2) of their control amplitude (see Figure 6). The greater effect of 150 μM pentobarbitone on the calcium transients recorded from the boutons compared to their effects on the axons was statistically significant (P<0.001 by unpaired two-tailed t-test).
Figure 5.
Pentobarbitone inhibits the calcium transients recorded from synaptic boutons. Panel a shows a single optical section of the labelled structures. The arrow indicates the position of the varicosity. (b) shows the calcium transients recorded from this synaptic bouton before, during and after exposure to 150 μM pentobarbitone. The dashed line indicates the prestimulus baseline. (c) shows the action potentials used to evoke the calcium transients shown in (b). The calcium transients were reversibly depressed by 150 μM pentobarbitone. Although the overshoot of the action potential was reduced during the wash, it was still sufficient to elicit a calcium transient comparable in amplitude to that seen in the control period. Note the expanded time base for the action potential records.
The time for the transients to reach their peak was <4 ms and this was not increased by either of the anaesthetics studied. However, as the time resolution of our line scans is 2 ms, the possibility that the anaesthetics might affect the time constant of the rise of the transients cannot be totally excluded. Neither of the anaesthetics studied had a consistent effect on the rate of decay of the calcium transients, whether they were recorded from the axon or the bouton.
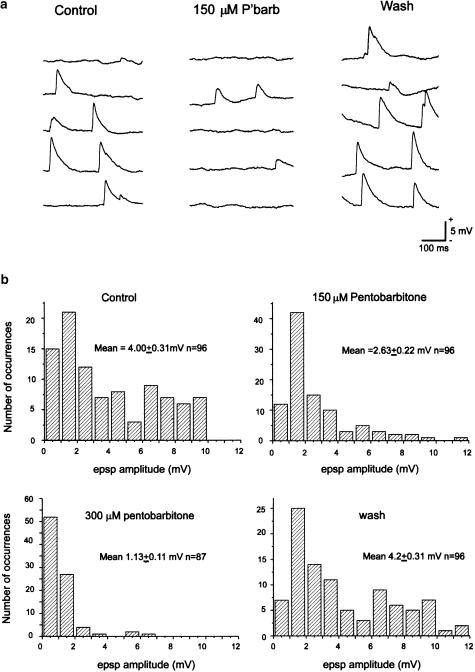
Spontaneous epsps occurred in most cells with a mean frequency of about 1–5 events s−1. In some cells, much higher frequencies were seen and the mean value was 5.1 epsps s−1 (range 0.93–13.4 s−1). Their amplitude varied between preparations, but typically the mean control amplitude was 3–4 mV (see Figure 7), although individual epsps ranged from 1 to 12 mV even in the same preparation. The epsps showed a similar concentration-dependent reduction in amplitude to that of the evoked calcium transients (see Figure 6). Their frequency fell in a concentration-dependent manner. It was 71±10% of the control in 75 μM pentobarbitone, 42±17% in 150 μM and 18±12% in 300 μM (n=4 for each concentration). The large errors reflect the wide variations in the frequency of epsps seen between individual cells. These effects of pentobarbitone could be reversed on washing.
Figure 7.
Examples of the spontaneous epsps recorded from hippocampal CA1 neurons in organotypic cultures and their modulation by pentobarbitone. (a) Examples of the epsps recorded from one neuron before, during and after exposure to 150 μM pentobarbitone. (b) Amplitude histograms of the spontaneous epsps recorded from the same cell. Pentobarbitone reversibly depressed the epsps.
Anaesthetic effects on calcium transients evoked by short trains of action potentials
To see whether anaesthetics could block action potential propagation during repetitive activity, we examined the effects of procaine and pentobarbitone on the calcium transients evoked by short 16 Hz trains of 4–6 action potentials. In the absence of anaesthetic, the calcium-induced fluorescence increased progressively with each action potential (Figure 8). In 10 (of 15) recordings, the calcium signal increased in a clear stepwise manner for the first two or three action potentials in the train. The increment of the calcium transients became progressively smaller during the later stages of the train as the calcium transient approached its maximum. This is to be expected for a dye obeying the law of mass action. At the end of the train, the rate of decline of the fluorescence signal could be fitted with a single exponential having a mean time constant of 475±92 ms (n=6). Even when recording distal to identified branch points, there was no failure of action potential invasion during the trains (49 trials in eight different preparations).
Figure 8.
Anaesthetics do not increase the likelihood of action potential failure during brief high-frequency trains. Panel a shows the calcium transients recorded from a 0.7 μm diameter axon during a 16 Hz train of five action potentials before and during the application of 150 μM pentobarbitone. The action potential train is shown below the calcium transients on the same time base. Step increments in fluorescence with the first three action potentials are clearly visible. Panel b, the effect of procaine on calcium transients recorded simultaneously from an axon of 0.6 μm diameter and a collateral (0.4 μm diameter) during a 16 Hz train of four action potentials shown in the electrophysiological records below the calcium transients. The increments in fluorescence during the train are largely masked by the noise, but the progressive growth in the signal during the trains is evident in both the parent axon and the collateral. Procaine had no significant depressant effect on the calcium transients, but the action potential was depressed and exhibited use-dependent inhibition (bottom row of records).
The actions of pentobarbitone and procaine on the amplitude of the transients recorded in response to the trains were similar to that seen in response to single action potentials. For 500 μM, procaine there was no significant reduction in the amplitude of the transients nor did exposure to the drug result in the failure of conduction through an axon branch point, even though procaine both depressed, and exhibited use-dependent inhibition of the action potential (see Figure 8b, middle panel). For pentobarbitone (150 μM), the amplitudes of the transients in response to trains of 4–6 action potentials and to a single action potential were 74±7% (n=3) and 78±3% (n=6) of the control, respectively. Nevertheless, there was no failure of impulse propagation into the axonal arbour during the trains (18 trials in three different preparations).
Discussion
The appearance of our calcium transients is very similar to that previously described both in this preparation (Emptage et al., 2001) and in slices of neocortex (Cox et al., 2000; Koester & Sakmann, 2000). Following an action potential, the calcium signal in the axons and collaterals abruptly rose from its resting level to reach a peak within 4 ms. The calcium level subsequently decayed exponentially to baseline with an average time constant of approximately 540 ms. The amplitude of the transients recorded from the boutons was significantly greater than those recorded from the axons, but the time course of the rise and fall were similar. The rise time was less than 4 ms and the decay of the fluorescence signal could generally be fitted with a single exponential, although in a number of instances, the fit was significantly improved using two exponentials. The time constants averaged about 460 ms for those transients that were adequately fitted by a single exponential. For those that required two exponentials, the fast time constant averaged 43 ms and the slow time constants averaged 825 ms. These values are similar to those previously reported by Koester & Sakmann (2000) for the calcium transients recorded from the axons of neocortical neurons. These authors used Oregon green BAPTA-1 and a number of other calcium-sensing dyes and concluded that the decay of the transients reflects the buffering characteristics of the dye rather than the intrinsic rate of calcium clearance.
The first aim of this study was to see whether anaesthetic agents increase the likelihood that an action potential will fail to invade a collateral or a synaptic bouton. In agreement with previous studies (Cox et al., 2000; Koester & Sakmann, 2000; Emptage et al., 2001) we find that, in the absence of anaesthetic, the action potential invasion of the axonal arbour is reliable. This is true both for the invasion of fine axon collaterals and for that of synaptic boutons. Blocking a substantial fraction of the sodium channels with 500 μM procaine broadened the action potential and reduced its amplitude by about a quarter, but this did not prevent it from invading the axonal arbour. This implies that even small diameter axons have a large safety factor for action potential propagation.
Pentobarbitone depresses excitatory synaptic transmission in the CNS (Richards, 1972; Nicoll & Wojtowicz, 1980; Richards & Strupinski, 1986) and, at higher concentrations, blocks voltage-gated sodium channels (McGivern & Scholfield, 1990). Although we found that pentobarbitone depressed the amplitude of the calcium transients (see below), it did not prevent action potential invasion of any part of the axonal arbour. We therefore conclude that the pentobarbitone-induced depression of excitatory synaptic transmission in the CNS synapses is not caused by a failure of the afferent action potential to invade every axon branch.
Our second aim was to determine whether pentobarbitone and procaine modulated the amplitude of the calcium transients. In agreement with an earlier study (Charlesworth et al., 1992), we found that procaine had little effect on the amplitude of the calcium transients, even though it was employed at a concentration (500 μM) that reduced the action potential by about 25%. This result implies that modest reductions in action potential amplitude do not affect the recruitment of voltage-gated calcium channels, provided that the depolarization is sufficient to activate them fully. Pentobarbitone also depressed the amplitude of the transients recorded from both axons and synaptic boutons. The degree of inhibition of the transients recorded from the axons is in line with the known action of this agent on calcium channels (Blaustein & Ector, 1975; Wertz & McDonald, 1985; Pocock & Richards, 1987). The depressant effect on the boutons closely paralleled the reduction in the amplitude of the spontaneous epsps recorded from the same preparation (see Figure 4). This reduction in epsp amplitude could arise from a reduction in the mean quantal content of the epsps and is consistent with a presynaptic action of pentobarbitone. Nevertheless, a contribution from a reduced sensitivity of the postsynaptic glutamate receptors cannot totally be excluded. The sensitivity of the calcium transients and the epsps to pentobarbitone is similar to that of evoked epsps in the olfactory cortex (Richards, 1972,1982; Richards & Strupinski, 1986) and occurs at concentrations within the range required for the induction and maintenance of general anaesthesia (50–300 μM, see Richards, 1972).
Although the effects of pentobarbitone on the amplitude of the calcium transients recorded from axons are in line with earlier studies on calcium channel currents and calcium influx (see above), the greater anaesthetic sensitivity of the calcium transients recorded from the boutons requires some additional explanation. Three possibilities present themselves:
The calcium channels in the synaptic boutons may be uniquely sensitive to anaesthetics. This seems unlikely as current evidence suggests that the calcium channels that give rise to the calcium transients in the synaptic boutons are predominantly of the P/Q and N subtypes (Koester & Sakmann, 2000), which are not strongly inhibited by anaesthetics (Charlesworth et al., 1994; Hall et al., 1994). Pharmacological studies are also consistent with a predominant role for P/Q and N subtypes in synaptic transmission (Takahashi & Momiyama, 1993; Wheeler et al., 1994).
A significant fraction of the calcium that gives rise to the transients recorded in the nerve terminals is not derived from calcium influx, but from internal stores (see Emptage et al., 2001). As this process involves calcium-induced calcium release, a positive feedback process, a small reduction in calcium influx could cause a disproportionate decrease in the amplitude of the final transient.
Pentobarbitone acts via presynaptic adenosine receptors to inhibit the evoked calcium transients of the boutons. Consistent with this view, Cox et al. (2000) have shown that adenosine attenuates the transients recorded from axonal varicosities in slices of the cerebral cortex and Tohdoh et al. (2000) have reported that the depressant action of pentobarbitone on evoked epsps in the CA1 region of the hippocampus is attenuated by prior treatment with antagonists of A1 receptors. Furthermore, Emptage et al. (1999) have shown that adenosine depresses the probability of a postsynaptic response to a presynaptic volley at single synaptic contacts.
Of these three possibilities, the second and third seem the most plausible. The transient nature of the effect of the A1 antagonists on the evoked epsps argues against the involvement of these receptors, so further experimental work will be required to settle this issue unequivocally. As the depressant action of pentobarbitone on hippocampal epsps is not affected by bicuculline, a γ-amino butyric acid (GABA)A antagonist, it appears that presynaptic GABAA receptors are not implicated (Tohdoh et al., 2000).
Some neurons were hyperpolarized by pentobarbitone (see Results). This probably reflects both a direct activation of GABAA receptors and a loss of excitatory tone following the barbiturate-induced depression of the spontaneous activity of the slices. Consistent with this, it has previously been shown that barbiturate-induced hyperpolarization of cultured hippocampal neurons can be blocked by GABAA receptor antagonists (Rho et al., 1996). Nevertheless, the pentobarbitone-induced hyperpolarization cannot explain the reduction in the amplitude of the calcium transients or that of the spontaneous epsps, as both occur to a similar degree in cells that were not hyperpolarized. Furthermore, hyperpolarization would be expected to offset any depressant action of pentobarbitone as the driving potential (the difference between the membrane potential and the epsp reversal potential) for the epsp would increase.
In contrast to its depressant effect on excitation, pentobarbitone and other general anaesthetics have been found to enhance inhibitory synaptic transmission in the hippocampus (Nicoll et al., 1975; Gage & Robertson, 1985). The fact that pentobarbitone depresses the presynaptic calcium transients of CA1 pyramidal neurons suggests that the release of GABA from inhibitory interneurons in response to an action potential will be depressed, as is GABA release in response to a high potassium stimulus (Jessell & Richards, 1977). This suggests that the pentobarbitone enhancement of inhibitory synaptic transmission is due entirely to its action on the postsynaptic GABAA receptors.
Although different groups of neurons may use rather different coding schemes, information within the CNS is coded in trains of action potentials (Gerstner et al., 1997). Moreover, there is evidence that synaptic activity may be based on highly structured temporal codes (Tsodyks & Markram, 1997), which may be subject to modulation by drugs, such as anaesthetics that interfere with information processing. Anaesthesia could result, at least in part, from a failure of an action potential to invade part of the axonal arbour during trains of action potentials. We have specifically tested this proposition and found no evidence of such a failure with either of the drugs tested.
In conclusion, these experiments show that neither pentobarbitone nor procaine alters the likelihood of the action potential invading axon collaterals or synaptic boutons. It is therefore unlikely that the small effects of general anaesthetics on voltage-gated sodium channels play any significant role in the blockade of synaptic transmission. However, pentobarbitone does cause a concentration-dependent reduction in the amplitude of the calcium transients recorded in the presynaptic boutons that closely parallels the reduction in epsp amplitude. It therefore appears that the depression of the presynaptic calcium transients contributes substantially to the depressant effect of pentobarbitone on excitatory synaptic transmission in the CNS. While this may reflect a direct action on presynaptic calcium channels, more indirect actions cannot yet be excluded.
Acknowledgments
We thank the BBSRC for funding this project through their Bio-Imaging initiative and Dr N.J. Emptage for help and advice, particularly during the early phase of the study. We also thank Dr D.A. Richards for the surface renderings in Figure 1 and for helpful comments on the manuscript.
Abbreviations
- Acsf
artificial cerebrospinal fluid
- CNS
central nervous system
- ΔF/F
fractional change in fluorescence
- epsps
excitatory postsynaptic potentials
- GABA
γ-amino butyric acid
References
- BAUDOUX S., EMPSON R.M., RICHARDS C.D.Action potential propagation in the hippocampus and its modulation by general anesthetics studied by two photon microscopy Society for Neuroscience 31st Annual Meeting, San Diego, California 2001(abstract 711.3)
- BERG-JOHNSEN J., LANGMOEN I.A. Isoflurane effects in rat hippocampal cortex: a quantitative evaluation of different cellular sites of action. Acta Physiol. Scand. 1986;128:613–618. doi: 10.1111/j.1748-1716.1986.tb08019.x. [DOI] [PubMed] [Google Scholar]
- BLAUSTEIN M.P., ECTOR A.C. Barbiturate inhibition of calcium uptake by depolarized nerve terminals in vitro. Mol. Pharmacol. 1975;11:369–378. [PubMed] [Google Scholar]
- CHARLESWORTH P., JACOBSON I., POCOCK G., RICHARDS C.D. The mechanism by which procaine inhibits catecholamine secretion from bovine chromaffin cells. Br. J. Pharmacol. 1992;106:802–812. doi: 10.1111/j.1476-5381.1992.tb14416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARLESWORTH P., POCOCK G., RICHARDS C.D. Calcium channel currents in bovine adrenal chromaffin cells and their modulation by anaesthetic agents. J. Physiol. 1994;481:543–553. doi: 10.1113/jphysiol.1994.sp020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX C.L., DENK W., TANK D.W., SVOBODA K. Action potentials reliably invade axonal arbors of rat neocortical neurons. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9724–9728. doi: 10.1073/pnas.170278697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE SOUSA S.L., DICKINSON R., LIEB W.R., FRANKS N.P. Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology. 2000;92:1055–1066. doi: 10.1097/00000542-200004000-00024. [DOI] [PubMed] [Google Scholar]
- DINGLEDINE R., SOMJEN G. Calcium dependence of synaptic transmission in the hippocampal slice. Brain Res. 1981;207:218–222. doi: 10.1016/0006-8993(81)90697-1. [DOI] [PubMed] [Google Scholar]
- EMPTAGE N.J., BLISS T.V.P., FINE A. Single synaptic events evoke NMDA receptor-mediated release of calcium from internal stores in hippocampal dendritic spines. Neuron. 1999;22:115–124. doi: 10.1016/s0896-6273(00)80683-2. [DOI] [PubMed] [Google Scholar]
- EMPTAGE N.J., REID C.A., FINE A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- FRENGUELLI B.G., MALINOW R. Fluctuations in intracellular calcium responses to action potentials in single en passage presynaptic boutons of layer V neurons in neocortical slices. Learn. Mem. 1996;3:150–159. doi: 10.1101/lm.3.2-3.150. [DOI] [PubMed] [Google Scholar]
- GAGE P.W., ROBERTSON B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br. J. Pharmacol. 1985;85:675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSTNER W., KREITER A.K., MARKRAM H., HERZ A.V. Neural codes: firing rates and beyond. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12740–12741. doi: 10.1073/pnas.94.24.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A.C., LIEB W.R., FRANKS N.P. Insensitivity of P-type calcium channels to inhalational and intravenous general anesthetics. Anesthesiology. 1994;81:117–123. doi: 10.1097/00000542-199407000-00017. [DOI] [PubMed] [Google Scholar]
- JESSELL T.M., RICHARDS C.D. Barbiturate potentiation of hippocampal ipsps is not mediated by blockade of GABA uptake. J. Physiol. (Lond.) 1977;269:42P. [PubMed] [Google Scholar]
- KINCAID A.E., ZHENG T., WILSON C.J. Connectivity and convergence of single corticostriatal axons. J. Neurosci. 1998;18:4722–4731. doi: 10.1523/JNEUROSCI.18-12-04722.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOESTER H.J., SAKMANN B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J. Physiol. 2000;529:625–646. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULLMANN D.M., MARTIN R.L., REDMAN S. Reduction by general anaesthetics of group Ia excitatory postsynaptic potentials and currents in the cat spinal cord. J. Physiol. 1989;412:277–296. doi: 10.1113/jphysiol.1989.sp017615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARRABEE M.G., POSTERNAK J.M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J. Neurophysiol. 1952;15:92–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- MARAVALL M., MAINEN Z.F., SABATINI B.L., SVOBODA K. Estimating intracellular calcium concentrations and buffering without wavelength ratioing. Biophys. J. 2000;78:2655–2667. doi: 10.1016/S0006-3495(00)76809-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGIVERN J., SCHOLFIELD C.N. General anaesthetics and field currents in unclamped, unmyelinated axons of rat olfactory cortex. Br. J. Pharmacol. 1990;101:217–223. doi: 10.1111/j.1476-5381.1990.tb12116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICOLL R.A., ECCLES J.C., OSHIMA T., RUBIA F. Prolongation of hippocampal inhibitory postsynaptic potentials by barbiturates. Nature (Lond.) 1975;258:625–627. doi: 10.1038/258625a0. [DOI] [PubMed] [Google Scholar]
- NICOLL R.A., WOJTOWICZ J.M. The effects of pentobarbital and related compounds on frog motoneurons. Brain Res. 1980;191:225–237. doi: 10.1016/0006-8993(80)90325-x. [DOI] [PubMed] [Google Scholar]
- POCOCK G., RICHARDS C.D. The action of pentobarbitone on stimulus–secretion coupling in adrenal chromaffin cells. Br. J. Pharmacol. 1987;90:71–80. doi: 10.1111/j.1476-5381.1987.tb16826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POCOCK G., RICHARDS C.D. The action of volatile anaesthetics on stimulus–secretion coupling in bovine adrenal chromaffin cells. Br. J. Pharmacol. 1988;95:209–217. doi: 10.1111/j.1476-5381.1988.tb16566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POCOCK G., RICHARDS C.D. Excitatory and inhibitory synaptic mechanisms in anaesthesia. Br. J. Anaesthol. 1993;71:134–147. doi: 10.1093/bja/71.1.134. [DOI] [PubMed] [Google Scholar]
- RHO J.M., DONEVAN S.D., ROGAWSKI M.A. Direct activation of GABAA receptors by barbiturates in cultured rat hippocampal neurons. J. Physiol. (Lond.) 1996;497:509–522. doi: 10.1113/jphysiol.1996.sp021784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS C.D. On the mechanism of barbiturate anaesthesia. J. Physiol. (Lond.) 1972;227:749–767. doi: 10.1113/jphysiol.1972.sp010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS C.D. The actions of pentobarbitone, procaine and tetrodotoxin on synaptic transmission in the olfactory cortex of the guinea-pig. Br. J. Pharmacol. 1982;75:639–646. doi: 10.1111/j.1476-5381.1982.tb09185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS C.D., SERCOMBE R. Calcium, magnesium and the electrical activity of guinea-pig olfactory coex in vitro. J. Physiol. 1970;211:571–584. doi: 10.1113/jphysiol.1970.sp009294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDS C.D., STRUPINSKI K. An analysis of the action of pentobarbitone on the excitatory postsynaptic potentials and membrane properties of neurones in the guinea-pig olfactory cortex. Br. J. Pharmacol. 1986;89:321–325. doi: 10.1111/j.1476-5381.1986.tb10263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPHERD G.M., HARRIS K.M. Three-dimensional structure and composition of CA3–CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J. Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOPPINI L., BUCHS P.A., MULLER D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI T., MOMIYAMA A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- TOHDOH Y., NARIMATSU E., KAWAMATA M., NAMIKI A. The involvement of adenosine neuromodulation in pentobarbital-induced field excitatory post-synaptic potentials depression in rat hippocampal slices. Anesthol. Analg. 2000;91:1537–1541. doi: 10.1097/00000539-200012000-00044. [DOI] [PubMed] [Google Scholar]
- TSODYKS M.V., MARKRAM H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc. Natl. Acad. Sci. U.S.A. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALL P.D. The mechanisms of general anesthesia. Anesthesiology. 1967;28:46–53. doi: 10.1097/00000542-196701000-00006. [DOI] [PubMed] [Google Scholar]
- WEAKLY J.N. Effect of barbiturates on ‘quantal' synaptic transmission in spinal motoneurones. J. Physiol. 1969;204:63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERTZ M.A., MCDONALD R.L. Barbiturates decrease voltage-dependent calcium conductance of mouse neurons in dissociated cell culture. Mol. Pharmacol. 1985;28:269–277. [PubMed] [Google Scholar]
- WHEELER D.B., RANDALL A., TSIEN R.W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- ZORYCHTA E., CAPEK R. Depression of spinal monosynaptic transmission by diethyl ether: quantal analysis of unitary synaptic potentials. J. Pharmacol. Exp. Ther. 1978;207:825–836. [PubMed] [Google Scholar]