Abstract
Moderate hypoxia in vivo and serum from rabbits subjected to moderate hypoxia (SHYPO) in vitro reduce CYP1A1 and 1A2 P450 isoforms and upregulate CYP3A6. The aim of this project was to investigate the signal transduction pathways implicated in the upregulation of CYP3A6 expression by hypoxia.
Hypoxia in vivo and SHYPO in vitro increased the expression of hypoxia-inducible factor-1α (HIF-1α) and c-jun, as well as CYP3A6. By electrophoresis mobility shift assay, it was shown that HIF-1 and activator protein-1 (AP-1) bind to CYP3A6 oligonucleotide probe after exposure to hypoxia in vivo and SHYPO in vitro. The effects of hypoxia in vivo or SHYPO in vitro were reproduced by CoCl2 and lead acetate, activators of HIF-1 and AP-1, respectively.
PD98059, a p42/44 MAPK inhibitor, prevented the increase of CYP3A6 and c-jun, but did not impede the increase of HIF-1α and binding to CYP3A6 oligonucleotide probe. Genistein, an inhibitor of protein tyrosine kinases (PTKs), prevented the increase in HIF-1α, c-jun and CYP3A6, as well as HIF-1 and AP-1 binding to CYP3A6 oligonucleotide probe. Moreover, hypoxia in vivo induced constitutive androstane receptor (CAR) as well as CAR binding to the CYP3A6 oligonucleotide probe, but not the pregnane X receptor.
In conclusion, hypoxia in vivo and SHYPO induce the expression of CYP3A6. The in vitro induction of CYP3A6 by SHYPO is PTK- and p42/44 MAPK-dependent. The present data support the hypothesis that HIF-1 and AP-1 are part of the signalling pathway leading to CYP3A6 induction by hypoxia.
Keywords: CYP3A6, CYP1A1, CYP1A2, hypoxia, HIF-1, AP-1, MAPKs, enzyme induction
Introduction
Moderate hypoxia in vivo and serum from rabbits subjected to moderate hypoxia (SHYPO) in vitro reduce the hepatic biotransformation of drugs eliminated through CYP1A1 and 1A2 P450 isoforms. On the other hand, the same experimental conditions induce CYP3A6 expression through a pretranslational mechanism (Kurdi et al., 1999; Fradette et al., 2002). Induction of CYP3A6 by xenobiotics and steroids is regulated by two ‘orphan' nuclear receptors, pregnane X receptor (PXR) and constitutive androstane receptor or constitutively activated receptor (CAR) (Sueyoshi & Negishi, 2001). However, the mechanisms underlying the upregulation of CYP3A6 under hypoxic conditions remain unknown.
Hypoxia activates transcription factors, which regulate the adaptive response of the cells to hypoxia (Semenza, 2000; Hur et al., 2001). Cells adapt to the low partial pressure of oxygen by upregulating the transcription of multiple genes, such as vascular endothelial growth factor (VEGF), erythropoietin (EPO) and several glycolytic enzymes. These target genes are induced by a common transcription factor, hypoxia-inducible factor 1 (HIF-1). HIF-1 is a heterodimer composed of α and β subunits. HIF-1β is the aryl hydrocarbon receptor (AhR) nuclear translocator (Arnt), which is also a heterodimerization partner of the AhR. Under normoxic conditions, HIF-1α is a very unstable protein, rapidly degraded. Hypoxia stabilizes HIF-1α, allowing the formation of the heterodimer HIF-1α/β that binds to DNA (Semenza, 2000).
Activator protein-1 complex (AP-1) is also activated by hypoxia, through a c-Jun N-terminal kinase (JNK)-dependent pathway. Activation of JNK results in the induction of the immediate-early-response proteins c-fos, c-jun and erg-1 (Gozal et al., 1999; Michiels et al., 2000). Dimerization of these early response proteins forms AP-1 that binds to DNA to modulate gene transcription. AP-1 activation by hypoxia cooperates with HIF-1 to increase the transcription of genes. The activation of both HIF-1 and AP-1 involves members of the mitogen-activated protein kinase (MAPK) signal-transduction pathways (Salnikow et al., 2002).
The aim of this study was to investigate the role of HIF-1, AP-1, CAR and PXR in the upregulation of CYP3A6 induced by moderate hypoxia. To this purpose, we assessed the effects of SHYPO, CoCl2 (an activator of HIF-1) and lead acetate (an activator of AP-1) on the expression of HIF-1α, c-jun and CYP3A6, as well as on the binding of HIF-1 and AP-1 to the CYP3A6 oligonucleotide probe. The results demonstrate that hypoxia in vivo as well as SHYPO, CoCl2 and lead increase the expression of HIF-1α, c-jun and CYP3A6, and activate HIF-1 and AP-1 to bind to CYP3A6 oligonucleotide probe. Inhibition of protein tyrosine kinases (PTKs) with genistein prevents the increase and binding of HIF-1 and AP-1 to the CYP3A6 oligonucleotide probe, as well as the increase of CYP3A6. On the other hand, inhibition of p42/44 MAPKs impedes the increase of c-jun and CYP3A6. The upregulation of CYP3A6 by serum from rabbits with hypoxia appears independent of CAR and PXR. Hypoxia in vivo, CoCl2 and lead downregulate CYP1A1 and 1A2.
Methods
Animals and hepatocyte collection
Male New Zealand White rabbits (1.8–2.2 kg) were obtained from Ferme Charles Rivers (St Constant, Québec, Canada). Rabbits were maintained on Purina Laboratory Chow and water ad libitum for at least 7 days before any experimental work was undertaken. To induce hypoxia, rabbits were introduced in a plexiglas chamber (0.75 × 1.20 × 1.25 m3) with a fractional concentration of inspired O2 (FiO2) of 8%, adjusted with an oxygen monitor (OM-15, Sensor Medics Corp., CA, U.S.A.) connected to an electrovalve (Asco Valves, Brantford, Ontario, Canada) that allowed the access of nitrogen into the chamber, which displaced the air off. Control rabbits were also placed into the chamber for the experiments, but breathing room air (FiO2=21%). All the rabbits remained for 48 h in the chamber, where they had access to Purina Laboratory Chow and water ad libitum. All the experiments were conducted according to the Canadian Council on Animal Care guidelines for use of laboratory animals.
Hepatocytes from rabbits with hypoxia (HHYPO) and from control rabbits (HCONT) were isolated 48 h after the induction of hypoxia or breathing room air, respectively, according to the two-step liver perfusion method of Seglen (1976), with minor modifications (El-Kadi et al., 1997). Cell cultures were always conducted under sterile conditions. Viability was assessed by trypan blue exclusion to ensure that it was greater than 90%; viability was not affected by in vivo hypoxia or any other experimental condition. Cell concentration was adjusted to 1 × 106 ml−1 with William's medium E (WME) supplemented with 10% calf serum and 1 μM insulin. Aliquots of 2 ml of the hepatocytes in suspension were transferred into six-well plastic culture plates (Falcon, Becton Dickinson Labware, Rutherford, NJ, U.S.A.) coated with type 1 rat tail collagen, and incubated for 2 h at 37°C in an atmosphere of 95% O2 and 5% CO2 to allow for stabilization. The medium was replaced with fresh WME supplemented with 10% calf serum and 1 μM insulin, and the HCONT were then incubated with 200 μl of serum from control rabbits (SCONT), 200 μl of SHYPO, 100 μM CoCl2 and 10 μM lead acetate for 24 h. The incubation of HCONT with CoCl2 and lead acetate was conducted in the absence of serum. Cells were also pretreated for 1 h with 100 μM PD98059 (Sigma Chemical, St Louis, MO, U.S.A.) and 150 μM genistein (Sigma Chemical) in 0.05% of DMSO at 37°C (Agani & Semenza, 1998; Hur et al., 2001).
Following 24 h of incubation, CYP3A activity and expression, and CYP1A1/1A2, HIF-1α and c-jun expression, as well as HIF-1 and AP-1 binding to CYP3A6 oligonucleotide probe, were documented.
Rabbit serum preparation
Blood samples (10 ml) were withdrawn from the rabbits 48 h after the induction of hypoxia and from control rabbits in a sterile Vacutainer Brand SST (Becton Dickinson, Mississauga, Ontario, Canada). Blood was allowed to clot at room temperature for 2 h; the samples were centrifuged at 2500 r.p.m. for 5 min, and the serum was decanted and stored frozen at −80°C in 1 ml aliquots until use. In samples handled as described, serum mediators conserved their activity for up to 12 months.
CYP3A activity
The activity of CYP3A was determined by measuring the ability of the hepatocytes to convert 3,4-difluorobenzyloxy-5,5 dimethyl-4-(4-methylsulfonyl phenyl)-(5H)-furan-2-one (DFB), a probe of CYP3A, to 3-hydroxy-4-(4-methylsulfonyl phenyl)-(5H)-furan-2-one (DFH), its fluorescent metabolite (Chauret et al., 1999). Briefly, 60 μM of DFB were incubated with the hepatocytes for 15 min, an aliquot of the supernatant was then transferred to a microtiter plate and quenched with an equal volume of acetonitrile containing 40% TRIS buffer (0.05 M). The fluorescence of the metabolite DFH was measured at excitation and emission wavelengths of 360 and 440 nm, respectively, using a fluorescent plate reader (Wallac Victor2 1420 multilabel counter), and expressed in arbitrary units (Silva & Nicoll-Griffith, 2001; Fradette et al., 2002).
Western blot analysis
Hepatocytes were washed, harvested in ice-cold PBS and centrifuged at 1500 × g for 5 min. The pellet was resuspended in cold lysis buffer (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol, protease inhibitor mixture), and cells were allowed to swell on ice for 15 min, and vortexed for 30 s. The amount of proteins in hepatocytes was measured in cell lysate by the method of Lowry (Lowry et al., 1951). For Western blot analysis, 50 μg of the cell lysate were separated by SDS–polyacrylamide gel electrophoresis (Fradette et al., 2002). The separated proteins were electrophoretically transferred to a nitrocellulose membrane, using a semidry transfer process (Bio-Rad, Hercules, CA, U.S.A.). CYP1A1 and 1A2 were detected with a polyclonal anti-rabbit CYP1A1 (Oxford Biochemical Research, Oxford, MI, U.S.A.) diluted 1 : 100 in 5% nonfat milk in TBS-T (0.1% Tween 20), and visualized with an alkaline phosphatase-conjugated secondary antibody using nitro blue tetrazolium as substrate. Membranes were incubated with specific antibodies against CYP3A6 diluted 1 : 500 (Oxford Biochemical Research, Oxford, MI, U.S.A.), HIF-1α diluted 1 : 400 (Novus Biological, Littleton, CO, U.S.A.), c-jun diluted 1 : 1000 (BD Biosciences, Mississauga, Canada), hCAR diluted 1 : 3000 (M. Negishi, National Institute on Environmental Health Sciences, Triangle Park, NC, U.S.A.) and PXR diluted 1 : 400 (P. Maurel, Centre National de la Recherche Scientifique, Montpellier, France), using a secondary antibody conjugated with a chemiluminescence reagent (horseradish peroxidase enzyme) and visualized by autoradiography. In each gel, 50 μg of proteins extracted from the same control hepatocytes, with constant amounts of CYP1A1, 1A2 and 3A6, were used as reference proteins. The assays were linear in the range of protein amounts assessed under the present experimental conditions. The intensity of the bands was quantified by means of the software Un-Scan-It-Gel (Silk Scientific Inc., Orem, UT, U.S.A.), and is represented in arbitrary units.
Analysis of the CYP3A6 5′ region
Inspection of the CYP3A6 5′ region revealed a potential HIF-1-binding-site sequence that was derived from bona fide HIF-1-binding sites previously identified by Wang et al. (1995), in genes encoding EPO and glycolytic enzymes. The CYP3A6 sequence 5′-CACGTGGG-3′ was located between −831 and −824 in the same orientation as the transcriptional unit. In the same way, inspection of the CYP3A6 promoter revealed a potential AP-1 binding-site sequence 5′-TGACTCC-3′ that was located between −109 and −103 bp.
Preparation of nuclear extracts
Hepatocytes were washed, harvested in ice-cold PBS and centrifuged at 1500 × g for 5 min. The pellet was resuspended in cold lyses buffer (10 mM HEPES pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol and protease inhibitor mixture), and cells were allowed to swell on ice for 15 min, to be vortexed for 30 s. After centrifugation, the nuclear pellet was resuspended in ice-cold nuclear suspension buffer (20 mM HEPES pH 7.9, 0.2 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol and protease inhibitor mixture). Finally, the nuclear extract was centrifuged for 5 min, and the supernatant was frozen at −80°C until use for the electrophoretic mobility shift assays (EMSAs).
Electrophoretic mobility shift assay (EMSAs)
The primers used for HIF-1α (5′-GGTCTCCCACGTGGG TGCA-3′), AP-1 (5′-GGGCACCTGACTCCCCGG-3′) and CAR (5′-ACATGAACTCAGAGGAGGTCACCACGG) oligo-nucleotides are all CYP3A6 promoter sequences, and were synthesized by Biocorp (Montréal, Québec, Canada). To generate double-stranded consensus oligonucleotides, the sense strands were annealed to a 10-fold excess of antisense strands by heating to 85°C for 10 min. The consensus oligonucleotides (5 pmol) were end-labelled with [γ-32P]ATP (Amersham Pharmacia Biotech, Piscataway, NJ, U.S.A.) using T4 polynucleotide kinase (Gibco Life Technologies, Burlington, Canada) and then purified on gel. Nuclear extracts (10–15 μg) were incubated on ice for 20 min with reaction buffer, containing 1 μg of poly(dI-dC) (Amersham Pharmacia Biotech), 50 mM Tris-HCl (pH 7.9), 5 mM MgCl2, 2.5 mM dithiothreitol, 250 mM NaCl, 20% glycerol and 2.5 mM EDTA in a total volume of 24 μl. A volume of 1 μm of 32P-labelled (30000 cpm) oligonucleotide probe was added to the reaction mixture and further incubated at room temperature for 20 min. For supershift assays, the antibodies were added to the reaction mixture and incubated on ice before loading. For all supershift assays, the serum from control rabbits was used as negative control. For competition studies, a 100-fold excess of unlabelled oligonucleotide was added to the reaction mixture during the incubation on ice before the addition of the labelled probe. DNA–protein complexes were resolved by polyacrylamide gel electrophoresis using a 4% nondenaturing gel, and run at 180 V in 0.25 × TBE (45 mM Tris borate and 1 mM EDTA). Gels were dried, and specific bands were quantified using the software Un-Scan-It-Gel (Silk Scientific Inc., Orem, UT, U.S.A.). The results are represented in arbitrary units.
Drugs and chemicals
Percoll, William's medium E, calf serum, type I rat tail collagen, NaCl, KCl, KH2PO4, HEPES, EDTA, EGTA, glucose, CoCl2 and lead acetate were purchased from Sigma Chemicals (Sigma, St Louis, MO, U.S.A.). DFB and DFH were provided by Merck Frosst Canada (Kirkland, Québec, Canada), and insulin was acquired from Boehringer Mannheim Biochemica (Mannheim, Germany).
Statistical analysis
All results are presented as mean±s.e. The activity of CYP3A and the amount of proteins assessed by Western blot or EMSA are presented in arbitrary units. The comparison of results from the various experimental groups and their corresponding controls was carried out by a one-way analysis of variance (ANOVA), followed by the Newman–Keuls post hoc test. Differences were considered significant when P<0.05.
Results
Effect of hypoxia in vivo on CYP3A6, HIF-1α and c-jun
Exposure of rabbits to an 8% FiO2 atmosphere generated a stable hypoxemia, with an average arterial partial pressure of O2 of 34.2±1.3 mmHg, without influencing the arterial partial pressure of CO2 (≈20.7±1.0) and arterial pH (7.47±0.05). Compared with HCONT, 48 h of hypoxia in vivo increased the amount of CYP3A6 (n=5), of HIF-1α (n=8) and of c-jun (n=5) (Figure 1a). With reference to HCONT, the increase in CYP3A6 expression in HHYPO was reflected by a greater activity, for example, 6589±250 vs 11571±295 (arbitrary units, n=5).
Figure 1.
Effect of moderate hypoxia for 48 h in vivo on CYP3A6, HIF-1α and c-jun expression in rabbit hepatocytes, and HIF-1 and AP-1 binding to the CYP3A6 oligonucleotide probe. (a) Average amounts of CYP3A6, HIF-1α and c-jun in hepatocytes from control (HCONT) and from rabbits with moderate hypoxia (HHYPO), and representative blots of these proteins. (b) Analysis of HIF-1 and AP-1 binding to the CYP3A6 oligonucleotide probe by EMSA in HCONT and HHYPO, and SS of HIF-1 and AP-1 in HHYPO. Vertical bars are mean±s.e. *P<0.05 compared with HCONT.
In the nuclear extracts of HHYPO, protein complexes were detected binding to CYP3A6 oligonucleotide probes (Figure 1b). The protein complexes were supershifted, using the anti-HIF-1α and anti-c-jun antibodies (Figure 1b, band SS). Competition studies with 100-fold excess of unlabelled probes efficiently competed with the labelled probes (data not shown).
Effect of SHYPO, CoCl2 and lead acetate in vitro on CYP3A6, HIF-1 and AP-1
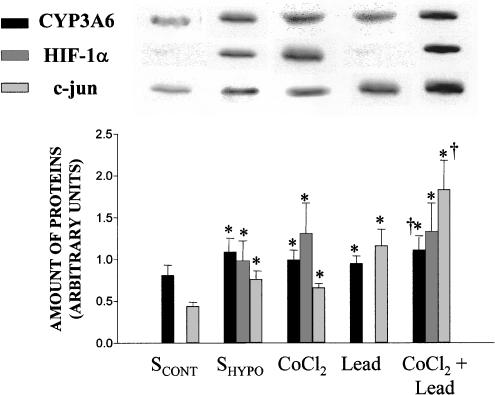
By reference to the effect of SCONT, incubation of HCONT with SHYPO for 24 h enhanced the amount of CYP3A6 (n=6), the amount of HIF-1α (n=8) and that of c-jun (n=6) (Figure 2). To confirm the role of HIF-1 and AP-1 on CYP3A6 expression, two activators of these transcription factors, CoCl2 and lead acetate, were used (Pyatt et al., 1996; Sultana et al., 1999). By comparison with saline, incubation of HCONT with CoCl2 for 24 h enhanced the expression of CYP3A6 (n=6), HIF-1α (n=10) and c-jun (n=7). On the other hand, lead acetate increased the expression of CYP3A6 (n=6) and that of c-jun (n=7). The effect of CoCl2 was additive to the effect of lead acetate, in the sense that the expression of CYP3A6 increased by 40% (n=6) when HCONT was incubated for 24 h with both substances simultaneously (Figure 2). Compared with the effect of SCONT, incubation of HCONT with SHYPO, CoCl2, lead acetate and the combination of CoCl2 and lead acetate increased the activity of CYP3A from 4952±186 to 6387±191, 6398±201, 6391±199 and 7184±219, respectively (n=6 for each group, P<0.05).
Figure 2.
Expression of CYP3A6, HIF-1α and c-jun in hepatocytes from control rabbits (HCONT). Average amounts of CYP3A6, HIF-1α and c-jun in hepatocytes from HCONT incubated for 24 h with the serum from rabbits with moderate hypoxia (SHYPO), CoCl2 and lead acetate, and representative blots of these proteins. Vertical bars are mean±s.e. *, †P<0.05 compared with SCONT and CoCl2 and lead, respectively.
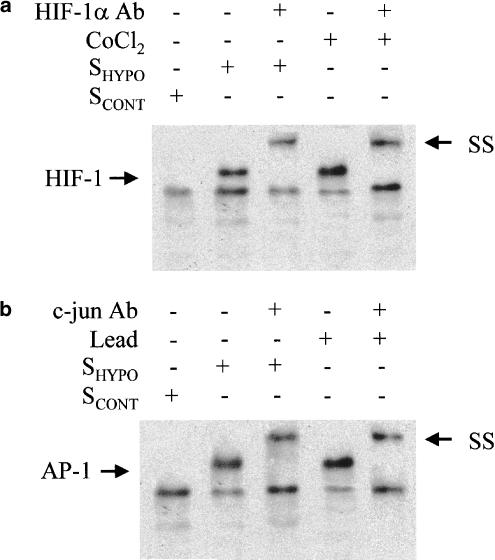
SHYPO induced the binding of transcription factors HIF-1 and AP-1 to the CYP3A6 oligonucleotide probe. In addition, CoCl2 and lead acetate promoted the binding of the transcription factors HIF-1 and AP-1, respectively, to the CYP3A6 oligonucleotide probe. Supershift analysis (SS) demonstrated that the protein complex included HIF-1α and c-jun (Figure 3a and b).
Figure 3.
EMSA of HIF-1 and AP-1. Effect of the serum from control rabbits (SCONT) and from rabbits with moderate hypoxia (SHYPO), CoCl2 and lead acetate on HIF-1 (a) and AP-1 (b) binding to the CYP3A6 oligonucleotide probe. SS indicates supershift analysis.
Signal transduction pathways implicated in CYP3A6 upregulation
HCONT were preincubated with PD98059, an inhibitor of p42/44 MAPK, and genistein, a nonspecific PTK inhibitor, and then exposed for 24 h to SCONT, SHYPO, CoCl2 and lead acetate. PD98059 or genistein did not affect the baseline values of HIF-1α, c-jun and CYP3A6 in HCONT (data not shown). However, PD98059 impeded the increment of CYP3A6 apoprotein induced by SHYPO, but not the increase in HIF-1α amount (Figure 4a) nor the binding of HIF-1 to the CYP3A6 oligonucleotide probe (Figure 4b). On the other hand, genistein prevented the effect of SHYPO on CYP3A6 and HIF-1α expression (Figure 4a), as well as the binding of HIF-1 to the CYP3A6 oligonucleotide probe (Figure 4b).
Figure 4.
Effect of the serum on CYP3A6 and HIF-1α expression, and HIF-1 binding to the CYP3A6 oligonucleotide probe in the presence of protein kinase inhibitors. (a) Average amount of CYP3A6 and HIF-1α, following incubation of hepatocytes from control rabbits (HCONT) with serum from control rabbits (SCONT), serum from rabbits with moderate hypoxia (SHYPO), SHYPO+PD98059 and SHYPO+genistein, and representative blots of CYP3A6 and HIF-1α. HCONT were preincubated with PD98059 (100 μM) and genistein (150 μM) for 1 h. (b) Effect of SHYPO, PD98059 and genistein on HIF-1 binding to the CYP3A6 oligonucleotide probe by EMSA, and SS of HIF-1. Vertical bars are mean±s.e. *P<0.05 compared with SCONT.
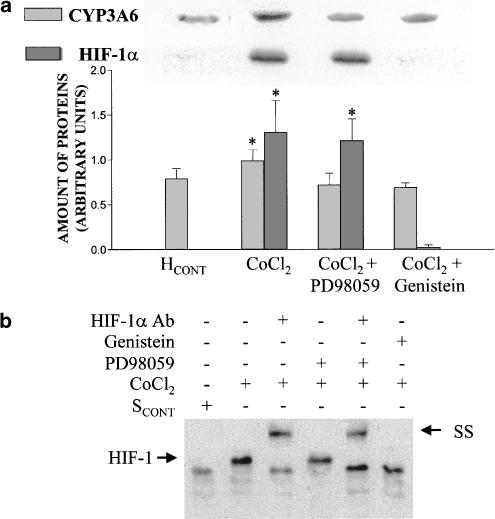
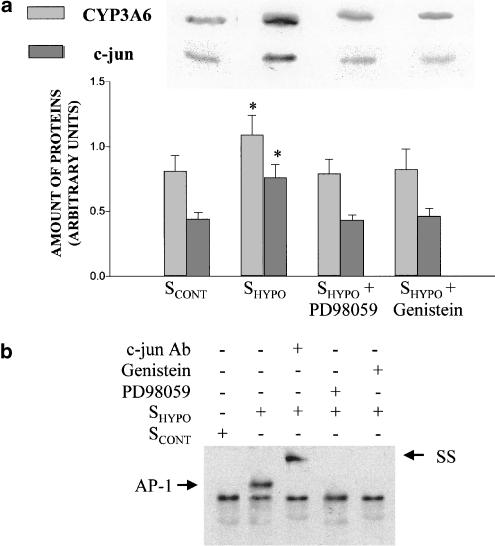
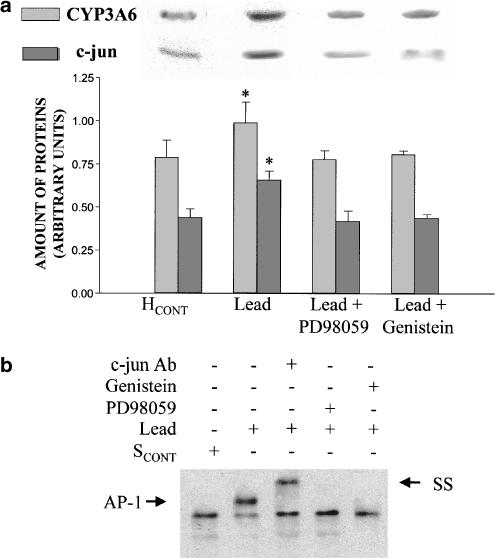
PD98059 impeded the increment of CYP3A6 apoprotein induced by CoCl2, but not the increase in HIF-1α amount (Figure 5a) nor the binding of HIF-1 to the CYP3A6 oligonucleotide probe (Figure 5b). On the other hand, genistein prevented the effect of CoCl2 on CYP3A6 and HIF-1α expression (Figure 5a), as well as the binding of HIF-1 to the CYP3A6 oligonucleotide probe (Figure 5b). Genistein and PD98059 prevented the effect of SHYPO (Figure 6a) and lead acetate (Figure 7a) on c-jun and CYP3A6 amounts, as well as AP-1 binding to the CYP3A6 oligonucleotide probe (Figures 6b and 7b).
Figure 5.
Effect of CoCl2 on CYP3A6 and HIF-1α expression and HIF-1 binding to the CYP3A6 oligonucleotide probe in the presence of protein kinase inhibitors. (a) Average amount of CYP3A6 and HIF-1α following incubation of hepatocytes from control rabbits (HCONT) with serum from control rabbits (SCONT), CoCl2, CoCl2+PD98059 and CoCl2+genistein, and representative blots of CYP3A6 and HIF-1α. HCONT were preincubated with PD98059 (100 μM) and genistein (150 μM) for 1 h. (b) Effect of CoCl2, PD98059 and genistein on HIF-1 binding to CYP3A6 oligonucleotide probe by EMSA, and SS of HIF-1. Vertical bars are mean±s.e. *P<0.05 compared with SCONT.
Figure 6.
Effect of serum on CYP3A6 and c-jun expression, and AP-1 binding to the CYP3A6 oligonucleotide probe in the presence of protein kinase inhibitors. (a) Average amount of CYP3A6 and c-jun following incubation of hepatocytes from control rabbits (HCONT) with the serum from control rabbits (SCONT), serum from rabbits with moderate hypoxia (SHYPO), SHYPO+PD98059 and SHYPO+genistein, and representative blots of CYP3A6 and c-jun. HCONT were preincubated with PD98059 (100 μM) and genistein (150 μM) for 1 h. (b) Effect of SHYPO, PD98059 and genistein on AP-1 binding to the CYP3A6 oligonucleotide probe by EMSA, and SS of AP-1. Vertical bars are mean±s.e. *P<0.05 compared with SCONT.
Figure 7.
Effect of lead acetate on CYP3A6 and c-jun expression, and AP-1 binding to the CYP3A6 oligonucleotide probe in the presence of protein kinase inhibitors. (a) Average amount of CYP3A6 and c-jun following incubation of hepatocytes from control rabbits (HCONT) with serum from control rabbits (SCONT), lead, lead+PD98059 and lead+genistein, and representative blots of CYP3A6 and c-jun. HCONT were preincubated with PD98059 (100 μM) and genistein (150 μM) for 1 h. (b) Effect of lead, PD98059 and genistein on AP-1 binding to the CYP3A6 oligonucleotide probe by EMSA, and SS of AP-1. Vertical bars are mean±s.e. *P<0.05 compared with SCONT.
Effect of hypoxia in vivo and of SHYPO on CAR and PXR expression
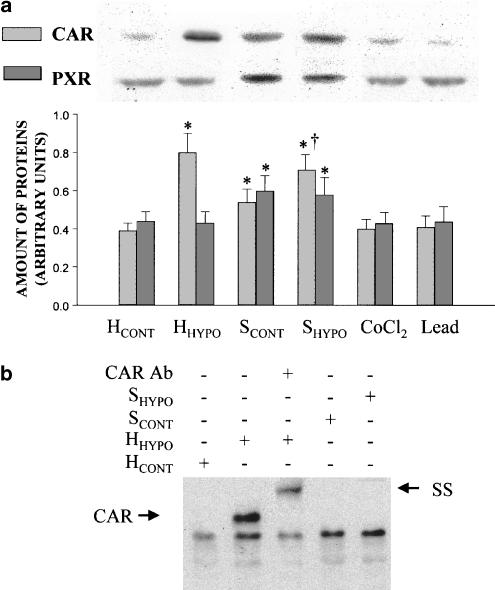
Compared with HCONT, 48 h of hypoxia in vivo increased the amount of CAR (n=5, P<0.05), but did not elicit any effect on PXR amount. When HCONT were exposed to SCONT for 24 h, the amounts of CAR and PXR increased (n=5). In the presence of SHYPO, the amount of CAR was 20% higher than that estimated after the incubation with SCONT (n=6). Incubation of HCONT for 24 h with CoCl2 or lead acetate did not change CAR and PXR amounts (Figure 8a). In vivo, hypoxia for 48 h promoted the binding of the nuclear receptor CAR to the CYP3A6 oligonucleotide probe (Figure 8b). SS demonstrated that the protein complex included CAR. On the other hand, SCONT or SHYPO did not activate CAR binding to the CYP3A6 oligonucleotide probe (Figure 8b), even in the presence of CYP3A6 upregulation. Competition studies with 100-fold excess of unlabelled probes efficiently competed with the labelled probes (data not shown).
Figure 8.
Effect of hypoxia in vivo and of serum from rabbits with moderate hypoxia (SHYPO) on the CAR and PXR expression, and CAR binding to the CYP3A6 oligonucleotide probe. (a) Average amount of CAR and PXR in hepatocytes from control rabbits (HCONT), hepatocytes from rabbits with moderate hypoxia (HHYPO) and in HCONT incubated with serum from control rabbits (SCONT), SHYPO, CoCl2 and lead acetate, and representative blots of CAR and PXR. (b) Effect of moderate hypoxia in vivo and SHYPO on CAR binding to the CYP3A6 oligonucleotide probe by EMSA, and SS of CAR. Vertical bars are mean±s.e. *, †P<0.05 compared with HCONT and SCONT, respectively.
Effect of hypoxia in vivo, and of SHYPO, CoCl2 and lead acetate in vitro on CYP1A1 and 1A2 expression
Compared to rabbits breathing a 21% FiO2, hypoxia reduced the amounts of CYP1A1 and 1A2 apoproteins (n=5) (Figure 9). No changes in the amount of CYP1A1 and 1A2 were observed following the incubation of HCONT with SHYPO. On the other hand, CoCl2 and lead acetate diminished the amount of CYP1A1 and 1A2 apoproteins (n=6) (Figure 9).
Figure 9.
Expression of CYP1A1 and CYP1A2 in hepatocytes from rabbits. The average amount of CYP1A1 and CYP1A2 in hepatocytes from control rabbits (HCONT), from rabbits with moderate hypoxia (HHYPO), and in HCONT incubated for 24 h with serum from control rabbits (SCONT) and from rabbits with moderate hypoxia (SHYPO), with CoCl2, and with lead acetate and representative blots of CYP1A1 and CYP1A2. Vertical bars are mean±s.e. *P<0.05 compared with HCONT.
Discussion
This study demonstrates that, in vivo, hypoxia enhances the expression of CYP3A6, CAR, HIF-1 and AP-1, and prompts HIF-1 and AP-1 binding to the CYP3A6 oligonucleotide probe. In vitro, SHYPO augments HIF-1 and AP-1 expression, and stimulates their binding to the CYP3A6 oligonucleotide probe. A p42/44 MAPK-dependent protein phosphorylation is required to increase CYP3A6 expression. In response to hypoxia, HIF-1 is detected in most cell types and activates the transcription of genes encoding proteins that will increase O2 delivery (EPO and VEGF), allow metabolic adaptation (glucose transporters, glycolytic enzymes), or promote cell survival (insulin-like growth factor 2) (Semenza, 1999a). The binding of HIF-1 and AP-1 to the CYP3A6 oligonucleotide probe suggests that CYP3A6 gene is another target for HIF-1 and AP-1. The present results do not discard that hypoxia and SHYPO, HIF-1 and AP-1 may modulate the CYP3A6 steady-state expression by mechanisms other than CYP3A6 transcription.
The underlying mechanisms by which cells sense a decrease in O2 concentration and transduce this signal to HIF-1α remain largely unknown (Semenza, 1999b). Several mechanisms have been proposed to mediate the hypoxic transcriptional response, such as: (a) changes in activity of the iron containing heme groups or in iron/sulfur clusters under hypoxic conditions (Goldberg et al., 1988), (b) release of reactive oxygen intermediates generated by a flavoprotein-containing NAD(P)H oxidase or by the mitochondria (Fandrey et al., 1994; Chandel et al., 1998) and (c) release of growth factors and cytokines, such as epidermal growth factor, interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), angiotensin II, transforming growth factor β1 and hepatocyte growth factor, capable of stabilizing HIF-1α (Wenger, 2002). Since the changes in cytochrome P450 induced by hypoxia are mediated by the release of IL-1β, IL-2, interferon-γ and circulating EPO in blood (Fradette et al., 2002), we may speculate that these circulations have contributed to increase HIF-1 and AP-1 expression.
The regulation of CYP3A6 expression by HIF-1 has similarities with the modulation of other proteins by HIF-1 (Salceda et al., 1997). Confirming the report of Wang et al. (1995), HIF-1 binding to the CYP3A6 oligonucleotide probe induced by SHYPO is prevented by genistein. In addition, the present results show that p42/44 MAPK is a required step for the increase in CYP3A6 expression, presumably by the transactivation of CYP3A6 gene by HIF-1, but not for HIF-1 binding to CYP3A6 promoter. On the other hand, p42/44 MAPK appears to modulate the transcriptional activity of HIF-1, but not the transactivation of VGEF gene (Richard et al., 1999). These observations suggest that the role of p42/44 MAPK activation may differ as a function of the gene transactivated.
Hypoxia in vivo and SHYPO in vitro increased the expression of c-jun and prompted AP-1 binding to the CYP3A6 oligonucleotide probe. Inhibition of PTK with genistein and p42/44 MAPK with PD98059 impeded AP-1 activation and binding to the CYP3A6 oligonucleotide probe. The expression of AP-1 is regulated by calmodulin-dependent mechanisms associated to the hypoxia-induced increase in intracellular Ca2+, either from the influx of Ca2+ through L-type voltage-gated Ca2+ channels (Berna et al., 2001), or via the Ca2+ released from intracellular stores (Hampl et al., 1995). The increase in intracellular Ca2+ activates extracellular-regulated kinase (ERK2), essential for c-fos and c-jun activation, and prompts AP-1-dependent transcription. Both the activation of c-jun expression and the AP-1-dependent transcription are sensitive to PTK inhibitors (Premkumar et al., 2000; Salnikow et al., 2002).
The effect of SHYPO was reproduced by CoCl2, which mimics the hypoxic environment by substituting for Fe2+ in the porphyrin ring of hemoglobin, thus causing a conformational change resulting in decreased oxygen affinity and effectively locking the heme molecule in a deoxygenated conformation (Goldberg & Schneider, 1994). By simulating hypoxia, CoCl2 increased the amount of HIF-1α and slightly that of c-jun. Binding of HIF-1 to the CYP3A6 oligonucleotide probe was also enhanced, a binding that was prevented by genistein, although not by PD98059. This may be explained by the fact that CoCl2 causes a time-dependent tyrosine phosphorylation of MAPK kinase isoform ERK2, which is inhibited by PD98059 (Sultana et al., 1999). According to our results, we postulate that the transactivation of CYP3A6 gene requires a phosphorylation, but not the binding of HIF-1 to the CYP3A6 promoter.
Lead acetate is a potent blocker of calcium channels; it activates calmodulin with a higher affinity than Ca2+ and, most importantly, picomolar concentrations can substitute for Ca2+ to activate protein kinase C (PKC) (Markovac & Goldstein, 1988), resulting in the activation of mitogen-activated protein kinase kinase (MEK) and MAPK (Ramesh et al., 1999), and in the induction of the immediate-early-response proteins c-fos, c-jun and erg-1 (Bressler et al., 1999), which will form AP-1 by dimerization (Halazonetis et al., 1988). Our results suggest that lead, by triggering the formation and nuclear transcription of AP-1, events requiring a PTK and p22/p44 MAPK, increases the expression of CYP3A6, subsequently, to the binding of AP-1 to CYP3A6 promoter.
By reproducing the effect of the mediators in serum, the experiments conducted with CoCl2 and lead acetate strongly support that HIF-1 and AP-1 are directly implicated in the hypoxia-induced upregulation of CYP3A6. Moreover, when hepatocytes were incubated with both CoCl2 and lead, the upregulation of CYP3A6 was greater than with any single agent, supporting the independent and cooperative effects on CYP3A6 expression. This observation is in agreement with reports showing that, in response to hypoxia, HIF-1 and AP-1 cooperate to increase the expression of the genes encoding for VEGF (Damert et al., 1997) and tyrosine hydroxylase (Norris & Millhorn, 1995).
The expression of CYP3A is modulated by several nuclear receptors, such as CAR, PXR, retinoid X receptor (RXR), hepatocyte nuclear factor 4 (HNF-4), and glucocorticoid receptor (GR) (Gibson et al., 2002). PXR forms a heterodimer with RXR, which activates CYP3A transcription through binding to an ER6 element in the proximal promoter (Lehmann et al., 1998). PXR is activated by numerous compounds known to induce CYP3A expression (Moore et al., 2000). CAR is sequestered in the cytosol and translocates into the nucleus upon activation, presumably through several steps of phosphorylation, where it dimerizes with RXR (Kawamoto et al., 1999). Since both CAR and PXR are involved in the regulation of CYP3A gene, it was of interest to assess whether hypoxia activates these nuclear receptors. In vivo, hypoxia induced CAR for 48 h, but not PXR protein expression; moreover, hypoxia enhanced CAR binding to the CYP3A6 oligonucleotide probe. In HCONT, SCONT and SHYPO induced the expression of both CAR and PXR, without prompting their binding to the CYP3A6 oligonucleotide probe. Since CoCl2 and lead acetate did not impact CAR and PXR expression, we may speculate that SCONT and SHYPO contain ligands to the GR, known to activate these nuclear receptors. Our results support the postulate that hypoxia increases CYP3A6 expression independently of PXR, although in vivo CAR may be implicated.
We have shown that in vivo hypoxia reduces the expression of CYP1A1/1A2, downregulation in part due to the cytokines present in SHYPO, such as interferon-γ, IL-1β and IL-2 (Fradette et al., 2002). In addition, in vitro CoCl2 downregulates CYP1A1/1A2 in control hepatocytes, for example, constitutive CYP1A1/1A2, suggesting that several mechanisms underlie the reduction of CYP1A1/2 by hypoxia. The effect of CoCl2 on CYP1A may be explained on the basis that stabilized HIF-1α competes with AhR for Arnt, resulting in the decrease in AhR/Arnt heterodimer and reduction in CYP1A1/1A2 expression (Chan et al., 1999). Lead acetate also downregulates CYP1A1/1A2, even if lead does not increase HIF-1α expression or HIF-1 binding to the CYP3A6 oligonucleotide probe. On the other hand, SHYPO did activate HIF-1, but did not downregulate CYP1A1/1A2. All these apparently contradictory results support the presence of various mechanisms, HIF-1-dependent and independent, modulating the expression of CYP1A1/1A2.
Promoter regions of various CYP3A genes may contain several response elements or nuclear receptor-binding sites with direct repeat-3 and -4, and everted repeat-6 motifs, such as the common regulatory elements found at −140 to −170 bp. Upstream, there is a 230 bp distal element from −7836 to −7607 bp, called the xenobiotic-responsive enhancer module (XREM) (Sueyoshi & Negishi, 2001). Both common positive regulatory elements and XREM are docking sites for CAR/PXR/RXR, and are implicated in the induction elicited by high doses of dexamethasone, rifampicin and pregnenolone 16α-carbonitrile. About −2 kb upstream, the glucocorticoid response element binds the GR, which is activated by low doses of dexamethasone (Quattrochi & Guzelian, 2001). XREM appears to be modulated by an upstream negative regulatory element, the chicken ovalbumin upstream promoter transcription factor (COUP-TF), and downstream, by a positive regulatory element, dNR3. In addition, HNF-4 and the upstream stimulatory factor-1 (USF-1) are positive regulatory elements present in the region from −146 to −156 bp (Nakayama et al., 2001). The present study allows one to hypothesize that the two binding sites, −109 bp to bind AP-1 and at −831 bp to bind HIF-1, identified on the CYP3A6 promoter are positive regulatory elements.
Acknowledgments
This work was supported by a grant from the Canadian Institutes of Health Research (MOP-43925). We are grateful to Professor Patrick Maurel (Centre National de la Recherche Scientifique, Montpellier, France) for providing PXR and CAR antibodies, Dr N. Chauret from Merck Frosst, Canada, for providing 3,4-difluorobenzyloxy-5,5 dimethyl-4-(4-methylsulfonyl phenyl)-(5H)-furan-2-one (DFB) and Dr M. Nemer laboratory for providing technical assistance for the gel shift. The technical assistance of Ms Lucie Héroux is also gratefully acknowledged.
Abbreviations
- AhR
aryl hydrocarbon receptor
- AP-1
activator protein-1
- Arnt
aryl hydrocarbon receptor nuclear translocator
- CAR
constitutively activated receptor
- COUP-TF
chicken ovalbumin upstream promoter transcription factor
- DFB
3,4-difluorobenzyloxy-5,5 dimethyl-4-(4-methylsulfonyl phenyl)-(5H)-furan-2-one
- DFH
3-hydroxy-4-(4-methylsulfonyl phenyl)-(5H)-furan-2-one
- EPO
erythropoietin
- ERK
extracellular-regulated kinase
- GR
glucocorticoid receptor
- HCONT
hepatocytes from control rabbits
- HHYPO
hepatocytes from rabbits with hypoxia
- HIF-1
hypoxia-inducible factor 1
- HNF-4
hepatocyte nuclear factor 4
- IL-1β
interleukin 1β
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MEK
mitogen-activated protein kinase kinase
- PKC
protein kinase C
- PTK
protein tyrosine kinase
- PXR
pregnane X receptor
- RXR
retinoid X receptor
- SCONT
serum from control rabbits
- SHYPO
serum from rabbits subjected to moderate hypoxia
- TNF-α
tumor necrosis factor α
- USF-1
upstream stimulatory factor-1
- VEGF
vascular endothelial growth factor
- WMR
William's medium E
- XREM
xenobiotic-responsive enhancer module
References
- AGANI F., SEMENZA G.L. Mersalyl is a novel vascular endothelial growth factor gene expression and hypoxia-inducible factor 1 activity. Mol. Pharm. 1998;54:749–754. doi: 10.1124/mol.54.5.749. [DOI] [PubMed] [Google Scholar]
- BERNA N., ARNOULD T., REMACLE J., MICHIELS C. Hypoxia-induced increase in intracellular calcium concentration in endothelial cells: role of the Na(+)-glucose cotransporter. J. Cell. BioChem. 2001;84:115–131. doi: 10.1002/jcb.1271. [DOI] [PubMed] [Google Scholar]
- BRESSLER J., KIM K.A., CHAKRABORTI T., GOLDSTEIN G. Molecular mechanisms of lead neurotoxicity. Neurochem. Res. 1999;24:595–600. doi: 10.1023/a:1022596115897. [DOI] [PubMed] [Google Scholar]
- CHAN W.K., YAO G., GU Y.Z., BRADFIELD C.A. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J. Biol. Chem. 1999;274:12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- CHANDEL N.S., MALTEPE E., GOLDWASSER E., MATHIEU C.E., SIMON M.C., SCHUMACKER P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5015–5019. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAURET N., TREMBLAY N., LACKMAN R.L., GAUTHIER J.Y., SILVA J.S., MAROIS J., YERGRY J.A., NICOLL-GRIFFITH D.A. Description of a 96-well plate assay to measure P4503A inhibition in human liver microsomes using a selective fluorescent probe. Anal. BioChem. 1999;276:215–225. doi: 10.1006/abio.1999.4348. [DOI] [PubMed] [Google Scholar]
- DAMERT A., IKEDA E., RISAU W. Activator-protein-1 binding potentiates the hypoxia-inducible factor-1-mediated hypoxia-induced transcriptional activation of vascular-endothelial growth factor expression in C6 glioma cells. Biochem. J. 1997;327:419–423. doi: 10.1042/bj3270419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-KADI A.O.S., MAURICE H., ONG H., DU SOUICH P. Down regulation of the hepatic cytochrome P450 by an acute inflammatory reaction: implication of human and animal serum, and intrahepatic mediators. Br. J. Pharmacol. 1997;121:1164–1170. doi: 10.1038/sj.bjp.0701232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANDREY J., FREDE S., JELKMANN W. Role of hydrogen peroxide in hypoxia-induced erythropoietin production. Biochem. J. 1994;303:507–510. doi: 10.1042/bj3030507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRADETTE C., BLEAU A.M., PICHETTE V., CHAURET N., DU SOUICH P. Hypoxia changes the expression of CYP1A1/1A2 and 3A6 by a mechanism involving the release of serum mediators. Br. J. Pharmacol. 2002;137:881–891. doi: 10.1038/sj.bjp.0704933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON G.G., PLANT N.J., SWALES K.E., AYRTON A., EL-SANKARY W. Receptor-dependent transcriptional activation of cytochrome P4503A genes: induction mechanisms, species differences and interindividual variation in man. Xenobiotica. 2002;32:165–206. doi: 10.1080/00498250110102674. [DOI] [PubMed] [Google Scholar]
- GOLDBERG M.A., DUNNING S.P., BUNN H.F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988;242:1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- GOLDBERG M.A., SCHNEIDER T.J. Similarities between the oxygen-sensing mechanisms regulating the expression of vascular endothelial growth factor and erythropoietin. J. Biol. Chem. 1994;269:4355–4359. [PubMed] [Google Scholar]
- GOZAL E., SIMAKAJORNBOON N., DAUSMAN J.D., XUE Y.D., CORTI M., EL-DAHR S.S., GOZAL D. Hypoxia induces selective SAPK/JNK-2-AP-1 pathway activation in the nucleus tractus solitarii of the conscious rat. J. NeuroChem. 1999;73:665–674. doi: 10.1046/j.1471-4159.1999.0730665.x. [DOI] [PubMed] [Google Scholar]
- HALAZONETIS T.D., GEORGOPOULOS K., GREENBERG M.E., LEDER P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988;55:917–924. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- HAMPL V., CORNFIELD D.N., COWAN N.J., ARCHER S.L. Hypoxia potentiates nitric oxide synthesis and transiently increases cytosolic calcium levels in pulmonary artery endothelial cells. Eur. Respir. J. 1995;8:515–522. [PubMed] [Google Scholar]
- HUR E., CHANG K.Y., LEE E., LEE S.K., PARK H. Mitogen-activated protein kinase kinase inhibitor PD98059 blocks the trans-activation but not the stabilization or DNA binding ability of hypoxia-inducible factor-1α. Mol. Pharmacol. 2001;59:1216–1224. doi: 10.1124/mol.59.5.1216. [DOI] [PubMed] [Google Scholar]
- KAWAMOTO T., SUEYOSHI T., ZELKO I., MOORE R., WASHBURN K., NEGISHI M. Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol. Cell. Biol. 1999;19:6318–6322. doi: 10.1128/mcb.19.9.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURDI J., MAURICE H., EL-KADI A.O.S., ONG H., DALKARA S., BÉLANGER P.M., DU SOUICH P. Effect of hypoxia alone or combined with inflammation and 3-methylcholanthrene on hepatic cytochrome P450 in conscious rabbits. Br. J. Pharmacol. 1999;128:365–373. doi: 10.1038/sj.bjp.0702795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEHMANN J.M., MCKEE D.D., WATSON M.A., WILLSON T.M., MOORE J.T., KLIEWER S.A. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MARKOVAC J., GOLDSTEIN G.W. Picomolar concentrations of lead stimulate brain protein kinase C. Nature. 1988;334:71–73. doi: 10.1038/334071a0. [DOI] [PubMed] [Google Scholar]
- MICHIELS C., ARNOULD T., REMACLE J. Endothelial cell responses to hypoxia: initiation of a cascade of cellular interactions. Biochim. Biophys. Acta. 2000;1497:1–10. doi: 10.1016/s0167-4889(00)00041-0. [DOI] [PubMed] [Google Scholar]
- MOORE L.B., PARKS D.J., JONES S.A., BLEDSOE R.K., CONSLER T.G., STIMMEL J.B., GOODWIN B., LIDDLE C., BLANCHARD S.G., WILLSON T.M., COLLINS J.L., KLIEWER S.A. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J. Biol. Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- NAKAYAMA K., SUDO Y., SASAKI Y., IWATA H., TAKAHASHI M., KAMATAKI T. Studies on transcriptional regulation of CYP3A16 gene in mouse livers by application of direct DNA injection method. Biochem. Biophys. Res. Commun. 2001;287:820–824. doi: 10.1006/bbrc.2001.5663. [DOI] [PubMed] [Google Scholar]
- NORRIS M.L., MILLHORN D.E. Hypoxia-induced protein binding to O2-responsive sequences on the tyrosine hydroxylase gene. J. Biol. Chem. 1995;270:23774–23779. doi: 10.1074/jbc.270.40.23774. [DOI] [PubMed] [Google Scholar]
- PREMKUMAR D.R., ADHIKARY G., OVERHOLT J.L., SIMONSON M.S., CHERNIACK N.S., PRABHAKAR N.R. Intracellular pathways linking hypoxia to activation of c-fos and AP-1. Adv. Exp. Med. Biol. 2000;475:101–109. doi: 10.1007/0-306-46825-5_10. [DOI] [PubMed] [Google Scholar]
- PYATT D.W., ZHENG J.H., STILLMAN W.S., IRONS R.D. Inorganic lead activates NF-kappa B in primary human CD4+ T lymphocytes. Biochem. Biophys. Res. Commun. 1996;227:380–385. doi: 10.1006/bbrc.1996.1516. [DOI] [PubMed] [Google Scholar]
- QUATTROCHI L.C., GUZELIAN P.S. CYP3A regulation: from pharmacology to nuclear receptors. Drug Metab. Dispos. 2001;29:615–622. [PubMed] [Google Scholar]
- RAMESH G.T., MANNA S.K., AGGARWAL B.B., JADHAV A.L. Lead activates nuclear transcription factor-kappaB, activator protein-1, and amino-terminal c-Jun kinase in pheochromocytoma cells. Toxicol. Appl. Pharmacol. 1999;155:280–286. doi: 10.1006/taap.1999.8624. [DOI] [PubMed] [Google Scholar]
- RICHARD D.E., BERRA E., POUYSSEGUR J. Angiogenesis: how a tumor adapts to hypoxia. Biochem. Biophys. Res. Commun. 1999;266:718–722. doi: 10.1006/bbrc.1999.1889. [DOI] [PubMed] [Google Scholar]
- SALCEDA S., BECK I., SRINIVAS V., CARO J. Complex role of protein phosphorylation in gene activation by hypoxia. Kidney Int. 1997;51:556–559. doi: 10.1038/ki.1997.78. [DOI] [PubMed] [Google Scholar]
- SALNIKOW K., KLUZ T., COSTA M., PIQUEMAL D., DEMIDENKO Z.N., XIE K., BLAGOSKLONNY M.V. The regulation of hypoxic genes by calcium involves c-Jun/AP-1, which cooperates with hypoxia-inducible factor 1 in response to hypoxia. Mol. Cell. Biol. 2002;22:1734–1741. doi: 10.1128/MCB.22.6.1734-1741.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGLEN P.O. Preparation of isolated rat liver cells. Methods Cell. Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- SEMENZA G.L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 1999a;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- SEMENZA G.L. Perspectives on oxygen sensing. Cell. 1999b;98:281–284. doi: 10.1016/s0092-8674(00)81957-1. [DOI] [PubMed] [Google Scholar]
- SEMENZA G.L. HIF-1: mediator of physiological and pathological responses to hypoxia. J. Appl. Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- SILVA J.M., NICOLL-GRIFFITH D.A.In vitro models for studying induction of cytochromes P450 enzymes Drug–drug Interactions 2001New York: Marcel Dekker; 199–201.ed. Rodrigues, A.D. pp [Google Scholar]
- SUEYOSHI T., NEGISHI M. Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu. Rev. Pharmacol. Toxicol. 2001;41:123–143. doi: 10.1146/annurev.pharmtox.41.1.123. [DOI] [PubMed] [Google Scholar]
- SULTANA C., SHEN Y., JOHNSON C., KALRA V.K. Cobalt chloride-induced signaling in endothelium leading to the augmented adherence of sickle red blood cells and transendothelial migration of monocyte-like HL-60 cells is blocked by PAF-receptor antagonist. J. Cell. Physiol. 1999;179:67–78. doi: 10.1002/(SICI)1097-4652(199904)179:1<67::AID-JCP9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- WANG G.L., JIANG B.H., SEMENZA G.L. Effect of protein kinase and phosphatase inhibitors on expression of hypoxia-inducible factor 1. Biochem. Biophys. Res. Commun. 1995;216:669–675. doi: 10.1006/bbrc.1995.2674. [DOI] [PubMed] [Google Scholar]
- WENGER R.H. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]