Abstract
This study investigated whether a single administration of a range of doses (1, 4 and 8 mg kg−1, i.p.) of paroxetine, citalopram or venlafaxine may simultaneously increase extracellular levels of 5-HT ([5-HT]ext) and noradrenaline ([NA]ext) by using in vivo microdialysis in the frontal cortex (FCx) of awake, freely moving Swiss mice.
In vivo, paroxetine induced similar increases in cortical [5-HT]ext at the three doses tested, and induced a statistically significant increase in cortical [NA]ext at 4 and 8 mg kg−1. Citalopram increased neither [5-HT]ext nor [NA]ext at the lowest dose, but increased both neurotransmitter levels at 4 and 8 mg kg−1. At these doses, citalopram induced greater increases in cortical [5-HT]ext than in [NA]ext. Venlafaxine increased [5-HT]ext and [NA]ext to about 400 and 140% of the respective basal values at 8 mg kg−1.
Citalopram and paroxetine have the highest potency to increase cortical [5-HT]ext and [NA]ext, respectively. In addition, the rank of order of efficacy of these antidepressant drugs to increase [5-HT]ext in vivo in the FCx of mice was as follows: venlafaxine>citalopram>paroxetine, while the efficacy to increase cortical [NA]ext in mice of paroxetine and citalopram is similar, and greater than that of venlafaxine.
In conclusion, extracellular levels of cortical [NA]ext increase with the highest doses of the very selective SSRI citalopram, as well as with the very potent SSRI paroxetine. Surprisingly, the SNRI venlafaxine increased cortical [5-HT]ext to a greater extent rather than [NA]ext in the range of doses studied in mice.
Keywords: Antidepressants, frontal cortex, microdialysis, noradrenaline, serotonin
Introduction
Decrease in the functional activity of central serotonergic and catecholaminergic systems is probably associated in major depressive episodes (Blier & De Montigny, 1994). Applying an appropriate strategy with drugs targeting both neurotransmitter systems may improve the efficacy of the antidepressant treatment. In recent years, new antidepressant drugs, with less adverse effects than imipramine derivatives, have been developed that selectively block either the serotonin (5-hydroxytryptamine, 5-HT) transporter, that is, selective serotonin reuptake inhibitors (SSRIs), or the noradrenaline (NA) transporter (e.g. reboxetine), or block both, that is, mixed serotonin/noradrenaline reuptake inhibitors (SNRIs – venlafaxine, duloxetine) (Artigas, 1995; Vetulani & Nalepa, 2000). SSRIs, such as paroxetine and citalopram, are effective in treating depressed patients (Delgado et al., 1992; Deakin & Dursun, 2002), but it has been suggested that SNRIs such as venlafaxine, a compound that increased both extracellular levels of 5-HT ([5-HT]ext) and NA ([NA]ext) in the rat frontal cortex (FCx) (Dawson et al., 1999; Millan et al., 2001), should be a greater therapeutic success than SSRI in depressed patients (Einarson et al., 1999).
The data obtained in vitro, in rat brain synaptosomal preparations (Bolden-Watson & Richelson, 1993; Barker et al., 1994) led to the classification of paroxetine as the most potent SSRI based on its very high affinity for the 5-HT transporter (KD 5-HT=0.29 nM). Citalopram is the most selective SSRI, being about 100-fold weaker in blocking the NA transporter than paroxetine in vitro (KD 5-HT/KD NA for citalopram=1.8 nM/6100 nM=1/3388; KD 5-HT/KD NA for paroxetine=0.29 nM/81 nM=1/279 (Sánchez & Hytell, 1999)). However, the 5-HT/NA selectivity ratios measured in vitro, vary considerably between SSRIs (Sánchez & Hytell, 1999). Animal studies are necessary to determine their pharmacological properties in vivo (potency (calculated ED50 values) and efficacy (maximal response in a preclinical test)). Recent preclinical studies indicate that the selectivity of SSRIs for 5-HT over NA transporters might be less apparent in vivo. Indeed, in the mouse forced swimming test (FST) in Swiss mice, an animal model of depression which predicts the efficacy of antidepressant drugs in humans, paroxetine, citalopram and venlafaxine dose-dependently increased the mobility time (Bourin et al., 1998; Redrobe et al., 1998a,1998c). Moreover, in this test, we demonstrated that paroxetine, as well as venlafaxine, display noradrenergic-like behavioural activity at a clinically relevant dose as low as 8 mg kg−1 in mice (Redrobe et al., 1998b,1998c).
The main advantage of intracerebral in vivo microdialysis is to allow the simultaneous measurement of [5-HT]ext and [NA]ext at nerve terminal regions of the brain. This technique has already been performed to measure [NA]ext in rats, following administration of a single dose of SSRI systemically, but the results are inconsistent (Thomas et al., 1998; Bymaster et al., 2002; Koch et al., 2002). Thus, the absence of intracerebral microdialysis data in mice led us to investigate the selectivity of both SSRIs, paroxetine and citalopram in vivo by using this technique in mice, to measure simultaneously cortical [5-HT]ext and [NA]ext. We used an experimental protocol similar to that used by Redrobe et al. (1998b) in the behavioral test (FST): awake, freely moving Swiss mice received a single dose of either paroxetine or citalopram (1, 4 or 8 mg kg−1) with the purpose of establishing the same dose–response relationship between the present results and those obtained in this previous behavioural experiment. The effects of SSRIs on dialysate [5-HT]ext and [NA]ext were compared to those of venlafaxine, a reference compound acting on both systems administered at similar doses (1, 4 or 8 mg kg−1).
Methods
Animals
Male Swiss mice, 4–6 weeks old, weighing 20–24 g, were housed in our animal care facility in groups of 3–6 and kept under standard conditions (room temperature of 22–23°C, 12 : 12 light–dark cycle, free access to food and water). Mice were tested between 10.00 a.m. and 4.00 p.m. during the light phase. Only mice with probes confined to the FCx were used for subsequent analysis of microdialysis data. Procedures involving animals and their care were conducted in conformity with the institutional guidelines that are in compliance with national and international laws and policies (Council directive #87–848, October 19, 1987, Ministère de l'Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale, permissions #005037 to A.M.G.).
Treatment
Extracellular 5-HT and NA levels were measured in the FCx of awake, freely moving mice treated with 1, 4 or 8 mg kg−1, not corrected for the salt : base ratio of paroxetine, citalopram or venlafaxine intraperitoneally (i.p.). Control mice received saline (NaCl 0.9%) treatment given by the same route of administration.
For each drug administration, the effects on [5-HT]ext and [NA]ext were simultaneously measured in the same animal by using microdialysis probe implanted in the right and the left FCx, respectively.
Drugs and reagents
Paroxetine HCl (Glaxo SmithKline, U.K.), citalopram HBr (Lundbeck, Denmark) and venlafaxine HCl (Wyeth, U.S.A.) were dissolved in saline in a volume of 25 ml kg−1.
The reagents used for analysis of dialysate levels of NA were sodium acetate trihydrate and citric acid monohydrate (Merck, Germany), 1-octanesulphonic acid sodium salt hydrate (Acros Organics, U.S.A.), disodium ethylenediaminetetraacetic acid (EDTA) (Sigma, U.S.A.), dibutylamine (Fluka chemie AG, Germany), methyl alcohol (SDS, France), noradrenaline bitartrate (Sigma, U.S.A.), perchloric acid and ascorbic acid (Merck, Germany).
Intracortical in vivo microdialysis studies in awake, freely moving mice
Concentric dialysis probes were made of cuprophan fibres and set up as described previously (Malagié et al., 2001). All probes presented an active length of 2 mm for the FCx (× 0.30 mm outer diameter). Animals were anaesthetized with chloral hydrate (400 mg kg−1, i.p.). They were placed in a stereotaxic frame and were implanted with two probes according to a mouse brain atlas (Franklin & Paxinos, 1997): coordinates from bregma (in mm) of the left and right FCx: A=+2.0, L=±1.2, V=−1.6. Animals were allowed to recover from the surgery overnight. The following day, approximately 20 h after the surgery, the probes were continuously perfused with an artificial cerebrospinal fluid (aCSF) (composition in mM: NaCl 147, KCl 3.5, CaCl2 1.26, MgCl2 1.2, NaH2PO4 1.0, NaHCO3 25.0, pH 7.4±0.2) at a flow rate of 1.5 μl/min using a CMA/100 pump (Carnegie Medicin, Stockholm, Sweden). At 1 h after the start of aCSF perfusion, four fractions were collected every 15 min to measure the basal values (mean±s.e.m. corresponding to B0 at t0) calculated for each mouse before systemic administration of either saline, paroxetine, citalopram or venlafaxine. For extracellular NA level measurements, 2 μl of an antioxidant mixture (100 μM perchloric acid 70–72%, 50 μM L(+) ascorbic acid and 270 μM EDTA) was added in Eppendorf tubes to protect NA from degradation. After collections, samples were immediately frozen at −80°C, then analysed with two different high-performance liquid chromatography (HPLC) methods, one for 5-HT as previously described (Malagié et al., 2000; Gardier et al., 2003), and another one for NA adapted from Malagié et al. (2001), as indicated below.
Analysis of 5-HT dialysats
Briefly, cortical dialysates were analysed for 5-HT by using a HPLC system (XL-ODS, 4.6 × 75 mm, particle size 3 μM; Beckman) coupled to an amperometric detector (HP1049A, Hewlett-Packard, Les Ullis, France). The column was maintained at room temperature (≈22°C) and the flow rate of the mobile phase was 0.7 ml min−1.
Analysis of NA in dialysates
The HPLC system consisted of a programmable solvent module 116, an isocratic pump (Beckman), in combination with a pulse damper, an automatic injector system (Carnegie Medicin, Stockholm, Sweeden) and a silica-based reverse-phase column (Nucleosil, spherical particles of 5 μm, packed in a column of 15 cm with internal diameter of 4.6 mm, Macherey-Nagel, Hoerdt, France) coupled to a coulometric detector Coulochem (ESA, Bedford, MA, U.S.A.). The potentials was set up at 0 mV for the guard cell, and at –10 mV (E1), +350 mV (E2) for the analytical cell. The mobile phase consisted of 100 mM sodium acetate trihydrate, 40 mM citric acid monohydrate, 7.4 mM 1-octanesulphonic acid sodium salt hydrate, 270 μM disodium EDTA, dihydrate ACS reagent with 0.17‰ dibutylamine and 7% methanol HPLC grade. The pH of the mobile phase was 4.55±0.05 U pH. The column was maintained at room temperature (≈22°C) and the flow rate of the mobile phase was 1.5 ml min−1.
As desipramine is considered the most selective noradrenergic reuptake inhibitor as yet available (Ki=0.83 nM, Sánchez & Hytell, 1999), we set up an experiment to validate the specificity of our HPLC assay for NA levels. We thus measured the effects of an acute desipramine administration on cortical [NA]ext levels in mice. In agreement with earlier studies performed in rats (Mateo et al., 1998; Seo et al., 1999), a preliminary experiment indicated that desipramine (15 mg kg−1, s.c.) increased [NA]ext in the FCx (mean±s.e.m. for area under the curve (AUC) values calculated for the amount of NA outflow collected in the FCx during the 0–120 min period postdrug administration and expressed as a percentage of basal values=163.7±6.6% (n=11) in Swiss mice).
Data presentation and statistical analysis
Statistical analyses were performed by using the computer software StatView 4.02. (Abacus Concepts, Inc., Berkley, CA, U.S.A.). Data (mean±s.e.m.) in Figures 1, 2 and 3(a–c) were standardized by transforming dialysate 5-HT concentrations into percentages of baseline values based on averages of the first four fractions uncorrected for in vitro probe recovery. In Figures 1, 2 and 3 groups of Swiss mice (n=6–10) were exposed to saline, paroxetine (1, 4, 8 mg kg−1), citalopram or venlafaxine. In Figures 1, 2 and 3 (d), the AUC (mean±s.e.m.) values were calculated for the amount of 5-HT or NA outflow collected from the FCx during the 0–120 min post-treatment period following the i.p. injection of antidepressant drugs, and were expressed as a percentage of baseline. Statistical analysis was carried out using a one-way ANOVA on AUC values, with the various treatments (NaCl 0.9%, paroxetine, citalopram or venlafaxine) at the three doses tested (1, 4, 8 mg kg−1) as main factor, followed by Fisher protected least significance difference (PLSD) post hoc test.
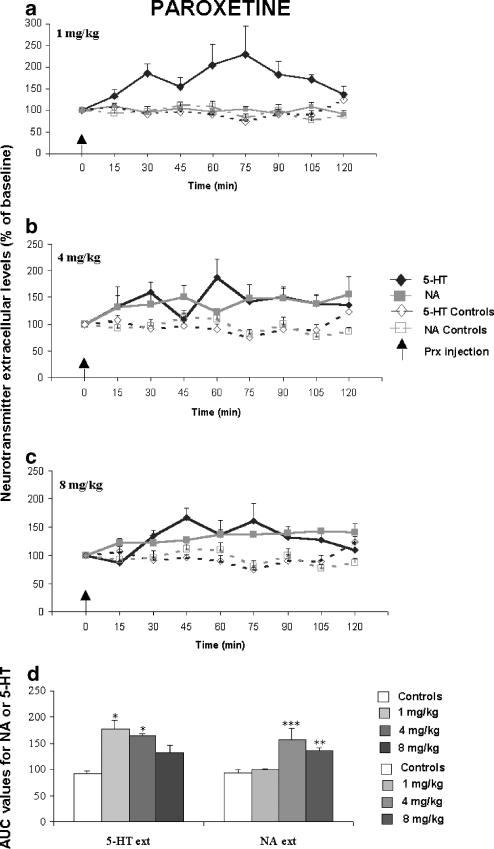
Figure 1.
Effect of a single administration of paroxetine (Prx) (a–c) (1, 4, 8 mg kg−1) on extracellular 5-HT [5-HT]ext and NA levels [NA]ext in the FCx of awake, freely moving naive Swiss mice. Data are means±s.e.m. (bars) values of extracellular 5-HT and NA levels expressed as percentages of baseline (B0) in Swiss mice following exposure to saline or antidepressant drugs, respectively (n=5–10 determinations for each group). The arrows indicate drug injection. In the FCx (1D) AUC (mean±s.e.m.), values were calculated for the amount of neurotransmitter 5-HT and NA outflow collected during 0–120 min post-treatment for paroxetine. AUCs are expressed as a percentage of basal values for all antidepressants. *P<0.05, **P<0.01, ***P<0.001 relative to the saline-treated control group.
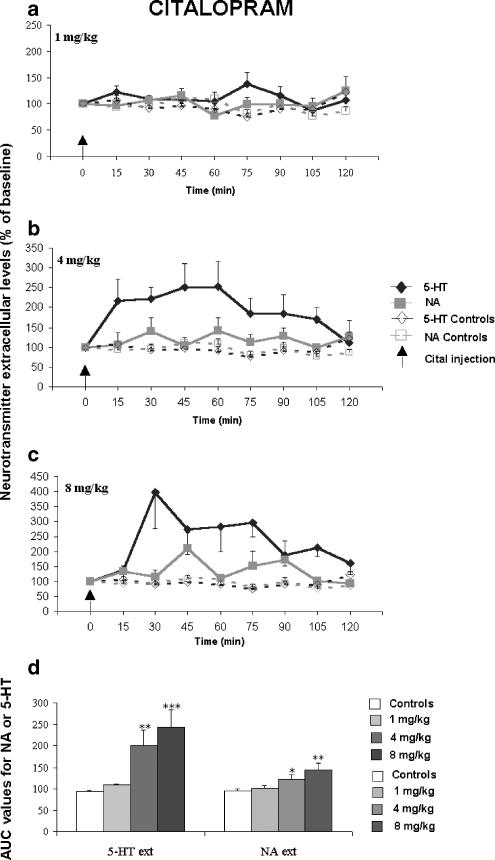
Figure 2.
Effect of a single administration of citalopram (Cital) (a–c) (1, 4, 8 mg kg−1) on extracellular 5-HT [5-HT]ext and NA levels [NA]ext in the FCx of awake, freely moving naive Swiss mice. Data are means±s.e.m. (bars) values of extracellular 5-HT and NA levels expressed as percentages of baseline (B0) in Swiss mice following exposure to saline or antidepressant drugs, respectively (n=5–10 determinations for each group). The arrows indicate drug injection. In the FCx (2D) AUC (mean±s.e.m.), values were calculated for the amount of neurotransmitter 5-HT and NA outflow collected during 0–120 min post-treatment for citalopram. AUCs are expressed as a percentage of basal values for all antidepressants. *P<0.05, **P<0.01, ***P<0.001 relative to the saline-treated control group.
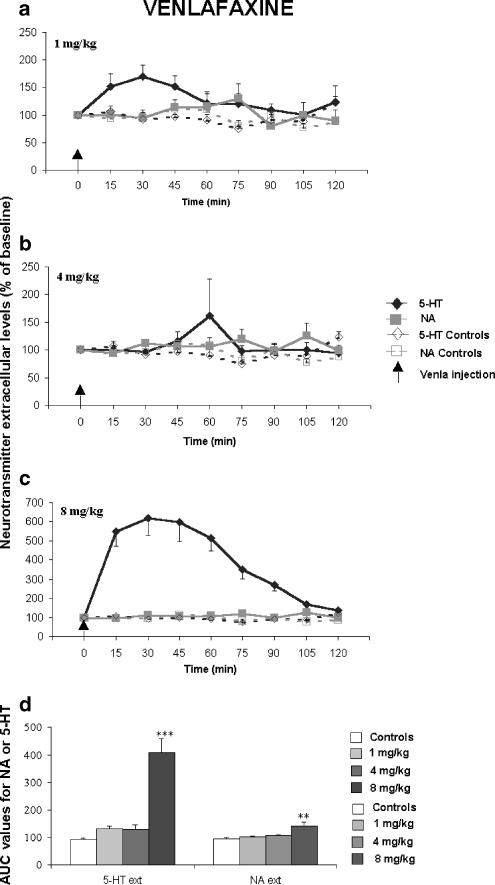
Figure 3.
Effect of a single administration of venlafaxine (Venla) (a–c) (1, 4, 8 mg kg−1) on extracellular 5-HT [5-HT]ext and NA levels [NA]ext in the FCx of awake, freely moving naive Swiss mice. Data are means±s.e.m. (bars) values of extracellular 5-HT and NA levels expressed as percentages of the baseline (B0) in Swiss mice, following exposure to saline or antidepressant drugs, respectively (n=5–10 determinations for each group). The arrows indicate drug injection. In the FCx (3D) AUC (mean±s.e.m.), values were calculated for the amount of neurotransmitter 5-HT and NA outflow collected during 0–120 min post-treatment for venlafaxine. AUCs are expressed as a percentage of basal values for all antidepressants. *P<0.05, **P<0.01, ***P<0.001 relative to the saline-treated control group.
In addition, the effects of antidepressant drugs on dialysate 5-HT and NA levels were calculated to yield a theoretical maximum effect (efficacy), as described by Tallarida & Murray (1987). The ED50 values (doses of antidepressant drugs that elicit a half-maximal increase in basal neurotransmitter levels) and 95% confidence intervals were calculated by using the computer software Prism 2.0 (GraphPad Software, Inc., U.S.A.). For this purpose, we used the two fractions in which we found a maximal effect in each mouse following antidepressant drug administration. The in vivo potency of these antidepressant drugs was calculated according to the method of Tallarida & Murray (1987). The statistical differences of Emax 5-HT or Emax NA between antidepressants were determined by a Sidak post hoc test following a one-way ANOVA. The comparison between Emax 5-HT and Emax NA for a particular antidepressant drug (i.e., paroxetine and citalopram) was determined by the Mann–Whitney U-test. Statistical significance was set at P<0.05.
Results
Effects of antidepressant drugs on extracellular 5-HT and NA levels in the FCx of Swiss mice
Basal extracellular levels of 5-HT and NA (in fmol 20 μl−1) in the FCx (mean experiments, n=60 animals) were 3.6±0.2 and 2.4±0.2, respectively.
A dose–response experiment was undertaken to determine the effects of a single administration of paroxetine, citalopram and venlafaxine on [5-HT]ext and [NA]ext in the FCx of Swiss mice. Thus, both SSRIs (i.e., paroxetine, citalopram) and the SNRI (venlafaxine) induced a statistically significant increase in cortical [5-HT]ext (F(9,76)=11.84, P=0.001). The maximal increase in AUC values for [5-HT]ext was 177.1±16.6% (P<0.05) of the respective basal values (100%) for the lowest paroxetine dose (1 mg kg−1) (Figures 1a, d). Citalopram significantly increased the cortical [5-HT]ext at the intermediate and the highest doses studied (4 and 8 mg kg−1, respectively): the maximal increase in AUC values was 243.6±41.0% (P<0.001) of the respective basal values (100%) induced by the 8 mg kg−1 dose (Figures 2c, d). Venlafaxine significantly increased cortical [5-HT]ext only at the highest dose (8 mg kg−1): the maximal increase in AUC values was to 410.5±47.1% of the respective basal values (100%; P<0.01) (Figures 3c, d). This increase was greater than those measured for the two SSRIs studied.
The SSRIs as well as the SNRI induced statistically significant increases in [NA]ext in the FCx of Swiss mice (F(9,76)=5.13, P=0.001). At the lowest dose tested, paroxetine (1 mg kg−1) increased cortical [5-HT]ext, but not [NA]ext. The 4 and 8 mg kg−1 paroxetine doses significantly increased AUC [NA]ext values (P<0.01; P<0.001) (Figures 1b–d). The maximal effect in AUC values was 158.1±20.7% of the respective basal values (100%) for the 4 mg kg−1 dose. Citalopram induced a dose-dependent increase in cortical [NA]ext and the maximal effect in AUC values was 144.4±15.19% of the respective basal values (100%; P<0.01) for the 8 mg kg−1 dose (Figures 2c, d). Venlafaxine did not alter cortical [NA]ext at 1 and 4 mg kg−1, but increased it at the highest dose tested: the increase in AUC values was 141.5±13.6% of the respective basal values (100%; P<0.01) (Figures 3c, d).
In vivo effects of various doses of antidepressant drugs (paroxetine, citalopram, venlafaxine) on [5-HT]ext and [NA]ext in the FCx of mice were compared by dose–response curves, by calculating pharmacological parameters such as ED50 values (potency) as well as the theoretical maximal responses (efficacy) of mice to antidepressant drugs in the intracerebral microdialysis test.
Potency of antidepressant drugs in vivo
The ED50 values and 95% confidence intervals (in parentheses) are given in Table 1 . The increase in cortical [5-HT]ext induced by a single administration of paroxetine was already maximal at the lowest dose studied (1 mg kg−1); so its potency to block the 5-HT transporter could not be determined in vivo in mice. Among the two other antidepressant drugs studied, citalopram was more potent than venlafaxine in increasing cortical [5-HT]ext in vivo (ED50 value=4.20 mg kg−1 with a 95% confidence interval 3.80–4.60, and 6.65 mg kg−1 with a 95% confidence interval 6.50–6.80, respectively). Among the three antidepressant drugs tested, paroxetine, having the lowest calculated ED50 value (1.8 mg kg−1 with a 95% confidence interval 1.70–1.90) was more potent than citalopram and venlafaxine in augmenting cortical [NA]ext in vivo in mice. Citalopram blocked 5-HT and NA transporters in vivo in mice with a comparable potency (calculated ED50 values and 95% confidence intervals were 4.20 (3.80–4.60) and 3.90 (3.30–4.50) mg kg−1, respectively). Venlafaxine did not alter cortical [NA]ext at 1 and 4 mg kg−1; so its potency to block the NA transporter could not be determined in vivo in mice.
Table 1.
Comparison–effect relationship for the acute paroxetine, citalopram and venlafaxine administration in the frontal cortex of Swiss mice
| Potency | Efficacy | |||
|---|---|---|---|---|
| ED50 (dialysate 5-HT) (mg kg−1) | ED50 (dialysate NA) (mg kg−1) | Emax (dialysate 5-HT) (% of maximal increase) | Emax (dialysate NA) (% of maximal increase) | |
| Paroxetine | ND | 1.80 (1.70–1.90) | 231.4±15.41***; +++ | 183.10±19.60 |
| Citalopram | 4.20 (3.80–4.60) | 3.90 (3.30–4.50) | 462.5±43.80++; @@@ | 245.0±45.15 |
| Venlafaxine | 6.65 (6.50–6.80) | ND | 634.45±59.59 | ND |
The potency was calculated as the ED50 values to induce increases in extracellular levels of 5-HT and NA with a 95% confidence interval (in parentheses). The efficacy was calculated as the maximal effects based on the AUC values of the antidepressant drugs on dialysate 5-HT and NA. Emax (mean±s.e.m.; dialysate 5-HT): F(2.37)=25.83, P<0.001.
P<0.001 when compared to citalopram.
P<0.01 and +++P<0.001 when compared to venlafaxine. Emax (dialysate): NA F(2.42)=0.303, P=0.74. Comparison between Emax 5-HT and Emax NA for paroxetine: F(1.29)=1.96, P=0.72. Comparison between Emax 5-HT and Emax NA for citalopram: F(1.28)=12.1, P<0.002; the Mann–Whitney U-test=34.0;
P<0.001 when compared to Emax NA. ND could not be determined by the computer software prism 2.0. ND indicates that the values could not be determined by the computer software Prism 2.0.
Efficacy of antidepressant drugs in vivo
The Emax values (% maximal increase±s.e.m.) are given in Table 1. The SNRI venlafaxine displayed a greater efficacy in increasing cortical [5-HT]ext compared to the two SSRIs studied, paroxetine (P<0.01) and citalopram (P<0.001). Citalopram gave a more efficient response than paroxetine on [5-HT]ext (P<0.001): Emax values indicate that cortical [5-HT]ext increased to 231, 462 and 634% of the baseline, following paroxetine, citalopram and venlafaxine administration, respectively (i.e. only 131, 362 and 534% increases were measured for the three antidepressant drugs studied, respectively) (Table 1). Thus, the rank order of the efficacy of the antidepressant effects on cortical [5-HT]ext in mice was as follows: venlafaxine>citalopram>paroxetine. In contrast, paroxetine and citalopram elicited a comparable efficacy on cortical [NA]ext as they both induced similar maximal increases of this parameter. Emax values indicate that cortical [NA]ext increased to 183 and 245% of the baseline, following paroxetine and citalopram administrations, respectively (i.e., only 83 and 145% increases were measured for the two SSRIs studied, respectively) (Table 1). Furthermore, paroxetine had a similar efficacy on both cortical [5-HT]ext and [NA]ext, while citalopram had a higher efficacy in increasing cortical [5-HT]ext than [NA]ext (P<0.002).
Discussion
In the present in vivo study, we found that a single administration of SSRIs (paroxetine, citalopram) and an SNRI (venlafaxine) induced increases in the extracellular levels of 5-HT and NA in the dialysates from the FCx of awake, freely moving mice. Depending on the antidepressant drugs studied, these increases occurred either at 1, 4 or 8 mg kg−1 given systemically. In our experimental conditions, citalopram and paroxetine have the highest potency to increase cortical [5-HT]ext and [NA]ext, respectively. These effects may have occurred via the blockade of 5-HT transporter and/or the NA transporter in vivo. The rank order of efficacy of these antidepressant drugs to increase [5-HT]ext in the FCx of mice was as follows: venlafaxine>citalopram>paroxetine. Furthermore, paroxetine was as efficient as citalopram in increasing cortical [NA]ext, and the efficacy of the two SSRIs studied on dialysate NA was greater than that of the SNRI, venlafaxine (paroxetine=citalopram>venlafaxine).
The FCx plays a crucial role in processes involved in the control of mood, cognition and motor behaviour, functions that are compromised in depression (Millan et al., 2000). A perturbation of corticolimbic serotonergic and noradrenergic transmissions is implicated in the aetiology of depression, and antidepressant drugs elevating the monoamine neurotransmission in the FCx likely plays a role in depression therapy (Tanda et al., 1994; Gobert et al., 1997). Preclinical studies have clearly shown that the 5-HT and NA systems interact extensively in the brain, and it would be difficult to investigate how a given molecule affects one system, without considering its influence on others (Gobert et al., 1997; Blier, 2001; Koch et al., 2002). By using in vivo microdialysis in the FCx of awake mice, we tested whether SSRIs, such as paroxetine and citalopram, may act at both noradrenergic and serotonergic systems in the brain. As it would be expected from their ability to block potently the 5-HT transporter, we demonstrated that paroxetine and citalopram enhanced the dialysate levels of 5-HT in the FCx. Similar results have been obtained in this brain region by others in rats (Arborelius et al., 1996; Millan et al., 2001; Bymaster et al., 2002) and by our group in mice (Malagié et al., 2001,2002), following administration of a single dose of SSRIs.
We also found that cortical [5-HT]ext looks stable in the range of paroxetine doses studied. Surprisingly, the effects of paroxetine decline at the highest dose tested. The absence of a statistically significant effect of the 8 mg kg−1 dose of paroxetine on cortical [5-HT]ext might be due to the randomization of our treated groups of mice.
In a previous behavioural study, we used the FST in Swiss mice to evaluate the possible noradrenergic-like activity of paroxetine (Redrobe et al., 1998b). We found that a pretreatment with p-chlorophenylalanine (PCPA, a serotonin synthesis inhibitor) attenuated the antidepressant-like effect of a low dose of paroxetine in the FST, whereas a higher dose of this SSRI remained active. These data suggest that paroxetine displays both serotonergic and noradrenergic activities in the preclinical psychopharmacological test employed. Here, our neurochemical results obtained by using the microdialysis technique in Swiss mice are in line with those obtained in the FST. Indeed, both SSRIs, especially paroxetine, simultaneously elevated [NA]ext and [5-HT]ext in the FCx. Thus, these results observed with two different in vivo assays, behavioural and neurochemical approaches, the FST and the microdialysis study, respectively, led to the same conclusion: paroxetine may have effects on noradrenergic system in vivo in Swiss brain mice.
In vitro data indicate that citalopram is the most selective SSRI for inhibiting the neuronal 5-HT transporter, whereas paroxetine is the most potent one (Sánchez & Hytell, 1999). Our experiment allowed us to characterize in vivo both SSRIs, analysing their potency and their efficacy in blocking 5-HT and NA transporters. In the dose range tested (i.e., 1, 4 and 8 mg kg−1), citalopram displayed a similar potency to increase cortical [5-HT]ext and [NA]ext, while the potency of paroxetine to increase cortical [5-HT]ext could not be determined. As previously observed in rats (Romero & Artigas, 1997), here, in mice we found that paroxetine reached a ceiling effect on [5-HT]ext when administered at relatively low doses, whereas citalopram and venlafaxine increased cortical [5-HT]ext dose-dependently. However, paroxetine displayed the highest potency to increase cortical [NA]ext. Thus, when measuring AUC values, we clearly demonstrate that, even if paroxetine and citalopram belong to the same class of antidepressant drugs, their pharmacological profiles were different in vivo, following a single acute administration. These in vivo data barely correlate with in vitro data. The clinical relevance of the in vivo findings obtained here in mice is currently unknown.
Our study is the first which demonstrates an influence of a single administration of SSRI simultaneously on extracellular levels of 5-HT and NA in the FCx using microdialysis in awake, freely moving mice. Indeed, the effects of a single dose of SSRI on [NA]ext levels using intracerebral in vivo microdialysis technique in the FCx have been mainly studied in rats. However, major discrepancies regarding these effects of acute treatment with various SSRIs have been reported in the literature in this species. Fluvoxamine (12 mg kg−1, i.p.) (Shachar et al., 1997) or, more recently, fluoxetine (10 mg kg−1, s.c.) augmented both [NA]ext and [5-HT]ext (by 90 and 110%, respectively) in the rat FCx (Gobert et al., 1997). The stereoisomer R-fluoxetine (20 mg kg−1, i.p.) increased both [5-HT]ext and [NA]ext levels in the prefrontal cortex (Koch et al., 2002). In addition, Millan et al. (1999),(2000) recently showed that citalopram increased [NA]ext in rat FCx, although an increase in [NA]ext was also found in saline-treated rats.
In contrast, sertraline did not alter noradrenaline release in rat cortex and hippocampus, when administered acutely (Thomas et al., 1998). Hajós-Korcsok et al. (2000) showed in awake, freely moving rats that a single paroxetine dose of 5 mg kg−1 did not elevate [NA]ext in the hippocampus, while [5-HT]ext was increased. Conversely, in the FCx, it has been demonstrated that paroxetine increased NA levels following administration of a very high dose to rats (30 mg kg−1) (Beyer et al., 2002). Different brain regions in the same species could lead to differences in the effect of the same SSRI on [NA]ext. The FCx is a complex neuronal system with widespread afferents of serotonergic, dopaminergic as well as noradrenergic neurons in rat (Lindvall et al., 1978), suggesting that this brain area could be preferable to demonstrate the noradrenergic effect of SSRIs. In addition, the effects of paroxetine and citalopram seem to differ between species, since we measured an increase in cortical [NA]ext in mice for both SSRIs, whereas recently Bymaster et al. (2002) found that, with the exception of fluoxetine, SSRIs (citalopram, paroxetine and sertraline) did not increase [NA]ext in the rat FCx. Consistent with these latter microdialysis results, but not with ours obtained in mice, an electrophysiological study found that paroxetine had no effect on the firing of noradrenaline neurones in rats (Szabo et al., 1999).
In our study, we compared the effects of the two SSRIs with those of venlafaxine because this SNRI was marketed as a dual 5-HT and NA reuptake inhibitor (Artigas, 1995). We found that venlafaxine was inactive at 1 and 4 mg kg−1 in mice since it did not increase cortical [5-HT]ext and [NA]ext levels. Only the 8 mg kg−1 dose had a significant effect on dialysate levels of these neurotransmitters, the peak accumulation of 5-HT (410%) in the FCx being considerably greater than that of NA (141%). These results are somewhat surprising because venlafaxine is assumed to block NA reuptake in vivo at the dose we used. They are clearly different from previous studies performed in rat FCx, showing a dose-dependent increase in [NA]ext, while [5-HT]ext did not increase following administration of a single venlafaxine dose (3–50 mg kg−1, s.c.) (Dawson et al., 1999). Thus, the effects of venlafaxine on cortical [5-HT]ext and [NA]ext levels seem to differ between species (rats versus mice). However, Gur et al. (1999) found that an acute administration of venlafaxine resulted in dose-dependent increases in cortical and hippocampal [5-HT]ext over the range 5–20 mg kg−1. Millan et al. (2001) indicated that basal [5-HT]ext and [NA]ext levels increase from the lower dose of venlafaxine in the rat FCx (0.63 mg kg−1). The potency and selectivity of venlafaxine for 5-HT/NA systems have also been questioned (Beïque et al., 1998). An in vivo study has shown that the affinity of venlafaxine for the 5-HT transporter is greater than its affinity for the NA transporter (Gur et al., 1999). Our neurochemical data suggest that, in mice, low doses of venlafaxine (<10 mg kg−1) are able to increase cortical [5-HT]ext more than [NA]ext levels in vivo.
Our neurochemical data are consistent with the behavioural study of Redrobe et al. (1998c) regarding the antidepressant-like effect of venlafaxine in Swiss mice in the FST: at low doses, this SNRI displayed an activity indicative of in vivo inhibition of 5-HT reuptake, while at 8 mg kg−1 and higher doses it mobilized both serotonergic and noradrenergic brain neurotransmissions in the Swiss mice (Redrobe et al., 1998c). However, in the present study, the profile of venlafaxine's effects differs from that of paroxetine at 8 mg kg−1. Indeed, an acute treatment with venlafaxine increased cortical [NA]ext, but the increase in cortical [5-HT]ext was more pronounced, while paroxetine equally increase cortical [5-HT]ext and [NA]ext in mice. Knowing that, at an 8 mg kg−1 dose, the magnitude of antidepressant-like effect of venlafaxine in the FST is slightly greater (20%) than the size effect of paroxetine (14%) (Redrobe et al., 1998b,1998c). Our microdialysis data suggest that the increase in cortical [5-HT] could play a more important role than NA in this antidepressant-like effect of venlafaxine.
Our microdialysis study clearly demonstrates that SSRIs, that is, paroxetine and citalopram, can not only increase cortical [5-HT]ext, but also [NA]ext in vivo. The effects of the very selective SSRI citalopram or the very potent SSRI paroxetine on cortical [NA]ext could be either a direct effect of the drug on the NA transporter or an indirect effect on brain noradrenergic function. The first hypothesis may involve increases in cortical [NA]ext via the direct blockade of NA transporter by paroxetine and citalopram. There are several lines of evidence suggesting that SSRIs can directly inhibit NA transporters, thus being devoid of selectivity for the brain serotonergic system in vivo. It is possible that the same dose of the three different antidepressant drugs administered to Swiss mice led to an equivalent brain exposure and therefore occupancy at the NA and 5-HT transporters in vivo. Thus, synaptosomal uptake of [3 H]noradrenaline could be used to test whether inhibition of uptake could contribute to SSRI-induced increases in [NA]ext in vivo. Positive results have already been obtained in rats (Hughes & Stanford, 1996; Stanford, 1996). The blockade of catecholamine reuptake by SSRIs has also been suggested by several studies (Owens et al., 2000; Shinkai et al., 2002). Indeed, Shinkai et al. (2002) demonstrated that paroxetine competitively inhibited the functional activity of the NA transporter by acting on a desipramine-binding site. Similarly, noradrenaline efflux was increased during local infusion of either citalopram or fluoxetine (5 or 50 μM for 3 h) in the FCx of anaesthetized rats (Hughes & Stanford, 1996,1998). This effect was abolished by a pretreatment with a selective noradrenergic neurotoxin, suggesting that SSRI can directly bind to NA transporters located on noradrenergic neurones (Hughes & Stanford, 1998). Since SSRIs were administered systemically in the present study, they may have blocked NA transporters either in the FCx (where the dialysis probe was implanted), or elsewhere in the brain. Further experiments studying the effects of a local infusion of these antidepressant drugs through a microdialysis probe implanted either into the FCx (at noradrenergic nerve terminals) or the locus coeruleus (the main source of noradrenergic cell bodies innervating the FCx) may help to clarify this issue. The NA transporter blockade seems to occur mainly in the locus coeruleus (LC), since local administration of citalopram by reverse dialysis in the LC increased [NA]ext in the LC, thus leading to the subsequent inhibition of LC neurones via alpha(2)-adrenoceptors in rats (Mateo et al., 2000). Then, the decreased firing rate of noradrenergic neurones leads to decreased release of NA in the cortex.
The indirect hypothesis suggests that an increase in [5-HT]ext, resulting from the systemic administration of a SSRI, could secondly increase NA release in various nerve terminal areas of the brain. It implies that the activation of 5-HT heteroreceptors located on noradrenergic nerve terminals may occur in the present study. Mateo et al. (2000) concluded from their results (described above) that SSRI-induced NA release is dependent on the presence of endogenous 5-HT in the LC, since a pretreatment with PCPA prevented the effects of a local administration of citalopram on [NA]ext in rats. We have not investigated this hypothesis in mice yet, but Stanford (1996) excluded the indirect hypothesis as an explanation for the results of similar in vivo experiments.
The effects of antidepressants on serotonergic and noradrenergic neurones are not independent, because there is a close anatomical and functional relationship between the two pathways in the brain (Nutt, 2002). In nerve terminal regions of the brain, through an unclear mechanism, SSRIs can produce an overall facilitation of noradrenergic transmission (Hughes & Stanford, 1996). Bymaster et al. (2002) have shown that the increase in [NA]ext measured in the prefrontal cortex following administration of fluoxetine in the awake, freely moving rat was not due to a nonselective blockade of NA uptake, but to an interaction with the 5-HT2C receptor subtype, blocking the serotonergic inhibitory control on NA release in the locus coeruleus. It has been also demonstrated that a systemic administration of 5-HT1A receptor agonists increases NA release in the rat FCx through activation of postsynaptic 5-HT1A heteroreceptors (Hajós-Korcsok et al., 1999).
In conclusion, our data confirm that SSRIs lack selectivity in vivo to block the 5-HT transporter because these antidepressant drugs can simultaneously increase cortical [5-HT]ext and [NA]ext in mice. Differences between the pharmacological properties of these drugs, that is, their potency and efficacy in increasing dialysate 5-HT and NA, clearly exist in vivo in this species. In the FCx of Swiss mice, following its acute administration, citalopram was the most potent SSRI to increase cortical [5-HT]ext, while paroxetine was more potent than citalopram and venlafaxine in increasing cortical [NA]ext. In the range of doses tested, a dose of one of the three compounds studied either (i) had no effects on dialysate 5-HT and NA (citalopram at 1 mg kg−1; venlafaxine at 1 and 4 mg kg−1), or (ii) selectively blocked a transporter (paroxetine for the 5-HT transporter at 1 mg kg−1), or (iii) in most of the cases, exhibited nonselective effects (paroxetine and citalopram at 4 and 8 mg kg−1; venlafaxine at 8 mg kg−1). Our results suggest that paroxetine and citalopram, currently classified as SSRIs, can act as mixed 5-HT/NA reuptake inhibitors in vivo. For example, paroxetine can block both 5-HT and NA transporters in vivo at doses ⩾4 mg kg−1 in mice, at least in the FCx. This idea has recently been supported by a clinical study in which NA and 5-HT transporter functions were assayed by using human transporter-transfected cells in the presence of serum collected from patients with major depressive disorder treated with paroxetine (Gilmor et al., 2002). The clinical significance of this action of paroxetine on the brain noradrenaline transporter is currently unknown, but it may contribute to its broad therapeutic efficacy since it can be prescribed either in the treatment of depression or in anxiety disorders. Our results are especially important because several clinical studies have revealed that using drugs that block both NA and 5-HT transporters in the brain may represent a more effective antidepressant treatment for severe or refractory depression than a single selective treatment (Nelson, 1998; Thase et al., 2001).
Acknowledgments
This work was supported by SmithKline Beecham Laboratories. The Department of Neuropharmacology is an ‘Équipe d'accueil' (EA 3544-2002-2005) from the ‘Ministère de la Jeunesse, de l'Éducation Nationale et de la Recherche' (MJENR-France). We are grateful to Bruno Guiard, Jean Philippe Guilloux and Bríd Áine. Nic Dhonnchadha from the two labs in Châtenay-Malabry and Nantes for critical reading of the manuscript.
Abbreviations
- FCx
frontal cortex
- 5-HT
5-hydroxytryptamine (serotonin)
- NA
noradrenaline
- SNRI
serotonin noradrenaline reuptake inhibitor
- SSRIs
selective serotonin reuptake inhibitors
References
- ARBORELIUS L., NOMIKOS G.G., HERTEL P., SALMI P., GRILLNER P., HOOK B.B., HACKSELL U., SVENSSON T.H. The 5-HT1A receptor antagonist (S)-UH-301 augments the increase in extracellular concentrations of 5-HT in the frontal cortex produced by both acute and chronic treatment with citalopram. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;353:630–640. doi: 10.1007/BF00167182. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F. Selective serotonin/noradrenaline reuptake inhibitors (SNRIs) CNS Drugs. 1995;4:79–89. [Google Scholar]
- BARKER E.L., KIMMEL H.L., BLAKELY R.D. Chimeric human and rat serotonin transporters reveal domains involved in recognition of transporter ligands. Mol. Pharmacol. 1994;46:799–807. [PubMed] [Google Scholar]
- BEÏQUE J.C., LAVOIE N., DE MONTIGNY C., DEBONNEL G. Affinities of venlafaxine and various reuptake inhibitors for the serotonin and norepinephrine transporters. Eur. J. Pharmacol. 1998;349:129–132. doi: 10.1016/s0014-2999(98)00241-6. [DOI] [PubMed] [Google Scholar]
- BEYER C.E., BOIKESS S., LUO B., DAWSON L.A. Comparison of the effects of antidepressants on norepinephrine and serotonin concentrations in the rat frontal cortex: an in-vivo microdialysis study. J. PsychoPharmacol. 2002;16:297–304. doi: 10.1177/026988110201600403. [DOI] [PubMed] [Google Scholar]
- BLIER P. Norepinephrine and selective norepinephrine reuptake inhibitors in depression and mood disorders: their pivotal roles. J. Psychiatry Neurosci. 2001;26 Suppl:S1–S2. [PMC free article] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- BOLDEN-WATSON C., RICHELSON E. Blockade by newly developed antidepressants of biogenic uptake into rat brain synaptosomes. Life Sci. 1993;52:1023–1029. doi: 10.1016/0024-3205(93)90194-8. [DOI] [PubMed] [Google Scholar]
- BOURIN M., COLOMBEL M.C., REDROBE J.P., NIZARD J., HASCOËT M., BAKER G.B. Evaluation of efficacies of different classes of antidepressants in the forced swimming test in mice at different ages. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1998;22:343–351. doi: 10.1016/s0278-5846(98)00009-8. [DOI] [PubMed] [Google Scholar]
- BYMASTER F.P., ZHANG W., CARTER P.A., SHAW J., CHERNET E., PHEBUS L., WONG D.T., PERRY K.W. Fluoxetine, but not other selective serotonin reuptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology. 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- DAWSON L.A., NGUYEN H.Q., GEIGER A. Effects of venlafaxine on extracellular concentrations of 5-HT and noradrenaline in the rat frontal cortex: augmentation via 5-HT1A receptor antagonism. Neuropharmacology. 1999;38:1153–1163. doi: 10.1016/s0028-3908(99)00052-0. [DOI] [PubMed] [Google Scholar]
- DEAKIN B., DURSUN S. Optimizing antidepressant treatment: efficacy and tolerability. Int. Clin. PsychoPharmacol. 2002. pp. S13–S24. [DOI] [PubMed]
- DELGADO P.L., PRICE L.H., HENINGER G.R., CHARNEY D.S.Neurochemistry of affective disorders Handbook of Affective Disorders 1992Livingston, NY: Churchill; 219–253.ed. Paytkel E.S. pp [Google Scholar]
- EINARSON T.R., ARIKIAN S.R., CASCIANO J., DOYLE J.J. Comparison of extended-release venlafaxine, selective serotonin reuptake inhibitors, and tricyclic antidepressants in the treatment of depression: a meta-analysis of randomized controlled trials. Clin. Ther. 1999;21:296–308. doi: 10.1016/S0149-2918(00)88287-9. [DOI] [PubMed] [Google Scholar]
- FRANKLIN K.B., PAXINOS G. The Mouse Brain in Stereotaxic Coordinates. Toronto/San Diego: Academic Press; 1997. [Google Scholar]
- GARDIER A.M., DAVID D.J., JEGO G., PRZYBYLSKI C., JACQUOT C., DURIER S., GRUWEZ B., DOUVIER E., BEAUVERIE P., POISSON N., HEN R., BOURIN M. Regulation of serotonin release in the frontal cortex and ventral hippocampus of homozygous mice lacking 5-HT1B receptors: in vivo microdialysis studies. J. NeuroChem. 2003;69:2019–2025. doi: 10.1046/j.1471-4159.1997.69052019.x. [DOI] [PubMed] [Google Scholar]
- GILMOR M.L., OWENS M.J., NEMEROFF C.B. Inhibition of norepinephrine uptake in patients with major depression treated with paroxetine. Am. J. Psychiatry. 2002;159:1702–1710. doi: 10.1176/appi.ajp.159.10.1702. [DOI] [PubMed] [Google Scholar]
- GOBERT A., RIVET J.M., CISTARELLI L., MELON C., MILLAN M.J. Alpha2-adrenoreceptor blockade markedly potentiates duloxetine- and fluoxetine-induced increases in noradrenaline, dopamine and serotonin levels in the frontal cortex of freely moving rats. J. NeuroChem. 1997;69:2616–2619. doi: 10.1046/j.1471-4159.1997.69062616.x. [DOI] [PubMed] [Google Scholar]
- GUR E., DREMENCOV E., LERER B., NEWMAN M.E. Venlafaxine: acute and chronic effects on 5-hydroxytryptamine levels in rat brain in vivo. Eur. J. Pharmacol. 1999;372:17–24. doi: 10.1016/s0014-2999(99)00164-8. [DOI] [PubMed] [Google Scholar]
- HAJÓS-KORCSOK E., MCQUADE R., SHARP T. Influence of 5-HT1A receptors on central noradrenergic system activity: microdialysis studies using (±)-MDL73005EF and its enantiomers. Neuropharmacology. 1999;38:299–306. doi: 10.1016/s0028-3908(98)00175-0. [DOI] [PubMed] [Google Scholar]
- HAJÓS-KORCSOK E., MCTAVISH S.F., SHARP T. Effect of a selective 5-hydroxytryptamine reuptake inhibitor on brain extracellular noradrenaline: microdialysis studies using paroxetine. Eur. J. Pharmacol. 2000;407:101–107. doi: 10.1016/s0014-2999(00)00723-8. [DOI] [PubMed] [Google Scholar]
- HUGHES Z.A., STANFORD S.C. Increased noradrenaline efflux induced by local infusion of fluoxetine in the rat frontal cortex. Eur. J. Pharmacol. 1996;317:83–90. doi: 10.1016/s0014-2999(96)00715-7. [DOI] [PubMed] [Google Scholar]
- HUGHES Z.A., STANFORD S.C. Evidence from microdialysis and synaptosomal studies of rat cortex for noradrenaline uptake sites with different sensitivities to SSRIs. Br. J. Pharmacol. 1998;124:1141–1148. doi: 10.1038/sj.bjp.0701947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH S., PERRY K.W., NELSON D.L., CONWAY R.G., THRELKELD P.G., BYMASTER F.P. R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: an in vivo microdialysis and receptor binding study. Neuropsychopharmacology. 2002;27:949–959. doi: 10.1016/S0893-133X(02)00377-9. [DOI] [PubMed] [Google Scholar]
- LINDVALL O., BJORKLUND A., DIVAC I. Organization of catecholamine neurons projecting to the frontal cortex in the rat. Brain Res. 1978;142:1–24. doi: 10.1016/0006-8993(78)90173-7. [DOI] [PubMed] [Google Scholar]
- MALAGIE I., DAVID D.J., JOLLIET P., HEN R., BOURIN M., GARDIER A.M. Improved efficacy of fluoxetine in increasing hippocampal 5-hydroxytryptamine outflow in 5-HT(1B) receptor knock-out mice. Eur. J. Pharmacol. 2002;443:99–104. doi: 10.1016/s0014-2999(02)01604-7. [DOI] [PubMed] [Google Scholar]
- MALAGIE I., DESLANDES A., GARDIER A.M. Effects of acute and chronic tianeptine administration on serotonin outflow in rats: comparison with paroxetine by using in vivo microdialysis. Eur. J. Pharmacol. 2000;403:55–65. doi: 10.1016/s0014-2999(00)00486-6. [DOI] [PubMed] [Google Scholar]
- MALAGIE I., TRILLAT A.C., BOURIN M., JACQUOT C., HEN R., GARDIER A.M. 5-HT1B autoreceptors limit the effects of selective serotonin re-uptake inhibitors in mouse hippocampus and frontal cortex. J. NeuroChem. 2001;76:865–871. doi: 10.1046/j.1471-4159.2001.00083.x. [DOI] [PubMed] [Google Scholar]
- MATEO Y., PINEDA J., UDEGO L., MEANA J.J. Somatodendritic 2-adrenoreceptors in the locus coeruleus are involved in the in vivo modulation of cortical noradrenaline release by the antidepressant desipramine. J. NeuroChem. 1998;71:790–798. doi: 10.1046/j.1471-4159.1998.71020790.x. [DOI] [PubMed] [Google Scholar]
- MATEO Y., RUIZ-ORTEGA J.A., UDEGO L., MEANA J.J. Inhibition of 5-hydroxytryptamine reuptake by the antidepressant citalopram in the locus coeruleus modulates the rat brain noradrenergic transmission in vivo. Neuropharmacology. 2000;39:2036–2043. doi: 10.1016/s0028-3908(00)00041-1. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J., GOBERT A., GIRARDON S., DEKEYNE A. Citalopram elicits a discriminative stimulus in rats at dose selectively increasing extracellular levels of serotonin vs dopamine and noradrenaline. Eur. J. Pharmacol. 1999;364:147–150. doi: 10.1016/s0014-2999(98)00802-4. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J., GOBERT A., LEJEUNE F., NEWMAN-TANCREDI A., RIVET J.M., AUCLAIR A., PEGLION J.L. S33005, a novel ligand at both serotonin and norepinephrine transporters : I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. J. Pharmacol. Exp. Ther. 2001;298:565–580. [PubMed] [Google Scholar]
- MILLAN M.J., LEJEUNE F., GOBERT A. Reciprocal autoreceptor and heteroreceptor control of serotonergic, dopaminergic and noradrenergic transmission in the frontal cortex: relevance to the actions of antidepressants agents. J. PsychoPharmacol. 2000;14:114–138. doi: 10.1177/026988110001400202. [DOI] [PubMed] [Google Scholar]
- NELSON J.C. Augmentation strategies with serotonergic–noradrenergic combinations. J. Clin. Psychiatry. 1998;59:65–68. [PubMed] [Google Scholar]
- NUTT D.J. The neuropharmacology of serotonin and noradrenaline in depression. Int. Clin. PsychoPharmacol. 2002;17:S1–S12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- OWENS M.J., KNIGHT D.L., NEMEROFF C.B. Paroxetine binding to the rat norepinephrine transporter in vivo. Biol. Psychiatry. 2000;47:842–845. doi: 10.1016/s0006-3223(99)00314-5. [DOI] [PubMed] [Google Scholar]
- REDROBE J.P., BOURIN M. Dose-dependant influence of buspirone on the activities of selective serotonin reuptake inhibitors in the mouse forced swimming test. Psychopharmacology. 1998a;138:198–206. doi: 10.1007/s002130050663. [DOI] [PubMed] [Google Scholar]
- REDROBE J.P., BOURIN M., COLOMBEL M.C., BAKER G.B. Psychopharmacological profile of the selective serotonin reuptake inhibitor, paroxetine: implication of noradrenergic and serotonergic mechanisms. J. PsychoPharmacol. 1998b;12:155–161. doi: 10.1177/026988119801200404. [DOI] [PubMed] [Google Scholar]
- REDROBE J.P., BOURIN M., COLOMBEL M.C., BAKER G.B. Dose-dependent noradrenergic and sertonergic properties of venlafaxine in animal models indicative of antidepressant activity. Psychopharmacology. 1998c;138:1–8. doi: 10.1007/s002130050638. [DOI] [PubMed] [Google Scholar]
- ROMERO L., ARTIGAS F. Preferential potentiation of the effects of serotonin uptake inhibitors by 5-HT1A receptor antagonists in the dorsal raphe pathway: role of somatodendritic autoreceptors. J. NeuroChem. 1997;68:2593–2603. doi: 10.1046/j.1471-4159.1997.68062593.x. [DOI] [PubMed] [Google Scholar]
- SÁNCHEZ C., HYTTEL J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell. Mol. NeuroBiol. 1999;19:467–489. doi: 10.1023/A:1006986824213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEO D.O., SHIN C.Y., LEE C.J., DAILEY J.W., REITH M.E.A., JOB P.C., KO H.H. Effect of alterations in extracellular norepinephrine on adrenoreceptors: a microdialysis study in freely moving rats. Eur. J. Pharmacol. 1999;365:39–46. doi: 10.1016/s0014-2999(98)00856-5. [DOI] [PubMed] [Google Scholar]
- SHACHAR D., KLEIN E., TABAK A., FINDBERG J.P. Effect of single and repeated administration of fluvoxamine on noradrenaline release in rat brain. Eur. J. Pharmacol. 1997;332:237–243. doi: 10.1016/s0014-2999(97)01084-4. [DOI] [PubMed] [Google Scholar]
- SHINKAI K., YOSHIMURA R., TOYOHIRA Y., YANAGIHARA N., NAKAMURA J. Inhibition mechanism of paroxetine, a selective serotonin reuptake inhibitor, on norepinephrine transporter activity. Int J. NeuropsychoPharmacol. 2002;5:S67. [Google Scholar]
- STANFORD S.C. Prozac: panacea or puzzle. Trends Pharmacol. Sci. 1996;17:150–154. doi: 10.1016/0165-6147(96)81591-4. [DOI] [PubMed] [Google Scholar]
- SZABO S.T., DE MONTIGNY C., BLIER P. Modulation of noradrenergic neuronal firing by selective serotonin reuptake blockers. Br. J. Pharmacol. 1999;126:568–571. doi: 10.1038/sj.bjp.0702343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALLARIDA R.J., MURRAY R.B. Manual of Pharmacological Calculations with Computer Programs. New York, U.S.A.: Springer-Verlag; 1987. [Google Scholar]
- TANDA G., CARBONI E., FRAU R., DI CHIARA G. Increase of extracellular dopamine in the prefrontal cortex: a trait of drugs with antidepressant potential. Psychopharmcology. 1994;115:285–288. doi: 10.1007/BF02244785. [DOI] [PubMed] [Google Scholar]
- THASE M.E., ENTSUAH A.R., RUDOLPH R.L. Remission rates during treatment with venlafaxine or selective serotonin reuptake inhibitors. Br. J. Psychiatry. 2001;178:234–241. doi: 10.1192/bjp.178.3.234. [DOI] [PubMed] [Google Scholar]
- THOMAS D.N., NUTT D.J., HOLMAN R.B. Sertraline, a selective serotonin reuptake inhibitor modulates extracellular noradrenaline in the rat frontal cortex. J. PsychoPharmacol. 1998;12:366–370. doi: 10.1177/026988119801200406. [DOI] [PubMed] [Google Scholar]
- VETULANI J., NALEPA I. Antidepressants: past, present and future. Eur. J. Pharmacol. 2000;405:351–363. doi: 10.1016/s0014-2999(00)00565-3. [DOI] [PubMed] [Google Scholar]