Abstract
An increased incidence of systemic hypertension has been documented in postmenopausal women and identified as an independent risk factor in the development of cerebrovascular stroke. The present study examined whether cerebrovascular reactivity was increased in the hypertensive ovariectomized rat, and explored the potential therapeutic benefit of the partial estrogen receptor agonist tamoxifen.
Female Sprague–Dawley rats were subjected to bilateral ovariectomy (OVX, n=16) or a sham operation (n=8). At 6-week postsurgery, rats were anesthetized to assess ventricular contractility and blood pressure. In a second series of experiments, OVX rats (n=8) were given tamoxifen starting 3 weeks postsurgery, and continued for 3 weeks. At the end of each protocol, the middle cerebral artery was harvested and rings were mounted in wire-myographs to measure isometric tension.
Systolic arterial pressure (SAP) was significantly increased (P<0.05) in the OVX rat (174±8 mmHg), as compared to sham (135±6 mmHg). The resting tension of isolated cerebral arteries from OVX rats (186±15 mg) was significantly elevated (P<0.05), as compared to sham (129±9 mg). Phenylephrine treatment did not elicit a constriction of cerebral arteries isolated from sham rats, whereas a potent response (P<0.05) was observed in OVX rats. Nitric oxide (NO) synthase inhibition with L-NNA led to a limited contraction in sham rats (8±3% of Emax), whereas a significant (P<0.05) increase was observed in OVX rats (34±12% of Emax). Lastly, vascular sensitivity (pD2) to sodium nitroprusside was significantly increased (P<0.05) in OVX rats, as compared to sham.
Tamoxifen therapy normalized the resting tension of isolated cerebral arteries from OVX rats, abrogated phenylephrine-mediated contraction, and modestly reduced SAP. By contrast, tamoxifen treatment of OVX rats did not attenuate L-NNA-mediated contractile response of cerebral arteries.
These data demonstrate that the cerebral artery isolated from the OVX rat was associated with an exaggerated vasoconstrictor response to phenylephrine, and altered NO-dependent vascular reactivity. The administration of tamoxifen to OVX rats normalized cerebral artery reactivity to phenylephrine. These findings provide the impetus to examine the potential therapeutic benefit of the partial estrogen receptor agonist tamoxifen to reduce the incidence of cerebrovascular stroke in postmenopausal women.
Keywords: Cerebral arteries, endothelin-1, α1-adrenergic receptors, nitric oxide, tamoxifen, ovariectomy
Introduction
Cerebrovascular stroke and coronary artery disease are two salient complications associated with menopause (Matthews et al., 1989; Greendale et al., 1999; Simon et al., 2001). An increased incidence of systemic hypertension has been documented in postmenopausal women and identified as an independent risk factor in the development of cerebrovascular stroke and coronary artery disease (Kannel et al., 1976; Stokes et al., 1987; Laurent et al., 2001; Reckelhoff, 2001). Based on these findings, hormonal replacement therapy (HRT; estrogen and progesterone regiment) in postmenopausal women has received considerable interest. However, at least two independent clinical studies have shown that long-term HRT in either normal postmenopausal women or those with established coronary heart disease failed to demonstrate an overall cardioprotective action or reduce the incidence of stroke (Hulley et al., 1998; Simon et al., 2001; Rossouw et al., 2002). Moreover, these latter clinical studies further highlighted that HRT was associated with the increased incidence of thromboembolism, coronary artery disease, as well as invasive breast cancer (Hulley et al., 1998; Rossouw et al., 2002).
Tamoxifen and raloxifene are representative of a family of molecules designated as selective estrogen receptor modulators (SERM). These molecules interact with the estrogen receptor, but are pharmacologically different from estrogen via its ability to act either as an agonist or antagonist depending on the target tissue and hormonal milieu. Tamoxifen is an effective antagonist in the treatment of estrogen-dependent breast cancer, whereas in bone, both tamoxifen and raloxifene mimic the beneficial action of estrogen (MacGregor & Jordan, 1998). Akin to estrogen, tamoxifen treatment promoted coronary artery relaxation, acutely and chronically (Jiang et al., 1991; Mugge et al., 1993; Chester et al., 1995; Sudhir et al., 1995). In vivo studies have demonstrated that SERM therapy reduced identifiable cardiovascular risk factors, including serum cholesterol, LDL, and fibrinogen B (Bjarnason et al., 1997; MacGregor & Jordan, 1998; Walsh et al., 1998; Clarke et al., 2001). Surprisingly, and in contrast to HRT, the incidence of coronary artery disease and fatal MI in breast cancer patients receiving tamoxifen were reduced, as compared to placebo (McDonald & Stewart, 1991; McDonald et al., 1995). In the Multiple Outcomes of Raloxifene Evaluation (MORE) study, raloxifene treatment significantly reduced the incidence of cardiovascular events in a subset of women characterized with increased cardiovascular risk (Barrett-Connor et al., 2001). Thus, SERM therapy may represent a surrogate approach to HRT in postmenopausal women.
A review of 14 prospective, randomized, controlled trials demonstrated a 42% risk reduction of stroke when the diastolic arterial pressure (DAP) was reduced by 5–6 mmHg (Collins et al., 1990). The Systolic Hypertension in the Elderly Program (SHEP) study found that treating isolated systolic hypertension in the elderly reduced stroke by 36% (SHEP Cooperative Research Group, 1991). These studies support a link between hypertension and stroke, albeit whether a similar relationship exists between hypertension-related menopause and cerebrovascular reactivity remains undefined. We have previously demonstrated that mean arterial pressure was significantly elevated in the ovariectomized (OVX) female rat, and normalized following the administration of an ETA receptor antagonist (Mercier et al., 2002). In this regard, the present study tested the hypothesis that cerebrovascular reactivity was altered in the hypertensive OVX female rat. Second, the potential therapeutic benefit of the partial estrogen receptor agonist tamoxifen on cerebral artery reactivity in the OVX rat was examined.
Methods
Ovariectomy model
Female Sprague–Dawley rats (9–11 weeks; Charles River, St Constant, Quebec, Canada) were anesthetized with a ketamine (50 mg kg−1)/xylazine (10 mg kg−1) mixture and underwent either a sham surgery or bilateral ovariectomy. In a parallel study, OVX rats (n=8) were given tamoxifen (10 mg kg−1 day−1) in standard rat chow starting 3 weeks postsurgery, and continued for 3 weeks. Previous studies have demonstrated that mean arterial pressure was elevated and significant uterine atrophy was evident in 3 week OVX rats (Mercier et al., 2002). At 6 weeks postsurgery, rats were anesthetized and hemodynamic parameters assessed, as previously described (Mercier et al., 2002). Briefly, rats were anesthetized with a ketamine (50 mg kg−1)/xylazine (10 mg kg−1) mixture. A microtip pressure transducer catheter (model SPR-407, 2F; Millar Instrument, Houston, TX, U.S.A.) was inserted in the carotid artery and advanced into the left ventricle. Systolic arterial pressure (SAP) and diastolic arterial pressure (DAP) were measured in the carotid artery prior to entry into the left ventricle. The pressures were recorded on a Gould 2600S recorder (Gould Instruments, Valley View, OH, U.S.A.). The uterus (uterine horn) was excised, immediately weighed, and rat middle cerebral arteries (MCA) were subsequently harvested from the brain. The use and care of laboratory animals was according to the Canadian Council for Animal Care and approved by the Animal Care Committee of the Montreal Heart Institute.
Isometric tension recording in isolated cerebral arteries
Following hemodynamic measurements, rat MCA were harvested from the brain and placed in ice-cold physiological salt solution (PSS) containing indomethacin (10 μmol l−1, cyclooxygenase inhibitor) and the following composition (mmol l−1): NaCl 130, KCl 4.7, KH2PO4 1.18, MgSO4 1.17, NaHCO3 14.9, CaCl2 1.6, EDTA 0.026, glucose 10 and aerated with 12% O2/5% CO2/83% N2 (pH 7.4). Segments 2 mm in length were mounted on 17 μm tungsten wires in microvessels myographs (IMF, University of Vermont, VT, U.S.A.) as previously described (Thorin et al., 1998b). Arterial segments were stretched (300 mg) and permitted to reach their resting tension. This resting tension was optimal to induce a stable and reproducible contraction to 40 mmol l−1 KCl-PSS. Resting tension was not affected by a Ca2+-free PSS containing 3 mmol l−1 EGTA and 3 μmol l−1 sodium nitroprusside (SNP) (data not shown). To prepare K+-rich solutions, equimolar amounts of NaCl were replaced with KCl.
Statistical analysis
Results are expressed as mean±s.e. In all experiments, n represents the number of rats. Contractions are expressed as a percentage of Emax obtained in the presence of 127 mmol l−1 KCl-PSS at the end of each experiment. Relaxations are expressed as a percentage of the initial precontraction. Half-maximum effective concentrations (EC50) of agonist-induced responses were measured from each individual dose–response curve using a logistic curve-fitting program (Allfit®, Dr Deléan, University of Montréal). The pD2 value is the negative log of the EC50. Statistical differences between means were determined by analysis of variance followed by a Scheffé's F test or a Student's t test when only two groups were compared. A probability value of <0.05 was accepted as significant for differences between groups of data. Hemodynamic measurements were analyzed by a two-way ANOVA and a significant difference was determined by the Scheffé's F test.
Chemicals
The following drugs were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.) indomethacin, N-nitro-L-arginine (L-NNA), phenylephrine, SNP, tamoxifen. Endothelin-1 was purchased from American Peptide Company (Sunnyvale, CA, U.S.A.). All drugs were dissolved in PSS except for indomethacin that was dissolved in ethanol; final concentration of ethanol in the bath was 0.1% (vol vol−1). Solutions were prepared freshly every day and kept on ice.
Results
Body weight, uterus atrophy, hemodynamic measurements, and the effect of tamoxifen
In 6-week OVX rats, an increase in body weight and uterus atrophy was evident, as compared to sham rats (Table 1). The administration of tamoxifen to OVX rats reversed the body weight gain, and partially attenuated uterus atrophy (Table 1). OVX significantly (P<0.05) increased SAP and DAP (Table 1). Left ventricular systolic pressure (LVSP), dp/dt indices, and left ventricular end diastolic pressure (LVEDP) were significantly increased in the OVX female rat, as compared to sham (Table 1). Tamoxifen treatment of OVX rats normalized LVEDP, −dp/dt, modestly reduced LVSP, +dp/dt, SAP, and had no effect on DAP (Table 1).
Table 1.
Hemodynamic parameters, body and uterus weights of the sham, ovarijectomized (OVX), and tamoxifen-treated OVX (OVX+TAM) rat
| Groups | n | Body weight (g) | Uterus weight (g) | DAP (mmHg) | SAP (mmHg) | LVSP (mmHg) | +dp/dt (mmHg s−1) | −dp/dt (mmHg s−1) | LVEDP (mmHg) |
|---|---|---|---|---|---|---|---|---|---|
| Sham | 8 | 280±9 | 0.50±0.02 | 96±2 | 135±6 | 116±4 | 6327±270 | 5331±282 | 6.3±0.8 |
| OVX | 8 | 362±11* | 0.11±0.01* | 114±3* | 174±8* | 150±6* | 7759±360* | 7032±284* | 10.1±1.0* |
| OVX+TAM | 8 | 278±8† | 0.22±0.01*† | 109±3 | 150±2 | 133±14 | 6750±267 | 5531±300† | 5.6±0.6† |
Results are expressed as mean±s.e.
:P<0.05 versus Sham;
P<0.05 versus OVX (ANOVA, Scheffé's F test).
Cerebral vascular reactivity and the effect of tamoxifen
At 6 weeks post ovariectomy, the resting tension and the Emax of isolated cerebral arteries were significantly (P<0.05) increased, and both parameters normalized with tamoxifen therapy (Table 2). The inhibition of nitric oxide (NO) synthase with L-NNA (100 μmol l−1) increased vascular tone in both groups, albeit the increase was quantitatively greater in the OVX rat, and was not reversed with tamoxifen treatment (Table 2). Lastly, a depolarizing PSS containing 40 mmol l−1 KCl induced a greater contraction of arteries isolated from OVX rats, as compared to sham rats, and tamoxifen therapy was without effect (Table 2).
Table 2.
The internal diameter (i.d.) and resting tension of isolated segments of MCA, and the contractile response to either 127 mmol l−1 KCl-PSS (Emax), 40 mmol l−1 KCl-PSS (40 K+), or N-nitro-L-arginine (L-NNA) of sham-operated (Sham), ovariectomized (OVX), and tamoxifen-treated OVX (OVX+TAM) rats
| Groups | n | i.d. (μm) | Resting tension (mg) | Emax (mg) | 40 K+ (%Emax) | L-NNA (%Emax) |
|---|---|---|---|---|---|---|
| Sham | 8 | 167±5 | 129±7 | 500±35 | 53±5 | 8±3 |
| OVX | 8 | 178±9 | 186±15* | 618±51* | 72±4* | 34±12* |
| OVX+TAM | 8 | 180±9 | 142±12† | 525±54† | 76±4* | 23±12 |
Results are expressed as mean±s.e. All PSS contained indomethacin (10 μmol l−1).
P<0.05 versus Sham;
P<0.05 versus OVX (ANOVA, Scheffé's F test).
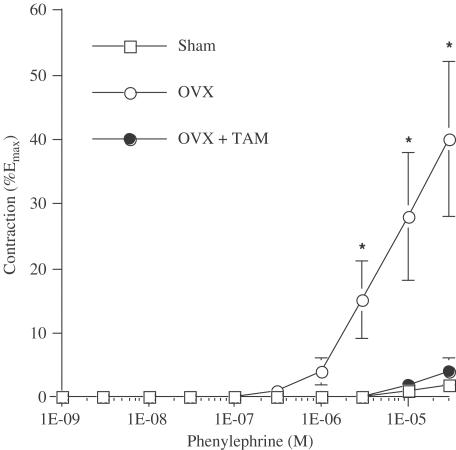
Phenylephrine treatment did not elicit a contraction of the isolated rat cerebral artery in the sham rat with an intact endothelium (Figure 1). By contrast, the α1-adrenoceptor agonist induced a potent contraction (P<0.05) of the MCA in the OVX rat, and was prevented with tamoxifen therapy (P<0.05). Following the administration of L-NNA, phenylephrine induced a potent contraction in all groups with a maximal response representing 57±6, 54±9, and 68±8% of Emax in sham-operated, OVX and OVX+tamoxifen rats, respectively. The vascular sensitivity (pD2 values) to phenylephrine was increased in arteries isolated from OVX rats and not reduced by tamoxifen (Table 3).
Figure 1.
Phenylephrine reactivity of cerebral arteries isolated from sham-operated (Sham; n=8), ovariectomized (OVX; n=8) rats, and tamoxifen-treated OVX (OVX+TAM; n=8) rats. Results are mean±s.e. *: P<0.05 versus all other groups (ANOVA, Scheffé's F test).
Table 3.
Sensitivity (pD2) of cerebral arteries isolated from sham-operated (Sham), ovariectomized (OVX), and tamoxifen-treated OVX (OVX+TAM) rats to phenylephrine (in the presence of L-NNA), endothelin-1, and SNP
| Groups | Phenylephrine | Endothelin-1 | SNP |
|---|---|---|---|
| Sham | 6.00±0.26 | 8.30±0.09 | 6.23±0.18 |
| OVX | 6.61±0.15* | 8.60±0.08* | 6.72±0.16* |
| OVX+TAM | 6.67±0.16* | 8.40±0.06 | 6.66±0.13* |
Results are expressed as mean±s.e. All PSS contained indomethacin (10 μmol l−1).
P<0.05 versus Sham (Student's t test).
Endothelin-1 induced a potent contraction of isolated cerebral arteries with an intact endothelium. The maximal contraction induced by the peptide was similar between sham-operated (105±5% of Emax), OVX (112±6% of Emax) and OVX+tamoxifen rats (102±3% of Emax). The sensitivity to endothelin-1 of arteries isolated from OVX rat was modestly increased and significant, as compared to arteries isolated from sham-operated rats (Table 3). This change in vascular sensitivity to endothelin-1 was reduced although not normalized by tamoxifen.
SNP-induced maximal relaxation was similar between sham (79±6%), OVX (89±3%) and OVX+tamoxifen (90±4%) rats. However, the vascular sensitivity to SNP was increased in arteries isolated from OVX rats and not normalized with tamoxifen therapy (Table 3).
Discussion
Clinical studies support the premise that postmenopausal women were associated with an increased incidence of stroke. Moreover, hypertension was prevalent in postmenopausal women, and represents an independent risk factor in the development of cerebrovascular stroke and coronary artery disease. Based on these observations, cerebrovascular reactivity may be altered during the progression of menopause, and thus may represent an underlying event contributing to the increased incidence of stroke. The present study has demonstrated that elevated blood pressure (SAP and DAP) in the OVX rat was associated with increased reactivity in response to contractile agents, phenylephrine (α1-AR agonist) and L-NNA (NO synthase blocker). Interestingly, whereas tamoxifen therapy abrogated phenylephrine-mediated vasoconstriction, and modestly reduced SAP, DAP and the hyper-reactivity to L-NNA remained elevated in the OVX rat.
It has been established that normal rat cerebral arteries were unresponsive to phenylephrine when the endothelium was intact (Thorin et al., 1998a). Likewise, in human cerebral arteries, norepinephrine induced a limited contraction, as compared to serotonin (Bevan et al., 1998; Thorin et al., 1998b). By contrast, in the presence of NO synthase blockade, phenylephrine induced a contraction of normal rat cerebral arteries (Thorin et al., 1998a). However, in the OVX rat, phenylephrine treatment promoted cerebral artery contraction in the absence of NO synthase inhibition, thereby supporting the premise that NO activity and/or synthesis was reduced. A strong predictive parameter to support this latter premise was the increased cerebrovascular sensitivity to SNP. It has been demonstrated that vascular smooth muscle sensitivity to NO negatively correlated to endogenous NO activity (Gilbert et al., 2001).
It is difficult to reconcile the exaggerated contractile response to L-NNA and the concomitant increased sensitivity to SNP of arterial rings isolated from OVX rats. However, it is important to note that baseline tension was Ca2+ independent, and thus arterial segments had no active tone at rest before the addition of L-NNA. Thus, the blockade of NO by L-NNA per se could not have contributed to the exaggerated contraction of cerebral arteries from OVX rats. The L-NNA-mediated inhibition of NO synthase may have provided the requisite environment for endothelium-derived contractile factors (EDCF) to induce a contraction (Nguyen et al., 1999; Gilbert et al., 2001). Conceivably, the augmented response to L-NNA may reflect a greater release of EDCF in the OVX rat. In this context, it is our contention that the increased vascular sensitivity in response to exogenous endothelin-1, high external K+, as well as to phenylephrine (in the presence of L-NNA) observed 6-weeks post-OVX may in part reflect increased EDCF production. In addition, the enhanced vascular sensitivity to neurotransmitters and hormones may have contributed to the rise in systemic blood pressure. This view is strengthened by our recent report demonstrating that ETA receptor inhibition prevented the rise in blood pressure associated with OVX (Mercier et al., 2002). Endothelin-1 is a likely EDCF candidate considering its important regulatory role in human and rat pial arteries (Thorin et al., 1998a, 1998b).
In OVX rats, tamoxifen behaved primarily as a partial estrogen receptor agonist as increased body weight was normalized, and uterus atrophy was partially reversed. Moreover, tamoxifen therapy normalized the increased baseline tension of cerebral arteries, and abrogated the exaggerated phenylephrine-mediated contraction in the OVX rat. By contrast, tamoxifen treatment caused a modest decrease of SAP, was without effect on elevated DAP, and failed to inhibit either high K+ or L-NNA-mediated contractile response of the cerebral artery in the OVX rat. The two latter observations suggest that at least in the present study when tamoxifen was given 3 weeks post-OVX, the partial estrogen receptor agonist was unable to influence either vascular NO- or EDCF-dependent activity. At present, it is not possible to discern whether the beneficial effect of tamoxifen on cerebral vascular reactivity represented either a direct action or attributed to the modest secondary decrease of SAP.
In a recent study, Simon et al. (2002) reported that ⩾1-year tamoxifen therapy reduced carotid intima-media thickness in menopausal women, an effect highly significant with an impact on intima-media thickness of −70 μm. In the present study, the experimental model of ovariectomy was associated with elevated blood pressure, and in this regard significant cerebrovascular vessel remodelling may be prevalent (Hajdu et al., 1991; Hajdu & Baumbach, 1994). Thus, it is tempting to speculate that changes in vessel structure may have contributed to the augmented resting tension and hyper-reactivity of cerebral arteries from OVX rats. Moreover, tamoxifen therapy normalized baseline tension and phenylephrine responsiveness of cerebral arteries from OVX rats, and partially reduced systolic blood pressure. It remains to be determined whether the beneficial action of tamoxifen may have occurred in part via the direct modulation of vessel remodelling.
Lacunar infarcts, which may account for a large proportion of strokes, are related to small vessel disease in the brain. Hypertension is a major risk factor in the pathogenesis of this form of vascular disease (Sacco et al., 1997). The premise that HRT was an effective therapeutic approach in preventing stroke has been examined in several observational studies, and the conclusions remain equivocal (Tolbert & Oparil, 2001). Moreover, as previously mentioned, several recent clinical studies have demonstrated that HRT (estrogen and progesterone regiment) was ineffective in the primary prevention of coronary artery disease in postmenopausal women (Hulley et al., 1998; Rossouw et al., 2002). It has been suggested that the coadministration of progesterone may limit the cardioprotective action of estrogen. Indeed, in surgically postmenopausal monkeys fed an atherogenic diet, coronary artery responsiveness to acetylcholine was improved in the estrogen-treated animals, whereas an amelioration was not observed in the estrogen–progesterone-treated group (Suparto et al., 2003). In the present study, the administration of the partial estrogen receptor agonist tamoxifen in the ovariectomized rat markedly improved cerebral vascular reactivity. Thus, this study at least in part supports the concept that the activation of an estrogen receptor-dependent pathway exerts a cardioprotective action. Lastly, although extrapolation from the experimental to the clinical setting is always subject to caution, the data of the present study suggest that SERMs may represent an alternative therapeutic approach in the treatment of cerebrovascular stroke in postmenopausal women.
Acknowledgments
This work was supported by the Heart and Stroke Foundation of Canada and Quebec, Canadian Institutes of Health Research, and Les Fonds de Recherche de l'Institut de Cardiologie de Montréal. A. Calderone is a Chercheur-Boursier Senior de la Fonds de Recherche de la Sante de Quebec. Eric Thorin is a research scholar of the Heart & Stroke Foundation of Canada.
Abbreviations
- DAP
diastolic arterial pressure
- HRT
hormonal replacement therapy
- L-NNA
N-nitro-L-arginine
- LVEDP
left ventricular end diastolic pressure
- LVSP
left ventricular systolic pressure
- MCA
middle cerebral arteries
- NO
nitric oxide
- OVX
ovariectomized
- SAP
systolic arterial pressure
- SNP
sodium nitroprusside
References
- BARRETT-CONNOR E., GRADY D., SASHEGYI A., ANDERSON P.W., COX D.A., HOSZOWSKI K., RAUTAHARJU P., HARPER K.D. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2001;287:847–857. doi: 10.1001/jama.287.7.847. [DOI] [PubMed] [Google Scholar]
- BEVAN R.D., DODGE J., NICHOLS P., PENAR P.L., WALTERS C.L., WELLMAN T., BEVAN J.A. Weakness of sympathetic neural control of human pial compared with superficial temporal arteries reflects low innervation density and poor sympathetic responsiveness. Stroke. 1998;29:212–221. doi: 10.1161/01.str.29.1.212. [DOI] [PubMed] [Google Scholar]
- BJARNASON N.H., HAARBO J., BYRJALSEN I., KAUFFMAN R.F., CHRISTIANSEN C. Raloxifen inhibits aortic accumulation of cholesterol in ovariectomized, cholesterol-fed rabbits. Circulation. 1997;96:1964–1969. doi: 10.1161/01.cir.96.6.1964. [DOI] [PubMed] [Google Scholar]
- CHESTER A.H., JIANG C., BORLAND J.A., YACOUB M.H., COLLINS P. Oestrogen relaxes human epicardial coronary arteries through non-endothelium-dependent mechanisms. Coron. Artery Dis. 1995;6:417–422. doi: 10.1097/00019501-199505000-00009. [DOI] [PubMed] [Google Scholar]
- CLARKE S.C., SHOFIELD P.M., GRACE A.A., METCALFE J.C., KIRSCHENLOHR H.L. Tamoxifen effects on endothelial function and cardiovascular risk factors in men with advanced atherosclerosis. Circulation. 2001;103:1497–1502. doi: 10.1161/01.cir.103.11.1497. [DOI] [PubMed] [Google Scholar]
- COLLINS R., PETO R., MACMAHON S., HEBERT P., FIEBACH N.H., EBERLEIN K.A., GODWIN J., QIZILBASH N., TAYLOR J.O., HENNEKENS C.H. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- GILBERT P., TREMBLAY J., THORIN E. Endothelium-derived endothelin-1 reduces cerebral artery sensitivity to nitric oxide by a protein kinase C-independent pathway. Stroke. 2001;32:2351–2355. doi: 10.1161/hs1001.096007. [DOI] [PubMed] [Google Scholar]
- GREENDALE G.A., LEE N.P., ARRIOLA E.R. The menopause. Lancet. 1999;353:571–580. doi: 10.1016/S0140-6736(98)05352-5. [DOI] [PubMed] [Google Scholar]
- HAJDU M.A., BAUMBACH G.L. Mechanics of large and small cerebral arteries in chronic hypertension. Am. J. Physiol. 1994;266:H1027–H1033. doi: 10.1152/ajpheart.1994.266.3.H1027. [DOI] [PubMed] [Google Scholar]
- HAJDU M.A., HEISTAD D.D., BAUMBACH G.L. Effects of antihypertensive therapy on mechanics of cerebral arterioles in rats. Hypertension. 1991;17:308–316. doi: 10.1161/01.hyp.17.3.308. [DOI] [PubMed] [Google Scholar]
- HULLEY S., GRADY D., BUSH T., FURBERG C., HERRINGTON D., RIGGS B., VITTINGHOFF E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- JIANG C.W., SARREL P.M., LINDSAY D.C., POOLE-WILSON P.A., COLLINS P. Endothelium-independent relaxation of rabbit coronary artery by 17 beta-oestradiol in vitro. Br. J. Pharmacol. 1991;104:1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANNEL W.B., MCGEE D., GORDON T. A general cardiovascular risk profile: the Framingham Study. Am. J. Cardiol. 1976;38:46–51. doi: 10.1016/0002-9149(76)90061-8. [DOI] [PubMed] [Google Scholar]
- LAURENT S., BOUTOUYRIE P., ASMAR R., GAUTIER I., LALOUX B., GUIZE L., DUCIMETIERE P., BENETOS A. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- MACGREGOR J.I., JORDAN V.C. Basic guide to the mechanisms of antiestrogen action. Pharmacol. Rev. 1998;50:152–196. [PubMed] [Google Scholar]
- MATTHEWS K.A., MEILAHN E., KULLER L.H., KELSEY S.F., CAGGIULA A.W., WING R.R. Menopause and risk factors for coronary heart disease. N. Engl. J. Med. 1989;321:641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- MCDONALD C.C., ALEXANDER F.E., WHYTE B.W., FORREST A.P., STEWART H.J. Cardiac and vascular morbidity in women receiving adjuvant tamoxifen for breast cancer in a fandomized trial. BMJ. 1995;311:977–980. doi: 10.1136/bmj.311.7011.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD C.C., STEWART H.J. Fatal myocardial infarction in the Scottish adjuvant tamoxifen trial. The Scottish breast cancer committee. BMJ. 1991;303:435–437. doi: 10.1136/bmj.303.6800.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERCIER I., PHAM-DANG M., CLEMENT R., GOSSELIN H., COLOMBO F., ROULEAU J.L., CALDERONE A. Elevated mean arterial pressure in the ovariectomized rat was normalized by ET(A) receptor antagonist therapy: absence of cardiac hypertrophy and fibrosis. Br. J. Pharmacol. 2002;136:685–692. doi: 10.1038/sj.bjp.0704765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUGGE A., RIEDEL M., BARTON M., KUHN M., LICHTLEN P.R. Endothelium independent relaxation of human coronary arteries by 17 beta-oestradiol in vitro. Cardiovasc. Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- NGUYEN T.-D., VÉQUAUD P., THORIN E. Effects of endothelin receptor antagonists and nitric oxide on myogenic tone and α1-adrenergic-dependent contraction of rabbit resistance arteries. Cardiovasc. Res. 1999;43:755–761. doi: 10.1016/s0008-6363(99)00170-4. [DOI] [PubMed] [Google Scholar]
- RECKELHOFF J.F. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- ROSSOUW J.E., ANDERSON G.L., PRENTICE R.L., LACROIX A.Z., KOOPERBERG C., STEFANICK M.L., JACKSON R.D., BERESFORD S.A., HOWARD B.V., JOHNSON K.C., KOTCHEN J.M., OCKENE J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principle results from the women's health initiative randomized control trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- SACCO R.L., BENJAMIN E.J., BRODERICK J.P., DYKEN M., EASTON J.D., FEINBERG W.M., GOLDSTEIN L.B., GORELICK P.B., HOWARD G., KITTNER S.J., MANOLIO T.A., WHISNANT J.P., WOLF P.A. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk Factors. Stroke. 1997;28:1507–1517. doi: 10.1161/01.str.28.7.1507. [DOI] [PubMed] [Google Scholar]
- SHEP Cooperative Research Group Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final Resuts of the Systolic Hypertension in the Elderly Program (SHEP) JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- SIMON J.A., HSIA J., CAULEY J.A., RICHARDS C., HARRIS F., FONG J., BARRETT–CONNOR E., HULLEY S.B. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen–Progestin Replacement Study (HERS) Circulation. 2001;103:638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- SIMON T., BOUTOUYRIE P., SIMON J.M., LALOUX B., TOURNIGAND C., TROPEANO A.I., LAURENT S., JAILLON P. Influence of tamoxifen on carotid intima-media thickness in postmenopausal women. Circulation. 2002;106:2925–2929. doi: 10.1161/01.cir.0000041044.93571.ca. [DOI] [PubMed] [Google Scholar]
- STOKES J., III, KANNEL W.B., WOLF P.A., CUPPLES L.A., D'AGOSTINO R.B. The relative importance of selected risk factors for various manifestations of cardiovascular disease among men and women from 35 to 64 years old: 30 years of follow-up in the Framingham Study. Circulation. 1987;75:V65–V73. [PubMed] [Google Scholar]
- SUDHIR K., CHOU T.M., MULLEN W.L., HAUSMANN D., COLLINS P., YOCK P.G., CHATTERJEE K. Mechanisms of estrogen-induced vasodilation: in vivo studies in canine coronary conductance and resistance arteries. J. Am. Coll. Cardiol. 1995;26:807–814. doi: 10.1016/0735-1097(95)00248-3. [DOI] [PubMed] [Google Scholar]
- SUPARTO I.H., WILLIAMS J.K., CLINE J.M., ANTHONY M.S., FOX J.L. Contrasting effects of two hormonal replacement therapies on the cardiovascular and mammary gland outcomes in surgically postmenopausal monkeys. Am. J. Obstet. Gynecol. 2003;188:1132–1140. doi: 10.1067/mob.2003.237. [DOI] [PubMed] [Google Scholar]
- THORIN E., CERNACEK P., DUPUIS J. Endothelin-1 regulates tone of isolated small arteries in the rat: effects of hyper-endothelinemia. Hypertension. 1998a;31:1035–1041. doi: 10.1161/01.hyp.31.4.1035. [DOI] [PubMed] [Google Scholar]
- THORIN E., NGUYEN T.D., BOUTHILLIER A. Control of vascular tone by endogenous endothelin-1 in human pial arteries. Stroke. 1998b;29:175–180. doi: 10.1161/01.str.29.1.175. [DOI] [PubMed] [Google Scholar]
- TOLBERT T., OPARIL S. Hormone replacement therapy and stroke: are the results surprising. Circulation. 2001;103:620–622. doi: 10.1161/01.cir.103.5.620. [DOI] [PubMed] [Google Scholar]
- WALSH B.W., KULLER L.H., WILD R.A., PAUL S., FARMER M., LAWRENCE J.B., SHAH A.S., ANDERSON P.W. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445–1451. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]