Abstract
Whole-cell patch recordings were made from substantia gelatinosa (SG) neurons in transverse lumbar spinal cord slices of 15- to 30-day-old rats.
Endomorphin 1 (EM-1) or EM-2 (⩽10 μM) hyperpolarized or induced an outward current in 26 of the 66 SG neurons. The I–V relationship showed that the peptide activates an inwardly rectifying K+ current.
EM-1 or EM-2 (0.3–10 μM) suppressed short-latency excitatory postsynaptic currents (EPSCs) and long-latency inhibitory postsynaptic currents (IPSCs) in nearly all SG neurons tested or short-latency IPSCs in six of the 10 SG neurons. [Met5] enkephalin or [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin (DAMGO) (1–10 μM) depressed EPSCs and IPSCs. EM-1 or EM-2 depressed synaptic responses without causing a significant change in holding currents or inward currents induced by glutamate.
Glutamate also evoked a short-latency outward current in five SG neurons or a biphasic current in two neurons; the outward current was blocked by tetrodotoxin (TTX, 0.3 μM) or bicuculline (10 μM). EM-1 or DAMGO (1 or 5 μM) attenuated the glutamate-evoked outward or biphasic currents in four of the seven SG neurons.
EM-1 (1 μM) reduced the frequency, but not the amplitude of miniature EPSCs or miniature IPSCs.
Naloxone (1 μM) or the selective μ-opioid receptor antagonist β-funaltrexamine (β-FNA, 25 μM) antagonized the action of EM; EM-induced hyperpolarizations persisted in the presence of the κ-opioid receptor antagonist (nor-binaltorphimine dihydrochloride, 1 μM) and/or σ-opioid receptor antagonist (naltrindole hydrochloride, 1 μM).
It may be concluded that EM acting on μ-opioid receptors hyperpolarizes a population of SG neurons by activating an inwardly rectifying K+ current, and attenuates excitatory and inhibitory synaptic currents evoked in a population of SG neurons, probably by a presynaptic site of action.
Keywords: Dorsal horn, enkephalin, μ-opioid receptors, opioid peptide, spinal cord
Introduction
Opioids inhibit neuronal activity by interacting with specific receptors located on post- and/or presynaptic membranes (North, 1992). Opioid receptors, which are all G-protein coupled, can be subdivided pharmacologically into three major types: μ, δ and κ. A fourth type of opioid receptor, which is pharmacologically distinct from other opioid receptors in its resistance to naloxone, was named ORL1 (Mollereau et al., 1994). There is a considerable interest in the pharmacology of μ-opioid receptors, because the analgesia and reinforced behavior are associated principally with the activation of μ-opioid receptors (Matthes et al., 1996). Endomorphin-1 (EM-1) and endomorphin-2 (EM-2), two tetrapeptides first isolated from bovine and human brains (Hackler et al., 1997; Zadina et al., 1997), are proposed to be the putative endogenous ligand for the μ-opioid receptors. These two peptides exhibit the highest selectivity and affinity for μ-opioid receptors among the putative endogenous opioid peptides known to date (Zadina et al., 1997). The selectivity of EM-1 and EM-2 for μ-opioid receptors is further indicated by the observations that these two ligands showed no binding to cell membranes from μ-opioid receptor-deficient mice and that they failed to stimulate [35S]GTPχS binding and inhibit adenylate cyclase (Monory et al., 2000).
Since the gate-control theory proposed by Melzack & Wall (1965), substantia gelatinosa (SG) neurons of the dorsal horn (lamina II of Rexed) are thought to play a critical role in nociceptive transmission from the periphery to the central nervous system (Kumazawa & Perl, 1978; Light et al., 1979; Cervero & Iggo, 1980; Fitzgerald, 1981). In the present context, immunoreactivity to EM-2 or EM-1 is detected in networks of fibers in the superficial layers of the rat dorsal horn (Martin-Schild et al., 1997,1999; Pierce et al., 1998; Schreff et al., 1998; Wu et al., 1999). EM-2 immunoreactivity is nearly abolished after the disruption of primary sensory afferents by mechanical (dorsal rhizotomy) or chemical (capsaicin pretreatment) means (Martin-Schild et al., 1998). Electron microscopy revealed axon terminals with dense-cored vesicles containing EM-2 immunoreactivity form asymmetrical synaptic contacts with μ-opioid receptor-immunoreactive profiles (Wang et al., 2003). Moreover, an EM-2-like substance is released upon electrical stimulation of the dorsal root entry zone in isolated rat spinal cords (Williams et al., 1999). Intrathecal injection of EM-1 or EM-2 injection produced a naloxone-sensitive antinociception as assessed by the tail-flick test and inhibited the nociceptive behavior elicited by intrathecally injected substance P (Stone et al., 1997). Intrathecal administration of EM-1 or EM-2 decreases all components of electrically evoked C-fiber responses of spinal neurons in a naloxone-reversible manner (Chapman et al., 1997). These observations indicate that the antinociceptive effects of EMs may be related to their inhibitory action on dorsal horn neurons. Our preliminary results identified two conspicuous effects of EM-1 and EM-2 on SG neurons, namely, suppression of excitatory postsynaptic potentials (EPSPs) and membrane hyperpolarization, both of which are naloxone sensitive (Wu et al., 1999).
The present study was undertaken to further characterize the cellular mechanism underlying EM-induced hyperpolarization, the site of synaptic depressant action and the pharmacologic profile of EM-1 and EM-2 relative to two other μ-opioid receptor agonists, [met5] enkephalin and [D-Ala2, N-Me-Phe4, Gly5-ol] enkephalin (DAMGO). Moreover, the results obtained with regard to the action of opioid peptides on inhibitory synaptic transmission in SG neurons vary with different investigators. For example, the μ-opioid receptor agonist DAMGO suppresses inhibitory synaptic responses evoked in SG neurons of the rat trigeminal nucleus pars caudalis (Grudt & Henderson, 1998), but not in SG neurons of the rat spinal cord (Kohno et al., 1999). We re-examined this issue by assessing the effects of opiate peptides on electrically, as well as glutamate-evoked, short-latency inhibitory postsynaptic currents (IPSCs) and on spontaneously occurring miniature IPSCs (mIPSCs).
Methods
The procedures used for obtaining 400-μm transverse lumbar spinal cord slices have been described (Wu & Dun, 1993; Wu et al., 1999). Procedures were reviewed and approved by the Institutional Animal Care and Use Committee. Briefly, 15- to 30-day-old Sprague–Dawley rats of either sex were anesthetized with urethane (1.2 g kg−1, i.p.) and decapitated. Following a laminectomy, L1–L5 segments were removed and immersed in cold Krebs solution saturated with 95% O2 and 5% CO2. After removing the pia-arachnoid membrane with a pair of fine forceps, the lumbar segment was sectioned to 400 μm thickness with the use of a Vibratome. Spinal slices with dorsal rootlets were incubated in oxygenated Krebs solution at room temperature (21±1°C) until use. One slice was placed between two pieces of nylon mesh in the recording chamber (<0.5 ml) and superfused with a Krebs solution of the following composition (in mM): 127 NaCl, 1.9 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgCl2, 26 NaHCO3 and 10 glucose; the solution was saturated with 95% O2 and 5% CO2. All experiments were performed in room temperature.
Patch pipettes were pulled from thin-walled fiber-filled borosilicate glasses (WPI, OD 2.0 mm). Pipettes filled with a solution of the following composition (in mM): 130 K+ gluconate, 1 MgCl2, 2 CaCl2, 4 ATP, 10 EGTA and 10 HEPES, had a resistance of 5–7 MΩ. In several experiments, K gluconate was replaced with cesium sulfate. Viewed under a dissecting microscope with transmitted illumination, the SG was readily discernable as a translucent band across the top of the dorsal horn. SG neurons were either ‘blind' patched or visually identified using differential interference contrast microscopy.
Signals were recorded using an Axopatch 1C (Axon Instruments, Foster City, CA, U.S.A.), low-pass filtered at 2 kHz and acquired using a PC and pCLAMP software (version 7.0, Axon Instruments) for later analysis. Signals were either displayed on a Nicolet Digital Oscilloscope and stored on diskettes for later analysis or output to a Gould pen recorder. Synaptic currents were evoked by electrical stimulation via either a bipolar concentric stimulating electrode (0.25 mm OD) (FHC, Bowdoinham, ME, U.S.A.) positioned close to the dorsal root entry zone or placed in close proximity to the recording neuron. The stimulus intensity varied from 3 to 5 V and the duration was 1–10 ms. Excitatory postsynaptic currents (EPSCs) and IPSCs were identified as being monosynaptic by their short latency (<3 ms) and/or ability to follow a high-frequency stimulation (10–20 Hz) with constant latency and absence of failures. EM and other pharmacological agents were dissolved in Krebs solution and applied to the slices by superfusion in known concentrations or by pressure ejection. Glutamate (10 mM) was applied to the neuron by pressure ejection (40 p.s.i., 10–30 ms pulse duration) with the use of a Picospritzer (General Valve Co., Fairfield, NJ, U.S.A.). EPSCs or IPSCs shown in the figures represent the average of 16–32 consecutive signals elicited at a frequency of 0.5 Hz. Data were expressed as means±s.e.m. and analyzed statistically with the use of the paired and unpaired t-test. Significance was accepted with P<0.05.
The following compounds were used: EM-1, EM-2, β-funaltrexamine, naltrindole hydrochloride and nor-binaltorphimine dihydrochloride from Tocris Cookson, Inc. (Ellisville, MO, U.S.A.); D(−)-2-amino-5-phosphonovaleric acid (APV), 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX), bicuculline methobromide, [met5] enkephalin and DAMGO from Research Biochemicals International (Natick, MA, U.S.A.); strychnine hydrochloride and L-glutamate from Sigma (St Louis, MO, U.S.A.) and naloxone hydrochloride from Endo Laboratories Inc (Garden City, NJ, U.S.A.).
Results
Whole-cell recordings in current- or voltage-clamp mode were obtained from 152 SG neurons in 152 slices. SG neurons with a resting membrane potential more negative than −50 mV and an action potential, in response to a depolarizing stimulus of 70 mV or higher, were included in the study. Neurons had a mean resting membrane potential of −63±3.8 mV (n=84) and input resistance of 1031±297 MΩ (n=41). The electrical membrane properties of SG neurons were comparable to those reported in earlier studies (Yoshimura & Nishi, 1993; Wu et al., 1999).
EM hyperpolarization
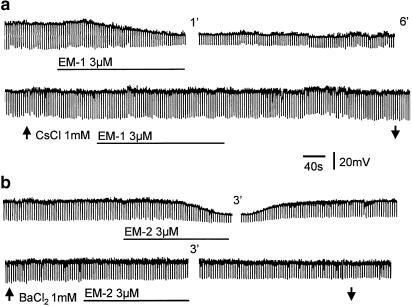
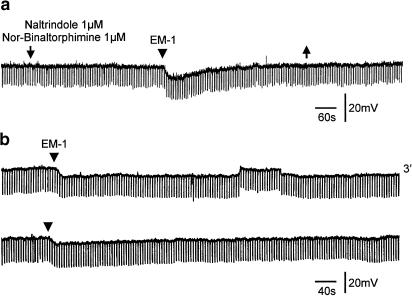
EM-1 and EM-2 (3 or 10 μM) hyperpolarized or produced an outward current in 18 of the 44 and eight of the 22 SG neurons, respectively. Hyperpolarizations, which persisted in a tetrodotoxin (TTX, 0.3 μM)–Krebs solution, varied from 3 to 15 mV, and were accompanied by a decrease in membrane resistance ranging from 30 to 70%; a representative response is shown in Figure 1. Pretreatment of the slices with the K+ channel blocker Cs+ or Ba2+ (1 mM) abolished the hyper-polarizing action of EM-1 or EM-2 (Figure 1). EM-1 or EM-2 hyperpolarizations were prevented by pretreating the slices with naloxone (1 μM) or the selective μ-opioid receptor antagonist β-funaltrexamine (β-FNA; 25 μM) (Takemori et al., 1981) in all six cells tested. EM-1 hyperpolarizations persisted in the presence of κ-opioid receptor antagonist (nor-binaltorphimine dihydrochloride, 1 μM) and/or σ-opioid receptor antagonist (naltrindole hydrochloride, 1 μM) in all six cells tested (Figure 2a). A second application of EM-1 produced a smaller (10–20%) response; an example is shown in Figure 2b, where the amplitude of the second response was 12% smaller than that of the first response.
Figure 1.
Hyperpolarization of SG neurons by EM-1 and EM-2. (a) EM-1 (3 μM) applied by superfusion produces a long-lasting hyperpolarization with a decrease in membrane resistance, which is blocked by pretreating the slice with CsCl2 (1 mM). (b) Bath application of EM-2 (3 μM) hyperpolarizes this SG neuron; the effect is reversible after wash and blocked by BaCl2 (1 mM). Numerals indicate minutes of wash.
Figure 2.
Pharmacological characteristics of EM-1-induced hyperpolarizations. (a) EM-1-induced hyperpolarization persisted in the presence of κ-opioid receptor antagonist nor-binaltorphimine dihydrochloride (1 μM) and σ-opioid receptor antagonist naltrindole hydrochloride (1 μM); the antagonists were applied between two arrows and EM-1 was applied by pressure ejection at the time indicated by the arrowhead. A control hyperpolarization (not shown) was elicited prior to the superfusion of antagonists. (b) Two consecutive applications of EM-1, as indicated by arrowheads, induced membrane hyperpolarizations, with the second response 12% smaller than the first one. Numeral indicates minutes of wash.
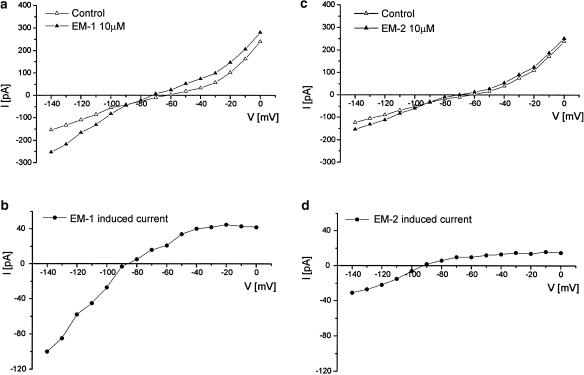
Steady-state I–V relationships of EM-1 and EM-2 were investigated in SG neurons voltage clamped to −60 mV, which is close to the resting membrane potential. The I–V relationship was obtained by applying a series of 1 s voltage command steps every 5 s from a holding potential of −60 mV to different potentials (−140–0 mV) with 10 mV increments, before and during the application of EM-1 or EM-2 (10 μM) in TTX–Krebs solution. EM-1 or EM-2 induced an outward current that showed an inward rectification and reversed at the membrane potential close to −90 mV (Figure 3).
Figure 3.
I–V relationships of EM-1- and EM-2-induced currents in two SG neurons. Panels a and c show the steady-state I–V curves obtained before (open triangle) and after (filled triangle) the application of EM-1 and EM-2. The difference between each set of I–V curves, representing the EM-induced current, is shown in panels b and d. The reversal potential of EM-1-and EM-2-induced currents is about −90 mV. All experiments were performed in TTX (0.3 μM); neurons were held at −60 mV.
Synaptic responses evoked by stimulation of dorsal root entry zone
Stimulation of the dorsal root entry zone evoked an EPSC in the great majority of SG neurons; an IPSC in eight SG neurons or a biphasic response consisted of an EPSC followed by an IPSC in 16 SG neurons. EPSCs recorded from SG neurons were judged to be monosynaptic on the basis of short latency (⩽3 ms) and their ability to follow high (10 or 20 Hz)-frequency stimulation with constant latency and no failures. EPSCs, which had an amplitude of 30–110 pA when recorded at the resting membrane potential of −60 to −70 mV, appeared to be mediated by the excitatory amino-acid glutamate acting predominantly on non-NMDA receptors, as CNQX (10 μM) nearly eliminated the EPSCs. The NMDA receptor antagonist APV (20 μM), although not significantly affecting the peak amplitude, shortened the decay phase of the EPSCs in some of the SG neurons tested. IPSCs, which had a synaptic latency of 5–15 ms and therefore di- or polysynaptic in nature, were abolished either by the glycine antagonist strychnine (1 μM) or the GABAA receptor antagonist bicuculline (10 μM) or by the combination of both antagonists (see also Yoshimura & Nishi, 1993).
Effects of EM on EPSCs
Bicuculline (10 μM) and strychnine (1 μM) were included in the perfusing Krebs solution to isolate EPSCs evoked in SG neurons pharmacologically in this series of experiments. EM-1 or EM-2 at concentrations ranging from 0.3 to 10 μM were applied to the spinal slices until a steady state of synaptic depression was reached, usually in 5–7 min. At lower concentration (0.3–3 μM), EM-1 or EM-2 reversibly depressed EPSCs without causing a significant change of holding currents in the large majority of SG neurons investigated (n=45). At higher concentrations (>3 μM), the peptide induced an outward current in five of the 12 SG neurons, and attenuated EPSCs in nearly all the cells tested.
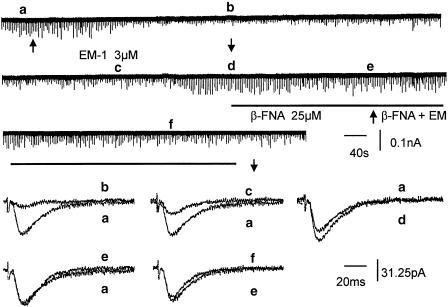
β-FNA (25 μM) antagonized the synaptic depressant effect of EM-1; an example is shown in Figure 4. The average suppression caused by EM-1 (3 μM) was 45.4±10.4% before and 11.8±5.2% (P<0.05, n=8) after pretreatment of the slices with β-FNA. Interestingly, β-FNA alone caused a small increase (104–126%) of EPSCs over the control responses in four of the eight cells tested. Naloxone (1 μM) also antagonized the synaptic inhibitory action of EM-1. The average suppression caused by EM-1 (3 μM) was 42.4±8.4% before and 12.3±2.1% after naloxone treatment (n=4). Both naloxone and β-FNA did not significantly affect the holding current.
Figure 4.
Antagonism of the synaptic depressant effect of EM by the selective μ-opioid receptor antagonist β-funaltrexamine. EM-1 (3 μM) applied by superfusion, as indicated by two arrows, depressed EPSCs to about 20% of control, without causing a change of holding current. Second application of EM-1 in the presence of β-FNA (25 μM, solid line) caused no appreciable change of EPSCs. Upper traces are continuous chart recordings. Lower traces are averaged EPSCs taken from upper traces at times marked by letters a–f. Averaged EPSCs before and after EM-1 are superimposed in a and b; a and c are averaged EPSCs before and after wash, a and d are averaged EPSCs before addition and after washout of EM-1; a and e are averaged EPSCs before first and second application of EM-1; e and f are superimposed EPSCs taken before and after application of EM-1 in the presence of β-FNA.
Effects of DAMGO and enkephalin
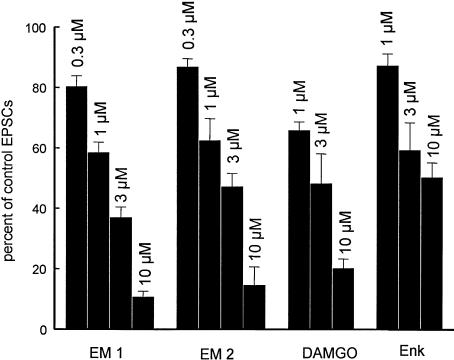
DAMGO and [met5] enkephalin have been shown to attenuate EPSCs and hyperpolarize SG neurons by interacting with pre- and postsynaptic opioid receptors (Yoshimura & North, 1983; Hori et al., 1992; Kohno et al., 1999). Here, [met5] enkephalin and DAMGO inhibited synaptic responses in a concentration-dependent manner (Figure 5). The synaptic depressant effect of [met5] enkephalin was less effective as compared to that of EM-1, EM-2 and DAMGO, and the latter three agents were about equipotent (Figure 5). DAMGO and [met5] enkephalin at lower concentrations (⩽3 μM), which depressed EPSCs, caused no significant shift of holding currents in the majority of neurons tested. At higher concentrations, [met5] enkephalin and DAMGO depressed synaptic responses and induced an outward current, the amplitude of which varied among different cells tested. The onset of synaptic depression preceded the development of outward current and the recovery of holding current preceded the recovery of EPSCs during the washout period (Figure 6).
Figure 5.
Concentration-dependent suppression of short-latency EPSCs evoked in SG neurons by EM-1, EM-2, DAMGO and [met5] enkephalin (Enk). Bars show percent inhibition of 0.3–10 μM (from left to right) of EM-1 and EM-2 and 1–10 μM DAMGO and Enk on EPSCs. Error bars indicate s.e.m. (n⩾4).
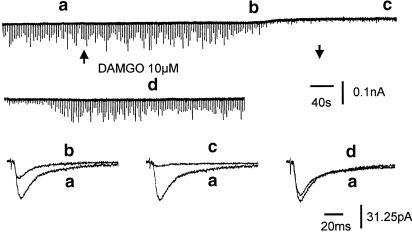
Figure 6.
Effects of DAMGO on holding current and EPSCs of an SG neuron. Superfusion of DAMGO (10 μM) reduced the EPSC amplitude to 47% of control at the time b and 12% of control at the time c, and induced a small outward current (as shown on the top chart recording). Arrows indicate the onset and offset of DAMGO superfusion. Note that synaptic depression occurred first followed by the outward current. The holding current recovered first followed by the recovery of EPSCs after a period of wash.
Effects of opioids on IPSCs
Electrical stimulation applied to the dorsal root entry zone elicited an IPSC with variable latency in eight SG neurons; the long (>5 ms) synaptic latency indicates that IPSCs were either di- or polysynaptic in nature (see Yoshimura & Nishi, 1993). EM-1 and other μ-receptor agonists consistently reduced IPSCs evoked in these neurons. Accordingly, IPSCs were reduced by an average of 62% (n=6), 67% (n=3) and 60% (n=3) by EM-1 (1 μM), DAMGO (5 μM) and [met5] enkephalin (10 μM), respectively (Figure 7).
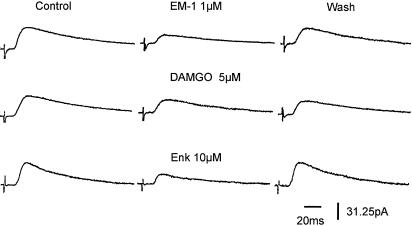
Figure 7.
Suppression of IPSCs by opioids in SG neurons of rat spinal cord. EM-1 (1 μM), DAMGO (5 μM) and [met5] enkephalin (10 μM) reduced IPSCs to 40, 57 and 45% of control in a reversible manner. The membrane potential was held at −60 mV. Recordings were obtained from three different SG neurons. IPSCs exhibited a long synaptic latency, indicating their polysynaptic nature.
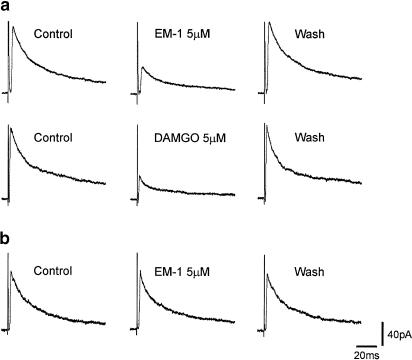
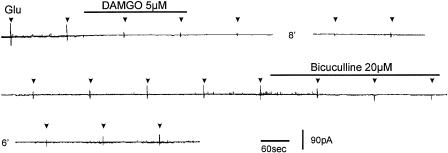
Owing to the di- or polysynaptic nature of IPSCs evoked by stimulation of dorsal root entry zone, attempts were made to evoke a short-latency IPSC by placing the stimulating electrode close to the recording SG neuron under visual guidance. A short-latency IPSC was elicited in five SG neurons. EM-1 (5 μM) or DAMGO (5 μM) reversibly suppressed IPSCs in three SG neurons (Figure 8a) and had no detectable effect on IPSCs in the remaining two neurons (Figure 8b).
Figure 8.
Suppression of short-latency IPSCs elicited in some of the SG neurons by opioids. Short-latency IPSCs were elicited by electrical stimulation close to the recording SG neuron. (a) EM-1 (5 μM) or DAMGO (5 μM) reversibly suppressed short-latency IPSCs evoked in SG neurons. (b) EM-1 (5 μM) had no detectable effect on IPSCs in this SG neuron. The membrane potential was held at 0 mV. K gluconate in the pipette solution was replaced with cesium sulfate to block the activation of K channels.
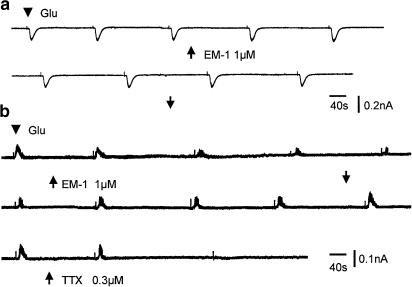
A third protocol to elicit IPSCs was by pressure ejection of glutamate to the vicinity of recording SG neurons. Glutamate elicited an inward current in 12 SG neurons, and an outward current in five SG neurons. The inward current induced by glutamate was not significantly affected by TTX, indicating that the response was due to glutamate acting directly on the SG neuron from which the recording was made. Superfusing the slices with EM-1 (1 μM) did not significantly change the amplitude of glutamate-induced inward currents or the holding currents in six neurons examined (Figure 9a). The mean amplitude in control and in the presence of EM-1 (1 μM) was 84.3±14.6 and 86±14.3 pA (n=6). The outward current could be reversibly abolished by 0.3 μM TTX (Figure 9b), suggesting that the outward current probably was caused by glycine and/or GABA released from inhibitory interneuron(s) activated by glutamate. EM-1 (1 μM) reversibly reduced glutamate outward currents in three of five SG neurons tested (Figure 9b).
Figure 9.
Effects of EM-1 on glutamate-induced currents from two different SG neurons. (a) Glutamate (10 mM solution, 10 ms pulse duration) applied by constant pressure, marked by an arrowhead, from a micropipette induced an inward current in this SG neuron. Bath application of EM-1 (1 μM) indicated by two arrows, has no detectable effect on glutamate-induced inward current in this cell. (b) Glutamate caused an outward current in this SG neuron, which is reversibly suppressed by EM-1 (1 μM). Bath application of TTX (0.3 μM) abolished the glutamate-induced outward current, indicating that glutamate depolarized an inhibitory interneuron, causing a release of inhibitory transmitters.
In two SG neurons where glutamate evoked a biphasic current, DAMGO attenuated the outward and inward components in one neuron (Figure 10). The outward current was abolished by bicuculline (20 μM) (Figure 10).
Figure 10.
Suppression of inward and outward currents induced by glutamate in this SG neuron by DAMGO. Glutamate applied by pressure close to the recording SG neuron, marked by arrowheads, evoked a biphasic current; DAMGO attenuated both the inward and outward components of this response. Additionally, the outward current was selectively abolished by bicuculline (20 μM). The membrane potential was held at −40 mV. K gluconate in the pipette solution was replaced with cesium sulfate.
Effects of EM on mEPSCs and mIPSCs
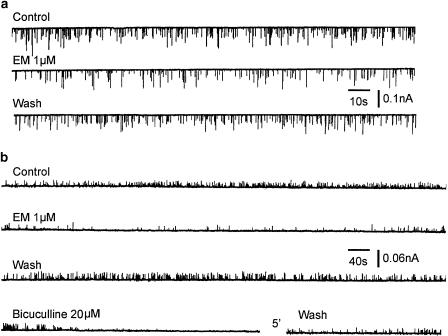
Miniature EPSCs (mEPSCs) or miniature IPSCs (mIPSCs) were recorded from SG neurons superfused with TTX (0.3 μM). The mean frequency of mEPSCs was 1.08±0.1 s−1 (n=10). mEPSCs were recorded for a period of 3 min before and 5 min after EM-1 application. In the presence of EM-1 (1 μM), the mEPSC frequency gradually decreased and approached a steady state in several minutes. After a wash period of 20 min, the mEPSC frequency recovered to the control level (Figure 11a). The mean mEPSC frequency before and after EM was 1.08±0.1 and 0.62±0.1 s−1 (n=10, P<0.01), respectively. The mean amplitude of mEPSCs was not significantly changed (30.77 vs 28.42 pA).
Figure 11.
Effects of EM-1 on miniature EPSCs and miniature IPSCs of SG neurons. (a) Chart recordings of mEPSCs, in the presence of TTX (0.3 μM), strychnine (1 μM) and bicuculline (20 μM), before, during and after the application of EM-1 (1 μM). (b) Representative recordings of mIPSCs before, during and after the application of EM-1 (1 μM), and their block by bicuculline (20 μM); the recordings were made in the presence of TTX (0.3 μM), CNQX (20 μM) and APV (10 μM).
EM-1 (1 μM) significantly depressed the frequency of mIPSCs (Figure 11b) recorded in the presence of TTX (0.3 μM), CNQX (20 μM) and APV (10 μM) in one out of four SG neurons exhibiting bicuculline (20 μM)-sensitive mIPSCs. The response recovered after a period of 20 min wash. The mean frequency of mIPSCs (counted on 10 periods of 1 min each before and during the EM-1 application) was 0.386±0.05 s−1 and decreased to 0.185±0.03 s−1 (47% of control) in the presence of EM-1. The mean amplitude of mIPSCs (counted for 100 events before and during the application of EM-1) was 19.5±0.8 pA in the control and 17.0±1.3 pA in the presence of EM-1.
Discussion
EM-1 and EM-2 were reported to be the putative endogenous ligands specific for the μ-opioid receptor (Zadina et al., 1997). The rat dorsal horn received a fairly dense innervation of EM-immunoreactive terminals, particularly EM-2 (Martin-Schild et al., 1999). An EM-2-like substance was released from the dorsal horn upon electrical stimulation of dorsal root entry zone in vitro (Williams et al., 1999). These, together with the demonstration that the rat dorsal horn is endowed with pre- and postsynaptic μ-opioid receptors (Besse et al., 1990), render the rat dorsal horn a suitable site to investigate the cellular action of EM.
EM-1 or EM-2 applied by superfusion to dorsal horn neurons in spinal slices caused two conspicuous effects: membrane hyperpolarization and attenuation of synaptic responses, similar to those reported in our earlier study (Wu et al., 1999). The ionic mechanism underlying the hyperpolarization has not been studied. EM-1 or EM-2 hyperpolarization was associated with a decrease of membrane resistance, and was eliminated by the nonselective K+ channel blocker Ba+ or Cs2+, indicating that EM hyperpolarizes SG neurons by increasing membrane permeability to K+. The response was sensitive to the μ-opioid receptor-selective antagonist β-FNA and not affected by the κ-opioid receptor antagonist nor-binaltorphimine dihydrochloride or the δ-opioid receptor antagonist naltrindole hydrochloride, suggesting that EM acted on μ-opioid receptors. The EM-induced current showed inward rectification and reversed at −90 mV, which is close to the calculated K+ equilibrium potential (−94 mV) of SG neurons under our experimental conditions. Together, our result suggests that EM-1 or EM-2 hyperpolarized SG neurons by activating an inwardly rectifying K current, similar to that reported for the activation of μ-opioid receptors on dorsal horn neurons (Grudt & Henderson 1998; Schneider et al., 1998).
EPSCs evoked in the majority of SG neurons were eliminated by CNQX. Thus, the putative transmitter mediating the dorsal root-evoked EPSCs appeared to be glutamate acting primarily on non-NMDA receptors (Yoshimura & Jessell, 1990; Yoshimura & Nishi, 1992,1993). Glutamate acting on NMDA receptors seems to mediate a part of the later component of EPSCs evoked in some SG neurons. IPSCs evoked in SG neurons were abolished by strychnine and/or bicuculline, supporting the contention that glycine and/or GABA are inhibitory transmitters to the SG neurons of the rat (Yoshimura & Nishi, 1993,1995).
EM-1, EM-2 and two other opioid receptor agonists, [met5] enkephalin and DAMGO, reversibly depressed EPSCs and IPSCs evoked by the stimulation of dorsal root entry zone of spinal cord slices. EM-1, EM-2 and DAMGO were about equipotent in suppressing excitatory synaptic transmission in dorsal horn neurons. Enkephalin, which was present in the dorsal horn (Hunt et al., 1980; Glazer & Basbaum, 1981), is the least effective of the four opioid receptor agonists examined here. As the extent of degradation of enkephalin and EM in the spinal slice is not known, the effectiveness of these two peptides, as extrapolated from the dose–response curve, may have been underestimated. As synaptic depression produced by these four opioid ligands was antagonized by the nonselective opiate receptor antagonist naloxone and the specific μ-opiate receptor antagonist β-FNA, the pharmacological profile of opioid receptors activated by these agonists are consistent with the preponderance of μ-opioid receptors in the rat superficial dorsal horn (Besse et al., 1990,1991). Although enkephalin is thought to be a more selective δ-opioid receptor agonist, the synaptic depressant effect appears to be mediated by μ-opioid receptors in the dorsal horn (Hori et al., 1992).
In the rat dorsal horn, μ-opioid receptors are distributed post- and presynaptically (Besse et al., 1990; Ding et al., 1996). Our results were consistent with a presynaptic inhibitory effect of EM on glutamatergic, glycinergic and/or GABAergic inputs to SG neurons. First, opioids including EM in concentrations attenuated synaptic responses, caused little or no change of holding currents. At higher concentrations, EM caused an outward current as well as synaptic depression. The synaptic depression and outward current exhibited different time courses, suggesting that the mechanisms underlying these two events differ. Second, while decreasing the EPSCs, EM had minimal effects on inward currents induced by glutamate, indicating that the synaptic depression cannot be attributed to a decrease of postsynaptic glutamate receptor sensitivity. Third, EM, in addition to depressing EPSCs and IPSCs, reduced the frequency of mEPSCs and mIPSCs without affecting their amplitude. As recordings were conducted in the presence of TTX, EM likely suppressed transmitter release by interacting with presynaptic opioid receptors. Collectively, our finding is in agreement with several studies that opiates, including EM, suppress synaptic transmission by a presynaptic inhibitory action in the rat dorsal horn (Hori et al., 1992; Glaum et al., 1994; Kohno et al., 1999; Wu et al., 1999).
It is worth noting that β-FNA alone increased the EPSC amplitude in four of eight SG neurons. Together with our previous observation that electrical stimulation evoked a release of EM-2-like substances from the dorsal horn of isolated rat spinal cords (Williams et al., 1999), the result suggests that μ-opioid receptors located on nerve endings may be under the influence of EM released endogenously within the superficial dorsal horn. As a corollary, μ-opioid receptor antagonists may enhance synaptic transmission by disinhibition.
Grudt & Henderson (1998) and more recently Moran & Smith (2002) reported that the μ-opioid receptor agonist DAMGO attenuated, respectively, IPSCs in SG neurons of trigeminal nucleus pars caudalis and spinal cord. The inability of DAMGO to suppress evoked IPSCs evoked or spontaneously occurring mIPSCs in SG neurons of the rat spinal cord led Kohno et al. (1999) to conclude that μ-opioid receptors are absent from inhibitory interneurons in the rat superficial dorsal horn. In our study, short-latency IPSCs were elicited by electrical or chemical stimulation applied close to the recording neurons. IPSCs, sensitive to bicuculline and/or strychnine, were suppressed by μ-opioid receptor agonists (EM, DAMGO and [met5] enkephalin) in six of the 10 SG neurons, while mIPSCs were depressed by EM in one of four SG neurons, suggesting that a subset of inhibitory interneurons in the superficial dorsal horn are endowed with μ-opioid receptors. The percentage of dorsal horn GABA-containing inhibitory interneurons expressing μ-opioid receptor immunoreactivity is reported to be about 6%, and they occur in the medial half of the dorsal horn (Kemp et al., 1996). Our result, albeit derived from a small sample, suggests a much higher percentage of the μ-opioid receptor bearing inhibitory interneurons in the rat dorsal horn. The fact that only a small percentage of inhibitory interneurons are endowed with μ-opioid receptors (Kemp et al., 1996) may explain the negative result of Kohno et al. (1999) where none of the dorsal horn neurons randomly selected in their study received inhibitory inputs from μ-opioid receptor bearing interneurons. The age of experimental animals used in these two studies may also contribute to the observed differences. The density of μ-opioid receptors appears to undergo developmental changes in the rat such that there were more μ-opioid receptors in the early postnatal periods as compared to the adult stage (Attali et al., 1990). Younger (15–30 days old) rather than adult (Kohno et al., 1999) rats were used in our study.
The mechanism whereby EM may suppress excitatory and/or inhibitory transmitter liberation is not known. Opiates may inhibit adenylate cyclase activity via G-protein-coupled receptors. Decreasing the level of cAMP causes a reduction in the cAMP-dependent protein kinase activity, which in turn may inhibit the phosphorylation of synapsins I and II (Childers et al., 1992). Whether or not EM may block voltage-gated Ca2+ channels in presynaptic nerve terminals through the activation of intracellular messengers remains to be elucidated. EM inhibits high-threshold Ca2+ currents in rodent NG108-15 cells overexpressing μ-opioid receptors (Higashida et al., 1998). Alternatively, the opioid peptide may decrease transmitter release by increasing K+ conductance of the presynaptic membrane.
A recent study suggests that the pharmacology of EM-1 and EM-2, as assessed by paw-withdrawal behavior in mice, differs in their sensitivity to opioid receptor antagonists (Sakurada et al., 2001). Future study with selective antagonists to subtypes of μ-opioid receptor may be needed to dissect out the pharmacological specificity of EM-1 and EM-2. Our result indicates that the cellular action of EM-1 and EM-2 on rat dorsal horn neurons is qualitatively similar.
Acknowledgments
This study was supported by NIH Grants NS 39646 and NS18710 from the Department of Health and Human Services.
Abbreviations
- DAMGO, [D-Ala2, N-Me-Phe4, Gly5-ol]-enkephalin∣EM-1
endomorphin 1
- EM-2
endomorphin 2
- EPSCs
excitatory postsynaptic currents
- β-FNA
β-funaltrexamine
- IPSCs
inhibitory postsynaptic currents
- mEPSCs
miniature EPSCs
- mIPSCs
miniature IPSCs
- SG
substantia gelatinosa
- TTX
tetrodotoxin
References
- ATTALI B., SAYA D., VOGEL Z. Pre- and postnatal development of opiate receptor subtypes in rat spinal cord. Dev. Brain Res. 1990;53:97–102. doi: 10.1016/0165-3806(90)90128-l. [DOI] [PubMed] [Google Scholar]
- BESSE D., LOMBARD M.C., BESSON J.M. Autoradiographic distribution of mu, delta and kappa opioid binding sites in the superficial dorsal horn, over the rostrocaudal axis of the rat spinal cord. Brain Res. 1991;548:287–291. doi: 10.1016/0006-8993(91)91134-m. [DOI] [PubMed] [Google Scholar]
- BESSE D., LOMBARD M.C., ZAJAC J.M., ROQUES B.P., BESSON J.M. Pre- and postsynaptic distribution of μ, δ and κ opioid receptors in the superficial layers of cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- CERVERO F., IGGO A. The substantia gelatinosa of the spinal cord. J. Neurol. 1980;103:717–772. doi: 10.1093/brain/103.4.717. [DOI] [PubMed] [Google Scholar]
- CHAPMAN V., DIAZ A., DICKENSON A.H. Distinct inhibitory effects of spinal endomorphin-1 and endomorphin-2 on evoked dorsal horn neuronal responses in the rat. Br. J. Pharmocol. 1997;122:1537–1539. doi: 10.1038/sj.bjp.0701594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHILDERS S.R., FLEMING L., KONKOY C., MARCKEL D., PACHECO M., SEXTON T., WARD S. Opioid and cannabinoid receptor inhibition of adenylyl cyclase in brain. Ann. NY Acad. Sci. 1992;654:33–51. doi: 10.1111/j.1749-6632.1992.tb25954.x. [DOI] [PubMed] [Google Scholar]
- DING Y.-Q., KANEKO T., NOMURA S., MIZUNO N. Immunohistochemical localization of μ-opiate receptors in the central nervous system of the rat. J. Comp. Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- FITZGERALD M. A study of the cutaneous afferent input to the substantia gelatinosa. Neuroscience. 1981;6:2229–2237. doi: 10.1016/0306-4522(81)90011-7. [DOI] [PubMed] [Google Scholar]
- GLAUM S.R., MILLER R.J., HAMMOND D.L. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. J. Neurosci. 1994;14:4965–4971. doi: 10.1523/JNEUROSCI.14-08-04965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAZER E.J., BASBAUM A.I. Immunohistochemical localization of leucine-enkephalin in the spinal cord of cat: enkephalin containing marginal neurons and pain modulation. J. Comp. Neurol. 1981;196:377–389. doi: 10.1002/cne.901960303. [DOI] [PubMed] [Google Scholar]
- GRUDT T.J., HENDERSON G. Glycine and GABAA receptor-mediated synaptic transmission in rat substantia gelatinosa: inhibition by mu-opioid and GABAB agonists. J. Physiol. 1998;507:473–483. doi: 10.1111/j.1469-7793.1998.473bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HACKLER L., ZADINA J.E., GE L.J., KASTIN A.J. Isolation of relatively large amounts of endogenous endomorphin-1 and endomorphin-2 from human brain cortex. Peptides. 1997;18:1635–1639. doi: 10.1016/s0196-9781(97)00259-3. [DOI] [PubMed] [Google Scholar]
- HIGASHIDA H., HOSHI N., KNIJNIK R., ZADINA J.E., KASTIN A.J. Endomorphins inhibit high-threshold Ca2+ channel currents in rodent NG108-15 cells overexpressing mu-opioid receptors. J. Physiol. 1998;507:71–75. doi: 10.1111/j.1469-7793.1998.071bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORI Y., ENDO K., TAKAHASHI T. Presynaptic inhibitory action of enkephalin on excitatory transmission in superficial dorsal horn. J. Physiol. 1992;450:673–685. doi: 10.1113/jphysiol.1992.sp019149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT S.P., KELLY J.S., EMSON P.C. The electron microscopic localization of methionine–enkephalin within the superficial layers (I and II) of spinal cord. Neuroscience. 1980;5:1871–1890. doi: 10.1016/0306-4522(80)90036-6. [DOI] [PubMed] [Google Scholar]
- KEMP T., SPIKE R.C., WATT C., TODD A.J. The mu-opioid receptor (MOR1) is mainly restricted to neurons that do not contain GABA or glycine in the superficial dorsal horn of the rat spinal cord. Neuroscience. 1996;75:1231–1238. doi: 10.1016/0306-4522(96)00333-8. [DOI] [PubMed] [Google Scholar]
- KOHNO T., KUMAMOTO E., HIGASHI H., SHIMOJI K., YOSHIMURA M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J. Physiol. 1999;518:803–813. doi: 10.1111/j.1469-7793.1999.0803p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAZAWA T., PERL E.R. Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: indications of their place in dorsal horn organization. J. Comp. Neurol. 1978;186:133–150. doi: 10.1002/cne.901770305. [DOI] [PubMed] [Google Scholar]
- LIGHT A.R., TREVINO D.L., PERL E.R. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J. Comp. Neurol. 1979;186:151–172. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- MARTIN-SCHILD S., GERALL A.A., KASTIN A.J., ZADINA J.E. Endomorphin-2 is an endogenous opioid in primary sensory afferent fibers. Peptides. 1998;19:1783–1789. doi: 10.1016/s0196-9781(98)00136-3. [DOI] [PubMed] [Google Scholar]
- MARTIN-SCHILD S., GERALL A.A., KASTIN A.J., ZADINA J.E. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J. Comp. Neurol. 1999;405:450–471. [PubMed] [Google Scholar]
- MARTIN-SCHILD S., ZADINA J.E., GERALL A.A., VIGH S., KASTIN A.J. Localization of endomorphin-2-like immunoreactivity in the rat medulla and spinal cord. Peptides. 1997;18:1641–1649. doi: 10.1016/s0196-9781(97)00320-3. [DOI] [PubMed] [Google Scholar]
- MATTHES H.W.D., MALDONADO R., SIMONIN F., VALVERDE O., SLOWE S., KITCHEN I., BEFORT K., DIERICH A., LE MEUR M., DOLLE P., TZAVARA E., HANOUNE J., ROQUE B.P., KIEFFER B.L. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- MELZACK R., WALL P.D. Pain mechanism: a new story. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- MOLLEREAU C., PARMENTIER M., MAILLEUX P., BUTOUR J.L., MOISAND C., CHALON P., CAPUT D., VASSART G., MEUNIER J.C. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- MONORY K., BOURIN M., SPETEA M., TÖMBÖLY C., TÓTH G., MATHES H.W., KIEFFER B.L., HANOUNE J., BORSODI A. Specific activation of the μ opioid receptor (MOR) by endomorphin 1 and endomorphin 2. Eur. J. Neurosci. 2000;12:577–584. doi: 10.1046/j.1460-9568.2000.00936.x. [DOI] [PubMed] [Google Scholar]
- MORAN T.D., SMITH P.A. Morphine-3-β-D-glucuronide suppresses inhibitory synaptic transmission in rat substantia gelatinosa. J. Pharmacol. Exp. Ther. 2002;302:568–576. doi: 10.1124/jpet.102.035626. [DOI] [PubMed] [Google Scholar]
- NORTH R.A. Cellular actions of opiates and cocaine. Ann. NY Acad. Sci. 1992;654:1–6. doi: 10.1111/j.1749-6632.1992.tb25951.x. [DOI] [PubMed] [Google Scholar]
- PIERCE T.L., GRAHEK M.D., WESSENDORF M.W. Immunoreactivity for endomoephin-2 occurs in primary afferents in rats and monkey. Neuroreport. 1998;9:385–389. doi: 10.1097/00001756-199802160-00005. [DOI] [PubMed] [Google Scholar]
- SAKURADA S., HAYASHI T., YUHKI M., ORITO T., ZADINA J.E., KASTIN A.J., FUJIMURA T., MURAYAMA K., SAKURADA C., SAKURADA T., NARITA M., SUZUKI T., TAN-NO K., TSENG L.F. Differential antinociceptive effects induced by intrathetically administered endomorphin-1 and endomorphin-2 in the mouse. Eur. J. Pharmacol. 2001;427:203–210. doi: 10.1016/s0014-2999(01)01238-9. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER S.P., ECKERT W.A., III, LIGHT A.R. Opioid-activated postsynaptic, inward rectifying potassium currents in whole cell recordings in substantia gelatinosa neurons. J. NeuroPhysiol. 1998;80:2954–2962. doi: 10.1152/jn.1998.80.6.2954. [DOI] [PubMed] [Google Scholar]
- SCHREFF M., SCHULZ S., WIBORNY D., HOLLT V. Immunofluorescent identification of endomorphin-2-containing nerve fibers and terminals in the rat brain and spinal cord. Neuroreport. 1998;9:1031–1034. doi: 10.1097/00001756-199804200-00014. [DOI] [PubMed] [Google Scholar]
- STONE L.S., FAIRBANKS C.A., LAUGHLIN T.M., NGUYEN H.O., BUSHY T.M., WESSENDORF M.W., WILCOX G.L. Spinal analgesic actions of the new endogenous opioid peptides endomorphin-1 and -2. Neuroreport. 1997;8:3131–3135. doi: 10.1097/00001756-199709290-00025. [DOI] [PubMed] [Google Scholar]
- TAKEMORI A.E., LARSON D.L., PORTOGHESE P.S. The irreversible narcotic antagonistic and eversible agonistic properties of the fumaramate methyl ester derivatives of natrexone. Eur. J. Pharmacol. 1981;70:445–451. doi: 10.1016/0014-2999(81)90355-1. [DOI] [PubMed] [Google Scholar]
- WANG Q.P., ZADINA J.E., GUAN J.L., SHIODA S. Morphological evidence of endomorphin as an agonist for the mu-opioid receptor in the rat spinal cord. Neurosci. Lett. 2003;341:107–110. doi: 10.1016/s0304-3940(03)00182-4. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C.A., WU S.Y., DUN S.L., KWOK E.H., DUN N.J. Release of endomorphin-2 like substances from the rat spinal cord. Neurosci. Lett. 1999;273:25–28. doi: 10.1016/s0304-3940(99)00613-8. [DOI] [PubMed] [Google Scholar]
- WU S.Y., DUN N.J. Excitatory amino acids depress synaptic currents in neonate rat sympathetic preganglionic neurons. J. NeuroPhysiol. 1993;69:2030–2038. doi: 10.1152/jn.1993.69.6.2030. [DOI] [PubMed] [Google Scholar]
- WU S.Y., DUN S.L., WRIGHT M.T., CHANG J.-K., DUN N.J. Endomorphin-like immunoreactivity in the rat dorsal horn and inhibition of substantia gelatinosa neurons in vitro. Neuroscience. 1999;89:317–321. doi: 10.1016/s0306-4522(98)00570-3. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA M., JESSELL T.M. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurons in the rat spinal cord. J. Physiol. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIMURA M., NISHI S. Excitatory amino acid receptors involved in primary afferent-evoked polysynaptic EPSCs of substantia gelatinosa neurons in the adult rat spinal cord slice. Neurosci. Lett. 1992;143:131–134. doi: 10.1016/0304-3940(92)90249-7. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA M., NISHI S. Blind patch-clamp recording from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53:519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]
- YOSHIMURA M., NISHI S. Primary afferent-evoked glycine- and GABA-mediated IPSPs in substantia gelatinosa neurons in the rat spinal cord in vitro. J. Physiol. 1995;482:29–38. doi: 10.1113/jphysiol.1995.sp020497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIMURA M., NORTH R.A. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983;305:529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]
- ZADINA J.E., HACKLER L., GE L.J., KASTIN A.J. A potent and selective endogenous agonist for the mu-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]