Abstract
The glycine-binding site of the glutamatergic N-methyl-D-aspartate receptor subtype (NMDAr) has been proposed as a putative target for treating cognitive impairments in neurodegenerative disorders and schizophrenia. Although behavioural evidence has been accumulated showing that the partial agonist D-cycloserine (DCS) facilitated learning and memory, physiological mechanisms of the drug still remained to be characterized. In the present study, we have investigated the effects of DCS on glutamatergic neurotransmission and synaptic plasticity in CA1 region of rat hippocampal slices, using extracellular field excitatory postsynaptic potentials.
We showed that DCS facilitated NMDAr-mediated synaptic potentials. In addition, we found that the magnitude of NMDAr-dependent long-term depression was significantly enhanced by the agonist, while the threshold for the induction of lasting potentiations was lowered.
We found that DCS decreased neurotransmission mediated by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate subtypes of glutamate receptors. This inhibition was not prevented by the γ-aminobutyric acid GABAA antagonist bicuculline, but was antagonized by the glycine antagonist strychnine.
These results, therefore, show opposite effects of DCS on NMDA and non-NMDA synaptic responses within the hippocampus. They also demonstrate that DCS facilitates long-term synaptic plasticity that may support the DCS-induced enhanced cognitive performances.
Keywords: Glutamate, hippocampus, glycine-binding site, long-term depression, learning, schizophrenia
Introduction
The potential usefulness of agonists and antagonists of N-methyl-D-aspartate subtype of glutamate receptors (NMDAr) as therapeutic agents has been extensively investigated (Rogawski, 2000). In particular, the use of drugs specifically acting at the strychnine-insensitive glycine-binding site indicates that the NMDAr-modulatory site should be involved in cognitive processes in normal subjects and might contribute to deficits in pathological states including neurodegenerative disorders, anxiety, depression and schizophrenia (for a review, see Danysz & Parsons, 1998). Originally used as an antibiotic agent for the treatment of tuberculosis, D-cycloserine (DCS) was later characterized as a partial agonist at the NMDAr glycine-binding site (Hood et al., 1989; Watson et al., 1990). DCS is now generally reported as a performance enhancer in a wide range of learning tasks involved in spatial memory and trace eye blink conditioning that are closely dependent on the hippocampus (Thompson et al., 1992; Quatermain et al., 1994; Lelong et al., 2001). Although beneficial effects of DCS have been shown in aged memory-deficient rats (Baxter et al., 1994; Aura & Riekkinen, 2000), DCS-induced improvement in Alzheimer's disease is still debated (Mohr et al., 1995). Similarly, DCS in schizophrenia has also been reported as beneficial (for a review, see Javitt, 2002), but a general consensus is still lacking since worsening of symptoms was observed at high doses (Goff et al., 1996). These data raise the question of the cellular mechanisms activated by DCS. For example, it may be postulated that DCS could produce different effects depending on the subunits composing the NMDAr (Priestley et al., 1995; Sheinin et al., 2001). Alternatively, the possibility exists of DCS-mediated side effects that should not be dependent on NMDAr. Interestingly, only one study investigated the effects of DCS in neuronal activity as compared to the extensive behavioural trials (Pitkänen et al., 1994). Considering this lack of physiological data and the questions that persist after behavioural investigations, we determined, in the CA1 hippocampal field, the effects of DCS on glutamatergic neurotransmission and calcium-dependent forms of synaptic plasticity considered as a basic neuronal activity underlying memory formation (Bliss & Collingridge, 1993).
Methods
Experiments were conducted in 2–4-month-old (n=33) male Sprague–Dawley rats purchased from IFFA-CREDO (France). All experiments were performed in compliance with French laws on animal care, using relevant INSERM guidelines. Rats were housed five per cage and were maintained on a controlled light–dark cycle at a constant temperature (22±2°C) with ad libitum access to food and water.
One animal was daily studied using extracellular recordings in the ex vivo slice preparation. The rat was anaesthetized with halothane and decapitated. The hippocampus was quickly removed and placed in a cold oxygenated medium. Slices (400 μM thick) were cut and placed in a holding chamber for at least 1 h. A single slice was transferred to the recording chamber where it was held between two nylon nets. The slice was then submerged beneath a continuously superfusing medium pregassed with 95% O2 and 5% CO2. The composition of the medium was (mM): NaCl, 124; KCl, 3.5; MgSO4, 1.5; CaCl2, 2.3; NaHCO3, 26.2; NaH2PO4, 1.2; glucose, 11.
Extracellular recordings were obtained at 20°C from the apical dendritic layer of CA1 area using micropipettes filled with 2 M NaCl (resistance of 2–6 MΩ). Field excitatory postsynaptic potentials were evoked every 10 s by electrical stimulation of afferent fibres (Schaffer collaterals and commissural fibres), located in the stratum radiatum. Stimuli (20–100 μs duration) were applied between the two poles of a bipolar electrode, one pole inserted into the slice and the other in the fluid just above the slice.
Synaptic transmission
Input/output (I/O) curves of NMDAr-mediated synaptic responses were constructed from slices perfused for 30 min in an Mg2+-free medium, supplemented with the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonist 6-cyano-7-nitroquinoxaline-2 (CNQX, 10 μM). In these experiments, a knife cut separating CA3 and CA1 was introduced to prevent the propagation of epileptiform discharges. I/O curves were constructed before and 15 min after DCS (2–100 μM) was added to the perfusate. The slope of three averaged field excitatory postsynaptic potentials (fEPSPs) was measured and plotted against different intensities of stimulation (from 0 to 500 μA) using the Acquis 1 software (CNRS, Paris). To estimate the effects of DCS, the changes in fEPSP slope expressed as the percentage of the control value were calculated and averaged for intensities from 200 to 500 μA. Under our experimental conditions, the variability of each fEPSP slope did not exceed 5% of the mean baseline value calculated from a 15 min recording. As a consequence, an fEPSP was considered to be drug responsive if the per cent change of its slope exceeded 10%.
I/O curves were also constructed in control medium to assess the responsiveness of AMPA/kainate subtypes of glutamate receptors (non-NMDAr) to DCS. The effects of the drug were quantified as previously described for NMDAr-mediated synaptic responses.
Pharmacological characterization of the response to DCS was investigated in the presence of the γ-aminobutyric acid (GABA)A receptor antagonist bicuculline (10 μM) and the glycine receptor antagonist strychnine (10–20 μM).
Synaptic plasticity
PPF of synaptic transmission was induced by electrical stimulation of the Schaffer collateral/commissural fibres with paired pulses at interstimulus interval (ISI) of 50 ms. The PPF was quantified as the ratio of the second slope over that of the first response.
In order to investigate the effects of DCS on long-term depression (LTD) of synaptic transmission, test stimuli were applied every 10 s and adjusted to get a baseline fEPSP slope of 0.1 mV s−1. The initial slope was measured for 10 min before the delivery of an LFS (900 pulses, 1 Hz at test intensity). Testing with single pulses was then resumed for 45 min after LFS. Synaptic potentiation was investigated using the same protocol, but following a conditioning stimulation of 50 Hz for 1 s at test intensity.
Drugs including DCS, CNQX, D-2-amino-5-phosphonovalerate (2-APV), glycine, strychnine, bicuculline and L689-560 were purchased from Tocris (Illkirch, France). They were dissolved in water, except CNQX, which was dissolved in dimethylsulphoxide (DMSO).
All results are expressed as mean±s.e.m. throughout. In order to take the correlation inherent in repeated measure data (experiments of synaptic plasticity) into account, the degree of statistical significance was calculated using multivariate analyses of variance (ANOVA). In cases of nonrepeated measures, paired t-test was used. Finally, to compare the per cent of synaptic responses sensitive to different doses of DCS, a χ2 test was used. In all cases, differences were considered significant when P⩽0.05.
Results
Effects of DCS on NMDAr-mediated synaptic responses
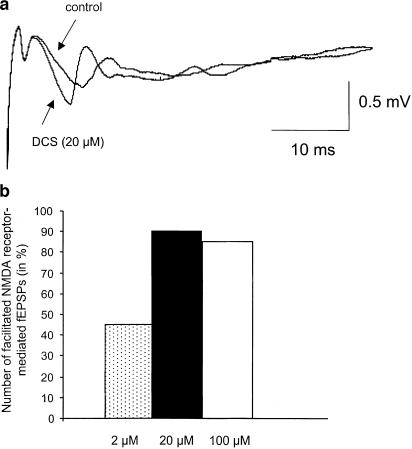
An fEPSP of prolonged duration (Figure 1a), totally abolished at the end of the recordings by the NMDAr competitive antagonist 2-APV (30 μM) or the antagonist of the glycine-binding site L689-560 (10 μM), was recorded in 20 slices from 14 animals. In 19 slices, DCS (2–100 μM) significantly increased the slope of NMDAr-mediated synaptic responses (P<0.0001) with an average of 37.9±5.1%. Considering a threshold of 10% increase (see Methods), half of responses were facilitated by the drug at the concentration of 2 μM (five over 11 slices). The per cent of slices sensitive to the drug increased relative to the concentration of the agonist (Figure 1b). It was significantly higher at 20 μM (nine over 10 slices) and 100 μM (nine over 11 slices) (P<0.05 for both concentrations).
Figure 1.
Facilitation of NMDA receptor-mediated synaptic responses by DCS. (a) Examples of fEPSPs recorded in a magnesium-free medium supplemented with CNQX (10 μM) before and 15 min after bath application of DCS (20 μM). (b) Histograms of the percentage of facilitated fEPSP as a function of DCS concentration.
When added to the bath supplemented with the glycine receptor antagonist strychnine (20 μM), DCS (20 μM: two slices; 100 μM: eight slices) also increased the magnitude of the NMDAr-mediated synaptic responses (seven animals). Interestingly, we found that in these conditions, the averaged increase (64.9±7.8%) was significantly higher than in control medium (P<0.01). These results, therefore, showed (i) that the facilitation of NMDAr-dependent neurotransmission by DCS involves the glycine-binding site of NMDAr and (ii) that it is prevented by the activation of glycinergic receptors.
Effects of DCS on AMPA/kainate-mediated synaptic responses
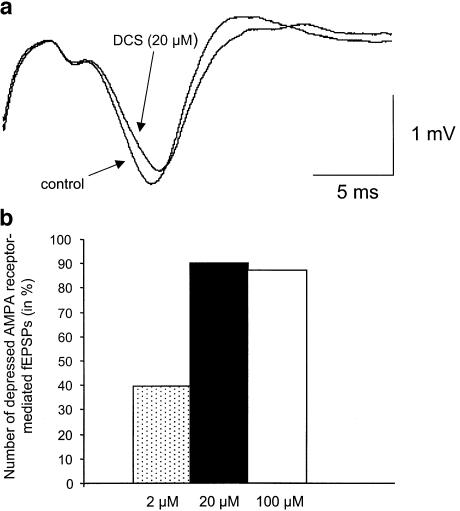
Stimulation of the stratum radiatum induced, in a control medium, an AMPA/kainate receptor-mediated fEPSP (Figure 2a), as confirmed by its complete suppression at the end of the recording by the AMPAr antagonist CNQX (10 μM). Experiments from 13 animals showed a significant decrease in fEPSP slope by DCS (2–100 μM) in 20 over 25 tested slices (P<0.0001) that reached an average of 24.3±2.4%. At 2 μM, only four over 10 slices were responsive to the drug, the slope decrease exceeded 10% (Figure 2b). The percentage of depressed fEPSPs significantly increased to nine over 10 slices and 12 over 15 slices for concentrations of 20 and 100 μM, respectively (P<0.05).
Figure 2.
Depression of AMPA/kainate receptor-mediated synaptic responses by DCS. (a) Examples of fEPSPs recorded in a control medium before and 15 min after bath application of DCS (20 μM). (b) Histograms of the percentage of depressed fEPSP regarding increased concentration of DCS.
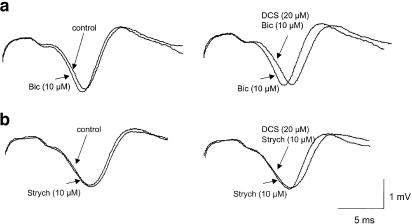
We then determined the mechanisms by which DCS decreased non-NMDAr-mediated fEPSPs. In the presence of the GABAA receptor antagonist bicuculline (10 μM), the magnitude of AMPA/kainate receptor-mediated synaptic potentials was significantly enhanced (P<0.05) in seven over nine tested slices (six animals). This in fact reflected a loss of GABAergic inhibition (Figure 3a, left). In these conditions (Figure 3a, right), DCS added to the perfusate still significantly depressed synaptic responses (P<0.02). On the contrary, when recorded in the presence of strychnine (20 μM), which had no significant effect on fEPSPs (Figure 3b, left), the depression by DCS (20 μM: six slices; 100 μM: four slices) was prevented in nine over the 10 tested slices (six animals) (Figure 3b, right). These results showed that the inhibitory effect of DCS on non-NMDAr glutamatergic neurotransmission was dependent on the activation of glycinergic but not GABAergic receptors. When glycine (20 μM: three slices; 100 μM: seven slices) was added to the perfusate, a significant decrease in AMPA/kainate receptor-mediated fEPSPs (p<0.05) was found in six over 11 tested slices (seven animals) with an averaged magnitude of 16.1±2.2%.
Figure 3.
Depressive effects of DCS on AMPA/kainate receptor-mediated synaptic responses is mediated by glycinergic receptors. (a) Left: examples of fEPSPs recorded before and in the presence of the GABAA receptor antagonist bicuculline (10 μM); right: examples of fEPSPs recorded in the presence of bicuculline before and after DCS (20 μM). (b) Left: examples of fEPSPs recorded before and in the presence of the glycinergic antagonist strychnine (10 μM); right: examples of fEPSPs recorded in the presence of strychnine before and after DCS (20 μM).
Inhibitory effects of DCS might be driven by glycinergic receptors located either on CA3 pyramidal cells, CA1 glutamatergic terminals and/or pyramidal cells. When applied on slices where CA3 was severed from CA1 with a knife cut, DCS (20 μM: three slices; 100 μM: two slices) still significantly depressed fEPSPs by 24.5 5% (P<0.05) in four over the five tested slices (four animals). This result indicated that glycinergic receptors were located within CA1 field. To test whether they were on presynaptic glutamatergic terminals, we used the paradigm of PPF that is usually used to assess the presynaptic control of neurotransmitter release (Creager et al., 1980). Our results showed that the facilitation ratio of the response evoked by the second pulse at 50 ms ISI (five animals/eight slices) was not significantly affected in the presence of DCS (100 μM) (1.21±0.13 vs 1.32±0.1) (P>0.05). This result indicated that the release of glutamate was not affected by the drug. Taking together, our data strongly suggest that the depressive effect of DCS is carried out by glycinergic receptors located postsynaptically on CA1 pyramidal cells rather than on presynaptic glutamatergic terminals.
Effects of DCS on NMDAr-dependent synaptic plasticity
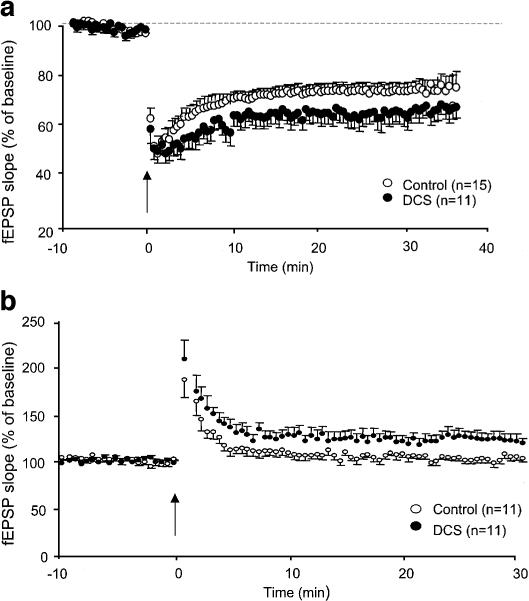
Figure 4a shows the effects of DCS on LTD induced by an LFS of glutamatergic afferents. When applied in a control medium (12 animals/15 slices), LFS produced a depression of the fEPSP, which stabilized around 30% and persisted for at least 40 min (baseline vs last 5 min: F(1,20)=85.4, P<0.0001). When delivered in the presence of the competitive NMDAr antagonist 2-APV (60 μM, six animals/nine slices) or L-689-560 (10 μM, three animals/five slices), LFS still depressed fEPSPs, but the response rapidly returned towards basal values (not illustrated). These results indicated that activation of the glycine-binding site of NMDAr was necessary to induce long lasting depression of synaptic transmission. In seven animals recorded in the presence of DCS (20 μM: nine slices; 100 μM: two slices), the decrease in fEPSP described above was compensated during the baseline by increasing the stimulus intensity. In these conditions, fEPSPs were significantly more depressed than in control medium in response to the conditioning stimulation (around 40%, see Figure 4a) as raised by a statistical analysis of the whole time course of the depression (F(1,23)=4.46, P<0.05).
Figure 4.
Facilitation of long-term synaptic plasticity by DCS. (a) LTD expressed as the per cent change in fEPSP slope versus time in slices recorded in control medium and in the presence of DCS (20–100 μM) before and after low-frequency conditioning stimulation (900 pulses at 1 Hz) of the stratum radiatum (arrow). (b) LTP expressed as the per cent change in fEPSP slope versus time in slices recorded in control medium and in the presence of DCS (100 μM) before and after 50 Hz conditioning stimulation. The arrows indicate the delivery of the conditioning stimulation.
In Figure 4b are illustrated the effects of DCS on synaptic potentiation induced by a 50 Hz conditioning stimulation. When delivered in a control medium (five animals/11 slices), a strong facilitation of synaptic transmission was induced, which progressively returned towards baseline values during the first 5 min post-tetanus, indicating that only short-term potentiation was generated. In the presence of DCS (100 μM, seven animals/11 slices), the post-tetanic potentiation, which developed in response to the conditioning stimulation, also decayed but then stabilized around 20% increase after 10 min (Figure 4b). As a consequence, fEPSPs remained significantly potentiated 30 min post-tetanus as compared to baseline values (baseline vs last 10 min: F(1.20)=12.5, P<0.002). This result indicated that the duration of the potentiation of synaptic transmission was facilitated by DCS.
Discussion
The aim of the present study was to determine the physiological effects of DCS in CA1 field of the hippocampus. Our results point out three major issues.
The first contribution is to show that DCS facilitated NMDAr in synaptic transmission. Indeed, we found that isolated NMDAr-mediated fEPSPs were significantly enhanced by DCS. Since recordings were carried out in the presence of CNQX, which, at 10 μM, produces a small antagonism at the NMDA glycine site (Kessler et al., 1989), some effects of DCS might be to reverse any small CNQX antagonism. Our study is the first physiological demonstration that DCS is indeed able to enhance NMDAr-dependent synaptic potentials in the brain by activating the glycine-binding sites of the receptors. This result found in CA1 hippocampal area correlates with a previous report indicating that DCS might increase in vivo the excitability of dentate granule cells, possibly by enhancing the NMDAr component of the response to the stimulation of the perforant pathway (Pitkänen et al., 1994).
As a second contribution, our study shows that DCS also facilitates synaptic plasticity of glutamatergic transmission, increasing the magnitude of LTD and lowering the threshold to induce a lasting potentiation. Different by the strength of the conditioning stimulation and consequently by the intracellular activated pathways, both forms of synaptic plasticity are closely dependent on the activation of the NMDAr glycine-binding site (Watanabe et al, 1992; Kew et al., 2000; Ballard et al., 2002). Accordingly, we showed in the present study that blocking the site by the specific antagonist L689,560 impaired LTD. Synaptic plasticity is now generally referred as a major property of neuronal networks involved in the formation of memory traces (Bliss & Collingridge, 1993; Abel & Lattal, 2001). Its facilitation by DCS in CA1 field of the hippocampus reported here may thus represent the physiological mechanism allowing DCS to improve cognitive performances, which has been reported for hippocampal-dependent memory tasks (Thompson et al., 1992; Quatermain et al., 1994; Lelong et al., 2001).
The third contribution of this work is to show that the glutamatergic neurotransmission, which involves the activation of AMPA/kainate receptors, is depressed by DCS. Based on the maintenance of the depression in the presence of bicuculline and the lack of effects in the presence of strychnine, we concluded that DCS reduced fast glutamatergic transmission by activating inhibitory glycinergic receptors. Accordingly, we found that glycine, although to a weaker extent, might also depress AMPA/kainate synaptic responses. Furthermore, we determined that presynaptic calcium-dependent mechanisms controlling glutamate release were not affected by DCS, showing that the depressive effect of the agonist should be mediated by glycinergic receptors located postsynaptically on pyramidal cells. Accordingly, immunohistochemical studies recently displayed the expression of glycinergic subunits in adult rat CA1 hippocampal area (Chattipakorn & McMahon, 2002), while patch-clamp recordings of pyramidal cells showed that glycine-gated chloride currents might be recorded in response to iontophoretic application of the agonist (Yoon et al., 1998; Chattipakorn & McMahon, 2002). In addition, inhibitory effects of endogenous β-alanine and taurine on glutamatergic neurotransmission were recently demonstrated in the hippocampus. This inhibition was also dependent on the activation of glycinergic receptors like the mechanism described in the present study for DCS (Chattipakorn & McMahon, 2002; Mori et al., 2002).
Clinical and preclinical studies indicate that DCS might find some use in the alleviation of negative symptoms in schizophrenia (Goff et al., 1996; Javitt, 2002). However, this benefit seems limited regarding the narrow range of effective doses and even the symptom worsening at high concentrations. Considering the depression of AMPA/kainate transmission by DCS characterized in the present study, we postulate that this mechanism might accentuate the glutamatergic deficit already present in schizophrenia (Ishimaru & Toru, 1997), thus preventing a simultaneous beneficial effect on NMDAr activation by the drug. According to this hypothesis, we found that the DCS-mediated facilitation of NMDAr synaptic potentials was larger in the presence of strychnine that blocks inhibitory glycinergic receptors. In addition, it was reported that the potency of DCS to facilitate spatial memory is particularly pronounced in aged animals (Baxter et al., 1994, Aura & Riekkinen, 2000) in which the depressive effect of the agonist on AMPA/kainate receptor-mediated synaptic responses is lacking (Rouaud, unpublished results). In addition, it may be postulated that the worsening of symptoms at high concentrations of DCS could be due to a general impairment of AMPA/kainate-dependent neurotransmission that mediates the functional properties of a large number of neuronal networks within the central nervous system. As a consequence, it will be important to reinvestigate the behavioural consequences of a treatment with DCS when given in conjunction with glycinergic antagonists that suppress its depressive effects on glutamatergic neurotransmission.
Abbreviations
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DCS
D-cycloserine
- DMSO
dimethylsulphoxide
- fEPSPs
field excitatory postsynaptic potentials
- I/O
input/output
- ISI
interstimulus interval
- LFS
low-frequency stimulation
- LTD
long-term depression
- Mg2+
magnesium
- PPF
paired-pulse facilitation
- 2-APV
D-2-amino-5-phosphonovalerate
References
- ABEL T., LATTAL M. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr. Opin. NeuroBiol. 2001;11:180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- AURA J., RIEKKINEN P., JR Pre-training blocks the improving effect of tetrahydroaminoacridine and D-cycloserine on spatial navigation performance in aged rats. Eur. J. Pharmacol. 2000;390:313–318. doi: 10.1016/s0014-2999(00)00054-6. [DOI] [PubMed] [Google Scholar]
- BALLARD T.M., PAULY-EVERS M., HIGGINS G.A., OUAGAZZA A.M., MUTEL V., BORRONI E., KEMP J.A., BLUETHMANN H., KEW J.N. Severe impairment of NMDA receptor function in mice carrying targeted point mutations in the glycine binding site results in drug-resistant nonhabituating hyperactivity. J. Neurosci. 2002;22:6713–6723. doi: 10.1523/JNEUROSCI.22-15-06713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAXTER M.G., LANTHORN T.H., FRICK K.M., GOLSKI S., WAN R-Q., OLTON D.S. D-Cycloserine, a novel cognitive enhancer, improves spatial memory in aged rats. Neurobiol. Aging. 1994;15:207–213. doi: 10.1016/0197-4580(94)90114-7. [DOI] [PubMed] [Google Scholar]
- BLISS T.V.P., COLLINGRIDGE G.L. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- CHATTIPAKORN S.C., MCMAHON L.L. Pharmacological characterization of glycine-gated chloride currents recorded in rat hippocampal slices. J. NeuroPhysiol. 2002;87:1515–1525. doi: 10.1152/jn.00365.2001. [DOI] [PubMed] [Google Scholar]
- CREAGER R., DUNWIDDIE T., LYNCH G. Paired-pulse and frequency facilitation in the CA1 region of the in vitro rat hippocampus. J. Physiol (Lond). 1980;299:409–424. doi: 10.1113/jphysiol.1980.sp013133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANYSZ W., PARSONS C.G. Glycine and N-methyl-D-aspartate receptors: physiological significance and possible therapeutic applications. Pharmacol. Rev. 1998;50:598–664. [PubMed] [Google Scholar]
- GOFF D.C., TSAI G.C., MANOACH D.S., FLOOD J., DARBY G.C., COYLE J.T. D-Cycloserine added to neuroleptics for negative symptoms in schizophrenia. Am. J. Psychiatry. 1996;153:1628–1630. doi: 10.1176/ajp.153.12.1628. [DOI] [PubMed] [Google Scholar]
- HOOD W.F., COMPTON R.P., MONAHAN J.B. D-Cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci. Lett. 1989;98:91–95. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- ISHIMARU M., TORU M. The glutamate hypothesis of schizophrenia: therapeutic implications. CNS Drugs. 1997;7:47–67. [Google Scholar]
- JAVITT D.C. Glycine modulators in schizophrenia. Curr. Opin. Invest. Drugs. 2002;3:1067–1072. [PubMed] [Google Scholar]
- KESSLER M., BAUDRY M., LYNCH G. Quinoxaline derivatives are high-affinity antagonist of the NMDA-associated glycine sites. Brain Res. 1989;489:377–382. doi: 10.1016/0006-8993(89)90875-5. [DOI] [PubMed] [Google Scholar]
- KEW J.N., KOESTER A., MOREAU J.L., JENK F., OUAGAZZA A.M., MUTEL V., RICHARDS J.G., TRUBE G., FISCHER G., MONTKOWSKI A., HUNDT W., REINSCHEID R.K., PAULY-EVERS M., KEMP J.A., BLUETHMANN H. Functional consequences of reduction in NMDA receptor glycine affinity in mice carrying targeted point mutations in the glycine binding site. J. Neurosci. 2000;20:4037–4049. doi: 10.1523/JNEUROSCI.20-11-04037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LELONG V., DAUPHIN F., BOULOUARD M. RS 67333 and D-cycloserine accelerate learning acquisition in the rat. Neuropharmacology. 2001;41:517–522. doi: 10.1016/s0028-3908(01)00085-5. [DOI] [PubMed] [Google Scholar]
- MOHR E., KNOTT V., SAMPSON M., WESNES K., HERTING R., MENDIS T. Cognitive and quantified electroencephalographic correlates of cycloserine tratment in Alzheimer's disease. Clin. NeuroPharmacol. 1995;18:28–38. doi: 10.1097/00002826-199502000-00004. [DOI] [PubMed] [Google Scholar]
- MORI M., GAHWILER B.H., GERBER U. Beta-alanine and taurine as endogenous agonists at glycine receptors in rat hippocampus in vitro. J. Physiol (Lond) 2002;539:191–200. doi: 10.1113/jphysiol.2001.013147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITKÄNEN M., SIRVÏO J., LAHTINEN H., KOIVISTO E., RIEKKINEN P. D-Cycloserine, a partial agonist at the glycine site, enhances the excitability of dentate granule cells in vivo in rats. Eur. J. Pharmacol. 1994;253:125–129. doi: 10.1016/0014-2999(94)90766-8. [DOI] [PubMed] [Google Scholar]
- PRIESTLEY T., LAUGHTON P., MYERS J., LEBOURDELLES B., KERBY J., WHITING P.J. Pharmacological properties of recombinant human N-methyl-D-aspartate receptors comprising NR1a/NR2a and NR1a/NR2b subunits assemblies expressed in permanently transfected mouse fibroplast cells. Mol. Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- QUATERMAIN D., MOWER J., RAFFERTY M.F., HERTING R.L., LANTHORN T.H. Acute but not chronic activation of NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur. J. Pharmacol. 1994;257:7–12. doi: 10.1016/0014-2999(94)90687-4. [DOI] [PubMed] [Google Scholar]
- ROGAWSKI M.A. Low affinity channel blocking (uncompetitive) NMDA receptor antagonists as therapeutic agents: toward an understanding of heir favorable tolerabiliy. Amino Acids. 2000;19:133–149. doi: 10.1007/s007260070042. [DOI] [PubMed] [Google Scholar]
- SHEININ A., SHAVIT S., BENVENISTE M. Subunit specificity and mechanism of action of NMDA partial agonist D-serine. Neuropharmacology. 2001;41:151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- THOMPSON L.T., MOSKAL J.R., DISTERHOFT J.F. Hippocampus-dependent learning facilitated by a monoclonal antibody or D-cycloserine. Nature. 1992;359:638–641. doi: 10.1038/359638a0. [DOI] [PubMed] [Google Scholar]
- WATSON G.B., BOLANOWSKI M.A., BAGANOFF M.P., DEPPELER C.J., LANTHORN H. D-Cycloserine acts as a partial agonist at the glycine modulatory site of the NMDA receptor expressed in Xenopus oocytes. Brain Res. 1990;510:158–160. doi: 10.1016/0006-8993(90)90745-w. [DOI] [PubMed] [Google Scholar]
- WATANABE Y., HIMI T., SAITO H., ABE K. Involvement of glycine site associated with the NMDA receptor in hippocampal long-term potentiation and acquisition of spatial memory in rats. Brain Res. 1992;582:58–64. doi: 10.1016/0006-8993(92)90316-2. [DOI] [PubMed] [Google Scholar]
- YOON K.W., WOTRING V.E., FUSE T. Multiple picrotoxinin effect on glycine channels in rat hippocampal neurons. Neuroscience. 1998;87:807–815. doi: 10.1016/s0306-4522(98)00158-4. [DOI] [PubMed] [Google Scholar]