Abstract
Cyclic GMP phosphodiesterase-5 inhibitors have been shown to alter blood flow in specific tissues by potentiating local NO-dependent vasodilatory mechanisms. Since the haemodynamic effects of physiologic insulin, particularly capillary recruitment, may be critical for muscle glucose uptake in vivo and are blocked by inhibitors of nitric oxide synthase, we have explored the acute effects of the specific cGMP phosphodiesterase-5 inhibitor T-1032 on physiologic insulin action in anaesthetized healthy rats in vivo.
Whole-body glucose infusion (GIR), femoral blood flow (FBF), hind leg vascular resistance (VR), hind leg glucose uptake (HGU), 2-deoxyglucose uptake into muscles of the lower leg (R′g), hind leg metabolism of infused 1-methylxanthine (1-MX), a measure of capillary recruitment, and muscle cGMP were determined. The experimental groups were T-1032 (10 μg min−1 kg−1) infused for 1 h before and during a euglycaemic insulin clamp (3 mU min−1 kg−1 × 2 h), T-1032 infused for 3 h with saline, T-1032 during a 2 h clamp, T-1032 with saline for 2 h, and a 2 h saline control.
Insulin increased GIR from zero to 13 mg min−1 kg−1, HGU from 0.1±0.01 to 0.43±0.05 μmol min−1, R′g and 1-MX, marginally increased FBF, and had no effect on blood pressure or heart rate. T-1032 alone had no effect on blood pressure, heart rate, FBF, VR, HGU, R′g or 1-MX, but increased muscle cGMP. T-1032 1 h before and during insulin completely blocked GIR (1 h), HGU (2 h), R′g (2 h), and 1-MX (2 h). T-1032 commenced with insulin had only partial blocking activity against insulin.
We conclude that T-1032 is a potent acutely acting inhibitor of the muscle effects of physiologic insulin on capillary recruitment and glucose uptake in vivo. These, together with inhibition of whole-body glucose infusion during insulin, may caution against the use of isoenzyme-5-specific cyclic GMP phosphodiesterase inhibitors as therapeutic agents.
Keywords: Capillary recruitment, muscle insulin action, femoral blood flow, 1-methyl xanthine metabolism, 2-deoxyglucose uptake
Introduction
The notion that nitric oxide (NO) may play a role in muscle glucose uptake, not only under basal conditions, but during exercise, has been raised by a number of workers (Reid, 1998; Balon, 1999; Stamler & Meissner, 2001). Arguments in favour of such a relationship have been based on findings that NO donors such as sodium nitroprusside increase 2-deoxyglucose (2-DG) uptake in a dose-dependent manner in isolated incubated soleus (Young et al., 1997a) and EDL (Balon & Nadler, 1996; 1997; Higaki et al., 2001) muscles. In addition, sodium nitroprusside has been shown to increase the rate of glucose oxidation in incubated soleus muscles (Young & Leighton, 1998). There are also data to indicate that the pathway for NO-mediated glucose uptake by muscle is distinct from that of insulin. Thus, wortmannin, a strong inhibitor of PI3-K of the insulin-signalling pathway, has little effect on sodium nitroprusside-stimulated glucose uptake by incubated muscle (Etgen et al., 1997; Higaki et al., 2001). Since NO signalling involves activation of the soluble form of guanylate cyclase to produce cGMP, the relationship between NO, cGMP, and muscle glucose uptake has been explored. For example, Young et al. (1997a) showed that cGMP was increased following sodium nitroprusside addition to incubated soleus muscle, and a similar finding was made for EDL muscle (Lau et al., 2000). An inhibitor of guanylate cyclase was also shown to decrease sodium nitroprusside-mediated increase in cGMP and 2-DG uptake by incubated soleus (Young et al., 1997a) and EDL (Higaki et al., 2001) muscles. In addition, dibutyryl cGMP increased glucose transport in isolated epitrochlearis muscle (Etgen et al., 1997) and zaprinast, an inhibitor of PDE, increased the net rate of lactate release in isolated soleus muscle from lean Zucker rats (Young & Leighton, 1998).
NO does not appear to be involved in insulin action in vitro (Kapur et al., 1997; Higaki et al., 2001) but, because of haemodynamic effects involving vasodilation, NO may be involved in insulin action in vivo to increase muscle glucose uptake. Thus, insulin stimulates glucose uptake by muscle in vivo and a component of insulin's action involves the haemodynamic effects of increased bulk (i.e. limb) blood flow (Steinberg et al., 1994) and capillary recruitment (Clark et al., 2003), that are each NO-dependent. Both are increased by insulin action, but limb blood flow, unlike capillary recruitment, requires relatively high doses of insulin and/or extended periods to occur (Yki-Jarvinen & Utriainen, 1998). It has been proposed that insulin and/or a product of insulin-mediated glucose metabolism induces vasodilation by recruiting previously unperfused or underperfused capillaries. The recruitment of these ‘new' capillaries increases the mass of tissue available for insulin-mediated glucose metabolism and, therefore, leads to an increase in insulin-mediated glucose uptake (Baron et al., 1990). Capillary recruitment in muscle can now be measured by specific methods (Clark et al., 2003) including the metabolism of infused 1-MX (Rattigan et al., 1997), a substrate targeted for capillary endothelial xanthine oxidase (Jarasch et al., 1986). When NO production is blocked by an inhibitor of NO synthase, insulin-stimulated capillary recruitment is totally blocked and insulin-mediated glucose uptake by muscle is inhibited by approximately 50% (Vincent et al., 2003).
NO of the muscle may originate from macrophages, neural cells, myocytes, or endothelial cells (Vallance & Moncada, 1994), but there is some evidence that insulin-mediated production of NO is endothelial (Shankar et al., 2000) and its target is the neighbouring vascular smooth muscle, where it activates soluble guanylate cyclase to produce cGMP. This cyclic nucleotide in turn activates a cascade of phosphorylations, leading to the lowering of intracellular calcium and vasorelaxation. Cyclic GMP phosphodiesterase-5 inhibitors, which slow the degradation of cGMP and increase its concentration, have been shown to alter blood flow in specific tissues by potentiating local NO-dependent vasodilatory mechanisms.
This paracrine link has been used to facilitate local blood flow in specific tissues by inhibiting the isoform of the PDE expressed in the vasculature of that tissue. Sildenafil and its effects on the vasculature of the corpus cavernosum is such an example. For skeletal muscle vasculature, the dominant form of PDE is yet to be identified.
Since the insulin-mediated haemodynamic effects are dependent on NO (Steinberg et al., 1994; Vincent et al., 2003), and NOS inhibitors reduce insulin-mediated glucose uptake in vivo (Vincent et al., 2003), inhibitors of PDE have the potential to enhance insulin's action on muscle glucose uptake by contributions from vascular (influencing access) and myocyte effects. Accordingly, in the present study, we have explored the effects of T-1032 on insulin action in vivo, as an example of PDE-5 inhibitors to which zaprinast and sildenafil are thought to belong (Kotera et al., 2000).
Methods
Animals
All procedures adopted and experiments undertaken were approved by the University of Tasmania Animal Ethics Committee. Male hooded Wistar rats weighing 245±3 g were raised on a commercial diet (Pivot, Launceston, Australia) containing 21.4% protein, 4.6% lipid, 68% carbohydrate, and 6% crude fibre with added vitamins and minerals, together with water ad libitum. Until the day of surgery, rats were housed at a constant temperature of 21±1°C with a 12h/12 h light/dark cycle. Food, but not water, was withdrawn at the beginning of the last dark cycle.
T-1032
T-1032, obtained from Sigma-Aldrich Co. (St Louis, MO, U.S.A.), was initially dissolved in dilute acid (1 ml 1 mM HCl per 10 mg T-1032) and diluted 1 in 40 or 400 in saline for infusion. In preliminary experiments, controls were conducted using diluted acidified saline, but since these did not differ from unacidified saline infusions, the latter were adopted as the controls.
Surgical preparation
Details were as essentially described previously (Rattigan et al., 1997). In brief, the overnight fasted rats were anaesthetized using Nembutal (50 mg kg−1 body weight), and had polyethylene cannulas (PE-50, Intramedic®) surgically implanted into the carotid artery, for arterial sampling and measurement of blood pressure (pressure transducer Transpac IV, Abbott Critical Systems), and into both the jugular veins for continuous administration of anaesthetic and other intravenous infusions. A tracheotomy tube was inserted, and the animal was allowed to spontaneously breathe room air throughout the course of the experiment. Small incisions (1.5 cm) were made in the skin overlaying the femoral vessels of both legs, and the femoral artery was separated from the femoral vein and saphenous nerve. The epigastric vessels were ligated, and an ultrasonic flow probe (Transonic Systems, VB series 0.5 mm) was positioned around the femoral artery of the right leg just distal to the rectus abdominis muscle. The probe was connected to the flow meter (Model T106 ultrasonic volume flow meter, Transonic Systems). This was in turn interfaced with an IBM compatible PC computer, which acquired the data (at a sampling frequency of 100 Hz) for femoral blood flow (FBF), heart rate, and blood pressure, using WINDAQ data acquisition software (DATAQ Instruments). The animals were maintained under anaesthesia for the duration of the experiment, using a continual infusion of Nembutal (0.6 mg min−1 kg−1) via the left jugular cannula. The body temperature was maintained using a water-jacketed platform and a heating lamp positioned above the rat.
Euglycaemic hyperinsulinaemic clamp studies
Once the surgery was completed, a 45–60 min equilibration period was allowed, so that leg blood flow and blood pressure could become stable and constant. Rats were then subjected to Protocol A (Figure 1), where they were infused with saline or T-1032 for 3 h (some received T-1032 for only 2 h starting at 0) and underwent euglycaemic insulin clamp (3 mU kg−1 min−1, Humulin R, Eli Lilly & Co., Indianapolis), or saline alone for the final 2 h. T-1032 was infused at 10 μg min−1 kg−1 unless indicated otherwise; some experiments were conducted using one-tenth of this amount for 2 h with insulin.
Figure 1.
Study design. The protocol involved the euglycaemic clamp at 3 mU min−1 kg−1 insulin, commencing at time=0 min, and either saline or T-1032, which was commenced at time=−60 min, or jointly with insulin. Duplicate arterial and femoral venous plasma samples were collected at 120 min, for HPLC analysis, plasma glucose, T-1032, and insulin determinations. Venous infusions are indicated by the bars. Bolus injections of allopurinol or 2-DG were made as indicated. The arterial samples were taken for glucose determinations as indicated. Venous infusions are indicated by the bars. Muscle samples were taken at 120 min for 2-DG and cGMP analyses.
Blood samples
The femoral vein of the left leg was used for venous sampling, using an insulin syringe with an attached 29G needle (Becton Dickinson). A duplicate venous sample (300 μl) was taken only on completion of the experiment (total time 180 min) to prevent alteration of the blood flow from the hind limb due to sampling, and to minimize the effects of blood loss. The blood samples were placed on ice, immediately centrifuged, and the plasma stored at −20°C until assayed. The total blood volume withdrawn from the animals before the final arterial and venous samples did not exceed 1.5 ml, and was easily compensated by the volume of fluid infused.
Capillary recruitment
Capillary recruitment was determined by measuring the metabolism of infused 1-MX, a substrate targeted for xanthine oxidase, that in the hindlimb muscle is located predominantly in the capillary endothelium (Jarasch et al., 1986). 1-MX (Sigma Aldrich Inc.) was infused at a constant rate (0.5 mg min−1 kg−1) to maintain a steady-state arterial level of approx. 20 μM. As noted previously (Rattigan et al., 1997; 1999), this was achieved by partly inhibiting the activity of xanthine oxidase with allopurinol (Emmerson et al., 1987) (10 μmol kg−1), administered as a bolus dose 5 min prior to commencing the 1-MX infusion (Figure 1). This not only allowed constant arterial concentrations of 1-MX to be maintained throughout the experiment, but also lowered the Km of the enzyme sufficiently, so that 20 μM 1-MX was well above saturation (unpublished observations).
Plasma (100 μl) from arterial and leg venous blood samples taken at the end of the experiment was mixed with 20 μl of 2 M perchloric acid and centrifuged for 10 min. The supernatant was used to determine 1-MX, allopurinol, and oxypurinol concentrations by reverse-phase HPLC, as previously described (Rattigan et al., 1997). Capillary recruitment, expressed as 1-MX disappearance, was calculated from arteriovenous plasma 1-MX difference, and multiplied by FBF (corrected for the volume accessible to 1-MX, 0.871, determined from plasma concentrations obtained after additions of standard 1-MX to whole rat blood) and expressed as nmol min−1.
Muscle glucose uptake
At 45 min prior to the completion of each experiment (Figure 1), a 50 μCi bolus of 2-deoxy-D-[2,6-3H]glucose (2DG) (specific activity 44.0 Ci mmol−1, Amersham Life Science) was administered in saline. Plasma samples (25 μl) were collected at 5, 10, 15, 30, and 45 min to determine the plasma clearance of the radioactivity. At the conclusion of the experiment, the soleus, plantaris, gastrocnemius white, gastrocnemius red, EDL, and tibialis muscles were removed, clamp frozen in liquid nitrogen, and stored at −20°C. Individual frozen muscles were ground under liquid nitrogen and homogenized using an Ultra Turrax™. Free and phosphorylated [3H]2DG were separated by ion exchange chromatography using an anion exchange resin (AG1-X8) (James et al., 1985; Kraegen et al., 1985). Biodegradable Counting Scintillant-BCA (Amersham, U.S.A.) was added to each radioactive sample and radioactivity determined using a scintillation counter (Beckman LS3801, U.S.A.). From this measurement and a knowledge of plasma glucose and the time course of plasma 2DG disappearance, R′g, which reflects glucose uptake into the muscle, was calculated as previously described by others (James et al., 1985; Kraegen et al., 1985).
Other assays
A glucose analyzer (Yellow Springs Instruments, Model 2300 Stat plus) was used to determine whole blood glucose (by the glucose oxidase method) during the insulin clamp. A blood sample of 25 μl was required for each determination. Human insulin levels at the end of the euglycaemic insulin clamp were determined from arterial plasma samples by ELISA assay (Dako Diagnostics Ltd, U.K.), using human insulin standards. Muscle cGMP levels were determined using a Biotrak enzymeimmuno assay kit (Amersham Pharmacia Biochem., U.K.) on trichloracetic acid extracts of muscle.
Plasma T-1032 was measured by reverse-phase HPLC, using a 5 μm C-18 column (Luna, Phenomenex, U.S.A.) with a 50 mM phosphate buffer in 64% methanol as the mobile phase, at a flow rate of 1.2 ml min−1. An aliquot of plasma (1 ml) was first protein precipitated with 3 ml of ethanol and 10.2 ml chloroform was added to 3.4 ml of supernatant, shaken and briefly centrifuged. The lower chloroform layer was removed and air-dried at 60°C. The residue was re-dissolved in buffer (100 μl) and an aliquot (50 μl) was used for HPLC. Standards were made up in plasma and treated as above.
Expression of results
All data are expressed as means±s.e. The numbers of animals in each group were saline, n=6; T-1032 infused at 10 μg min−1 kg−1 for 3 h, n=5; 3 mU min−1 kg−1 insulin, n=7; insulin+T-1032, n=8; T-1032 for 1 h before and during the insulin clamp, n=5. The mean FBF, mean heart rate, and mean arterial blood pressure were calculated from 5 s subsamples of the data, representing approximately 500 flow and pressure measurements every 15 min. Vascular resistance (VR) in the hind leg was calculated as the mean arterial blood pressure in millimetres of mercury divided by FBF in millilitres per minute, and expressed as resistance units (RUs). Glucose uptake in the hind limb was calculated from A−V glucose difference, and multiplied by FBF and expressed as μmol min−1.
Statistical analysis
In order to ascertain the differences between treatment groups at the end of the experiment (120 min), repeated measures ANOVA was used and Dunn's post hoc tests used for those parameters that were not normally distributed. This was used for FBF, arterial blood pressure, femoral VR, and GIR. For HGU, R′g, and 1-MX metabolism, one-way ANOVA was applied, followed by pairwise comparisons using the Student–Newman–Keuls Method. All tests were performed using the SigmaStat™ statistical program (Jandel Software Corp.).
Results
Plasma levels of insulin and T-1032
The basal plasma level of insulin was 143±11 pM (saline) and this was unaffected by 3 h infusion of T-1032 at 10 μg min−1 kg−1 (159±22 pM). Insulin infusion at 3 mU min−1 kg−1 for 2 h increased plasma levels to 592±91 pM, and this was unaffected by 3 h infusion of T-1032 at 10 μg min−1 kg−1 (518±57 pM). The plasma concentration of T-1032 was 0.217±0.003 μM when T-1032 was infused at 10 μg min−1 kg−1for 3 h.
Systemic effects of insulin and T-1032
Figure 2 shows the data for blood pressure and heart rate, as well as changes in FBF and hind limb VR as a result of physiologic insulin. Although there was some variability in steady-state blood pressure and heart rate prior to the commencement of infusions, changes over the infusion period for either 3 mU insulin min−1 kg−1 alone or 10 μg T-1032 min−1 kg−1 alone (for 2 or 3 h) were minimal. Similarly, the combinations of insulin and T-1032 resulted in no significant change to either blood pressure or heart rate (Figure 2). The starting values for FBF were more variable than either heart rate or blood pressure. Thus. at −60 min, the mean values±s.e. for FBF were 0.90±0.14 (saline), 0.64±0.14 (T-1032, 3 h), 0.69±0.08 (insulin), 0.85±0.05 (insulin+T-1032), and 0.97±0.11 ml min−1 (T-1032 before and during insulin). However, insulin alone marginally increased FBF, and this was increased significantly by approx. 0.35 ml min−1 relative to the saline control at 120 min (P<0.05). T-1032 for 3 h alone had no effect, but when infused for 3 h (1 h before and for 2 h with insulin) blocked the increase in FBF due to insulin, suggesting that the major effect of T-1032 required it to be infused before the insulin. Thus, when the same dose of T-1032 was infused jointly with the insulin, there was essentially no inhibitory effect and the increase in FBF was not significantly different from insulin alone (Figure 2).
Figure 2.
Cardiovascular changes elicited by infusion of insulin, T-1032, and combinations of the two. Time courses from the protocol of Figure 1 are shown for mean arterial blood pressure (BP), heart rate (HR), and changes in FBF and VR. The values are means±s.e. *Significantly different (P<0.05) from saline infusion, and #significantly different (P<0.05) from insulin+T-1032.
Since there was little change in blood pressure due to either insulin, T-1032 or a combination of the two, changes in calculated VR gave the same outcomes as were noted for FBF.
GIR
The whole-body GIR to maintain euglycaemia is shown in Figure 3. Insulin (3mU min−1 kg−1) increased GIR from zero to approx. 13 mg min−1 kg−1. Coinfusion of T-1032 at 1 μg min−1 kg−1 with insulin for 2 h took approx. 1 h to have an effect, and the inhibition gradually increased until by 120 min GIR was inhibited 50%. At 10 μg min−1 kg−1, T-1032 prevented about 40% of the rise in GIR over the initial 0.5 h, and resulted in a steady decline to approx. 4 mg min−1 kg−1 at 2 h (Figure 3). Prior infusion of the same high dose of T-1032 for 1 h before, and for the 2 h with insulin, resulted in the most marked inhibition. Thus, by 40 min after commencement of insulin infusion, the requirement for glucose infusion to maintain euglycaemia was zero. Plasma glucose analysis for saline or T-1032 alone indicated no change from basal at any time point; thus infusion of glucose for either of these protocols was not required.
Figure 3.
Glucose infusion rate (GIR) for clamps at 3 mU min−1 kg−1 insulin with and without T-1032. Data were collected from samples taken, as shown in the protocol of Figure 1. Values are means±s.e. *Significantly different (P<0.05) from insulin infusion.
Muscle metabolism
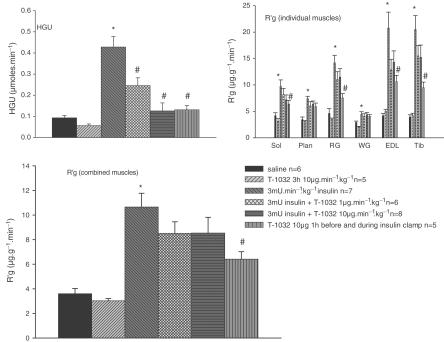
Figure 4 shows the data for HGU, and R′g for combined and individual muscles of the lower leg. Insulin (3 mU min−1 kg−1) alone significantly increased HGU from 0.1±0.01 to 0.43±0.05 μmol min−1, a factor of approx. four-fold. Insulin alone also increased R′g combined, as well as R′g for each of the individual muscles, including soleus, plantaris, red gastrocnemius, EDL, and tibialis, but not the white gastrocnemius. The increase in R′g for individual muscles ranged from 5.5- and 4.8-fold for tibialis and EDL to two-fold for soleus and plantaris and zero for gastrocnemius white. When combined, the increase represented 2.9-fold basal. T-1032 (10 μg min−1 kg−1) for 3 h alone had no significant effect on either HGU or R′g, but when added for 1 h before and during insulin, completely inhibited the stimulation of HGU and R′g due to insulin. When added with insulin for 2 h, T-1032 was less effective and the increase in R′g due to insulin was only partly inhibited (Figure 4). T-1032 at the lower dose of 1 μg min−1 kg−1 for 2 h with insulin was even less effective and inhibited the insulin-mediated increase in HGU from 0.42±0.05 to 0.25±0.03 μmol min−1, P<0.05; n=5 (data not shown).
Figure 4.
HGU and [3H]2-DG uptake (R′g) values for combined and individual muscles for clamps at 3 mU min−1 kg−1 insulin with and without T-1032, using the protocol of Figure 1 and determined at 120 min. Individual R′g for soleus, plantaris, gastrocnemius red, gastrocnemius white, EDL, and tibialis at 120 min have been combined. The values shown are means±s.e. *Significantly different (P<0.05) from saline infusion, and #significantly different (P<0.05) from insulin.
The muscle content of cGMP was 37.8±8.0 (saline), 30.6±4.9 (insulin), 89.5±17.6 (T-1032, 10 μg min−1 kg−1), and 89.7±11.4 pmol g wet weight−1 muscle (T-1032, 10 μg min−1 kg−1+insulin). The increase due to T-1032 (10 μg min−1 kg−1) was significant (P<0.05) and unaffected by insulin.
A change in the vascular smooth muscle content of cGMP, as might be expected with insulin alone, was not detected by analysis of these muscle homogenates.
Capillary recruitment
As shown previously, 1-MX metabolism, determined by the product of arteriovenous difference × FBF, is an indicator of capillary recruitment (Rattigan et al., 1997). However, 1-MX metabolism is not always linked to changes in FBF (Rattigan et al., 1997). Figure 5 shows the data for 1-MX metabolism from the present study. Neither insulin nor T-1032 alone had any significant effect on 1-MX extraction (data not shown). However, insulin but not T-1032 alone significantly increased 1-MX metabolism (Figure 5). T-1032 (10 μg min−1 kg−1) completely blocked the insulin-mediated increase in 1-MX metabolism, whether present before and during insulin (3 h) or only during insulin (2 h).
Figure 5.
Hind leg 1-MX metabolism for clamps at 3 mU min−1 kg−1 insulin with and without T-1032 using the protocol of Figure 1 and determined at 120 min. The values shown are means±s.e. *Significantly different (P<0.05) from saline infusion, and #significantly different (P<0.05) from insulin.
Discussion
The main finding emerging from this study was that T-1032, a specific PDE-5 inhibitor, antagonized, rather than enhanced, the vasodilatory and muscle glucose uptake effects of physiologic insulin. Thus, T-1032 had no effect on its own, but when added with insulin it inhibited insulin-mediated increases in FBF, GIR, HGU, R′g (type I and IIa muscles), and capillary recruitment determined by 1-MX metabolism.
The data for GIR (Figure 3) show that, when infused with insulin, the effects of T-1032 were in part dose-dependent. Thus, exposure for 2 h to T-1032 infused at 1 μg min−1 kg−1 produced inhibitory effects that were considerably less than those of a dose of 10 μg min−1 kg−1 for 2 h.
As the vascular effect of insulin to recruit capillary blood flow is both an early (Vincent & Barrett, 2002) and highly sensitive (Vincent et al., 2002) process for insulin action in vivo and is NO-dependent (Vincent et al., 2003), it is probable that T-1032 has negatively affected insulin's action at the endothelium. This view is based on previous studies, where complete inhibition of insulin-mediated capillary recruitment has resulted in approx. 50% of muscle glucose uptake being inhibited (Clark et al., 2003). Thus, the complete blockade of capillary recruitment in the present study may account for the partial inhibition of lower leg R′g and some of the inhibition of HGU. Since the fall in small vessel resistance evoked by insulin was blocked by T-1032, it is not unexpected that the associated increase in FBF was also blocked by T-1032.
T-1032 is regarded as a specific inhibitor of PDE-5, and there are data from isolated rat aortas to suggest that it is more specific for PDE-5 than sildenafil (Mochida et al., 2002), and much more specific than zaprinast (Wang et al., 2001). For example, for PDE-5, T-1032 has an IC50 of 1.0 nM (sildenafil, 6.6 nM), and for PDE-1, -2, -3, and -4 the IC50 for T-1032 is greater than 1 μM, compared to sildenafil which has an IC50 of 396 nM (PDE-1) and 44 nM (PDE-6) (Kotera et al., 2000; Saenz et al., 2001). Zaprinast has an IC50 of 53 nM (rabbit), 332 nM (human) or 217 nM (dog) for corpus cavernosum PDE-5, depending on the species (Wang et al., 2001). In addition, Mochida et al. (2002) showed, using isolated rat aorta, that sildenafil and T-1032 produced a similar, moderate, relaxation at 10−10–10−7 M, but sildenafil and not T-1032 produced further relaxation at higher concentrations. Moreover, sildenafil but not T-1032 at the higher concentrations of 10−5 and 10−4 M produced a more potent relaxation than did T-1032 in endothelium-denuded preparations, caused a rightward shift of the concentration–response curve for calcium chloride in K+-depolarized endothelium-denuded preparations, and further increased cGMP levels. These results suggested that high concentrations of sildenafil had additional vasorelaxant properties through mechanisms other than phosphodiesterase type 5 inhibition.
As an inhibitor of PDE-5, T-1032 might be expected to have enhanced, rather than inhibited, insulin's action to increase muscle glucose uptake on two grounds. First, there is the consideration of insulin's haemodynamic effects, particularly capillary recruitment. This microvascular effect of insulin was totally inhibited by the systemically infused NO synthase inhibitor, L-NAME (Vincent et al., 2003), making it likely that this process is NO-dependent. Moreover, the inhibition of insulin-mediated capillary recruitment was accompanied by approx. 50% inhibition of muscle glucose uptake (Vincent et al., 2003), suggesting that access for glucose and insulin is a rate-limiting process for glucose uptake by muscle in vivo (Clark et al., 2003), which is overcome by insulin. The microvascular NO of endothelial origin is thought to act as a paracrine signal and, after entering the nearby vascular smooth muscle cells of the terminal arterioles that control blood flow entry to further capillary networks, activates guanylate cyclase to produce cGMP. The cyclic GMP so formed activates a phosphorylation cascade to lower the intracellular calcium ions and relax the smooth muscle. Cyclic GMP is destroyed by a number of isoforms of PDE, which can be expressed in a tissue-specific manner. For example, PDE-5 is found in vascular smooth muscle generally and although it is concentrated in the vessels of the corpus cavernosum (Wallis et al., 1999), systemic administration of T-1032 to noradrenaline-treated anaesthetized rats lowered the mean arterial pressure (Inoue et al., 2001). Thus, it could reasonably be expected that T-1032 administered to anaesthetized rats, as in the present study, would have vascular effects in the hindlimb, consistent with vasodilation. In fact, this did not occur either for T-1032 administered alone or when administered with insulin. Thus, an insulin-mediated increase in NO in endothelial cells and vasodilation, which should have been potentiated, was inhibited.
The second basis focuses on the myocyte and metabolism, where there is evidence for an NO-dependent mechanism for glucose uptake by muscle. For example, Higaki et al. (2001) have shown that the NO donor nitroprusside increases glucose uptake by muscle, through a mechanism that is distinct from those of insulin and contraction. Isolated soleus and EDL muscles from rats were treated with various combinations of sodium nitroprusside, insulin, electrical stimulation to produce contractions, wortmannin (a PI-3 K inhibitor), and/or L-NMMA. The glucose uptake due to sodium nitroprusside was additive to that of either insulin or contractions, largely insensitive to wortmannin, but associated with activation of the alpha-1 subunit of 5′AMP kinase, an indicator of fuel depletion. In addition, others have shown that NO donors stimulate glucose transport and the rates of lactate release and glucose oxidation in isolated incubated rat skeletal muscle preparations (Young et al., 1997b). Whereas the effector mechanism used by NO donors to stimulate glucose metabolism in this tissue is yet to be defined, the neuronal form of NO synthase is expressed in the myocytes (Kobzik et al., 1994; Brenman et al., 1995), and synthetic NO donors such as sodium nitroprusside stimulate the generation of cGMP in isolated rat skeletal muscle as well as stimulate the rate of glucose utilization (Young et al., 1997b). Muscle homogenates have been found to contain the activities of PDE-5, along with PDE-2 and PDE-4 (Wallis et al., 1999). While it is not clear whether the PDE-5 activity is due in part or whole to homogenized vascular components of the homogenate, there are compelling data that at least one other PDE-5 inhibitor, zaprinast, causes a build-up of cGMP and increases glucose metabolism by isolated incubated muscle of lean Zucker rats (Young & Leighton, 1998), keeping in mind that glucose uptake by isolated incubated muscle, unlike muscle in vivo, cannot be influenced by vascular events as delivery of insulin and/or glucose is by diffusion only. To the authors' knowledge, neither zaprinast nor sildenafil have been tested in vivo for their effects on insulin action, where vascular effects are involved.
Another important observation emerging from the study was that, to have its maximal effect, the T-1032 needed to be infused prior to the insulin. Thus, 1 h prior infusion before commencement of insulin as well as during the insulin led to complete inhibition of GIR by 40 min. This contrasted with the same dose of T-1032 infused for only 2 h with insulin, where GIR was inhibited by 50%. Such a finding indicates that, to have its full effects, the T-1032 either needs a long time or alternatively needs to alter a pathway that cannot be modified in the same way once physiologic levels of insulin are present. It may suggest either that T-1032 has other non-PDE-mediated effects, or high levels of cGMP may antagonize insulin's non-NO-dependent effects. One of the non-NO-dependent effects of insulin that has been reported is hyperpolarization. Okubo et al. (1999) reported that insulin dilated small arteries of the mesenteric from normotensive rats, and that this was significantly suppressed by denudation, tetrabutylammonium, apamin, and charybdotoxin, and not by L-NAME, indomethacin or ouabain. Similarly, in animal models where insulin resistance occurs, such as the fructose-fed rat (Katakam et al., 1999) and the streptozotocin-treated rat (Makino et al., 2000), there is evidence that EDHF-mediated relaxation is impaired. Moreover, exercise training of type II diabetic rats was shown to improve hyperglycaemia and insulin resistance, and to prevent the impairment of EDHF (Minami et al., 2002). However, inhibition by T-1032 of insulin's effect involving EDHF in the present study would appear to be minor. A recent finding shows that, in the presence of L-NAME, insulin-mediated capillary recruitment and FBF were totally blocked (Vincent et al., 2003), suggesting that these processes are mediated solely by NO.
In the present study, no attempt was made to assess the relative contribution of the effects of T-1032 to muscle and liver glucose metabolism. Yet, since GIR was completely inhibited by T-1032 after 3 h and the combined muscle R′g was only inhibited by 50%, an effect of the PDE-5 inhibitor to block insulin-mediated inhibition of hepatic glucose output would seem likely. Clearly, this warrants further investigation.
Finally, the possibility that T-1032 may have interacted with other pathways unrelated to cGMP cannot be ruled out. Such ‘nonspecific' interaction could account for the present findings and result from the particular chemistry of T-1032. For example, in preliminary studies (unpublished) using another PDE-5 inhibitor, zaprinast, which differs in structure from T-1032, we found insulin-mediated glucose uptake by muscle in vivo not to be inhibited, even though it increased lactate release, as reported by others (Young & Leighton, 1998). Clearly, additional members of this class of PDE-5 inhibitors will need to be tested before a general conclusion can be made regarding the potential diabetogenic nature of these substances. However, HPLC analysis of the T-1032 revealed only one component, and thus the inhibitory effects reported herein are unlikely to be attributable to a contaminant.
In conclusion, the acute effects of T-1032 on insulin action in vivo were strongly diabetogenic and completely blocked the haemodynamic effects of physiologic insulin to recruit capillary blood flow and to increase bulk blood flow. A likely ramification of these vascular effects was a marked inhibition of muscle glucose uptake, although a direct inhibitory effect on muscle glucose uptake cannot be ruled out.
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council and National Heart Foundation of Australia.
Abbreviations
- cGMP
cyclic 3′,5′ guanosine monophosphate
- 2-DG
2-deoxyglucose
- EDL
extensor digitorum longus
- FBF
femoral blood flow
- GIR
whole-body glucose infusion rate
- HGU
hind leg glucose uptake
- L-NAME
Nϖ-nitro-L-arginine methyl ester
- 1-MX
1-methylxanthine
- NMMA
NG-methyl-L-arginine
- NO
nitric oxide
- PDE-x
cyclic 3′,5′ guanosine monophosphate phosphodiesterase isoenzyme-x
- PI3-K
phosphatidyl inositol 3-kinase
- R′g
2-deoxyglucose uptake
- T-1032
methyl 2-(4-aminophenyl)-1,2-dihydro-1-oxo-7-(2-pyridylmethoxy)-4-(345-trimethoxy-phenyl)-3-isoquinoline carboxylate sulphate
- VR
vascular resistance
References
- BALON T.W. Integrative biology of nitric oxide and exercise. Exerc. Sport Sci. Rev. 1999;27:219–253. [PubMed] [Google Scholar]
- BALON T.W., NADLER J.L. Nitric oxide mediates skeletal glucose transport. Am. J. Physiol. 1996;270:E1058–E1059. doi: 10.1152/ajpendo.1996.270.6.E1058. [DOI] [PubMed] [Google Scholar]
- BALON T.W., NADLER J.L. Evidence that nitric oxide increases glucose transport in skeletal muscle. J. Appl. Physiol. 1997;82:359–363. doi: 10.1152/jappl.1997.82.1.359. [DOI] [PubMed] [Google Scholar]
- BARON A.D., LAAKSO M., BRECHTEL G., HOIT B., WATT C., EDELMAN S.V. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J. Clin. Endocrinol. Metab. 1990;70:1525–1533. doi: 10.1210/jcem-70-6-1525. [DOI] [PubMed] [Google Scholar]
- BRENMAN J.E., CHAO D.S., XIA H., ALDAPE K., BREDT D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- CLARK M.G., WALLIS M.G., BARRETT E.J., VINCENT M.A., RICHARDS S.M., CLERK L.H., RATTIGAN S. Blood flow and muscle metabolism: a focus on insulin action. Am. J. Physiol. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- EMMERSON B.T., GORDON R.B., CROSS M., THOMSON D.B. Plasma oxipurinol concentrations during allopurinol therapy. Br. J. Rheumatol. 1987;26:445–449. doi: 10.1093/rheumatology/26.6.445. [DOI] [PubMed] [Google Scholar]
- ETGEN G.J.J., FRYBURG D.A., GIBBS E.M. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction- and phosphatidylinositol-3-kinase-independent pathway. Diabetes. 1997;46:1915–1919. doi: 10.2337/diab.46.11.1915. [DOI] [PubMed] [Google Scholar]
- HIGAKI Y., HIRSHMAN M.F., FUJII N., GOODYEAR L.J. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes. 2001;50:241–247. doi: 10.2337/diabetes.50.2.241. [DOI] [PubMed] [Google Scholar]
- INOUE H., YANO K., IKEO T., NOTO T., KIKKAWA K. T-1032, a novel specific phosphodiesterase type 5 inhibitor, increases venous compliance in anesthetized rats. Eur. J. Pharmacol. 2001;422:109–114. doi: 10.1016/s0014-2999(01)01044-5. [DOI] [PubMed] [Google Scholar]
- JAMES D.E., JENKINS A.B., KRAEGEN E.W. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am. J. Physiol. 1985;248:E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- JARASCH E.D., BRUDER G., HEID H.W. Significance of xanthine oxidase in capillary endothelial cells. Acta Physiol. Scand. 1986;548 Suppl:39–46. [PubMed] [Google Scholar]
- KAPUR S., BEDARD S., MARCOTTE B., COTE C.H., MARETTE A. Expression of nitric oxide synthase in skeletal muscle: a novel role for nitric oxide as a modulator of insulin action. Diabetes. 1997;46:1691–1700. doi: 10.2337/diab.46.11.1691. [DOI] [PubMed] [Google Scholar]
- KATAKAM P.V., UJHELYI M.R., MILLER A.W. EDHF-mediated relaxation is impaired in fructose-fed rats. J. Cardiovasc. Pharmacol. 1999;34:461–467. doi: 10.1097/00005344-199909000-00022. [DOI] [PubMed] [Google Scholar]
- KOBZIK L., REID M.B., BREDT D.S., STAMLER J.S. Nitric oxide in skeletal muscle. Nature. 1994;372:546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- KOTERA J., FUJISHIGE K., MICHIBATA H., YUASA K., KUBO A., NAKAMURA Y., OMORI K. Characterization and effects of methyl-2-(4-aminophenyl)-1, 2-dihydro-1-oxo-7-(2-pyridinylmethoxy)-4-(3,4,5-trimethoxyphenyl)-3-isoquinoline carboxylate sulfate (T-1032), a novel potent inhibitor of cGMP-binding cGMP-specific phosphodiesterase (PDE5) Biochem. Pharmacol. 2000;60:1333–1341. doi: 10.1016/s0006-2952(00)00457-3. [DOI] [PubMed] [Google Scholar]
- KRAEGEN E.W., JAMES D.E., JENKINS A.B., CHISHOLM D.J. Dose–response curves for in vivo insulin sensitivity in individual tissues in rats. Am. J. Physiol. 1985;248:E353–E362. doi: 10.1152/ajpendo.1985.248.3.E353. [DOI] [PubMed] [Google Scholar]
- LAU K.S., GRANGE R.W., ISOTANI E., SARELIUS I.H., KAMM K.E., HUANG P.L., STULL J.T. nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol. Genomics. 2000;2:21–27. doi: 10.1152/physiolgenomics.2000.2.1.21. [DOI] [PubMed] [Google Scholar]
- MAKINO A., OHUCHI K., KAMATA K. Mechanisms underlying the attenuation of endothelium-dependent vasodilatation in the mesenteric arterial bed of the streptozotocin-induced diabetic rat. Br. J. Pharmacol. 2000;130:549–556. doi: 10.1038/sj.bjp.0703354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINAMI A., ISHIMURA N., HARADA N., SAKAMOTO S., NIWA Y., NAKAYA Y. Exercise training improves acetylcholine-induced endothelium-dependent hyperpolarization in type 2 diabetic rats, Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis. 2002;162:85–92. doi: 10.1016/s0021-9150(01)00685-2. [DOI] [PubMed] [Google Scholar]
- MOCHIDA H., INOUE H., TAKAGI M., NOTO T., YANO K., KIKKAWA K. Sildenafil and T-1032, phosphodiesterase type 5 inhibitors, showed a different vasorelaxant property in the isolated rat aorta. Eur. J. Pharmacol. 2002;440:45–52. doi: 10.1016/s0014-2999(02)01339-0. [DOI] [PubMed] [Google Scholar]
- OKUBO K., KUSHIRO T., TAKAHASHI A., KANMATSUSE K. Role of endothelium-derived hyperpolarizing factor in insulin-induced vasodilation in rat mesenteric artery. Nippon Jinzo Gakkai Shi. 1999;41:685–691. [PubMed] [Google Scholar]
- RATTIGAN S., CLARK M.G., BARRETT E.J. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes. 1997;46:1381–1388. doi: 10.2337/diab.46.9.1381. [DOI] [PubMed] [Google Scholar]
- RATTIGAN S., CLARK M.G., BARRETT E.J. Acute vasoconstriction-induced insulin resistance in rat muscle in vivo. Diabetes. 1999;48:564–569. doi: 10.2337/diabetes.48.3.564. [DOI] [PubMed] [Google Scholar]
- REID M.B. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol. Scand. 1998;162:401–409. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- SAENZ D.T., IANGULO J., CUEVAS P., FERNANDEZ A., MONCADA I., ALLONA A., LLEDO E., KORSCHEN H.G., NIEWOHNER U., HANING H., PAGES E., BISCHOFF E. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE5 inhibitor vardenafil. Int. J. Impot. Res. 2001;13:282–290. doi: 10.1038/sj.ijir.3900726. [DOI] [PubMed] [Google Scholar]
- SHANKAR R.R., WU Y., SHEN H.Q., ZHU J.S., BARON A.D. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000;49:684–687. doi: 10.2337/diabetes.49.5.684. [DOI] [PubMed] [Google Scholar]
- STAMLER J.S., MEISSNER G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- STEINBERG H.O., BRECHTEL G., JOHNSON A., FINEBERG N., BARON A.D. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J. Clin. Investig. 1994;94:1172–1179. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLANCE P., MONCADA S. Nitric oxide – from mediator to medicines. J. R. Coll. Physicians Lond. 1994;28:209–219. [PMC free article] [PubMed] [Google Scholar]
- VINCENT M.A., BARRETT E.J. Insulin-induced capillary recruitment precedes changes in skeletal muscle glucose uptake. Diabetes. 2002;51:A31. [Google Scholar]
- VINCENT M.A., BARRETT E.J., LINDNER J.R., CLARK M.G., RATTIGAN S. Inhibiting NOS blocks microvascular recruitment and blunts glucose uptake in response to insulin. Am. J. Physiol. 2003;285:E123–E129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- VINCENT M.A., DAWSON D., CLARK A.D., LINDNER J.R., RATTIGAN S., CLARK M.G., BARRETT E.J. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes. 2002;51:42–48. doi: 10.2337/diabetes.51.1.42. [DOI] [PubMed] [Google Scholar]
- WALLIS R.M., CORBIN J.D., FRANCIS S.H., ELLIS P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am. J. Cardiol. 1999;83:3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- WANG P., WU P., MYERS J.G., STAMFORD A., EGAN R.W., BILLAH M.M. Characterization of human, dog and rabbit corpus cavernosum type 5 phosphodiesterases. Life Sci. 2001;68:1977–1987. doi: 10.1016/s0024-3205(01)00989-4. [DOI] [PubMed] [Google Scholar]
- YKI-JARVINEN H., UTRIAINEN T. Insulin-induced vasodilatation: physiology or pharmacology. Diabetologia. 1998;41:369–379. doi: 10.1007/s001250050919. [DOI] [PubMed] [Google Scholar]
- YOUNG M.E., CLARK J.F., LEIGHTON B. Zaprinast raises glucose utilisation in SHR skeletal muscle. Biochem. Soc. Trans. 1997a;25:401S. doi: 10.1042/bst025401s. [DOI] [PubMed] [Google Scholar]
- YOUNG M.E., LEIGHTON B. Evidence for altered sensitivity of the nitric oxide/cGMP signalling cascade in insulin-resistant skeletal muscle. Biochem. J. 1998;329:73–79. doi: 10.1042/bj3290073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG M.E., RADDA G.K., LEIGHTON B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem. J. 1997b;322:223–228. doi: 10.1042/bj3220223. [DOI] [PMC free article] [PubMed] [Google Scholar]