Abstract
The sphingolipid ceramide, a primary building block for all other sphingolipids, is associated with growth arrest, apoptosis, and lipotoxic dysfunction. Interestingly, ceramide may attenuate high glucose-induced myocyte dysfunction, produce Ca2+ influx, and augment smooth muscle contraction. To determine the role of ceramide on cardiac excitation–contraction (E–C) coupling, electrically paced adult rat ventricular myocytes were acutely exposed to a cell-permeable ceramide analog (10 pM–100 μM) and the following indices were determined: peak shortening (PS), time-to-P., time-to-90% relengthening, and the maximal velocity of shortening and relengthening (±dLdt). Intracellular Ca2+ properties were assessed using fura-2AM fluorescent microscopy.
Our results revealed a concentration- and time-dependent increase of PS in ventricular myocytes in response to ceramide associated with an increase in ±dLdt. The maximal increase in PS was ∼35% from control value and was maintained throughout the first 20 min of ceramide exposure. However, the ceramide-induced increase in PS was not maintained once the exposure time was beyond 20 min. Acute exposure of ceramide significantly enhanced intracellular Ca2+ release, although at a much lower concentration range. The ceramide-induced augmentation of PS was not significantly affected by inhibition of phosphatidylinositol (PI)-3-kinase, protein kinase C (PKC), ceramide-activated protein phosphatase (CAPP), and nitric oxide (NO) synthase.
Our data suggest that ceramide acutely augments the contractile function of cardiac myocytes through an alternative mechanism(s) rather than PI-3-kinase, PKC, CAPP, or NO.
Keywords: Ceramide, cardiac myocyte, shortening and relengthening, cell signaling
Introduction
Sphingolipids possess many cellular functions including stimulation of proliferation and differentiation, growth arrest, apoptosis, and stress activation (Mathias et al., 1998; Liu et al., 1999; Hernandez et al., 2000). Ceramide, a sphingolipid, is central to the synthesis and metabolism of nearly all other sphingolipids (Mathias et al., 1998; Liu et al., 1999). Intriguingly, a role for ceramide-associated cellular dysfunction has been implicated in ischemia reperfusion and lipotoxic cardiac dysfunction (Hernandez et al., 2000; Zhou et al., 2000). The metabolism of ceramide results in elevated sphingosine and sphingosine-1-phosphate levels which may also alter the cardiac function (Yokoyama et al., 1993; McDonough et al., 1994; Friedrichs et al., 2002). Sphingosine is thought to mediate the depression of cardiac myocyte contractile function associated with the proinflammatory cytokine tumor necrosis factor α (TNFα) (Oral et al., 1997; Schereur & Liu, 1997; Huwiler et al., 1999a). On the contrary, recent study has also shown that ceramide may play a role in the protection of cardiac myocytes, such as in high glucose-induced cardiomyocyte contractile dysfunction (Colligan et al., 2002).
Although ceramide itself has been speculated as a second messenger (Yokoyama et al., 1993; Zu Heringdorf et al., 1997; Mathias et al., 1998; Liu et al., 1999), acute ceramide exposure to smooth muscle or carcinoma cells results in an immediate activation of cell signaling pathways such as phosphatidylinositol (PI)-3-kinase (Mathias et al., 1991; Ibitayo et al., 1998). The ceramide-induced activation of PI-3-kinase occurs within seconds and may be responsible for enhanced tension in colonic smooth muscle (Ibitayo et al., 1998). Interestingly, previous investigations regarding the role of ceramide in cardiac function examined only prolonged exposure of ceramide analogs or endogenous ceramide (Oral et al., 1997). In addition, the possible mechanisms of ceramide signaling have not been fully elucidated in ventricular cardiac myocytes (Mathias et al., 1991; Dobrowsky & Hannun, 1992). Therefore, the aim of the present study was to determine the direct effects of acute ceramide exposure in isolated ventricular myocyte contractile function and signaling mechanisms involved by utilizing a cell membrane-permeable ceramide analog, C2-ceramide.
Methods
Isolation of ventricular myocytes
The experimental procedure used in this study was approved by the University of North Dakota Animal Use and Care Committee (Grand Forks, ND, U.S.A.). Briefly, adult male Sprague–Dawley rats (body weight=422.4±15.8 g, n=14) were anesthetized with ketamine/xylazine (5 : 3, 1.32 mg kg−1 i.p.). Hearts were rapidly removed and perfused (at 37°C) with Krebs–Henseleit bicarbonate (KHB) buffer (mM: NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, N-[2-hydro-ethyl]-piperazine-N′-[2-ethanesulfonic acid] (HEPES) 10, glucose 11.1, pH 7.4). The heart was then perfused for 20 min with KHB containing 223 U ml−1 collagenase (type II, Worthington Biochemical Corp., Freehold, NJ, U.S.A.) and 0.5 mg ml−1 hyaluronidase (Sigma Chemical, St Louis, MO, U.S.A.). After perfusion, the left ventricle was removed and minced. The cells were further digested with 0.02 mg ml−1 trypsin (Sigma, St Louis, MO, U.S.A.) before being filtered through a nylon mesh (300 μm). Extracellular Ca2+ was added incrementally back to 1.25 mM. Isolated myocytes were maintained in a serum-free medium for up to 18 h prior to experimentation (Nickola et al., 2000).
Cell shortening/relengthening
The mechanical properties of ventricular myocytes were assessed using an IonOptix MyoCam® soft-edge system (IonOptix Corporation, Milton, MA, U.S.A.) (Ren et al., 1996). In brief, cells were superfused with a buffer containing (in mM): NaCl 131, KCl 4, CaCl2 1, MgCl2 1, glucose 10, HEPES 10, pH 7.4. The cells were field stimulated at 0.5 Hz. The myocyte was displayed on a computer monitor using an IonOptix MyoCam® camera, which rapidly scans the image area every 8.3 ms such that the amplitude and velocity of shortening/relengthening are recorded with good fidelity.
Intracellular fluorescence measurement
Myocytes were loaded with fura-2AM (0.5 μM) for 10 min and fluorescence measurements were recorded with a dual-excitation fluorescence photomultiplier system (Ionoptix) (Ren et al., 1996; Nickola et al., 2000). Myocytes were imaged through an Olympus (Model IX-70) fluor × 40 oil objective. Cells were exposed to light emitted by a 75 W lamp and passed through either a 360 or a 380 nm filter (bandwidths were ±15 nm), while being stimulated to contract at 0.5 Hz. Fluorescence emissions were detected between 480 and 520 nm after first illuminating cells at 360 nm for 0.5 s, and then at 380 nm for the duration of the transient period (1000 Hz sampling rate). The 360 nm excitation scan was repeated at the end of the protocol and qualitative changes in intracellular Ca2+ concentration were inferred from the ratio of the fluorescence intensity at two wavelengths (Nickola et al., 2000).
Experimental protocols
Myocytes (either fura-2AM loaded or nonloaded) were stimulated to contract at 0.5 Hz. A cell-permeable ceramide analog, C2-ceramide (N-acetyl-D-sphingosine, Sigma, St Louis, MO, U.S.A.), was added to the contractile buffer to determine the concentration- (0–100 μM) and time- (0–30 min) dependent changes on myocyte contractile characteristics. Furthermore, in some studies, selective chemical inhibitors were utilized to elucidate the role of PI-3-kinase, protein kinase C (PKC), ceramide-activated protein phosphatase (CAPP), and nitric oxide synthase (NOS). Wortmannin (100 nM), chelerythrine chloride (1 μM), okadaic acid (100 nM) or Nω-nitro-L-arginine methyl ester (L-NAME) (100 μM) (all from Sigma) were used to inhibit PI-3-kinase, PKC, CAPP, and NOS, respectively (Mathias et al., 1991; Dobrowsky & Hannun, 1992; Ren, 2000).
Statistical analyses
For each experimental series, data are presented as mean±s.e.m. The statistical significance (P<0.05) for each variable was determined by repeated measures analysis of variance (ANOVA) for concentration- and time-dependent response data, ANOVA for the effects of inhibitors on the ceramide response, or t-test where appropriate. Tukey's post hoc test was used where appropriate.
Results
Effect of ceramide on myocyte peak shortening (PS)
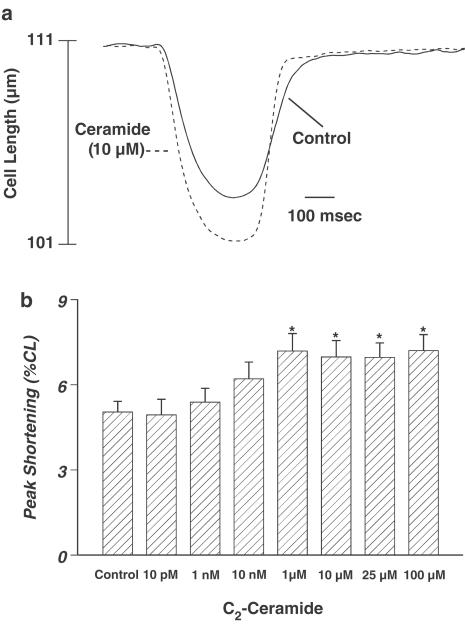
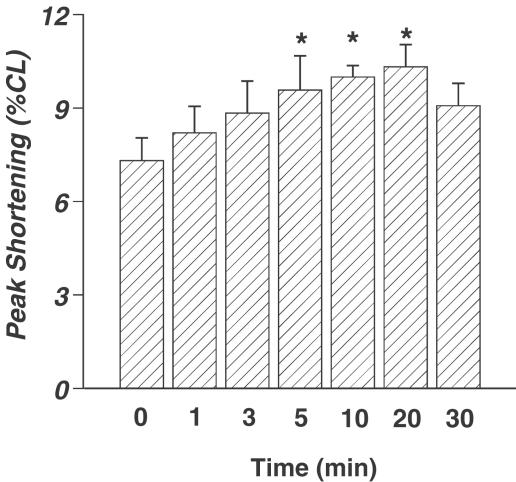
The average cell length used in this study was 113.1±1.5 μm (n=201). Acute exposure of ceramide did not affect the cell shape, percentage of variable cells (∼70%), and resting cell length over the range of time durations and concentrations tested (data not shown). A representative trace depicting the effect of ceramide (10 μM) on myocyte shortening (PS) after 5 min exposure is shown in Figure 1a. Acute ceramide exposure elicited a concentration-dependent increase of PS, with a maximal increase of 34.6±7.7% (Figure 1b). The ceramide-induced augmentation in PS reached a plateau between 1 and 100 μM, suggesting the saturation of the response to acute ceramide application. Additionally, acute ceramide application resulted in a time-dependent increase of PS. After 5 min of exposure, 10 μM ceramide increased PS by 36.1±13.6% (Figure 2). The augmented PS was maintained throughout the first 20 min of ceramide exposure, but the response returned toward basal levels and became insignificant after 30 min of ceramide exposure (Figure 2). Approximately 17% of the 41 myocytes tested became unresponsive to electrical stimulation beyond 10 min of ceramide exposure. The ceramide-induced augmentation of PS was partially reversible upon washout (data not shown). Finally, ceramide application increased TPS, decreased TR90, and increased ±dLdt (Table 1 ). Therefore, it appears that acute ceramide improves the contractile characteristics of the isolated cardiac myocytes.
Figure 1.
(a) Typical experiment showing the effect of ceramide (10 μM) on myocyte shortening in isolated ventricular myocytes. Myocyte shortening and relengthening were recorded at 25°C before and 5 min after ceramide application. (b) Concentration-dependent response to ceramide (0–100 μM) on myocyte shortening in ventricular myocytes. Mean±s.e.m., n=33–41 cells per group, *P<0.05 vs control, as determined by repeated measures ANOVA and subsequent Tukey's post hoc test.
Figure 2.
Time-dependent augmentation of PS in cardiac ventricular myocytes treated with 10 μM cell-permeable C2-ceramide. Mean±s.e.m., n=8–16 cells per group, *P<0.05 vs control, as determined by repeated measures ANOVA and subsequent Tukey's post hoc test.
Table 1.
Effect of ceramide on baseline mechanical characteristics in cells from adult Sprague–Dawley rat hearts
| TPS (ms) | TR90 (ms) | +dLdt (μm s−1) | −dLdt (μm s−1) | |
|---|---|---|---|---|
| Control | 174±6 | 356±18 | 72.9±5.2 | −66.1±6.7 |
| Ceramide 10 pM | 167±8 | 367±20 | 70.6±7.8 | −58.0±9.5 |
| Ceramide 1 nM | 189±10* | 296±21 | 89.8±6.8 | −79.5±7.2 |
| Ceramide 10 nM | 212±8* | 318±18 | 91.2±7.0* | −79.7±7.1 |
| Ceramide 1 μM | 218±8* | 296±18* | 97.4±7.7* | −102.4±11.0* |
| Ceramide 10 μM | 213±9* | 295±20* | 93.1±6.9* | −91.4±8.1* |
| Ceramide 25 μM | 211±9* | 289±18* | 96.3±6.5* | −97.5±8.3* |
| Ceramide 100 μM | 209±9* | 271±17* | 101.2±7.6* | −98.6±7.8* |
Time-to-PS (TPS), time-to-90% relengthening (TR90), maximal velocities of shortening and relengthening (±dLdt). Data represent mean±s.e.m. n=32–41 cells per group.
P<0.05 vs control, as determined by repeated measures ANOVA and subsequent Tukey's post hoc test.
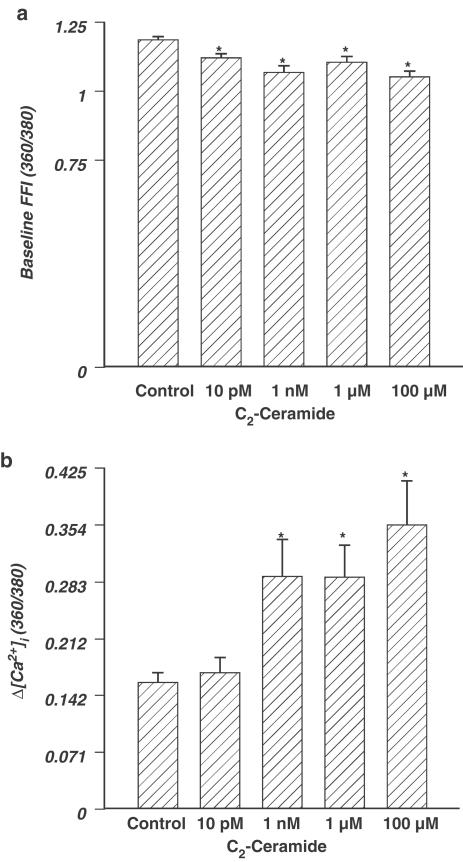
Effect of ceramide on intracellular Ca2+ properties
The Ca2+ fluorescence indicator fura-2AM was utilized to evaluate the role of acute ceramide application (5 min) on intracellular Ca2+ handling. Resting and peak intracellular Ca2+ levels were assessed during electrical stimulation of the cells. Increasing concentrations of ceramide resulted in depressed resting levels of intracellular Ca2+, as determined by baseline fura fluorescence intensity (FFI) (Figure 3a). At the highest concentration of ceramide, 100 μM, there was a 11% depression of FFI. On the contrary, the change in fura fluorescence intensity (ΔFFI), in response to the electrical stimuli, was increased with increasing concentrations of ceramide (Figure 3b). Intracellular Ca2+ clearance (evaluated by the intracellular Ca2+ transient decay rate τ) was facilitated at 1 nM and 100 μM (ceramide at 1 nM: τ=476±30 ms; ceramide at 100 μM: τ=469±32 ms, n=22–23 cells, P<0.05 vs baseline τ=592±27 ms).
Figure 3.
(a) Effect of ceramide on baseline [Ca2+]i, represented by FFI. (b) Concentration-dependent response of ceramide (0–100 μM) on intracellular Ca2+ transient changes (ΔFFI) in ventricular myocytes. Mean±s.e.m., n=21–24 cells per group, *P<0.05 vs control value, as determined by repeated measure ANOVA and subsequent Tukey's post hoc test.
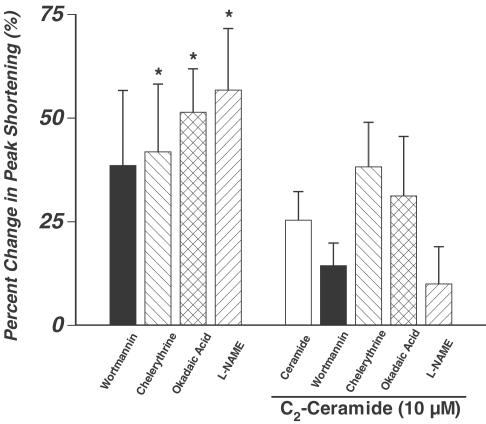
Role of PI-3-kinase, PKC, CAPP, and NOS in ceramide-induced cardiac contractile response
Several signaling pathways have been implicated in ceramide-induced cellular effects. Ceramide has been shown to activate PI-3-kinase (Ibitayo et al., 1998), the atypical protein kinase Cζ (PKCζ) (Mathias et al., 1998), CAPP (Dobrowsky & Hannun, 1992), and the ceramide-activated protein kinase (CAPK) (Mathias et al., 1991). Additionally, ceramide can augment NOS mRNA and protein levels in certain cell types (Li et al., 2002). To examine if these pathways play any role in the ceramide-induced acute response in myocyte shortening, the acute ceramide-induced response of PS was re-examined in the presence of selective inhibitors of the above-mentioned signaling pathways. Wortmannin (100 nM), a PI-3-kinase inhibitor (Powis et al., 1994; Wymann et al., 1996; Ren, 2000), was incubated with cells for 5 min before the cells were exposed to 10 μM ceramide. The same process was repeated for the selective PKC inhibitor chelerythrine (1 μM) (Herbert et al., 1990), the CAPP inhibitor okadaic acid (100 nM) (Dobrowsky & Hannun, 1992), or the NOS inhibitor L-NAME (100 μM). Wortmannin and L-NAME decreased the ceramide-induced augmentation of PS, but were found insignificant. Chelerythrine and okadaic acid also failed to exert any effect on the ceramide-induced cardiac response (Figure 4). Interestingly, myocytes incubated with chelerythrine, okadaic acid, or L-NAME alone exhibited significant increases of 41.8±16.4, 51.4±10.5, and 56.7±14.8% in PS, respectively, compared to their respective controls (n=11–15 cells per group, P<0.05 vs the respective control value when evaluated using a paired t-test). Wortmannin alone had no effect on peak cell shortening (PS for control=5.2±2.1% vs wortmannin=6.8±3.0%, n=11 cells per group) (Ren, 2000).
Figure 4.
Effect of the PI-3-kinase inhibitor wortmannin (100 nM), the CAPP inhibitor okadaic acid (100 nM), the PKC inhibitor chelerythrine (1 μM), or the NOS inhibitor L-NAME (100 μM) on per cent change in PS in the absence or presence of ceramide (10 μM) (5 min exposure). Mean±s.e.m., n=11–16 cells per group for inhibitors in the absence of ceramide (left four bars) and n=11–30 cells per group for inhibitors in the presence of 10 μM ceramide (right five bars), *P<0.05 vs the respective baseline control value for each inhibitor when assessed using a paired t-test. For the rest of the groups, P>0.05 vs 10 μM ceramide exposure (empty bar), determined by one-way ANOVA.
Discussion
Our study has provided evidence that acute exposure of ceramide, a central molecule in sphingolipid synthesis and metabolism, augments cardiac contraction in isolated ventricular myocytes. The ceramide-induced enhancement of contractile function was impervious to the inhibitory effects of wortmannin, okadaic acid, chelerythrine, and L-NAME, suggesting an alternative mechanism other than the ones tested here in the enhanced contractile function.
In the current study, acute ceramide exposure to ventricular myocytes directly enhanced PS in both a concentration- and time-dependent manner. The rapid ceramide-induced increase in myocyte PS was associated with an augmentation of the maximal velocity of cell shortening and relengthening (±dLdt) as well as intracellular Ca2+ release (Δ[Ca2+]i) in response to electrical stimuli. Interestingly, prolonged ceramide exposure up to 20 min resulted in a sustained augmentation of PS, whereas longer duration of exposure (30 min) appeared to cause a reversal of the positive PS response to ceramide. The loss in the cardiac contractile response to ceramide following prolonged exposure is somewhat consistent with the earlier report that the cardiac contractile response became negligible after only 15–20 min of ceramide exposure (Yokoyama et al., 1993). The slight discrepancy in the duration of exposure, although not clear, may be related to the difference in contractile buffer, cell culture medium, or other experimental conditions. Interestingly, our present study revealed that ∼17% of cardiomyocytes became unresponsive to electrical stimulation once the exposure time reached beyond 10 min, suggesting the potential cellular toxicity of ceramide. This time-related cellular toxicity and contractile depression associated with prolonged ceramide exposure is congruous to other sphingolipids (Oral et al., 1997; Hernandez et al., 2000; Zhou et al., 2000; Friedrichs et al., 2002), and may be attenuated by the ceramidase blocker n-oleoylethanolamine, which prevents the metabolism of ceramide to sphingosine (Friedrichs et al., 2002). This suggests that the negative inotropic effect of ceramide may be a result of the metabolic product sphingosine or sphingosine-1-phosphate rather than ceramide alone (Yokoyama et al., 1993; Friedrichs et al., 2002). Therefore, ceramide exposure may have a biphasic effect whereby acute application may augment cardiac myocyte contractile function, while prolonged exposure may result in an accumulation of metabolic products leading to depressed cardiac function (Oral et al., 1997; Ibitayo et al., 1998; Friedrichs et al., 2002).
Although the mechanism(s) for ceramide-induced augmentation of cardiomyocyte contractile function is not clear, the effect of ceramide on intracellular Ca2+ transients may play an integral role. Elevation of intracellular Ca2+ appears to be necessary for a ceramide-induced effect lasting greater than a few seconds (Ibitayo et al., 1998). However, in a Ca2+-free environment, colonic smooth muscle contracts in response to ceramide application, suggesting a Ca2+-independent effect of ceramide (Ibitayo et al., 1998). Therefore, ceramide may trigger smooth muscle contraction within seconds, but requires the presence of extracellular Ca2+ for sustained tension (Ibitayo et al., 1998; Zheng et al., 2000). Results from our current study demonstrated that ceramide significantly increased ΔFFI at a concentration range (>1 nM) significantly lower than that found in cell-shortening response (>1 μM). These disparate observations seem to be consistent with the notion that ceramide may elicit both Ca2+-dependent and -independent responses, suggesting that other mechanisms such as myofilament Ca2+ responsiveness may also be affected by ceramide and play a role in ceramide-induced cardiac contractile response (Ibitayo et al., 1998; Zheng et al., 2000). Ceramide has been shown to work through both Ca2+-dependent and -independent mechanisms to increase the smooth muscle contractile function (Ibitayo et al., 1998; Zheng et al., 2000). The fact that ceramide reduced resting intracellular Ca2+ may be related to the facilitated intracellular Ca2+ clearance (indicated by a smaller τ value), reduced L-type Ca2+ current (McDonough et al., 1994; Schereur & Liu, 1997), inactivated efflux of Ca2+ by the reverse Na+/Ca2+ exchanger (Condrescu & Reeves, 2001), and augmented sarcolemmal Ca2+-ATPase activity (Colina et al., 2002), in response to acute ceramide application. While resting intracellular Ca2+ decreased, the ceramide-induced increase of ΔFFI may be a result of facilitated Ca2+ release from intracellular Ca2+-storage organelles, such as the sarcoplasmic reticulum (SR) and mitochondria. This is consistent with the ability of ceramide to rapidly facilitate the release of intracellular Ca2+ stores (Mihai et al., 2000). The above-mentioned regulatory mechanisms of the ceramide, especially SR Ca2+ release, and membrane Ca2+ channel may contribute in part to the depressed resting intracellular Ca2+ and elevated ΔFFI in response to acute ceramide exposure. Nevertheless, failure to provide such key information in ventricular myocytes is considered the main limitation of the present study, and should warrant further investigation to depict the precise mechanisms of action for the cardiac actions for ceramide and other sphingolipids.
The mechanism of ceramide action within cardiac myocytes has not been well defined. Although PI-3-kinase (Ibitayo et al., 1998), the atypical PKCζ (Mathias et al., 1998), CAPP (Dobrowsky & Hannun, 1992), CAPK (Mathias et al., 1991), and NO (Huwiler et al., 1999a,1999b; Li et al., 2002) have all been shown to participate in ceramide-induced cell signaling, our data show that acute ceramide-induced increased contractile function of isolated cardiac myocytes appears to be mediated through a mechanism(s) other than PI-3-kinase, PKC, CAPP, or NO. A positive inotropic effect of PI-3-kinase has been identified in the colonic smooth muscle (Ibitayo et al., 1998) and the stretch-induced augmentation of cardiac contractile function (Vila Petroff et al., 2001). However, wortmannin, a specific blocker of PI-3-kinase activity, was unable to suppress the ceramide-induced effect on PS. Similarly, specific blockers of CAPP, PKC, and NOS were unable to overcome the ceramide-induced augmentation of the ventricular myocyte contractile function. Nevertheless, caution should be taken regarding the involvement of CAPP, PKC, and NOS in ceramide-induced cardiac contractile response, since okadaic acid, chelerythrine, and L-NAME themselves exert positive cardiac contractile responses.
Metabolic products of ceramide, such as sphingosine and sphingosine-1-phosphate, are associated with depression of cardiac myocyte contractile function (Yokoyama et al., 1993; Oral et al., 1997). This may suggest that synthesis and metabolism of ceramide must be considered to fully understand the integral role of ceramide in the cardiac contractile response (Oral et al., 1997; Levade et al., 2001). While ceramide is often associated with depressed cardiac function (McDonough et al., 1994; Delpy et al., 1999; Hernandez et al., 2000), sphingosine exposure results in a robust deterioration of cardiac contractile function (Yokoyama et al., 1993; Mc-Donough et al., 1994; Condrescu & Reeves, 2001). Furthermore, inhibiting the metabolism of ceramide to sphingosine with a ceramidase inhibitor, n-oleoylethanolamine, attenuates the negative inotropicity and mortality in a model of ischemia reperfusion (Friedrichs et al., 2002).
In conclusion, our study reveals an acute ceramide exposure-induced augmentation of ventricular myocyte contractile function at least partially mediated by increased intracellular Ca2+ transients, although a certain intracellular Ca2+-independent mechanism(s) is also likely involved. The precise mechanism(s) of action remains to be fully elucidated. Further study of the influence of acute ceramide application on membrane ion channels, intracellular Ca2+-regulating proteins and signaling in ventricular myocytes should be essential to understand the precise role of ceramide on cardiac contractile function.
Acknowledgments
We gratefully acknowledge the technical assistance of Faye L. Norby, Grant E. McFadden, and Bonnie H. Ren. This work was supported by a Predoctoral Fellowship award (DPR) from the American Heart Association Northland Affiliate, the North Dakota Experimental Program to Stimulate Competitive Research (EPSCoR) program, Max Baer Heart Fund, and the American Diabetes Association. This work was included in the PhD dissertation for DPR, and was presented at the Scientific Session of the North American Section of the International Society for Heart Research (Madison, WI, U.S.A.) in July 2002.
Abbreviations
- CAPK
ceramide-activated protein kinase
- CAPP
ceramide-activated protein phosphatase
- ±dLdt
maximal velocity of shortening and relengthening
- FFI
fura fluorescence intensity
- ΔFFI
change in fura fluorescence intensity
- NOS
nitric oxide synthase
- PS
peak shortening
- TPS
time-to-peak shortening
- TR90
time-to-90% relengthening
- τ
intracellular Ca2+ transient decay rate
References
- COLINA C., CERVINO V., BENAIM G. Ceramide and sphingosine have an antagonistic effect on the plasma-membrane Ca2+-ATPase from human erythrocytes. Biochem. J. 2002;362:247–251. doi: 10.1042/0264-6021:3620247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIGAN P.B., RELLING D.P., REN J.Ceramide attenuates high glucose-induced cardiac contractile abnormalities in cultured adult rat ventricular myocytes Cell Mol. Biol. 200248OL251–OL257.(online) [PubMed] [Google Scholar]
- CONDRESCU M., REEVES J.P. Inhibition of sodium–calcium exchange by ceramide and sphingosine. J. Biol. Chem. 2001;276:4046–4054. doi: 10.1074/jbc.M006862200. [DOI] [PubMed] [Google Scholar]
- DELPY E., HATEM S.N., ANDRIEU N., DE VAUMAS C., HENAFF M., RUCKER-MARTIN C., JAFFREQOU J.-P., LAURENT G., LEVADE T., MERCADIER J.-J. Doxorubicin induces slow ceramide accumulation and late apoptosis in cultured adult rat ventricular myocytes. Cardiovasc. Res. 1999;43:398–407. doi: 10.1016/s0008-6363(99)00142-x. [DOI] [PubMed] [Google Scholar]
- DOBROWSKY R.T., HANNUN Y.A. Ceramide stimulates a cytosolic protein phosphatase. J. Biol. Chem. 1992;267:5048–5051. [PubMed] [Google Scholar]
- FRIEDRICHS G.S., SWILLO R.E., JOW B., BRIDAL T., NUMANN R., WARNER L.M., KILLAR L.M., SIDEK K. Sphingosine modulates myocyte electrophysiology, induces negative inotropy, and decreases survival after myocardial ischemia. J. Cardiovasc. Pharmacol. 2002;39:18–28. doi: 10.1097/00005344-200201000-00003. [DOI] [PubMed] [Google Scholar]
- HERBERT J.M., AUGEREAU J.M., GLEYE J., MAFFRAND J.P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ O.M., DISCHER D.J., BISHOPRIC N.H., WEBSTER K.A. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Circ. Res. 2000;86:198–204. doi: 10.1161/01.res.86.2.198. [DOI] [PubMed] [Google Scholar]
- HUWILER A., DORSCH S., BRINER V.A., VAN DEN BOSCH H., PFEILSCHIFTER J. Nitric oxide stimulates chronic ceramide formation in glomerular endothelial cells. Biochem. Biophys. Res. Commun. 1999a;258:60–65. doi: 10.1006/bbrc.1999.0582. [DOI] [PubMed] [Google Scholar]
- HUWILER A., PFEILSCHIFTER J., VAN DEN BOSCH H. Nitric oxide donors induce stress signaling via ceramide formation in rat renal mesangial cells. J. Biol. Chem. 1999b;274:7190–7195. doi: 10.1074/jbc.274.11.7190. [DOI] [PubMed] [Google Scholar]
- IBITAYO A.I., TSUNODA Y., NOZU F., OWYANG C., BITAR K.N. Src kinase and PI 3-kinase as a transduction pathway in ceramide-induced contraction of colonic smooth muscle. Am. J. Physiol. 1998;275:G705–G711. doi: 10.1152/ajpgi.1998.275.4.G705. [DOI] [PubMed] [Google Scholar]
- LEVADE T., AUGE N., VELDMAN R.J., CUVILLIER O., NEGRE-SALVAYRE A., SALVAYRE R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ. Res. 2001;89:957–968. doi: 10.1161/hh2301.100350. [DOI] [PubMed] [Google Scholar]
- LI H., JUNK P., HUWILER A., BURKHARDT C., WALLERATH T., PFEILSCHIFTER J., FORSTERMANN U. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002;106:2250–2256. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- LIU G., KLEINE L., HEBERT R.L. Advances in the signal transduction of ceramide and related sphingolipids. Crit. Rev. Clin. Lab. Sci. 1999;36:511–573. doi: 10.1080/10408369991239240. [DOI] [PubMed] [Google Scholar]
- MATHIAS S., DRESSLER K.A., KOLESNICK R.N. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor alpha. Proc. Natl. Acad. Sci. U.S.A. 1991;88:10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHIAS S., PENA L.A., KOLESNICK R.N. Signal transduction of stress via ceramide. Biochem. J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONOUGH P., YASUI K., BETTO R., SALVIATI G., GLEMBOTSKI C., PALADE P., SABBADINI R. Control of cardiac Ca2+ levels. Inhibitory actions of sphingosine on Ca2− levels. Inhibitory actions of sphingosine on Ca2+ transients and L-type Ca2− channel conductance. Circ. Res. 1994;75:981–989. doi: 10.1161/01.res.75.6.981. [DOI] [PubMed] [Google Scholar]
- MIHAI R., LAI T., SCHOFIELD G., FARNDON J. C2-ceramide increases cytoplasmic calcium concentrations in human parathyroid cells. Biochem. Biophys. Res. Commun. 2000;268:636–641. doi: 10.1006/bbrc.2000.2159. [DOI] [PubMed] [Google Scholar]
- NICKOLA M.W., WOLD L.E., COLLIGAN P.B., WANG G.-J., SAMSON W.K., REN J. Leptin attenuates cardiac contraction in rat ventricular myocytes: role of NO. Hypertension. 2000;36:501–505. doi: 10.1161/01.hyp.36.4.501. [DOI] [PubMed] [Google Scholar]
- ORAL H., DORN G.W., II, MANN D.L. Sphingosine mediates the immediate negative inotropic effects of tumor necrosis factor-α in the adult mammalian cardiac myocyte. J. Biol. Chem. 1997;272:4836–4842. doi: 10.1074/jbc.272.8.4836. [DOI] [PubMed] [Google Scholar]
- POWIS G., BONJOUKLIAN R., BERGGREN M.M., GALLEGOS A., ABRAHAM R., ASHENDEL C., ZALKOW L., MATTER W.F., DODGE J., GRINDEY G., VLAHOS C.J. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- REN J. Attenuated cardiac contractile responsiveness to insulin-like growth factor I in ventricular myocytes from biobreeding spontaneous diabetic rats. Cardiovasc. Res. 2000;46:162–171. doi: 10.1016/s0008-6363(00)00011-0. [DOI] [PubMed] [Google Scholar]
- REN J., DOMINGUEZ L., SOWERS J.R., DAVIDOFF A. Troglitazone attenuates high glucose-induced abnormalities in relaxation and intracellular calcium in rat ventricular myocytes. Diabetes. 1996;45:1822–1825. doi: 10.2337/diab.45.12.1822. [DOI] [PubMed] [Google Scholar]
- SCHEREUR K.D., LIU S. Involvement of ceramide in inhibitory effect of IL-1B on L-type Ca2+ current in adult rat ventricular myocytes. Am. J. Physiol. 1997;272:H2591–H2598. doi: 10.1152/ajpheart.1997.272.6.H2591. [DOI] [PubMed] [Google Scholar]
- VILA PETROFF M.G., KIM S.H., PEPE S., DESSY C., MARBAN E., BALLINGAND J.-L., SOLLOTT S.J. Endogenous nitric oxide mechanisms mediate the stretch dependence of Ca2+ release in cardiomyocytes. Nat. Cell Biol. 2001;3:867–873. doi: 10.1038/ncb1001-867. [DOI] [PubMed] [Google Scholar]
- WYMANN M.P., BULGARELLI-LEVA G., ZVELEBIL M.J., PIROLA L., VANHAESEBROECK B., WATERFIELD M.D., PANAYOTOU G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOYAMA T., VACA L., ROSSEN R.D., DURANTE W., HAZARIKA P., MANN D.L. Cellular basis for the negative inotropic effects of tumor necrosis factor-alpha in the adult mammalian heart. J. Clin. Investig. 1993;92:2303–2312. doi: 10.1172/JCI116834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG T., LI W., WANG J., ALTURA B.T., ALTURA B.M. Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca2+]i in canine cerebral vascular muscle. Am. J. Physiol. 2000;278:H1421–H1428. doi: 10.1152/ajpheart.2000.278.5.H1421. [DOI] [PubMed] [Google Scholar]
- ZHOU Y.-T., GRAYBURN P., KARIM A., SHIMABUKURO M., HIGA M., BAETENS D., ORCI L., UNGER R.H. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZU HERINGDORF D.M., VAN KOPPEN C.J., JAKOBS K.H. Molecular diversity of sphingolipid signalling. FEBS Lett. 1997;410:34–38. doi: 10.1016/s0014-5793(97)00320-7. [DOI] [PubMed] [Google Scholar]