Abstract
The current study examined the hypothesis that endothelial production of hydrogen peroxide (H2O2) mediates relaxations to acetylcholine (ACh) in aorta and small mesenteric arteries (SMA) from mice.
Relaxations to ACh (0.01–10 μM) and H2O2 (0.1–1000 μM) were produced in aorta and SMA isolated from wild-type C57BL/6 mice and type II diabetic mice (db/db). In SMA, relaxations to ACh were produced in the presence of Nω-nitro-L-arginine methyl ester (100 μM) and indomethacin (Indo, 10 μM).
1-H[1,2,4]oxadiazolo[4,3-]quinoxalin-1-one (10 μM) significantly reduced ACh-induced relaxations in SMA, abolished responses in aorta, but had no effect on relaxations induced by H2O2. Catalase (2500 U ml−1) abolished responses to H2O2, but did not alter relaxations to ACh in the SMA and only caused a small rightward shift in responses to ACh in the aorta.
ACh-, but not H2O2-, mediated relaxations were significantly reduced by tetraethylammonium (10 mM), the combination of apamin (1 μM) and charybdotoxin (100 nM), and 25 mM potassium chloride (KCl). Higher KCl (60 mM) abolished relaxations to both ACh and H2O2. Polyethylene glycolated superoxide dismutase (100 U ml−1), the catalase inhibitor 3-amino-1,2,4-triazole (3-AT, 50 mM) and treatment with the copper chelator diethyldithiolcarbamate (3 mM) did not affect relaxations to ACh.
H2O2-induced relaxations were endothelium-independent and were not affected by ethylene diamine tetraacetic acid (EDTA 0.067 mM), 4-aminopyridine (1 mM), ouabain (100 μM) and barium (30 μM), 3-AT or Indo.
Although the data from this study show that H2O2 dilates vessels, they do not support the notion that H2O2 mediates endothelium-dependent relaxations to ACh in either aorta or SMA from mice.
Keywords: Endothelium, endothelium-derived hyperpolarizing factor (EDHF), hydrogen peroxide (H2O2), small mesenteric arteries (SMA), relaxation
Introduction
The release of endothelium-derived substances, such as nitric oxide (NO) and prostacyclin, is important in mediating vasodilatation and maintaining blood pressure homeostasis. The reactive oxygen species (ROS) superoxide and hydrogen peroxide (H2O2) are also increasingly gaining acceptance as endogenously derived modulators of vascular tone. While the NO scavenging properties of superoxide are well known, it appears that H2O2 exerts a direct modulatory effect on vasomotor tone to mediate relaxation. As H2O2 relaxes smooth muscle (Burke & Wolin, 1987; Wei & Kontos, 1990; Barlow & White, 1998; Hayabuchi et al., 1998; Iida & Katusic, 2000) and endothelial cells can generate significant amounts of ROS (Arroyo et al., 1990; Holland et al., 1998; Stroes et al., 1998; Zhang et al., 1999), it is conceivable that endogenously produced H2O2 might also regulate vascular tone. Indeed, several studies support this concept and have demonstrated a catalase-sensitive and, therefore, an assumed H2O2-mediated component to endothelium-dependent relaxation in some vascular beds (Kontos et al., 1984; Rubanyi & Vanhoutte, 1986; Yang et al., 1991; Sobey et al., 1997; Cosentino et al., 2001; Rabelo et al., 2003). Since the relaxant effects of H2O2 have been associated with an action on potassium channels (Barlow & White, 1998; Hayabuchi et al., 1998; Bychkov et al., 1999), it was proposed that H2O2 mediated the relaxations attributed to endothelium-derived hyperpolarizing factor (EDHF) in mouse mesenteric arteries (Matoba et al., 2000). EDHF-mediated relaxations are characteristically resistant to inhibitors of nitric oxide synthase (NOS), cyclooxygenase or soluble guanylate cyclase, and are attributed to an endothelial-derived hyperpolarization that is conducted to adjacent vascular smooth muscle cells to cause relaxation (Triggle et al., 1999; McGuire et al., 2001; Feletou & Vanhoutte, 2003). Depolarizing stimuli such as high extracellular concentrations of potassium, or a combination of the calcium-activated potassium channel blockers apamin (Ap) and charybdotoxin (ChTx), can inhibit EDHF relaxations (Edwards et al., 1998; Busse et al., 2002). Matoba et al. (2000) drew comparisons between relaxations resistant to inhibitors of NOS and cyclooxygenase and those to H2O2 in mouse mesenteric arteries because both were similarly sensitive to the nonselective calcium-activated potassium channel inhibitor tetrabutylammonium (TBA). Furthermore, both the endothelium-dependent hyperpolarizations and relaxations in these vessels were inhibited by catalase. It was also recently proposed that endothelium-dependent relaxations in the mouse aorta were mediated by H2O2 and that impaired endothelium-dependent dilatation in hypercholesterolemic mice resulted from a reduced output of H2O2 (Rabelo et al., 2003). In the current study, we sought to further investigate the prospect that endothelium-dependent relaxations in mouse vessels are mediated at least in part by the ROS H2O2. The approach we took was to compare endothelium-dependent relaxations induced by acetylcholine (ACh) to those induced by H2O2 in the presence of agents that modify either the production or site of action of these dilators.
Methods
Animals
A total of 6- to 8-week-old male mice (C57BL/6, Charles River Laboratories, Montreal, PQ, Canada; or type II diabetic db/db and their respective wild-type controls db +/?, Jackson Laboratories, Bar Harbour, ME, U.S.A.) were killed by cervical dislocation. The mesenteric bed was isolated and placed in physiological saline solution (PSS) for dissection. First-order small mesenteric arteries (SMA) were cleared of any adherent connective tissue and fat, and cut into 2 mm segments. These were then mounted onto a wire myograph chamber, set at a resting tension of 1 mN and allowed to equilibrate for 60 min with intermittent washes every 15 min. The thoracic aorta was isolated and cleared of connective tissue and fat, and cut into 2 mm segments. Rings were placed onto tissue hooks in a wire myograph chamber and set at a resting tension of 4.5 mN. The experimental use of animals in this study conformed to guidelines as set out by the Health Sciences Animal Care Committee at the University of Calgary.
Experimental protocol
At the end of the equilibration period, the vessels were tested for responsiveness with a bolus concentration of potassium chloride (KCl, 120 mM) and promptly washed with PSS. To assess the integrity of the endothelium, tissues were contracted with a submaximal concentration of prostaglandin F2α (PGF2α, 0.01–0.1 μM) in SMA and phenylephrine (Phe, 0.1–0.3 μM) in aorta, and if the relaxation to ACh (10 μM) was greater than 70% of the contraction they were considered to be endothelium-intact. In some vessels, the endothelium was removed by passing a wire through the lumen several times. If vessels failed to relax to ACh, they were considered to be devoid of endothelial cells.
Endothelium-dependent relaxations
Aortic rings were constricted with submaximal concentrations of Phe (0.1–0.3 μM), and control concentration–response curves to ACh (0.01–10 μM) were produced and compared to those produced in rings treated with catalase (2500 U ml−1; activity: 19,990 U mg−1 solid; catalog number: C40, Sigma) or 1-H[1,2,4]oxadiazolo[4,3-]quinoxalin-1-one (ODQ, 10 μM). SMA were pretreated with Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM) and indomethacin (Indo, 10 μM) (incubated for 15 min), and control concentration–response curves to ACh (0.01–10 μM) were produced during a submaximal contraction to PGF2α (0.01–0.3 μM). These responses were then repeated in the presence of catalase, high KCl (25 and 60 mM), tetraethylammonium (TEA, 10 mM), ODQ, a combination of Ap (1 μM) and ChTx (100 nM), polyethylene-glycolated superoxide dismutase (PEG-SOD, 100 U ml−1) and in tissues pretreated with the copper chelator diethyldithiocarbamate (DETCA, 3 mM, 30 min incubation followed by wash) or the catalase inhibitor 3-amino-1,2,4-triazole (3-AT, 50 mM, 1 h incubation followed by wash). In some experiments, SMA were treated with the combination of L-NAME, Indo and ODQ or L-NAME, Indo and 2-[4-carboxyphenyl]-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (carboxy-PTIO, 300 μM) to assess any further effects that catalase might have on responses to ACh under these conditions. The effects of the different agents on relaxations to ACh or H2O2 were tested following a 30 min incubation period unless otherwise stated.
H2O2-induced relaxations
Endothelium-intact or denuded mouse SMA and aorta were constricted with PGF2α or Phe to observe concentration–response curves to H2O2 (1–1000 μM). Tissues that were exposed to an initial concentration–response curve to H2O2 did not contract as effectively with PGF2α or Phe following washout; therefore, each treatment was performed in a separate vessel preparation. Relaxations to H2O2 were produced in the presence of high KCl (25 and 60 mM), Indo, ODQ, catalase, TEA (10 mM), Ap/ChTx (1 μM and 100 nM, respectively), ouabain and BaCl2 (100 and 30 μM, respectively), 4-aminopyridine (4-AP, 1 mM) or ethylene diamine tetraacetic acid (EDTA, 0.067 mM).
Drugs and materials
The composition of the PSS was as follows (mM): NaCl (118.0), KCl (4.7), KH2PO4 (0.8), MgSO4 (1.2), NaHCO3 (24.9), dextrose (11.1) and CaCl2 (2.5). The PSS was maintained at a temperature of 37°C and bubbled with carbogen (95% O2, 5% CO2). All drugs and reagents, except TEA, were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.), while TEA was obtained from BDH Chemicals Ltd (Poole, U.K.). Drugs were prepared as stock solutions, which were then diluted to appropriate concentrations in PSS or distilled water. H2O2 was purchased as a 30% w w−1 concentrated solution and was subsequently diluted in distilled water. All drugs except prostaglandin F2α, Indo and ODQ were dissolved in water. Indo and prostaglandin F2α were dissolved in ethanol, and ODQ was prepared in DMSO.
Statistical analysis
Data are expressed as a percentage of PGF2α-, Phe- or KCl-induced contractions. Symbols represent means and T-bars are standard error of means (s.e.m.). Some data are also displayed as pEC50 values, which are derived according to the equation pEC50=−log(EC50). Comparison of the effects of different treatments on responses to ACh and H2O2 was determined by one-way analysis of variance followed by Dunnett's multiple comparison test (Prism, Graph Pad). Asterisks represent P-values less than 0.05 and indicate statistical significance. The n values in parentheses indicate the number of animals used for an experiment.
Results
Endothelium-dependent relaxations
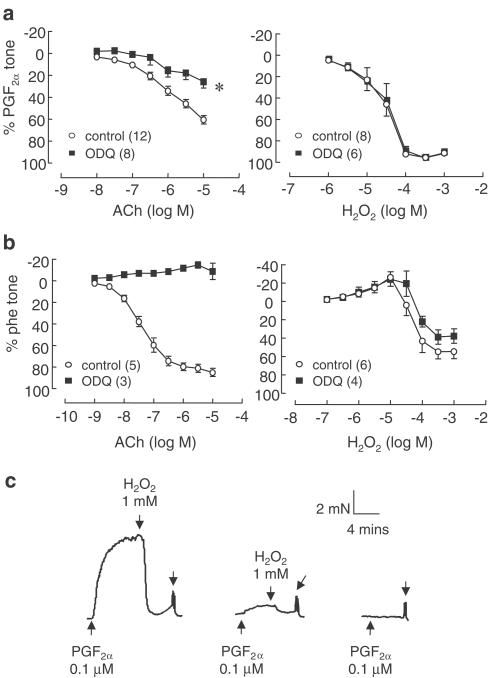
In the presence of L-NAME (100 μM) and Indo (10 μM), ACh (0. 01–10 μM) relaxed C57BL/6 mouse SMA to a maximum of 62.6±3.6% (n=32) of the PGF2α-induced contraction. The addition of the soluble guanylate cyclase inhibitor ODQ (10 μM) significantly reduced the relaxations to 25.9±6.2% (n=9) (Figure 1a). In the aorta, ACh (0.001–10 μM) produced relaxations that reached a maximum of 84.2±3.0% (n=8) of the Phe-induced contraction. Relaxations were abolished in rings treated with ODQ (Figure 1b).
Figure 1.
Relaxations produced to ACh (0.01–10 μM) or H2O2 (0.1–1000 μM) in (a) SMA and (b) aorta, in the absence and presence of ODQ (10 μM). Relaxations to ACh in SMA were performed in the presence of L-NAME (100 μM) and Indo (10 μM). (c) Trace depicting the effect of repeated exposures of 1 mM H2O2 on contractile tone in SMA. Symbols represent means and t-bars are standard error of means (s.e.m.). *P<0.05 when compared to control (one-way analysis of variance). Numbers in parentheses refer to number of experiments performed.
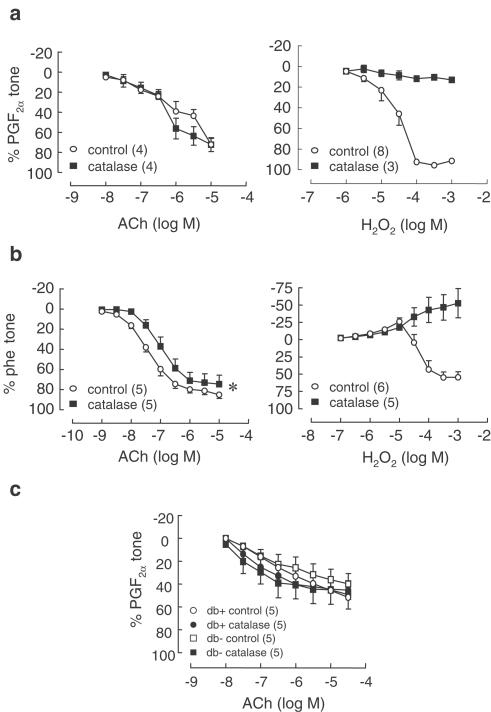
L-NAME- and Indo-resistant relaxations to ACh in SMA were not affected by catalase (2500 U ml−1) in either SMA from C57BL/6 or db/db mice (Figure 2a and c). In aorta, catalase (2500 U ml−1) caused a significant rightward shift of the concentration–response curve to ACh (pEC50 control: 7.38±0.1, catalase: 6.98±0.1, n=5, P<0.05, paired t-test) but caused no significant change to maximal relaxation (Figure 2b).
Figure 2.
Effect of catalase (2500 U ml−1) on relaxations to ACh (0.01–10 μM) in (a) SMA and (b) aorta from C57BL/6 mice, and (c) SMA from type II diabetic mice db/db (and respective wild-type controls db +/?; n=5). Relaxations to H2O2 (1–1000 μM) were abolished by catalase in both aorta and SMA. Symbols represent means and t-bars are standard error of means (s.e.m.). *P<0.05 when compared to control (comparison of pEC50 values, paired t-test). Numbers in parentheses refer to number of experiments performed.
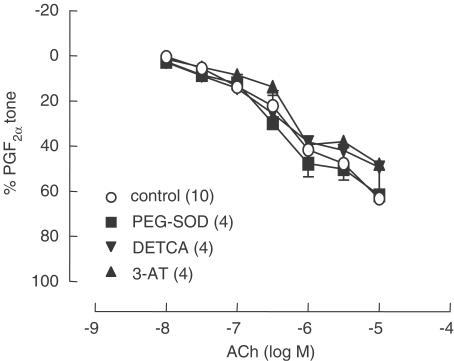
Both ODQ and the NO scavenger carboxy-PTIO (300 μM) further reduced the L-NAME- and Indo-resistant relaxations to ACh in SMA; however, catalase did not have any additional effects on the residual relaxations that remained in the presence of these agents (Figure 3). Relaxations to ACh were significantly reduced by 25 mM KCl and were abolished by 60 mM KCl (Figure 4a). Relaxations were also significantly reduced by the potassium channel inhibitors TEA (10 mM) and by the combination of Ap/ChTx (Figure 4b). Treatment of SMA with DETCA (3 mM) to inhibit the production of H2O2 by endogenous Cu/Zn SOD, with 3-AT (50 mM), to inhibit endogenous catalases, or the addition of cell-permeable PEG-SOD (100 U ml−1) to augment endogenous formation of H2O2, all had no effect on ACh-induced relaxations (Figure 5).
Figure 3.
L-NAME/Indo-resistant relaxations to ACh (0.01–10 μM) in C57BL/6 mouse SMA were further reduced by (a) ODQ (10 μM) and (b) carboxy-PTIO (C-PTIO, 300 μM). However, the subsequent addition of catalase (2500 U ml−1) did not have an additional effect on residual relaxation that persisted in the presence of these agents. Symbols represent means and t-bars are standard error of means (s.e.m.). *P<0.05 when compared to control (one-way analysis of variance). Numbers in parentheses refer to number of experiments performed.
Figure 4.
Comparative effects of (a) varied concentrations of KCl (25 and 60 mM), or (b) the potassium channel inhibitors TEA (10 mM) and Ap (1 μM) and ChTX (100 nM), on L-NAME/Indo-resistant relaxations to ACh (0.01–10 μM) and those induced by H2O2 (1–1000 μM) in C57BL/6 mouse SMA. Symbols represent means and t-bars are standard error of means (s.e.m.). *P<0.05 when compared to control (one-way analysis of variance). Numbers in parentheses refer to number of experiments performed.
Figure 5.
L-NAME/Indo-resistant relaxations to ACh (0.01–10 μM) in C57BL/6 mouse SMA were not affected by DETCA pretreatment (3 mM, 30 min incubation), the addition of PEG-SOD (100 U ml−1) or the treatment of vessels with 3-amino-1,2,4-triazole (3-AT, 50 mM, 1 h incubation). Symbols represent means and t-bars are standard error of means (s.e.m.). Numbers in parentheses refer to number of experiments performed.
Hydrogen peroxide-induced relaxations
H2O2 (1–1000 μM) produced concentration-dependent relaxations, which fully relaxed SMA. In aortic rings, H2O2 induced relaxations that reached a maximum of 57.6±7.1% of the Phe-induced contraction, which were abolished by catalase but were not affected by ODQ (Figures 1b and 2b, n=4–6). Following washout, H2O2 impaired the ability of the aorta and SMA to contract again to their respective contractile agonists (Figure 1c). Relaxations were not dependent on the presence of endothelial cells since there was no difference in the concentration–response curve to H2O2 between endothelium-intact or denuded SMA (Table 1 ). Relaxations were abolished by catalase (2500 U ml−1) and 60 mM KCl; however, lower KCl (25 mM) did not significantly reduce responses to H2O2 (Figure 4). Furthermore, Indo, ODQ, TEA, Ap/ChTx, 4-AP, ouabain/BaCl2, pretreatment of vessels with 3-AT and the inclusion of EDTA in the PSS all failed to alter H2O2-induced relaxations in SMA (Table 1).
Table 1.
Summary of the effects of different agents or treatments on pEC50 values and maximal relaxations to H2O2 in PGF2α contracted mouse SMA
| Treatment | n | pEC50 | Maximal relaxationa (%) |
|---|---|---|---|
| Control (EC+) | 8 | 4.54±0.13 | 94.4±1 |
| Control (EC−) | 5 | 4.6±0.2 | 95.1±1 |
| Indomethacin 10 μM | 4 | 4.57±0.14 | 93.7±1 |
| ODQ 10 μM | 6 | 4.51±0.18 | 94.8±1 |
| EDTA 0.067 mM | 4 | 4.37±0.04 | 96.6±1 |
| 3-AT 50 mM | 4 | 4.58±0.03 | 93.2±1 |
| KCl 25 mM | 4 | 4.31±0.06 | 87.1±1b |
| TEA 10 mM | 4 | 4.27±0.06 | 94.8±1 |
| Ap 1 μM/ChTx 100 nM | 4 | 4.57±0.04 | 97.6±1 |
| 4-AP 1 mM | 4 | 4.38±0.11 | 93.8±1 |
| TEA/4-AP | 4 | 4.79±0.05 | 91.2±2 |
| Oua 100 μM/Ba2+ 30 μM | 4 | 4.52±0.1 | 90.3±5 |
Maximal relaxations are expressed as percentages of the contraction to PGF2α (0.01–0.1 μM).
P<0.05 when compared to control (EC+), ANOVA, followed by Dunnett's multiple comparison test.
3-AT: 3-amino-1,2,4-triazole; 4-AP: 4-aminopyridine; Ap: apamin; Ba2+: barium chloride; ChTx: charybdotoxin; EC+: endothelium-intact; EC−: endothelium-denuded; EDTA: ethylene diamine tetraacetic acid; H2O2: hydrogen peroxide; KCl: potassium chloride; ODQ: 1-H[1,2,4]oxadiazolo[4,3-]quinoxalin-1-one; oua: ouabain; pEC50: −log[EC50]; TEA: tetraethylammonium.
Discussion
The findings of the current study do not support the contention that endothelium-dependent relaxations to ACh in either aorta or SMA from mouse are mediated by H2O2. Catalase, which metabolizes H2O2 into water and oxygen, did not inhibit relaxations to ACh in SMA and only slightly reduced responses in aorta. The irreversible nature of the relaxations to H2O2 coupled with the fact that very high concentrations were required to induce a response, indicate that H2O2 is unlikely to function as a physiological endothelium-derived dilator in mouse vessels.
Endothelium-dependent relaxation
In the aorta, ACh produced relaxations that were abolished by the soluble guanylate cyclase (sGC) inhibitor ODQ. In SMA, relaxations to ACh persisted in the presence of L-NAME and Indo. ODQ further reduced responses, indicating that ACh relaxations in these vessels are mostly mediated by the activation of sGC by a residual source of NO, whose synthesis cannot be fully inhibited by L-NAME alone. The possibility that the endothelium-dependent relaxations in these vessels were mediated by H2O2 was discounted in this study because catalase (2500 U ml−1) did not inhibit relaxations in SMA and only caused a small rightward shift in response curves to ACh in aorta. In SMA, both ODQ and the NO scavenger carboxy-PTIO further reduced the relaxations to ACh, and catalase did not have any additional effect on the residual relaxations seen under these conditions.
In order to determine whether the synthesis of H2O2 by Cu/Zn SOD could contribute to endothelium-dependent relaxation, SMA were also pretreated with the copper chelator diethyldithiocarbamate (DETCA, 3 mM), commonly used to inhibit the Cu/Zn form of superoxide dismutase (Cu/Zn SOD) (Omar et al., 1991; Martin et al., 1994). DETCA treatment of vessels did not reduce ACh-mediated relaxations, indicating that endogenous Cu/Zn SOD is unlikely to synthesize sufficient amounts of H2O2 to relax vessels. Nonetheless, non-copper-containing forms of SOD are also expressed by endothelial cells that could also produce H2O2, thus the lack of effect by DETCA does not entirely exclude a role for H2O2. On the other hand, if endogenously synthesized H2O2 contributes to ACh-mediated relaxation, supplementing vessels with SOD would be expected to augment the production of H2O2; however, the addition of PEG-SOD did not enhance ACh-mediated relaxations in SMA. The possibility that endogenous catalases were contributing to the destruction of endothelial H2O2, thus explaining the lack of effect of exogenously applied catalase, is unlikely because the catalase inhibitor 3-AT did not significantly alter relaxations to ACh.
Oxidative stress is a common characteristic of diabetes (De Vriese et al., 2000), and studies from our laboratory have demonstrated that elevated production of ROS contributes to endothelial dysfunction in the leptin-receptor-deficient, type II diabetic mouse db/db (Pannirselvam et al., 2002). Thus, the prospect of H2O2 being the mediator of EDHF highlighted the possibility that its role in vasodilatory tone might be more pronounced in conditions characterized by oxidative stress. Given the smooth muscle relaxant effects of H2O2, we wondered whether increased endothelial production of H2O2 in blood vessels from diabetic animals might compensate for impaired endothelium-dependent dilatation that results from the reduced bioavailability of NO. Thus, we initially set out to investigate the contribution of H2O2 to endothelium-dependent vasodilation in db/db mice. However, catalase did not inhibit endothelium-dependent relaxations in SMA from these mice. As, Matoba et al. (2000) showed that L-NAME/Indo-resistant relaxations in wild-type C57BL/6 mice were sensitive to catalase, our next step was to see if we could at least observe catalase sensitivity in SMA from these mice; however, catalase again did not affect relaxations. Overall, even in conditions where increased production of ROS is expected to occur, H2O2 does not appear to mediate relaxant tone in the mesenteric vasculature.
Hydrogen peroxide-induced relaxations
H2O2 produced concentration-dependent relaxations in both aorta and SMA and these were abolished by catalase. In order to induce a relaxation to H2O2, it was necessary to exceed concentrations of 100 μM, which may have irreversibly damaged smooth muscle cells, as vessels did not contract effectively on subsequent challenges with PGF2α or Phe (see Figure 1c). H2O2 has been reported to damage the calcium ATPase of the sarcoplasmic reticulum (SR) in smooth muscle (Grover et al., 1992), reducing the ability of cells to replenish the SR with calcium and thus affecting their ability to respond to a contractile agonist (Grover et al., 1995). In the present study, relaxations to H2O2 were prevented by high extracellular concentrations of KCl (60 mM), suggesting that relaxations could be mediated by the activation of potassium channels. However, when a lower concentration of KCl (25 mM) was applied, relaxation to H2O2 remained largely unchanged whereas relaxations to ACh were substantially reduced. Also, incubation with TEA, ouabain and barium, apamin and ChTx, or 4-AP all failed to inhibit relaxations to H2O2, suggesting the lack of involvement of KCa, KV, KIR and the Na+/K+/ATPase in mediating responses to H2O2. Some studies have suggested that the effects of H2O2 may be cyclic GMP (cGMP)-mediated (Burke & Wolin, 1987; Burke-Wolin et al., 1991; Fujimoto et al., 2002) or endothelium-dependent (Zembowicz et al., 1993); however, in the present study ODQ did not inhibit relaxations to H2O2, and removal of endothelium did not significantly alter relaxations to H2O2 in SMA.
EDTA did not significantly affect responses to H2O2, indicating that these responses were unlikely to be due to the metal-catalyzed transformation of H2O2 into other ROS (e.g. hydroxyl radicals) that might mediate the relaxation. Some of the vasoactive effects of H2O2 have also been associated with arachidonic acid-dependent pathways, resulting in elevated formation of prostanoids (Clyman et al., 1989; Iida & Katusic, 2000). However, the conditions required to observe EDHF relaxations (i.e. the presence of L-NAME and Indo) would exclude the possibility that prostanoids contribute to the relaxant effects of endogenous H2O2 because any formation of prostanoids would be inhibited by Indo. Indo itself did not prevent relaxations to H2O2 in the present study; therefore, cyclooxygenase-derived metabolites are unlikely to be involved in these responses.
The findings of the present study suggest that relaxations mediated by H2O2 result from nonspecific effects on vascular smooth muscle tone. The fact that, aside from catalase, only depolarizing concentrations of KCl (60 mM) were able to prevent the ‘relaxations' to H2O2 indicates that the effects might be associated with alterations in intracellular calcium signaling. High KCl (60 mM) produces contractions by depolarizing smooth muscle cells, which activates a voltage-dependent calcium influx. Contractions to PGF2α are mediated by the activation of thromboxane receptors, which, depending on the tissue, raise tone either by stimulating an influx of calcium from extracellular sources (Godfraind & Miller, 1982) or by calcium sensitization (Nobe & Paul, 2001). Although we did not further investigate the possible mechanisms that might account for the decrease in tone by H2O2, recent evidence suggests that it may relax smooth muscle by interfering with calcium sensitization processes. For example, H2O2 increased cytosolic calcium in canine tracheal smooth muscle, but paradoxically also caused a decrease in the amount of myosin phosphorylated in response to ACh, causing a loss in tone (Lorenz et al., 1999). Fujimoto et al. (2002) reported that H2O2 reduced the amount of myosin phosphorylated by noradrenaline in guinea pig aorta partly through a cGMP-mediated mechanism. It has also been reported that H2O2 irreversibly inhibited myosin ATPase activity to prevent cross-bridge formation during contractions (Perkins et al., 2003); thus it is possible that H2O2 elicits relaxation, or a loss in tone, by indiscriminately impairing smooth muscle contractile processes.
Discrepancy with other studies
In contrast to some recent reports (Matoba et al., 2000; Rabelo et al., 2003), we were unable to establish the existence of catalase-sensitive, and therefore H2O2-mediated, endothelium-dependent relaxations in the SMA and aorta from mouse. Other studies have also reported negligible inhibitory effects by catalase on endothelium-dependent relaxations (Beny & von der Weid, 1991; Fulton et al., 1997; Pomposiello et al., 1999; Hamilton et al., 2001; Itoh et al., 2003). Both Beny & von der Weid (1991) and Pomposiello et al. (1999) found that catalase did not affect L-NAME- and Indo-resistant relaxations to bradykinin in porcine coronary arteries, indicating that H2O2 is not an endogenous vasodilator in this preparation. Subsequent studies by another group proposed that an epoxyeicosatrienoic acid was the mediator of EDHF-type relaxations in these vessels (Fisslthaler et al., 1999), and although the enzyme that synthesizes this substance, CYP-2C, was also reported to generate significant amounts of ROS in response to bradykinin, these ROS do not appear to mediate vasorelaxation (Fleming et al., 2001). Itoh et al. (2003) reported that even though SOD enhanced ACh-induced relaxations, neither SOD nor catalase significantly affected ACh-induced hyperpolarizations in rabbit SMA, ruling out the likelihood that H2O2 is an EDHF in this vessel preparation. We also found that in the rat SMA, catalase has no effect on L-NAME/Indo-resistant relaxations to ACh (data not shown). On the other hand, Matoba et al. (2000) reported that catalase effectively inhibited both ACh-induced relaxations and hyperpolarizations in both wild-type and eNOS-deficient mouse SMA. The reason for the discrepancy between the data from Matoba et al. (2000) and those from our laboratory is unclear. Conceivably, differences in the activity of the catalase used in the studies might contribute to the discrepancies or, alternatively, variations in the experimental procedure might suitably explain these differences. The form of catalase used in our studies had a higher activity than the one used by Matoba and others (Sigma catalog number C40 – 19,900 U mg−1 solid cf. Sigma catalog number C9322 – 2390 U mg−1 solid). Thus, one point to consider is whether the lower activity catalase has contaminants that themselves might have an inhibitory effect on relaxations (Halliwell & Gutteridge, 1999). However, when we performed experiments with this other form of catalase, we found that other than causing excessive foaming when in the myograph chamber, it did not significantly affect ACh-induced relaxations (data not shown). Indirect approaches that were used (e.g. inhibition of synthesis and metabolism of H2O2 and supplementation with enzymatic sources of H2O2) also failed to confirm the involvement of H2O2 as an endothelium-derived dilator in SMA. Although our study does not support the idea of H2O2 as an endothelium-derived dilator in the mesenteric vasculature, it may be worthwhile to consider if the contribution of H2O2 is greater in vascular beds that are noted for their sensitivity to changes in oxygen tension and redox conditions such as the cerebral or pulmonary vasculature (Burke & Wolin, 1987; Yang et al., 1991).
Our main reservation against the suggestion that H2O2 is a physiological vasodilator is based on our observation that high, and likely supraphysiological, concentrations of H2O2 were required to induce dilatations, which progressively impaired contractile tone. In contrast, although high concentrations of exogenous NO (10- to 100-fold higher than those generated physiologically) are routinely used in studies to compare with endogenous NO processes, under these conditions the responses to NO (either as NO donors or NO solutions) are reversible and do not usually impair contractile function.
In summary, the data from the current study demonstrate that H2O2 relaxes aorta and SMA from mouse possibly through nonspecific alterations to smooth muscle function. We also conclude that H2O2 is unlikely to mediate endothelium-dependent relaxations in these vessels.
Acknowledgments
We gratefully acknowledge support provided by the Heart and Stroke Foundation of Canada (A. Ellis), Alberta Heritage Foundation/Canadian Institute of Health Research (M. Pannirselvam), Canadian Diabetes Association (T.J. Anderson) and Canadian Institute of Health Research (C.R. Triggle).
Abbreviations
- ACh
acetylcholine
- Ap
apamin
- 4-AP
4-aminopyridine
- 3-AT
3-amino-1,2,4-triazole
- carboxy-PTIO
2-[4-carboxyphenyl]-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide
- ChTx
charybdotoxin
- DETCA
diethyldithiolcarbamate
- EDHF
endothelium-derived hyperpolarizing factor
- EDTA
ethylene diamine tetraacetic acid
- H2O2
hydrogen peroxide
- Indo
indomethacin
- KCl
potassium chloride
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1-H[1,2,4]oxadiazolo[4,3-]quinoxalin-1-one
- PEG-SOD
polyethylene glycolated superoxide dismutase
- Phe
phenylephrine
- PSS
physiological saline solution
- ROS
reactive oxygen species
- SMA
small mesenteric arteries
- TEA
tetraethylammonium
References
- ARROYO C.M., CARMICHAEL A.J., BOUSCAREL B., LIANG J.H., WEGLICKI W.B. Endothelial cells as a source of oxygen-free radicals. An ESR study. Free Radic. Res. Commun. 1990;9:287–296. doi: 10.3109/10715769009145687. [DOI] [PubMed] [Google Scholar]
- BARLOW R.S., WHITE R.E. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am. J. Physiol. 1998;275:H1283–H1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- BENY J.L., VON DER WEID P.Y. Hydrogen peroxide: an endogenous smooth muscle cell hyperpolarizing factor. Biochem. Biophys. Res. Commun. 1991;176:378–384. doi: 10.1016/0006-291x(91)90935-z. [DOI] [PubMed] [Google Scholar]
- BURKE T.M., WOLIN M.S. Hydrogen peroxide elicits pulmonary arterial relaxation and guanylate cyclase activation. Am. J. Physiol. 1987;252:H721–H732. doi: 10.1152/ajpheart.1987.252.4.H721. [DOI] [PubMed] [Google Scholar]
- BURKE-WOLIN T., ABATE C.J., WOLIN M.S., GURTNER G.H. Hydrogen peroxide-induced pulmonary vasodilation: role of guanosine 3′,5′-cyclic monophosphate. Am. J. Physiol. 1991;261:L393–L398. doi: 10.1152/ajplung.1991.261.6.L393. [DOI] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concepts together. Trends Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- BYCHKOV R., PIEPER K., RIED C., MILOSHEVA M., BYCHKOV E., LUFT F.C., HALLER H. Hydrogen peroxide, potassium currents, and membrane potential in human endothelial cells. Circulation. 1999;99:1719–1725. doi: 10.1161/01.cir.99.13.1719. [DOI] [PubMed] [Google Scholar]
- CLYMAN R.I., SAUGSTAD O.D., MAURAY F. Reactive oxygen metabolites relax the lamb ductus arteriosus by stimulating prostaglandin production. Circ. Res. 1989;64:1–8. doi: 10.1161/01.res.64.1.1. [DOI] [PubMed] [Google Scholar]
- COSENTINO F., BARKER J.E., BRAND M.P., HEALES S.J., WERNER E.R., TIPPINS J.R., WEST N., CHANNON K.M., VOLPE M., LUSCHER T.F. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2001;21:496–502. doi: 10.1161/01.atv.21.4.496. [DOI] [PubMed] [Google Scholar]
- DE VRIESE A.S., VERBEUREN T.J., DE VAN V., LAMEIRE N.H., VANHOUTTE P.M. Endothelial dysfunction in diabetes. Br. J. Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- FELETOU M., VANHOUTTE P.M. Third pathway: endothelium-dependent hyperpolarization. Drug Dev. Res. 2003;58:18–22. [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- FLEMING I., MICHAELIS U.R., BREDENKOTTER D., FISSLTHALER B., DEHGHANI F., BRANDES R.P., BUSSE R. Endothelium-derived hyperpolarizing factor synthase (cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ. Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO S., MORI M., TSUSHIMA H. Mechanisms underlying the hydrogen peroxide-induced, endothelium-independent relaxation of the norepinephrine-contraction in guinea-pig aorta. Eur. J. Pharmacol. 2002;60992:1–9. doi: 10.1016/s0014-2999(02)02825-x. [DOI] [PubMed] [Google Scholar]
- FULTON D., MCGIFF J.C., WOLIN M.S., KAMINSKI P., QUILLEY J. Evidence against a cytochrome P450-derived reactive oxygen species as the mediator of the nitric oxide-independent vasodilator effect of bradykinin in the perfused heart of the rat. J. Pharmacol. Exp. Ther. 1997;280:702–709. [PubMed] [Google Scholar]
- GODFRAIND T., MILLER R.C. Actions of prostaglandin F2 alpha and noradrenaline on calcium exchange and contraction in rat mesenteric arteries and their sensitivity to calcium entry blockers. Br. J. Pharmacol. 1982;75:229–236. doi: 10.1111/j.1476-5381.1982.tb08777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROVER A.K., SAMSON S.E., FOMIN V.P. Peroxide inactivates calcium pumps in pig coronary artery. Am. J. Physiol. 1992;263:H537–H543. doi: 10.1152/ajpheart.1992.263.2.H537. [DOI] [PubMed] [Google Scholar]
- GROVER A.K., SAMSON S.E., FOMIN V.P., WERSTIUK E.S. Effects of peroxide and superoxide on coronary artery: ANG II response and sarcoplasmic reticulum Ca2+ pump. Am. J. Physiol. 1995;269:C546–C553. doi: 10.1152/ajpcell.1995.269.3.C546. [DOI] [PubMed] [Google Scholar]
- HALLIWELL B., GUTTERIDGE J.M.C.The chemistry of free radicals and related ‘reactive' species Free Radicals in Biology and Medicine 1999New York: Oxford University Press; 3rd edn. ed. Halliwell, B. & Gutteridge, J.M.C. [Google Scholar]
- HAMILTON C.A., MCPHADEN A.R., BERG G., PATHI V., DOMINICZAK A.F. Is hydrogen peroxide an EDHF in human radial arteries. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2451–H2455. doi: 10.1152/ajpheart.2001.280.6.H2451. [DOI] [PubMed] [Google Scholar]
- HAYABUCHI Y., NAKAYA Y., MATSUOKA S., KURODA Y. Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. Heart Vessels. 1998;13:9–17. doi: 10.1007/BF02750638. [DOI] [PubMed] [Google Scholar]
- HOLLAND J.A., MEYER J.W., CHANG M.M., O'DONNELL R.W., JOHNSON D.K., ZIEGLER L.M. Thrombin stimulated reactive oxygen species production in cultured human endothelial cells. Endothelium. 1998;6:113–121. doi: 10.3109/10623329809072198. [DOI] [PubMed] [Google Scholar]
- IIDA Y., KATUSIC Z.S. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke. 2000;31:2224–2230. doi: 10.1161/01.str.31.9.2224. [DOI] [PubMed] [Google Scholar]
- ITOH T., KAJIKURI J., HATTORI T., KUSAMA N., YAMAMOTO T. Involvement of H2O2 in superoxide dismutaseinduced enhancement of endothelium-dependent relaxation in rabbit mesenteric resistance artery. Br. J. Pharmacol. 2003;139:444–456. doi: 10.1038/sj.bjp.0705255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONTOS H.A., WEI E.P., POVLISHOCK J.T., CHRISTMAN C.W. Oxygen radicals mediate the cerebral arteriolar dilation from arachidonate and bradykinin in cats. Circ. Res. 1984;55:295–303. doi: 10.1161/01.res.55.3.295. [DOI] [PubMed] [Google Scholar]
- LORENZ R.R., WARNER D.O., JONES K.A. Hydrogen peroxide decreases Ca(2+) sensitivity in airway smooth muscle by inhibiting rMLC phosphorylation. Am. J. Physiol. 1999;277:L816–L822. doi: 10.1152/ajplung.1999.277.4.L816. [DOI] [PubMed] [Google Scholar]
- MARTIN W., MCALLISTER K.H., PAISLEY K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacology. 1994;33:1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- MATOBA T., SHIMOKAWA H., NAKASHIMA M., HIRAKAWA Y., MUKAI Y., HIRANO K., KANAIDE H., TAKESHITA A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J. Clin. Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGUIRE J.J., DING H., TRIGGLE C.R. Endothelium-derived relaxing factors: a focus on endothelium-derived hyper-polarizing factor(s) Can. J. Physiol. Pharmacol. 2001;79:443–470. [PubMed] [Google Scholar]
- NOBE K., PAUL R.J. Distinct pathways of Ca2+ sensitization in porcine coronary artery: effect of Rho-related kinase and protein kinase C inhibition on force and intracellular Ca2+ Circ. Res. 2001;88:1283–1290. doi: 10.1161/hh1201.092035. [DOI] [PubMed] [Google Scholar]
- OMAR H.A., CHERRY P.D., MORTELLITI M.P., BURKE-WOLIN T., WOLIN M.S. Inhibition of coronary artery superoxide dismutase attenuates endothelium-dependent and -independent nitrovasodilator relaxation. Circ. Res. 1991;69:601–608. doi: 10.1161/01.res.69.3.601. [DOI] [PubMed] [Google Scholar]
- PANNIRSELVAM M., VERMA S., ANDERSON T.J., TRIGGLE C.R. Cellular basis of endothelial dysfunction in small mesenteric arteries from spontaneously diabetic (db/db −/−) mice: role of decreased tetrahydrobiopterin bioavailability. Br. J. Pharmacol. 2002;136:255–263. doi: 10.1038/sj.bjp.0704683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS W.J., LORENZ R.R., BOGOGER M., WARNER D.O., CREMO C.R., JONES K.A. A novel mechanism by which hydrogen peroxide decreases calcium sensitivity in airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;284:L324–L332. doi: 10.1152/ajplung.00159.2002. [DOI] [PubMed] [Google Scholar]
- POMPOSIELLO S., RHALEB N.E., ALVA M., CARRETERO O.A. Reactive oxygen species: role in the relaxation induced by bradykinin or arachidonic acid via EDHF in isolated porcine coronary arteries. J. Cardiovasc. Pharmacol. 1999;34:567–574. doi: 10.1097/00005344-199910000-00014. [DOI] [PubMed] [Google Scholar]
- RABELO L.A., CORTES S.F., ALVAREZ-LEITE J.I., LEMOS V.S. Endothelium dysfunction in LDL receptor knockout mice: a role for H2O2. Br. J. Pharmacol. 2003;138:1215–1220. doi: 10.1038/sj.bjp.0705164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G.M., VANHOUTTE P.M. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am. J. Physiol. 1986;250:H815–H821. doi: 10.1152/ajpheart.1986.250.5.H815. [DOI] [PubMed] [Google Scholar]
- SOBEY C.G., HEISTAD D.D., FARACI F.M. Mechanisms of bradykinin-induced cerebral vasodilatation in rats. Evidence that reactive oxygen species activate K+ channels. Stroke. 1997;28:2290–2294. doi: 10.1161/01.str.28.11.2290. [DOI] [PubMed] [Google Scholar]
- STROES E., HIJMERING M., VAN ZANDVOORT M., WEVER R., RABELINK T.J., VAN FAASSEN E.E. Origin of superoxide production by endothelial nitric oxide synthase. FEBS Lett. 1998;438:161–164. doi: 10.1016/s0014-5793(98)01292-7. [DOI] [PubMed] [Google Scholar]
- TRIGGLE C.R., DONG H., WALDRON G.J., COLE W.C. Endothelium-derived hyperpolarizing factor(s): species and tissue heterogeneity. Clin. Exp. Pharmacol. Physiol. 1999;26:176–179. doi: 10.1046/j.1440-1681.1999.03007.x. [DOI] [PubMed] [Google Scholar]
- WEI E.P., KONTOS H.A. H2O2 and endothelium-dependent cerebral arteriolar dilation. Implications for the identity of endothelium-derived relaxing factor generated by acetylcholine. Hypertension. 1990;16:162–169. doi: 10.1161/01.hyp.16.2.162. [DOI] [PubMed] [Google Scholar]
- YANG S.T., MAYHAN W.G., FARACI F.M., HEISTAD D.D. Mechanisms of impaired endothelium-dependent cerebral vasodilatation in response to bradykinin in hypertensive rats. Stroke. 1991;22:1177–1182. doi: 10.1161/01.str.22.9.1177. [DOI] [PubMed] [Google Scholar]
- ZEMBOWICZ A., HATCHETT R.J., JAKUBOWSKI A.M., GRYGLEWSKI R.J. Involvement of nitric oxide in the endothelium-dependent relaxation induced by hydrogen peroxide in the rabbit aorta. Br. J. Pharmacol. 1993;110:151–158. doi: 10.1111/j.1476-5381.1993.tb13785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H., SCHMEISSER A., GARLICHS C.D., PLOTZE K., DAMME U., MUGGE A., DANIEL W.G. Angiotensin II-induced superoxide anion generation in human vascular endo-thelial cells: role of membrane-bound NADH-/NADPH-oxidases. Cardiovasc. Res. 1999;44:215–222. doi: 10.1016/s0008-6363(99)00183-2. [DOI] [PubMed] [Google Scholar]