Abstract
We investigated the relative effectiveness of L-arginine, L-ascorbate and pyridoxine in preventing the impairment of endothelium-mediated vasorelaxation induced by native low-density lipoprotein (nLDL) from healthy subjects, oxidised LDL (oxLDL, formed by oxidation of nLDL) or nLDL from type II diabetic patients (dLDL).
Rabbit aortic rings were exposed to nLDL, dLDL or oxLDL (50–200 mg protein l−1), or corresponding vehicle, following which they were constricted with noradrenaline 10−6 M; concentration–relaxation curves were determined to acetylcholine (ACh), A23187, or sodium nitroprusside (NP), in the absence or presence of L-arginine (10−5–10−3 M), L-ascorbate (10−5–10−3 M) and pyridoxine (0.5–2.0 mM).
nLDL, dLDL and oxLDL all inhibited relaxant responses to ACh and A23187, but not to NP, in a concentration-dependent manner (oxLDL>dLDL>nLDL).
In the presence of all LDL preparations, L-arginine, L-ascorbate or pyridoxine each improved ACh and A23187 responses, although none completely normalised endothelium-dependent relaxations. The maximal effect of L-arginine occurred at 10−4 M. The combination of L-arginine 10−4 M, L-ascorbate 10−5 M and pyridoxine 2.0 mM was equally effective as L-arginine 10−4 M alone.
Our results confirm that nLDL, dLDL and oxLDL exert inhibitory effects on endothelium dependent, but not endothelium independent, relaxation of rabbit aorta. ACh and A23187 responses in the presence of any LDL species can be ameliorated by supplementation with L-arginine, L-ascorbate or pyridoxine, either singly or in combination, with no agent or combination proving superior to L-arginine alone. Nevertheless, ACh and A23187 responses are not completely normalised with such supplements, suggesting that there also exists a component of LDL-induced inhibition of endothelium-mediated vasorelaxation that is independent of the nitric oxide system.
Keywords: Low-density lipoprotein, oxidised low-density lipoprotein, type II diabetes mellitus, endothelial function, L-arginine, L-ascorbate, pyridoxine
Introduction
The vascular endothelium produces a variety of vasoactive substances, which have important effects on vascular tone, haemostasis, leucocyte and platelet adhesion, vascular permeability, and atherogenesis (reviewed recently by Anderson, 2003). One of the most important of these mediators is nitric oxide (NO). NO is synthesised in vascular endothelial cells by the action of the enzyme nitric oxide synthase (NOS), which oxidises the amino-acid L-arginine to generate L-citrulline and NO. NO acts on vascular smooth muscle to promote vasorelaxation, on leucocytes and platelets to prevent adhesion to the endothelium and platelet aggregation, on endothelial cells themselves to inhibit vascular permeability, and on a host of cellular components of the vascular wall to inhibit atherogenesis (Anderson, 2003). It reacts rapidly with superoxide anion (O2−) to form peroxynitrite (Beckman & Koppenol, 1996), so that conditions associated with increased oxidative stress give rise to an accelerated scavenging and removal of NO.
Endothelial dysfunction is found in a variety of cardiovascular disease states, such as type II diabetes and hypercholesterolaemia as well as in established atherosclerosis, and studies suggest that the degree of endothelial dysfunction increases with the number of risk factors present in an individual (Vita et al., 1990; Celermajer et al., 1994). This dysfunction is characterised by reduced bioactivity of NO; this may be due either to suppression of NOS activity or to increased NO scavenging attributable to enhanced O2− production, since increased oxidative stress is a characteristic feature of these conditions (Cai & Harrison, 2000). Indeed, it has been suggested that endothelial dysfunction and impairment of NO availability may play a pathogenetic role in atherosclerosis (Kinlay & Ganz, 1997; Ross, 1999).
Cholesterol is mainly transported in the circulation in the form of low-density lipoprotein (LDL) particles, and increased LDL concentration is associated both with the stimulation of atherogenesis and impairment of vascular endothelium-dependent relaxation (Ludmer et al., 1986; Chowienczyk et al., 1992; Casino et al., 1995). This impairment can largely be attributed to a decrease in NO availability, although the precise mechanism by which LDL exerts this effect is unclear at present. Additionally, LDL can itself undergo oxidation in the presence of reactive oxygen species; oxidised (including minimally oxidised) LDL exerts a greater impairment of endothelium-dependent relaxation than does nonoxidised LDL (Galle et al., 1991; Chin et al., 1992), as does glyc-oxidised LDL, as found for example in patients with type II diabetes (Galle et al., 1998). Thus, qualitative as well as quantitative differences in LDL can be important as regards effects on vascular endothelial function. Recently, McNeill et al. (2000) have reported that incubation of rabbit aortic rings in the organ bath with LDL isolated from type II diabetic patients causes a greater reduction in acetylcholine (ACh)-induced vasorelaxation than does an equivalent concentration of LDL from healthy control subjects. Furthermore, Posch et al. (1999) have demonstrated that glycated LDL reduces the shear stress-induced increase in endothelial cell NO synthesis, by inhibition of shear stress-stimulated L-arginine uptake and NO bioactivity through increased endothelial cell superoxide anion release; and the same group has recently shown that both LDL taken from diabetic patients and LDL glycated in vitro increase endothelial cell apoptosis (Artwohl et al., 2003).
Supplementation with L-arginine, the precursor for NO synthesis, has been shown to improve endothelium-dependent vasorelaxation in hypercholesterolaemic subjects in vivo (Drexler et al., 1991; Creager et al., 1992; Clarkson et al., 1996). L-Ascorbate (vitamin C) can also improve endothelium-dependent relaxation, due to removal of reactive oxygen species (Fontana et al., 1999). In addition, pyridoxine (vitamin B6) has been demonstrated in one study to ameliorate endothelial dysfunction in vivo in cardiac transplant recipients (Miner et al., 2001), and this may be attributable to the essential role of pyridoxine as a cofactor in the metabolism of homocysteine, which is associated with the generation of oxidative stress in vivo. Therefore, L-arginine, L-ascorbate and pyridoxine are potentially promising as therapies in cardiovascular diseases associated with endothelial dysfunction and impaired NO availability.
The aim of the present study, therefore, was to evaluate the effectiveness of L-arginine, L-ascorbate and pyridoxine, administered either singly or in combination, in preventing the impairment of endothelium-mediated vasorelaxation induced by LDL, in rabbit aortic rings in vitro. We assessed the effectiveness of supplementation with these substances in aortic tissues incubated with native LDL (nLDL) taken from healthy subjects, LDL from the same healthy subjects following in vitro oxidation (oxLDL), and nLDL taken from subjects with type II diabetes (dLDL). To date, no studies have compared the effectiveness of L-arginine and L-ascorbate in healthy arteries exposed to LDL of different oxidation and/or glycation states, nor have any studies examined the effect of pyridoxine on LDL-induced endothelial dysfunction.
Methods
Animals and preparation of vascular rings
New Zealand White rabbits, weighing 2–2.5 kg, were from the Nanjing Experimental Animals Centre of Jiangsu Province, China. They were provided with food and water ad libitum and, on the day of experimentation, were anaesthetised with sodium pentobarbital (60 mg kg−1); the descending thoracic aorta was dissected out carefully, following which the animal was killed by pentobarbital overdose. The aorta was trimmed of adherent connective tissue and fat, and then cut into 2-mm-long rings, which were continuously oxygenated with 95% O2/5% CO2.
Subjects and LDL preparation
Seven healthy control subjects (five male, two female) and 11 subjects with type II diabetes (eight male, three female) were recruited for these experiments. Their characteristics are shown in Table 1. Verbal consent was obtained for blood sampling in all cases, and the study was approved by the institutional review board for human studies of Nanjing Medical University and by the King's College London Research Ethics Committee. Venous blood (100 ml) was drawn from an antecubital vein and collected into EDTA (1.3 mM final concentration). Plasma was separated by centrifugation (500 × g, 10 min, room temperature), and nLDL (1.019–1.063 g ml−1) isolated from the plasma by discontinuous density gradient ultracentrifugation as described previously (Chung et al., 1980), followed by extensive dialysis with phosphate-buffered saline (PBS), pH 7.4, containing 0.3 mM EDTA (48 h, 4°C). Within each subject group, isolated LDL were pooled, in order to obtain sufficient LDL to carry out all the experiments outlined below. All LDL isolates were adjusted to a protein concentration of 0.5 mg ml−1, as measured using the method of Bradford (1976), and were stored at 4°C and used within 1 month.
Table 1.
Subject characteristics
| Controls | Type II diabetic subjects | |
|---|---|---|
| Number and sex | Five male, two female | Eight male, three female |
| Age (years) | 46.7±2.9 | 48.2±7.3 |
| Total cholesterol (mM) | 5.5±0.5 | 5.7±0.9 |
| HDL cholesterol (mM) | 1.4±0.5 | 1.1±0.2 |
| LDL cholesterol (mM) | 3.2±0.4 | 3.1±0.8 |
| Triglycerides (mM) | 1.7±0.2 | 2.1±0.5 |
| Glucose (mM) | 4.9±0.3 | 9.1±1.6** |
| HbA1c (%) | 4.9±0.2 | 8.8±1.8** |
P<0.01 as compared with control subjects.
In order to obtain oxLDL, copper oxidation was performed on nLDL as follows. Prior to oxidation, a sample of nLDL was dialysed overnight at 4°C against 2000 volumes of PBS without EDTA; protein concentration was adjusted to 0.5 mg ml−1, and CuSO4 was added to a final concentration of 20 μM for 24 h at 37°C. EDTA was then added at a final concentration of 2.7 mM to stop the reaction. Again, the resultant oxLDL was extensively dialysed with PBS, pH 7.4, containing 0.3 mM EDTA (48 h, 4°C), stored at 4°C and used within 1 month.
Oxidation and glycation status of isolated LDL
LDL oxidation status was determined by measuring the concentrations of thiobarbituric acid reactive substances (TBARS) in the different LDL samples according to the method of Ohkawa et al. (1979). In brief, 1.5 ml 20% acetic acid (pH 3.5) and 1.5 ml of a 0.8% thiobarbituric acid solution were added to a sample of the LDL, and the volume brought to 4.0 ml with distilled water. The mixture was shaken thoroughly and heated in an oil bath at 95°C for 60 min. After the mixture was cooled with tap water, 1.0 ml of distilled water and 5.0 ml of butyl alcohol and pyridine (15 : 1) were added, and the sample was shaken gently for 5 min. After centrifugation at 1500 × g for 10 min, the butyl alcohol-pyridine phase containing the TBARS was separated and its absorbance was measured at 532 nm. The results were expressed as nmol equivalent malondialdehyde per mg protein, with malondialdehyde from tetramethoxypropane used as standard and double-distilled water as a control.
LDL glycation was quantified in our samples by competitive ELISA, using a proprietary assay kit (Glycacor), from which the percentage of total LDL in the glycated state was calculated.
Organ bath pharmacology
Rabbit aortic rings were mounted in 3 ml organ baths containing Krebs buffer of the following composition (mM): NaCl 125, KCl 4.8, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, glucose 11, EDTA 0.3, CaCl2 2.5, pH 7.4 (gassed with 95% O2/5% CO2 at 37°C). Preliminary length–tension curves suggested that a resting tension of 2 g gave optimal contractile responses to KCl 45 mM, therefore this level of resting tension was used in all experiments.
Once stable and reproducible contractions were obtained with KCl 45 mM, rings were washed extensively and subsequently contracted with noradrenaline 10−6 M. Following attainment of plateau constriction, ACh 1 μM was added; only rings which demonstrated ⩾60% relaxation to ACh were considered to have functional endothelium, and were used for further experiments.
Following further extensive washing, some rings were exposed to nLDL, oxLDL or dLDL (protein concentration 50, 100 or 200 mg l−1), in the absence or presence of one or more of the following: L-arginine (10−5, 10−4 or 10−3 M), sodium L-ascorbate (10−5, 10−4 or 10−3 M), pyridoxine hydrochloride (0.5, 1.0 or 2.0 mM) or superoxide dismutase (SOD, 250 U ml−1). After 15 min incubation, noradrenaline 10−6 M was reapplied and, following attainment of plateau of contraction, cumulative concentration–relaxation curves were performed to either ACh (concentration range 10−9–10−5 M, log-unit increments) or to the calcium ionophore A23187 (concentration range 10−9–10−7 M, half log-unit increments). For comparison, concentration–effect curves were also performed to the endothelium-independent NO-releasing vasodilator sodium nitroprusside (NP, concentration range 10−8–10−5 M, log-unit increments), following noradrenaline 10−6 M preconstriction.
Materials and drugs
The Glycacor kit was from BioGnosis Ltd (Hailsham, East Sussex, U.K.). All other drugs and chemicals were from Sigma (Dorset, U.K.).
Statistical analysis
All results are presented as mean±s.e.m. of six experiments, and plotted using GraphPad Prism version 3.03. Data were analysed by repeated measures two-way ANOVA using StatView version 5.0.1. Statistical significance was taken as P<0.05 (two-tailed).
Results
Oxidation and glycation state of LDL preparations
nLDL was prepared from plasma from healthy subjects, and a portion of this was subjected to Cu2+ oxidation, to yield oxLDL. dLDL was obtained from plasma taken from type II diabetic subjects. Controls and type II diabetic subjects were of similar age and sex distribution, and had comparable lipid profiles; however, both plasma glucose and HbA1C were significantly higher in type II diabetic subjects than in controls (Table 1).
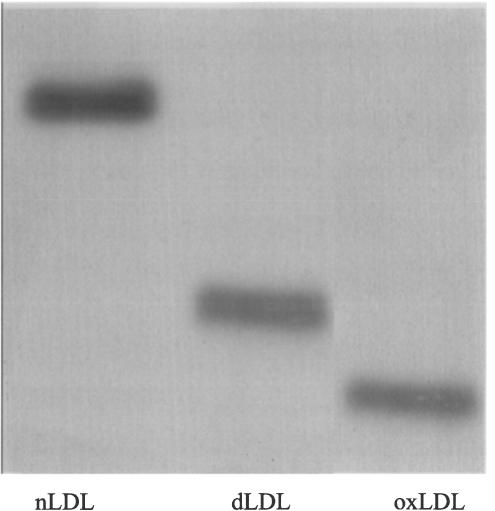
TBARS measurement revealed that dLDL was more oxidised than nLDL, and oxLDL even more so, as evidenced by an approximately 10- and 20-fold increase in malondialdehyde equivalents in dLDL and oxLDL, respectively, as compared with nLDL (nLDL: 2.7, dLDL: 28.1 and oxLDL: 58.5 nmol (mg protein)−1). Storage of each of our LDL preparations for up to 1 month was not associated with an increase in measured TBARS (data not shown). Since LDL oxidation (including in vitro oxidation of nLDL) is associated with a shift towards a smaller denser profile, thus resulting in an electrophoretic mobility change (Sobal et al., 2000), we also examined the electrophoretic profile of our LDL preparations. As seen in Figure 1, oxLDL had a greater mobility than did nLDL, with the mobility of dLDL being intermediate between the two.
Figure 1.
Nondenaturing polyacrylamide gradient gel electrophoresis of LDL samples, showing differences in mobility between nLDL, dLDL and oxLDL. oxLDL migrates fastest and nLDL slowest, and dLDL is intermediate.
Glycation state of our LDL preparations was assessed by competitive ELISA. nLDL and oxLDL were glycated to only a small degree (14.3 and 15.0% of total LDL, respectively), whereas dLDL exhibited a much greater degree of glycation (49.0% of total LDL).
Effect of LDL preparations on endothelium-dependent and endothelium-independent relaxation of rabbit aortic rings
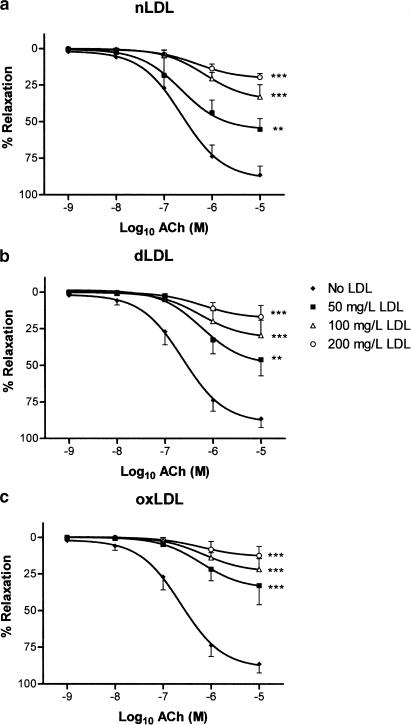
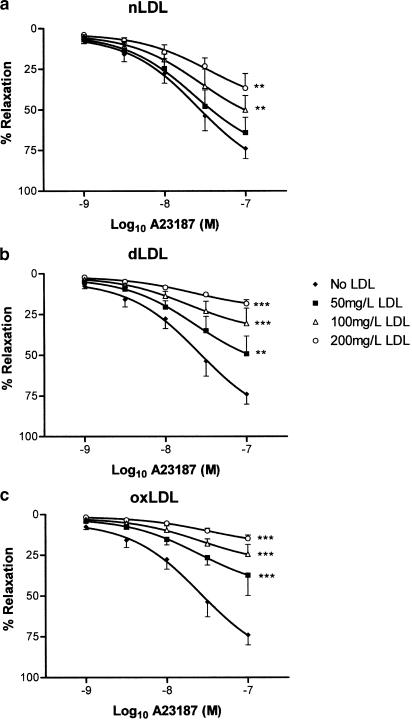
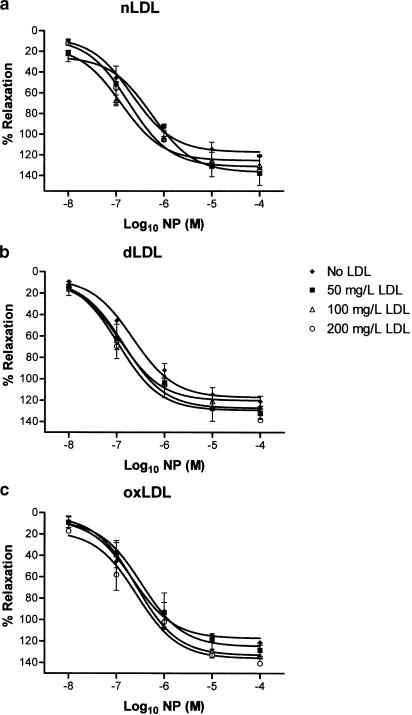
Tensioned rings of rabbit aorta with intact endothelium were preconstricted with noradrenaline in organ baths, following which endothelium-dependent relaxant responses were studied to either ACh or A23187. For comparison, endothelium-independent relaxation to NP was also examined. All three agents elicited concentration-dependent relaxation in aortic rings in the absence of LDL (Figures 2, 3 and 4). nLDL, oxLDL and dLDL had no effect on NP-induced relaxation (Figure 4); by contrast, each induced a concentration-dependent inhibition of relaxation to both ACh (Figure 2) and A23187 (Figure 3). None of these LDL species affected the level of preconstriction to noradrenaline (Table 2). The inhibitory effects of the different LDL preparations on ACh-dependent responses were in the rank order: oxLDL>dLDL>nLDL (Table 3).
Figure 2.
Effects of (a) nLDL, (b) dLDL and (c) oxLDL on relaxant responses to ACh. Responses are shown in the absence of LDL, and in the presence of 50, 100 and 200 mg protein l−1 of each type of LDL. **,***P<0.01 and <0.001 as compared with the absence of LDL.
Figure 3.
Effects of (a) nLDL, (b) dLDL and (c) oxLDL on relaxant responses to A23187. Responses are shown in the absence of LDL, and in the presence of 50, 100 and 200 mg protein l−1 of each type of LDL. **,***P<0.01 and <0.001 as compared with the absence of LDL.
Figure 4.
Effects of (a) nLDL, (b) dLDL and (c) oxLDL on relaxant responses to NP. Responses are shown in the absence of LDL, and in the presence of 50, 100 and 200 mg protein l−1 of each type of LDL.
Table 2.
Magnitude of contractions to noradrenaline 10−6 M (expressed as developed tension (g)) in the absence and presence of nLDL, dLDL or oxLDL (each at 100 mg l−1), and of L-arginine 10−4 M, L-ascorbate 10−5 M or pyridoxine 2.0 mM
| No LDL | nLDL | dLDL | oxLDL | |
|---|---|---|---|---|
| Vehicle | 7.6±2.1 | 7.3±2.7 | 7.6±1.0 | 6.5±2.1 |
| L-arginine | 7.5±1.8 | 5.8±1.2 | 8.6±2.1 | 7.5±0.6 |
| L-ascorbate | 5.6±1.1 | 9.6±3.5 | 5.6±1.5 | 7.6±3.1 |
| Pyridoxine | 7.0±1.4 | 7.9±1.9 | 7.9±1.6 | 6.5±1.5 |
Table 3.
Maximal relaxation (expressed as % relaxation of noradrenaline precontraction) to acetylcholine in the presence of different concentrations of nLDL, dLDL and oxLDL
| Maximal relaxation | |
|---|---|
| nLDL 50 mg l−1 | 55.5±4.4 |
| nLDL 100 mg l−1 | 35.2±3.6 |
| nLDL 200 mg l−1 | 20.4±1.5 |
| dLDL 50 mg l−1 | 49.6±3.3* |
| dLDL 100 mg l−1 | 31.2±3.0* |
| dLDL 200 mg l−1 | 17.9±1.2* |
| oxLDL 50 mg l−1 | 35.1±1.5**, # |
| oxLDL 100 mg l−1 | 23.5±2.3**, # |
| oxLDL 200 mg l−1 | 13.2±1.2 **, # |
P<0.05 and <0.01, respectively, as compared with the same concentration of nLDL.
P<0.05 as compared with the same concentration of dLDL.
Effect of L-arginine, L-ascorbate, pyridoxine and SOD on endothelium-dependent relaxation of rabbit aortic rings
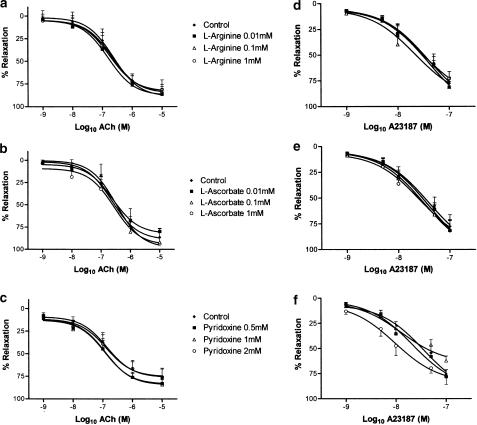
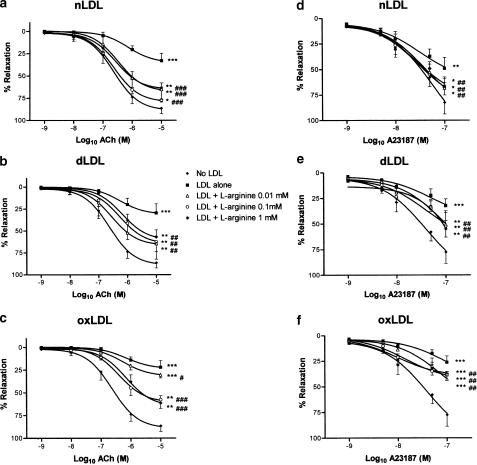
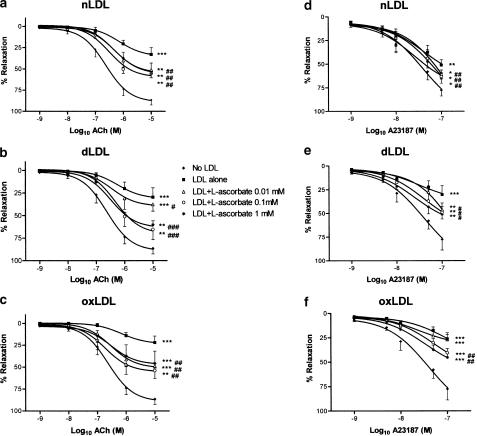
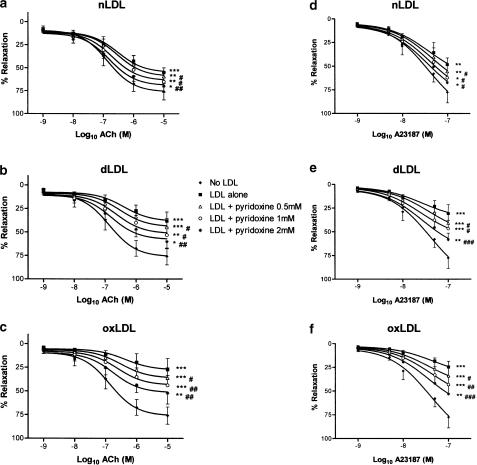
Since nLDL, dLDL and oxLDL were all found to inhibit endothelium-dependent responses in a concentration-dependent manner, in the range 50–200 mg protein l−1, an LDL concentration of 100 mg protein l−1 was used in all the following experiments, to assess whether L-arginine, L-ascorbate and/or pyridoxine could prevent the impairment of endothelium-dependent relaxation. Rabbit aortic rings were incubated with nLDL, dLDL or oxLDL (100 mg l−1), in the absence or presence of different concentrations of L-arginine (10−5–10−3 M), L-ascorbate (10−5–10−3 M) or pyridoxine (0.5–2.0 mM). After 15 min incubation, rings were preconstricted with noradrenaline, and concentration–relaxation responses determined to ACh or to A23187, as before. L-arginine, L-ascorbate and pyridoxine did not affect the degree of preconstriction to noradrenaline, either in the absence or presence of different LDL species (Table 2). In the absence of LDL, L-arginine and L-ascorbate did not affect ACh- or A23187-dependent responses (Figure 5a, b, d and e). On the other hand, pyridoxine 0.5 mM significantly increased the relaxation to ACh 10−9 and 10−6 M (P<0.05), and pyridoxine 1.0 mM significantly increased the relaxation to ACh 10−6 and 10−5 M (P<0.05); furthermore, pyridoxine 2.0 mM significantly increased the relaxation to A23187 10−9 M, 5 × 10−9 M, 10−8 M and 5 × 10−8 M (P<0.05 for each). While these increases to ACh and to A23187 responses in the presence of these concentrations of pyridoxine reached statistical significance, as did the corresponding curves overall, these changes were small in magnitude (Figure 5c and f). By contrast, in the presence of any of our LDL preparations, L-arginine, L-ascorbate or pyridoxine each improved ACh- and A23187-dependent responses considerably, although none of these agents could restore ACh or A23187 maximal relaxations to those seen in the absence of LDL (Figures 6, 7 and 8). The maximal effect of L-arginine was seen at 10−4 M, and higher concentrations had no additional effect; moreover, the effects of L-arginine on ACh- or A23187-induced relaxations in the presence of any of our LDL samples could be completely inhibited by coincubation with L-NAME 10−4 M (data not shown). All concentrations of L-ascorbate tested (10−5–10−3 M) improved ACh responses to the same degree in the presence of nLDL and oxLDL approximately, although in the case of dLDL 10−5 M L-ascorbate was less effective than the higher concentrations; in the presence of nLDL or dLDL, all concentrations of L-ascorbate tested improved A23187 relaxant responses to a similar degree, but in the presence of oxLDL 10−5 M L-ascorbate was less effective than higher concentrations. On the other hand, the improvement in both ACh and A23187 relaxations by pyridoxine was progressive as the concentration of pyridoxine was increased (0.5, 1.0 and 2.0 mM). L-arginine, L-ascorbate and pyridoxine had no effect on NP-induced relaxant responses, either in the absence or presence of any LDL species (data not shown).
Figure 5.
Effects of L-arginine, L-ascorbate and pyridoxine on relaxant responses to ACh (a–c) and to A23187 (d–f), in the absence of LDL. Responses are shown in the absence and in the presence of incremental concentrations of L-arginine (a and d), L-ascorbate (b and e) or pyridoxine (c and f). *P<0.05 as compared with control
Figure 6.
Effects of L-arginine supplementation on ACh (a–c) and on A23187 (d–f) relaxant responses in the presence of 100 mg protein l−1 nLDL (a and d), dLDL (b and e) and oxLDL (c and f). Responses are shown in the absence of LDL, and in the presence of LDL either alone or with L-arginine 10−5, 10−4 or 10−3 M. *,**,***P<0.05, <0.01 and <0.001 as compared with the absence of LDL. #,##,###P<0.05, <0.01 and <0.001 as compared with LDL alone.
Figure 7.
Effects of L-ascorbate supplementation on ACh (a–c) and on A23187 (d–f) relaxant responses in the presence of 100 mg protein l−1 nLDL (a and d), dLDL (b and e) and oxLDL (c and f). Responses are shown in the absence of LDL, and in the presence of LDL either alone or with L-ascorbate 10−5, 10−4 or 10−3 M. **,***P<0.01 and <0.001 as compared with the absence of LDL. #,##,###P<0.05, <0.01 and <0.001 as compared with LDL alone.
Figure 8.
Effects of pyridoxine supplementation on ACh (a–c) and on A23187 (d–f) relaxant responses in the presence of 100 mg protein l−1 nLDL (a and d), dLDL (b and e) and oxLDL (c and f). Responses are shown in the absence of LDL, and in the presence of LDL either alone or with pyridoxine 0.5, 1.0 or 2.0 mM. *,**,***P<0.05, <0.01 and <0.001 as compared with absence of LDL. #,##P<0.05 and <0.01 as compared with LDL alone.
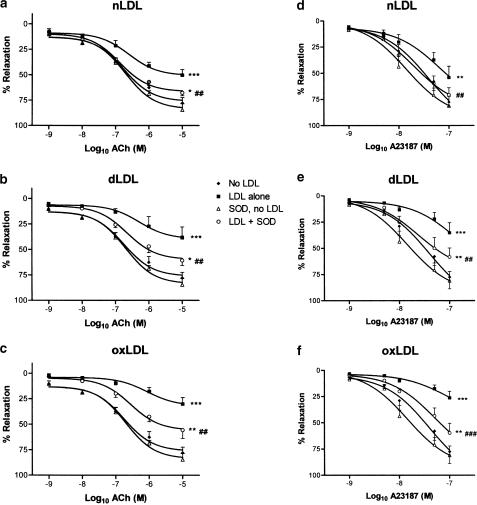
In order to confirm that the amelioration of endothelium-dependent responses by L-ascorbate in the presence of nLDL, dLDL and oxLDL was indeed due to scavenging of reactive oxygen species, we also tested the effect of SOD 250 U ml−1 (a concentration that we have previously found to exert maximal effects on superoxide anion scavenging in rat aortic rings in vitro) on vasorelaxant responses to ACh and to A23187. We found that SOD had no significant effect on ACh or A23187 responses in the absence of LDL. However, in the presence of any of these LDL species, SOD improved ACh and A23187 relaxations, to a similar degree as did L-ascorbate (Figure 9).
Figure 9.
Effects of supplementation with SOD 250 U ml−1 on ACh (a–c) and on A23187 (d–f) relaxant responses, in the absence and presence of 100 mg protein L−1 nLDL (a and d), dLDL (b and e) and oxLDL (c and f). *,**,***P<0.05, <0.01 and <0.001 as compared with absence of LDL. ##,###P<0.01 and <0.001 as compared with LDL alone.
We also wished to determine whether coincubation of aortic rings with the combination of L-arginine 10−4 M, L-ascorbate 10−5 M and pyridoxine 2.0 mM gave rise to a greater improvement in ACh-mediated relaxations than L-arginine 10−4 M alone. However we found that, in the presence of nLDL, dLDL or oxLDL (each at 100 mg protein l−1), the EC50 and Emax to ACh were in fact the same in the presence either of the combination or of L-arginine alone (Table 4), although both regimes improved Emax as compared with values in the absence of these supplements.
Table 4.
Potency (expressed as −log10 EC50) and maximal relaxation (Emax, expressed as a percentage of noradrenaline preconstriction) to acetylcholine in the presence of nLDL, dLDL and oxLDL (each at 100 mg l−1), and L-arginine 10−4 M either alone or in combination with L-ascorbate 10−5 M and pyridoxine 2.0 mM
| −log10EC50 | Emax | |
|---|---|---|
| NLDL | 6.6±0.2 | 35.2±3.6 |
| nLDL+L-arginine | 6.6±0.2 | 58.1±3.8*** |
| nLDL+L-arginine+L-ascorbate+pyridoxine | 6.8±0.1 | 57.9±2.7** |
| dLDL | 6.4±0.2 | 31.2±3.0 |
| dLDL+L-arginine | 6.9±0.1 | 73.3±1.8** |
| dLDL+L-arginine+L-ascorbate+pyridoxine | 6.8±0.1 | 73.4±2.2** |
| OxLDL | 6.4±0.2 | 23.5±2.3 |
| oxLDL+L-arginine | 6.5±0.1 | 61.3±2.1*** |
| oxLDL+L-arginine+L-ascorbate+pyridoxine | 6.5±0.1 | 53.9±1.8** |
P<0.01 and <0.001, respectively, as compared with the relevant LDL species alone.
Discussion
Previous studies have demonstrated that LDL exerts an inhibitory effect on endothelium-dependent vascular relaxation, and this is largely due to impairment of endothelial NO availability (Ludmer et al., 1986; Chowienczyk et al., 1992; Casino et al., 1995), either due to decreased synthesis or to increased scavenging by reactive oxygen species. This is likely to be a major contributor to the endothelial dysfunction observed in patients with hypercholesterolaemia, and this endothelial dysfunction may in turn contribute to the pathogenesis of atherosclerosis (Kinlay & Ganz, 1997; Ross, 1999). LDL that has undergone in vitro oxidation or glyc-oxidation exerts a more marked inhibitory effect on endothelial function than does nonoxidised LDL (Galle et al., 1991; 1998; Chin et al., 1992), and indeed glycated LDL, such as that found in the circulation of type II diabetic patients, is more susceptible to oxidation than is nonglycated LDL (Sobal et al., 2000), a finding confirmed in our study by high TBARS measurement in dLDL; furthermore, nLDL isolated from patients with type II diabetes also exerts a more marked inhibitory effect on endothelium-dependent vasorelaxation than does nLDL from healthy controls (McNeill et al., 2000). These data are borne out by the results of the present study. nLDL, oxLDL and dLDL all inhibited endothelium-dependent relaxation of rabbit aortic rings to both ACh and A23187 in a concentration-dependent manner, whereas they had no effect on endothelium-independent relaxations to NP. The rank order of inhibitory effect, for a given LDL protein concentration, was oxLDL>dLDL>nLDL, and this was the same as the rank order of LDL lipid peroxidation (as determined from TBARS). Strategies to prevent the deleterious effects of LDL on vascular endothelial function may be expected to improve clinical end points in patients at risk of atherosclerosis, although this remains to be proven in clinical outcome studies.
In this study, we examined the effects of three therapies that might be expected to prevent the inhibitory effects of LDL on endothelium-dependent relaxation: L-arginine, a substrate for NOS and therefore a precursor of NO; L-ascorbate (vitamin C), an antioxidant vitamin that scavenges reactive oxygen species; and pyridoxine (vitamin B6), a cofactor involved in homocysteine metabolism. We found that each of these gave rise to an improvement in rabbit aortic ACh and A23187 relaxant responses in the presence of nLDL, dLDL or oxLDL (100 mg protein l−1), although none restored ACh and A23187 responses to those seen in the absence of LDL. The effect of L-arginine was maximal at 10−4 M, and concentrations of L-ascorbate higher than 10−5 M similarly gave rise to no additional improvement in ACh responses (except in the case of dLDL coincubation). Although this action of L-ascorbate can be explained by scavenging of reactive oxygen species, it can also exert other effects that may give rise to improvement in endothelial function independently of its antioxidant action, namely increasing the intracellular availability of the NOS cofactor tetrahydrobiopterin (Baker et al., 2001) or catalysing the release of NO from endogenous S-nitrosothiols (Al-Sa'doni & Ferro, 2000). Nevertheless, since we also found that 250 U ml−1 SOD (which removes O2−) improved both ACh and A23187 responses, and did so to a similar degree as L-ascorbate, in the presence of all LDL species, it is likely that oxidative stress is indeed important in the causation of LDL-induced endothelial dysfunction, and also that the beneficial effect of L-ascorbate on endothelium-dependent responses is indeed due to scavenging of reactive oxygen species. Our data therefore support the notion that nLDL, dLDL and oxLDL exert their inhibitory effects on endothelial function both through suppression of NO synthesis and enhancement of oxidative stress.
In the case of pyridoxine, a progressive improvement in both ACh and A23187 responses was seen in the concentration range 0.5–2.0 mM. The highest concentration of pyridoxine improved ACh and A23187 responses to a similar degree as did 10−4 M L-arginine or L-ascorbate, suggesting that homocysteine may play an important role in LDL-induced endothelial dysfunction. On the other hand, pyridoxine has been previously demonstrated to exert other effects that may improve endothelial function, namely inhibition of O2− formation and of lipid peroxidation (Jain & Lim, 2001), and scavenging of singlet oxygen (Ohta & Foote, 2002). The precise mechanism by which pyridoxine improves endothelium-dependent vasorelaxation in our model therefore remains to be determined.
The combination of L-arginine 10−4 M, L-ascorbate 10−5 M and pyridoxine 2.0 mM was no more effective in improving ACh responses, in the presence of nLDL, dLDL or oxLDL, than was L-arginine 10−4 M alone. This suggests that oxidative stress plays a minor role in LDL-induced endothelial dysfunction when the NOS substrate L-arginine is present in excess. Indeed, it has been previously shown that LDL can uncouple endothelial cell NOS from NO generation, such that it generates O2−, and that the enzyme can be recoupled to NO formation by supplementation with L-arginine (Pritchard et al., 1995). Our experiments therefore lend support to the view that nLDL, oxLDL and dLDL uncouple endothelial NOS in this way, and that L-arginine supplementation both increases NO generation and decreases O2− production. Furthermore, since pyridoxine was as effective in restoring vascular ACh and A23187 responses as L-arginine or L-ascorbate, in the presence of any of our LDL preparations, it is possible that LDL induces accumulation of homocysteine, which is predominantly acting through suppression of NO production rather than through generation of reactive oxygen species. In agreement with this, hyperhomocysteinemia has been previously demonstrated to reduce both L-arginine uptake and NOS activity in vascular endothelial cells (Jin et al., 2001). On the other hand, pyridoxine may be exerting antioxidant effects, as discussed above. It is worth noting that previous reports have suggested that pyridoxine analogues may chelate metal ions (Kesel et al., 1999), such as Ca2+ (an essential cofactor for the endothelial isoform of NOS), Fe2+ (essential for soluble guanylyl cyclase function) and Zn2+ (essential for the activity of endogenous cytosolic Cu–Zn SOD), and that L-arginine and pyridoxine may exert mutually antagonistic effects in a mouse model of nociception (Abacioglu et al., 2001); such effects may contribute to the lack of additive action by pyridoxine seen in the present study.
LDL may exert additional inhibitory effects on endothelial function that are unrelated to NO generation or oxidative stress, particularly when lipid peroxidation is prominent. The major component of oxidised forms of LDL that inhibits endothelial function is believed to be lysophosphatidylcholine, since removal of lysophosphatidylcholine with phospholipase B or albumin renders oxLDL inactive in this respect (Kugiyama et al., 1990; Yokoyama et al., 1990; Mangin et al., 1993), and conversely direct treatment of arterial rings with lysophosphatidylcholine mimics the inhibitory effect of oxLDL on endothelium-dependent relaxation (Kugiyama et al., 1990; Yokoyama et al., 1990; Tanner et al., 1991; Mangin et al., 1993). Although it is generally held that endothelium-derived hyperpolarising factor (EDHF) is of lesser importance in mediating endothelium-dependent vasorelaxation in large arteries as compared with resistance vessels, lysophosphatidylcholine has been previously demonstrated to inhibit relaxation not only to endothelium-derived NO but also to EDHF in rabbit aorta (Cowan & Steffen, 1995). Our results are consistent with these findings, since L-arginine was capable of restoring endothelium-dependent relaxations almost to normal in the presence of nLDL, whereas it was much less effective in this regard in the presence either of dLDL or of oxLDL, both of which exhibit a greater degree of lipid peroxidation. It is likely, therefore, that nLDL, dLDL and oxLDL all inhibit endothelial NO-dependent relaxation of rabbit aorta, which is reversible by L-arginine, pyridoxine and L-ascorbate, but that they additionally inhibit EDHF-dependent relaxation, especially in the case of dLDL and oxLDL.
Our study is the first to demonstrate similar effects of L-arginine and of L-ascorbate on endothelial function in arterial rings exposed to nLDL, dLDL or oxLDL. It is also the first to show that pyridoxine can favourably affect LDL-induced endothelial dysfunction. Our results suggest that combining L-arginine with L-ascorbate and pyridoxine is unlikely to produce additional benefit on endothelial function as compared with L-arginine alone; if this is borne out by future studies of combinations of such supplements on endothelial function in vivo, this will have important implications for therapy in patients with vascular disease and endothelial dysfunction.
In conclusion, our experiments confirm that nLDL, dLDL and oxLDL exert inhibitory effects on endothelium-dependent, but not endothelium-independent, relaxation of rabbit aorta, and that these effects are more marked for oxLDL and dLDL than for nLDL. Their inhibitory effects on endothelium-mediated vasorelaxation are largely mediated through a decrease in NO availability, both through suppression of NO synthesis and augmentation of NO scavenging by reactive oxygen species, and can be ameliorated by supplementation with L-arginine, L-ascorbate or pyridoxine, either singly or in combination. In addition, there exists a component of inhibition of endothelium-mediated vasorelaxation that is not ameliorated by these agents, particularly in the presence of dLDL and oxLDL. Whether the favourable effects of L-arginine, L-ascorbate and pyridoxine on endothelial function translate into clinical benefit remains to be proven.
Acknowledgments
Yong Ji is funded by a Wellcome Trust Travelling Research Fellowship.
Abbreviations
- ACh
acetylcholine
- dLDL
native low-density lipoprotein from type II diabetic subjects
- EDHF
endothelium-derived hyperpolarising factor
- LDL
low-density lipoprotein
- nLDL
native low-density lipoprotein from healthy subjects
- NO
nitric oxide
- NOS
nitric oxide synthase
- NP
sodium nitroprusside
- oxLDL
oxidised nLDL
- TBARS
thiobarbituric acid reactive substances
References
- ABACIOGLU N., TUNCTAN B., CAKICI I., AKBULUT E., ULUDAG O., KANZIK I. The role of L-arginine/nitric oxide pathway in the antinociceptive activity of pyridoxine in mouse. Arzneimittelforschung. 2001;51:832–838. doi: 10.1055/s-0031-1300122. [DOI] [PubMed] [Google Scholar]
- AL-SA'DONI H., FERRO A. S-Nitrosothiols: a class of nitric oxide-donor drugs. Clin. Sci. 2000;98:507–520. [PubMed] [Google Scholar]
- ANDERSON T.J. Nitric oxide, atherosclerosis and the clinical relevance of endothelial dysfunction. Heart Fail Rev. 2003;8:71–86. doi: 10.1023/a:1022199021949. [DOI] [PubMed] [Google Scholar]
- ARTWOHL M., GRAIER W.F., RODEN M., BISCHOF M., FREUDENTHALER A., WALDHAUSL W., BAUMGARTNER-PARZER S.M. Diabetic LDL triggers apoptosis in vascular endothelial cells. Diabetes. 2003;52:1240–1247. doi: 10.2337/diabetes.52.5.1240. [DOI] [PubMed] [Google Scholar]
- BAKER T.A., MILSTIEN S., KATUSIC Z.S. Effect of vitamin C on the availability of tetrahydrobiopterin in human endothelial cells. J. Cardiovasc. Pharmacol. 2001;37:333–338. doi: 10.1097/00005344-200103000-00012. [DOI] [PubMed] [Google Scholar]
- BECKMAN J.S., KOPPENOL W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CAI H., HARRISON D.G. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- CASINO P.R., KILCOYNE C.M., CANNON R.O., III, QUYYUMI A.A., PANZA J.A. Impaired endothelium-dependent vascular relaxation in patients with hypercholesterolemia extends beyond the muscarinic receptor. Am. J. Cardiol. 1995;75:40–44. doi: 10.1016/s0002-9149(99)80524-4. [DOI] [PubMed] [Google Scholar]
- CELERMAJER D.S., SORENSEN K.E., BULL C., ROBINSON J., DEANFIELD J.E. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J. Am. Coll. Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- CHIN J.H., AZHAR S., HOFFMAN B.B. Inactivation of endothelial derived relaxing factor by oxidized lipoproteins. J. Clin. Invest. 1992;89:10–18. doi: 10.1172/JCI115549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOWIENCZYK P.J., WATTS G.F., COCKCROFT J.R., RITTER J.M. Impaired endothelium-dependent vasodilation of forearm resistance vessels in hypercholesterolaemia. Lancet. 1992;340:1430–1432. doi: 10.1016/0140-6736(92)92621-l. [DOI] [PubMed] [Google Scholar]
- CHUNG B.H., WILKINSON T., GEER J.C., SEGREST J.P. Preparative and quantitative isolation of plasma lipoproteins: rapid, single discontinuous density gradient ultracentrifugation in a vertical rotor. J. Lipid Res. 1980;21:284–291. [PubMed] [Google Scholar]
- CLARKSON P., ADAMS M.R., POWE A.J., DONALD A.E., MCCREDIE R., ROBINSON J., MCCARTHY S.N., KEECH A., CELERMAJER D.S., DEANFIELD J.E. Oral L-arginine improves endothelium-dependent dilation in hypercholesterolaemic young adults. J. Clin. Invest. 1996;97:1989–1994. doi: 10.1172/JCI118632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWAN C.L., STEFFEN R.P. Lysophosphatidylcholine inhibits relaxation of rabbit abdominal aorta mediated by endothelium-derived nitric oxide and endothelium-derived hyperpolarizing factor independent of protein kinase C activation. Arterioscler. Thromb. Vasc. Biol. 1995;15:2290–2297. doi: 10.1161/01.atv.15.12.2290. [DOI] [PubMed] [Google Scholar]
- CREAGER M.A., GALLAGHER S.J., GIRERD X.J., COLEMAN S.M., DZAU V.J., COOKE J.P. L-Arginine improves endothelium-dependent vasodilation in hypercholesterolemic humans. J. Clin. Invest. 1992;90:1248–1253. doi: 10.1172/JCI115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREXLER H., ZEIHER A.M., MEINZER K., JUST H. Correction of endothelial dysfunction in the coronary microcirculation of hypercholesterolaemic patients by L-arginine. Lancet. 1991;338:1546–1550. doi: 10.1016/0140-6736(91)92372-9. [DOI] [PubMed] [Google Scholar]
- FONTANA L., MCNEILL K.L., RITTER J.M., CHOWIENCZYK P.J. Effects of vitamin C and of a cell permeable superoxide dismutase mimetic on acute lipoprotein induced endothelial dysfunction in rabbit aortic rings. Br. J. Pharmacol. 1999;126:730–734. doi: 10.1038/sj.bjp.0702331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLE J., MÜLSCH A., BUSSE R., BASSENGE E. Effects of native and oxidized low density lipoproteins on formation and inactivation of EDRF. Arterioscler. Thromb. 1991;11:198–203. doi: 10.1161/01.atv.11.1.198. [DOI] [PubMed] [Google Scholar]
- GALLE J., SCHNEIDER R., WINNER B., LEHMANN-BODEM C., SCHINZEL R., MUNCH G., CONZELMANN E., WANNER C. Glyc-oxidized LDL impair endothelial function more potently than oxidized LDL: role of enhanced oxidative stress. Atherosclerosis. 1998;138:65–77. doi: 10.1016/s0021-9150(98)00005-7. [DOI] [PubMed] [Google Scholar]
- JAIN S.K., LIM G. Pyridoxine and pyridoxamine inhibits superoxide radicals and prevents lipid peroxidation, protein glycosylation, and (Na++K+)-ATPase activity reduction in high glucose-treated human erythrocytes. Free Radic. Biol. Med. 2001;30:232–237. doi: 10.1016/s0891-5849(00)00462-7. [DOI] [PubMed] [Google Scholar]
- JIN L., ABOU-MOHAMED G., CALDWELL R.B., CALDWELL R.W. Endothelial cell dysfunction in a model of oxidative stress. Med. Sci. Monit. 2001;7:585–591. [PubMed] [Google Scholar]
- KESEL A.J., SONNENBICHLER I., POLBORN K., GURTLER L., KLINKERT W.E., MODOLELL M., NUSSLER A.K., OBERTHUR W. A new antioxidative vitamin B6 analogue modulates pathophysiological cell proliferation and damage. Bioorg. Med. Chem. 1999;7:359–367. doi: 10.1016/s0968-0896(98)00229-6. [DOI] [PubMed] [Google Scholar]
- KINLAY S., GANZ P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am. J. Cardiol. 1997;80:11I–16I. doi: 10.1016/s0002-9149(97)00793-5. [DOI] [PubMed] [Google Scholar]
- KUGIYAMA K., KERNS S.A., MORRISETT J.D., ROBERTS R., HENRY P.D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990;344:160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- LUDMER P.L., SELWYN A.P., SHOOK T.L., WAYNE R.R., MUDGE G.H., ALEXANDER R.W., GANZ P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N. Engl. J. Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- MANGIN E.L., KUGIYAMA K., NGUY J.H., KERNS S.A., HENRY P.D. Effects of lysolipids and oxidatively modified low density lipoprotein on endothelium-dependent relaxation of rabbit aorta. Circ. Res. 1993;72:161–166. doi: 10.1161/01.res.72.1.161. [DOI] [PubMed] [Google Scholar]
- MCNEILL K.L., FONTANA L., RUSSELL-JONES D.L., RAJMAN I., RITTER J.M., CHOWIENCZYK P.J. Inhibitory effects of low-density lipoproteins from men with type II diabetes on endothelium-dependent relaxation. J. Am. Coll. Cardiol. 2000;35:1622–1627. doi: 10.1016/s0735-1097(00)00607-0. [DOI] [PubMed] [Google Scholar]
- MINER S.E., COLE D.E., EVROVSKI J., FORREST Q., HUTCHISON S., HOLMES K., ROSS H.J. Pyridoxine improves endothelial function in cardiac transplant recipients. J. Heart Lung Transplant. 2001;20:964–969. doi: 10.1016/s1053-2498(01)00293-5. [DOI] [PubMed] [Google Scholar]
- OHKAWA H., OHISHI N., YAGI K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- OHTA B.K., FOOTE C.S. Characterization of endoperoxide and hydroperoxide intermediates in the reaction of pyridoxine with singlet oxygen. J. Am. Chem. Soc. 2002;124:12064–12065. doi: 10.1021/ja0205481. [DOI] [PubMed] [Google Scholar]
- POSCH K., SIMECEK S., WASCHER T.C., JURGENS G., BAUMGARTNER-PARZER S., KOSTNER G.M., GRAIER W.F. Glycated low-density lipoprotein attenuates shear stress-induced nitric oxide synthesis by inhibition of shear stress-activated L-arginine uptake in endothelial cells. Diabetes. 1999;48:1331–1337. doi: 10.2337/diabetes.48.6.1331. [DOI] [PubMed] [Google Scholar]
- PRITCHARD K.A., JR, GROSZEK L., SMALLEY D.M., SESSA W.C., WU M., VILLALON P., WOLIN M.S., STEMERMAN M.B. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ. Res. 1995;77:510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- ROSS R. Atherosclerosis; an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- SOBAL G., MENZEL J., SINZINGER H. Why is glycated LDL more sensitive to oxidation than native LDL? A comparative study. Prostaglandins Leukot. Essent. Fatty Acids. 2000;63:177–186. doi: 10.1054/plef.2000.0204. [DOI] [PubMed] [Google Scholar]
- TANNER F.C., NOLL G., BOULANGER C.M., LÜSCHER T.F. Oxidized low density lipoproteins inhibit relaxations of porcine coronary arteries. Circulation. 1991;83:2012–2020. doi: 10.1161/01.cir.83.6.2012. [DOI] [PubMed] [Google Scholar]
- VITA J.A., TREASURE C.B., NABEL E.G., McLENACHAN J.M., FISH R.D., YEUNG A.C., VEKSHTEIN V.I., SELWYN A.P., GANZ P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- YOKOYAMA M., HIRATA K., MIYAKE R., AKITA H., ISHIKAWA Y., FUKUZAKI H. Lysophosphatidylcholine: essential role in the inhibition of endothelium-dependent vasorelaxation by oxidized low density lipoprotein. Biochem. Biophys. Res. Commun. 1990;168:301–308. doi: 10.1016/0006-291x(90)91708-z. [DOI] [PubMed] [Google Scholar]