Abstract
Two molecularly distinct rat P2Y receptors activated equally by adenosine-5′-triphosphate (ATP) and uridine-5′-triphosphate (UTP) (rP2Y2 and rP2Y4 receptors) were expressed in Xenopus oocytes and studied extensively to find ways to pharmacologically distinguish one from the other.
Both P2Y subtypes were activated fully by a number of nucleotides. Tested nucleotides were equipotent at rP2Y4 (ATP=UTP=CTP=GTP=ITP), but not at rP2Y2 (ATP=UTP>CTP>GTP>ITP). For dinucleotides (ApnA, n=2–6), rP2Y4 was only fully activated by Ap4A, which was as potent as ATP. All tested dinucleotides, except for Ap2A, fully activated rP2Y2, but none were as potent as ATP. ATPγS and BzATP fully activated rP2Y2, whereas ATPγS was a weak agonist and BzATP was inactive (as an agonist) at rP2Y4 receptors.
Each P2Y subtype showed different sensitivities to known P2 receptor antagonists. For rP2Y2, the potency order was suramin>>PPADS= RB-2>TNP-ATP and suramin was a competitive antagonist (pA2, 5.40). For rP2Y4, the order was RB-2>>suramin>PPADS> TNP-ATP and RB-2 was a competitive antagonist (pA2, 6.43). Also, BzATP was an antagonist at rP2Y4 receptors.
Extracellular acidification (from pH 8.0 to pH 5.5) enhanced the potency of ATP and UTP by 8–10-fold at rP2Y4 but did not affect agonist responses at rP2Y2 receptors.
Extracellular Zn2+ ions (0.1–300 μM) coapplied with ATP inhibited agonist responses at rP2Y4 but not at rP2Y2 receptors.
These two P2Y receptors differ significantly in terms of agonist and antagonist profiles, and the modulatory activities of extracellular H+ and Zn2+ ions. These pharmacological differences will help to distinguish between rP2Y2 and rP2Y4 receptors, in vivo.
Keywords: GPCR, purinoceptor, P2Y receptor, P2Y2 receptor, P2Y4 receptor, P2U receptor, extracellular pH, Zinc, oocyte
Introduction
Prior to expression cloning, the P2U receptor was defined as a G-protein-coupled receptor activated equally by the purine and pyrimidine nucleotides adenosine-5′-triphosphate (ATP) and uridine-5′-triphosphate (UTP) (Dubyak, 1991; O'Connor et al., 1991; Dubyak & El-Moatassim, 1993). Cell-surface receptors sensitive to these nucleotides (and, in some cases, other naturally occurring nucleoside triphosphates) have a widespread distribution in tissues and cell types of various species – including astrocytes, leukocytes, epithelium, endothelium, endocrine and exocrine glands, hepatocytes, vascular and visceral smooth muscle and others (Ralevic & Burnstock, 1998). A subdivision of ATP/UTP sensitive receptors was proposed on the basis that suramin hexasodium salt (suramin) blocked some, but not all, P2U receptors in mammalian tissues (Dainty et al., 1994; Ralevic & Burnstock, 1998).
With the introduction of expression cloning, an ATP/UTP receptor was isolated from mouse neuroblastoma (NG108-15) cells and human airway epithelial (CF/T43) cells (Lustig et al., 1993; Parr et al., 1994). Heterologous expression of these P2 receptors, in either Xenopus laevis oocytes or human 1321N1 astrocytoma cells, resulted in ATP- and UTP-mediated responses that signalled through the Gq/PLCβ/IP3/Ca2+ pathway (Lustig et al., 1993; Parr et al., 1994). The encoding cDNAs for these P2 receptors were defined as genes for the mouse and human P2Y2 receptor, whereas cDNAs for another two P2 purinoceptors isolated from chick brain were classed as P2Y1 and P2Y3 (Barnard et al., 1994; Von Kügelgen et al., 1987). This nomenclature system still remains in place and has been expanded to include cDNAs for P2Y4 to P2Y14, most of which encode functional nucleotide receptors (Abbracchio et al., 2003).
Shortly after the molecular identification of the P2Y2 receptor, the human P2Y4 receptor was cloned and, when expressed in 1321N1 cells, this metabotropic receptor was shown to be sensitive to UTP and insensitive to ATP (Communi et al., 1995; Nguyen et al., 1995). This human P2Y4 receptor was viewed as a pyrimidinoceptor that was both structurally and phenotypically distinct from the ATP/UTP-sensitive P2Y2 receptors. However, the subsequent cloning of rat and mouse P2Y4 receptors – each greater than 80% identical to the human isoform of P2Y4 – resulted in a metabotropic receptor that was activated equally by ATP and UTP in all expression systems employed (Xenopus oocytes, Jurkat cells and 1321N1 cells) (Bogdanov et al., 1998; Webb et al., 1998; Kennedy et al., 2000; Lazarowski et al., 2001; Suarez-Heurta et al., 2001). Therefore, the rodent P2Y4 was found to be phenotypically similar to the rat and human P2Y2 receptor.
From the functional evidence available at the time, it was proposed that several pharmacological features might help to distinguish P2Y2 isoforms from the rat P2Y4 receptor (King et al., 1998). These features included: (i) the potency of UTP and ATP seemed higher at P2Y2 receptors; (ii) adenosine-5′-O-(thiotriphosphate) (ATPγS) was a full agonist at P2Y2, but weakly stimulated P2Y4; (iii) in contrast, inosine-5′-triphosphate (ITP) was a full agonist at P2Y4, but weakly stimulated P2Y2; (iv) suramin blocked P2Y2, but not P2Y4 receptors. However, many of these distinguishing pharmacological features were gleaned from published data taken from unrelated studies and have not been tested directly.
In the present study, the issue of differentiating rat P2Y2 from rat P2Y4 receptors has been revisited. For the first time, we demonstrate the pharmacological means to distinguish between these two ATP/UTP-sensitive P2Y receptor subtypes. A part of this study has been presented elsewhere (Wildman et al., 2002).
Methods
Oocyte preparation
Defolliculated Xenopus oocytes were selected to express recombinant P2Y receptors because they do not possess endogenous P2 receptors, lack functional P1 receptors and, furthermore, surface ATPase activity is exceedingly low with no effective breakdown of superfused UTP, ATP and adenine-based dinucleotides (P1-P(n)-di(adenosine-5′)phosphate (ApnA) family) for the superfusion periods used in this study (King et al., 1996a; Pintor et al., 1996). To prepare these cells, Xenopus laevis frogs were anaesthetized with Tricaine (0.4% w v−1 in tap water), killed by decapitation, and the ovarian lobes surgically removed. Oocytes (stages V and VI) were defolliculated by a two-step process involving collagenase treatment (Type IA, 1 mg ml−1 in a Ca2+-free Ringer solution, for 2 h) followed by stripping away the follicular layer with fine forceps (in double-strength Ca2+-free Ringer solution to help shrink oocytes).
Prepared oocytes were stored in a Barth's solution (pH 7.50) containing 110 mM NaCl, 1 mM KCl, 7.5 mM Tris-HCl, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.4 mM NaHCO3, supplemented with 50 μg l−1 gentamycin sulphate. Defolliculated oocytes were injected cytosolically (40 nl) with either rP2Y4 or P2Y2 cRNA (1 μg μl−1), then incubated for 48 h at 18°C in Barth's solution and, thereafter, kept at 4°C in Barth's solution for up to 7 days and until used in electrophysiological experiments.
Electrophysiological recordings
Agonist-activated membrane currents were recorded under voltage-clamp conditions (Vh=−60 mV) from cRNA-injected oocytes using twin-electrode amplifiers (Axoclamp 2A and 2B; Axon Instruments, Union City, CA, U.S.A.). The voltage-recording and current-recording microelectrodes (1–5 MΩ tip resistance) were filled with 3.0 M KCl. The evoked membrane currents were calcium-activated chloride currents (ICl,Ca), which resulted from P2Y receptor stimulation of the Gq/PLCβ/IP3/Ca2+ pathway in oocytes (Lustig et al., 1993). Oocytes were superfused with a Ca2+-Ringer solution (12 ml min−1, pH 7.50, at 18°C) containing 110 mM NaCl, 2.5 mM KCl, 5 mM HEPES and 1.8 mM CaCl2. Where stated, the pH of the bathing solution was adjusted using either 1.0 N HCl or 1.0 N NaOH to achieve the desired level. Electrophysiological data were stored on a computer using an MP100 WSW interface (Biopac Systems, Goleta, CA, U.S.A.) and analysed using the software package Acqknowledge III (Biopac Systems).
Analysis of drug actions
All drugs were superfused by a gravity-fed continuous flow system, which allowed for rapid addition and washout. Agonists were added for 90 s or until the evoked current reached a peak, then washed out with Ringer solution for a period of 1 h. For all agonist concentration–response (C/R) curves, data were normalised to the maximum current (Imax) evoked by ATP (30 μM or 100 μM for rP2Y4 and rP2Y2, respectively; at pH 7.5) in the oocyte under study. The agonist concentration that evoked 50% of the maximum response (EC50) was taken from Hill plots of the transform, log (I/Imax−I), where I is the current evoked by each concentration of agonist. The Hill coefficient (nH) was taken from the slope of the Hill plots.
The inhibitory activity of P2 antagonists was tested by adding each antagonist in increasing concentrations, each applied 20 min prior to and during the addition of ATP (∼EC70 concentration, 3 or 10 μM ATP for rP2Y4 and rP2Y2, respectively). Where the activity of antagonists was tested at a lower pH level (pH 5.5), the EC70 concentrations of ATP were 300 nM and 10 μM for rP2Y4 and rP2Y2, respectively. The antagonist concentration that reduced ATP responses by 50% (IC50) was taken from the inhibition curves. The reversibility of receptor blockade was tested after prolonged (>2 h) washout of antagonists. pA2 values were determined by Schild analysis.
C/R curves for the modulatory activity of Zn2+ ions were constructed against submaximal concentrations of ATP (3 or 10 μM ATP for rP2Y4 and rP2Y2, respectively) and thereafter data were normalized to the amplitude of control ATP responses.
Data are presented as mean±s.e.m. of three or more sets of data obtained from different oocyte batches. Significant differences were determined by Student's t-test (using Instat v2.05A, GraphPad Software, San Diego, CA, U.S.A.). Concentration–response curves and inhibition curves were fitted by nonlinear regression analysis using commercial software (Prism v3.0, GraphPad).
Drugs
ATP, other nucleotides and drugs were purchased from Sigma-Aldrich (Poole, Dorset, U.K.). Suramin was a gift from Bayer plc (Newbury, Berkshire, U.K.), while 2′,3′-O-(2,4,6-trinitrophenyl)ATP (TNP-ATP) was obtained from Molecular Probes (Eugene, OR, U.S.A.). All reagents, including ZnCl2, were AnalaR grade from Sigma-Aldrich. Drugs were prepared in a Ca2+-Ringer solution and the extracellular pH was adjusted to the desired level.
DNA constructs
The rat P2Y2 receptor cDNA (U56839) was kindly provided by Dr Claude Desgranges and Dr Cheikh Seye (INSERM U441, Pessac, FRANCE) (as described in Seye et al., 1997). We have previously isolated the rat P2Y4 receptor cDNA (Y14705) (as described in Bogdanov et al., 1998).
Results
Activation by nucleoside triphosphates
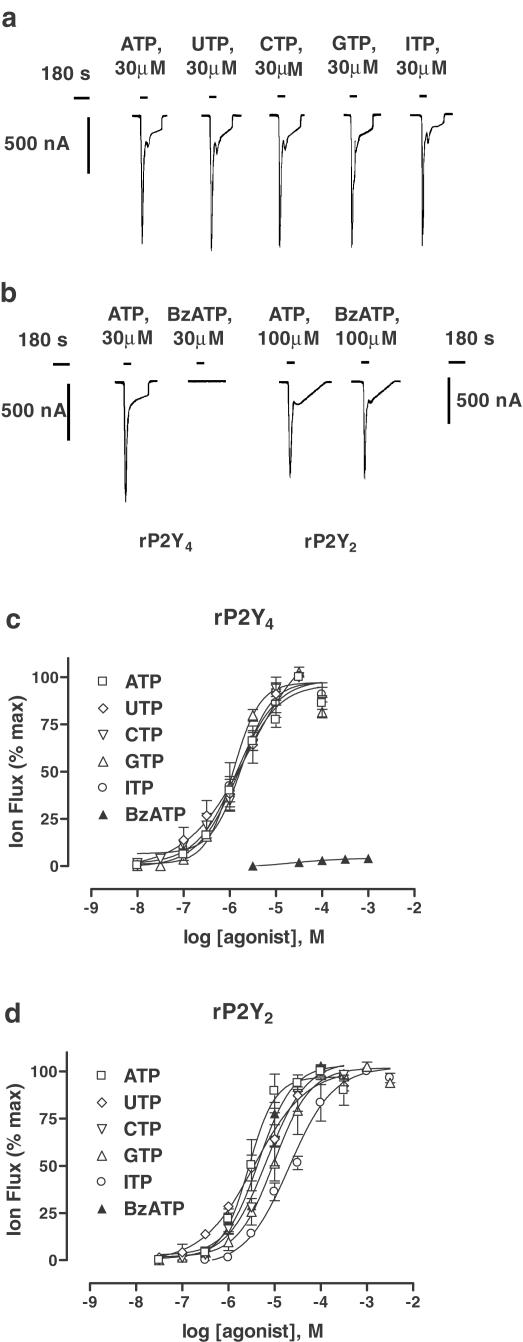
Previously, rat P2Y4 (rPY4) receptors expressed in human 1321N1 astrocytoma cells were shown to be activated by a wide range of nucleoside triphosphates (Kennedy et al., 2000). Here, we have confirmed that UTP, ATP, cytosine-5′-triphosphate (CTP), guanosine-5′-triphosphate (GTP) and ITP were active at rP2Y4 expressed in Xenopus oocytes (Figure 1a,c), also established that the same range of nucleotides were active at rat P2Y2 (rP2Y2) receptors expressed in oocytes (Figure 1d) and, in both cases, all tested nucleotides were found to be full agonists. These five nucleotides were equipotent at rP2Y4 (Table 1 ), whereas at rP2Y2 only UTP and ATP were equipotent and CTP, GTP and ITP – in this order – were progressively less potent (Table 1). It has already been shown that BzATP (2′,3′-O-(4-benzoylbenzoyl)ATP) is an agonist at human P2Y2 expressed in 1321N1 cells (Erb et al., 1993) and, therefore, we confirmed that BzATP also activated rat P2Y2 in oocytes (Figure 1b,d). Here BzATP was as potent as UTP and ATP (Table 1), but it proved to be virtually inactive at rP2Y4 receptors (at 1 mM, 6.4±0.9% of the maximum ATP response; n=3) (Figure 1b,c).
Figure 1.
Activation by nucleoside triphosphates. (a) Naturally occurring nucleoside triphosphates (ATP, UTP, CTP, GTP and ITP; at 30 μM, the EC100) activated rP2Y4 receptors and evoked calcium-dependent chloride currents (ICl,Ca) in Xenopus oocytes. Water-injected control oocytes failed to respond to any of these nucleotides. However, the same range of agonists also activated rP2Y2 receptors (records not shown). (b) The synthetic nucleotide BzATP (30 μM) was inactive at rP2Y4 receptors, but the same compound (100 μM, the EC100) was fully active at rP2Y2 receptors. (c, d) The concentration/response (C/R) curves for agonists at each of the two P2Y receptor subtypes. Data are expressed as mean± s.e.m. (n=3–6). The EC50 values of agonists and Hill coefficients (nH) for C/R curves are given in Table 1. In (a), all records were evoked from the same oocyte whereas in (b), paired records were from separate oocytes (Vh=−60 mV).
Table 1.
Agonist and antagonist potency at recombinant P2Y receptors
| rP2Y4 | rP2Y2 | |||
|---|---|---|---|---|
| EC50 | nH | EC50 | nH | |
| (μM) | (μM) | |||
| UTP | 1.8±0.9 | 1.3±0.3 | 3.6±0.3 | 0.9±0.3 |
| ATP | 1.5±0.8 | 1.0±0.2 | 2.7±0.9 | 1.1±0.3 |
| CTP | 1.2±0.1 | 1.1±0.2 | 6.8±0.5 | 1.0±0.2 |
| GTP | 1.4±0.1 | 1.0±0.3 | 9.7±0.8 | 1.1±0.2 |
| ITP | 1.6±0.3 | 1.2±0.2 | 20.9±0.9 | 0.9±0.3 |
| Ap2A | Inactive | 1,029±21a | 1.1±0.2 | |
| Ap3A | 5.7±1.9a | 1.4±0.7 | 23.8±1.2 | 1.0±0.2 |
| Ap4A | 2.6±1.6 | 0.7±0.2 | 6.5±0.7 | 1.1±0.3 |
| Ap5A | 3.4±1.3a | 1.4±0.5 | 20.3±0.6 | 1.3±0.1 |
| Ap6A | Inactive | 26.5±0.8 | 1.0±0.2 | |
| BzATP | nda | nd | 4.7±0.2 | 1.1±0.2 |
| ATPγS | 5.4±0.3a | 1.9±0.4 | 10.5±0.7 | 1.1±0.3 |
| 2-MeSATP | 1.4±0.2a | 1.5±0.3 | 9.4±0.5a | 1.4±0.3 |
| IC50 | nH | IC50 | nH | |
| (μM) | (μM) | |||
| BzATP | 159±19 | 1.2±0.4 | Nd | nd |
| PPADS | >1,000 | nd | >10,000 | nd |
| RB-2 | 18.5±1.4 | 0.8±0.2 | >10,000 | nd |
| Suramin | 1027±32 | 0.9±0.2 | 8.9±3.3 | 1.3±0.5 |
| TNP-ATP | >1000 | nd | >10,000 | nd |
Partial agonist; nd, not determined.
Potency indices (EC50, IC50) and Hill coefficients (nH) for a range of agonists and antagonists at rP2Y4 receptors (left) and rP2Y2 receptors (right) expressed in Xenopus oocytes. Data are expressed as mean±s.e.m. (n=3–6).
Activation by adenine dinucleotides
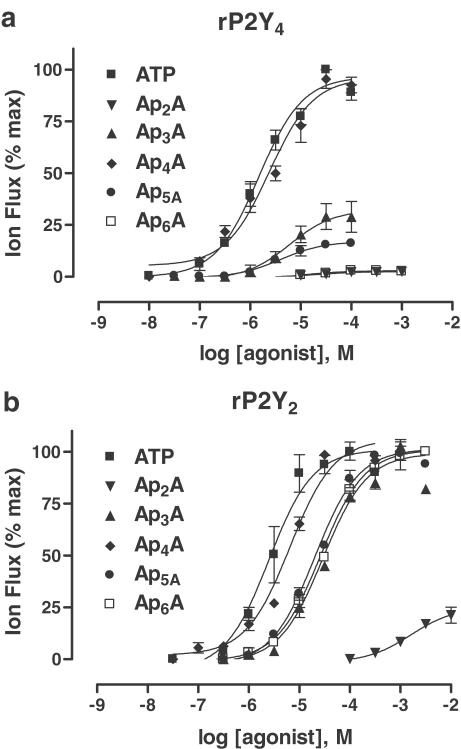
Diadenosine polyphosphates (ApnAs, n=2–6) are active at a number of native and recombinant P2 receptors (Hoyle et al., 2001) and, so, the ApnA family was tested at rat P2Y receptor subtypes. At rP2Y4, the tetraphosphate molecule Ap4A (EC50, 2.6±1.6 μM, n=4) was as potent as ATP, whereas the remainder of the tested dinucleotides were either partial agonists (<25% of the maximum ATP response) or inactive (Figure 2a; Table 1). The rP2Y2 receptor was found to be less selective and was fully activated by all of the tested adenine dinucleotides, except for the diphosphate molecule Ap2A, which was a partial agonist (at 10 mM, 21.2±3.9% of the maximum ATP response) (Figure 2b; Table 1). At rP2Y2, the most potent dinucleotide was also Ap4A (EC50, 6.5±0.7 μM, n=4).
Figure 2.
Activation by adenine dinucleotides. Concentration/response (C/R) relationships (a, b) for the adenine dinucleotide series (ApnA, n=2–6) as agonists of rP2Y4 receptors (top) and rP2Y2 receptors (bottom) expressed in Xenopus oocytes. ApnA responses were normalised to the maximum ATP response evoked at each P2Y subtype. Data are expressed as mean±s.e.m. (n=3–7). Further details are given in the text and Table 1.
Activation by synthetic nucleotides
To conclude the study of agonists, some well-known synthetic nucleotides were tested at both P2Y receptor subtypes (Table 1). At rP2Y2, ATPγS was a full agonist but only a partial agonist at rP2Y4 (24.2±5.1% of maximum ATP response; n=4). 2-methylthioATP (2-MeSATP) was a partial agonist at rP2Y2 (33.8±4.9% of maximum ATP response; n=4) and rP2Y4 (23.5±3.9% of maximum ATP response; n=4). Both α,β-methyleneATP (αβmeATP) and β,γ-methyleneATP (βγmeATP) (up to 100 μM) were inactive at each P2Y receptor subtype.
Blockade of P2Y receptors
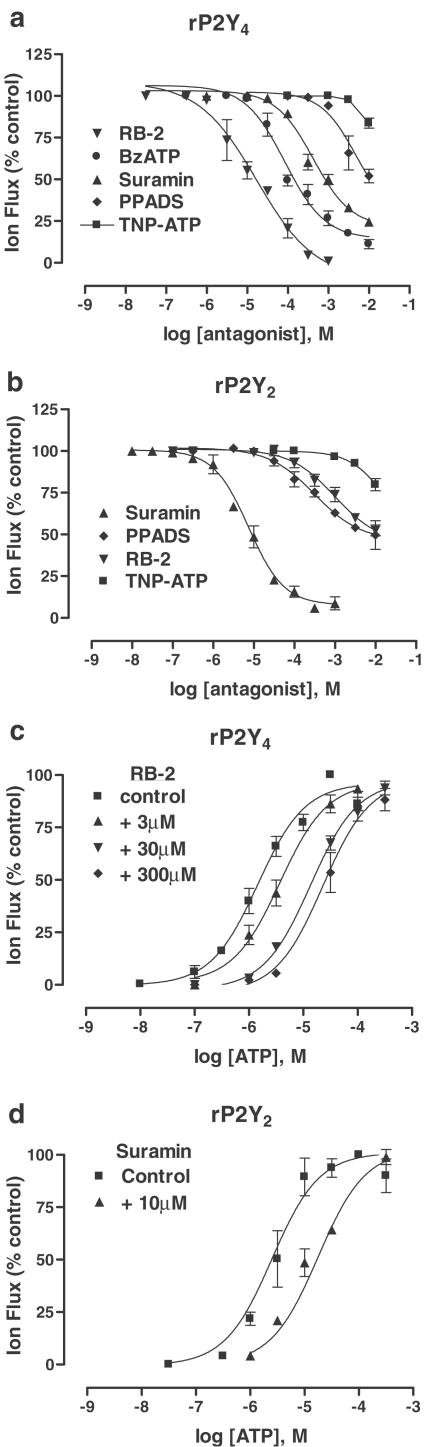
Comparatively little is known about the potency of classical P2 receptor antagonists at native P2U receptors in mammalian tissues – other than that some are suramin sensitive and some are not (Dainty et al., 1994; Ralevic & Burnstock, 1998). Here, a short series of known P2 antagonists – Reactive blue 2 (RB-2), suramin, PPADS (pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid) and TNP-ATP – was tested at rP2Y4 and rP2Y2 receptors (Figure 3a,b).
Figure 3.
Blockade of P2Y receptors. (a, b) Inhibition curves for a range of P2 receptor antagonists superfused (for 20 min) prior to and during (90 s) the superfusion of ATP (EC70 value, 3 or 10 μM for rP2Y4 and rP2Y2, respectively) at rP2Y4 receptors (a) and rP2Y2 receptors (b) expressed in Xenopus oocytes. Data are expressed as mean±s.e.m. (n=3–4). IC50 values and, where possible, Hill coefficients (nH) are given in Table 1. (c, d) Concentration/responses (C/R) curves for ATP activation of rP2Y4 receptors (c) and rP2Y2 receptors (d) in the presence of either Reactive blue 2 (RB-2, in (c) or suramin (in D) at the given concentrations. In the presence these antagonists, the C/R curves were shifted to the right, without reducing the maximum agonist response, indicating competitive antagonism. From these rightward shifts, pA2 values (RB-2, 6.43; suramin, 5.40) were determined by Schild analysis (not shown).
For rP2Y4, RB-2 was the most potent blocking agent (IC50, 18.5±1.4 μM, n=4) and, in further experiments, it behaved as a competitive antagonist (pA2, 6.43)–displacing the, C/R curve for ATP to the right without reducing its maximum response (Figure 3c). Suramin and PPADS were relatively weak antagonists, requiring very high concentrations to block rP2Y4 (Figure 3a), and each antagonist yielded predictive IC50 values in the region of 1–10 mM (Table 1). TNP-ATP was inactive at 1 mM, although it did reduce ATP responses at higher concentrations (Figure 3a). Since BzATP was an agonist of very low efficacy, it was tested for antagonist activity and, at high concentrations, it blocked rP2Y4 receptors (IC50, 159±19 μM, n=4) (Figure 3a).
For rP2Y2, suramin was the most potent blocking agent (IC50, 8.9±3.3 μM), and, in further experiments, it behaved as a competitive antagonist (pA2, 5.40) – displacing the C/R curve for ATP to the right without reducing its maximum response (Figure 3d). PPADS, RB-2 and TNP-ATP were very weak antagonists (between 20 and 50% inhibition at 10 mM).
The blocking actions of suramin at both P2Y receptor subtypes, as well as that of BzATP and TNP-ATP, were reversible following a 1 h washout period. However, blockade by either RB-2 or PPADS required in excess of 2 h washout.
Modulatory actions of pH
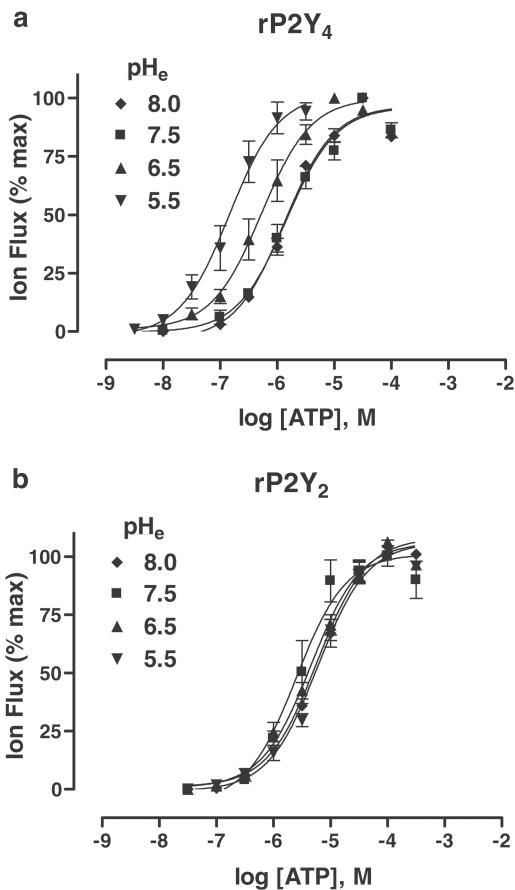
It has been previously reported that changing extracellular pH altered the potency of agonists at native P1 (adenosine) receptors (Hiley et al., 1995) and recombinant P2X receptors (King et al., 1996b). Thus the effects of changing extracellular pH – over the range of pH 8.0–5.5 – were investigated for rP2Y4 and rP2Y2 receptors (Figure 4a,b).
Figure 4.
Modulatory actions of extracellular pH. (a, b) Concentration/response (C/R) curves for ATP activation of rP2Y4 receptors (a) and rP2Y2 receptors (b) expressed in Xenopus oocytes and examined at the levels of extracellular pH indicated. Data are expressed as mean±s.e.m. (n=3–7). The EC50 values and Hill coefficients (nH) are given in Table 2. Further details on the forms of ATP-ion species activating these two P2Y receptors are given in the text and Table 3.
With rP2Y2, there were no significant differences in agonist potency at the four pH levels investigated (Figure 4b). However, a different picture emerged for rP2Y4, where agonist potency was significantly altered under acidic conditions (Figure 4a, Table 2 ). ATP potency was increased three-fold at pH 6.5 and 10-fold at pH 5.5 (compared to agonist activity at pH 7.5), with similar changes in potency when using UTP (Table 2), and also for Ap4A (data not shown). Use of the program “Bound and Determined” (v.4.35) (Brooks & Storey, 1992) showed that the proportional changes in agonist potency at rP2Y4 were unrelated to the levels of free ATP (i.e. ATP4−) (Table 3 ). The calculated levels for ATP4− ion species progressively decreased with acidification, although agonist potency had increased. Also, changes in the calculated levels of other major ATP-ion species did not match the proportional increase in EC50 values upon changing to lower extracellular pH levels. These computational results suggested that the effect of pH was on the receptor and not on the ligand. Changing extracellular pH had no significant effect (P>0.1) on the potency of either RB-2 or suramin at rP2Y4 and P2Y2 receptors.
Table 2.
H+ modulation of agonist potency at recombinant P2Y receptors
| rP2Y4 | rP2Y2 | |||
|---|---|---|---|---|
| EC50 (μM) | nH | EC50 (μM) | nH | |
| ATP | ||||
| pH 8.0 | 1.33±0.47 | 1.2±0.2 | 5.80±0.63 | 1.2±0.2 |
| pH 7.5 | 1.46±0.82 | 1.0±0.2 | 2.51±0.93 | 1.1±0.3 |
| pH 6.5 | 0.53±0.09 | 1.0±0.2 | 4.57±0.60 | 1.0±0.3 |
| pH 5.5 | 0.15±0.13 | 1.3±0.3 | 5.96±0.73 | 1.1±0.3 |
| UTP | ||||
| pH 7.5 | 1.79±0.92 | 1.3±0.3 | 3.63±0.28 | 0.9±0.3 |
| pH 5.5 | 0.23±0.08 | 0.9±0.1 | 3.04±0.49 | 1.1±0.3 |
Potency indices (EC50) and Hill coefficients (nH) for ATP/UTP activation of rP2Y4 receptors (left) and rP2Y2 receptors (right) expressed in Xenopus oocytes and examined under different levels of extracellular pH (pH 8.0−5.5). Data are expressed as mean±s.e.m. (n=3−6).
Table 3.
Analysis of amounts of ATP-ion species in EC50 concentrations
| rP2Y4 | RP2Y2 | |||||||
|---|---|---|---|---|---|---|---|---|
| pH 8.0 | pH 7.5 | pH 6.5 | pH 5.5 | pH 8.0 | pH 7.5 | pH 6.5 | pH 5.5 | |
| Mean EC50 (nM) | 1332 | 1458 | 529 | 145 | 5801 | 2511 | 4566 | 5955 |
| ATP species (nM) | ATP species (nM) | |||||||
| ATP4− | 106 | 115 | 38 | 6 | 460 | 197 | 329 | 230 |
| HATP3− | 4 | 13 | 43 | 63 | 16 | 22 | 373 | 2594 |
| H2ATP2− | 0 | 0 | 0 | 3 | 0 | 0 | 2 | 130 |
| NaATP3− | 182 | 198 | 66 | 10 | 794 | 340 | 568 | 395 |
| KATP3− | 3 | 4 | 1 | 0 | 15 | 6 | 10 | 7 |
| CaATP2− | 849 | 922 | 307 | 45 | 3698 | 1590 | 2648 | 1842 |
| Ca2ATP | 187 | 203 | 68 | 10 | 817 | 352 | 585 | 407 |
| CaHATP− | 1 | 2 | 6 | 9 | 2 | 3 | 50 | 351 |
| Total | 1332 | 1457 | 529 | 146 | 5802 | 2510 | 4565 | 5956 |
Computational determinations of the amounts (in nM) of each ATP-ion species present in the oocyte bathing solution (Ringer's solution; composition in Methods, temperature 18°C) for the mean concentration of ATP (in nM) evoking the 50% maximum response (EC50) at rP2Y4 receptors (left) and rP2Y2 receptors (right) examined at four extracellular pH levels (pH 8.0−5.5). The amounts of each ATP-ion species were determined by the programme “Bound and Determined” (Brooks & Storey, 1992).
Modulatory actions of divalent cations
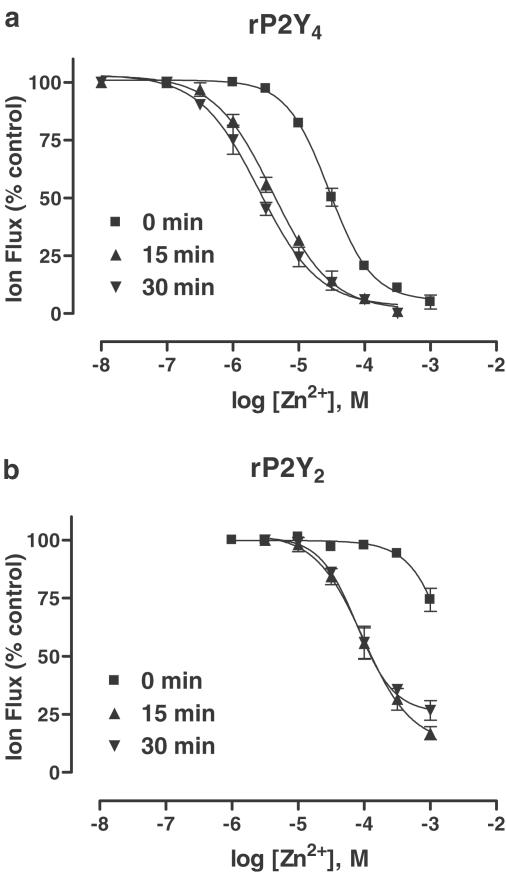
Where extracellular pH has a strong modulatory effect, it is not uncommon for metal cations (especially Zn2+) to modulate the same receptor subtype – whether they are nonpurinergic G protein-coupled receptors (LGICs) and G protein-coupled receptors (GPCRs) (Smart et al., 1994) or purinergic LGICs (Wildman et al., 1998) and GPCRs (Hiley et al., 1995; Mundell & Kelly, 1998). Here, a modulatory effect by extracellular Zn2+ ions was revealed at both P2Y subtypes (Figure 5a,b).
Figure 5.
Modulatory actions of Zn2+ ions. (a, b) Inhibition curves for Zn2+ ions (0.01–1000 μM) applied in the superfusate, either prior to (15, 30 min) or simultaneously (0 min), with ATP (EC70 value, 3 or 10 μM for rP2Y4 and rP2Y2, respectively) at rP2Y4 receptors (a) and rP2Y2 receptors (b) expressed in Xenopus oocytes. Data are expressed as mean±s.e.m. (n=4). IC50 values and further details are given in the text.
For rP2Y4, Zn2+ ions (0.1–1000 μM) coapplied with the ATP inhibited agonist-evoked responses in a concentration-dependent manner (IC50, 29.8±3.1 μM; slope, −1.2±0.1; n=4) (Figure 5a). ATP responses were further inhibited by Zn2+ ions, if this cation was applied either for 15 min (IC50, 4.0±0.4 μM; slope, −1.0±0.1; n=4) or for 30 min (IC50, 2.5±0.6 μM; slope, −1.0±0.1; n=4) prior to addition of the agonist. The inhibitory effect of Zn2+ ions (10 μM) was abolished by the Zn2+ chelator, N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenedi-amine (TPEN) (30 μM, 15 min preincubation), and restored upon washout of TPEN (data not shown). The modulatory effects of Zn2+ were reversed after washout.
For rP2Y2, the modulatory effect of extracellular Zn2+ ions was much less pronounced (Figure 5b). Without preincubation, Zn2+ ions (1 mM) barely inhibited ATP responses, whereas a more potent inhibitory effect was seen following preincubation periods of either 15 min (IC50, 186±24 μM; slope, −1.2±0.2; n=4) or 30 min (IC50 198±19μM; slope, −1.3±0.1; n=4). This inhibitory effect was reversed after washout.
Discussion
The present investigations provided for the first time a direct comparison of two structurally distinct rat P2Y receptor subtypes that are known to be activated equally by ATP and UTP yet, at a superficial level, appear to share the same phenotype. In the past, some pharmacological procedures have been proposed to separate these two P2Y receptors (King et al., 1998; also see a list of procedures in the Introduction), but few of these suggestions have been tested directly, or validated, until now.
The rat P2Y4 receptor did not select for UTP, ATP, CTP, GTP or ITP when expressed in oocytes. All five of these nucleotides were equipotent at rP2Y4 in oocytes, but not at rat P2Y4 nor murine P2Y4 expressed in 1321N1 cells, where only ATP and UTP are equipotent and CTP, GTP and ITP are significantly less potent (Kennedy et al., 2000; Lazarowski et al., 2001). In contrast, the human P2Y4 receptor expressed in 1321N1 cells is only stimulated by three of these five nucleotides (UTP>ITP=GTP), while ATP is an antagonist (Kennedy et al., 2000). Our EC50 values for ATP and UTP at rP2Y4 were approximately two-fold lower than equivalent data for other P2Y4 isoforms expressed in 1321N1 cells, suggesting that we had not overexpressed rP2Y4 and unduly altered the potency of ATP, UTP and other nucleotides by affecting receptor reserve (Kenakin, 2002). Others have shown that UTP potency can be higher than other nucleotides at rP2Y4 receptors, depending on the type of G protein to which it is coupled (Filippov et al., 2003). Thus, if the present findings are to be used as discriminatory tools of P2Y subtypes in vivo, it is important to apply our findings to P2Y receptors in rat tissues and coupling through the Gq/PLCβ/IP3/Ca2+ pathway.
In the present study, rat P2Y2 was fully activated by the above five nucleotides, but only ATP and UTP were equipotent and the remainder were significantly less potent (i.e. ATP=UTP>CTP>GTP>ITP). UTP and ATP also are equipotent at mouse P2Y2 (Lustig et al., 1993) and human P2Y2 (Parr et al., 1994), although only CTP and GTP have been tested at murine P2Y2 where they were found to be inactive (Erb et al., 1993). The agonist activity of some or all of the above five nucleotides at species orthologues of P2Y2 and P2Y4 receptors suggests that the negatively charged triphosphate-ribose moiety common to each nucleotide is critically important for receptor activation, while the nature of the purine or pyrimidine moiety is much less important. In accordance with this suggestion, neutralization of positively charged amino-acid residues in TM6 and TM7 to limit triphosphate binding greatly reduced the potency of ATP and UTP at the mutated murine P2Y2 receptor (Erb et al., 1995).
In our experiments, we tried to match the levels of receptor expression for both P2Y subtypes by adjusting the amounts of injected cRNA so that the maximum ATP response by oocytes was of similar amplitude (about 1 μA). While this is a relatively crude and imperfect procedure to control the P2Y receptor number, it helped us nonetheless to avoid the worst effects of differences in the receptor reserve on agonist potency (Kenakin, 2002) and allowed us to broadly compare the relative potencies of nucleotides at these two P2Y subtypes. Our findings indicate that rP2Y4 was slightly more sensitive to ATP and UTP than at rP2Y2 when receptor reserve appears to be similar, a conclusion which ran counter to earlier suggestions that P2Y2 is more sensitive. This earlier misconception probably resulted from one of us (King et al., 1998) comparing potency data from different cell types expressing significantly different P2Y receptor numbers. Our findings also showed that the two P2Y receptor subtypes can be distinguished by differences in the relative potency of CTP (five-fold), GTP (seven-fold) and, notably, ITP (13-fold). In particular, the lower potency of ITP at rP2Y2 is in agreement with earlier proposed procedures to separate these two P2Y receptor subtypes.
The pharmacological activity of BzATP was more clear-cut in our experiments: it activated rP2Y2 and inhibited rP2Y4. BzATP has been shown to activate murine P2Y2 receptors (Erb et al., 1993), but it has not been tested beforehand on other isoforms of P2Y4 receptors. This synthetic compound also activates human P2Y11 (Communi et al., 1999) and inhibits rat and human P2Y1 (Vigne et al., 1999), so the power of BzATP to discriminate between P2Y2 and P2Y4 receptors in native tissues is not wholly reliable, although for that purpose BzATP could still be combined with other pharmacological tools. For example, human P2Y11 is activated by α,β-meATP (van der Weyden et al., 2000) and rat and human P2Y1 receptors are fully activated by either 2-MeSADP or 2-MeSATP and blocked by PPADS. In the present study, α,β-meATP was inactive at both rP2Y2 and rP2Y4 and 2-MeSATP was a partial agonist, whereas neither receptor was potently inhibited by PPADS. We also found that ATPγS was a full agonist at rP2Y2 receptors and a partial agonist at rP2Y4. The mouse P2Y2 receptor is also fully activated by ATPγS (Erb et al., 1993). Taken together, ATPγS may also help to distinguish P2Y2 and P2Y4 subtypes in vivo, but with the caveat that ATPγS can also activate other P2Y (P2Y1,11) and P2X (P2X1–3,5) receptor subtypes. Thus, ATPγS (like BzATP) could be used as a discriminatory tool for P2Y2 and P2Y4 receptors in vivo, but only in conjunction with other pharmacological agents to exclude the presence of other P2 receptor subtypes.
The observed actions of the adenine dinucleotides (ApnA family) revealed some similarities, and differences, between the two P2Y receptor subtypes. First, in the oocyte system, Ap4A fully activated both P2Y receptor subtypes. Ap4A also fully activated rat and human P2Y4 receptors expressed in 1321N1 cells (Kennedy et al., 2000). Ap4A has never before been tested at rat and mouse P2Y2 isoforms, but is known to be equipotent with ATP and UTP at human P2Y2 (Hoyle et al., 2001). In the present study, rat P2Y2 was also activated by several other dinucleotides (Ap3A, Ap5A and Ap6A). We have already shown in an earlier study that Xenopus oocytes do not rapidly break down ApnA molecules (Pintor et al., 1996), and so the activity of many of the ApnA family at rat P2Y2 cannot be explained by their breakdown to ATP. Therefore, the activity profile of the ApnA series might also be considered when trying to distinguish between P2Y2 and P2Y4 subtypes in rat tissues. Our data for the ApnA series mirrored the structure–activity relationship (SAR) for the P1-P(n)-di(uridine-5′)phosphate (UpnU) series (n=2–6) at the equivalent human P2Y receptors (Pendergast et al., 2001). Thus human and rat P2Y4 isoforms are only activated fully by the tetraphosphates of the UpnU and ApnA series, respectively, and these tetraphosphates are as potent as either UTP or ATP. On the other hand, human and rat P2Y2 isoforms are activated by the same range of UpnU and ApnA compounds – with the tetraphosphates most active and almost as potent as either UTP and ATP, other dinucleotides marginally less potent and the diphosphates being either weakly active or inactive.
Prior to this study, it had been shown that suramin shifted the C/R curve to the right for UTP acting on rat aortic strips but had no effect on the C/R for UTP acting on canine tracheal epithelium, indicating that there may be two subtypes of P2U receptors in vivo (Dainty et al., 1994). Examples of P2U/P2Y2-like receptors in other tissues have also been reported to show different sensitivities to suramin (Ralevic & Burnstock, 1998). Rat P2Y2 was competitively antagonised by suramin (pA2, 5.40) (this study) and also at human P2Y2 (pA2, 4.26) (Charlton et al., 1996). Suramin has not yet been tested on canine P2Y2 (Zambon et al., 2000). In contrast, suramin was effective only at high concentrations at rat P2Y4 receptors (mean IC50, 1 mM). It is possible that blockade by high concentrations of suramin (100 μM and greater) was due to the inhibition of G-protein signalling rather than, or as well as, P2Y receptor blockade (Freissmuth et al., 1999). The observed differences in suramin sensitivity at rP2Y2 and rP2Y4 might account for the possibility of two P2U receptors, in vivo.
Of the other antagonists tested, rat P2Y4 was competitively antagonised by RB-2 (pA2, 6.43), which contrasted with negative data for rP2Y2 and provided another way of differentiating between these two P2Y subtypes. The P2X receptor antagonist TNP-ATP also was inactive at both rP2Y2 and rP2Y4 receptors – raising the possibility that TNP-ATP might be one of the few P2 antagonists selective only for ATP-gated ion channels. We found that rat P2Y4 was incompletely blocked by PPADS (by 47% at 10 mM), as also seen with PPADS at human P2Y4 receptors expressed in 1321N1 cells (30% at 100 μM) (Charlton et al., 1996). In contrast, it has been reported that PPADS blocked both the rat P2Y4 (mean IC50, 25 M) and mouse P2Y4 receptors (mean IC50, 45 μM) expressed in 1321N1cells (Suarez-Heurta et al., 2001). We did protect PPADS for white light, which promotes the breakdown of the azo-bridge in PPADS, and its limited activity in our hands cannot be due to degradation. Also, differences in PPADS activity at rP2Y4 cannot be due to the oocyte expression system since the antagonist is not active at hP2Y4 in 1321N1 cells. Also, we did not find any significant differences in antagonist activity when the extracellular pH was changed. Therefore, at this time, we cannot explain the differences in the activity of PPADS at rP2Y4 seen in separate studies and, therefore, caution against the use of PPADS to identify the P2Y4 subtype.
Another new aspect of the present study involved the modulation of agonist activity at P2Y receptors. This is the first time that extracellular pH has been shown to affect recombinant P2Y receptors, although H+ ions are known to enhance agonist potency at native P1(A2A) receptors (Hiley et al., 1995) and recombinant P2X subtypes – especially rat P2X2 receptors (King et al., 1996b). This potentiating effect was peculiar to the rP2Y4 subtype, whereas agonist potency at the rP2Y2 subtype was unaffected. This latter negative finding agreed with an earlier study of native P2U receptors in adenosine-5′-triphosphate (BPAE) cells, which were believed to be activated by free ATP (ATP4−) and unaffected by changing pH (Lustig et al., 1992).
We carried out a computational analysis of the available ATP-ion species, using “Bound and Determined” (Brooks & Storey, 1992), and, at the four pH levels tested, the amounts of ATP4− present (197–460 nM) were proportionally similar to the EC50 ratios determined for rP2Y2 receptors. However, in our experiments with rP2Y4, the available ATP4− levels progressively decreased (106–6 nM) with extracellular acidification – even though agonist potency was progressively enhanced. Thus, free ATP (ATP4-) may be able to activate rP2Y2 receptors, as suggested for P2U receptors in BPAE cells (Lustig et al., 1992), but this did not seem to be the case for rP2Y4 receptors since our EC50 values were not in proportion with the available amounts of free ATP.
It was not possible to identify the ATP-ion species that activated rP2Y4 receptors and, instead, the observed pH-induced changes in agonist potency may involve an action on the receptor itself. The pH modulation of agonist potency at the P2X2 receptor is thought to be due to the acidification of histidine residue(s) close to the ATP-binding site (Clyne et al., 2002a). However, the amino-acid sequences of rP2Y4 and rP2Y2 show eight conserved histidine residues and only 1 unique histidine in the intracellular C-terminus of rP2Y4 – a site unlikely to be instantly accessible to extracellular pH. The substitution of cysteine with alanine residues can also blunt the pH modulation of agonist potency at P2X2 receptors (Clyne et al., 2002b). Of the 13 cysteine residues found in rP2Y4, 10 are shared with rP2Y2 receptors. The three unique cysteine residues are located on IL2, TM4 and C-terminus of rP2Y4. The precise contribution of these cysteine residues – particularly in TM4 – awaits mutational analysis of the P2Y4 receptor.
The inhibitory action of Zn2+ ions on agonist activity represented another novel finding for P2Y receptors. This divalent cation caused an inhibition of rP2Y4 receptors when coapplied with ATP, and the immediacy of its effect suggested an extracellular site-of-action – perhaps one of the histidine and/or cysteine residues on rP2Y4, since these residues have also been causally linked with the modulatory actions of Zn2+ ions at P2X2 receptors (Clyne et al., 2002a, 2002b). However, the actions of Zn2+ ions were not duplicated by H+ ions at rP2Y4 receptors and it seems unlikely that the same histidine and cysteine residues can be involved in the modulatory effects of both ion species yet still have divergent effects. Zn2+ effects were time dependent and enhanced by preincubation, which might indicate additional sites-of-action – either deeper in the agonist-binding pocket or at an intracellular site. Intracellular Zn2+ ions can potentiate agonist responses at A2A and A2B receptors in NG108-15 cells, by slowing down the rate of GRK2 kinase-mediated receptor desensitisation, and can also affect the rate of desensitisation at β2-adrenoceptors and 5-HT4 receptors by a similar mechanism (Mundell & Kelly, 1998, and references therein). However, we observed an inhibitory effect – not a potentiation – with Zn2+ ions at P2Y receptors, suggesting that these cations might not easily gain access to the inside of oocytes.
In summary, the pharmacological profiles of rat P2Y2 and rat P2Y4 were more divergent than previously supposed. There were differences in agonist potency for naturally occurring nucleotides, greater differences in the selectivity of dinucleotides and significant differences in the pharmacological actions of some synthetic nucleotides. The actions of extracellular pH on ATP/UTP activity were significantly different at these two P2Y receptors, as was the blocking activity of a range of P2 receptor antagonists on ATP/UTP responses. Finally, agonist activity was modulated by Zn2+ ions at the rP2Y4 receptor, which was much more sensitive than rP2Y2. Collectively, these pharmacological differences should help to determine the presence of either of the P2Y receptor subtype in rat cell lines and tissues.
Acknowledgments
We are grateful to Dr Claude Desgranges (INSERM U441, Pessac, FRANCE) and Dr Cheikh Seye (now University of Missouri, Columbia, U.S.A.) for the provision of the rat P2Y2 cDNA. This work was supported by funding from the Les Clark Fund (U.K.), St Peter's Trust for Kidney, Bladder and Prostate Research (U.K.) and BBSRC (U.K.).
Abbreviations
- ATP
adenosine-5′-triphosphate
- ATPγS
adenosine-5′-O-(thiotriphosphate)
- 2-MeSATP
2-methylthioATP
- αβ-meATP
α,β-methyleneATP
- βγ-meATP
β,γ-methyleneATP
- BPAE
bovine pulmonary-artery endothelium
- BzATP
2′,3′-O-(4-benzoylbenzoyl)ATP
- CTP
cytosine-5′-triphosphate
- GTP
guanosine-5′-triphosphate
- ITP
inosine-5′-triphosphate
- UTP
uridine-5′-triphosphate
- ApnA
P1-P(n)-di(adenosine-5′)phosphate
- GPCR
G protein-coupled receptor
- LGIC
ligand-gated ion-channel
- PPADS
pyridoxal-5-phosphate-6-azophenyl-2′,4′-disulphonic acid
- RB-2
Reactive blue 2
- SAR
structure-activity relationship
- TNP-ATP
2′,3′-O-(2,4,6-trinitrophenyl)ATP
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenedi-amine
- suramin
suramin hexasodium salt
- UpnU
P1-P(n)-di(uridine-5′)phosphate
References
- ABBRACCHIO M.P., BOEYNAEMS J.M., BARNARD E.A., BOYER J.L., KENNEDY C., MIRAS-PORTUGAL M.P., KING B.F., GACHET C., JACOBSON K.A., WEISMAN G.A., BURNSTOCK G. Characterization of the UDP-glucose receptor (renamed here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNARD E.A., BURNSTOCK G., WEBB T.E. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol. Sci. 1994;15:67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- BOGDANOV Y.D., WILDMAN S.S., CLEMENTS M.P., KING B.F., BURNSTOCK G. Molecular cloning and characterization of rat P2Y4 nucleotide receptor. Br. J. Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS S.P., STOREY K.B. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal. Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- CHARLTON S.J., BROWN C.A., WEISMAN G.A., TURNER J.T., ERB L., BOARDER M.R. Cloned and transfected P2Y4 receptors: characterization of a suramin and PPADS-insensitive response to UTP. Br. J. Pharmacol. 1996;119:1301–1303. doi: 10.1111/j.1476-5381.1996.tb16038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLYNE J.D., LAPOINTE L.D., HUME R.I. The role of histidine residues in modulation of the rat P2X2 purinoceptor by zinc and pH. J. Physiol. (Lond.) 2002a;539:347–359. doi: 10.1113/jphysiol.2001.013244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLYNE J.D., WANG L.F., HUME R.I. Mutational analysis of the conserved cysteines of the rat P2X2 purinoceptor. J. Neurosci. 2002b;22:3873–3880. doi: 10.1523/JNEUROSCI.22-10-03873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMUNI D., PIROTTON S., PARMENTIER M., BOEYNAEMS J.M. Cloning and functional expression of a human uridine nucleotide receptor. J. Biol. Chem. 1995;270:30849–30852. doi: 10.1074/jbc.270.52.30849. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., ROBAYE B., BOEYNAEMS J.M. Pharmacological characterization of the human P2Y11 receptor. Br. J. Pharmacol. 1999;128:1199–1206. doi: 10.1038/sj.bjp.0702909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAINTY I.A., POLLARD C.E., ROBERTS S.M., FRANKLIN M., MCKECHNIE K.C.W., LEFF P. Evidence for subdivision of P2U-purinoceptors based on suramin sensitivity. Br. J. Pharmacol. 1994;112:578P. [Google Scholar]
- DUBYAK G.R. Signal transduction by P2-purinergic receptors for extracellular ATP. Am. J. Respir. Cell. Mol. Biol. 1991;4:295–300. doi: 10.1165/ajrcmb/4.4.295. [DOI] [PubMed] [Google Scholar]
- DUBYAK G.R., El-MOATASSIM C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993;265:C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- ERB L., LUSTIG K.D., SULLIVAN D.M., TURNER J.T., WEISMAN G.A. Functional expression and photoaffinity labeling of a cloned P2U purinergic receptor. Proc. Nat. Acad. Sci. U.S.A. 1993;90:10449–10453. doi: 10.1073/pnas.90.22.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERB L., GARRAD R., WANG Y., QUINN T., TURNER J.T., WEISMAN G.A. Sited-directed mutagenesis of P2U purinoceptors. Positively charged amino acids in transmembrane helices 6 and 7 affect agonist potency and specificity. J. Biol. Chem. 1995;270:4185–4188. doi: 10.1074/jbc.270.9.4185. [DOI] [PubMed] [Google Scholar]
- FILIPPOV A.K., SIMON J., BARNARD E.A., BROWN D.A. Coupling of the nucleotide P2Y4 receptor to neuronal ion channels. Br. J. Pharmacol. 2003;138:400–406. doi: 10.1038/sj.bjp.0705043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREISSMUTH M., WALDHOER M., BOFILL-CARDONA E., NANOFF C. G protein antagonists. Trends Pharmacol. Sci. 1999;20:237–245. doi: 10.1016/s0165-6147(99)01337-1. [DOI] [PubMed] [Google Scholar]
- HILEY C.R., BOTTRILL F.E., WARNOCK J., RICHARDSON P.J. Effects of pH on responses to adenosine, CGS 21680, carbachol and nitroprusside in the isolated perfused superior mesenteric arterial bed of the rat. Br. J. Pharmacol. 1995;116:2641–2646. doi: 10.1111/j.1476-5381.1995.tb17220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYLE C.H.V., HILDERMAN R.H., PINTOR J.J., SCHLUTER H., KING B.F. Diadenosine polyphosphates as extracellular signal molecules. Drug Devel. Res. 2001;52:260–273. [Google Scholar]
- KENAKIN T. Drug efficacy at G protein-coupled receptors. Ann. Rev. Pharmacol. Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- KENNEDY C., QI A.D., HEROLD C.L., HARDEN T.K., NICHOLAS R.A. ATP, an agonist at the rat P2Y4 receptor, is an antagonist at the human P2Y4 receptor. Mol. Pharmacol. 2000;57:926–931. [PubMed] [Google Scholar]
- KING B.F., TOWNSEND-NICHOLSON A., BURNSTOCK G. Metabotropic receptors for ATP and UTP: exploring the correspondence between native and recombinant nucleotide receptors. Trends Pharmacol. Sci. 1998;19:506–514. doi: 10.1016/s0165-6147(98)01271-1. [DOI] [PubMed] [Google Scholar]
- KING B.F., WANG S., BURNSTOCK G. P2 purinoceptor-activated inward currents in follicular oocytes of Xenopus laevis. J. Physiol. (Lond.) 1996a;494:17–28. doi: 10.1113/jphysiol.1996.sp021472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING B.F., ZIGANSHINA L.E., PINTOR J., BURNSTOCK G. Full sensitivity of P2X2 purinoceptor to ATP revealed by changing extracellular pH. Br. J. Pharmacol. 1996b;117:1371–1373. doi: 10.1111/j.1476-5381.1996.tb15293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., ROCHELLE L.G., O'NEAL W.K., RIBEIRO C.M., GRUBB B.R., ZHANG V., HARDEN T.K., BOUCHER R.C. Cloning and functional characterization of two murine uridine nucleotide receptors reveal a potential target for correcting ion transport deficiency in cystic fibrosis gallbladder. J. Pharmacol. Exp. Ther. 2001;297:43–49. [PubMed] [Google Scholar]
- LUSTIG K.D., SHIAU A.K., BRAKE A., JULIUS D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc. Nat. Acad. Sci. U.S.A. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUSTIG K.D., SPORTIELLO M.G., ERB L., WEISMAN G.A. A nucleotide receptor in vascular endothelial cells is specifically activated by the fully ionized forms of ATP and UTP. Biochem. J. 1992;284:733–739. doi: 10.1042/bj2840733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNDELL S.J., KELLY E. Evidence for co-expression and desensitization of A2A and A2B adenosine receptors in NG108-15 cells. Biochem. Pharmacol. 1998;55:595–603. doi: 10.1016/s0006-2952(97)00466-8. [DOI] [PubMed] [Google Scholar]
- NGUYEN T., ERB L., WEISMAN G.A., MARCHESE A., HENG H.H., GARRAD R.C., GEORGE S.R., TURNER J.T., O'DOWD B.F. Cloning, expression, and chromosomal localization of the human uridine nucleotide receptor gene. J. Biol. Chem. 1995;270:30845–30848. doi: 10.1074/jbc.270.52.30845. [DOI] [PubMed] [Google Scholar]
- O'CONNOR S.E., DAINTY I.A., LEFF P. Further subclassification of ATP receptors based on agonist studies. Trends Pharmacol. Sci. 1991;12:137–141. doi: 10.1016/0165-6147(91)90530-6. [DOI] [PubMed] [Google Scholar]
- PARR C.E., SULLIVAN D.M., PARADISO A.M., LAZAROWSKI E.R., BURCH L.H., OLSEN J.C., ERB L., WEISMAN G.A., BOUCHER R.C., TURNER J.T. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Nat. Acad. Sci. U.S.A. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENDERGAST W., YERXA B.R., DOUGLASS-III J.G., SHAVER S.R., Dougherty R.W., REDICK C.C., SIMS I.F., RIDEOUT J.L. Synthesis and P2Y receptor activity of a series of uridine dinucleoside 5′-polyphosphates. Bioorg. Med. Chem. Lett. 2001;11:157–160. doi: 10.1016/s0960-894x(00)00612-0. [DOI] [PubMed] [Google Scholar]
- PINTOR J., KING B.F., ZIGANSHIN A.U., MIRAS-PORTUGAL M.T., BURNSTOCK G. Diadenosine polyphosphate-activated inward and outward currents in follicular oocytes of Xenopus laevis. Life Sci. 1996;59:179–184. doi: 10.1016/0024-3205(96)00409-2. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SEYE C.I., GADEAU A.P., DARET D., DUPUCH F., ALZIEU P., CAPRON L., DESGRANGES C. Overexpression of P2Y2 purinoceptor in intimal lesions of the rat aorta. Arterioscler. Thromb. Vasc. Biol. 1997;17:3602–3610. doi: 10.1161/01.atv.17.12.3602. [DOI] [PubMed] [Google Scholar]
- SMART T.G., XIE X., KRISHEK B.J. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog. Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- SUAREZ-HUERTA N., POUILLON V., BOEYNAEMS J.M., ROBAYE B. Molecular cloning and characterization of the mouse P2Y4 nucleotide receptor. Eur. J. Pharmacol. 2001;416:197–202. doi: 10.1016/s0014-2999(01)00875-5. [DOI] [PubMed] [Google Scholar]
- VAN DER WEYDEN L., ADAMS D.J., LUTTRELL B.M., CONIGRAVE A.D., MORRIS M.B. Pharmacological characterisation of the P2Y11 receptor in stably transfected haematological cell lines. Mol. Cell. Biochem. 2000;213:75–81. doi: 10.1023/a:1007168215748. [DOI] [PubMed] [Google Scholar]
- VIGNE P., HECHLER B., GACHET C., BREITTMAYER J.P., FRELIN C. Benzoyl ATP is an antagonist of rat and human P2Y1 receptors and of platelet aggregation. Biochem. Biophys. Res. Commun. 1999;256:94–97. doi: 10.1006/bbrc.1999.9558. [DOI] [PubMed] [Google Scholar]
- VON KÜGELGEN I., HAUSSINGER D., STARKE K. Evidence for a vasoconstriction-mediating receptor for UTP, distinct from the P2 purinoceptor, in rabbit ear artery. Naunyn Schmiedeberg's Arch. Pharmacol. 1987;336:556–560. doi: 10.1007/BF00169313. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., HENDERSON D.J., ROBERTS J.A., BARNARD E.A. Molecular cloning and characterization of the rat P2Y4 receptor. J. Neurochem. 1998;71:1348–1357. doi: 10.1046/j.1471-4159.1998.71041348.x. [DOI] [PubMed] [Google Scholar]
- WILDMAN S.S., KING B.F., BURNSTOCK G. Zn2+ modu-lation of ATP-responses at recombinant P2X2 receptors and its dependence on extracellular pH. Br. J. Pharmacol. 1998;123:1214–1220. doi: 10.1038/sj.bjp.0701717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDMAN S.S., TURNER C., BURNSTOCK G., UNWIN R.J., KING B.F.P2Y4 abundance in rat kidney epithelial cysts and its properties in vitro J. Am. Soc. Nephrol. 200213F-P0272(abstract) [Google Scholar]
- ZAMBON A.C., HUGHES R.J., MESZAROS J.G., WU J.J., TORRES B., BRUNTON L.L., INSEL P.A. P2Y2 receptor of MDCK cells: cloning, expression, and cell-specific signalling. Am. J. Physiol. 2000;279:1049–1052. doi: 10.1152/ajprenal.2000.279.6.F1045. [DOI] [PubMed] [Google Scholar]