Abstract
Investigation into the modulatory effects of chlormethiazole at human recombinant γ-aminobutyric acid A receptor (GABAA) and N-methyl-D-aspartate (NMDA) receptors was undertaken to gain insight into its mechanism of action and determine if the drug exhibited any subtype-selective activity.
Despite a structural similarity to the β-subunit-selective compound loreclezole, chlormethiazole did not show any difference in maximum efficacy and only a slight difference in EC50 in its potentiating action at α1β1γ2 and α1β2γ2 GABAA receptor subtypes with preference for α1β1γ2.
Similar to the previously reported subtype-dependent activity of pentobarbital, chlormethiazole elicited a significantly greater degree of maximum potentiation on receptors lacking a γ2 subunit, and also those receptors containing an α4 or α6 subunit. This also demonstrates that chlormethiazole does not act via the benzodiazepine binding site.
Unlike pentobarbital and propofol, chlormethiazole elicited only a slight direct GABAA receptor activation at concentrations up to 1 mM. In addition, the drug did not potentiate anaesthetic-mediated currents elicited by pentobarbital or propofol, suggesting that chlormethiazole may be acting via an anaesthetic binding site.
Chlormethiazole produced weak nonselective inhibition of human NMDA NR1a+NR2A and NR1a+NR2B receptors. IC50's were approximately 500 μM that likely exceed the therapeutic dose range for chlormethiazole, indicating that the primary mechanism of the compounds in vivo activity is via GABAA receptors.
Keywords: Chlormethiazole, GABAA receptor, NMDA receptor, anaesthetic, barbiturate, subtype
Introduction
Chlormethiazole is a sedative, hypnotic compound that has long been used for the treatment of alcohol-withdrawal symptoms (Morgan, 1995), status epilepticus and pre-eclampsia (Fulton & Park, 1992). The compound has also recently been shown to have neuroprotective properties in models of ischaemia (Green, 1998). The sedative/anxiolytic properties are consistent with the potentiation of γ-aminobutyric acid A receptors (GABAA) (Harrison & Simmonds, 1983), and chlormethiazole has been demonstrated to potentiate directly GABAA receptors in neuronal cultures (Hales & Lambert, 1992), rat brain slices (Zhong & Simmonds, 1997) and recently using recombinantly expressed GABAA receptors (Nelson et al., 2002). While the GABA mechanism is well established, the site of action of chlormethiazole is still unknown and the recent neuroprotective effects have raised the question of activity at N-methyl-D-aspartate (NMDA) receptors. Here, we have used recombinant human GABAA receptors to look at the subtype specificity of chlormethiazole and provide evidence for interactions similar to the barbiturate pentobarbital. We also demonstrate for the first time nonsubtype-selective inhibition at recombinant human NMDA receptors expressed in Xenopus oocytes.
Methods
cDNAs for human α1, α4, α6, β1, β2, γ2S, NR1a, NR2A and NR2B were injected into Xenopus oocytes and incubated at 20°C. Adult female Xenopus laevis were anaesthetised by immersion in a 0.4% solution of 3-aminobenzoic acid ethylester for 30–45 min (or until unresponsive). Ovary tissue was removed via a small abdominal incision and stage V and stage VI oocytes were isolated with fine forceps. After mild collagenase treatment to remove follicle cells (Type IA, 0.5 mg ml−1, for 6 min), the oocyte nuclei were directly injected with 10–20 nl of injection buffer (88 mM NaCl, 1 mM KCl, 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES), at pH 7, filtered through nitrocellulose) containing different combinations of human GABAA subunit cDNAs (20 ng μl−1) engineered into the expression vector pCDM8 or pcDNAI/Amp. Following incubation for 24–72 h, oocytes were placed in a 50 μl bath and perfused at 4–6 ml min−1 with modified Barth's medium consisting of 88 mM NaCl, 1 mM KCl, 10 mM HEPES, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.91 mM CaCl2 and 2.4 mM NaHCO3, at pH 7.5. Cells were impaled with two 1–3 MΩ electrodes containing 2 M KCl and voltage clamped at −70 mV. Experiments conducted with NMDA receptors were performed using magnesium-free buffer of composition: 115 mM NaCl, 2.5 mM KCl, 10 mM HEPES and 1.8 mM BaCl2, pH 7.2.
Currents could be recorded after 24 h. In all experiments drugs were applied in the perfusate until the peak of the response was observed. Noncumulative concentration–response curves examining the modulatory effect of chlormethiazole were constructed using a control GABA EC20 concentration. A preapplication time of 30 s for chlormethiazole and a 3-min interval, upon recovery to baseline, between each GABA EC20 application was employed. Concentration–response curves were fitted to the Hill equation f(x)=EMAX/[1+(EC50/x)]n, where x is the drug concentration, EMAX is the maximum response, EC50 is the concentration of drug eliciting a half-maximal response and n is the Hill coefficient (Prism, Graphpad Software). The same equation was used to fit the NMDA inhibition curves, constraining the minimum parameter to 0, since higher concentrations of chlormethiazole could not be used.
Data are shown as either mean (95% confidence limits) or as the arithmetric mean±s.e.m. of at least four individual oocytes from a minimum of two Xenopus toads. Differences between means were evaluated by Student's t-test and considered significant if P<0.05.
Inhibition curves to chlormethiazole (0.3–3000 μM) were constructed using oocytes expressing NR1a+NR2A and NR1a+NR2B versus maximum concentrations of glutamate and glycine concentration (10 μM/10 μM). Chlormethiazole was preapplied for 30 s before coaddition of the Glut/Gly. A minimum of two Xenopus toads were used for each data set.
Results
Several allosteric modulators have recently been shown to be selective for β2/3-containing GABAA receptors versus β1-containing receptors, for example, loreclezole, etomidate, β-carbolines and tracazolate. Since chlormethiazole bears some structural resemblance to loreclezole, we first looked for any β-subunit selectivity of the compound. Chlormethiazole exhibited a small degree of β-subunit selectivity, showing EC50 values of 21.7 μM (19.2, 24.4) (n=4) and 43.3 μM (36.9, 44.3) (n=8)) at α1β1γ2 and α1β2γ2 GABAA receptors, respectively. While this was significant (P<0.05), the difference was small and unlike loreclezole, more potent at α1β1γ2. The maximum efficacy was equivalent on both subtypes with values of 264±31% (α1β1γ2) and 298±24% (α1β2γ2) (Figure 1, Table 1 ).
Figure 1.
Comparison of chlormethiazole potentiation at GABAA receptors containing different β-subunits. Concentration–response curves to GABA on α1β1γ2 and α1β2γ2 GABAA receptors. Data represent the mean±s.e. of four and eight cells in each case. Inset shows the structure of chlormethiazole.
Table 1.
Concentration–response curve data from chlormethiazole application to different human GABAA receptor subtypes
| Subtype | EC50 (μM) | Max potentiation (% of control) | Hill coefficient | n |
|---|---|---|---|---|
| α1β2 | 81.7 (76.1, 87.8) | 526±61 | 1.6 | 4 |
| α1β2γ2 | 43.3 (36.9, 44.3) | 298±24 | 1.1 | 8 |
| α1β1γ2 | 21.7 (19.2, 24.4) | 264±31 | 1.0 | 4 |
| α4β2γ2 | 87.1 (78.6, 96.5) | 646±57 | 1.5 | 4 |
| α6β2γ2 | 162 (121, 218) | 695±188 | 1.1 | 4 |
EC50 is shown as mean and 95% confidence limits. Maximum potentiation of an EC20 control GABA concentration is shown as arithmetic mean±s.e. Hill coefficient is that of the curve fitted through the mean data points for each subtype.
Unlike benzodiazepines, the γ2 subunit was not required for potentiation by chlormethiazole. However, the maximum degree of potentiation observed at α1β2 (526±61% (n=4)) was significantly greater than that at α1β2γ2 (298±24% (n=8)), P<0.005 (Figure 2). The EC50 for chlormethiazole was also significantly increased at α1β2 receptors (81.7 μM (76.1, 87.8)) compared to α1β2γ2, P<0.05 (Table 1).
Figure 2.
Comparison of chlormethiazole potentiation at α1β2 and α1β2γ2 GABAA receptors. Data represent the mean±s.e. of four and eight cells, respectively.
Similar to pentobarbital, chlormethiazole elicited a significantly greater maximum potentiation of both α4β2γ2 (646±57%, P<0.0001) and α6β2γ2 (695±188%, P<0.05) receptors than α1β2γ2 receptors (298±25%) (Figure 3). EC50's were also significantly different, being approximately two- and four-fold higher for α4 and α6, respectively (Table 1).
Figure 3.
Concentration–response curves for chlormethiazole potentiation at α4β2γ2- and α6β2γ2-containing GABAA receptors. Data are compared with α1β2γ2 and represent the mean±s.e. of four cells in each case, with eight cells for α1β2γ2.
The coapplication of 300 μM chlormethiazole elicited a seven-fold shift to the left of the GABA concentration–response curve at α1β2γ2 receptors, with an EC50 of 10.4 (9.4, 20.3) μM in the absence and 1.6 (1.0, 2.5) μM in the presence of chlormethiazole, P<0.001 (paired t-test). Chlormethiazole had little effect on the maximum GABA current, since maximum response in the presence of chlormethiazole was 104±3.6% of that in the absence of drug (Figure 4).
Figure 4.
Concentration–response curve to GABA in the absence and presence of 300 μM chlormethiazole at α1β2γ2 GABAA receptors. The curve is shifted seven-fold to the left. Data were plotted relative to the maximum GABA concentration in the absence of chlormethiazole and represents mean±s.e. for four cells.
Surprisingly, unlike both pentobarbital and propofol, chlormethiazole did not elicit a large direct current at α1β2γ2 GABA receptors. Example traces from cells using these three compounds are illustrated (Figure 5a), with mean data from at least four cells compared below (Figure 5b). Peak current amplitudes were plotted relative to the response elicited by 3 mM GABA on the same cell. At a concentration of 1 mM, a very small current was evoked by chlormethiazole, which was less than 5% of the total GABA current. Both pentobarbital and propofol elicited currents directly, and at high concentrations appeared to block the response as has been reported previously (Figure 5a; Thompson et al., 1996).
Figure 5.
Direct activation of α1β2γ2 GABAA receptors by increasing concentrations of (i) chlormethiazole, (ii) pentobarbital and (iii) propofol. (a) Example traces illustrate currents activated by each agonist. Drug application period is indicated above each response, and scale bars represent amplitude and time in each case. At the highest concentration indications of channel blockade and rebound current can be observed with pentobarbital and propofol. (b) Mean data representing concentration–response curves for direct activation of α1β2γ2 receptors. Peak current amplitude is plotted relative to that of 3 mM GABA on the same cell. Data represents mean±s.e. for 4–5 cells.
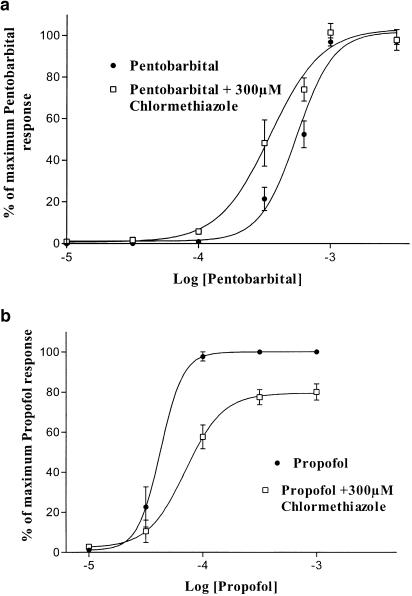
To determine if the effects of chlormethiazole were additive to those of the anaesthetics pentobarbital or propofol, concentration–response curves to the direct activation of α1β2γ2 GABA receptors by these agents were performed in the absence and presence of 300 μM chlormethiazole. Since the potentiation of GABA responses by these anaesthetics would be saturated before direct activation occurred, a lack of effect of chlormethiazole on anaesthetic-gated currents would suggest an interaction at an anaesthetic binding site. Compared to the seven-fold shift of the GABA concentration–response curve in Figure 4, a small shift was observed, with an EC50 for pentobarbital of 556 (490, 630) μM (n=5) compared to 350 (306, 400) μM (n=5) in the presence of 300 μM chlormethiazole, but this did not reach significance (Figure 6a). When a propofol concentration–response curve was carried out in the presence of 300 μM chlormethiazole, the coapplication of the drug produced no significant shift in EC50 (48 μM (41, 56) n=4) compared to 69 μM (56, 87) (n= 4) in the presence of chlormethiazole). The compound did, however, produce inhibition of the maximal response of propofol to 82±4.2% of the control (P<0.05) (Figure 6b).
Figure 6.
Chlormethiazole does not potentiate receptor activation by pentobarbital or propofol. Concentration–response curves to the direct activation of α1β2γ2 GABA receptors by (a) pentobarbital and (b) propofol in the absence and presence of 300 μM chlormethiazole. Data represents the mean±s.e. from five cells in (a) and four cells in (b).
The effects of chlormethiazole were also investigated on the expressed human NMDA receptors NR1a+NR2A and NR1a+NR2B. The compound produced complete inhibition of both subtypes with IC50's of approximately 485 and 683 μM, respectively (Figure 7). These inhibitory effects were much weaker (>20-fold) than those at the GABAA receptor, and chlormethiazole is neuroprotective at plasma concentrations of 10–30 μM (Green, 1998), suggesting this effect is unlikely to be mediated through the NMDA receptor.
Figure 7.
Chlormethiazole inhibition of the human NMDA receptors NR1a+NR2A and NR1a+NR2B expressed in Xenopus oocytes. Data represents percent inhibition of peak current amplitude in response to 10 μM glutamate/10 μM glycine and is expressed as mean±s.e. from 6–7 cells.
Discussion
Chlormethiazole has been in clinical use for over 30 years and has been used for several indications, including sedation and anxiolysis, the management of acute ethanol withdrawal, status epilepticus, pre-eclampsia and eclampsia (Fulton & Park, 1992). It was established that the compound interacts with GABAA receptors using brain slices of the rat cuneate nucleus and demonstrating potentiation of the depolarizing response to muscimol (Harrison & Simmonds, 1983). Chlormethiazole was shown to potentiate directly currents induced by GABA and glycine in both bovine chromaffin cells and murine spinal neurons where the potentiation was insensitive to the benzodiazepine antagonist flumazenil, hence not via the benzodiazepine binding site (Hales & Lambert, 1992). The compound has also recently been shown to potentiate recombinant human α1β2γ2 receptors expressed in HEK cells (Nelson et al., 2002). We have looked in more detail at the subunit selectivity of chlormethiazole with a view to investigating further its site of action and comparing this profile to other allosteric modulators. Despite having some structural resemblance to loreclezole, which is selective for receptors containing β2 and β3 versus β1, we found no difference in maximum efficacy and only a small difference in EC50 for chlormethiazole between α1β1γ2 and α1β2γ2, in this case with slight selectivity for α1β1γ2, suggestive of a different site of action to loreclezole. This would agree with the findings of Zhong & Simmonds (1997) using modulation of [3H]flunitrazepam binding to study interactions between loreclezole, chlormethiazole and pentobarbitone. In this study, loreclezole was found to potentiate [3H]flunitrazepam binding whereas chlormethiazole had no effect. The maximum efficacy of chlormethiazole potentiation was greater on α1β2 GABA receptors compared to α1β2γ2, confirming that the compound does not act via the benzodiazepine site, and the enhanced efficacy is similar to that reported for pentobarbital (Thompson et al., 1998). Chlormethiazole also exhibited significantly greater potentiation at α4β2γ2- and α6β2γ2-containing receptors compared to α1β2γ2. Again this profile is very reminiscent of pentobarbital on α4- and α6-containing subtypes, where potentiation of ∼700% of EC20 was observed compared to ∼300% at α1-containing receptors (Wafford et al., 1996). Both α4- and α6-containing receptors are located extrasynaptically, and are more sensitive to GABA (Nusser et al., 1998; Gustincich et al., 1999). It is interesting to note that after an ischaemic attack extracellular GABA concentrations are decreased, which would have major effects on extrasynaptically located receptors. By having greater effect at α4 and α6, chlormethiazole may serve to restore function at this population of receptors preferentially.
Similar to the reported findings in HEK cells, we observed a significant shift in GABA EC50 with no significant enhancement of peak currents. The seven-fold shift in this study is slightly greater than the three-fold shift in a previous study (Nelson et al., 2002). In contrast to some previous studies, we observed very little direct activation of GABAA receptors by chlormethiazole in the absence of GABA. A direct comparison was carried out with pentobarbital and propofol, as both these anaesthetics produce robust direct activation in addition to potentiating receptors. While pentobarbital and propofol produced activation up to 50% of a saturating GABA current, chlormethiazole at 1 mM only elicited a response 4% that of maximum GABA. Previous studies have shown some direct receptor activation (Hales & Lambert, 1992; Nelson et al., 2002), however, only at concentrations between 100 μM–10 mM, beyond the saturating concentration required for potentiation, and these were not compared to maximal elicited GABA currents. With the exception of the direct effects, these subunit-selective actions of chlormethiazole suggest similarities to that of pentobarbital. Previous studies have compared chlormethiazole with other allosteric modulators with mixed results. In binding studies using [35S]TBPS, a ligand that binds nonselectively to all GABA receptors both chlormethiazole and pentobarbital bound to receptors composed only of β3 subunits, suggesting some commonality to the binding site for these compounds (Slany et al., 1995) and more specifically an interaction with the β-subunit. Other studies comparing binding in rat brain have suggested that there are differences in how these compounds interact with the receptor (Cross et al., 1989; Zhong & Simmonds, 1997). To evaluate this further, we looked to see if direct activation of the receptor by pentobarbital or propofol could be potentiated in a manner similar to GABA. Chlormethiazole produced no potentiation of either pentobarbital- or propofol-activated GABA receptors, in fact significant inhibition of propofol responses was observed, suggesting that there may be some interaction between these sites, and that saturating doses of pentobarbital or propofol prevents allosteric modulation by chlormethiazole. This is in contrast to potentiation by benzodiazepines that have previously been shown to potentiate responses elicited by propofol (Hara et al., 1993).
There is evidence that the potentiating effect of chlormethiazole is shared by glycine receptors (Harrison & Simmonds, 1983; Hales & Lambert, 1992); however, this has not yet been demonstrated in recombinant glycine receptors. Interestingly this property is also shared by pentobarbital and propofol and is dependent on residues in the transmembrane spanning domain (Belelli et al., 1999).
The recent evidence that chlormethiazole is neuroprotective in several animal models suggests that chlormethiazole might have additional properties independent of its other actions at the GABAA receptor (Green, 1998). Voltage-gated channels are unaffected by chormethiazole as demonstrated by both ligand binding (Green et al., 1998) and electrophysiological techniques (Hope et al., 1995). Previous investigations using radioligand binding have shown no interaction at binding sites located on glutamate receptors (Green et al., 1998) and little activity against excitatory amino-acid responses when directly applied to rat cerebral cortex (Addae & Stone, 1988). Here, we have demonstrated that at high concentrations there does appear to be an inhibitory effect at the two major subtypes of the NMDA receptor, NR1a+NR2A and NR1a+NR2B. This is at much higher concentrations than chormethiazole's effects at GABA receptors. The neuroprotective effects are reported to correspond with plasma concentrations of 10–30 μM (Cross et al., 1995), which correlate better with the effects on GABA receptors than those required to inhibit NMDA receptors. It should be borne in mind, however, that chlormethiazole is highly brain penetrant and that brain concentrations may be up to 40% higher than in plasma (Green et al., 2000). Since there is some evidence that other GABA modulators can under some circumstances be neuroprotective (Schwartz-Bloom & Sah, 2001), it seems more likely that this phenomenon is GABA mediated.
In summary, we have demonstrated that there is some subunit dependency to the action of chlormethiazole on GABAA receptors. The profile of this compound is most similar to that of pentobarbital, and interaction studies suggest a mechanistic link to pentobarbital and propofol, although this is not direct evidence that chlormethiazole binds to the same site on the receptor. Very little direct GABA receptor activation was observed in this study, and it is likely that at therapeutic doses chlormethiazole acts by potentiating GABAA receptors. There also appear to be inhibition of NMDA receptors by high concentrations of chlormethiazole, but this is unlikely to occur at therapeutic doses.
Abbreviations
- GABAA receptor
γ-aminobutyric acid A receptor
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid
- NMDA
N-methyl-D-aspartate
References
- ADDAE J.I., STONE T.W. Effects of anticonvulsants on responses to excitatory amino acids applied topically to rat cerebral cortex. Gen. Pharmacol. 1988;19:455–462. doi: 10.1016/0306-3623(88)90047-x. [DOI] [PubMed] [Google Scholar]
- BELELLI D., PISTIS M., PETERS J.A., LAMBERT J.J. The interaction of general anaesthetics and neurosteroids with GABAA and glycine receptors. Neurochem. Int. 1999;34:447–452. doi: 10.1016/s0197-0186(99)00037-6. [DOI] [PubMed] [Google Scholar]
- CROSS A.J., JONES J.A., SNARES M., JOSTELL K.G., BREDBERG U., GREEN A.R. The protective action of chlormethiazole against ischaemia-induced neurodegeneration in gerbils when infused at doses having little sedative or anticonvulsant activity. Br. J. Pharmacol. 1995;114:1625–1630. doi: 10.1111/j.1476-5381.1995.tb14949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS A.J., STIRLING J.M., ROBINSON T.N., BOWEN D.M., FRANCIS P.T., GREEN A.R. The modulation by chlormethiazole of the GABAA-receptor complex in rat brain. Br. J. Pharmacol. 1989;98:284–290. doi: 10.1111/j.1476-5381.1989.tb16893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULTON B., PARK G.R. Intravenous chlormethiazole. Br. J. Hosp. Med. 1992;48:742–747. [PubMed] [Google Scholar]
- GREEN A.R. Clomethiazole (Zendra) in acute ischemic stroke: basic pharmacology and biochemistry and clinical efficacy. Pharmacol. Ther. 1998;80:123–147. doi: 10.1016/s0163-7258(98)00024-2. [DOI] [PubMed] [Google Scholar]
- GREEN A.R., HAINSWORTH A.H., JACKSON D.M. GABA potentiation: a logical pharmacological approach for the treatment of acute ischaemic stroke. Neuropharmacology. 2000;39:1483–1494. doi: 10.1016/s0028-3908(99)00233-6. [DOI] [PubMed] [Google Scholar]
- GREEN A.R., MISRA A., HEWITT K.E., SNAPE M.F., CROSS A.J. An investigation of the possible interaction of clomethiazole with glutamate and ion channel sites as an explanation of its neuroprotective activity. Pharmacol. Toxicol. 1998;83:90–94. doi: 10.1111/j.1600-0773.1998.tb01449.x. [DOI] [PubMed] [Google Scholar]
- GUSTINCICH S., FEIGENSPAN A., SIEGHART W., RAVIOLA E. Composition of the GABAA receptors of retinal dopaminergic neurons. J. Neurosci. 1999;19:7812–7822. doi: 10.1523/JNEUROSCI.19-18-07812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALES T.G., LAMBERT J.J. Modulation of GABAA and glycine receptors by chlormethiazole. Eur. J. Pharmacol. 1992;210:239–246. doi: 10.1016/0014-2999(92)90410-6. [DOI] [PubMed] [Google Scholar]
- HARA M., KAI Y., IKEMOTO Y. Propofol activates GABAA receptor-chloride ionophore complex in dissociated hippocampal pyramidal neurons of the rat. Anesthesiology. 1993;79:781–788. doi: 10.1097/00000542-199310000-00021. [DOI] [PubMed] [Google Scholar]
- HARRISON N.L., SIMMONDS M.A. Two distinct interactions of barbiturates and chlormethiazole with the GABAA receptor complex in rat cuneate nucleus in vitro. Br. J. Pharmacol. 1983;80:387–394. doi: 10.1111/j.1476-5381.1983.tb10045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPE P.J., MACMILLAN S., PATMORE L., SHERIDEN R.D. Actions of chlormethiazole on voltage-gated sodium currents in mouse neuroblastoma N1E-115 cells. Br. J. Pharmacol. 1995;116:440P. doi: 10.1111/j.1476-5381.1995.tb14965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN M.Y. The management of alcohol withdrawal using chlormethiazole. Alcohol Alcohol. 1995;30:771–774. [PubMed] [Google Scholar]
- NELSON R.M., GREEN A.R., HAINSWORTH A.H. Electrophysiological actions of gamma-aminobutyric acid and clomethiazole on recombinant GABAA receptors. Eur. J. Pharmacol. 2002;452:255–262. doi: 10.1016/s0014-2999(02)02233-1. [DOI] [PubMed] [Google Scholar]
- NUSSER Z., SIEGHART W., SOMOGYI P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ-BLOOM R.D., SAH R. Gamma-aminobutyric acidA neurotransmission and cerebral ischemia. J. NeuroChem. 2001;77:353–371. doi: 10.1046/j.1471-4159.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- SLANY A., ZEZULA J., TRETTER V., SIEGHART W. Rat beta 3 subunits expressed in human embryonic kidney 293 cells form high affinity [35S]t-butylbicyclophosphorothionate binding sites modulated by several allosteric ligands of gamma-aminobutyric acid type A receptors. Mol. Pharmacol. 1995;48:385–391. [PubMed] [Google Scholar]
- THOMPSON S.A., BONNERT T.P., WHITING P.J., WAFFORD K.A. Functional characteristics of recombinant human GABAA receptors containing the epsilon-subunit. Toxicol. Lett. 1998;100–101:233–238. doi: 10.1016/s0378-4274(98)00190-8. [DOI] [PubMed] [Google Scholar]
- THOMPSON S.A., WHITING P.J., WAFFORD K.A. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br. J. Pharmacol. 1996;117:521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAFFORD K.A., THOMPSON S.A., THOMAS D., SIKELA J., WILCOX A.S., WHITING P.J. Functional characterization of human gamma-aminobutyric acidA receptors containing the alpha 4 subunit. Mol. Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- ZHONG Y., SIMMONDS M.A. Interactions between loreclezole, chlormethiazole and pentobarbitone at GABAA receptors: functional and binding studies. Br. J. Pharmacol. 1997;121:1392–1396. doi: 10.1038/sj.bjp.0701269. [DOI] [PMC free article] [PubMed] [Google Scholar]