Abstract
The present study has evaluated the effect of two phenanthrene alkaloids, uvariopsine and stephenanthrine, on angiotensin II (Ang-II)-induced leukocyte–endothelial cell interactions in vivo and the mechanisms involved in their activity. Intravital microscopy within the rat mesenteric microcirculation was used.
A 60 min superfusion with 1 nM Ang-II induced a significant increase in the leukocyte–endothelial cell interactions that were completely inhibited by 1 μM uvariopsine cosuperfusion. A lower dose of 0.1 μM significantly reduced Ang-II-induced leukocyte adhesion by 75%.
When Ang-II was cosuperfused with 1 and 0.1 μM stephenanthrine, Ang-II-induced leukocyte responses were significantly diminished. A lower dose of 0.01 μM only affected Ang-II-induced leukocyte adhesion.
Both alkaloids inhibited Ang-II-induced endothelial P-selectin upregulation and the generation of reactive oxygen species (ROS) in endothelial cells stimulated with Ang-II, in fMLP-stimulated human neutrophils (PMNs) and in the hypoxanthine–xanthine oxidase system. However, cyclic AMP levels in PMNs stimulated with fMLP were not affected.
Uvariopsine and stephenanthrine inhibited PAF-induced elevations in intracellular calcium levels in PMNs (IC50 values: 15.1 and 6.1 μM respectively) and blocked the binding of [3H]PAF to these leukocytes. They also reduced PAF-induced increases in intracellular levels of superoxide anion and hydrogen peroxide.
In conclusion, stephenanthrine and uvariopsine are potent inhibitors of Ang-II-induced leukocyte accumulation in vivo. This effect appears to be mediated through ROS scavenging activity and blockade of PAF receptor. Thus, they have potential therapeutic interest for the control of leukocyte recruitment that occurs in cardiovascular disease states in which Ang-II is involved.
Keywords: Angiotensin II, phenanthrene alkaloids, leukocyte, endothelium, intravital microscopy
Introduction
Atherosclerosis is the main contributor to the pathogenesis of myocardial and cerebral infarction and loss of function in the extremities, accounting for nearly half of all mortality in developed countries. This process bears several histopathologic similarities to chronic inflammation. One of the earliest stages of atherogenesis is endothelial dysfunction that is thought to cause an inflammatory response consisting of intimal accumulation of T lymphocytes and lipid-laden macrophages, which occurs continuously throughout the entire atherogenic process (Cybulsky & Gimbrone, 1991; Ross, 1993; Price & Loscalzo, 1999).
In this way, vascular endothelium is the principal controller of leukocyte traffic between the blood stream, the arterial intima and the extravascular space (Adams & Shaw, 1994). In fact, the migration of leukocytes from the blood to sites of extravascular injury in response to locally produced stimuli is mediated through a sequential cascade of leukocyte–endothelial cell adhesive interactions, which involve an array of cell adhesion molecules (CAMs) present on leukocytes and on the endothelial cells. This multistage process is initiated by the tethering of leukocytes to the endothelium, followed by transient adhesive interactions manifested as leukocyte rolling, leading ultimately to firm leukocyte adhesion to and subsequent transmigration through the vascular endothelium (Butcher, 1991; Springer, 1994).
Angiotensin II (Ang-II) is the main effector peptide of the renin–angiotensin system and, in addition to its role as a potent vasoconstrictor and blood pressure and fluid homeostasis regulator, it has been shown to exert proinflammatory activity. Ang-II receptors have been demonstrated on human monocytes and are capable of promoting monocyte adhesion and activation in vitro (Shimada & Yazaki, 1978; Hahn et al., 1994; Kim et al., 1996; Gräfe et al., 1997). In addition, Ang-II can release a neutrophil chemoattractant factor from cultures of human and bovine arterial endothelial cells (Farber et al., 1990). This may be directly relevant as hypertension is associated with migration of monocytes into the vessel wall, a critical event leading to the development of the atherosclerotic lesion, which can be attenuated by angiotensin-converting enzyme (ACE) inhibition or by pretreatment with an Ang-II AT1 receptor antagonist (Mügge et al., 1991; Hernández-Presa et al., 1997; Mervaala et al., 1999). Interestingly, we have recently revealed that acute Ang-II superfusion of the rat mesenteric microcirculation provokes an inflammatory response in vivo at sub-vasoconstrictor doses. In particular, it induces leukocyte recruitment in postcapillary venules through endothelial P-selectin upregulation in the vessel wall, and this effect is primarily mediated via an Ang-II AT1 receptor interaction (Piqueras et al., 2000).
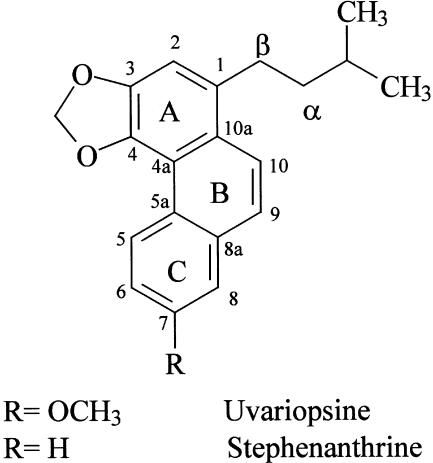
On the other hand, some natural and synthetic phenanthrene alkaloids have proved to be effective inhibitors of platelet aggregation induced by different stimuli (Cheng et al., 1996; Teng et al., 1997). This effect is sometimes associated with increases in adenosine 3′:5′-cyclic monophosphate (cyclic AMP) levels and we have demonstrated that cyclic AMP elevating agents can reduce leukocyte–endothelial cell interactions induced by Ang-II (Álvarez et al., 2001). In addition, alkaloids that present a methylenedioxy group in their structure can display antioxidant activity (Yim & Ko, 1998), and we have also proved that antioxidants can reduce the leukocyte recruitment elicited by this peptide hormone (Álvarez & Sanz, 2001). Our group has isolated two phenanthrene alkaloids, uvariopsine and stephenanthrine (Figure 1), from the fresh root of Dennettia tripetala (López-Martín et al., 2002). Therefore, in the present study, we have evaluated the effect of uvariopsine and stephenanthrine on Ang-II-induced leukocyte–endothelial cell interactions in vivo. For this purpose, we used intravital microscopy within the rat mesenteric microcirculation and tested their effect on leukocyte responses elicited by Ang-II. Furthermore, we have also characterized in the present study the mechanisms that may contribute to their anti-inflammatory activity.
Figure 1.
Chemical structure of uvariopsine and stephenanthrine.
Methods
Isolation and structural determination of uvariopsine and stephenanthrine
The fresh root of D. tripetala was first extracted with methanol. The methanolic extract was further purified by several silica column chromatographies and the alkaloids isolated. Their structures were determined by physical and spectroscopical one-dimensional (1D) and 2D-nuclear magnetic resonance analysis (López-Martín et al., 2002).
Intravital microscopy
The experimental preparation used in this study was similar to that previously described (Álvarez et al., 2001). Male Sprague–Dawley rats (200–250 g) were fasted for 24 h and anesthetized with sodium pentobarbital (50 mg kg−1, i.p.). A tracheostomy was performed to maintain a patent airway throughout the experiment. A polyethylene catheter was inserted in the right carotid artery to monitor mean arterial blood pressure (MABP) through a pressure transducer (Spectramed Stathan P-23XL Quincy, MA, U.S.A.) connected to a recorder (GRASS RPS7C8B, Quincy, MA, U.S.A.), and a second catheter was placed in the contralateral jugular vein to permit the intravenous administration of anesthetic. A midline abdominal incision was made and the rats were then placed in a supine position on an adjustable Plexiglass microscope stage. A segment of the mid-jejunum was exteriorized and draped over an optically clear viewing pedestal that allowed transillumination of a 2 cm2 segment of the tissue. The temperature of the pedestal was maintained at 37°C and the exposed tissue was covered with saline-soaked gauze to minimize tissue dehydration. The exposed mesentery was suffused continuously at a rate of 1 ml min−1 with a warmed bicarbonate-buffered salt solution (pH 7.4).
The mesenteric preparation was then observed using an intravital orthostatic microscope (Nikon Optiphot-2, SMZ1, Badhoevedorp, The Netherlands) equipped with a × 20 objective lens (Nikon SLDW, Badhoevedorp, The Netherlands) and a × 10 eyepiece. A video camera (Sony SSC-C350P, Koeln, Germany) mounted on the microscope projected the image onto a color monitor (Sony Trinitron PVM-14N2E, Koeln, Germany) and the images were video recorded (Sony SVT-S3000P, Koeln, Germany) for playback analysis. The final magnification of the video screen was × 1300. Animal temperature, monitored using a rectal electrothermometer, was maintained at 37°C with an infrared heat lamp.
Single unbranched mesenteric venules (20–40 μm in diameter) were selected for study and their diameters were measured on-line using a video caliper (Microcirculation Research Institute, Texas A&M University, College Station, TX, U.S.A.). Centerline red blood cell velocity (Vrbc) was also measured on-line using an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University, College Station, TX, U.S.A.). Venular blood flow was calculated from the product of mean red blood cell velocity (Vmean=Vrbc × 1.6−1) and cross-sectional area, assuming cylindrical geometry. Venular wall shear rate (γ) was calculated based on the Newtonian definition: γ=8 × (VmeanDv−1) s−1, in which Dv is the venular diameter (House & Lipowsky, 1987).
The number of rolling, adherent and emigrated leukocytes was determined off-line during playback of videotaped images. Rolling leukocytes were defined as white blood cells moving at a slower velocity than erythrocytes. Rolling leukocyte flux was determined by counting the number of rolling leukocytes per minute passing a reference point in the microvessel. The same reference point was used throughout the experiment as leukocytes may roll for only a section of the vessel before rejoining the blood flow or becoming firmly adherent. Leukocyte rolling velocity (Vwbc) was determined as the time required for a leukocyte to move along 100 μm length of the microvessel and is expressed as μm s−1. A leukocyte was defined as adherent to venular endothelium if it was stationary for at least 30 s. Leukocyte adhesion was expressed as the number of cells adhered per 100 μm length of venule. Leukocyte emigration was expressed as the number of white blood cells per microscopic field surrounding the venule. The rate of emigration was determined from the difference between the number of any interstitial leukocytes present at the beginning of the experiment and those present at the end of the experiment.
Experimental protocol
After a 30 min stabilization period, baseline measurements (time 0) of MABP, Vrbc, Dv, shear rate, leukocyte rolling flux and velocity and leukocyte adhesion and emigration were taken. The buffered-saline superfusion was either continued or supplemented with Ang-II (1 nM) and recordings were performed for 5 min at 15 min intervals over a 60 min period during which the aforementioned leukocyte and hemodynamic parameters were measured.
In the first group of experiments, uvariopsine (0.1–1 μM) was applied topically 10 min prior to Ang-II suffusion, then it was cosuperfused with Ang-II, and responses evaluated for 5 min at 15 min intervals over a 60 min period. In the second group of experiments, stephenanthrine (0.01–1 μM) was similarly applied topically 10 min prior to Ang-II suffusion and then it was cosuperfused with Ang-II, again measurements were performed for 5 min at 15 min intervals over a 1 h period. Alkaloids were dissolved in dimethylsulfoxide (DMSO) and diluted further. The same percentage of DMSO (0.01% at 1 μM) was used in the control groups (buffer or Ang-II 1 nM).
Immunohistochemistry
Immunohistochemistry was used to examine the expression of P-selectin. Once the experiment using intravital microscopy was completed, the portion exposed to buffer or Ang-II with or without the phenanthrene alkaloids (1 μM concentration) for 1 h was then isolated and further fixed in 4% paraformaldehyde for 90 min at 4°C as previously described (Sanz et al., 2002). After fixation, the tissue was dehydrated using graded acetone washes at 4°C, embedded in paraffin wax and 4 μm-thick sections were cut.
Immunohistochemical localization of P-selectin was accomplished using a modified avidin and biotin immunoperoxidase technique as previously described by Sanz et al. (2002). After different previous procedures, tissue sections were incubated with the anti-rat-P-selectin mAb (RP-2) for 24 h at 200 μg ml−1. Control preparations consisted in the incubation with the isotype matched murine antibody MOPC 21 (IgG1) as primary antibody for the same period of time at 200 μg ml−1. Positive staining was defined as a venule displaying brown reaction product.
Cytotoxicity studies in human polymorphonuclear leukocytes (PMNs)
Human PMNs were isolated as previously described (Dasi et al., 2000) and the cytoplasmic marker enzyme lactate dehydrogenase was determined by measuring the rate of NADH oxidation (Bergmeyer & Bernt, 1974).
Measurement of cyclic AMP levels from PMNs
The procedure followed was similar to that described by Derian et al. (1995). The human PMNs number was adjusted to 5 × 106 cells ml−1 in PBS with 1 mM CaCl2 and 2 mM glucose. Samples of 480 μml of cells were preincubated with 0.5 μM cytochalasin B, alone or in combination with either rolipram (10 μM), forskolin (30 μM), uvariopsine (1–100 μM), stephenanthrine (1–100 μM) for 5 min at 37°C. After the addition of N-formyl-methionyl-leucyl-phenylalanine (fMLP, 30 nM) samples were incubated for an additional 5 min at 37°C. Control assays with DMSO were also performed (0.01–1%). Cold ethyl alcohol (65% final) was added to each sample and samples were kept at –20°C for 1 h. Samples were centrifuged at 10,000 × g for 5 min at 4°C and supernatants were evaporated and kept at –80°C until being assayed. After reconstitution in assay buffer, samples were assayed for cyclic AMP in duplicate by using a cyclic AMP enzyme immunoassay kit following the manufacturer's instructions.
Measurement of reactive oxygen species (ROS) generation in human umbilical vein endothelial cells (HUVECs)
HUVECs were isolated by collagenase treatment (Jaffe et al., 1973) and maintained in human endothelial cell-specific medium EBM-2 supplemented with EGM-2 and 10% FCS. HUVECs were grown to confluence and used up to passage 2 for the experiment. HUVECs were placed on 24-well culture plates, and prior to every experiment they were incubated for 16 h in medium containing 1% FCS. Cells were washed twice with Hank's balance salt solution. Then, 250 μl of phenol-red free medium 199 with 0.25% of bovine serum albumin (BSA) containing 140 μM ferricytochrome c with or without Ang-II 0.1 μM was added to each well according to the protocol described by Zhang et al. (1999). In some wells, the cells were pretreated with uvariopsine or stephenanthrine (10 and 100 μM) for 30 min before Ang-II was added. The plate was kept in the cell culture incubator for 1 h. The supernatant from each reaction was pipetted out and analysed by using a Wallac 1420 Victor2 Multilabel Counter (EG&G, Turku, Finland) at a wavelength of 550 nm. Superoxide dismutase (SOD) was used as positive control (1000 U ml−1). The amount of superoxide released was calculated by dividing the difference in absorbance of the samples with or without SOD by the extinction coefficient for reduction of ferricytochrome c to ferrocytochrome c (ɛ=21.1 cm−1 M).
Measurement of ROS generation from human PMNs
The formation of ROS by human PMNs was assessed by luminol-enhanced chemiluminescence with a modified method of that described by Schudt et al. (1991). Assay was carried out in opaque 96 well plates, 105 cells per well were suspended in an assay volume of 180 μl of Krebs-HEPES buffer containing 5.6 mM glucose, 0.05% (w/v) BSA, 2 μM microperoxidase with 0.2% gelatine pH 7.4, alone or in combination with uvariopsine or stephenanthrine (final concentration in 200 μl, 1–100 μM) for 30 min at 37°C. All the assays were performed in duplicate. Plates were placed in a Wallac 1420 Victor2 Multilabel Counter (EG&G, Turku, Finland). Then 20 μl of a mixture containing luminol, CaCl2 and fMLP, to give a final concentration in 200 μl of 5 μM, 1 mM and 100 nM, respectively, was added sequentially to each well, except in the blank group. Experiments with the appropriate DMSO concentration were also carried out (0.01–1%). Chemiluminescence was recorded at 4 s intervals over a 100 s period per well and the area under the curve (AUC) was integrated. Drug-induced reduction was expressed as % inhibition. Inhibitory concentration 50% (IC50) values were then calculated from the concentration–inhibition curves by nonlinear regression analysis.
Measurement of ROS scavenging activity
The formation of ROS was carried out by the hypoxanthine–xanthine oxidase system and detected by luminol-enhanced chemiluminescence using a modified method previously employed by Sekiguchi & Nagamine (1994). Assay was carried out in opaque 96-well plates. A volume of 180 μl of Krebs-HEPES buffer containing 0.1 mM hypoxanthine, 5.6 mM glucose, with 0.2% gelatine pH 7.4, alone or in combination with uvariopsine or stephenanthrine (final concentration in 200 μl, 1–100 μM), was added to the wells for 5 min at 37°C, all the assays were performed in duplicate. Plates were placed in a Wallac 1420 Victor2 Multilabel Counter (EG&G, Turku, Finland). Then, 20 μl of a mixture containing the following reagents, 5 μM luminol, 1 mM CaCl2 and 0.02 U ml−1 xanthine oxidase, which would have the indicated concentrations in 200 μl was added sequentially to each well except in the blank group. Experiments with the appropriate DMSO concentration were also carried out (0.01–1%). Chemiluminescence was recorded at 4 s intervals over a 100 s period per well and the AUC was integrated. The concentration of hypoxanthine and xanthine oxidase was adjusted to obtain the same AUC control values as those reached with stimulated human PMNs. Drug-induced reduction was expressed as % inhibition. IC50 values were calculated from the concentration–inhibition curves by nonlinear regression analysis. A direct inhibitory effect on xanthine oxidase activity was tested by measuring uric acid formation from xanthine by following the rate of absorbance change at 295 nm.
Determination of PAF-induced intracellular calcium elevations in human PMNs
Inhibition by uvariopsine and stephenanthrine of increases in intracellular calcium levels induced by platelet-activating factor (PAF) was determined by flow cytometric analysis in isolated human PMNs. Human PMNs were incubated with saturating amounts of CD45-PC5 monoclonal antibody (mAb), a marker of human leukocytes (excitation wavelength 488 nm and emission wavelength 675 nm; 1 μl per 20 μl of PMNs suspension) for 15 min at 20°C in the dark. Samples of CD45-stained leukocytes were diluted 1 : 50 in Tyrode's buffer (pH 7.4) to have 2.5 × 106 cells ml−1 and incubated with the calcium indicator fluo-4 (2 μM) for 15 min at 37°C. Then, 1 ml samples were incubated for a further 5 min alone or in combination with uvariopsine or stephenanthrine (final concentration, 1–100 μM). Samples were run in an EPICS XL (Beckman-Coulter, Hialea, FL, U.S.A.) and baseline fluo-4 fluorescence in the leukocyte populations (gated on side scatter vs CD45+ events) were recorded for 10–15 s. Then, run was paused and 1 μM PAF or 100 nM fMLP added and run continued up to 5 min. Increases in intracellular calcium concentrations were determined as maximal increase in mean fluorescence intensity over the basal levels. Drug-induced reduction was expressed as % inhibition. IC50 values were again calculated from the concentration–inhibition curves by nonlinear regression analysis.
In vitro [3H]PAF binding studies
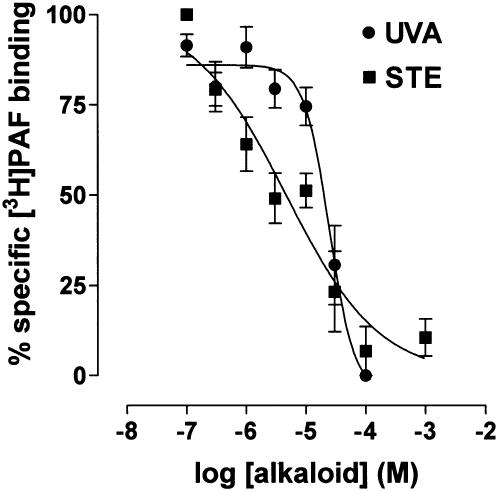
[3H]PAF (C18; 1-O-[3H] octadecyl-2-acetyl-sn-3-phosphocholine) receptor binding was studied in human PMNs (4 × 106 cells ml−1) by use of a modified method previously described by Vasange et al. (1997). Incubations were performed for 120 min at 20°C in a total volume of 250 μl of incubation buffer (0.6 mM NaH2PO4, 25 mM Tris HCl, 130 mM NaCl, 5.5 mM KCl, BSA 0.5%, pH 7.4), with [3H]PAF (0.4 nM) and different concentrations of inhibitors (uvariopsine or stephenanthrine, final concentration: 0.1–100 μM). The incubation was initiated by the addition of the cells. Nonspecific binding was determined with unlabeled PAF (2.0 μM). The binding reactions were terminated by rapid vacuum filtration using a Brandel cell harvester (M24R, Gaithesburg, MD, U.S.A.) with glass fiber filters (Schleicher and Schuell, no. 30) presoaked in 0.3% polyethylenimine. The filters were then washed with 3 × 2 ml of incubation buffer containing 0.1% BSA, and the filter-bound radioactivity was determined by liquid scintillation counting. Assays were conducted in triplicate. The IC50 (concentration of the compound needed to produce 50% inhibition of [3H]PAF binding) values for uvariopsine or stephenanthrine were calculated from a nonlinear regression plot (Graph Pad Software; San Diego, CA, U.S.A.).
Determination of PAF-induced intracellular superoxide anion and hydrogen peroxide elevations in human PMNs
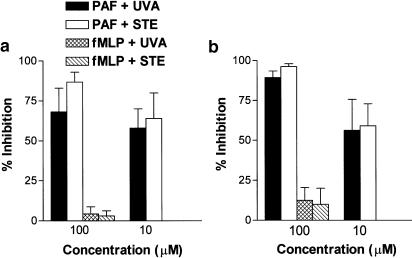
Inhibition by uvariopsine and stephenanthrine of increases in intracellular superoxide anion and hydrogen peroxide levels induced by PAF was determined by flow cytometric analysis in isolated human PMNs using a modified method previously described by Szucs et al. (1998). Human PMNs were incubated with saturating amounts of CD45-PC5 monoclonal antibody (mAb) (1 μl/20 μl of PMNs suspension) for 15 min at 20°C in the dark. Samples of CD45-stained leukocytes were diluted 1 : 50 in Tyrode's buffer (pH 7.4) to have 2.5 × 106 cells ml−1 and incubated with 3 μM hydroethidine (HE) and 3 μM dihydrorhodamine 123 (DHR) for 15 min at 37°C. Then, 1 ml samples were incubated for a further 5 min alone or in combination with uvariopsine or stephenanthrine (final concentration, 10 and 100 μM) and stimulated with 1 μM PAF or 100 nM fMLP except in the blank group for another 30 min. Samples were run in an EPICS XL (Beckman-Coulter, Hialea, FL, U.S.A.) and HE and DHR fluorescences in the PMNs (gated on side scatter vs CD45+ events) were recorded. Increases in intracellular superoxide anion and hydrogen peroxide concentrations were determined as maximal increase in HE and DHR mean fluorescence intensity over the levels in the blank group, respectively. Drug-induced reduction was expressed as % inhibition.
Materials
Ang-II, BSA, cytochalasin B, DMSO, fMLP, forskolin, gelatin, glucose, hypoxanthine, luminol, microperoxidase, PAF, rolipram, MOPC 21, ferricytochrome c, SOD and xanthine oxidase were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.). EBM-2 medium supplemented with EGM-2 was from Clonetics (Barcelona, Spain). Biotrak cyclic AMP enzyme immunoassay and [3H]PAF (specific activity 159 Ci mmol−1) were from Amersham Pharmacia Biotech (Uppsala, Sweden). Phenol-red free medium 199 was from Life Technologies (Paisley, U.K.). Hydroethidine and dihydrorhodamine 123 were from Molecular Probes (Leiden, The Netherlands) and CD45-PC5 from Immunotech (Prague, Czech Republique). Monoclonal antibody against rat-P-selectin (RP-2) was acquired as previously stated (Walter et al., 1997).
Statistical analysis
All data are expressed as mean±s.e.m. The data within groups were compared using a two-way analysis of variance (ANOVA) with a Newman–Keuls post hoc correction for multiple comparisons. A P-value <0.05 was considered to be statistically significant.
Results
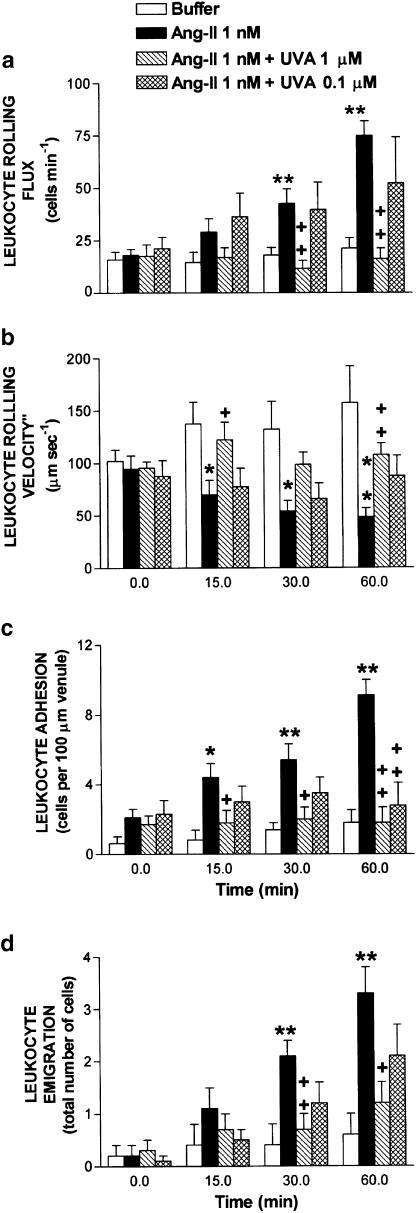
Figure 2 illustrates Ang-II-induced leukocyte responses. Leukocyte rolling flux, adhesion and emigration were significantly increased within 30 min of 1 nM Ang-II superfusion. After 60 min superfusion with Ang-II, increases in leukocyte rolling flux (74.9±6.9 vs 21.2±5.1 cells min−1) and concomitant significant decreases in the leukocyte rolling velocity (48.2±8.9 vs 157.0±35.3 μm s−1) were observed vs buffer (Figure 2). Similarly, at the same time point, Ang-II induced increases in leukocyte adhesion (9.1±0.9 vs 1.8±0.7 cells per 100 μm) and emigration (3.3±0.5 vs 0.6±0.4 cells field−1) as shown in Figure 2. Cosuperfusion with uvariopsine 1 μM markedly reduced Ang-II-induced increase in the leukocyte rolling flux, adhesion and emigration, which were inhibited by 100, 100 and 78%, respectively (Figure 2). In addition, the decrease in the leukocyte rolling velocity induced by Ang-II at 60 min was reversed by the coadministration of uvariopsine 1 μM (Figure 2). In contrast, when Ang-II was cosuperfused with uvariopsine 0.1 μM, increases in leukocyte rolling flux and emigration caused by Ang-II were attenuated by 42 and 44%, respectively (Figure 2), but at this dose there was no longer a significant effect in these parameters. Similar behavior was observed in the reduction of leukocyte rolling velocity exerted by Ang-II superfusion, which was partly reversed by uvariopsine 0.1 μM cosuperfusion (Figure 2). Interestingly, uvariopsine at the same dose significantly reduced Ang-II-induced leukocyte adhesion by 75% (Figure 2). None of these treatments affected circulating leukocyte counts, 9.3±0.7 × 106 cells ml−1 after 60 min buffer superfusion, 10.8±0.5 × 106 cells ml−1 after 60 min Ang-II superfusion and 8.7±0.9 × 106 cells ml−1 after 60 min Ang-II+uvariopsine 1 μM cosuperfusion.
Figure 2.
Effect of uvariopsine (UVA) on Ang-II-induced leukocyte rolling flux (a), leukocyte rolling velocity (b), leukocyte adhesion (c) and leukocyte emigration (d) in rat mesenteric postcapillary venules. Parameters were measured 0, 15, 30 and 60 min after superfusion with buffer (n=5) or with Ang-II (1 nM) in animals untreated (n=7) or cosuperfused with uvariopsine 1 μM (n=7) or with uvariopsine 0.1 μM (n=7). Results are represented as mean±s.e.m. *P<0.05 or **P<0.01 relative to values in the buffer group. +P<0.05 or ++P<0.01 relative to the Ang-II untreated group.
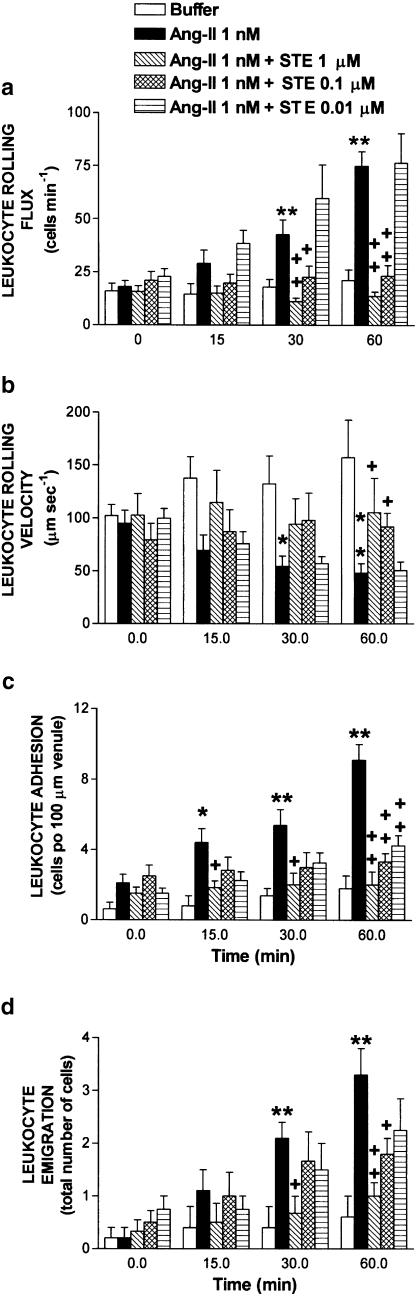
Figure 3 presents the effects of stephenanthrine 0.01–1 μM on the leukocyte–endothelial cell interactions elicited by mesenteric exposure to 1 nM Ang-II. Buffer and Ang-II data were the same as those presented in Figure 2. Cosuperfusion of Ang-II with stephenanthrine 1 μM resulted in a significant reduction of the leukocyte rolling flux (100%), adhesion (97%) and emigration (85%) provoked by 60 min superfusion with Ang-II (Figure 3). Similarly, when Ang-II was cosuperfused with a 10-fold lower concentration of stephenanthrine (0.1 μM), these three parameters were significantly inhibited by 96, 80 and 56%, respectively (Figure 3). Decrease in leukocyte rolling velocity elicited by Ang-II was again totally reversed by administration of stephenanthrine at these two doses (0.1 and 1 μM, Figure 3). However, cosuperfusion of Ang-II with 0.01 μM stephenanthrine, while having no effect on the increase of the flux of rolling leukocytes or on the decrease of the leukocyte rolling velocity provoked by 60 min superfusion with Ang-II, it caused a significant reduction in Ang-II-induced leukocyte adhesion (66%, Figure 3). In addition, this dose of stephenanthrine provoked a small reduction in the leukocyte emigration (37%) induced by the superfusion of this peptide hormone (Figure 3). As found with uvariopsine, cosuperfusion of Ang-II with stephenanthrine 1 μM for 60 min did not affect the number of circulating leukocytes, 11.2±1.0 × 106 cells ml−1.
Figure 3.
Effect of stephenanthrine (STE) on Ang-II-induced leukocyte rolling flux (a), leukocyte rolling velocity (b), leukocyte adhesion (c) and leukocyte emigration (d) in rat mesenteric postcapillary venules. Parameters were measured 0, 15, 30 and 60 min after superfusion with buffer (n=5) or with Ang-II (1 nM) in animals untreated (n=7) or cosuperfused with stephenanthrine 1 μM (n=7), with stephenanthrine 0.1 μM (n=7) or with stephenanthrine (0.01 μM, n=5). Results are represented as mean±s.e.m. *P<0.05 or **P<0.01 relative to values in the buffer group. +P<0.05 or ++P<0.01 relative to the Ang-II untreated group. Buffer and Ang-II data were the same as those presented in Figure 2.
Table 1 summarizes the results obtained for MABP and shear rate prior to (0 min) and following (60 min) buffer or Ang-II superfusion in animals untreated or treated with uvariopsine (0.1–1 μM) or stephenanthrine (0.01–1 μM). MABP and shear rate were unaffected by phenanthrene alkaloids cosuperfusion at the different doses assayed.
Table 1.
Hemodynamic parameters in animals untreated and treated with uvariopsine (UVA) or stephenanthrine (STE) before (0 min) and after (60 min) Ang-II superfusion (1 nM)
| MABP (mmHg) | Shear rate (s−1) | |||
|---|---|---|---|---|
| Treatment | 0min | 60min | 0min | 60min |
| Buffer | 122.5±7.5 | 128.3±6.1 | 677.3±80.7 | 731.1±40.3 |
| Ang-II | 117.4±3.5 | 120.7±4.0 | 688.3±74.9 | 674.9±78.6 |
| Ang-II+UVA 1 μM | 133.6±7.3 | 141.3±8.0 | 625.1±90.3 | 640.2±72.1 |
| Ang-II+UVA 0.1 μM | 110.4±4.2 | 124.7±5.5 | 694.3±90.1 | 657.8±35.1 |
| Ang-II+STE 1 μM | 129.9±7.2 | 139.3±9.3 | 552.3±50.8 | 587.8±47.6 |
| Ang-II+STE 0.1 μM | 119.0±14.8 | 132.0±14.2 | 691.9±30.2 | 650.8±57.9 |
| Ang-II+STE 0.01 μM | 127.1±7.1 | 120.8±5.9 | 613.0±73.1 | 699.0±33.2 |
All values are mean±s.e.m. of n=5–7 experiments.
Once the inhibitory effect displayed by uvariopsine and stephenanthrine on leukocyte–endothelial cell interactions induced by Ang-II in vivo was established, we investigated the possible mechanism by which these phenanthrene alkaloids elicit this anti-inflammatory activity. In order to discard their potential cytotoxic effect, human PMNs were incubated with uvariopsine or stephenanthrine at 100 μM. At this dose, they did not cause the release of cytoplasmic lactate dehydrogenase.
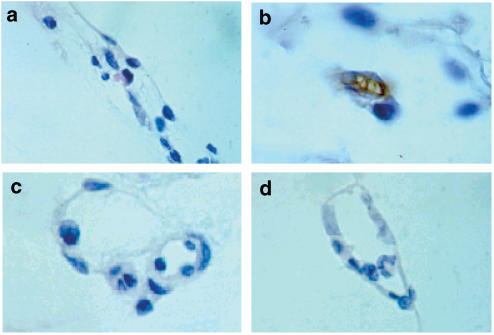
Immunohistochemical experiments revealed that when the mesenteric tissue was subjected to 60 min buffer superfusion, no increase in endothelial P-selectin expression was observed (Figure 4a). Ang-II superfusion caused a significant upregulation of P-selectin (Figure 4b) that was totally inhibited by cosuperfusion with uvariopsine or stephenanthrine at 1 μM (Figures 4c and d).
Figure 4.
Effect of uvariopsine and stephenanthrine on Ang-II-induced endothelial P-selectin upregulation. P-selectin expression after buffer (a), Ang-II (b), Ang-II 1 nM+uvariopsine 1 μM (c) and Ang-II 1 nM+stephenanthrine (d) 60 min superfusion. Brown reaction product indicates positive immunoperoxidase localization for P-selectin on the vascular endothelium. All four panels are lightly counterstained with hematoxylin and have the same magnification (× 400). Results are representative of n=4 experiments with each treatment.
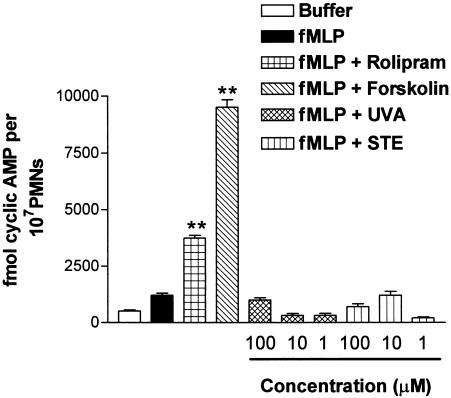
Since phenanthrene alkaloids can inhibit platelet aggregation via their ability to increase cyclic AMP levels, such possibility was investigated. As displayed in Figure 5, the phosphodiesterase-4 inhibitor rolipram, and the direct adenylate cyclase activator forskolin, significantly increased the levels of cyclic AMP in human PMNs stimulated with fMLP by 3751.3±125.6 and 9480.2±340.7 fmol of cyclic AMP per 107 cells, respectively, compared with the amount detected in PMNs stimulated with fMLP 1982.7±99.5 fmol of cyclic AMP per 107 cells. In contrast, uvariopsine and stephenanthrine at the three doses assayed (1–100 μM) did not increase the levels of this cyclic nucleotide (Figure 5).
Figure 5.
Effect of uvariopsine (UVA) and stephenanthrine (STE) on cyclic AMP levels in fMLP-stimulated PMNs. PMNs were incubated in the absence or presence of test compounds as described in Methods. fMLP (30 nM) was added for an additional 5 min at 37°C. Cyclic AMP was extracted from cells and quantified by enzyme immunoassay as described in Methods. Rolipram (10 μM) and forskolin (30 μM) were used as reference drugs. Uvariopsine and stephenanthrine were assayed at three different concentrations from 1 to 100 μM. Results are represented as mean±s.e.m. of n=4 preparations run in duplicate . **P<0.01 relative to values in fMLP group.
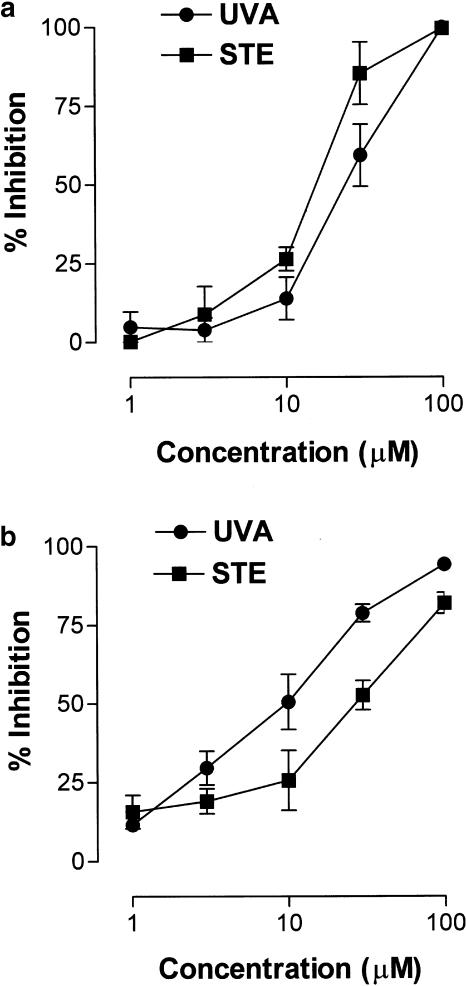
Ang-II-induced leukocyte–endothelial cell interactions are partly mediated through the production of ROS (Álvarez & Sanz, 2001). Therefore, uvariopsine and stephenanthrine probably inhibit leukocyte responses elicited by Ang-II through inhibition of superoxide anion generation. HUVECs were stimulated with Ang-II 100 nM for 1 h in the absence or presence of uvariopsine and stephenanthrine. Both alkaloids at the two doses assayed, 10 and 100 μM, inhibited the release of superoxide anion induced by Ang-II 100 nM in a concentration-dependent manner (Figure 6). Since Ang-II cannot directly stimulate humans PMNs to provoke the respiratory burst (Piqueras et al., 2000), we wanted to investigate whether these natural products were also capable of inhibiting ROS generation in human PMNs stimulated with fMLP. As shown in Figure 7a, both phenanthrene alkaloids inhibited fMLP-induced ROS generation in a concentration-dependent manner. The calculated IC50 values of both phenanthrene alkaloids were 25.1±1.6 μM for uvariopsine and 15.1±1.8 μM for stephenanthrine, the latter being slightly more potent than the former.
Figure 6.
Effect of uvariopsine (UVA) and stephenanthrine (STE) on superoxide anion release in HUVECs stimulated with Ang-II. HUVECs were grown to confluence in 24-well culture plate, then incubated in the absence or presence of uvariopsine or stephenanthrine (10 and 100 μM) for 30 min and stimulated with 100 nM Ang-II for 1 h as described in Methods. The assay of superoxide release was performed by measurement of SOD-inhibitable reduction of ferricytochrome c. Results are expressed as percentage inhibition of the control from duplicate determinations. Results are represented as mean±s.e.m. of n=4 preparations.
Figure 7.
Effect of uvariopsine (UVA) and stephenanthrine (STE) on ROS generation in fMLP-stimulated PMNs (a) and by hypoxanthine–xanthine oxidase system (b). PMNs were incubated in the absence or presence of uvariopsine and stephenanthrine (1–100 μM) at 37°C as described in Methods. After 30 min, fMLP 100 nM was added to each well and chemiluminiscence recorded at 4 s intervals over a 100 s period. Hypoxanthine was incubated in the absence or presence of uvariopsine and stephenanthrine (1–100 μM) at 37°C as described in Methods. After 5 min, xanthine oxidase 0.02 U ml−1 was added to each well and chemiluminescence recorded at 4 s intervals over a 100 s period. Results are expressed as percentage inhibition of ROS generation in PMNs stimulated with fMLP (n=5–7 preparations) or as percentage inhibition of increases in ROS generated by hypoxanthine–xanthine oxidase system (n=4–6 preparations). Results are represented as mean±s.e.m.
Since both phenanthrene alkaloids may exert ROS scavenger activity, a cell-free system for ROS generation was employed. As illustrated in Figure 7b, uvariopsine and stephenanthrine showed scavenger activity for ROS generated in a hypoxanthine–xanthine oxidase system. In this case, uvariopsine (IC50 value of 8.0±1.8 μM) was slightly more potent than stephenanthrine (IC50 value of 24.3±1.4 μM). None of these compounds inhibited xanthine oxidase activity when they were assayed at 100 μM.
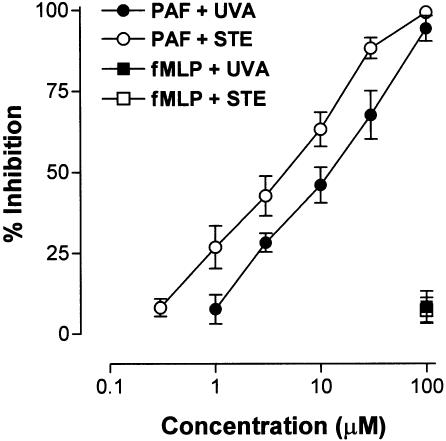
PAF is involved in Ang-II-induced leukocyte adhesion (Álvarez & Sanz, 2001), therefore we investigated whether PAF-induced intracellular calcium elevations could be inhibited by preincubation of CD45-stained PMNs with both phenanthrene alkaloids. On stimulation with PAF, the cells exhibited a rapid and concentration-dependent increase in intracellular calcium levels (data not shown). Based on these results, PAF 1 μM was chosen to stimulate human neutrophils. Figure 8 shows that both uvariopsine and stephenanthrine inhibited intracellular calcium elevations induced by PAF in a concentration-dependent manner. The calculated IC50 values were 15.1±1.6 μM for uvariopsine and 6.1±1.3 μM for stephenanthrine. None of these alkaloids at the highest dose were capable of inhibiting intracellular calcium elevations induced by 100 nM fMLP. To test whether uvariopsine and stephenanthrine could behave as PAF receptor antagonist, a [3H]PAF binding assay was carried out on human PMNs. As shown in Figure 9, both alkaloids inhibited [3H]PAF binding to human PMNs in a concentration-dependent manner. The calculated IC50 values were 15.5±3.6 μM for uvariopsine and 4.4±1.7 μM for stephenanthrine. Finally, as illustrated in Figure 10, both alkaloids at the two doses assayed, 10 and 100 μM, inhibited the increases in intracellular superoxide anion and hydrogen peroxide production induced by 1 μM PAF in a concentration-dependent manner. In contrast, they showed no effect when PMNs were stimulated with 100 nM fMLP (Figure 10).
Figure 8.
Effect of uvariopsine (UVA) and stephenanthrine (STE) on elevations in intracellular calcium induced by PAF in CD45-stained PMNs. CD45-stained PMNs were incubated with fluo-4 (2 μM) for 15 min at 37°C. Then, samples were incubated in the absence or presence of uvariopsine and stephenanthrine (1–100 μM) for 5 min at 37°C as described in Methods. Samples were run in an EPICS XL and baseline fluo-4 fluorescence in gated PMNs was recorded for 10–15 s. Then, 1 μM PAF or 100 nM fMLP was added and run continued up to 5 min. Results are expressed as percentage inhibition of PAF or fMLP-induced increases in mean fluorescence intensity over the basal levels. Results are represented as mean±s.e.m. of n=5–6 preparations.
Figure 9.
Effect of uvariopsine (UVA) and stephenanthrine (STE) on [3H]PAF binding to human PMNs. PMNs were incubated in the absence or presence of uvariopsine and stephenanthrine (0.1–100 μM) and [3H]PAF (0.4 nM) for 120 min at 20°C as described in Methods. Nonspecific binding was determined with unlabeled PAF (2.0 μM). Assays were conducted in triplicate. The IC50 (concentration of the compound needed to produce 50% inhibition of [3H]PAF binding) values for uvariopsine or stephenanthrine were calculated from a nonlinear regression plot. Results are represented as mean±s.e.m. of n=4–5 preparations.
Figure 10.
Effect of uvariopsine (UVA) and stephenanthrine (STE) on elevations in intracellular superoxide anion (a) and hydrogen peroxide (b) induced by PAF and fMLP in CD45-stained PMNs. CD45-stained PMNs were incubated with 3 μM HE and 3 μM DHR for 15 min at 37°C. Then, 1 ml samples were incubated in the absence or presence of uvariopsine or stephenanthrine (10 and 100 μM) for 5 min and stimulated with 1 μM PAF or 100 nM fMLP for another 30 min as described in Methods. Samples were run in an EPICS XL and HE and DHR fluorescences in gated PMNs were recorded. Results are expressed as percentage inhibition of PAF or fMLP-induced increases in mean fluorescence intensity over the levels in the blank group. Results are represented as mean±s.e.m. of n=6–7 preparations.
Discussion
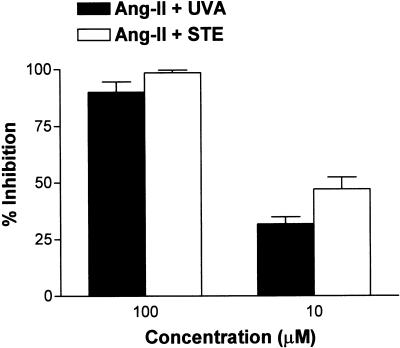
In the present study, we have demonstrated that two phenanthrene alkaloids from D. tripetala, uvariopsine and stephenanthrine, can inhibit the increase in the leukocyte–endothelial cell interactions induced by Ang-II 1 nM superfusion in the rat mesenteric postcapillary venules in a concentration-dependent manner. None of these treatments affect the different hemodynamic parameters. In vivo, stephenanthrine exerted greater inhibitory effect than uvariopsine. In fact, when the alkaloids were assayed at 0.1 μM concentration, stephenanthrine was capable of inhibiting leukocyte rolling flux, adhesion and emigration induced by Ang-II, while uvariopsine only significantly reduced Ang-II induced-leukocyte adhesion. Furthermore, a concentration of 0.01 μM of stephenanthrine provoked similar effects as 0.1 μM of uvariopsine. Although both compounds present similar structure, the smaller inhibitory effect provoked by uvariopsine could be due to the existence of a methoxy group in its structure. The only explanation for this different behavior seems to rely on the solubility of the product in water. In this context, chromatographic behavior under different conditions indicates that although both alkaloids bear similar lipophilicity, uvariopsine is slightly less polar than stephenanthrine, and, therefore, the latter may be more soluble in the aqueous superfusion medium and reach more easily the intravascular space to exert its action.
Once the in vivo anti-inflammatory activity of these two alkaloids was assessed, we next investigated the possible mechanism by which stephenanthrine and uvariopsine inhibit leukocyte–endothelial cell interactions induced by Ang-II. In this regard, some phenanthrene alkaloids can inhibit platelet aggregation (Cheng et al., 1996; Teng et al., 1997) and in some cases, this effect is the result of an increase in platelet cyclic AMP levels. In this context, we have recently demonstrated that cyclic AMP elevating agents, such as iloprost, salbutamol, forskolin or rolipram, can effectively inhibit leukocyte–endothelial cell interactions induced by Ang-II regardless of the mechanisms used to elevate the intracellular levels of this cyclic nucleotide (Álvarez et al., 2001). Therefore, we first evaluated whether these compounds can increase cyclic AMP levels in human PMNs. Since Ang-II cannot directly stimulate human neutrophils (Piqueras et al., 2000), fMLP was used to stimulate them. As shown in Figure 4, while forskolin and rolipram significantly increased cyclic AMP levels, none of the alkaloids at the three concentrations assayed were capable of enhancing the levels of this cyclic nucleotide in human PMNs stimulated with fMLP. Thus, the mechanism of action by which these phenanthrene alkaloids inhibited leukocyte responses induced by Ang-II does not involve an increase in intracellular cyclic nucleotides.
On the other hand, it has been illustrated that chronically elevated Ang-II-induced hypertension is mediated in part by superoxide anion production (Laursen et al., 1997). In addition, we extended these findings to demonstrate that free radical generation was also involved in acute Ang-II-induced leukocyte–endothelial cell interactions in vivo (Álvarez & Sanz, 2001). Therefore, we next examined whether the inhibition of Ang-II-induced leukocyte responses provoked by uvariopsine and stephenanthrine could be due to the inhibition of ROS generation. Figure 6 shows clearly that superoxide anion generation by HUVECs stimulated with Ang-II was inhibited by preincubation with uvariopsine and stephenanthrine. In addition, both compounds inhibited the ROS generation in human PMNs stimulated with fMLP in a concentration-dependent manner. Again, uvariopsine with an IC50 of 25.1 μM was slightly less potent than stephenanthrine with an IC50 of 15.1 μM. This action seems to rely on the presence of a methylenedioxy group in the structure of both alkaloids. In this regard, Yim & Ko (1998) have proved that the presence of a methylenedioxy group in the molecule of certain natural products such as schisandrins is an important structural determinant in mediating protection against myocardial ischemia–reperfusion injury. In this context, ischemia–reperfusion injury is a commonly used model for assessing antioxidant activities of synthetic and naturally occurring compounds, particularly in the myocardium. Indeed, schisandrins present free radical scavenging properties that may be responsible for the protection encountered against myocardial ischemia–reperfusion injury (Li et al., 1990). Likewise, both uvariopsine and stephenanthrine exerted free radical scavenging activity, the calculated IC50 values for ROS generation in a hypoxanthine–xanthine oxidase system being 8.0 and 24.3 μM, respectively. In this system, uvariopsine was slightly more potent than stephenanthrine. Previous studies have demonstrated that both superoxide anions produced via an hypoxantine–xanthine oxidase generating system and hydrogen peroxide have been shown to induce leukocyte influx through increased P-selectin expression (Gaboury et al., 1994; Johnston et al., 1996). Since uvariopsine and stephenanthrine were capable of inhibiting Ang-II-induced P-selectin upregulation in vivo, it is likely that these phenanthrene alkaloids preventing the generation of ROS would provoke the downregulation of P-selectin expression induced by Ang-II and thus inhibiting the subsequent infiltration of leukocytes.
Another interesting observation encountered in the present study is that both alkaloids were capable of preventing Ang-II-induced leukocyte adhesion even when they were assayed at doses where no effect in other leukocyte parameters was detected. We know that Ang-II does not appear to have direct effects on leukocytes since it does not cause the upregulation of β2 integrins or L-selectin shedding in the surface of rat granulocytes or monocytes stimulated by this peptide hormone (Piqueras et al., 2000). Therefore, Ang-II-induced leukocyte adhesion may be also due to the release of endogenous generated chemotactic mediators. In this regard, Ang-II-induced leukocyte recruitment is in part mediated by the release of PAF, as the administration of the PAF receptor antagonist WEB2086 can ameliorate this response (Álvarez & Sanz, 2001). In this context, some phenanthrene alkaloids can bind to the PAF receptor and behave as an antagonist of this inflammatory mediator as it has been demonstrated using binding assays in rabbit platelets (Jantan et al., 2001) or inhibiting platelet aggregation induced by PAF (Teng et al., 1997). In fact, in the present study, we have encountered that both phenanthrene alkaloids can inhibit intracellular calcium elevations in PMNs stimulated with PAF, with stephenanthrine (IC50 6.1 μM) being slightly more potent than uvariopsine (IC50 15.1 μM). In addition, both alkaloids inhibited the binding of [3H]PAF to PMNs in a concentration-dependent manner, and again stephenanthrine was found to be more potent than uvariopsine. Furthermore, both alkaloids were also capable of inhibiting PAF-induced increases in intracellular superoxide anion and hydrogen peroxide in human PMNs, while they had no effect when the cells were stimulated with fMLP. These results indicates that this inhibition of intracellular ROS generation may be mediated through a PAF receptor blockade. Hence, the possible antagonism of PAF-induced leukocyte adhesion may explain the inhibition in this parameter caused by these phenanthrene alkaloids at doses where they are devoid of other anti-inflammatory effects.
Finally, we would like to point out that although both alkaloids seem to be more potent inhibiting the parameters determined in intravital microscopy studies than those performed in isolated human PMNs, this is not the case. These results suggest that first, these compounds may require an intact vascular microenviroment to manifest their real activity and second, the effects observed in vivo are likely the result of all the effects detected in vitro; therefore, a decrease in their concentration is needed to clearly demonstrate their inhibitory effect in vivo. In fact, this apparent discrepancy between in vitro and in vivo concentrations has been encountered in other systems with different physiological or pharmacological relevant agents (Simoncini et al., 2000).
In conclusion, in the present study, we have demonstrated that two phenanthrene alkaloids isolated from D. tripetala, uvariopsine and stephenanthrine, can inhibit Ang-II-induced leukocyte–endothelial cell interactions in vivo. This effect is partly mediated through inhibition of the generation of ROS and the subsequent downregulation of P-selectin expression on the endothelial cell. Additionally, since the most powerful effects of these alkaloids were observed on leukocyte adhesion, it may be possible that they inhibit the synthesis and the release of PAF and leukotrienes and also block PAF-induced responses by interacting with its receptor. In this way, the use of these phenanthrene alkaloids could constitute an alternative therapy for the control of the subendothelial–leukocyte infiltration associated with the vascular damage in cardiovascular disease states where Ang-II plays a critical role.
Acknowledgments
The present study has been supported by Grant SAF 2002-01482 from CICYT, Spanish Ministerio de Ciencia y Tecnologia, GV01-292 from Generalitat Valenciana and has been awarded with the 2002 prize of the Spanish Pharmacological Society and Almirall-Prodesfarma Laboratories. L. Milian was supported by a grant from Spanish, Ministerio de Educacion, Cultura y Deporte.
Abbreviations
- ACE
angiotensin-converting enzyme
- Ang-II
angiotensin II
- AUC
area under the curve
- CAM
cell adhesion molecule
- cyclic AMP
adenosine 3′:5′-cyclic monophosphate
- DMSO
dimethylsulfoxide
- Dv
venular diameter
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- MABP
mean arterial blood pressure
- PAF
platelet activating factor
- PMNs
polymorphonuclear leukocytes
- ROS
reactive oxygen species
- Vmean
mean red blood cell velocity
- Vrbc
centerline red blood cell velocity
- Vwbc
leukocyte rolling velocity
References
- ADAMS D.H., SHAW S. Leukocyte–endothelial interactions and regulation of leukocyte migration. Lancet. 1994;343:831–836. doi: 10.1016/s0140-6736(94)92029-x. [DOI] [PubMed] [Google Scholar]
- ÁLVAREZ A., PIQUERAS L., BLÁZQUEZ M.A., SANZ M.J. Cyclic AMP elevating agents and nitric oxide modulate angiotensin II-induced leukocyte–endothelial cell interactions in vivo. Br. J. Pharmacol. 2001;133:485–494. doi: 10.1038/sj.bjp.0704096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ÁLVAREZ A., SANZ M.J. Reactive oxygen species mediate angiotensin II-induced leukocyte–endothelial cell interactions in vivo. J. Leukoc. Biol. 2001;70:199–206. [PubMed] [Google Scholar]
- BERGMEYER H.U., BERNT E.Lactate dehydrogenase: UV assay with pyruvate and NADH Methods of Enzymatic Analysis 1974New York: Academic Press; 574–577.ed. Bergmeyer H.U., pp [Google Scholar]
- BUTCHER E.C. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- CHENG K.S., KO F.N., TENG C.M., WU Y.C. Antiplatelet and vasorelaxing actions of some benzylisoquinoline and phenanthrene alkaloids. J. Nat. Prod. 1996;59:531–534. doi: 10.1021/np960354x. [DOI] [PubMed] [Google Scholar]
- CYBULSKY M.I., GIMBRONE M.A., JR Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- DASI F.J., ORTIZ J.L., CORTIJO J., MORCILLO E.J. Histamine up-regulates phosphodiesterase 4 activity and reduces prostaglandin E2-inhibitory effects in human neutrophils. Inflamm. Res. 2000;49:600–609. doi: 10.1007/s000110050637. [DOI] [PubMed] [Google Scholar]
- DERIAN C.K., SANTULLI R.J., RAO P.E., SOLOMON H.F., BARRETT J.A. Inhibition of chemotactic peptide-induced neutrophil adhesion to vascular endothelium by cyclic AMP modulators. J. Immunol. 1995;154:308–317. [PubMed] [Google Scholar]
- FARBER H.W., CENTER D.M., ROUNDS S., DANILOV S.M. Components of the angiotensin system cause the release of a neutrophil chemoattractant from cultured bovine and human endothelial cells. Eur. Heart J. 1990;11 Suppl. B:100–107. doi: 10.1093/eurheartj/11.suppl_b.100. [DOI] [PubMed] [Google Scholar]
- GABOURY J.P., ANDERSON D.C., KUBES P. Molecular mechanisms involved in superoxide-induced leukocyte–endothelial cell interactions in vivo. Am. J. Physiol. 1994;266:H637–H642. doi: 10.1152/ajpheart.1994.266.2.H637. [DOI] [PubMed] [Google Scholar]
- GRÄFE M., AUCH-SCHWELK W., ZAKRZEWICZ A., REGITZ-ZAGROSEK V., BARTSCH P., GRAF K., LOEBE M., GAEHTGENS P., FLECK E. Angiotensin II-induced leukocyte adhesion on human coronary endothelial cells is mediated by E-selectin. Circ. Res. 1997;81:804–811. doi: 10.1161/01.res.81.5.804. [DOI] [PubMed] [Google Scholar]
- HAHN A.W., JONAS U., BÜHLER F.R., RESINK T.J. Activation of human peripheral monocytes by angiotensin II. FEBS Lett. 1994;347:178–180. doi: 10.1016/0014-5793(94)00531-1. [DOI] [PubMed] [Google Scholar]
- HERNÁNDEZ-PRESA M., BUSTOS C., ORTEGO M., TUÑON J., RENEDO G., RUIZ-ORTEGA M., EGIDO J. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-kappa B activation, monocyte chemoattractant protein-1 expression and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–1541. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- HOUSE S.D., LIPOWSKY H.H. Leukocyte–endothelium adhesion: microhemodynamics in mesentery of the cat. Microvasc. Res. 1987;34:363–379. doi: 10.1016/0026-2862(87)90068-9. [DOI] [PubMed] [Google Scholar]
- JAFFE E.A., NACHMAN R.L., BECKER C.G., MINICK C.R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANTAN I., RAFI I.A., JALIL J. Inhibition of platelet-activating factor receptor binding by aporphine and phenanthrenoid alkaloids from Aromadendron elegans. Planta Med. 2001;67:466–467. doi: 10.1055/s-2001-15819. [DOI] [PubMed] [Google Scholar]
- JOHNSTON B., KANWAR S., KUBES P. Hydrogen peroxide induces leukocyte rolling: modulation by endogenous antioxidant mechanisms including NO. Am. J. Physiol. 1996;271:H614–H621. doi: 10.1152/ajpheart.1996.271.2.H614. [DOI] [PubMed] [Google Scholar]
- KIM J.A., BERLINER J.A., NADLER J.R. Angiotensin II increases monocyte binding to endothelial cells. Biochem. Biophys. Res. Commun. 1996;226:862–868. doi: 10.1006/bbrc.1996.1441. [DOI] [PubMed] [Google Scholar]
- LAURSEN J.B., RAJAGOPALAN A., GALIS Z., TARPEY M., FREEMAN B.A., HARRISON D.G. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- LI X.J., ZHAO B.L., LIU G.T., XING W.J. Scavenging effects on active oxygen free radicals by schisandrins with different structures and configurations. Free Radical Biol. Med. 1990;9:99–104. doi: 10.1016/0891-5849(90)90111-u. [DOI] [PubMed] [Google Scholar]
- LÓPEZ-MARTÍN J., ANAM E.M., BOIRA H., SANZ M.J., BLÁZQUEZ M.A. Chromone and phenanthrene alkaloids from Dennettia tripetala. Chem. Pharm. Bull. 2002;50:1613–1615. doi: 10.1248/cpb.50.1613. [DOI] [PubMed] [Google Scholar]
- MERVAALA E.M.A., MÜLLER D.M., PARK J.K., SCHMIDT F., LÖHN M., BREU V., DRAGUN D., GANTEN D., HALLER H., LUFT F.C. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension. 1999;33:389–395. doi: 10.1161/01.hyp.33.1.389. [DOI] [PubMed] [Google Scholar]
- MÜGGE A., ELWELL J.H., PETERSON T.E., HOFMEYER T.G., HEISTAD D.D., HARRISON D.G. Chronic treatment with polyethylene-glycolated superoxide dismutase partially restores endothelium-dependent vascular relaxations in cholesterol-fed rabbits. Circ. Res. 1991;69:1293–1300. doi: 10.1161/01.res.69.5.1293. [DOI] [PubMed] [Google Scholar]
- PIQUERAS L., KUBES P., ALVAREZ A., O'CONNOR E., ISSEKUTZ A.C., ESPLUGUES J.V., SANZ M.J. Angiotensin II induces leukocyte–endothelial cell interactions in vivovia AT1 and AT2 receptor-mediated P-selectin upregulation. Circulation. 2000;102:2118–2123. doi: 10.1161/01.cir.102.17.2118. [DOI] [PubMed] [Google Scholar]
- PRICE D.T., LOSCALZO J. Cellular adhesion molecules and atherogenesis. Am. J. Med. 1999;107:85–97. doi: 10.1016/s0002-9343(99)00153-9. [DOI] [PubMed] [Google Scholar]
- ROSS R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- SANZ M.J., ALVAREZ A., PIQUERAS L., CERDA M., ISSEKUTZ A.C., LOBB R.R., CORTIJO J., MORCILLO E.J. Rolipram inhibits leukocyte–endothelial cell interactions in vivo through P- and E-selectin downregulation. Br. J. Pharmacol. 2002;135:1872–1881. doi: 10.1038/sj.bjp.0704644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUDT C., WINDER S., FORDERKUNZ S., HATZELMANN A., ULLRICH V. Influence of selective phosphodiesterase inhibitors on human neutrophil functions and levels of cAMP and Ca. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:682–690. doi: 10.1007/BF00174752. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI T., NAGAMINE T. Inhibition of free radical generation by biotin. Biochem. Pharmacol. 1994;47:594–596. doi: 10.1016/0006-2952(94)90195-3. [DOI] [PubMed] [Google Scholar]
- SHIMADA K., YAZAKI Y. Binding sites for angiotensin II in human mononuclear leukocytes. J. BioChem. 1978;84:1013–1015. doi: 10.1093/oxfordjournals.jbchem.a132183. [DOI] [PubMed] [Google Scholar]
- SIMONCINI T., MAFFEI S., BASTA G., BARSACCHI G., GENAZZANI A.R., LIAO J.K., DE CATERINA R. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ. Res. 2000;87:19–25. doi: 10.1161/01.res.87.1.19. [DOI] [PubMed] [Google Scholar]
- SPRINGER T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- SZUCS S., VAMOSI G., POKA R., SARVARY A., BARDOS H., BALAZS M., KAPPELMAYER J., TOTH L., SZOLLOSI J., ADANY R. Single-cell measurement of superoxide anion and hydrogen peroxide production by human neutrophils with digital imaging fluorescence microscopy. Cytometry. 1998;33:9–31. doi: 10.1002/(sici)1097-0320(19980901)33:1<19::aid-cyto3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- TENG C.M., HSUEH C.M., CHANG Y.L., KO F.N., LEE S.S., LIU K.C. Antiplatelet effects of some aporphine and phenanthrene alkaloids in rabbits and man. J. Pharm. Pharmacol. 1997;49:706–711. doi: 10.1111/j.2042-7158.1997.tb06096.x. [DOI] [PubMed] [Google Scholar]
- VASANGE M., ROLFSEN W., BOHLIN L. A sulphonoglycolipid from the fern Polypodium decumanum and its effect on the platelet activating-factor receptor in human neutrophils. J. Pharm. Pharmacol. 1997;49:562–566. doi: 10.1111/j.2042-7158.1997.tb06842.x. [DOI] [PubMed] [Google Scholar]
- WALTER U.M., AYER L.M., WOLITZKY B.A., WAGNER D.D., HYNES R.O., MANNING A.M., ISSEKUTZ A.C. Characterization of a novel adhesion function blocking monoclonal antibody to rat/mouse P-selectin generated in P-selectin-deficient mouse. Hybridoma. 1997;16:249–257. doi: 10.1089/hyb.1997.16.249. [DOI] [PubMed] [Google Scholar]
- YIM T.K., KO K.M. Methylenedioxy group and cyclooctadiene ring as structural determinants of schisandrin in protecting against myocardial ischemia–reperfusion injury in rats. Biochem. Pharmacol. 1998;57:77–81. doi: 10.1016/s0006-2952(98)00297-4. [DOI] [PubMed] [Google Scholar]
- ZHANG H., SCHMEISSER A., GARLICHS C.D., PLOTZE K., DAMME U., MUGGE A., DANIEL W.G. Angiotensin II-induced superoxide anion generation in human vascular endothelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc Res. 1999;44:215–222. doi: 10.1016/s0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]