Abstract
G-protein-coupled receptor 100 (GPR100) was discovered by searching the human genome database for novel G-protein-coupled peptide receptors.
Full-length GPR100 was amplified from a cDNA library of the neuroendocrine cell line BON, which is derived from a human pancreas carcinoid. The open-reading frame, present on a single exon, coded for a protein of 374 amino acids with highest sequence identity (43%) to the human orphan somatostatin- and angiotensin-like peptide receptor. The analysis of chromosomal localisation mapped the GPR100 gene to chromosome 1q21.2–q21.3.
The stable expression of GPR100 in Chinese hamster ovary cells together with aequorin as calcium sensor and the promiscuous G-protein subunit α16 as signal transducer revealed bradykinin and kallidin as effectors to elicit a calcium response. Dose–response curves yielded EC50 values for both ligands in the low nanomolar range, while the respective analogues without arginine at the carboxy-terminus were inactive. Calcium mobilisation was inhibited by the phospholipase C blocker U73122, but not by pertussis toxin, suggesting the involvement of the G-protein subunit αq and not αi or αo in signal transduction.
In line with the main function of kinins as peripheral hormones, we found that GPR100 was expressed predominantly in tissues like pancreas, heart, skeletal muscle, salivary gland, bladder, kidney, liver, placenta, stomach, jejunum, thyroid gland, ovary, and bone marrow, but smaller amounts were also detected in the brain and in cell lines derived from tumours of various origins.
Keywords: Ligands for orphan, GPCRs, SALPR, GPR100, bradykinin, kallidin, kinin, aequorin, Ca2+ mobilisation
Introduction
G-protein-coupled receptors (GPCRs) represent the largest known family of receptors interacting at the plasma membrane with extracellular ligands. They are characterised by seven transmembrane domains with an extracellular N-terminus, a cytoplasmic C-terminus, and several conserved structural motifs (Bockaert & Pin, 1999). Members of this large family of transmembrane proteins are responsible for the transduction of extracellular signals to intracellular G proteins, which act as effectors to generate second messengers (Lefkowitz, 2000). GPCRs are divided into distinct subfamilies according to the types of ligands and sequence homologies (Joost & Methner, 2002; Fredriksson et al., 2003). At present, many open-reading frames encoding putative members of the GPCR family can be identified from public databases, for which the ligands are not known. These receptors, commonly known as orphan receptors, are of interest for the pharmaceutical industry as drug targets (Howard et al., 2001).
Here, we describe the discovery and tissue distribution of a novel human gene encoding the G-protein-coupled receptor 100 (GPR100), which shows highest homology to human SALPR, an orphan somatostatin- and angiotensin-like peptide receptor (Matsumoto et al., 2000). Astonishingly, we found that GPR100 is a new high-affinity receptor for bradykinin and kallidin, which may explain some of the pharmacological discrepancies observed with bradykinin and its antagonists (Drube & Liebmann, 2000; Howl & Payne, 2003).
Methods
Database searching and chromosomal localisation
The human genomic database (NCBI) was searched for sequences related to the melanin-concentrating hormone receptor and SALPR using the TBLASTN algorithm. The search revealed a novel GPCR gene, which we submitted as GPR100 to GenBank under the accession number AY170824, and which was contained in the genomic BAC clone AL355388. GPR100 has an intronless open-reading frame of 374 amino acids. The TMHMM program (http://www.cbs.dtu.dk/services/TMHMM) was used to predict the transmembrane domains. Amino-acid sequences were aligned with ClustalW (http://www.ebi.ac.uk/CLUSTALW), and a phylogenetic analysis was performed using the program puzzle 5.0 (Schmidt et al., 2002).
For chromosomal localisation of GPR100 in the human genome, the genomic BAC clone AL355388 and the genomic contig NT_004858 were screened for markers with the electronic PCR program from NCBI (http://www.ncbi.nlm.nih.gov/genome/sts/epcr.cgi).
Cloning and plasmid construction
Oligonucleotide primers were designed based on sequences close to the start (5′-AGC-ACCACCAATCTCTGATCGCCTG-3′) and stop (5′-GAGGAGTGTGTTCAGCTTGCG-CCCT-3′) codons for the human GPR100 gene. These primers were used to amplify GPR100 from a marathon cDNA library constructed from RNA of the human neuroendocrine cell line BON (Evers et al., 1994). For heterologous expression, the full-length GPR100 PCR product was subcloned into the pGEM-T-easy vector (Promega, Heidelberg, Germany). Subsequently, GPR100 was transferred into the EcoRI/XbaI sites of pSecTag (Invitrogen, Karlsruhe, Germany), into which we had introduced a sequence coding for a hemaglutinin (HA) tag to allow control of surface expression (Lintzel et al., 2002). All constructs were sequenced to ensure correct insertion and sequence.
Northern blot analysis
A human 12-lane multiple-tissue Northern blot and the human multiple-tissue expression array 2 were obtained from Clontech (Heidelberg, Germany). From several human cell lines RNA was extracted with the QuickPrep mRNA purification kit from Amersham Biosciences (Freiburg, Germany), denatured, and size fractionated on a formaldehyde–agarose gel, and transferred onto nylon membranes. The blots were hybridised with [α32P]dATP-labelled DNA encoding human GPR100 (nucleotides −27 to 1150 relative to the start codon, GenBank AY170824). Prehybridisation and hybridisation were carried out at 68°C using ExpressHyb from Clontech (Heidelberg, Germany).
Cell culture, transfection, and immunostaining
Human embryonal kidney (HEK293) and COS-7 cells were cultured in DMEM supplemented with 10% fetal calf serum (FCS), Chinese hamster ovary (CHO-K1) cells in DMEM-F12 supplemented with 5% FCS. For routine culture 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, and 10 mM HEPES, pH 7.2 were added to the basal media. CHO-K1 cells stably expressing apoaequorin as Ca2+ sensor and Gα16 for signal transduction (Stables et al., 1997) required the presence of 750 μg ml−1 geneticin and 200 μg ml−1 hygromycin. They were transfected with 5 μg of the GPR100-pSecTag-HA vector using lipofectamine 2000 (Invitrogen, Karlsruhe, Germany), and stable cell lines were selected with 300 μg ml−1 zeocin. In all, 12 individual clones were picked and analysed for GPR100 expression by immunostaining. One clone with good expression was selected for further experiments.
For immunocytochemistry cells were fixed with 4% formaldehyde in 7% acetic acid and 7% glycerol for 1 h at room temperature, washed, blocked with 1% BSA, incubated with anti-HA (Roche, Mannheim, Germany), and visualised with an alkaline-phosphatase-coupled secondary antibody.
Aequorin-based bioluminescence assay
For ligand-binding assays, the CHO cells, stably expressing GPR100, apoaequorin, and Gα16, were cultured for 24 h in 96-well plates in the selection medium, followed by 24 h in serum-free DMEM, supplemented with 5 μg ml−1 insulin, 30 μg ml−1 transferrin, 1 μM sodium pyruvate, 20 μM ethanolamine, 1% nonessential amino acids, 30 nM sodium selenite, and 2 mM glutamine. For Ca2+ measurements cells were preincubated for 4 h in this medium with 2.5 μM coelenterazine (100 μl well−1), before ligands were added in extracellular medium consisting of 125 mM NaCl, 5 mM KCl, 2 mM MgCl2, 0.5 mM NaH2PO4, 5 mM NaHCO3, 10 mM HEPES, pH 7.4, 10 mM glutamine, and 0.1% fatty-acid-free BSA. Luminescence was measured with a bioluminometer (Berthold Technologies, Bad Wildbad, Germany) at 37°C for 15 s.
Peptides and signal-transduction inhibitors
Bradykinin, desArg-bradykinin, kallidin, desArg-kallidin, (Pyr1)-apelin-13, formyl peptide (fMLP), head activator, and chromostatin were from Bachem (Heidelberg, Germany), and somatostatin, pancreastatin, and the inhibitors pertussis toxin, and U73122 from Sigma-Aldrich (Taufkirchen, Germany).
Statistical analysis
The results are expressed as means of three to six determinations±s.d. Curve fittings were performed with the Prism program. Each experiment was repeated at least three times.
Results
Cloning of human GPR100 and structure analysis
A search of the human genomic database with the sequences of the melanin-concentrating hormone receptor and SALPR retrieved a previously unrecognised intronless GPCR, which was part of the BAC clone AL355388, localised on chromosome 1. The start methionine was preceded by a stop codon at position −4 of the amino-acid sequence. Primers close to the start and stop codons for this GPCR were designed to amplify cDNA from the human neuroendocrine cell line BON (Evers et al., 1994). We obtained a product with an expected size of 1177 bp in length. This DNA was subcloned, sequenced, and found to be identical in sequence with the genomic BAC clone. It coded for a full-length GPCR of 374 amino acids, and was named GPR100 (GenBank AY170824). To determine the chromosomal localisation of the GPR100 gene, AL355388 was electronically screened for markers. On the BAC clone, the next marker to the GPR100 gene (stSG3119) mapped to 1q21–q22. A detailed chromosomal localisation with the genomic contig NT_004858 revealed the following microsatellite markers for precise linkage to chromosome 1q21.2–q21.3: D1S303, D1S2777, D1S2721, and D1S2624. Interestingly, this chromosomal region contained the locus for an axonal form of autosomal recessive Charcot–Marie–Tooth disease (Bouhouche et al., 1999).
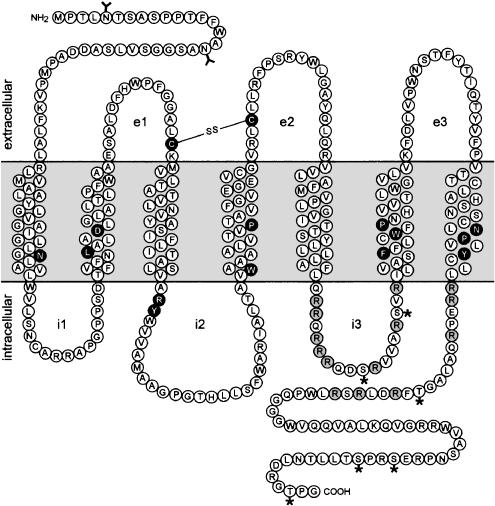
GPR100 shows, in addition to seven hydrophobic α-helical domains, conserved amino acids or amino-acid motifs, typical for members of the rhodopsin family of GPCRs (Figure 1). This includes an asparagine in the first transmembrane domain, an LXXXD motif in the second transmembrane domain, tryptophan and proline in the fourth transmembrane domain, an FXXXWXP motif in the sixth transmembrane domain, an NPXXY motif in the seventh transmembrane domain, conserved cysteine residues in the first two extracellular loops, which form an S–S bridge and contribute to protein stability, and a partially conserved GPCR signature triplet sequence (typically DRY, here ARY), found downstream of the third transmembrane domain, which is supposed to be involved in G-protein coupling (Bockaert & Pin, 1999; Chung et al., 2002). GPR100 has N-linked glycosylation sites in the N-terminal extracellular domain and phosphorylation sites, as well as basic amino-acid clusters in the third intracellular loop and in the C-terminal cytoplasmic domain.
Figure 1.
Schematic representation of the GPR100 protein showing the seven putative transmembrane domains. The extracellular (e) and intracellular domains (i) are indicated. Conserved amino acids or amino-acid motifs typical for members of the GPCR family class A are highlighted in black, and basic amino-acid clusters are shown in grey. Glycosylation sites are marked by a Y and phosphorylation sites by an asterisk, and the line between the two cysteine residues indicates a possible disulphide bond, typical for GPCRs, between the first and the second extracellular loops.
Comparison of receptor sequences
A search of the GenBank database with GPR100 revealed 43% amino-acid identity to the human orphan GPCR SALPR (Figure 2), with notable conservations in the transmembrane regions (56% identity and 76% similarity) and within the extracellular loops (53% identity and 72% similarity). Given these regions of conservation, we propose that GPR100 and SALPR may bind the same or similar cognate ligands. The intracellular loops and the C-termini are less related, suggestive of different signal-transduction pathways and/or sequestration.
Figure 2.
Alignment of human GPR100 (hGPR100) with human SALPR (hSALPR). Identical residues are shaded in black, similar residues in grey. The putative transmembrane domains (TMs) are overlined and the intracellular and extracellular loops indicated by i1–i3 and by e1–e3, respectively.
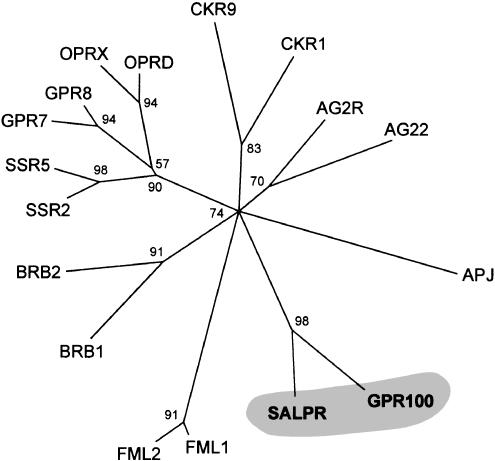
A phylogenetic analysis revealed that the two receptors, GPR100 and SALPR, constitute a novel subfamily of GPCRs, pertaining to a branch with short peptides as ligands. GPR100 and SALPR are most closely related to angiotensin, bradykinin, somatostatin, opioid, and apelin receptors (Figure 3), with which they share between 27–33% sequence identity.
Figure 3.
Phylogenetic tree of the human peptide receptors GPR100, SALPR, and their closest relatives. The phylogenetic analysis assigned a support value to each internal branch, representing in percent the likelihood of finding a connection.
Tissue distribution of human GPR100
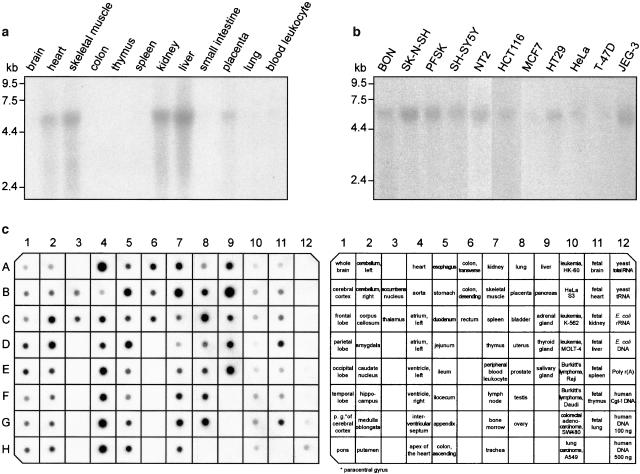
We investigated the expression profile of GPR100 by Northern blot analysis. GPR100 was found as a single transcript of approximately 5.4 kb length (Figures 4a and b). The strongest GPR100 expression was detected in human pancreas (Figure 4c), in accordance with the finding that the cDNA was amplified from BON cells, a cell line derived from a human pancreas carcinoid (Evers et al., 1994). The receptor was mainly expressed in peripheral tissues, including heart, skeletal muscle, salivary gland, bladder, kidney, liver, placenta, stomach, jejunum, thyroid gland, ovary, and bone marrow (Figures 4a and c). Lower concentrations of GPR100 were found in the corpus callosum and in the amygdala of the human brain, and still lower levels in the parietal and occipital lobes, the caudate nucleus, putamen, and thalamus. In the human fetus expression was detected in liver and lung. Northern analysis with GPR100 probes were also performed on blots containing RNA from various tumour cell lines (Figure 4b). A single transcript was seen in nearly all human cell lines probed, suggesting a role for GPR100 in tumour growth. This may be supported by an electronic Northern, which showed that in addition to lung (BG547647) and bone marrow (BF900226), GPR100 was expressed in ESTs originating from a very malignant prostate cancer (AW261853) and from brain meningioma (AA659574).
Figure 4.
GPR100 mRNA expression in various human tissues and cell lines. (a) A multiple-tissue Northern blot, (b) a Northern blot of cell lines derived from various tissues, and (c) a multiple-tissue expression array were probed with a radiolabelled GPR100 fragment covering the total coding sequence and 27 nucleotides upstream of the start codon. BON, neuroendocrine cell line derived from a pancreas carcinoid; HCT116 and HT29 from colon carcinoma; HeLa from cervix epitheloid carcinoma; JEG-3 from chorio carcinoma; MCF7 from breast carcinoma; NT2 from embryonal carcinoma; PFSK from a primitive ectodermal tumour of the brain; SH-SY5Y and SK-N-SH from neuroblastoma; and T-47D from ductal breast carcinoma.
Heterologous expression and ligand screening
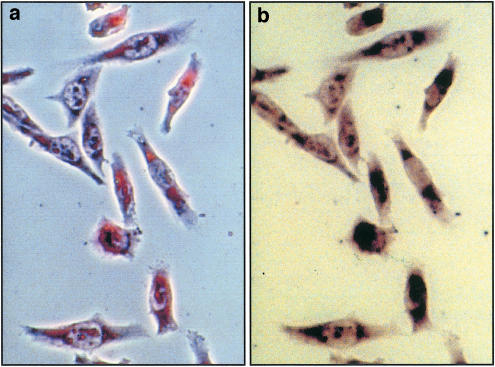
For heterologous expression GPR100 was inserted into a mammalian vector, which provided a signal peptide and a HA tag to monitor cell surface expression. The inclusion of a signal peptide had proven useful for other GPCRs to improve membrane insertion, folding, and transport to the outer cell membrane (Guan et al., 1992; Ignatov et al., 2003). Three cell lines were transiently transfected and monitored for GPR100 expression, CHO-K1 cells, HEK293 cells, and COS-7 cells, which are SV40-transformed kidney cells from the African green monkey. In all three cell lines transfection efficiencies were below 2% and proved inadequate for ligand screening. To improve expression, GPR100 was stably transfected into CHO cells, which already contained apoaequorin as calcium sensor and Gα16 to improve signalling (Stables et al., 1997). Several cell clones were obtained, one of which grew very slowly, but showed satisfactory GPR100 expression (Figure 5). CHO cells without GPR100 showed no staining.
Figure 5.
Stable expression of GPR100 in CHO cells. HA-tagged GPR100 was transfected into CHO cells, containing Gα16 and apoaequorin, and protein expression was probed with an antibody against the HA tag. Shown in (a) is a phase-contrast and in (b) a bright-field micrograph.
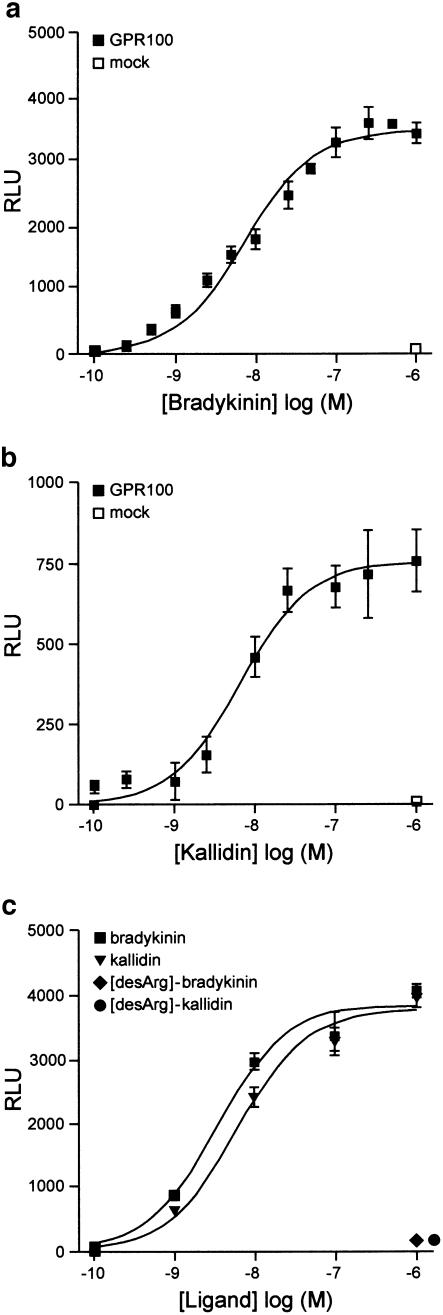
Since GPR100 is phylogenetically most related to GPCRs reacting with peptides as ligands, we assayed peptides and peptide extracts from cells and cell supernatants for their effect on Ca2+ mobilisation. No effect was obtained with somatostatin, apelin, fMLP, head activator, chromostatin, pancreastatin, and peptide extracts. We found that only bradykinin evoked a significant increase in Ca2+-induced luminescence in GPR100-expressing CHO cells. CHO cells without GPR100 showed no response (Figure 6a), indicating that GPR100 was responsible for binding. A dose–response curve yielded an EC50 value of 7.2 nM for bradykinin (Figure 6a). The other naturally occurring kinins were also assayed for their effect on GPR100. We found that kallidin was as effective as bradykinin with an EC50 value of 6.6 nM (Figure 6b), whereas desArg-bradykinin and desArg-kallidin, even at micromolar concentrations, were inactive (Figure 6c).
Figure 6.
Bradykinin and kallidin are high-affinity ligands for GPR100, stably expressed in CHO cells. CHO cells containing Gα16 and apoaequorin, stably expressing GPR100 (GPR100) or not (mock), were treated with increasing concentrations of ligands, and Ca2+-induced bioluminescence was measured at 469 nm, and expressed as relative light units (RLUs), from which the basal response to the injection stimulus was subtracted. (a) Dose–response curves yielded EC50 values of 7.2 nM for bradykinin and (b) of 6.6 nM for kallidin. (c) Even at micromolar concentration desArg-bradykinin and desArg-kallidin did not evoke a calcium response in GPR100-expressing cells.
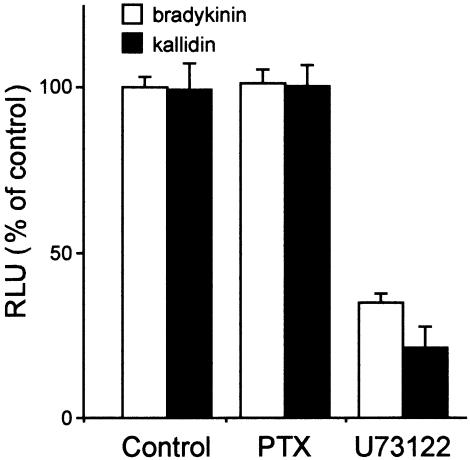
The bradykinin- and kallidin-induced Ca2+ mobilisation in CHO cells, stably transfected with GPR100, was not inhibited by pretreatment for 24 h with pertussis toxin (Figure 7), indicating that the inhibitory G proteins, Gαi or Gαo, did not contribute to signal transduction mediated by Gα16 and the endogenously relevant G protein. The Ca2+ luminescence was strongly reduced by a 5 min pretreatment with the phospholipase C inhibitor U73122 (Figure 7). This suggests that in CHO cells bradykinin/GPR100 signal transduction is mediated exclusively over the Gα16 and the endogenous Gαq pathway.
Figure 7.
Inhibition of GPR100 signalling in CHO cells. CHO cells expressing GPR100 were pretreated with 50 ng ml−1 pertussis toxin (PTX) for 24 h at 37°C, or with 5 μM of U73122, an inhibitor of phospholipase C, for 15 min at 37°C, before Ca2+ mobilisation was induced by treatment with 10−7 M bradykinin or kallidin. Data are expressed as percentage of CHO cells stimulated with the kinins in the absence of inhibitors.
Discussion
By searching the human genomic database with GPCRs, which interact with peptides as ligands, we discovered GPR100 as a new orphan receptor. Sequence comparison revealed a 43% identity and 59% similarity at the amino-acid level with the orphan receptor SALPR (Matsumoto et al., 2000). Phylogenetic analysis placed both receptors in close neighbourhood to bradykinin, angiotensin, and apelin receptors, hinting at peptides as ligands (Joost & Methner, 2002). Indeed, we found that GPR100 is a new receptor for bradykinin with binding features resembling those of the bradykinin B2 receptor. Like the bradykinin B2 receptor, GPR100 reacted equally well with bradykinin and kallidin with EC50 values in the low nanomolar range, whereas desArg-bradykinin and desArg-kallidin, which are specific ligands for the bradykinin B1 receptor, even at micromolar concentrations, showed no activity.
The homology between the human bradykinin B1 and B2 receptor sequences amounts to only 39% at the amino-acid level. Nevertheless, they map in close proximity to the human chromosome 14q32.1–q32.2 (Prado et al., 2002), suggestive of a common evolutionary origin. We localised GPR100 to chromosome 1q21.2–q21.3, and SALPR was described to be present on chromosome 5p15.1–p14 (Matsumoto et al., 2000). This hints at a more divergent origin in evolution between GPR100 and SALPR and of both to the bradykinin B1 and B2 receptors. In spite of the fact that GPR100 and SALPR share only 27% amino-acid identity with the bradykinin B1 and B2 receptors, a very recent phylogenetic analysis placed SALPR between the bradykinin B1 and B2 receptors (Fredriksson et al., 2003). We interpret this as conformation of our finding that GPR100 is a novel bradykinin receptor.
Binding of bradykinin and kallidin to GPR100 in CHO cells stably expressing Gα16 led to Ca2+ mobilisation from intracellular stores, which was completely insensitive to pertussis toxin, but blockable by U73122, a selective inhibitor of phospholipase C. This indicates that GPR100 couples in addition to Gα16 to Gαq and not to Gαi or Gαo. For the bradykinin B2 receptor, it was shown that signal transduction is mediated by Gαq and Gαi (Blaukat & Dikic, 2001; Prado et al., 2002), which suggests that the two receptors may differ in their response to bradykinin.
Kinins are important mediators for cardiovascular events, inflammation, and nociception. They possess a wide range of pharmacological actions, predominantly in peripheral organs and tissues, where they control blood flow, mediate inflammatory responses, and are involved in oedema formation, angiogenesis, pain sensation, muscle contraction, and cell proliferation (Howl & Payne, 2003). These actions were thought to be mediated by the two known bradykinin B1 and B2 receptors, although heterogeneities in response to agonists and antagonists hinted at additional receptors, especially of the B2 type (Drube & Liebmann, 2000; Howl & Payne, 2003). GPR100 is present in locations, where kinins act, such as heart, skeletal muscle, some endocrine glands, and in neural locations. In addition, GPR100 is highly expressed in a number of cell lines derived from various tumours, hinting at a role for GPR100 in mediating the effect of bradykinin on cell proliferation and tumourigenesis.
Acknowledgments
We thank Dr Jenny Stables for originally providing us with CHO cells, stably expressing Gα16 and apoaequorin, Simon Hempel for help with the figures, and Dr Axel Methner for comments on the manuscript.
Abbreviations
- GPCR
G-protein-coupled receptor
- GPR100
G-protein-coupled receptor 100
- HA
hemaglutinin
- SALPR
somatostatin- and angiotensin-like peptide receptor
References
- BLAUKAT A., DIKIC I. Activation of sphingosine kinase by the bradykinin B2 receptor and its implication in regulation of the ERK/MAP kinase pathway. Biol. Chem. 2001;382:135–139. doi: 10.1515/BC.2001.020. [DOI] [PubMed] [Google Scholar]
- BOCKAERT J., PIN J.P. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;18:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUHOUCHE A., BENOMAR A., BIROUK N., MULARONI A., MEGGOUH F., TASSIN J., GRID D., VANDENBERGHE A., YAHYAOUI M., CHKILI T., BRICE A., LEGUERN E. A locus for an axonal form of autosomal recessive Charcot–Marie–Tooth disease maps to chromosome 1q21.2–q21.3. Am. J. Hum. Genet. 1999;65:722–727. doi: 10.1086/302542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUNG D.A., WADE S.M., FOWLER C.B., WOODS D.D., ABADA P.B., MOSBERG H.I., NEUBIG R.R. Mutagenesis and peptide analysis of the DRY motif in the alpha2A adrenergic receptor: evidence for alternate mechanisms in G protein-coupled receptors. Biochem. Biophys. Res. Commun. 2002;293:1233–1241. doi: 10.1016/S0006-291X(02)00357-1. [DOI] [PubMed] [Google Scholar]
- DRUBE S., LIEBMANN C. In various tumour cell lines the peptide bradykinin B(2) receptor antagonist, Hoe 140 (Icatibant), may act as mitogenic agonist. Br. J. Pharmacol. 2000;131:1553–1560. doi: 10.1038/sj.bjp.0703764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVERS B.M., ISHIZUKA J., TOWNSEND C.M., JR, THOMPSON J.C. The human carcinoid cell line, BON. A model system for the study of carcinoid tumors. Ann. NY Acad. Sci. 1994;733:393–406. doi: 10.1111/j.1749-6632.1994.tb17289.x. [DOI] [PubMed] [Google Scholar]
- FREDRIKSSON R., LAGERSTROM M.C., LUNDIN L.G., SCHIOTH H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- GUAN X.M., KOBILKA T.S., KOBILKA B.K. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J. Biol. Chem. 1992;267:21995–21998. [PubMed] [Google Scholar]
- HOWARD A.D., MCALLISTER G., FEIGHNER S.D., LIU Q., NARGUND R.P., VAN DER PLOEG L.H., PATCHETT A.A. Orphan G-protein-coupled receptors and natural ligand discovery. Trends Pharmacol. Sci. 2001;22:132–140. doi: 10.1016/s0165-6147(00)01636-9. [DOI] [PubMed] [Google Scholar]
- HOWL J., PAYNE S.J. Bradykinin receptors as a therapeutic target. Expert. Opin. Ther. Targets. 2003;7:277–285. doi: 10.1517/14728222.7.2.277. [DOI] [PubMed] [Google Scholar]
- IGNATOV A., LINTZEL J., HERMANS-BORGMEYER I., KREIENKAMP H.J., JOOST P., THOMSEN S., METHNER A., SCHALLER H.C. Role of the G-protein-coupled receptor GPR12 as high-affinity receptor for sphingosylphosphorylcholine and its expression and function in brain development. J. Neurosci. 2003;23:907–914. doi: 10.1523/JNEUROSCI.23-03-00907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOOST P., METHNER A.Phylogenetic analysis of 277 human G-protein-coupled receptors as a tool for the prediction of orphan receptor ligands Genome Biol. 200231–16.RESEARCH0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFKOWITZ R.J. The superfamily of heptahelical receptors. Nat. Cell. Biol. 2000;2:E133–E136. doi: 10.1038/35017152. [DOI] [PubMed] [Google Scholar]
- LINTZEL J., FRANKE I., RIEDEL I.B., SCHALLER H.C., HAMPE W. Characterization of the VPS10 domain of SorLA/LR11 as binding site for the neuropeptide HA. Biol. Chem. 2002;383:1727–1733. doi: 10.1515/BC.2002.193. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO M., KAMOHARA M., SUGIMOTO T., HIDAKA K., TAKASAKI J., SAITO T., OKADA M., YAMAGUCHI T., FURUICHI K. The novel G-protein coupled receptor SALPR shares sequence similarity with somatostatin and angiotensin receptors. Gene. 2000;248:183–189. doi: 10.1016/s0378-1119(00)00123-2. [DOI] [PubMed] [Google Scholar]
- PRADO G.N., TAYLOR L., ZHOU X., RICUPERO D., MIERKE D.F., POLGAR P. Mechanisms regulating the expression, self-maintenance, and signaling-function of the bradykinin B2 and B1 receptors. J. Cell. Physiol. 2002;193:275–286. doi: 10.1002/jcp.10175. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.A., STRIMMER K., VINGRON M., VON HAESELER A. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18:502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- STABLES J., GREEN A., MARSHALL F., FRASER N., KNIGHT E., SAUTEL M., MILLIGAN G., LEE M., REES S. A bioluminescent assay for agonist activity at potentially any G-protein-coupled receptor. Anal. Biochem. 1997;252:115–126. doi: 10.1006/abio.1997.2308. [DOI] [PubMed] [Google Scholar]