Abstract
We previously demonstrated that a balance of Ca2+-activated Cl− current (ICl(Ca)) and K+ current activity sets the resting membrane potential of opossum lower esophageal sphincter (LES) circular smooth muscle at ∼−41 mV, which leads to continuous spike-like action potentials and the generation of basal tone. Ionic mechanisms underlying this basal ICl(Ca) activity and its nitrergic regulation remain unclear. Recent studies suggest that spontaneous Ca2+ release from sarcoplasmic reticulum (SR) and myosin light chain kinase (MLCK) play important roles. The current study investigated this possibility. Conventional intracellular recordings were performed on circular smooth muscle of opossum LES. Nerve responses were evoked by electrical square wave pulses of 0.5 ms duration at 20 Hz.
In the presence of nifedipine (1 μM), substance P (1 μM), atropine (3 μM) and guanethidine (3 μM), intracellular recordings demonstrated a resting membrane potential (MP) of −38.1±0.7 mV (n=25) with spontaneous membrane potential fluctuations (MPfs) of 1–3 mV. Four pulses of nerve stimulation induced slow inhibitory junction potentials (sIJPs) with an amplitude of 6.1±0.3 mV and a half-amplitude duration of 1926±147 ms (n=25).
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), a specific guanylyl cyclase inhibitor, abolished sIJPs, but had no effects on MPfs. Caffeine, a ryanodine receptor agonist, hyperpolarized MP and abolished sIJPs and MPfs. Ryanodine (20 μM) inhibited the sIJP and induced biphasic effects on MP, an initial small hyperpolarization followed by a large depolarization. sIJPs and MPfs were also inhibited by cyclopiazonic acid, an SR Ca2+ ATPase inhibitor. Specific ICl(Ca) and MLCK inhibitors hyperpolarized the MP and inhibited MPfs and sIJPs.
These data suggest that (1) spontaneous release of Ca2+ from the SR activates ICl(Ca), which in turn contributes to resting membrane potential; (2) MLCK is involved in activation of ICl(Ca); (3) inhibition of ICl(Ca) is likely to underlie sIJPs induced by nitrergic innervation.
Keywords: Sarcoplasmic reticulum, ryanodine receptors, myosin light chain kinase, nitric oxide, Ca2+-activated Cl− channels, niflumic acid, wortmannin, lower esophageal sphincter, intracellular microelectrode recordings
Introduction
Circular smooth muscle of lower esophageal sphincter (LES) is characterized by spontaneously generated basal tone, which creates a pressure barrier at the gastroesophageal junction to prevent reflux of gastric contents into the esophagus. The abnormal dynamics of LES function have been considered to be the most important factors in the pathogenesis of gastroesophageal reflux disease (Goyal & Paterson, 1989). Basal tone of LES is considered to be largely myogenic in origin as it can occur without neuronal and circulating factors (Goyal & Paterson, 1989). However, the ionic mechanism for this myogenic tone and its nitrergic regulation is incompletely understood.
We previously demonstrated that in opossum LES smooth muscle, the K+ channel blockers tetraethylammonium (TEA) and 4-aminopyridine (4-AP) enhanced, whereas the Ca2+-activated Cl− current (ICl(Ca)) blocker, niflumic acid (NFA), decreased basal tone. Conventional intracellular recordings revealed that basal tone was associated with a relatively more positive membrane potential (MP) of ∼−41 mV and ongoing, spike-like action potentials, which were potentiated by K+ channel blockers and attenuated by ICl(Ca) blockers (Zhang et al., 2000). This suggests that opposing activity of K+ and Cl− channels set the MP at a more positive level compared to the adjacent esophageal body (Zhang & Paterson, 2002), which in turn activates L-type Ca2+ channels, leading to ongoing action potentials and the generation of resting tone. Patch-clamp studies showed that delayed rectifier and Ca2+-activated large conductance K+ channels (BK(Ca)) were the major K+ channels in the LES (Zhang & Paterson, 2001). Furthermore, our recent observation in circular smooth muscle of opossum esophageal body suggests that basal activity of the ICl(Ca), which requires myosin light chain kinase (MLCK), also contributes to resting MP, and that suppression of the ICl(Ca) by nitrergic innervation leads to the slow inhibitory junction potential (sIJP). However, a mechanism for genesis of basal activity of ICl(Ca) remains undefined.
Nelson et al. (1995) showed that a local increase in intracellular Ca2+, which presumably results from spontaneous release of Ca2+ from the sarcoplasmic reticulum (SR), appears to mediate hyperpolarization of vascular smooth muscle via activation of BK(Ca) channels. Furthermore, ZhuGe et al. (1998) using freshly dissociated guinea-pig tracheal myocytes reported that depending on membrane potential, Ca2+ release from SR triggers either spontaneous transient inward currents (STICS) due to ICl(Ca), or spontaneous transient outward currents (STOCS), via Ca2+-activated large conductance K+ current (IBK(Ca)). This results in biphasic currents, with the outward phase always preceding the inward. These observations imply that spontaneous Ca2+ release plays an important role in the genesis of basal activity of ion channels in smooth muscle. We hypothesize that in LES circular smooth muscle, spontaneous Ca2+ release from the SR activates ICl(Ca) via MLCK, which maintains resting MP at a relatively more positive level, and that inhibition of the basal activity of ICl(Ca) by nitrergic innervation produces the sIJP. The current study was designed to test this hypothesis.
Methods
Tissue preparation
The protocols were approved by the Animal Care Committee of Queen's University. Opossums (Didelphis Virginiana) of either sex and weighing between 2.5 and 5 kg were anesthetized by tail vein injection of sodium phenobarbital (40 mg kg−1). The chest and abdominal cavities were then exposed via a midline incision, and the lower part of esophagus and a short segment of attached stomach were removed and placed in preoxygenated Kreb's solution. The opossum was then killed by intracardiac injection of sodium phenobarbital. The lower part of esophagus and esophago-gastric junction were opened longitudinally and pinned out with mucosa side up in a dissecting dish. Using a binocular microscope, the mucosa and connective tissue layers were carefully removed by sharp dissection. The LES was visible as a distinct thickening of circular muscle in the resultant tissue, located just on the gastric side of the squamocolumnar junction (Sengupta et al., 1987). A sheet of LES of about 2 × 3 × 15 mm was excised for intracellular recordings.
Electrical recordings
Conventional intracellular microelectrode recording techniques were employed to study the electrical properties of LES circular smooth muscle. The sheet of LES tissue was pinned on the silicon-coated bottom of a 2 ml electrophysiological recording chamber mounted on the stage of an Olympus IX-70 inverted microscope (Olympus, Japan). The chamber was continuously perfused at 2.1 ml min−1 with prewarmed and preoxygenated Kreb's solution and maintained at 35°C. Nifedipine (1 μM), atropine (3 μM), quanethidine (3 μM) and SP (1 μM) were included in the Kreb's solution. SP desensitization was tested by short-duration exposure (4 min) to additional 1 μM SP, to ensure that the excitatory effect of this agent was no longer present. The tissue was allowed to equilibrate for 2 h prior to the experiment. Glass microelectrodes were pulled using a vertical microelectrode puller (Model P-87, Sutter Instrument, U.S.A.) and filled with 3 M KCl. Microelectrode resistance was 50–70 MΩ. The microelectrode was positioned for the impalement under the guidance of the inverted microscope. The criterion for acceptance of a successful impalement was a sharp voltage drop on penetration that was maintained for at least 2 min. Silver wire electrodes, placed on either side of the muscle strip, were used to electrically stimulate intramural nerves using square wave pulses of 0.5 ms duration and 80 V generated by a Grass S88 Stimulator (Grass Instrument Co., MA, U.S.A.). Transmembrane potential was amplified and measured with an intracellular electrometer (Model IE-210, Warner Instrument Corporation, U.S.A.). An agar bridge (2% agar in 3 M KCl) was used to minimize junction potentials. MP was calibrated upon withdrawal of the microelectrode from the impaled cell. The output of the signal was displayed on an oscilloscope (Tektronix Model 5103N,Tektronix, U.S.A.) and coupled to the Axon Digidata-1200 acquisition system (Axon Instruments, U.S.A.). Data were digitized at a frequency of 500 Hz and stored in a Pentium computer for later analysis using Axon Scope 7.0 software (Axon Instruments, U.S.A.). The following parameters were used to quantitatively analyze the electrical properties of LES circular smooth muscle: (1) MP; (2) variance of MP fluctuations (MPfs) obtained by calculating the standard deviation of a 12 s MP sample; (3) amplitude of the sIJP; and (4) duration of the sIJP at 1/2 amplitude.
Solutions and drugs
The modified Kreb's solution contained (mM) NaCl 118.07, NaHCO3 25.00, D(+)-glucose 11.10, KCl 4.69, CaCl2 2.52, MgSO4 1.00 and NaH2PO4 1.01. NFA was purchased from ICN Biochemicals Inc., ryanodine from Molecular Probes Inc. and all others from Sigma. Nifedipine, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), cyclopiazonic acid (CPA), ryanodine, NFA, 9-anthroic acid (A-9-C), 1-(5-chloronaphthalene-1-sulfonyl)-1-H-hexahydro-1,4-diazepine (ML-9) and wortmannin were dissolved in dimethyl sulfoxide (DMSO) as stock solutions, and others in distilled water. These were diluted to final concentrations with Kreb's solution. Final concentration of DMSO in Kreb's solution was no more than 1%, which did not produce any effect on the electrical activity of the tissue. The Kreb's solution containing diluted drugs was fully bubbled with 5% CO2 + 95% O2 to restore pH prior to the application.
Statistical analysis
Data are shown as mean±s.e. n refers to number of animals. If more than one cell was successfully impaled in the preparation of an animal, parameters would be averaged. Only recordings in which a full protocol was completed in the same cell are included in the statistical analysis. Pre- and postdrug comparisons were made using the paired Student's t-test, and a P-value of <0.05 was considered statistically significant.
Results
General resting membrane electrical properties
Under the nonadrenergic and noncholinergic (NANC) conditions (atropine 3 μM and guanethidine 3 μM), nifedipine (1 μM) was used to immobilize muscle contraction and stabilize the intracellular impalements, while SP (1 μM) desensitization was used to eliminate neurokinin responses. Intracellular recordings revealed a resting MP of −38.1±0.7 mV (n=25) that was characterized by spontaneous MPfs of 1–3 mV (Figure 1A, a). MPfs were quantitated by calculating the standard deviation of MP sample over a 12 s recording period. The resulting variance value was 0.44±0.03 mV (n=25). Electrical nerve stimulation with four square wave pulses (0.5 ms, 20 Hz, 80 V) evoked sIJPs with a mean amplitude of 6.1±0.3 mV and a half-amplitude duration of 1926±147 ms (n=25). ODQ (10 μM, 10 min), a specific guanylyl cyclase inhibitor, decreased the amplitude of sIJPs to 0.5±0.1 mV from 6.5±1.3 mV (n=3, P<0.05), but had no effect on resting MP and MPfs (Figure 1B), demonstrating that a cGMP-dependent signal cascade is involved in the sIJP. The inhibition of the sIJP by ODQ was reversed 60 min after washing out.
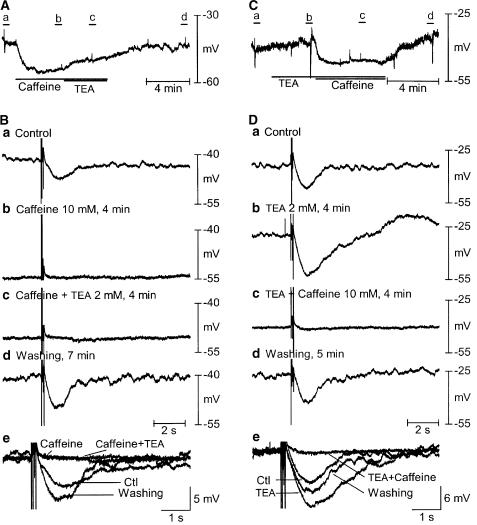
Figure 1.
Effects of pharmacological intervention on MPfs and MP. A (a, b) Snapshots of raw recordings of MPfs before and after application of caffeine (10 mM). (B) Histograms demonstrated statistical analysis of effects of pharmacological intervention on MPfs (a) and MP (b) (n=3–8). Neither ODQ (10 μM), a specific guanylyl cyclase inhibitor, nor 8-Br-cAMP (1 mM), a membrane-permeable cAMP analog, significantly affected MPf and MP. TEA (2 mM), a BK(Ca) blocker, depolarized MP. SR function inhibitors (caffeine 10 mM; CPA 10 μM), ICl(Ca) blockers (A-9-C 2 mM, NFA 300 μM) and MLCK inhibitors (ML-9 200 μM, wortmannin 30 μM) greatly suppressed MPfs. Both of the ICl(Ca) blockers and MLCK inhibitors hyperpolarized MP. Caffeine hyperpolarized, but CPA depolarized, MP.
Effects of caffeine, ryanodine and CPA on MP and sIJP
Caffeine has been reported to be an agonist of ryanodine receptors in the SR of different smooth muscles (Janssen & Sims, 1994; Wang et al., 1996; 1997; Cotton et al., 1997; Curtis & Scholfield, 2001). Application of caffeine causes Ca2+ release from SR, but with continuous application, Ca2+ stores are eventually depleted. Caffeine (10 mM) hyperpolarized MP by 11.6±2.7 mV (n=6, P<0.05) and abolished MPfs and the sIJP (amplitude of sIJP 0.3±0.3 mV versus control of 5.6±0.5 mV, n=6, P<0.05) 5 min after application (Figures 1 and 2A). The inhibitory effects reached a peak within a minute and reversed completely within 5 min of washing. In arteriolar and gallbladder smooth muscle, it was previously reported that Ca2+ release from SR activated BK(Ca) channels, resulting in membrane hyperpolarization (Nelson et al., 1995; Pozo et al., 2002). We therefore used TEA, a putative BK(Ca) channel blocker, to test this possibility in consecutive experiments. In the presence of caffeine (10 mM), TEA produced only slight depolarization (2.1±1.0 mV; n=3). Administration of TEA alone induced MP depolarization of 2.2±0.5 mV over control and potentiated the sIJP amplitude from 7.3±0.6 to 10.6±1.1 mV (n=8, P<0.05) (Figure 2C and D), but did not affect MPfs (Figure 1B). However, the concomitant application of caffeine (10 mM) still abolished MPfs and the sIJP, and hyperpolarized MP by 8.2±1.2 mV (n=5), a degree of hyperpolarization that was not significantly different from that induced by caffeine alone. Caffeine has also been reported to be a potent inhibitor of cAMP phosphodiesterase in different tissues (Rivedal & Sanner, 1985; Riley & Barclay, 1990; Tesarik et al., 1992; Pozo et al., 2002), resulting in intracellular cAMP accumulation, which activates protein kinase C-dependent signal cascade. A membrane-permeable cAMP analog, 8-bromo-cAMP (8-Br-cAMP), was used to exclude this possibility. Bath application of 8-Br-cAMP (1 mM) for up to 10 min did not evoke any measurable alterations of electrical parameters, but TEA (2 mM) significantly potentiated the sIJP in the presence of 8-Br-cAMP (n=3, Figure 1B).
Figure 2.
Effects of combined application of caffeine and TEA on the sIJP. sIJPs were evoked by four square wave electrical pulses of 0.3 ms duration at 20 Hz. (A, C) Original recordings of combined application of caffeine (10 mM) with TEA (2 mM) (A) or vice versa (C). (B, D) Original recordings labelled in (A, C) before (a), during (b, c) and after (d) combined application of caffeine and TEA, respectively. (e) Superimposed sIJPs using the periods prior to nerve stimulation as guides before, during and after application of caffeine and TEA for better comparison of sIJPs. Caffeine produced MP hyperpolarization, inhibited MPfs and abolished the sIJP. The effects of caffeine reached a peak in ∼1 min and stayed steady until the termination of the application. The alterations of electrical properties fully recovered 5–10 min after washout of caffeine. Concomitant application of TEA could not restore the alterations of electrical properties. However, application of TEA alone depolarized MP by 2.2±0.5 mV versus control (n=8, P<0.05) and significantly augmented the sIJP. Hyperpolarization of MP and inhibition of MPfs and the sIJP induced by caffeine were not affected by preapplication of TEA (2 mM).
Ryanodine is reported to be a putative agonist of ryanodine receptors in the SR (Fill & Copello, 2002), which locks the channel in a slow-gating subconductance state. Bath application of ryanodine (20 μM) produced biphasic effects on MP, namely an initial small hyperpolarization of 2.3±0.1 mV over control (n=3, P<0.05) in 4–4.5 min, followed by a large depolarization of 8.6±2.5 mV (n=3, P<0.05), which reached a steady state in 20–25 min (Figure 3A). The sIJP was decreased from 4.2±0.4 to 2.6±0.1 mV (n=3, P<0.05) and the MPf variance declined from 0.32±0.08 to 0.20±0.02 mV (n=3, P>0.05) 20 min after ryanodine. CPA has been demonstrated to be a specific Ca2+ ATPase inhibitor of the SR, and continuous application also depletes Ca2+ stores in the SR (Cohen et al., 1999; Ethier et al., 2001; Ng & Gurney, 2001; Albert & Large, 2002). Application of CPA (10 μM) depolarized MP by 12.9±4.1 mV (n=3, P<0.05) and abolished MPfs and the sIJP 15 min after bath application (Figure 3B). The effects of CPA reached the maximum in 5 min, but did not recover up to 30 min after washing.
Figure 3.
An example of the sIJP induced by nerve stimulation in the presence of ryanodine and CPA. (A.I) Original 40 min recording. (A.II) (a) Control sIJP; (b) ryanodine 20 μM, 20 min; (c) overlapped sIJPs. (B.I) Original recording. (B.II) (a) Control sIJP; (b) CPA 10 μM, 5 min; (c) overlapped sIJPs. Ryanodine produced biphasic effects on MP, initial small hyperpolarization followed by large depolarization, and inhibited the sIJP and MPfs, while CPA induced large depolarization and abolished the sIJP and MPfs. The effects of ryanodiine and CPA were irreversible.
Inhibitory effects of ICl(Ca) blockers on sIJP
Two putative ICl(Ca) blockers, A-9-C and NFA, were used to test the role of ICl(Ca) in the sIJP. The maximal A-9-C effect (2 mM) was reached within 5–10 min, when MPfs were abolished and the sIJP was significantly suppressed. This effect could be reversed within 10–15 min of washing out. MP was hyperpolarized by 7.9±2.6 mV and the amplitude of the sIJP was inhibited to 3.4±0.6 mV from 5.9±0.7 mV (n=4, P<0.05, Figures 1B and 4). NFA had a similar time course of action as A-9-C. NFA at a concentration of 300 μM, which was reported to be the maximal effective concentration in circular smooth muscle of esophageal body (Zhang & Paterson, 2002), abolished MPfs, hyperpolarized MP by 9.8±2.6 mV versus control and decreased the amplitude of the sIJP from 6.7±0.4 to 1.9±0.3 mV (n=5, P<0.05). In the presence of NFA (300 μM), TEA (2 mM) produced MP depolarization of only 3.2±1.8 mV (n=3) (Figure 5A and B). However, in the presence of TEA, the concomitant application of NFA (300 μM) still abolished MPfs, hyperpolarized MP by 10.5±3.3 mV and decreased the amplitude of the sIJP from 8.9±0.5 to 2.1±0.8 mV (n=4, P<0.05) (Figure 5C and D), suggesting that BK(Ca) channels do not contribute to the NFA-induced MP hyperpolarization. It has also been reported that NFA inhibits cation channels of rabbit SA node myocytes (Accili & DiFrancesco, 1996). A possible contribution of cation channels to MPfs, MP and the sIJP was tested by bath application of gadolinium, a putative nonselective cation channel blocker (Yang & Sachs, 1989; Zou et al., 1999; Kirber et al., 2000). Gadolinium (500 μM) did not produce measurable changes in electrical properties up to 10 min after bath application.
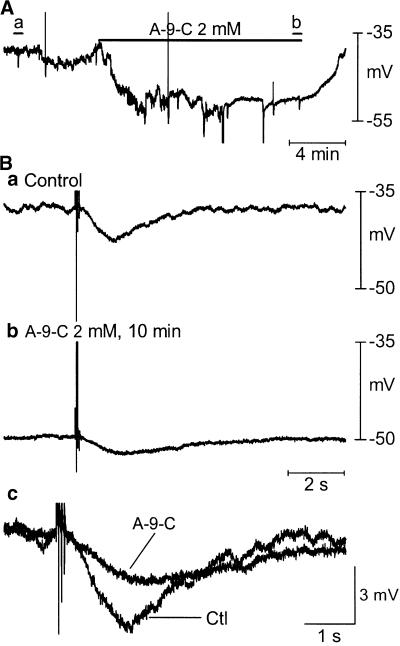
Figure 4.
Effects of A-9-C on the sIJP. (A) Original recording showed that A-9-C hyperpolarized MP and abolished MPfs. Large up- and downward deflections are nerve stimulation artifacts. (B) sIJP displayed in expanded timescale. The sIJP was greatly suppressed.
Figure 5.
Example of the inhibitory effects of NFA on membrane electrical properties. (A) Raw recordings demonstrated that NFA (300 μM) significantly hyperpolarized MP and inhibited MPfs. Concomitant application of TEA (2 mM) only depolarized MP by 3.2±1.8 mV (n=3, P<0.05). (B) Snapshots of sIJPs labelled in (A) in expanded timescale. NFA significantly suppressed sIJP, which was not restored by TEA. (C) Recording demonstrating that application of TEA depolarized MP by 2.2±0.5 mV versus control (n=8, P<0.05). In the presence of TEA, NFA still produced MP hyperpolarization and inhibition of MPfs, which were no different from that produced by NFA alone. (D) sIJPs from (C) in expanded timescale. Application of TEA augmented the sIJP. However, NFA-induced MP hyperpolarization and inhibition of MPfs and the sIJP were not affected by preapplication of TEA, suggesting that inhibitory effects of NFA were not due to nonspecific actions, such as opening of BK(Ca).
Inhibitory effects of MLCK inhibitors on electrical properties
We previously demonstrated the possible involvement of MLCK in activation of the ICl(Ca) and generation of the sIJP in circular smooth muscle of esophageal body (Zhang & Paterson, 2002). This possibility was also tested in the current studies by application of ML-9, an inhibitor of MLCK and protein kinase A and C, and wortmannin, a specific inhibitor of PI-3 kinase and MLCK. Time course studies showed that both inhibitors had maximal effects in about 5–10 min. The effect of ML-9 was reversible 15–17 min after washing out, but the effect of wortmannin was not reversible for up to 60 min after washing out. This is consistent with our previous studies (Zhang & Paterson, 2002). MPfs were inhibited by either ML-9 (200 μM) or wortmannin (30 μM). ML-9 and wortmannin hyperpolarized MP by 13.6±2.6 and 9.1±2.2 mV versus control, and significantly decreased the amplitude of the sIJP from 6.7±0.5 and 5.4±0.4 to 1.4±0.5 and 1.2±0.3 mV (n=5, P<0.05, Figure 6), respectively.
Figure 6.
Inhibitory effects of the MLCK inhibitors ML-9 (200 μM) and wortmannin (30 μM). (A) ML-9 induced reversible MP hyperpolarization. (B) (a,b) sIJPs before and 5 min after application of ML-9, which abolished MPfs and the sIJP. (B) (c) Superimposed sIJP before and after ML-9. (C) Wortmannin produced MP hyperpolarization that was not reversed after washout. (D) Snap views of sIJPs labelled in (C) demonstrated that wortmannin abolished MPfs and the sIJP.
Discussion
The major findings of the current study are that in the opossum LES circular smooth muscle (1) spontaneous MPfs of 1–3 mV are recorded, which are suppressed by either depletion of SR Ca2+ stores, inhibition of the ICl(Ca) or inhibition of MLCK; (2) inhibition of guanylyl cyclase abolishes the sIJP, but has no effect on MP and MPfs; (3) either depletion of SR Ca2+ stores, inhibition of the ICl(Ca) or inhibition of MLCK hyperpolarizes MP by 9–12 mV and nearly abolishes the sIJP. These data support our hypothesis that spontaneous Ca2+ release from SR activates the ICl(Ca) via a Ca2+-dependent signal cascade in which MLCK is pivotal. Basal activity of the ICl(Ca) maintains resting MP at relatively more positive levels and inhibition of the basal activity of the ICl(Ca) by nitrergic innervation may produce the sIJP, leading to LES relaxation.
Role of SR in control of MP and LES basal tone
We previously provided evidence that basal activity of the ICl(Ca) contributes to the resting MP of LES (Zhang et al., 2000), but the mechanisms underlying this basal ICl(Ca) activity were unclear. Spontaneous Ca2+ release from the SR via the opening of ryanodine-sensitive Ca2+-release channels has recently been proposed to play an important role in the regulation of membrane channel activity in cardiac (Cheng et al., 1993; 1995), skeletal (Valdivia et al., 1995) and smooth (Fay, 1995; Nelson et al., 1995) muscle cells. In stretched rat cerebral arterioles (Nelson et al., 1995; Jaggar et al., 1998) and guinea-pig gallbladder myocytes (Pozo et al., 2002), spontaneous Ca2+ release briefly hyperpolarizes cell membranes by activation of BK(Ca) channels, producing a relaxation. In guinea-pig tracheal smooth muscle cells, spontaneous Ca2+ transients activate either K+ or Cl− channels, depending on membrane potential (ZhuGe et al., 1998). Because the reversal potential of Cl− conductance is between −30 and −20 mV in smooth muscle (Aickin & Brading, 1982; 1983), activation of the ICl(Ca) would depolarize MP under the physiological conditions.
The aforementioned findings are not easily reconciled with the results of the current study. The resting MP recorded in LES circular smooth muscle is ∼−41 mV (Zhang et al., 2000) in the normal state and ∼−38.1 mV in the presence of atropine, guanethidine, nifedipine and SP desensitization. Depletion of SR Ca2+ stores by continuous application of caffeine abolishes MPfs and hyperpolarizes MP. These effects are unlikely to be due to opening of BK(Ca) channels, as they were not affected by preapplication of the BK(Ca) channel blocker TEA (2 mM) (Figure 2). Caffeine has been reported to be a potent cAMP phosphodiesterase inhibitor, resulting in intracellular cAMP accumulation (Rivedal & Sanner, 1985; Riley & Barclay, 1990; Tesarik et al., 1992; Sinha et al., 1993). However, our finding that 8-Br-cAMP, a membrane-permeable cAMP analog, had no effect on MP or MPfs precludes this mechanism as playing a role in the observed findings. On the other hand, the observations that TEA only produced MP depolarization of ∼2 mV and 8-Br-cAMP had no measurable effect imply that BK(Ca) is not a major contributor to resting MP.
Ryanodine has been reported to have different effects in different muscles. It produces rigid paralysis in skeletal muscle but flaccid paralysis in cardiac muscle, whereas in smooth muscles it produces depolarization/excitation (Knot et al., 1998; Herrera et al., 2000; Haddock & Hill, 2002). In the current study, ryanodine (20 μM) initially hyperpolarized MP. Continuous application then depolarized MP significantly, which reached a steady state in 20–25 min (Figure 3A). This biphasic MP response is consistent with our preliminary tension recordings; that is, ryanodine induced an initial slight decrease, followed by a significant increase in basal tone (data not shown). However, these results are difficult to interpret. We speculate that the initial small hyperpolarization may be due to either activation of BK(Ca) or diminution of the ICl(Ca), and that the large depolarization may be similar to that produced by CPA. Depletion of Ca2+ stores by CPA, via inhibition of Ca2+ ATPase in the SR, also abolishes MPfs, but in contrast to caffeine, CPA significantly depolarizes MP (Figures 1B and 3B), which is similar to the second phase of the observed ryanodine effect. This suggests that activation of nonselective cation channels by CPA may be predominant (Trepakova et al., 2001).
Taken together, the current findings suggest that spontaneous Ca2+ release from the SR mainly activates the ICl(Ca), which in turn results in a relatively more positive resting MP. This interpretation is further supported by the experiments in which the ICl(Ca) blockers NFA and ML-9 inhibit MPfs and produce comparable MP hyperpolarization. It is also consistent with a recent study (Suzuki et al., 2003) demonstrating that A-9-C reduces membrane noise and hyperpolarizes MP in mouse gastric antrum smooth muscle.
Previous studies have reported that NFA has nonspecific effects, including inhibition of Ca2+ channels and Ca2+-dependent contractile processes (Kato et al., 1999), opening of BK(Ca) channels (Large & Wang, 1996) and blockade of nonselective cation channels (Accili & DiFrancesco, 1996). However, such nonspecific mechanisms cannot explain the observed abolition of MPfs and membrane hyperpolarization in our study because (1) intracellular recordings were conducted in the presence of nifedipine, thus L-type Ca2+ channels were already blocked; (2) abolition of MPfs and membrane hyperpolarization was not affected by preapplication of TEA, and the alterations of membrane electrical properties were not restored by concomitant administration of TEA (Figure 5A); (3) gadolinium, a nonselective cation channel blocker (Zou et al., 1999), did not produce any measurable changes of electrical properties. In addition, in cerebral artery, cation channels were not affected by NFA at concentrations of up to 100 μM (Nelson et al., 1997; Welsh et al., 2000). More recent studies by Piper et al. (2002) demonstrated dual effects of NFA on the ICl(Ca) in acutely dissociated smooth muscle cells of rabbit pulmonary artery. NFA inhibited the outward part of the ICl(Ca) with intracellular Ca2+ concentrations fixed at 500 nM and 1 μM. Interestingly, NFA enhanced the inward part of the ICl(Ca). In the LES, the opening of Cl− channels would conduct inward currents, as recorded MP is ∼−38.1 mV. If this were the case in the tissues currently studied, it would be predicted that NFA would produce MP depolarization. In our studies, NFA actually hyperpolarized MP by ∼10 mV over control (Figure 5).
It has also been reported that NFA can affect skeletal muscle ryanodine receptor channels, with either activation or inhibition being produced depending on the channel subtype and concentration of NFA used (Oba, 1997). Whether this also applies to smooth muscle ryanodine receptors, which are quite distinct from those in skeletal muscle (Fill & Copello, 2002), is unknown. Nevertheless, we cannot fully exclude the possibility that some of the observed effects of NFA were via a nonspecific action on ryanodine receptors.
The processes of signal transduction in activation of the ICl(Ca) remain unclear. Ca2+-dependent calmodulin kinase II is proposed to be involved in the regulation of the ICl(Ca) in vascular smooth muscle (Greenwood et al., 2001) and in guinea-pig ileum circular smooth muscle (He et al., 1999). Our recent observation suggests that MLCK is pivotal to the activation of the ICl(Ca) in circular smooth muscle of opossum esophageal body (Zhang & Paterson, 2002). In the current study, the observation that MLCK inhibitors ML-9 and wortmannin suppress the MPfs and hyperpolarize MP to a similar degree to that induced by the ICl(Ca) blockers and the depletion of Ca2+ stores in SR, further supports a role for MLCK in activation of the ICl(Ca). Wortmannin has also been reported to inhibit PI-3 kinase (Arcaro & Wymann, 1993). However, it is less likely that PI-3 is involved in the regulation of ICl(Ca), because MP, MPfs and the sIJP in opossum esophageal body are not affected by LY 294002 (10 μM), a specific PI-3 kinase inhibitor (Zhang & Paterson, 2002). The effects of wortmannin on MP appear to be different in circular smooth muscle of esophageal body and LES. Wortmannin (30 μM) produces MP hyperpolarization by ∼2 mV in opossum esopageal body (Zhang & Paterson, 2002), while it hyperpolaizes MP by ∼9.1 mV in LES (Figure 6C). The reason for this difference is unknown. A greater degree of hyperpolarization may imply greater activity of the ICl(Ca) in LES than in esophageal body. In addition, the 9.1–13 mV of membrane hyperpolarization produced by the ICl(Ca) blockers, MLCK inhibitors and depletion of SR Ca2+ stores, strongly suggests that the ICl(Ca) is responsible for at least this degree of voltage in MP of LES smooth muscle.
A number of previous studies have reported that MLCK inhibitors may affect activity of other ion channels, including reduction of the M-currents in bullfrog sympathetic neurons (Akasu et al., 1993) and nonselective cation currents in gastric myocytes (Kim et al., 1997) and rabbit portal vein (Aromolaran et al., 2000). The current studies confirm our previous report of an interaction between MLCK and the ICl(Ca). However, the physiological significance of this interaction remains unclear. It is likely that MLCK serves as one of the link points between channel activity and the contractile element process.
Nitrergic regulation
It is well established that intrinsic nitrergic neurons provide the predominant inhibitory innervation to the LES of several species (Jury et al., 1985; Allescher et al., 1988; Goyal, 1989; Yamato et al., 1992; Conklin et al., 1993; Preiksaitis et al., 1994; Preiksaitis & Diamant, 1995; Paterson et al., 1992; Kim et al., 1999). However, the ionic mechanisms that underlie nitrergic inhibition remain unclear. It was previously proposed that NO relaxed GI smooth muscle by either opening of K+ channels or closing of Ca2+-activated Cl− channels (Sanders & Ozaki, 1994; Goyal, 2000). Indeed, patch-clamp studies demonstrated that NO and NO donors activate several kinds of K+ channels in arterial (Bolotina et al., 1994; Yuan et al., 1996), colonic (Koh et al., 1995) and esophageal smooth muscle (Jury et al., 1996), and that these channels are sensitive to either TEA, apamin or 4-AP. Unfortunately, the NO-mediated sIJP is not blocked by any of the classic K+ channel blockers in esophageal smooth muscle. We previously showed that NFA and A-9-C, putative ICl(Ca) blockers, abolished the sIJP in circular smooth muscle of opossum esophageal body (Zhang & Paterson, 2002), supporting the hypothesis that suppression of the ICl(Ca) by nitrergic innvervation mediates the sIJP. The current studies in LES confirm the inhibitory effects of ICl(Ca) blockers on sIJP, and are consistent with a recent study (Suzuki et al., 2003) in mouse antrum in which A-9-C was reported to inhibit the sIJP. It is less likely that inhibition of sIJP by NFA is due to its previously reported nonspecific actions, such as BK channel opening or blockade of cation channels, as abolition of the sIJP was not affected by either preapplication or concomitant application of TEA or gadolinium (see above). Interestingly, application of TEA potentiates the sIJP. This may be due to increased smooth muscle excitability and/or more neurotransmitter release by TEA (Jiang et al., 2001).
Jury et al. (2001) recently reported that in canine LES circular smooth muscle, NFA (60 μM) caused near-complete inhibition of basal tone, yet did not prevent electrical field stimulation (nitrergic innervation)-induced LES relaxation when tone was restored by addition of carbachol to the bath. This observation suggests that Cl− channels are not involved in NO-mediated LES relaxation. However, we previously demonstrated that in opossum esophageal body, the IC50 of NFA for inhibition of sIJP is ∼80 μM, while NFA completely abolished sIJP at concentrations of 300 μM (Zhang & Paterson, 2002). The discrepant findings in these two studies likely relate to the different concentrations of NFA used. Nevertheless, in the presence of either of caffeine, CPA, NFA and wortmannin, there remains a residual sIJP with amplitude varying between 0.3 and 1.9 mV (versus ∼6.1 mV in control cells). It is possible that the opening of unidentified K+ channels is responsible for this residual MP hyperpolarization induced by nitrergic innervation.
Cayabyab & Daniel (1996) reported a role for the SR in sIJPs and hyperpolarizations induced by NO donors in opossum oesophagus. They demonstrated that the selective SR Ca2+-ATPase inhibitors CPA and thapsigargin, and the SR Ca2+ release channel activator ryanodine caused depolarization and spontaneous contractions, which were diminished after prolonged (>30 min) incubation with these agents in Ca2+-containing medium. Moreover, these agents inhibited both the sIJP and NO-donor induced hyperpolarization. They interpreted these results to indicate that functional SR Ca2+ uptake is necessary for the response to endogenous or exogenous NO. Our studies confirm that CPA depolarizes MP and abolishes the sIJP and MPfs. However, we believe that these results can also be explained by activation of the ICl(Ca) by spontaneous Ca2+ release from caffeine-sensitive Ca2+ stores of the SR. The depletion of Ca2+ stores by CPA and caffeine abolishes spontaneous release of Ca2+ and thus basal activity of the ICl(Ca). Without the ICl(Ca) activity, the sIJP evoked by nitrergic innervation and hyperpolarization produced by NO donors would be prevented. The opposing effects on resting MP, that is depolarization by CPA and hyperpolarization by caffeine, may suggest the predominant effects of CPA on native cation channels activated by Ca2+ store depletion (Trepakova et al., 2001).
In summary, these data strongly support a role of the SR in control of resting MP and nitrergic responses in opossum LES circular smooth muscle. Spontaneous Ca2+ release from SR via activation of ryanodine receptors activates the ICl(Ca) in which MLCK is pivotal. NO released from nitrergic nerves may inactivate the ICl(Ca) via intracellular elevation of cGMP, leading to the sIJP and ensuing relaxation. The site(s) of action of NO and the nature of the interaction between the ICl(Ca) and MLCK require further investigation.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR) Grant # MOP 9978.
Abbreviations
- 4-AP
4-aminopyridine
- 8-Br-cAMP
8-bromo-cAMP
- A-9-C
9-anthroic acid
- BK(Ca)
Ca2+-activated large conductance K+ channel
- CPA
cyclopiazonic acid
- IBK(Ca)
Ca2+-activated large conductance K+ current
- ICl(Ca)
Ca2+-activated Cl− current
- IP3
inositol 1,4,5-triphosphate
- LES
lower esophageal sphincter
- MLCK
myosin light chain kinase
- ML-9
1-(5-chloronaphthalene-1-sulfonyl)-1-H-hexahydro-1,4-diazepine
- MP
resting membrane potential
- MPfs
resting membrane potential fluctuations
- NANC
nonadrenergic and noncholinergic
- NFA
niflumic acid
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- PLC
phospholipase C
- sIJP
slow inhibitory junction potential
- SR
sarcoplasmic reticulum
- TEA
tetraethylammonium
References
- ACCILI E.A., DIFRANCESCO D. Inhibition of the hyperpolarization-activated current (if) of rabbit SA node myocytes by niflumic acid. Pflugers Arch. 1996;431:757–762. doi: 10.1007/BF02253840. [DOI] [PubMed] [Google Scholar]
- AICKIN C.C., BRADING A.F. Measurement of intracellular chloride in guinea-pig vas deferens by ion analysis, 36chloride efflux and micro-electrodes. J. Physiol. 1982;326:139–154. doi: 10.1113/jphysiol.1982.sp014182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AICKIN C.C., BRADING A.F. Towards an estimate of chloride permeability in the smooth muscle of guinea-pig vas deferens. J. Physiol. 1983;336:179–197. doi: 10.1113/jphysiol.1983.sp014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKASU T., ITO M., NAKANO T., SCHNEIDER C.R., SIMMONS M.A., TANAKA T., TOKIMASA T., YOSHIDA M. Myosin light chain kinase occurs in bullfrog sympathetic neurons and may modulate voltage-dependent potassium currents. Neuron. 1993;11:1133–1145. doi: 10.1016/0896-6273(93)90226-h. [DOI] [PubMed] [Google Scholar]
- ALBERT A.P., LARGE W.A. Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes. J. Physiol. 2002;544:113–125. doi: 10.1113/jphysiol.2002.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLESCHER H.D., BEREZIN I., JURY J., DANIEL E.E. Characteristics of canine lower esophageal sphincter: a new electrophysiological tool. Am. J. Physiol. 1988;255:G441–G453. doi: 10.1152/ajpgi.1988.255.4.G441. [DOI] [PubMed] [Google Scholar]
- ARCARO A., WYMANN M.P.Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses Biochem. J. 1993296297–301.(Part 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- AROMOLARAN A.S., ALBERT A.P., LARGE W.A.Evidence for myosin light chain kinase mediating noradrenaline-evoked cation current in rabbit portal vein myocytes J. Physiol. 2000524853–863.(Part 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- CAYABYAB F.S., DANIEL E.E. Role of sarcoplasmic reticulum in inhibitory junction potentials and hyperpolarizations by nitric oxide donors in opossum oesophagus. Br. J. Pharmacol. 1996;118:2185–2191. doi: 10.1111/j.1476-5381.1996.tb15661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG H., FILL M., VALDIVIA H., LEDERER W.J. Models of Ca2+ release channel adaptation. Science. 1995;267:2009–2010. doi: 10.1126/science.7701326. [DOI] [PubMed] [Google Scholar]
- CHENG H., LEDERER W.J., CANNELL M.B. Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., WEISBROD R.M., GERICKE M., YAGHOUBI M., BIERL C., BOLOTINA V.M. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ. Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- CONKLIN J.L., DU C., MURRAY J.A., BATES J.N. Characterization and mediation of inhibitory junction potentials from opossum lower esophageal sphincter. Gastroenterology. 1993;104:1439–1444. doi: 10.1016/0016-5085(93)90353-e. [DOI] [PubMed] [Google Scholar]
- COTTON K.D., HOLLYWOOD M.A., MCHALE N.G., THORNBURY K.D. Ca2+ current and Ca2+-activated chloride current in isolated smooth muscle cells of the sheep urethra. J. Physiol. 1997;505:121–131. doi: 10.1111/j.1469-7793.1997.121bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS T.M., SCHOLFIELD C.N. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J. Physiol. 2001;532:609–623. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ETHIER M.F., YAMAGUCHI H., MADISON J.M. Effects of cyclopiazonic acid on cytosolic calcium in bovine airway smooth muscle cells. Am. J. Physiol. 2001;281:L126–L133. doi: 10.1152/ajplung.2001.281.1.L126. [DOI] [PubMed] [Google Scholar]
- FAY F.S. Calcium sparks in vascular smooth muscle: relaxation regulators. Science. 1995;270:588–589. doi: 10.1126/science.270.5236.588. [DOI] [PubMed] [Google Scholar]
- FILL M., COPELLO J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- GOYAL R.K. Muscarinic receptor subtypes. Physiology and clinical implications. New Engl. J. Med. 1989;321:1022–1029. doi: 10.1056/NEJM198910123211506. [DOI] [PubMed] [Google Scholar]
- GOYAL R.K. Targets of enteric motor neurones: smooth muscle cells. Gut. 2000;47 Suppl. 4:iv38–iv39. doi: 10.1136/gut.47.suppl_4.iv38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOYAL R.K., PATERSON W.G.Esophageal motility Hand Book of Physiology 1989Bethesda, MD: Oxford University Press; 865–908.ed. Schultz, S.G., Wood, J.D. & Rauner, B.B. pp [Google Scholar]
- GREENWOOD I., LEDOUX J., LEBLANC N. Differential regulation of Ca2+-activated Cl− currents in rabbit arterial and portal vein smooth muscle cells by Ca2+-calmodulin-dependent kinase. J. Physiol. 2001;534:395–408. doi: 10.1111/j.1469-7793.2001.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADDOCK R.E., HILL C.E. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J. Physiol. 2002;545:615–627. doi: 10.1113/jphysiol.2002.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE X.D., AKBARALI H.I., GOYAL R.K. Modulation of resting potential and nitrergic slow IJP by CAM kinase II and MAP kinase in guinea-pig ileum circular muscle. Gastroenterology. 1999;166 Suppl. 4:A1004. [Google Scholar]
- HERRERA G.M., HEPPNER T.J., NELSON M.T. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am. J. Physiol. 2000;279:R60–R68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- JAGGAR J.H., STEVENSON A.S., NELSON M.T. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am. J. Physiol. 1998;274:C1755–C1761. doi: 10.1152/ajpcell.1998.274.6.C1755. [DOI] [PubMed] [Google Scholar]
- JANSSEN L.J., SIMS S.M. Spontaneous transient inward currents and rhythmicity in canine and guinea-pig tracheal smooth muscle cells. Pflugers Arch. 1994;427:473–480. doi: 10.1007/BF00374263. [DOI] [PubMed] [Google Scholar]
- JIANG Z.G., SI J.Q., LASAREV M.R., NUTTALL A.L. Two resting potential levels regulated by the inward-rectifier potassium channel in the guinea-pig spiral modiolar artery. J. Physiol. 2001;537:829–842. doi: 10.1111/j.1469-7793.2001.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JURY J., BOEV K.R., DANIEL E.E. Nitric oxide mediates outward potassium currents in opossum esophageal circular smooth muscle. Am. J. Physiol. 1996;270:G932–G938. doi: 10.1152/ajpgi.1996.270.6.G932. [DOI] [PubMed] [Google Scholar]
- JURY J., JAGER L.P., DANIEL E.E. Unusual potassium channels mediate nonadrenergic noncholinergic nerve-mediated inhibition in opossum esophagus. Can. J. Physiol. Pharmacol. 1985;63:107–112. doi: 10.1139/y85-020. [DOI] [PubMed] [Google Scholar]
- JURY J., PATEL M., BOWES T., DANIEL E.E. Actions of putative chloride channel blocking agents on canine lower esophageal sphincter (LES) Can. J. Physiol. Pharmacol. 2001;79:1007–1014. [PubMed] [Google Scholar]
- KATO K., EVANS A.M., KOZLOWSKI R.Z. Relaxation of endothelin-1-induced pulmonary arterial constriction by niflumic acid and NPPB: mechanism(s) independent of chloride channel block. J. Pharmacol. Exp. Ther. 1999;288:1242–1250. [PubMed] [Google Scholar]
- KIM C.D., GOYAL R.K., MASHIMO H. Neuronal NOS provides nitrergic inhibitory neurotransmitter in mouse lower esophageal sphincter. Am. J. Physiol. 1999;277:G280–G284. doi: 10.1152/ajpgi.1999.277.2.G280. [DOI] [PubMed] [Google Scholar]
- KIM Y.C., KIM S.J., KANG T.M., SUH S.H., SO I., KIM K.W. Effects of myosin light chain kinase inhibitors on carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Pflugers Arch. 1997;434:346–353. doi: 10.1007/s004240050407. [DOI] [PubMed] [Google Scholar]
- KIRBER M.T., GUERRERO-HERNANDEZ A., BOWMAN D.S., FOGARTY K.E., TUFT R.A., SINGER J.J., FAY F.S. Multiple pathways responsible for the stretch-induced increase in Ca2+ concentration in toad stomach smooth muscle cells. J. Physiol. 2000;524:3–17. doi: 10.1111/j.1469-7793.2000.t01-4-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOT H.J., STANDEN N.B., NELSON M.T.Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels J. Physiol. 1998508211–221.(Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOH S.D., CAMPBELL J.D., CARL A., SANDERS K.M.Nitric oxide activates multiple potassium channels in canine colonic smooth muscle J. Physiol. 1995489735–743.(Part 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARGE W.A., WANG Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am. J. Physiol. 1996;271:C435–C454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., CHENG H., RUBART M., SANTANA L.F., BONEV A.D., KNOT H.J., LEDERER W.J. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., CONWAY M.A., KNOT H.J., BRAYDEN J.E. Chloride channel blockers inhibit myogenic tone in rat cerebral arteries. J. Physiol. 1997;502:259–264. doi: 10.1111/j.1469-7793.1997.259bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NG L.C., GURNEY A.M. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ. Res. 2001;89:923–929. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- OBA T. Niflumic acid differentially modulates two types of skeletal ryanodine-sensitive Ca2+-release channels. Am. J. Physiol. 1997;73:C1588–C1595. doi: 10.1152/ajpcell.1997.273.5.C1588. [DOI] [PubMed] [Google Scholar]
- PATERSON W.G., ANDERSON M.A., ANAND N. Pharmacological characterization of lower esophageal sphincter relaxation induced by swallowing, vagal efferent nerve stimulation, and esophageal distention. Can. J. Physiol. Pharmacol. 1992;70:1011–1015. doi: 10.1139/y92-139. [DOI] [PubMed] [Google Scholar]
- PIPER A.S., GREENWOOD I.A., LARGE W.A. Dual effect of blocking agents on Ca2+-activated Cl− currents in rabbit pulmonary artery smooth muscle cells. J. Physiol. 2002;539:119–131. doi: 10.1113/jphysiol.2001.013270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POZO M.J., PEREZ G.J., NELSON M.T., MAWE G.M. Ca2+ sparks and BK currents in gallbladder myocytes: role in CCK-induced response. Am. J. Physiol. 2002;282:G165–G174. doi: 10.1152/ajpgi.00326.2001. [DOI] [PubMed] [Google Scholar]
- PREIKSAITIS H.G., DIAMANT N.E. Phasic contractions of the muscular components of human esophagus and gastroesophageal junction in vitro. Can. J. Physiol. Pharmacol. 1995;73:356–363. doi: 10.1139/y95-045. [DOI] [PubMed] [Google Scholar]
- PREIKSAITIS H.G., TREMBLAY L., DIAMANT N.E. Cholinergic responses in the cat lower esophageal sphincter show regional variation. Gastroenterology. 1994;106:381–388. doi: 10.1016/0016-5085(94)90596-7. [DOI] [PubMed] [Google Scholar]
- RILEY B.B., BARCLAY S.L. Conditions that alter intracellular cAMP levels affect expression of the cAMP phosphodiesterase gene in Dictyostelium. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4746–4750. doi: 10.1073/pnas.87.12.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIVEDAL E., SANNER T. Caffeine and other phosphodiesterase inhibitors are potent inhibitors of the promotional effect of TPA on morphological transformation of hamster embryo cells. Cancer Lett. 1985;28:9–17. doi: 10.1016/0304-3835(85)90086-2. [DOI] [PubMed] [Google Scholar]
- SANDERS K.M., OZAKI H.Excitation–contraction coupling in gastrointestinal smooth muscles Pharmacology of Smooth Muscle 1994Berlin: Springer Verlag; 331–404.ed. Szekeres, L. & Papp, J.Gy. pp [Google Scholar]
- SENGUPTA A., PATERSON W.G., GOYAL R.K. Atypical localization of myenteric neurons in the opossum lower esophageal sphincter. Am. J. Anat. 1987;180:342–348. doi: 10.1002/aja.1001800404. [DOI] [PubMed] [Google Scholar]
- SINHA S., KUMAR P., LALORAYA M. Methyl xanthine and altered biomembrane dynamics: demonstration of protein mobility and enzyme inhibition by caffeine in sperm model system. Biochem. Mol. Biol. Int. 1993;31:1141–1148. [PubMed] [Google Scholar]
- SUZUKI H., WARD S.M., BAYGUINOV Y.R., EDWARDS F.R., HIRST G.D. Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum. J. Physiol. 2003;546:751–763. doi: 10.1113/jphysiol.2002.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESARIK J., MENDOZA C., CARRERAS A. Effects of phosphodiesterase inhibitors caffeine and pentoxifylline on spontaneous and stimulus-induced acrosome reactions in human sperm. Fertil. Steril. 1992;58:1185–1190. doi: 10.1016/s0015-0282(16)55567-8. [DOI] [PubMed] [Google Scholar]
- TREPAKOVA E.S., GERICKE M., HIRAKAWA Y., WEISBROD R.M., COHEN R.A., BOLOTINA V.M. Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J. Biol. Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- VALDIVIA H.H., KAPLAN J.H., ELLIS-DAVIES G.C., LEDERER W.J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Q., AKBARALI H.I., HATAKEYAMA N., GOYAL R.K. Caffeine- and carbachol-induced Cl− and cation currents in single opossum esophageal circular muscle cells. Am. J. Physiol. 1996;271:C1725–C1734. doi: 10.1152/ajpcell.1996.271.5.C1725. [DOI] [PubMed] [Google Scholar]
- WANG Q., WANG Y.X., YU M., KOTLIKOFF M.I. Ca2+-activated Cl− currents are activated by metabolic inhibition in rat pulmonary artery smooth muscle cells. Am. J. Physiol. 1997;273:C520–C530. doi: 10.1152/ajpcell.1997.273.2.C520. [DOI] [PubMed] [Google Scholar]
- WELSH D.G., NELSON M.T., ECKMAN D.M., BRAYDEN J.E. Swelling-activated cation channels mediate depolarization of rat cerebrovascular smooth muscle by hyposmolarity and intravascular pressure. J. Physiol. 2000;527:139–148. doi: 10.1111/j.1469-7793.2000.t01-1-00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMATO S., SPECHLER S.J., GOYAL R.K. Role of nitric oxide in esophageal peristalsis in the opossum. Gastroenterology. 1992;103:197–204. doi: 10.1016/0016-5085(92)91113-i. [DOI] [PubMed] [Google Scholar]
- YANG X.C., SACHS F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- YUAN X.J., TOD M.L., RUBIN L.J., BLAUSTEIN M.P. NO hyperpolarizes pulmonary artery smooth muscle cells and decreases the intracellular Ca2+ concentration by activating voltage-gated K+ channels. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10489–10494. doi: 10.1073/pnas.93.19.10489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Y., MILLER D.V., PATERSON W.G. Opposing roles of K+ and Cl− channels in maintenance of opossum lower esophageal sphincter tone. Am. J. Physiol. 2000;279:G1226–G1234. doi: 10.1152/ajpgi.2000.279.6.G1226. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., PATERSON W.G. Role of Ca2+-activated Cl− channels and MLCK in opossum esophageal smooth muscle. Am. J. Physiol. 2002;283 Suppl. 1:G104–G114. doi: 10.1152/ajpgi.00052.2002. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., PATERSON W.G. Diversity of K+ channels in circular smooth muscle of opossum lower esophageal sphincter. Can. J. Physiol. Pharmacol. 2001;79:608–620. [PubMed] [Google Scholar]
- ZHUGE R., SIMS S.M., TUFT R.A., FOGARTY K.E., WALSH J.V.J.Ca2+ sparks activate K+ and Cl− channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes J. Physiol. 1998513711–718.(Part 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZOU H., LIFSHITZ L.M., TUFT R.A., FOGARTY K.E., SINGER J.J. Imaging Ca2+ entering the cytoplasm through a single opening of a plasma membrane cation channel. J. Gen. Physiol. 1999;114:575–588. doi: 10.1085/jgp.114.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]