Abstract
Although capsaicin analogs might be a potential strategy to manipulate inflammation, the mechanism is still unclear. In this study, the effects and action mechanisms of vanilloid analogs on iNOS and COX-2 expression were investigated in RAW264.7 macrophages.
Capsaicin and resiniferatoxin (RTX) can inhibit LPS- and IFN-γ-mediated NO production, and iNOS protein and mRNA expression with similar IC50 values of around 10 μM.
Capsaicin also transcriptionally inhibited LPS- and PMA-induced COX-2 expression and PGE2 production. However, this effect exhibited a higher potency (IC50: 0.2 μM), and RTX failed to elicit such responses at 10 μM.
Interestingly, we found that capsazepine, a competitive TRPV1 antagonist, did not prevent the inhibition elicited by capsaicin or RTX. Nevertheless, it mimicked vanilloids in inhibiting iNOS/NO and COX-2/PGE2 induction with an IC50 value of 3 μM. RT–PCR and immunoblotting analysis excluded the expression of TRPV1 in RAW264.7 macrophages.
The DNA binding assay demonstrated the abilities of vanilloids to inhibit LPS-elicited NF-κB and AP-1 activation and IFN-γ-elicited STAT1 activation. The reporter assay of AP-1 activity also supported this action.
The kinase assay indicated that ERK, JNK, and IKK activation by LPS were inhibited by vanilloids.
In conclusion, vanilloids can modulate the expression of inflammatory iNOS and COX-2 genes in macrophages through interference with upstream signalling events of LPS and IFN-γ. These findings provide new insights into the potential benefits of the active ingredient in hot chilli peppers in inflammatory conditions.
Keywords: Capsaicin, capsazepine, TRPV1, macrophages, NO, COX-2, ERK, JNK, IKK
Introduction
Capsaicin, which is responsible for the piquancy of hot chilli peppers, is widely used in Asian diets as food additives. Capsaicin has been used for several therapeutic indications including amelioration of neuropathic pain or itching, inhibition of neurogenic inflammation, and suppression of urinary bladder hyper-reflexia (Winter et al., 1995; Szallasi & Blumberg, 1999). Similar to the action of capsaicin, resiniferatoxin (RTX) is another compound isolated from a cactus-like plant (Szallasi & Blumberg, 1999). Various effects of capsaicin and RTX are mediated through vanilloid receptor-1 (TRPV1) (Wood, 1993; Szallasi et al., 1999; Szallasi & Blumberg, 1999). TRPV1 possesses membrane topology with a possible pore–loop domain (Caterina et al., 1997; 1999; Schumacher et al., 2000; Delany et al., 2001).
Following stimulation of peripheral terminals of sensory dorsal root ganglion (DRG) neurons, with capsaicin and RTX, TRPV1 becomes permeable to Na+ and Ca2+, causing the neurons to depolarize and fire action potentials (Tominaga et al., 1998). In addition to expression in primary sensory neurons, TRPV1 is also present throughout the CNS (Sasamura et al., 1998), and non-neuronal cells, such as mast cells (Biro et al., 1998a), and glial cells (Biro et al., 1998b). In addition to the multiple actions mediated by TRPV1 in the nervous system (Mezey et al., 2000), capsaicin has been shown to be immunomodulatory. In this context, treatment with capsaicin analogs might be a potential strategy to manipulate inflammation, particularly for treatment of arthritis (Brand et al., 1990; Jarreau et al., 1994; Joe & Lokesh, 1997a; 2000).
The direct inhibitory action of capsaicin on the release of inflammatory cytokines was suggested. Two well-defined eucaryotic transcription factors, nuclear factor-kappa B (NF-κB) and activator protein 1 (AP-1), which have been implicated in many inflammatory diseases, were demonstrated to be inhibited by capsaicin (Singh et al., 1996; Surh et al., 2000) and RTX (Singh et al., 1996). Studies delineated that the suppression of NF-κB activation by capsaicin occurs through inhibition of IκBα kinase (IKK) and IκBα degradation, leading to a subsequent blockade of the nuclear translocation of p65 (Han et al., 2002; Sancho et al., 2002). Associated with this mechanism, capsaicin attenuated cytokines (granulocyte–macrophage colony-stimulating factor, interferon-γ (IFN-γ), and interleukin-2) transcription from Jurkat T cells (Gertsch et al., 2002). We demonstrated that anandamide, an endogenous agonist for both TRPV1 and cannabinoid CB1, also inhibited lipopolysaccharide (LPS)-induced expression of inducible nitric oxide synthase (iNOS) and interleukin-6 genes in murine macrophages (Chang et al., 2001). Inhibition of NF-κB activation through a non-TRPV1 action accounts for this anti-inflammatory effect of anandamide (Sancho et al., 2003). In rats fed with capsaicin, the release of nitric oxide (NO) and eicosanoids from peritoneal macrophages was also reduced (Joe & Lokesh, 1994; 1997b; 2000). A recent report seems to refute the involvement of TRPV1 in the anti-inflammation effect of capsaicin. A TRPV1 antagonist, capsazepine (CZP), like the effect of capsaicin, was able to inhibit the expression of iNOS gene in LPS-stimulated murine macrophages through inactivation of NF-κB (Oh et al., 2001).
Given that the roles of TRPV1 in vanilloids' action and the signalling mechanism contributing to AP-1 inhibition are still unclear, this study was undertaken to investigate the effects of TRPV1 agonists (capsaicin and RTX) and antagonist (CZP) on iNOS and cyclooxygenase-2 (COX-2) gene expression. In addition to investigating the effects on LPS-induced NF-κB and AP-1 activation, we were interested in exploring whether IFN-γ-induced upregulation of the iNOS gene was affected by vanilloids. Herein, we compared the effects of vanilloids on signal transducer and activator of transcription 1 (STAT1) signalling induced by IFN-γ.
Methods
Materials
Phenol-extracted LPS (L8274) from Escherichia coli was obtained from Sigma Aldrich (St Louis, MO, U.S.A.) and its protein content measured by Bradford protein assay was found to be 0.07% (w w−1). The prostaglandin E2 (PGE2) assay kit was obtained from Cayman Chemicals (Ann Arbor, MI, U.S.A.). Capsaicin, RTX, CZP and phorbol 12-myristate 13-acetate (PMA) were obtained from Sigma Aldrich (St Louis, MO, U.S.A.). IFN-γ was purchased from R&D systems (Minneapolis, MN, U.S.A.). Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum, penicillin, and streptomycin were obtained from Gibco BRL (Grand Island, NY, U.S.A.). Rabbit antibodies against iNOS, COX-2, STAT1, p65 NF-κB, c-fos, c-jun, IKKα, IKKβ, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and protein A/G beads were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Plasmids of pGEX-IκBα (amino acids 5–55) and pGEX-c-Jun were provided by Dr Frank S. Lee (Pennsylvania Medical Center, PA, U.S.A.) and Dr Min-L. Kuo (National Taiwan University, Taipei, Taiwan), respectively. AP-1-Luc construct was provided by Dr G. Haegeman (University of Ghent-VIB, Ghent, Belgium). Horseradish peroxidase-coupled second antibodies and the ECL agent were purchased from Amersham Biosciences (Piscataway, NJ, U.S.A.). All materials for SDS–PAGE were obtained from Bio-Rad (Hercules, CA, U.S.A.). Oligonucleotides sequences as previously reported (Chen et al., 1998) used to detect the DNA-binding activities of NF-κB, AP-1 and STAT1 were synthesized on a PS250 CRUACHEM DNA synthesizer using the cyanoethyl phosphoroamidate method. [α-32P]dATP (3000 Ci mmol−1) and [γ-32P]ATP (5000 Ci mmol−1) were obtained from NEN (Boston, MA, U.S.A.).
Cell culture
RAW264.7 macrophages obtained from American Type Culture Collection (Manassas, VA, U.S.A.) were cultured in DMEM containing 10% (v v−1) fetal bovine serum, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin at 37°C in a 5% CO2/air environment. Adult male Sprague–Dawley rats were exsanguinated under trichloroacetaldehyde (400 mg kg−1, i.p.) anesthesia. The lumbar ganglia (L4, L5) were excised after freeing the nerve trunks and connective tissue sheath. Pooled DRG was washed twice with ice-cold PBS and homogenized by cell lysis buffer as indicated below for immunoblotting analysis.
Nitrite and PGE2 measurement
Nitrite and PGE2 production were measured in the RAW264.7 macrophage supernatants. Briefly, the cells were cultured in 24-well plates in 500 μl of culture medium until confluence. The cells were treated with LPS, IFN-γ or PMA for the time indicated, then the culture media were collected. Nitrite was measured by adding 100 μl of Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamide in 5% phosphoric acid) to 100 μl samples of culture medium. The optical density at 550 nm (OD550) was measured using a microplate reader and the nitrite concentration calculated by comparison with the OD550 produced using standard solutions of sodium nitrite in the culture medium. PGE2 was measured by enzyme-linked immunosorbent assay (ELISA) kit following the manufacturer's instructions.
Immunoblotting analysis
After stimulation, cells were rinsed twice with ice-cold PBS, and 100 μl of cell lysis buffer (20 mM Tris-HCl, pH 7.5, 125 mM NaCl, 1% Triton X-100, 1 mM MgCl2, 25 mM β-glycerophosphate, 50 mM NaF, 100 μM Na3VO4, 1 mM PMSF, 10 μg ml−1 leupeptin, 10 μg ml−1 aprotinin) was then added to each plate. Protein was denatured in SDS, electrophoresed on a 10% SDS/polyacrylamide gel, and transferred to nitrocellulose membrane. Nonspecific binding was blocked with TBST (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) containing 5% nonfat milk for 1 h at room temperature. After incubation with the appropriate first antibodies, membranes were washed three times with TBST. The secondary antibody was incubated for 1 h. Following three washes with TBST, the protein bands were detected with the ECL reagent.
Reverse transcription–polymerase chain reaction (RT–PCR)
To amplify iNOS, COX-2 and TRPV1 mRNA, their specific primers for RT–PCR analysis were synthesized. Macrophages treated with indicated agents were homogenized with 1 ml of RNAzol B reagent (Gibco), and total RNA was extracted by acid guanidinium thiocyanate-phenol–chloroform extraction. RT was performed using StrataScript RT-PCR Kit, and 10 μg of total RNA was reverse transcribed to cDNA following the manufacturer's recommended procedures. RT-generated cDNA encoding iNOS, COX-2, TRPV1, and β-actin genes were amplified using PCR. The oligonucleotide primers used correspond to the mouse iNOS (5′-CCC TTC CGA AGT TTC TGG CAG CAG C-3′ and 5′-GGC TGT CAG AGC CTC GTG GCT TTG G-3′), COX-2 (5′-CAG CAA ATC CTT GCT GTT CC-3′ and 5′-TGG GCA AAG AAT GCA AAC ATC-3′), TRPV1 (5′-GCA CTG CAC ATT GCC ATT GAA-3′ and 5′-CAC CAG GGC ATG AAG CAC CGT GTT-3′), and β-actin (5′-GAC TAC CTC ATG AAG ATC CT-3′ and 5′-CCA CAT CTG CTG GAA GGT GG-3′). PCR was performed in a final volume of 50 μl containing: Taq DNA polymerase buffer, all four dNTPs, oligonucleotide primers, Taq DNA polymerase, and RT products. After an initial denaturation for 2 min at 94°C, 35 cycles of amplification (94°C for 45 s, 65°C for 45 s, and 72°C for 2 min) were performed followed by a 10-min extension at 72°C. PCR products were analyzed on 2% agarose gel. The mRNA of β-actin served as an internal control for sample loading and mRNA integrity.
Preparation of nuclear extracts and electrophoretic mobility shift assays (EMSA)
Nuclear extracts from stimulated or nonstimulated macrophages were prepared by cell lysis followed by nuclear lysis; cells were suspended in 30 μl of buffer containing 10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol, and 0.2 mM phenylmethylsulfonyl fluoride; vigorously vortexed for 15 s; allowed to stand at 4°C for 10 min; and centrifuged at 2000 r.p.m. for 2 min. The pelleted nuclei were resuspended in buffer containing 20 mM HEPES (pH 7.9), 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, and 0.2 mM phenylmethylsulfonyl fluoride for 20 min on ice, and then the lysates were centrifuged at 15,000 r.p.m. for 2 min. The supernatants containing the solubilized nuclear proteins were stored at −70°C until used for EMSAs. Binding tests for NF-κB, AP-1 and STAT1 were performed. Briefly, binding reaction mixtures (15 μl) contained 0.25 μg of poly(dI-dC) (Amersham Biosciences) and 20,000 d.p.m. of 32P-labeled DNA probe in binding buffer consisting of 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 4% Ficoll, 1 mM dithiothreitol, and 75 mM KCl; the binding reaction was started by the addition of cell extracts and continued for 30 min. Samples were analyzed on native 5% polyacrylamide gels. For supershift experiments, 5 μg of p65, c-fos, c-jun or STAT-1 antibody was mixed with the nuclear extract proteins.
Immunoprecipitation and kinase assay
To determine the effects of vanilloid analogs on protein kinases, anti-IKKα, IKKβ, ERK, and JNK antibodies (each of 1 μg) with protein A/G-agarose beads were added to the prepared cell extracts as mentioned above. Immunoprecipitation proceeded at 4°C overnight. The precipitated beads were washed three times with 1 ml of ice-cold cell lysis buffer and twice with kinase buffer (25 mM HEPES, pH 7.4, 20 mM MgCl2, 0.1 mM Na3VO4, 2 mM dithiothreitol). The immune-complex kinase assay of one half of the immunoprecipitates was performed at 30°C for 30 min in 20 μl of kinase reaction buffer containing 1 μg GST-IκBα, myelin basic protein (MBP), or GST-c-Jun, 25 μM ATP, and 3 μCi [γ-32P]ATP. The reaction was terminated with 5 × Laemmli sample buffer, and the products were resolved by 12% SDS–PAGE gel electrophoresis. The phosphorylated IκBα, MBP, or GST-c-Jun were visualized by autoradiography. The other half of the immunoprecipitates was subjected to SDS–PAGE and immunoblotting to verify equal amount of kinases undergoing kinase reaction.
Transfection and reporter gene assay
RAW264.7 cells seeded into 24-well plates were transfected with 0.5 μg AP-1 promoter plasmid and 1 μg β-galactosidase expression vector by Lipofectamine/Plus reagents (Invitrogen) according to the manufacturer's instructions. After 24 h, after transfection, cells were treated with indicated agents. After another 24-h incubation, the media were removed, and cells were washed once with cold PBS. To prepare lysates, 50 μl of reporter lysis buffer (Promega) was added to each well, and cells were scraped from dishes. The supernatant was collected after centrifugation at 13,000 r.p.m. for 30 s. Aliquots of cell lysates (5 μl) containing equal amounts of protein (10–20 μg) were placed into wells of an opaque black 96-well microplate. An equal volume of luciferase substrate (Promega) was added to all samples, and the luminescence was measured in a microplate luminometer (Meriden, CT, U.S.A.). The luciferase activity value was normalized to transfection efficiency monitored by the cotransfected β-galactosidase expression vector (pCR3lacZ; Pharmacia, Sweden). The level of induction of luciferase activity was determined as a ratio in comparison to cells with no stimulation.
Statistical evaluation
Values were expressed as the mean±s.e.m. of at least three experiments. Analysis of variance (ANOVA) was used to assess the statistical significance of the differences, and a P-value of less than 0.05 was considered to be statistically significant.
Results
Capsaicin inhibited NO and PGE2 production in response to LPS, IFN-γ and/or PMA
In mouse RAW264.7 macrophages, LPS (100 ng ml−1) and IFN-γ (3 ng ml−1) treatment for 24 h resulted in a large amount of NO release, from a basal level of 5±1 to 45±6 and 34±5 μM, respectively. The increased NO release was accompanied by the induction of iNOS. Cotreatment of capsaicin with LPS or IFN-γ markedly reduced NO and iNOS induction (Figure 1). The inhibitory effects of capsaicin exhibited concentration-dependency, with IC50 value of 7.2±1.4 μM (n=4) for LPS-induced NO release and 8.3±1.3 μM (n=3) for IFN-γ-induced NO release. The IC50 values for inhibition of LPS- and IFN-γ-induced iNOS induction were comparable at 9.7±1.3 μM (n=3). Using 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) assay as an index of mitochondria activity, capsaicin incubation at concentrations up to 100 μM for 24 h did not cause cell toxicity (data not shown).
Figure 1.
Inhibitory effects of capsaicin on LPS- and IFN-γ-induced NO release and iNOS expression. RAW264.7 cells were treated with LPS (100 ng ml−1), IFN-γ (3 ng ml−1) and/or capsaicin at the concentrations indicated for 24 h. Culture medium was collected for NO assay (a), and cell lysate was subjected to SDS–PAGE and immunoreactivities of iNOS and α-tubulin were measured by immunoblotting with a specific antibody. Changes in iNOS protein levels normalized by α-tubulin were quantified and are represented as percentages of the LPS response in the absence of capsaicin treatment. The data represent the mean±s.e.m. from at least three independent experiments, and the trace in (b) is representative of three separate experiments with similar results, which were quantified and shown. *P<0.05 as compared to the control response of LPS or IFN-γ without vanilloid treatment.
Not only did capsaicin inhibit stimuli-elicited iNOS/NO induction but also reduced LPS- and PMA-elicited COX-2/PGE2 induction (Figure 2). Both inhibitory effects of capsaicin were also exhibited in concentration-dependent manner. Interestingly, we found that capsaicin was much more potent in inhibiting COX-2/PGE2 production than iNOS/NO production. The IC50 values for the inhibition of LPS-induced PGE2 and COX-2 responses were 0.23±0.08 μM (n=3) and 0.31±0.09 μM (n=3), respectively.
Figure 2.
Inhibitory effects of capsaicin on LPS- and PMA-induced PGE2 release and COX-2 expression. RAW264.7 cells were treated with LPS (100 ng ml−1), PMA (10 nM) and/or capsaicin at the concentrations indicated for 24 or 10 h respectively, then the culture medium was collected for PGE2 assay (a), and cell lysate was subjected to SDS–PAGE and measured for COX-2 and α-tubulin immunoreactivities (b). PGE2 and COX-2 levels are calculated as percentages of LPS response in the absence of capsaicin treatment. The data represent the mean±s.e.m. from at least three independent experiments, and the trace in (b) is representative of three separate experiments with similar results, which were quantified and are shown. *P<0.05 as compared to the control response of LPS or PMA without vanilloid treatment.
RTX and capsazepine inhibited iNOS/NO and COX-2/PGE2 induction
In order to understand whether the action of capsaicin results from the interaction with TRPV1, we examined another TRPV1 agonist, RTX, and the TRPV1 antagonist, capsazepine. As shown in Figure 3a and c, we found that RTX, like the action of capsaicin, caused inhibition of NO release and iNOS induction. At 10 μM of RTX, the highest concentration tested, 67±10% (n=4) and 52±6% (n=4) inhibition of LPS-induced NO production was observed (IC50: 7.9±1.7 μM (n=3) for the LPS response and 9.7±0.4 μM (n=4) for the IFN-γ response). With respect to capsazepine, we found that it also attenuated both stimuli-elicited NO production (IC50: 3.2±0.9 μM (n=4) for the LPS response and 4.9±1.0 μM (n=3) for the IFN-γ response) (Figure 3b), and iNOS expression (IC50: 4.1±0.8 μM (n=4) for the LPS response and 7.0±1.1 μM (n=3) for the IFN-γ response) (Figure 3c). Since capsazepine and RTX each at 30 μM caused MTT reduction by 37±8% (n=3) and 32±7% (n=3), respectively, we used 10 μM of capsazepine and RTX in the following experiments. Upon exploring the effects on COX-2/PGE2 induction, we found that RTX at 10 μM failed to affect LPS-induced COX-2 and PGE2 responses (Figure 3d). Compared to the dramatic and potent inhibition elicited by capsaicin, capsazepine showed a weak potency for inhibiting LPS-induced COX-2 expression and PGE2 release, which was similar to that for iNOS/NO inhibition. In the presence of 10 μM capsazepine, PGE2 release was inhibited by 64±4% (n=4), while the COX-2 increase was inhibited by 42±4% (n=3) (Figure 3d). The IC50 value was 3.9±0.8 μM (n=4) for LPS-induced PGE2 response.
Figure 3.
Effects of RTX and capsazepine on LPS- and IFN-γ-induced NO release, iNOS expression, PGE2 release and/or COX-2 expression. RAW264.7 cells were treated with LPS (100 ng ml−1), IFN-γ (3 ng ml−1), RTX and/or capsazepine at the concentrations indicated for 24 h, then the culture medium was collected for NO assay (a, b), PGE2 assay (d), and cell lysate, as described above, was subjected to SDS–PAGE and measured and quantified for iNOS (c) or COX-2 immunoreactivities (d). The data represent the mean±s.e.m. from at least three independent experiments. *P<0.05 as compared to the control response without vanilloid treatment.
TRPV1-independent action of vanilloids
Since the vanilloid antagonist capsazepine exhibited similar inhibitory effect as TRPV1 agonists, we wonder whether these effects of vanilloids are linked to TRPV1. To address this point, RT–PCR and immunoblotting analysis were, respectively, carried out with specific primers and antibody. Figure 4 shows that when oligonucleotides in DNA sequences of TRPV1 was used as PCR primers, although TRPV1 mRNA was expressed in DRG, which is known as TRPV1-positive neurons (Szallasi et al., 1999), they were not detected in basal, LPS-, or vanilloid-treated RAW264.7 macrophages. In agreement with mRNA expression, TRPV1 protein can be observed in DRG but not in RAW264.7 cells.
Figure 4.
No TRPV1 expression by RAW264.7 macrophages. After treatment with agents as indicated for 24 h, cells were lysed. Total cell lysates and mRNA from RAW264.7 and DRG were, respectively, prepared for immunoblotting analysis (a) and RT–PCR (b). The results are representative of three separate experiments.
Inhibition of mRNA expression of iNOS and COX-2
Inducible NOS and COX-2 are two inducible gene products, whose expression requires several transcriptional factors, and are elicited by several coordinated signalling pathways. To understand whether the effects of vanilloids are due to transcriptional regulation, we treated cells with vanilloids at different time intervals after LPS stimulation. The results shown in Figure 5a and b indicated that the inhibition of iNOS/NO and PGE2 production by capsaicin displayed a time-dependency, which revealed a gradual decrease as capsaicin treatment was delayed by up to 8 h after LPS stimulation. Similar to the time-dependent effect of capsaicin, the delayed addition of RTX and capsazepine led to reduced inhibition of LPS-mediated iNOS/NO production (Figure 5c). These results suggest that the inhibitory action of vanilloids is located at the gene transcription level.
Figure 5.
Vanilloid time-dependent inhibition of LPS-induced NO, PGE2 production, and iNOS expression. Vanilloids at 10 μM was added to the cell cultures at the same time as, or at different periods after LPS (100 ng ml−1). At 24 h after LPS addition, NO (a, c), and PGE2 (b) production in the medium, and iNOS protein in cell lysates (a, c) were determined. Data are presented as the mean±s.e.m. of three experiments. The traces in (a, c) are representative of three separate experiments. The data in parentheses indicate the percentages of iNOS protein induction as compared to the control response of LPS alone without vanilloid treatment.
To direct verify the effect on gene transcription, we determined the total mRNA levels of iNOS and COX-2 after LPS and vanilloid treatment. As shown in Figure 6a, LPS-induced upregulation of iNOS mRNA within 12 h were inhibited by the presence of each vanilloid at 10 μM. With regard to COX-2 mRNA induction by LPS, it was 58±3, 88±2, and 65±10% inhibited by 1 μM capsaicin, 10 μM capsaicin, and 10 μM capsazepine, respectively. In contrast, no inhibition was seen with 10 μM RTX (Figure 6b).
Figure 6.
Vanilloids reduced iNOS and COX-2 mRNA expression. Following the treatment with drugs as indicated for 12 h, changes in iNOS (a) and COX-2 (b) mRNA levels were measured by the PCR products. RNA isolation and the RT–PCR process were carried out as described. The β-actin mRNA level was considered the internal control.
Inhibition of LPS-induced NF-κB and AP-1 activation and IFN-γ-induced STAT1 activation
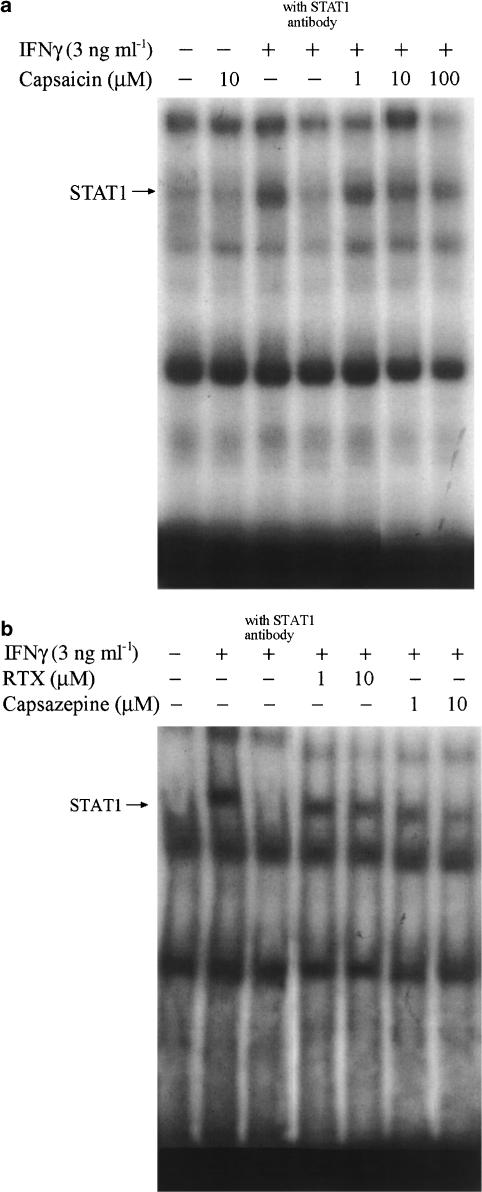
After observing the inhibitory effects of vanilloids on iNOS and COX-2 transcription, we next investigated the underlying mechanisms involved. Since activation of transcription factors NF-κB and AP-1 are crucial for the action of LPS, and activation of STAT-1 is involved in the action of IFN-γ, we determined the effects of vanilloids on these molecules. DNA binding abilities of these transcription factors, which exist in the nuclear extract following LPS, IFN-γ , and/or vanilloids treatment, were analyzed with EMSA. Vanilloids themselves at concentrations up to 10 μM did not alter the basal activities of NF-κB, AP-1, or STAT1. However, LPS-activated NF-κB (Figure 7a) and AP-1 (Figure 7b), as well as IFN-γ-activated STAT1 (Figure 8) were diminished with different degrees by these vanilloid analogs. Capsaicin significantly reduced NF-κB and AP-1 activities at 1 μM. Compared to the marginal effect at 1 μM, RTX significantly inhibited NF-κB at 10 μM. In contrast, at 10 μM, RTX weakly decreased AP-1 activity. In the presence of capsazepine (1 and 10 μM), LPS-induced NF-κB and AP-1 activations were also concentration-dependently reduced, and the inhibition extents were similar to those of capsaicin at the same concentrations tested (1 and 10 μM). Additionally, the effect of vanilloids on AP-1 activation was verified by a reporter assay using the AP-1 luciferase construct. Figure 7c revealed a similar effect as that shown from the experiment of transcription factor-DNA binding. With regard to IFN-γ-mediated STAT-1 activation, concentration-dependent inhibition by capsaicin (1–100 μM) (Figure 8a), RTX (1–10 μM) and capsazepine (1–10 μM) was shown (Figure 8b).
Figure 7.
Effects of vanilloids on DNA binding activity of NF-κB and AP-1. RAW264.7 cells were treated with 100 ng ml−1 LPS and/or vanilloids at the concentrations indicated for 1 h. Nuclear extracts from cell lysates were prepared and assayed for binding activity with specific oligonucleotides containing respective binding sequences for NF-κB (a) or AP-1 (b). In some experiments, specific antibodies of these transcription factors were included in the binding mixture to analyze the binding specificity. The results are representative of three different experiments. In (c), RAW264.7 cells were transfected with AP-1 reporter and treated with 100 ng ml−1 LPS and vanilloids. Luciferase activity normalized with LacZ expression was determined 24 h later. Data are presented as the mean±s.e.m. of three experiments. *P<0.05 as compared to the control response of LPS without vanilloid treatment.
Figure 8.
Effects of vanilloids on DNA binding activity of STAT-1. RAW264.7 cells were treated with 3 ng ml−1 IFN-γ and/or vanilloids at the concentrations indicated for 1 h. Nuclear extracts from cell lysates were prepared and assayed for binding activity with specific oligonucleotides containing binding sequences for STAT-1. In some experiments, a specific antibody of STAT1 was included in the binding mixture to analyze the binding specificity. The results are representative of three different experiments.
Vanilloids inhibition of LPS-induced IKK, ERK, and JNK activation
To understand whether the inhibitory effects of vanilloids on LPS-induced NF-κB and AP-1 are associated with uncoupling of the upstream signalling pathways, the kinase activities of IKK, ERK, and JNK were measured. Using GST-IκBα as a substrate for the IKK activity assay, immunoprecipitation accompanying the kinase assay showed that LPS-induced IKK activity was 50±8% (n=3), 30±6% (n=3), and 58±10% (n=3) inhibited by 10 μM capsaicin, RTX, and capsazepine, respectively (Figure 9a). Using MBP as a substrate for ERK activity assay, immunoprecipitation accompanying the kinase assay showed that LPS-induced ERK activity was 50±11% (n=3), 20±5% (n=3), and 65±12% (n=3) inhibited by 10 μM capsaicin, RTX, and capsazepine, respectively (Figure 9b). Using GST-c-Jun as a substrate for the JNK activity assay, immunoprecipitation accompanying the kinase assay showed that LPS-induced JNK activity was 60%, 20%, and 70% inhibited by 10 μM capsaicin, RTX, and capsazepine, respectively (Figure 9c).
Figure 9.
Effects of vanilloids on LPS-induced IKK, ERK and JNK activation. Cells were lysed after treatment with agents at the concentrations as indicated for 30 min. Immunoprecipitation with IKKα, IKKβ (a), ERK (b) or JNK (c) antibody together with protein A/G-agarose beads was performed overnight. The immunoprecipitates were then equally divided into two parts; one was used for kinase assay (upper panel), using IκBα (for IKK assay), MBP (for ERK assay) or GST-c-Jun (for JNK assay) as the substrate, and the other was subjected to SDS–PAGE for immunoblotting of IKK, ERK, or JNK (lower panel). The traces are representative of three different experiments, which are presented as the mean±s.e.m. from three independent experiments. *P<0.05 as compared to the control response of LPS without vanilloid treatment.
Discussion
In this study, we find that the spice principles from the chilli pepper, capsaicin, and two vanilloid analogs can reduce iNOS/NO induction caused by LPS and IFN-γ, and COX-2/PGE2 induction caused by LPS and PMA. All these effects display concentration- and time-dependencies. From the diminished inhibition with delayed treatment of vanilloid analogs after LPS stimulation, as well as the inhibition of mRNA level of both genes, we suggest that transcriptional regulation accounts for the major mechanism for vanilloids' actions.
Although specific actions through TRPV1 receptors have been extensively demonstrated for micromolar concentration ranges of capsaicin and capsazepine, TRPV1-unrelated nonspecific actions of vanilloids have also been suggested (Docherty et al., 1997; Liu & Simon, 1997; Oh et al., 2001). Capsaicin-mediated inhibition of various enzymes, induction of pseudochannel formation in lipid bilayers, alteration of membrane fluidity, and blockade of K+ channels belong to these actions (Szallasi & Blumberg, 1999). Based on this idea, we compared the effects of capsaicin, RTX, and capsazepine with the aim of elucidating the possible role of TRPV1 in macrophages. Previous studies have shown the effectiveness of capsazepine in antagonizing both capsaicin and RTX responses in the spinal cord, sensory neuron, and trachea (Bevan et al., 1991; Ellis & Undem 1994; Szallasi & Blumberg, 1999). Our present results in macrophages, however, do not prove the ability of capsazepine to prevent capsaicin and RTX actions. In contrast, capsazepine induces similar action as capsaicin and RTX, and exhibits even twofold more potent inhibition of NO production than capsaicin. These results suggest that the inhibitory action of vanilloids on the induced NO and PGE2 formation is not mediated by TRPV1. In support of this notion, TRPV1 mRNA and protein respectively assessed by RT–PCR and immunoblotting are undetectable in RAW264.7 macrophages.
In contrast to the comparable potency for NO reduction by these vanilloids, a markedly distinction in potency profile of PGE2 inhibition was demonstrated. The potency order for inhibition of NO production (IC50 values) was capsazepine (3±1 μM)⩾capsaicin (7±1 μM)=RTX (8±2 μM). This action potency is parallel to their effects on changes in iNOS mRNA and protein levels. However, regarding COX-2 and PGE2 inhibition, the potency order (IC50 values) was capsaicin (0.2±0.1 μM)>capsazepin (4±1 μM)>>RTX (>10 μM). Currently, we do not have sufficient and direct evidence to explain the distinct potencies of these vanilloids, particularly capsaicin and RTX, in the regulation of iNOS- and COX-2-related events. The possibility for the existence of other TRPV1 subtypes, which are more specific to capsaicin than RTX, cannot be excluded. Here we considered the supply of arachidonic acid substrate for COX-2-induced metabolism and production of PGE2. Joe & Lokesh (2000) reported that animals fed with capsaicin (5 mg kg−1 body wt day−1) for 2 weeks can decrease fatty acid diet-induced eicosanoid secretion from peritoneal macrophages and decrease the incorporation of [3H]arachidonic acid in macrophage lipids. The reduced PGE2 production resulting from a smaller supply of precursor thus regulated COX-2 mRNA expression in a negative manner (Faour et al., 2001; Diaz et al., 2002). To address this point, we analyzed the effect of vanilloids on [3H]arachidonic acid incorporation into RAW264.7 cells. In this aspect, we observed no effects of capsaicin, RTX, or capsazepine, each at 10 μM, on [3H]arachidonic acid uptake in RAW264.7 macrophages within 12 h (data not shown). In addition, we asked if there is a direct effect of these vanilloids on enzyme activity of COXs. In experiments using an in vitro enzyme assay, we find that capsaicin at concentrations up to 10 μM does not alter the enzyme activities of COX-1 and COX-2 (data not shown). Another notion of whether the higher lipid solubility of capsaicin than RTX and capsazepine is related to their differential effects on COX-2 inhibition remains unclear.
In previous in vivo study where it was determined that feeding capsaicin (5 mg kg−1 body wt day−1) to rats for 15 days can lower the generation of reactive oxygen species by macrophages (Joe & Lokesh, 1994), we ask whether the changes in the cellular redox state by vanilloids contribute to their actions in iNOS and COX-2 inhibition. Accumulating studies have demonstrated that reactive oxygen species may contribute to the complex processes regulating gene expressions of iNOS and COX-2, and treatment with antioxidants lead to inhibition of the expression of both inflammatory genes (Hecker et al., 1996; Subbaramaiah et al., 1998). To clarify this concern, we performed an in vitro assay using 1,1-diphenyl-2-picryl-hydrazyl as a substrate to address the possible antioxidant ability of vanilloids. We find that in contrast to the action of antioxidant vitamin C, capsaicin, RTX and capsazepine at concentrations up to 10 μM possess no antioxidant activity (data not shown).
In previous studies, although induced iNOS and COX-2 gene expressions are regulated by several transcription factors, distinct requirements and contributions of transcriptional factors for both genes expression has been reported. For iNOS expression, NF-κB is a more important and prerequisite transcription factor than AP-1 (Chen et al., 1998; Kristof et al., 2001). Although IKK is the most crucial kinase responsible for NF-κB activation by LPS, ERK also contributes to NF-κB transactivation (Chen & Lin, 2001). On the contrary, although NF-κB and AP-1 collaborate to induce maximal transcriptional activity of COX-2 gene expression, there is redundancy in the NF-κB promoter site for regulating COX-2 transcription in LPS-treated RAW264.7 cells (Wadleigh et al., 2000; Mestre et al., 2001). Previous studies indicated that activation of the AP-1 transcription factor is sufficient for COX-2 gene expression, and this accounts for the action of PMA (Xie & Herschman, 1995; Subbaramaiah et al., 1998). Consistent to previous results demonstrating the ability of capsaicin and capsazepine to suppress NF-κB activation by diverse agents (Singh et al., 1996; Surh et al., 2000; Oh et al., 2001; Patel et al., 2002), and the ability of capsaicin to suppress AP-1 activation by PMA (Surh et al., 2000), our results showed differential potency of vanilloid analogs in the inhibition of LPS-induced NF-κB, AP-1, IKK, ERK, and JNK activations. The potency for IKK and ERK inhibition displayed relative correlations with their inhibitory effects on NF-κB and NO production. In contrast, JNK inhibition seemed to be attributed to the decreased AP-1 activity and partially explains the low sensitivity of RTX to COX-2 gene transcription. Since the significant effect on AP-1 inhibition by capsaicin (1 and 10 μM) does not correlate with the observed reduction in COX-2 protein and PGE2 production at 0.1–1 μM, other action mechanisms of capsaicin are possibly involved. Understanding actions on transcription factors other than NF-κB and AP-1, and signalling events involved in regulating COX-2 expression at the post-transcriptional and/or post-translational level requires further investigation.
In addition to the ability of NF-κB and AP-1 to regulate iNOS gene expression, the STAT1 signal transduction pathway mediated by IFN-γ is also associated with iNOS induction. In murine and rat iNOS genes, its 5′-flanking region of the promoter contains several copies of interferon response element (γ-IRE), interferon-stimulated regulatory element (ISRE), and γ-activated site (GAS) (Xie et al., 1993; Eberhardt et al., 1996). Substantial evidence has demonstrated that the transcriptional induction of iNOS by IFN-γ depends on JAK/STAT activation (Lowenstein et al., 1993; Kamijo et al., 1994). Consistent with previous studies (Riese et al., 1994; Giroux & Descoteaux, 2000), we find that although murine RAW264.7 cell line can accumulate COX-2 mRNA by LPS, IFN-γ has no such effect (data not shown). In this study, consistent with the inhibition of IFN-γ-mediated NO production, all the vanilloid analogs tested can attenuate DNA binding activity of STAT1 induced by IFN-γ. Thus, in this study, we can extend the anti-inflammatory mechanisms of vanilloids to modulation of multiple biological functions of IFN-γ. Except for stimulation of iNOS induction, several aspects of the regulatory properties of IFN-γ in the immune response include stimulation of bactericidal activity of phagocytes, stimulation of antigen presentation through class I and class II major histocompatibility complex molecules, orchestration of leukocyte–endothelium interactions, and effects on cell proliferation and apoptosis (Boehm et al., 1997).
In conclusion, we have demonstrated a novel action mechanism by which the vanilloid products of capsaicin, resiniferatoxin, and capsazepine can inhibit LPS-, IFN-γ-, and PMA-induced NO and/or PGE2 production in macrophages. These effects of vanilloids are independent of the known vanilloid receptor, but due to their ability to interrupt the NF-κB and AP-1 signalling pathways of LPS and the STAT1 signalling pathway of IFN-γ. All the results herein shed a new light on the action mechanism for the beneficial uses of natural vanilloid products as anti-inflammatory agents.
Acknowledgments
This work was supported by the grants from National Science Council of Taiwan (NSC91-2320-B002-210) and Academia Sinica (IBMS-CRC91-T05, IBMS-CRC92-T03).
Abbreviations
- AP-1
activator protein-1
- COX-2
cyclooxygenase-2
- CZP
capsazepine
- DMEM
Dulbecco's modified Eagle's medium
- DRG
dorsal root ganglion
- ELISA
enzyme-linked immunosorbent assay
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- IFN-γ
interferon-γ
- JNK
c-Jun N-terminal kinase
- IKK
IκB kinase
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MBP
myelin basic protein
- MTT
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- PGE2
prostaglandin E2
- PMA
phorbol 12-myristate 13-acetate
- RT–PCR
reverse transcription–polymerase chain reaction
- RTX
resiniferatoxin
- STAT
signal transducer and activator of transcription
- TRPV1
vanilloid receptor-1
References
- BEVAN S., HOTHI S., HUGHES G.A., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S.J., YEATS J.C. Capsazepine: a competitive antagonist of the sensory neuron excitant capsaicin. Br. J. Pharmacol. 1991;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRO T., BRODIE C., MODARRES S., LEWIN N.E.D., ACS P., BLUMBER P.M. Specific vanilloid responses in C6 rat glioma cells. Mol. Brain Res. 1998b;56:89–98. doi: 10.1016/s0169-328x(98)00033-3. [DOI] [PubMed] [Google Scholar]
- BIRO T., MAURER M., MODARRES S., LEWIN N.E., BRODIE C., ACS G., ACS P., PAUS R., BLUMBERG P.M. Characterization of functional vanilloid receptors expressed by mast cells. Blood. 1998a;91:1332–1340. [PubMed] [Google Scholar]
- BOEHM U., KLAMP T., GROOT M., HOWARD J.C. Cellular responses to interferon-γ. Annu. Rev. Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- BRAND L.M., SKARE K.L., LOOMANS M.E., RELLER H.H., SCHWEN R.J., LADE D.A., BOHNE R.L., MADDIN C.S., MOOREHEAD D.P., FANELI R. Anti-inflammatory pharmacology and mechanism of the orally active capsaicin analogs, NE-19550 and NE-28345. Agents Actions. 1990;31:329–340. doi: 10.1007/BF01997628. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., ROSEN T.A., TOMINAGA M., BRAKE A.J., JULIUS D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHANG Y.H., LEE S.T., LIN W.W. Effects of cannabinoids on LPS-stimulated inflammatory mediator release from macrophages: involvement of eicosanoids. J. Cell. BioChem. 2001;81:715–723. doi: 10.1002/jcb.1103. [DOI] [PubMed] [Google Scholar]
- CHEN B.C., CHOU C.F., LIN W.W. Pyrimidinoceptor-mediated potentiation of inducible nitric-oxide synthase induction in J774 macrophages. J. Biol. Chem. 1998;273:29754–29763. doi: 10.1074/jbc.273.45.29754. [DOI] [PubMed] [Google Scholar]
- CHEN B.C., LIN W.W. PKC- and ERK-dependent activation of IκB kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br. J. Pharmacol. 2001;134:1055–1065. doi: 10.1038/sj.bjp.0704334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELANY N.S., HURLE M., FACER P., ALNADAF T., PLUMPTON C., KINGHORN I., SEE C.G., COSTIGAN M., ANAND P., WOOLF C.J., CROWTHER D., SANSEAU P., TATE S.N. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol. Genomics. 2001;4:165–174. doi: 10.1152/physiolgenomics.2001.4.3.165. [DOI] [PubMed] [Google Scholar]
- DIAZ B.L., FUJISHIMA H., KANAOKA Y., URADE Y., ARM J.P. Regulation of prostaglandin endoperoxide synthase-2 and IL-6 expression in mouse bone marrow-derived mast cells by exogenous but not endogenous prostanoids. J. Immunol. 2002;168:1397–1404. doi: 10.4049/jimmunol.168.3.1397. [DOI] [PubMed] [Google Scholar]
- DOCHERTY R.J., YEATS J.C., PIPER A.S. Capsazepine block of voltage-gated calcium channels in adult rat dorsal root ganglion neurones in culture. Br. J. Pharmacol. 1997;121:1461–1467. doi: 10.1038/sj.bjp.0701272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBERHARDT W., KUNZ D., HUMMEL R., PFEILSCHIFTER J. Molecular cloning of the rat inducible nitric oxide synthase gene promoter. Biochem. Biophys. Res. Commun. 1996;223:752–756. doi: 10.1006/bbrc.1996.0968. [DOI] [PubMed] [Google Scholar]
- ELLIS J.L., UNDEM B.J. Inhibition by capsazepine of resinferatoxin- and capsaicin-induced contractions of guinea pig trachea. J. Pharmacol. Exp. Ther. 1994;268:85–89. [PubMed] [Google Scholar]
- FAOUR W.H., HE Y., HE Q.W., LADURANTAYE M., QUINTERO M., MANCINI A., DI BATTISTA J.A. Prostaglandin E2 regulates the level and stability of cyclooxygenase-2 mRNA through activation of p38 mitogen-activated protein kinase in interleukin-1β-treated human synovial fibroblasts. J. Biol. Chem. 2001;276:31720–31731. doi: 10.1074/jbc.M104036200. [DOI] [PubMed] [Google Scholar]
- GERTSCH J., GUTTINGER M., STICHER O., HEILMANN J. Relative quantification of mRNA levels in Jurkat T cells with RT-real time-PCR (RT-rt-PCR): new possibilities for the screening of anti-inflammatory and cytotoxic compounds. Pharm. Res. 2002;19:1236–1243. doi: 10.1023/a:1019818814336. [DOI] [PubMed] [Google Scholar]
- GIROUX M., DESCOTEAUX A. Cyclooxygenase-2 expression in macrophages: modulation by protein kinase C-α. J. Immunol. 2000;165:3985–3991. doi: 10.4049/jimmunol.165.7.3985. [DOI] [PubMed] [Google Scholar]
- HAN S.S., KEUM Y.S., CHUN K.S., SURH Y.J. Suppression of phorbol ester-induced NF-κB activation by capsaicin in cultured human promyelocytic leukemia cells. Arch. Pharmacol. Res. 2002;25:475–479. doi: 10.1007/BF02976605. [DOI] [PubMed] [Google Scholar]
- HECKER M., PREIB C., KLEMM P., BUSSE R. Inhibition by antioxidants of nitric oxide synthase expression in murine macrophages: role of nuclear factor κB and interferon regulatory factor 1. Br. J. Pharmacol. 1996;118:2178–2184. doi: 10.1111/j.1476-5381.1996.tb15660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARREAU P.H., D'ORTHO M.P., BOYER V., HARF A., MACQUIN-MAVIER I. Effects of capsaicin on the airway responses to inhaled endotoxin in the guinea pig. Am. J. Res. Crit. Care Med. 1994;149:128–133. doi: 10.1164/ajrccm.149.1.8111569. [DOI] [PubMed] [Google Scholar]
- JOE B., LOKESH B.R. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim. Biophys. Acta. 1994;1224:255–263. doi: 10.1016/0167-4889(94)90198-8. [DOI] [PubMed] [Google Scholar]
- JOE B., LOKESH B.R. Prophylactic and therapeutic effects of n-3 polyunsaturated fatty acids, capsaicin and curcumin on adjuvant induced arthritis in rats. J. Nutr. BioChem. 1997a;8:397–407. [Google Scholar]
- JOE B., LOKESH B.R. Effect of curcumin and capsaicin on arachidonic acid metabolism and lysosomal enzyme secretion by rat peritoneal macrophages. Lipids. 1997b;32:1173–1180. doi: 10.1007/s11745-997-0151-8. [DOI] [PubMed] [Google Scholar]
- JOE B., LOKESH B.R. Dietary n-3 fatty acids, curcumin and capsaicin lower the release of lysosomal enzymes and eicosanoids in rat peritoneal macrophages. Mol. Cell. BioChem. 2000;203:153–161. doi: 10.1023/a:1007005605869. [DOI] [PubMed] [Google Scholar]
- KAMIJO R., HARADA H., MATSUYAMA T., BOSLAND M., GERECITANO J., SHAPIRO D., LE J., KOH S.I., KIMURA T., GREEN S.J., MAK T.W., TANIGUCHI T., VILCEK J. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- KRISTOF A.S., MARKS-KONCZALIK J., MOSS J. Mitogen-activated protein kinases mediate activator protein-1-dependent human inducible nitric-oxide synthase promoter activation. J. Biol. Chem. 2001;276:8445–8452. doi: 10.1074/jbc.M009563200. [DOI] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganalia. Neurosci. Lett. 1997;228:29–32. doi: 10.1016/s0304-3940(97)00358-3. [DOI] [PubMed] [Google Scholar]
- LOWENSTEIN C.J., ALLEY E.W., RAVAL P., SNOWMAN A.M., SYNDER S.H., RUSSEL S.W., MURPHY W.J. Macrophage nitric oxide synthase gene: two upstream regions mediated induction by interferon-γ and lipopolysaccharide. Proc. Natl. Acad. Sci. USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MESTRE J.R., MACKRELL P.J., RIVADENEIRA D.E., SAPLETON P.P., TANABE T., DALY J.M. Redundancy in the signalling pathways and promoter elements regulating cyclooxygenase-2 gene expression in endotoxin-treated macrophages/monocytic cells. J. Biol. Chem. 2001;276:3977–3982. doi: 10.1074/jbc.M005077200. [DOI] [PubMed] [Google Scholar]
- MEZEY É., TÓTH Z.E., CORTRIGHT D.N., ARZUBI M.K., KRAUSE J.E., ELDE R., GUO A., BLUMBERG P.M., SZALLASI A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OH G.S., PAE H.O., SEO W.G., KIM N.Y., PYUN K.H., KIM I.K., SHIN M.K., CHUNG H.T. Capsazepine, a vanilloid receptor antagonist, inhibits the expression of inducible nitric oxide synthase gene in lipopolysaccharide-stimulated RAW 264.7 macrophages through the inactivation of nuclear transcription factor-kappa B. Int. ImmunoPharmacol. 2001;1:777–784. doi: 10.1016/s1567-5769(01)00012-1. [DOI] [PubMed] [Google Scholar]
- PATEL P.S., VARNEY M.L., DAVE B.J., SINGH R.K. Regulation of constitutive and induced NF-κB activation in malignant melanoma cells by capsaicin modulates interleukin-8 production and cell proliferation. J. Interferon Cytokine Res. 2002;22:427–435. doi: 10.1089/10799900252952217. [DOI] [PubMed] [Google Scholar]
- RIESE J., HOFF T., NORDHOFF A., DEWITT D.L., RESCH K., KAEVER V. Transient expression of prostaglandin endoperoxide synthase-2 during mouse macrophage activation. J. Leuk. Biol. 1994;55:476–482. doi: 10.1002/jlb.55.4.476. [DOI] [PubMed] [Google Scholar]
- SANCHO R., CALZADO M.A., DI MARZO V., APPENDINO G., MUNOZ E. Anandamide inhibits nuclear factor-κB activation through a cannabinoid receptor-independent pathway. Mol. Pharmacol. 2003;63:429–438. doi: 10.1124/mol.63.2.429. [DOI] [PubMed] [Google Scholar]
- SANCHO R., LUCENA C., MACHO A., CALZADO M.A., BLANCO-MOLINA M., MINASSI A., APPENDINO G., MUNOZ E. Immunosuppressive activity of capsaicinoids: capsiate derived from sweet peppers inhibits NF-κB activation and is a potent anti-inflammatory compound in vivo. Eur. J. Immunol. 2002;32:1753–1763. doi: 10.1002/1521-4141(200206)32:6<1753::AID-IMMU1753>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- SASAMURA T., SASAKI M., TOHDA C., KURAISHI Y. Existence of capsaicin-sensitive glutamatergic terminal in rat hypothalamus. NeuroReport. 1998;9:2045–2048. doi: 10.1097/00001756-199806220-00025. [DOI] [PubMed] [Google Scholar]
- SCHUMACHER M.A., MOFF I., SUDANAGUNTA S.P., LEVINE J.D. Molecular cloning of an N-terminal splice variant of the capsaicin receptor. J. Biol. Chem. 2000;275:2756–2762. doi: 10.1074/jbc.275.4.2756. [DOI] [PubMed] [Google Scholar]
- SINGH S., NATARAJAN K., AGGARWAL B.B. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a potent inhibitor of nuclear transcription factor-κB activation by diverse agents. J. Immunol. 1996;157:4412–4420. [PubMed] [Google Scholar]
- SUBBARAMAIAH K., CHUNG W.J., MICHALUART P., TELANG N., TANABE T., INOUE H., JANG M., PEZZUTO J.M., DANNENBERG A.J. Resveratrol inhibits cyclooxygenase-2 transcription and activity in phorbol ester-treated human mammary epithelial cells. J. Biol. Chem. 1998;273:21875–21882. doi: 10.1074/jbc.273.34.21875. [DOI] [PubMed] [Google Scholar]
- SURH Y.J., HAN S.S., KEUM Y.S., SEO H.J., LEE S.S. Inhibitory effects of curcumin and capsaicin on phorbol ester-induced activation of eukaryotic transcription factors, NF-κB and AP-1. Biofactors. 2000;12:107–112. doi: 10.1002/biof.5520120117. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M., ANNICELLI L.L., KRAUSE J.E., CORTRIGHT D.N. The cloned rat vanilloid receptor VR1 mediates both R-type binding and C-type calcium response in dorsal root ganglion neurons. Mol. Pharmacol. 1999;56:581–587. doi: 10.1124/mol.56.3.581. [DOI] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.J., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- WADLEIGH D.J., REDDY S.T., KOPP E., GHOSH S., HERSCHMAN H.R. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J. Biol. Chem. 2000;275:6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- WINTER J., BEVAN S., CAMPBELL E.A. Capsaicin and pain mechanisms. Br. J. Anaesth. 1995;75:157–168. doi: 10.1093/bja/75.2.157. [DOI] [PubMed] [Google Scholar]
- WOOD J.N. Capsaicin in the Study of Pain. San Diego, CA: Academic Press; 1993. [Google Scholar]
- XIE Q.W., WHISNANT R., NATHAN C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon γ and bacterial lipopolysaccharide. J. Exp. Med. 1993;177:1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE W., HERSCHMAN H.R. v-Src induces prostaglandin synthase 2 gene expression by activation of the c-Jun-N-terminal kinase and the c-Jun transcription factor. J. Biol. Chem. 1995;270:27622–27628. doi: 10.1074/jbc.270.46.27622. [DOI] [PubMed] [Google Scholar]