Abstract
In response to pancreatic injury and in cell culture, pancreatic stellate cells (PSCs) are transformed (‘activated') into highly proliferative myofibroblast-like cells, which express α-smooth muscle actin (α-SMA), and produce type I collagen and other extracellular matrix components. There is accumulating evidence that activated PSCs play important roles in pancreatic fibrosis and inflammation.
The small GTP-binding protein Rho has emerged as an important regulator of the actin cytoskeleton and cell morphology through the downstream effector Rho kinase (ROCK). But, the roles of Rho-ROCK pathway in PSCs are unknown. Here, we examined the effects of (+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide (Y-27632) and HA-1077 (fasudil), specific inhibitors of ROCK, on the activation of PSCs.
PSCs were isolated from the pancreas of male Wistar rats after perfusion with collagenase P. The actin cytoskeleton was analyzed by phalloidin staining. Expression of RhoA and ROCK was examined by immunostaining and Western blotting. Effects of Y-27632 and HA-1077 on α-SMA expression, platelet-derived growth factor-induced proliferation and chemotaxis, and collagen production were assessed.
Culture-activated PSCs developed a well-spread cell shape, with extended stress fiber formation. PSCs expressed RhoA, ROCK-1, and ROCK-2.
Y-27632 caused disassembly of stress fibers. Y-27632 and HA-1077 inhibited α-SMA expression, proliferation, chemotaxis, and type I collagen production in culture-activated PSCs.
In addition, Y-27632 and HA-1077 inhibited spontaneous activation of freshly isolated PSCs in culture on plastic.
These findings suggest a role of Rho-ROCK pathway in the activation process of PSCs by regulating the actin cytoskeleton, and a potential application of Rho-ROCK pathway inhibitors for the treatment of pancreatic inflammation and fibrosis.
Keywords: pancreatitis, pancreatic fibrosis, pancreatic stellate cells, Rho, Rho kinase, ROCK, actin cytoskeleton, Y-27632, HA-1077, fasudil
Introduction
Chronic pancreatitis as well as pancreatic cancer are accompanied by progressive fibrosis that is characterized by the loss of functional tissue and its replacement by extracellular matrix-rich connective tissue (Etemad & Whitcomb, 2001; Lankisch, 2001). In contrast to liver fibrosis, the molecular mechanisms of pancreatic fibrosis remain largely unknown. In 1998, star-shaped cells in the pancreas, namely pancreatic stellate cells (PSCs), were identified and characterized (Apte et al., 1998; Bachem et al., 1998). In normal pancreas, stellate cells are quiescent and can be identified by the presence of vitamin A-containing lipid droplets in the cytoplasm. In response to pancreatic injury, they are transformed (‘activated') from their quiescent phenotype into highly proliferative myofibroblast-like cells that express the cytoskeletal protein α-smooth muscle actin (α-SMA), and produce type I collagen and other extracellular matrix components. Many of the morphological and metabolic changes associated with the activation of PSCs in animal models of fibrosis also occur when these cells are grown in culture on plastic in serum-containing medium. Therefore, culture of primary PSCs on plastic has been accepted as an established model that mimics the phenotypic changes that occur during the process of PSC activation following pancreatic injury. There is accumulating evidence that PSCs, like hepatic stellate cells, are responsible for the development of pancreatic fibrosis (Apte et al., 1998; Bachem et al., 1998; Haber et al., 1999; Masamune et al., 2002b). It has also been suggested that PSCs may participate in the pathogenesis of acute pancreatitis (Haber et al., 1999; Masamune et al., 2002c). The molecular mechanisms responsible for PSC activation remain to be elucidated, but the activation of signaling pathways such as p38 mitogen-activated protein kinase (Masamune et al., 2003b) is likely to play a central role. Obviously, the signaling mechanisms regulating the activation of PSCs are potential targets for the development of new treatments for pancreatic fibrosis and inflammation.
Rho is a member of the Ras superfamily of small GTP-binding proteins, and regulates the organization of the actin cytoskeleton by promoting the assembly of focal adhesions and actin stress fibers (Takai et al., 1995; Amano et al., 1997; Hall, 1998). The Rho receives upstream signals, and is converted from the GDP-bound inactive form to the GTP-bound active form, which then interacts with downstream target molecules and transduces signals to each downstream pathway (Takai et al., 1995; Hall, 1998). Among the several effector molecules of Rho, the Rho kinase (ROCK) has been shown to mediate RhoA-induced assembly of focal adhesions and stress fibers (Takai et al., 1995; Hall, 1998). ROCK exists in two isoforms: p160ROCK (also known as ROKβ or ROCK-1) and ROKα (also called ROCK-2) (Takai et al., 1995; Hall, 1998). ROCK-1 has 64% sequence identity with ROCK-2 throughout the structure, and the kinase domain of ROCK-1 is highly conserved with that of ROCK-2 (90% identical) (Takai et al., 1995; Hall, 1998). It has been shown that ROCK phosphorylates and inactivates myosin light chain (MLC) phosphatase in vitro, and thereby regulates MLC phosphorylation at Ser 19 (Kimura et al., 1996). (+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide (Y-27632) is a specific inhibitor of both isoforms of ROCK, and has been shown to inhibit the formation of stress fibers and focal adhesions induced by ROCK (Uehata et al., 1997). Y-27632 has contributed to clarifying the roles of Rho-ROCK pathway for various cell functions such as cell morphology, smooth muscle contraction, platelet aggregation, cell motility, and cytokinesis (Amano et al., 1997). In addition, it has been shown that the Rho-ROCK pathway plays critical roles in diverse cellular events such as membrane trafficking, transcriptional regulation, cell growth, and development (Van Aelst & D'Souza-Schorey, 1997). However, the roles of Rho-ROCK pathway in PSCs are unknown.
In this study, we examined the effects of Y-27632 as well as another ROCK inhibitor HA-1077 (fasudil) (Nagumo et al., 2000) on the activation of PSCs. Here, we report that Rho-ROCK pathway is involved in the morphological changes during the activation process, through the reorganization of actin cytoskeleton. Y-27632 and HA-1077 inhibited several parameters of PSC activation including α-SMA expression, proliferation, chemotaxis, and collagen production. In addition, Y-27632 and HA-1077 inhibited spontaneous activation of freshly isolated PSCs in culture, suggesting a potential application of Rho-ROCK inhibitors for the treatment of pancreatic fibrosis and inflammation.
Methods
Materials
Y-27632 was generously provided by Mitsubishi Pharma Co. (Osaka, Japan), and resuspended at 10 mM in water. Mouse anti-RhoA and goat anti-ROCK-1 (C-19) and anti-ROCK-2 (C-20) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). These anti-ROCK antibodies have been previously shown to specifically differentiate the two isoforms of ROCK (Sebbagh et al., 2001). Collagenase P was from Roche Diagnostics (Mannheim, Germany). Rat recombinant platelet-derived growth factor (PDGF)-BB was from R&D Systems (Minneapolis, MN, U.S.A.). Rhodamine-labeled phalloidin was purchased from Molecular Probes (Eugene, OR, U.S.A.). Rabbit antibodies against phosphorylated MLC II, phosphorylated extracellular signal-regulated kinase (ERK), total ERK, phosphorylated Akt, total Akt, and phosphorylated p70 S6 kinase were purchased from Cell Technologies (Beverly, MA, U.S.A.). Rabbit antibody against glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was from Trevigen (Gaithersburg, MD, U.S.A.). All other reagents were from Sigma-Aldrich (St Louis, MO, U.S.A.), unless specifically described.
Cell culture
All animal procedures were performed in accordance with the National Institutes of Health Animal Care and Use Guidelines. Rat PSCs were prepared from the pancreas tissues of male Wistar rats (Japan SLC Inc., Hamamatsu, Japan), weighing 200–250 g as previously described (Masamune et al., 2003a). Rats were anesthetized by intraperitoneal injection of sodium pentobarbital, the abdomen was opened, and a cannula was inserted into the right jugular vein. After perfusion with Hanks' balanced salt solution without Ca2+ or Mg2+ and containing 0.5 mM EGTA at 2.5 ml min−1 for 10 min, perfusion with 0.03% collagenase P with in Hanks' balanced salt solution with Ca2+ and Mg2+ at 2.5 ml min−1 for 12 min was performed. The distended pancreas was resected and minced with scissors, and shaken with 0.03% collagenase P solution for 10 min at 37°C. The digested tissue was pipetted through narrow orifices, filtered through a 150-μm mesh, and centrifuged. Cells were then resuspended in 8 ml of Gey's balanced salt solution containing 0.3% BSA. The cell suspension was mixed with 9 ml of 28.7% (wt vol−1) of the Nycodenz solution (Nycomed Pharma, Oslo, Norway) in Gey's balanced salt solution. The Nycodenz gradient was prepared by laying the cell suspension in Nycodenz underneath 6 ml of Gey's balanced salt solution with BSA in a 50-ml centrifuge tube. The gradient was centrifuged at 1400 × g for 20 min. Stellate cells separated into a fuzzy band just above the interface of the Nycodenz solution and the aqueous buffer. This band was harvested, and the cells were washed and resuspended in Ham's F-12 containing 10% heat-inactivated fetal bovine serum (ICN Biomedicals, Aurora, OH, U.S.A.), penicillin sodium, and streptomycin sulfate. Cell purity was always more than 90%, as assessed by a typical star-like configuration and by detecting vitamin A autofluorescence. All experiments were performed using cells between passages two and five, except for those using freshly isolated PSCs. Unless specifically described, we incubated PSCs in serum-free medium for 24 h before the addition of experimental reagents.
Fluorescence microscopy
Stress fibers were stained with rhodamine-labeled phalloidin, as previously described (Kaneko et al., 2002). Briefly, after fixation with 4% paraformaldehyde for 5 min, PSCs were permeabilized with 0.15% Triton X-100 in phosphate-buffered saline for 5 min. Cells were then incubated with rhodamine-labeled phalloidin in blocking buffer (1% BSA in phosphate-buffered saline) for 2 h at room temperature in the dark. Cells were then washed three times with phosphate-buffered saline for 10 min each, and coverslipped with Fluoromount (Vector Laboratories, Burlingame, CA, U.S.A.). Cells were observed with a Leica fluorescence microscope. Images were captured with a Leica QFISH system (Wetzlar, Germany).
Immunostaining
Serum-starved PSCs were grown on slides, and immunostaining for RhoA was performed as previously described (Masamune et al., 1999), using a streptavidin–biotin–peroxidase complex detection kit (Histofine Kit; Nichirei, Tokyo, Japan). Briefly, the cells were fixed with ice-cold methanol, and then endogenous peroxidase activity was blocked by incubation in methanol with hydrogen peroxide for 5 min. After immersion in normal rabbit serum, the slides were incubated with mouse anti-RhoA antibody overnight. The slides were incubated with biotinylated goat anti-mouse immunoglobulin antibody, followed by peroxidase-conjugated streptavidin. Finally, color was developed by incubating the slides for several minutes with diaminobenzidine (Dojindo, Kumamoto, Japan). As a control, the primary antibody was replaced with phosphate-buffered saline. Expression of ROCK-1, ROCK-2, and glial fibrillary acidic protein (GFAP) was examined in a similar manner.
Western blot analysis
Western blot analysis was performed as previously described (Masamune et al., 2002d). Cells were lysed in sodium dodecyl sulfate buffer, and cellular proteins (approximately 100 μg) were fractionated on a 10% sodium dodecyl sulfate–polyacrylamide gel. They were transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA, U.S.A.), and the membrane was incubated overnight at 4°C with rabbit phospho-specific antibodies against ERK (at Thr 222 and Tyr 204), Akt (at Ser 473), p70 S6 kinase (at Thr 389), or MLC II (at Ser 19). After incubation with peroxidase-conjugated goat anti-rabbit secondary antibody for 1 h, proteins were visualized by using an ECL kit (Amersham Biosciences U.K., Ltd, Buckinghamshire, England). The levels of total ERK, total Akt, G3PDH, RhoA, ROCK1, and ROCK2 were determined in a similar manner.
Pull-down assay
Pull-down assay was performed using the Rho activation assay kit (Upstate Biotechnology Inc, Lake Placid, NY, U.S.A.), according to the manufacturer's instruction. Briefly, after treatment with PDGF-BB (at 25 ng ml−1) for 30 min, cells were lysed, and the total proteins from clarified lysates were incubated with a glutathione S-transferase (GST)-Rho-binding domain of rhotekin-agarose slurry to precipitate GTP-bound RhoA, or with agarose beads with GST alone. Retained GTP-bound RhoA was subjected to Western blotting using anti-RhoA antibody. The level of RhoA in the cell lysates was also determined.
Rho translocation assay
The membrane fraction was prepared as previously described (Wang & Bitar, 1998). Briefly, after treatment with PDGF-BB (at 25 ng ml−1) for 30 min, cells were washed, lysed, and ultracentrifuged at 100,000 × g for 60 min. The pellet was resuspended by sonication for 30 s in the lysis buffer containing 1% Triton X-100, and then ultracentrifuged at 100,000 × g for 30 min. The supernatant was collected as a soluble membrane fraction, and subjected to Western blotting using anti-RhoA antibody, as described earlier.
Cell proliferation assay
Serum-starved PSCs (approximately 20–30% density) were treated with Y-27632 or HA-1077 for 30 min, and then stimulated with PDGF-BB (at 25 ng ml−1) or 10% fetal bovine serum in serum-free medium. Cell proliferation was assessed using a commercial kit (CellTiter nonradioactive cell proliferation assay; Promega, Madison, WI, U.S.A.), according to the manufacturer's instruction. This assay is a colorimetric method for determining the number of viable cells, and there is a good correlation between this assay and the [3H]thymidine incorporation assay. Briefly, after 72-h incubation with PDGF-BB or fetal bovine serum, the dye solution was added to cells and incubation continued at 37°C for 4 h. Then, the formazan product was solubilized with the stop solution. Cell number was determined by differences in absorbance at wavelength 570 minus 690 nm.
Analysis of cell cycle
The cell cycle of PSCs was analyzed by flow cytometry as previously described (Masamune et al., 2001). Briefly, serum-starved PSCs (approximately 60–70% density) were treated with Y-27632 (at 25 μM) for 30 min and then exposed to PDGF-BB (at 25 ng ml−1). After 24 h, cells were harvested and washed twice with phosphate-buffered saline. Cells were suspended in phosphate-buffered saline solution containing 40 μg ml−1 propidium iodide, 0.02% Triton X-100, and 50 μg ml−1 ribonuclease A. Samples were incubated in the dark at room temperature for 30 min and stored at 4°C until analysis. Cell fluorescence was measured by a FACSCaliber flow cytometer (Becton Dickinson Co. Ltd, Tokyo, Japan), and analyzed using ModFit LT software (Verity Software House, Topsham, ME, U.S.A.) to determine the distribution of cells in the various phases of the cell cycle.
Cell migration assay
PSCs were serum-starved for 24 h, trypsinized, and resuspended in serum-free medium containing 1% BSA at a concentration of 3 × 105 cells ml−1. For the assay, we used modified Boyden chambers with 8-μm-pore filters (Iwaki glass Co. Ltd, Funabashi, Japan). PDGF-BB (at 25 ng ml−1) was added to the lower chamber and 100 μl of cell suspension was added to the upper chamber. The chambers were then incubated for 24 h at 37°C. At the end of the incubation, the cell suspension was aspirated, and the upper part of the filter was cleaned with cotton plugs. The culture inserts were removed, stained with Difquick (Sysmex, Kobe, Japan), and viewed at × 200 magnification. Cell counts were obtained in six randomly chosen fields.
Collagen assay
PSCs were incubated in serum-free medium in the presence or absence of Y-27632 or HA-1077 in serum-free medium for 48 h. Type I collagen released into the culture supernatant was quantified by enzyme-linked immunosorbent assay, as previously described (Moshage et al., 1990). Briefly, immunoassay plates (Becton Dickinson, Franklin Lakes, NJ, U.S.A.) were coated with diluted samples overnight at 4°C. After blocking with 5% drymilk in phosphate-buffered saline, plates were incubated with rabbit anti-rat type I collagen antibody (LSL Cosmo Bio, Tokyo, Japan). After washes, goat anti-rabbit IgG antibody conjugated with alkaline phosphatase was added, and incubated. Finally, P-nitrophenylphosphate was added as a substrate, and the collagen levels were determined by differences in absorbance at wavelength 405 minus 690 nm. Rat tail collagen type I was used as a standard. The collagen levels in each sample were normalized to the cellular DNA content, which was determined by a fluorometric assay, according to the method of Brunk et al. (1979). The results are expressed as a percentage of the untreated control.
Effect of Y-27632 and HA-1077 on spontaneous activation of PSCs in culture
Freshly isolated PSCs were incubated with or without Y-27632 (at 10 or 25 μM) or HA-1077 (at 25 μM) in serum-free medium for 1 h; then fetal bovine serum was added at the final concentration of 5%. After 7-days incubation, morphological changes characteristic of PSC activation were assessed after staining with GFAP. In addition, total cellular proteins (approximately 25 μg) were prepared, and the level of α-SMA was determined by Western blotting.
Statistical analysis
The results were expressed as mean+s.d. Luminograms and photographs are representative of at least three experiments. Differences between experimental groups were evaluated by the two-tailed unpaired Student's t-test. A P-value less than 0.05 was considered statistically significant.
Results
Cytoskeletal changes during PSC activation
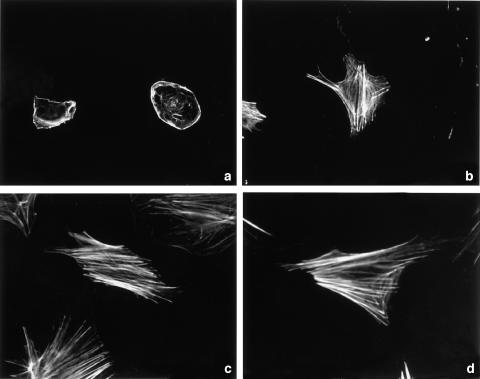
To assess the time-dependent changes of actin cytoskeleton during the activation of PSCs, freshly isolated PSCs were cultured for 1, 3, 8, and 14 days, followed by rhodamine-labeled phalloidin staining. Actin fibers were only detected at the cell surface and filopodia in cells on the day 1 (Figure 1a). During the culture, PSCs increasingly showed stress fiber formation (Figure 1b, c). Finally, almost all cells showed the activated phenotype, with well-spread cell shape and extended stress fiber formation (Figure 1d). These phenotypic changes occurred in a time-dependent manner, indicating that cytoskeletal reorganization was involved in the activation of PSCs.
Figure 1.
Stress fiber formation during the activation of PSCs. Freshly isolated PSCs were cultured for 1 (a), 3 (b), 8 (c), or 14 (d) in 10% serum-containing medium, and the time-dependent changes of actin cytoskeleton were assessed by rhodamine-labeled phalloidin staining. Original magnification: × 60 objective.
Expression of RhoA and ROCK
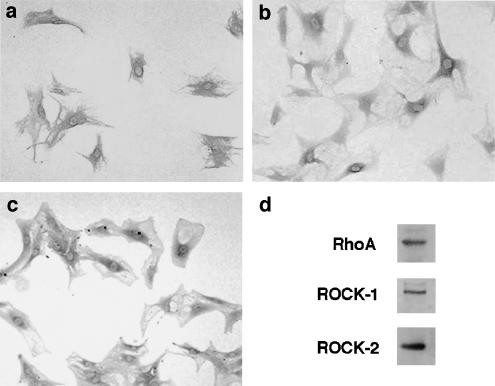
We next examined the expression of RhoA and ROCK in culture-activated PSCs by immunostaining. RhoA expression was diffusely detected in the cytoplasm of culture-activated PSCs (Figure 2a). Immunoreactivity for both isoforms of ROCK was observed mainly in the perinuclear cytoplasm in culture-activated cells (Figure 2b, c). Expression of RhoA and ROCK was confirmed by Western blotting (Figure 2d).
Figure 2.
Expression of RhoA and ROCK in PSCs. (a–c) Culture-activated PSCs (passage 2) were grown directly on slides. Immunostaining for RhoA (a), ROCK-1 (b), or ROCK-2 (c) was performed in serum-starved PSCs using a streptavidin–biotin–peroxidase complex detection kit. Original magnification: × 10 objective. (d) Total cell lysates (approximately 100 μg) were prepared from PSCs (passage 2), and the levels of RhoA and ROCK were determined by Western blotting.
Effects of Y-27632 on the cell shape and actin cytoskeleton
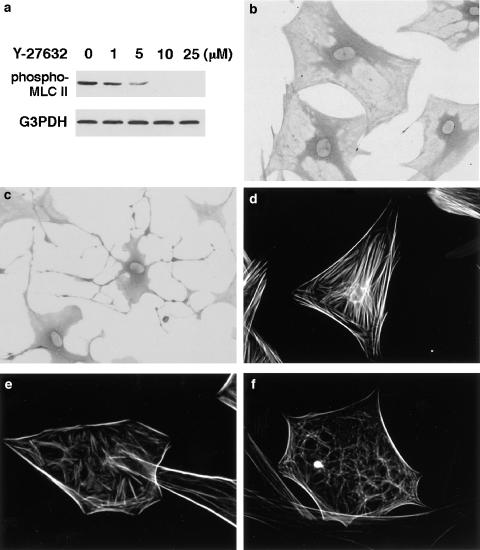
To clarify the role of Rho-ROCK pathway in the cytoskeletal reorganization, we employed Y-27632, a specific ROCK inhibitor (Uehata et al., 1997). We first examined the effects of Y-27632 on the phosphorylation of MLC II at Ser 19, which is a downstream target of ROCK (Kimura et al., 1996). Treatment of PSCs with Y-27632 resulted in the decrease of endogenous phosphorylation of MLC II (Figure 3a). When cells were treated with Y-27632, cells changed to an elongated, fusiform morphology with prominent dendritic processes (Figure 3b, c), suggesting that ROCK was necessary as a downstream effector of Rho for the cell spreading in PSCs. The effect of Y-27632 on the actin cytoskeleton was assessed by phalloidin staining. When cells were treated with Y-27632 at 10 or 25 μM for 24 h, disassembly of stress fibers was observed (Figure 3d–f). In these experiments, Y-27632 up to 25 μM did not affect the cell viability during the incubation, as assessed by trypan blue exclusion test (data not shown). In addition, the effects of Y-27632 were fully reversible within 48 h after the removal of Y-27632 (data not shown). However, when PSCs were treated with Y-27632 above 50 μM, cytotoxic effects were observed during the incubation.
Figure 3.
Decreased stress fiber formation. (a) Culture-activated PSCs were incubated with Y-27632 at the indicated concentrations for 30 min in serum-free medium. Then, total cell lysates (approximately 100 μg) were prepared, and the level of phosphorylated MLC II was determined by Western blotting. The level of G3PDH was also determined as a loading control. (b, c) PSCs were incubated in the absence (b) or presence (c) of Y-27632 (at 25 μM) for 4 h, and morphological changes were examined under light microscopy. Original magnification: × 20 objective. (d–f) PSCs were incubated in the absence (d) or presence of Y-27632 (at 10 (e) or 25 μM (f)) for 24 h, and stained with rhodamine-labeled phalloidin. Original magnification: × 60 objective.
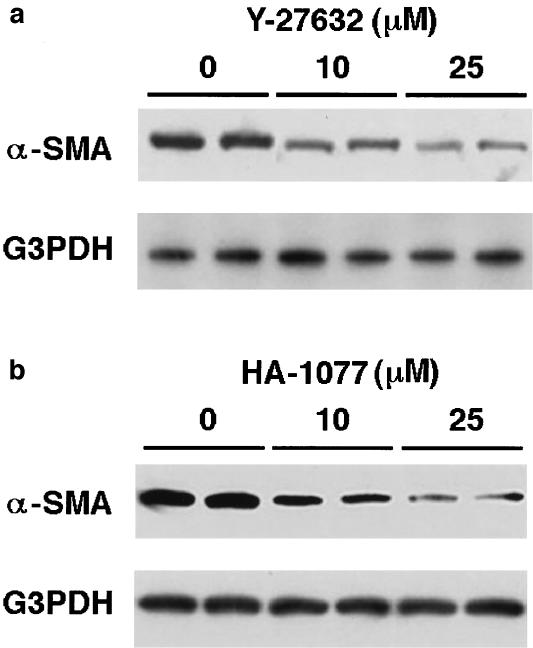
Y-27632 and HA-1077 decreased α-SMA expression
Culture-activated PSCs express α-SMA, and α-SMA expression has been accepted as a marker of PSC activation (Apte et al., 1998). Expression of α-SMA was confirmed in culture-activated PSCs by Western blotting, and the treatment of PSCs with Y-27632 at 10 or 25 μM for 48 h reduced the α-SMA expression (Figure 4a). Another ROCK inhibitor HA-1077, which is structurally unrelated to Y-27632 (Nagumo et al., 2000), was used to confirm the effect of inhibition of ROCK on α-SMA expression. Like Y-27632, HA-1077 (at 10 and 25 μM) decreased the α-SMA expression (Figure 4b). In these experiments, HA-1077 up to 25 μM did not affect the cell viability during the incubation, as assessed by trypan blue exclusion test (data not shown). However, when PSCs were treated with HA-1077 above 50 μM, cytotoxic effects were observed during the incubation.
Figure 4.
Y-27632 and HA-1077 decreased α-SMA expression. Culture-activated PSCs were incubated in the absence or presence of Y-27632 (at 10 or 25 μM) (a) or HA-1077 (at 10 or 25 μM) (b) for 48 h in serum-free medium. Then, total cell lysates (approximately 100 μg) were prepared, and the level of α-SMA was determined by Western blotting. The level of G3PDH was also determined as a loading control.
Y-27632 and HA-1077 inhibited PSC proliferation
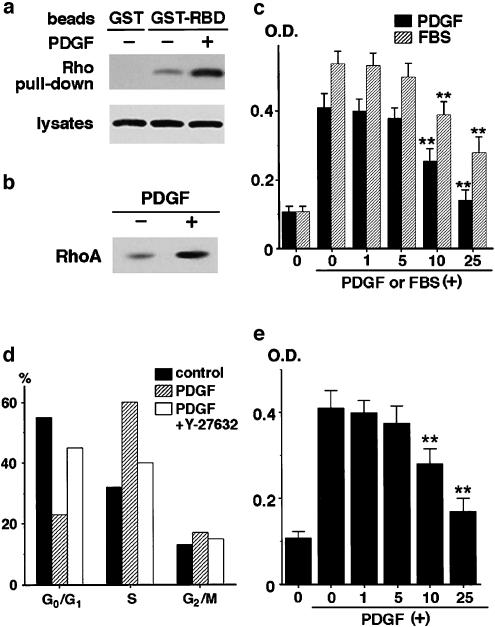
RhoA was activated in culture-activated PSCs, and the PDGF treatment augmented the activation, as assessed by the pull-down assay (Figure 5a) and the translocation of RhoA from the cytosol to the membrane (Figure 5b). We examined the effects of Y-27632 on PDGF-induced proliferation of PSCs. As previously reported (Masamune et al., 2003a), PDGF-BB induced approximately four-fold increase of cell proliferation in serum-free medium after 72 h (Figure 5c). Y-27632 inhibited PDGF-induced PSC proliferation in a dose-dependent manner. At 25 μM, Y-27632 almost completely abolished the stimulation of cell proliferation by PDGF. The inhibitory effect of Y-27632 on PSC proliferation was confirmed by measurement of bromodeoxyuridine incorporation during DNA synthesis (data not shown). Y-27632 also inhibited cell proliferation in response to 10% fetal bovine serum (Figure 5c).
Figure 5.
Y-27632 and HA-1077 inhibited proliferation of PSCs. (a) After treatment with PDGF-BB (at 25 ng ml−1) for 30 min, PSCs were lysed, and the total proteins from clarified lysates were incubated with GST-Rho-binding domain of rhotekin-agarose slurry (‘GST-RBD') to precipitate GTP-bound RhoA, or with agarose beads with GST alone. Precipitated RhoA was subjected to Western blotting using anti-RhoA antibody (‘Rho pull-down'). The level of RhoA in the lysates was also determined. (b) After treatment with PDGF-BB (at 25 ng ml−1) for 30 min, cells were lysed, and ultracentrifuged. The pellet was resuspended by sonication in the lysis buffer containing 1% Triton X-100, and ultracentrifuged. The supernatant was collected as a soluble membrane fraction, and subjected to Western blotting using anti-RhoA antibody. (c) Serum-starved PSCs were pretreated with Y-27632 at indicated concentrations for 30 min before exposure to PDGF-BB (at 25 ng ml−1) or 10% fetal bovine serum. After 72-h incubation with PDGF or fetal bovine serum, cell proliferation was assessed. The differences in absorbance at wavelength 570 minus 690 nm are shown (‘OD'). Data are shown as mean+s.d. (n=7). P<0.01 vs PDGF-BB or fetal bovine serum only. (d) PSCs were treated with Y-27632 (at 25 μM) for 30 min and then stimulated with PDGF-BB (at 25 ng ml−1). After 24-h incubation, the cells were harvested, and cell cycle was analyzed by flow cytometry after staining with propidium iodide. Data show the percentage of cells in each phase of the cell cycle in a representative experiment. (e) PSCs were pretreated with HA-1077 at indicated concentrations for 30 min before exposure to PDGF-BB (at 25 ng ml−1). After 72-h incubation with PDGF, cell proliferation was assessed. The differences in absorbance at wavelength 570 minus 690 nm are shown (‘OD'). Data are shown as mean+s.d. (n=6). **P<0.01 vs PDGF-BB only.
We analyzed the cell cycle in PSCs in the presence or absence of Y-27632. Exposure to PDGF was associated with a marked decrease in the percentage of cells in the G0 and G1 phase, together with an increase in the number of cells in the S phase (Figure 5d). The addition of Y-27632 before PDGF reduced the number of cells in the S phase, and the percentage of cells in the G0 and G1 phase was roughly similar to the percentage observed in untreated cells. Thus, the addition of Y-27632 inhibited PDGF-induced progression of the cell cycle beyond the G1 phase.
Like Y-27632, HA-1077 inhibited PDGF-induced proliferation of PSCs (Figure 5e).
Y-27632 and HA-1077 inhibited PDGF-induced chemotaxis of PSCs
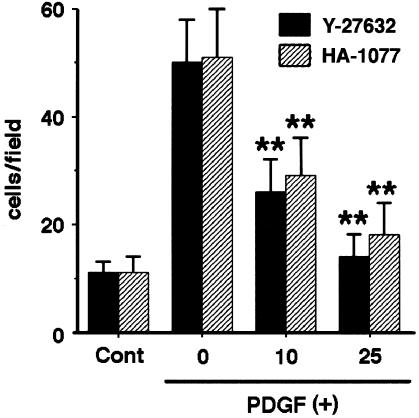
We have recently found that PDGF-BB induced chemotaxis of PSCs (Masamune et al., 2003a). We examined the effect of Y-27632 and HA-1077 on PDGF-induced chemotaxis. PDGF-BB (at 25 ng ml−1) induced approximately four-fold increase in chemotaxis of PSCs (Figure 6). Both Y-27632 and HA-1077 inhibited PDGF-induced PSC migration (Figure 6).
Figure 6.
Y-27632 and HA-1077 inhibited PDGF-induced chemotaxis. Cell migration was assessed using modified Boyden chambers with 8-μm pore filters. Serum-starved PSCs were left untreated (‘Cont') or were treated with PDGF-BB (at 25 ng ml−1) in the lower chamber in the absence or presence of Y-27632 or HA-1077 (at 10 or 25 μM). After 24-h incubation with PDGF-BB, the cells migrated to the underside of the filter were stained, and viewed at × 200 magnification. Cell counts were obtained in six randomly chosen fields. Data are shown as mean+s.d. (n=6). **P<0.01 vs PDGF-BB only.
Y-27632 did not affect the activation of ERK, Akt, and p70 S6 kinase
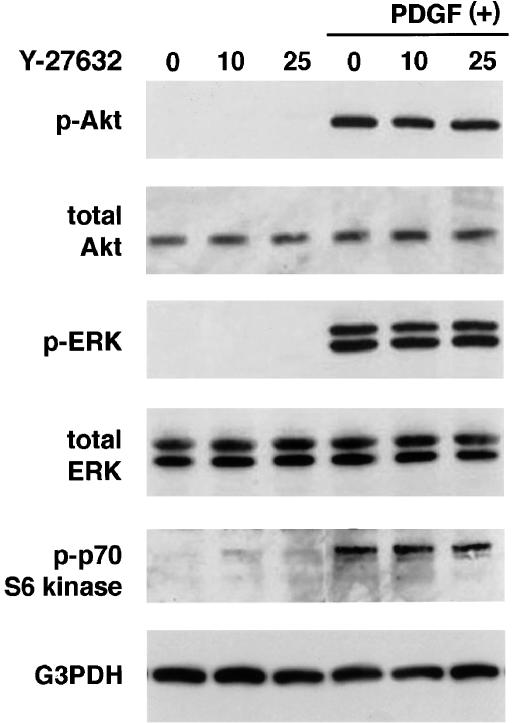
To elucidate the mechanisms by which Y-27632 inhibited proliferation and chemotaxis of PSCs, we examined the effects of Y-27632 on the activation of ERK and phosphatidylinositol 3-kinase-Akt pathways. These pathways have been shown to play central roles in the proliferation and migration of PSCs, respectively (Jaster et al., 2002; Masamune et al., 2003a). We assessed the activation of ERK and Akt by Western blotting using anti-phosphospecific antibodies. PDGF-BB rapidly activated ERK and Akt, but Y-27632 did not affect the PDGF-induced phosphorylation of ERK and Akt (Figure 7a, b). In addition, Y-27632 did not affect PDGF-induced phosphorylation of p70 S6 kinase (Figure 7c).
Figure 7.
Y-27632 did not affect PDGF-induced activation of Akt, ERK, and p70 S6 kinase. PSCs were preincubated with Y-27632 at the indicated concentrations (μM) for 30 min before exposure to PDGF-BB (at 25 ng ml−1). After 5-min incubation with PDGF, total cell lysates (approximately 100 μg) were prepared, and the levels of phosphorylated Akt, ERK, and p70 S6 kinase were determined by Western blotting. The levels of total ERK, Akt, and G3PDH were also determined.
Y-27632 inhibited collagen production
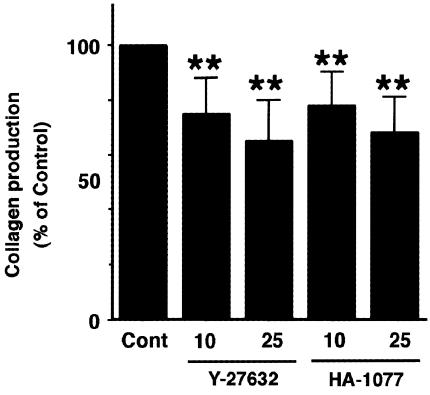
It has been accepted that PSCs are the principal source of collagen in the fibrotic pancreas (Haber et al., 1999). We examined the effect of ROCK inhibitors on the type I collagen release from PSCs into the culture supernatants by enzyme-linked immunosorbent assay. Y-27632 and HA-1077 decreased the collagen production (Figure 8).
Figure 8.
Y-27632 and HA-1077 decreased collagen production. PSCs were treated with Y-27632 or HA-1077 (at 25 μM) in serum-free medium for 48 h. The type I collagen released into the culture supernatant was quantified by enzyme-linked immunosorbent assay. The collagen levels in each sample were normalized to the cellular DNA content. The results are expressed as a percentage of the untreated control (‘Con'). Data are shown as mean+s.d. (n=6). **P<0.01 vs control.
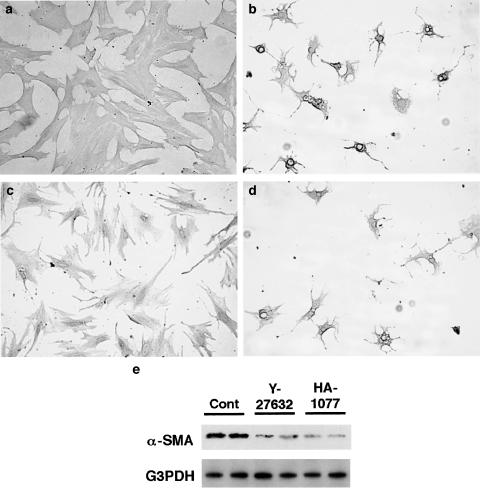
Y-27632 and HA-1077 inhibited the spontaneous activation of PSCs in culture
Finally, we examined whether Y-27632 and HA-1077 blocked the transformation of PSCs from quiescent to myofibroblast-like phenotype in culture. Freshly isolated PSCs were incubated with Y-27632 or HA-1077 in 5% serum-containing medium for 7 days. Morphological changes characteristic of PSC activation were assessed after staining with GFAP. After 7 days, PSCs cultured without Y-27632 or HA-1077 showed transformation into cells with a myofibroblast-like phenotype (Figure 9a). In contrast, PSCs cultured in the presence of Y-27632 (at 25 μM) were small and circular, with lipid droplets present in many cells and with slender dendritic processes (Figure 9b). Similar effect was observed with Y-27632 at 10 μM (data not shown). To rule out the possibility that the effects of Y-27632 might have been due to cytotoxicity, Y-27632 was withdrawn from PSCs that had been treated with it for 7 days. Within 48 h after the withdrawal of Y-27632, PSCs had acquired the activated phenotype (Figure 9c). Like Y-27632, HA-1077 inhibited the transformation into myofibroblast-like cells (Figure 9d). In agreement with the morphological changes, significant expression of α-SMA was observed on day 7 in culture-activated PSCs, and the expression was much lower in the presence of Y-27632 or HA-1077 (Figure 9e).
Figure 9.
Y-27632 and HA-1077 inhibited spontaneous activation of freshly isolated PSCs. (a–d) Freshly isolated PSCs were incubated in the absence (a) or presence of Y-27632 (at 25 μM) (b) or HA-1077 (at 25 μM) (d) for 7 days in 5% serum-containing medium, and morphological changes were assessed under phase-contrast microscopy after staining with GFAP. Original magnification: × 10 objective. (c) Y-27632 was withdrawn from PSCs that had been treated with it for 7 days. At 2 days after the withdrawal of Y-27632, PSCs showed the typical phenotype of activated PSCs. Original magnification: × 10 objective. (e) Total cell lysates (approximately 25 μg) were prepared from cells treated with or without ROCK inhibitors (at 25 μM) for 7 days, and the level of α-SMA was determined by Western blotting. The level of G3PDH was also determined as a loading control.
Discussion
Following pancreatic injury, PSCs undergo transformation from quiescent cells to activated myofibroblast-like cells. During the process of activation, the cells changed shape from small, rounded cells to well-spread cells. Here, we showed that the activation process of PSCs was accompanied by distinct changes in the actin cytoskeleton, that is, time-dependent formation of stress fibers. Activated PSCs expressed both isoforms of ROCK as well as RhoA. Inhibition of ROCK by Y-27632 caused disassembly of stress fibers, and inhibited key parameters of PSC activation including α-SMA expression, proliferation, migration, and collagen production in culture-activated PSCs. In addition, Y-27632 blocked the spontaneous activation of freshly isolated PSCs in culture. These effects were also observed with another ROCK inhibitor HA-1077, which is structurally unrelated to Y-27632 (Nagumo et al., 2000), suggesting a role of the Rho-ROCK pathway in the activation process of PSCs by regulating the actin cytoskeleton. These results are in agreement with the previous study in rat hepatic stellate cells that RhoA directs activation-associated alternations in the acto-myosin cytoskeleton (i.e., formation of actin stress fibers and focal adhesions) (Yee, 1998). The effects of Y-27632 in this study are not due to the cytotoxicity of Y-27632, because the concentrations of the reagent (up to 25 μM) used in this study did not reduce cell viability and the effects were reversible after the removal of Y-27632. Y-27632 has been extensively used as a specific inhibitor of ROCK in a variety of cell types. Sahai et al. (1999) reported that Y-27632 exhibited selectivity for ROCK in vitro and did not inhibit other kinases tested, including other Rho effectors such as PRK1/PKN and citron kinase, and protein kinases A and C. Furthermore, we showed that Y-27632 inhibited the phosphorylation of MLC as well as the formation of stress fibers, which had been shown to be ROCK-dependent in vitro (Hall, 1994; Takai et al., 1994). Culture-activated PSCs expressed both ROCK-1 and ROCK-2. Since Y-27632 inhibits both isoforms of ROCK, it remains unknown which of the isoforms plays a central role in the activation of PSCs.
In addition to the inhibition of spontaneous activation of PSCs in culture, Y-27632 and HA-1077 decreased α-SMA expression, which has been accepted as a marker of PSC activation (Apte et al., 1998) in culture-activated PSCs. This is in agreement with the previous studies showing that Y-27632 inhibited α-SMA expression or promoter activity in several types of cells including rat vascular smooth muscle cells (Mack et al., 2001), mesangial cells (Patel et al., 2003), and hepatic stellate cells (Tada et al., 2001). The Rho-mediated regulation of α-SMA gene expression has been investigated in detail in NIH3T3 cells (Sotiropoulos et al., 1999) and rat vascular smooth muscle cells (Mack et al., 2001); RhoA regulates the serum response factor-dependent transcription of α-SMA, and the effects of Rho on the serum response factor-mediated transcription is secondary to the actions of RhoA on the actin cytoskeleton. As Y-27632 only inhibited the α-SMA promoter activity by 50–60%, it was suggested that other Rho effectors, such as PKN, citron kinase, rhotekin, and the diaphanous family of proteins, might be involved (Mack et al., 2001). This is in agreement with the result of the current study showing that Y-27632 and HA-1077 significantly, but not completely, inhibited α-SMA expression in culture-activated PSCs. It should be noted that Buck et al. (2000) reported that c-Myb might be a molecular mediator of oxidative stress on stellate cell activation, and that c-Myb modulates transcription of α-SMA gene in activated hepatic stellate cells. Further studies will clarify whether the Rho-ROCK pathway modulates α-SMA expression through the interaction with c-Myb in PSCs.
There is accumulating evidence that RhoA activation may serve as a convergence point for multiple environmental factors known to regulate smooth muscle cell-specific gene expression and differentiation. For example, integrin–matrix interactions, mechanical stretch, and contractile agonists, such as endothelin-1 and angiotensin II, are known to regulate smooth muscle cell-specific gene expression as well as the activity of RhoA (Kim et al., 1997; Yamakawa et al., 2000). Although it remains to be elucidated whether these environmental factors regulate cellular functions of PSCs through RhoA-dependent signaling mechanisms and/or changes in actin dynamic, inhibition of Rho-ROCK pathway might be useful to regulate a variety of cellular functions of PSCs. Indeed, we have recently found that endothelin-1 stimulates the contraction and migration of PSCs, both of which were mediated by the Rho-ROCK pathway (Masamune et al., unpublished observation). Along this line, Kawada et al. (1999) showed that Y-27632 attenuated phosphorylation of MLC and formation of actin stress fiber, resulting in the impairment of contractile force in hepatic stellate cells.
Stellate cell proliferation and the expansion of their pool are a fundamental feature of pancreatic fibrosis (Haber et al., 1999). Accumulation of PSCs may result not only from proliferation but also from migration. Here, we showed that Y-27632 and HA-1077 inhibited proliferation and migration in response to PDGF, suggesting a role of the Rho-ROCK pathway for these cellular functions of PSCs. The effects of Y-27632 on cellular functions of PSCs appear not to be specific for PDGF-BB, because Y-27632 also inhibited serum-induced proliferation of PSCs. This is in agreement with the previous report showing that Y-27632 attenuated fetal bovine serum-induced growth (Iwamoto et al., 2000), and endothelin-1-induced migration (Tangkijvanich et al., 2001) in rat hepatic stellate cells. Inhibition of cellular migration by Y-27632 and HA-1077 was not unexpected, because cellular migration is known to be a complex process involving dynamic changes in the actin–myosin cytoskeleton (Lauffenburger & Horwitz, 1996). Inhibition of proliferation was accompanied by the arrest of the cell cycle at the G1 phase, as in the case of hepatic stellate cells, where Y-27632 increased p27kip1 expression and reduced cyclin D1 expression (Iwamoto et al., 2000). Along this line, Welsh et al. (2001) reported that Rho is crucial for maintaining the correct timing of cyclin D1 expression in G1 phase, suggesting a new role for cytoskeletal integrity in the regulation of cell cycle progression. The signaling pathways responsible for PDGF's actions on PSCs remain largely unknown, but we and others have recently shown that activation of ERK is crucial for proliferation and of phosphatidylinositol 3-kinase-Akt pathway for migration (Jaster et al., 2002; Masamune et al., 2003a). However, in this study, Y-27632 did not affect PDGF-induced phosphorylation of ERK and Akt, suggesting that the target of ROCK is, if any, located downstream of ERK and Akt. Previous studies have shown that the relation between Rho, Akt, and ERK might depend on the cell types and stimuli. In hepatic stellate cells, Y-27632 inhibited integrin-mediated, but not PDGF-induced, phosphorylation of ERK (Iwamoto et al., 2000). Rho-ROCK pathway regulated mechanical stretch-induced ERK activation in vascular smooth muscle cells (Numaguchi et al., 1999), whereas Y-27632 did not affect angiotensin II-induced ERK phosphorylation in this type of cells (Takeda et al., 2001). In Swiss 3T3 cells, the activation of phosphatidylinositol 3-kinase is dependent on Rho (Kumagai et al., 1993). Involvement of p70 S6 kinase was also unlikely, because Y-27632 did not affect the PDGF-induced activation of this kinase in PSCs. On the other hand, intracellular calcium and protein kinase C might play a role because the previous study in hepatic stellate cells showed that the cytoskeletal reorganization was dependent on intracellular calcium and protein kinase C (Di Sario et al., 2002). Obviously, further experiments, which are beyond the scope of the current study, are necessary to clarify the mechanisms by which Y-27632 and HA-1077 inhibited proliferation and chemotaxis of PSCs.
In this study, Y-27632 and HA-1077 blocked spontaneous activation of freshly isolated PSCs in culture. In addition, Y-27632 and HA-1077 inhibited several parameters of PSC activation including α-SMA expression, proliferation, migration, and collagen production, suggesting a pharmacological application of ROCK inhibitors in the treatment of pancreatic fibrosis and inflammation. Indeed, Y-27632 reportedly prevented dimethylnitrosamine- and carbon tetrachloride-induced hepatic fibrosis in rats (Murata et al., 2001; Tada et al., 2001). Previously, we reported that troglitazone, a ligand of the peroxisome proliferator-activated receptor-γ, blocked the activation of rat PSCs in vitro in a similar manner as that of Y-27632 (Masamune et al., 2002a). Recently, Shimizu et al. (2002) have reported that troglitazone prevented the progression of pancreatic inflammatory process and fibrosis in an animal model of chronic pancreatitis, suggesting that PSCs are potential targets of antifibrogenic and anti-inflammatory strategies in vivo. Experiments to test this hypothesis are under way in our laboratory.
Acknowledgments
This work was supported in part by Grant-in-Aid for Encouragement of Young Scientists from the Japan Society for the Promotion of Science (to A.M.), and by the Pancreas Research Foundation of Japan (to A.M.). This was presented in part at the annual meeting of the American Gastroenterological Association in Orlando, FL in May 2003. We thank Dr. Kenzo Kaneko for fluorescence microscopy, and Mitsubishi Pharma Co. for Y-27632.
Abbreviations
- α-SMA
α-smooth muscle actin
- ERK
extracellular signal-regulated kinase
- GFAP
glial fibrillary acidic protein
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
- GST
glutathione S-transferase
- MLC
myosin light chain
- PDGF
platelet-derived growth factor
- PSCs
pancreatic stellate cells
- ROCK
Rho kinase
References
- AMANO M., CHIHARA K., KIMURA K., FUKUTA Y., NAKAMURA N., MATSUURA Y., KAIBUCHI K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- APTE M.V., HABER P.S., APPLEGATE T.L., NORTON I.D., MCCAUGHAN G.W., KORSTEN M.A., PIROLA R.C., WILSON J.S. Periacinar stellate-shaped cells in rat pancreas: identification, isolation and culture. Gut. 1998;43:128–133. doi: 10.1136/gut.43.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACHEM M.G., SCHNEIDER E., GROSS H., WEIDENBACH H., SCHMIDT R.M., MENKE A., SIECH M., BEGER H., GRUNERT A., ADLER G. Identification, culture, and characterization of pancreas stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. doi: 10.1016/s0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- BRUNK K.C., JONES K.C., JAMES T.W. Assay for nanogram quantities of DNA in cellular homogenates. Anal. Biochem. 1979;2:497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- BUCK M., KIM D.J., HOUGLUM K., HASSANEIN T., CHOJKIER M. c-Myb modulates transcription of the alpha-smooth muscle actin gene in activated hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;278:G321–G328. doi: 10.1152/ajpgi.2000.278.2.G321. [DOI] [PubMed] [Google Scholar]
- DI SARIO A., BENDIA E., SVEGLIATI-BARONI G., MARZIONI M., RIDOLFI F., TROZZI L., UGILI L., SACCOMANNO S., JEZEQUEL A.M., BENEDETTI A. Rearrangement of the cytoskeletal network induced by platelet-derived growth factor in rat hepatic stellate cells: role of different intracellular signalling pathways. J. Hepatol. 2002;36:179–190. doi: 10.1016/s0168-8278(01)00242-2. [DOI] [PubMed] [Google Scholar]
- ETEMAD B., WHITCOMB D.C. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120:682–707. doi: 10.1053/gast.2001.22586. [DOI] [PubMed] [Google Scholar]
- HABER P.S., KEOGH G.W., APTE M.V., MORAN C.S., STEWART N.L., CRAWFORD D.H., PIROLA R.C., MCCAUGHAN G.W., RAMM G.A., WILSON J.S. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am. J. Pathol. 1999;155:1087–1095. doi: 10.1016/S0002-9440(10)65211-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALL A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- IWAMOTO H., NAKAMUTA M., TADA S., SUGIMOTO R., ENJOJI M., NAWATA H. A p160Rho kinase-specific inhibitor, Y-27632, attenuates rat hepatic stellate cell growth. J. Hepatol. 2000;32:762–770. doi: 10.1016/s0168-8278(00)80245-7. [DOI] [PubMed] [Google Scholar]
- JASTER R., SPARMANN G., EMMRICH J., LIEBE S. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut. 2002;51:579–584. doi: 10.1136/gut.51.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEKO K., SATOH K., MASAMUNE A., SATOH A., SHIMOSEGAWA T. Myosin light chain kinase inhibitors can block invasion and adhesion of human pancreatic cancer cell lines. Pancreas. 2002;24:34–41. doi: 10.1097/00006676-200201000-00005. [DOI] [PubMed] [Google Scholar]
- KAWADA N., SEKI S., KUROKI T., KANEDA K. ROCK inhibitor Y-27632 attenuates stellate cell contraction and portal pressure increase induced by endothelin-1. Biochem. Biophys. Res. Commun. 1999;266:296–300. doi: 10.1006/bbrc.1999.1823. [DOI] [PubMed] [Google Scholar]
- KIM J.H., CHO Y.S., KIM B.C., KIM Y.S., LEE G.S. Role of Rho GTPase in the endothelin-1-induced nuclear signaling. Biochem. Biophys. Res. Commun. 1997;232:223–226. doi: 10.1006/bbrc.1997.6261. [DOI] [PubMed] [Google Scholar]
- KIMURA K., ITO M., AMANO M., CHIHARA K., FUKATA Y., NAKAFUKU M., YAMAMORI B., FENG J., NAKANO T., OKAWA K., IWAMATSU A., KAIBUCHI K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- KUMAGAI N., MORII N., FUJISAWA K., NEMOTO Y., NARUMIYA S. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. J. Biol. Chem. 1993;268:24535–24538. [PubMed] [Google Scholar]
- LANKISCH P.G. Natural course of chronic pancreatitis. Pancreatology. 2001;1:3–14. doi: 10.1159/000055786. [DOI] [PubMed] [Google Scholar]
- LAUFFENBURGER D.A., HORWITZ A.F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- MACK C.P., SOMLYO A.V., HAUTMANN M., SOMLYO A.P., OWENS G.K. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- MASAMUNE A., KIKUTA K., SATOH M., SAKAI Y., SATOH A., SHIMOSEGAWA T. Ligands of peroxisome proliferator-activated receptor-γ block activation of pancreatic stellate cells. J. Biol. Chem. 2002a;277:141–147. doi: 10.1074/jbc.M107582200. [DOI] [PubMed] [Google Scholar]
- MASAMUNE A., KIKUTA K., SATOH M., KUME K., SHIMOSEGAWA T. Differential roles of signaling pathways for proliferation and migration of rat pancreatic stellate cells. Tohoku J. Exp. Med. 2003a;199:69–84. doi: 10.1620/tjem.199.69. [DOI] [PubMed] [Google Scholar]
- MASAMUNE A., KIKUTA K., SATOH M., SATOH A., SHIMOSEGAWA T. Alcohol activates activator protein-1 and MAP kinases in rat pancreatic stellate cells. J. Pharmacol. Exp. Ther. 2002b;302:36–42. doi: 10.1124/jpet.302.1.36. [DOI] [PubMed] [Google Scholar]
- MASAMUNE A., SAKAI Y., KIKUTA K., SATOH M., SATOH A., SHIMOSEGAWA T. Lysophosphatidylcholine induces apoptosis in AR42J cells. Pancreas. 2001;22:75–83. doi: 10.1097/00006676-200101000-00014. [DOI] [PubMed] [Google Scholar]
- MASAMUNE A., SAKAI Y., KIKUTA K., SATOH M., SATOH A., SHIMOSEGAWA T. Activated rat pancreatic stellate cells express intercellular adhesion molecule-1 in vitro. Pancreas. 2002c;25:78–85. doi: 10.1097/00006676-200207000-00018. [DOI] [PubMed] [Google Scholar]
- MASAMUNE A., SATOH K., SAKAI Y., YOSHIDA M., SATOH A., SHIMOSEGAWA T. Ligands of peroxisome proliferator-activated receptor-γ induce apoptosis in AR42J cells. Pancreas. 2002d;24:130–138. doi: 10.1097/00006676-200203000-00003. [DOI] [PubMed] [Google Scholar]
- MASAMUNE A., SATOH M., KIKUTA K., SAKAI Y., SATOH A., SHIMOSEGAWA T. Inhibition of p38 mitogen-activated protein kinase blocks activation of rat pancreatic stellate cells. J. Pharmacol. Exp. Ther. 2003b;304:8–14. doi: 10.1124/jpet.102.040287. [DOI] [PubMed] [Google Scholar]
- MASAMUNE A., SHIMOSEGAWA T., KIMURA K., FUJITA M., SATOH A., KOIZUMI M., TOYOTA T. Specific induction of adhesion molecules in human vascular endothelial cells by rat experimental pancreatitis-associated ascetic fluids. Pancreas. 1999;18:141–150. doi: 10.1097/00006676-199903000-00005. [DOI] [PubMed] [Google Scholar]
- MOSHAGE H., CASINI A., LIEBER C.S. Acetaldehyde selectively increases collagen synthesis in cultured rat fat-storing cells but not in hepatocytes. Hepatology. 1990;12:511–518. doi: 10.1002/hep.1840120311. [DOI] [PubMed] [Google Scholar]
- MURATA T., ARII S., NAKAMURA T., MORI A., KAIDO T., FURUYAMA H., FURUMOTO K., NAKAO T., ISOBE N., IMAMURA M. Inhibitory effect of Y-27632, a ROCK inhibitor, on progression of rat liver fibrosis in association with inactivation of hepatic stellate cells. J. Hepatol. 2001;35:474–481. doi: 10.1016/s0168-8278(01)00169-6. [DOI] [PubMed] [Google Scholar]
- NAGUMO H., SASAKI Y., ONO Y., OKAMOTO H., SETO M., TAKUWA Y. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am. J. Physiol. Cell Physiol. 2000;278:C57–C65. doi: 10.1152/ajpcell.2000.278.1.C57. [DOI] [PubMed] [Google Scholar]
- NUMAGUCHI K., EGUCHI S., YAMAKAWA T., MOTLEY E.D., INAGAMI T. Mechanotransduction of rat aortic vascular smooth muscle cells requires RhoA and intact actin filaments. Circ. Res. 1999;85:5–11. doi: 10.1161/01.res.85.1.5. [DOI] [PubMed] [Google Scholar]
- PATEL K., HARDING P., HANEY L.B., GLASS W.F., II Regulation of the mesangial cell myofibroblast phenotype by actin polymerization. J. Cell Physiol. 2003;195:435–445. doi: 10.1002/jcp.10267. [DOI] [PubMed] [Google Scholar]
- SAHAI E., ISHIZAKI T., NARUMIYA S., TREISMAN R. Transformation mediated by RhoA requires activity of ROCK kinases. Curr. Biol. 1999;11:136–145. doi: 10.1016/s0960-9822(99)80067-0. [DOI] [PubMed] [Google Scholar]
- SEBBAGH M., RENYOIZE C., HAMELIN J., RICHE N., BERTOGLIO J., BREARD J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat. Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- SHIMIZU K., SHIRATORI K., HAYASHI N., KOBAYASHI M., FUJIWARA T., HORIKOSHI H. Thiazolidinedione derivatives as novel therapeutic agents to prevent the development of chronic pancreatitis. Pancreas. 2002;24:184–190. doi: 10.1097/00006676-200203000-00010. [DOI] [PubMed] [Google Scholar]
- SOTIROPOULOS A., GINEITIS D., COPELAND J., TREISMAN R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- TADA S., IWAMOTO H., NAKAMUTA M., SUGIMOTO R., ENJOJI M., NAKASHIMA Y., NAWATA H. A selective Rho kinaase inhibitor, Y-27632, prevents dimethylnitrosamine-induced hepatic fibrosis in rats. J. Hepatol. 2001;34:529–536. doi: 10.1016/s0168-8278(00)00059-3. [DOI] [PubMed] [Google Scholar]
- TAKAI Y., SASAKI T., TANAKA K., NAKANISHI H. Rho as a regulator of the cytoskeleton. Trends Biochem. Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- TAKEDA K., ICHIKI T., TOKUNOU T., IINO N., FUJII S., KITABATAKE A., SHIMOKAWA H., TAKESHITA A. Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler. Thromb. Vasc. Biol. 2001;21:868–873. doi: 10.1161/01.atv.21.5.868. [DOI] [PubMed] [Google Scholar]
- TANGKIJVANICH P., TAM S.P., YEE H.F., JR Wound-induced migration of rat hepatic stellate cells is modulated by endothelin-1 through rho-kinase-mediated alterations in the acto-myosin cytoskeleton. Hepatology. 2001;33:74–80. doi: 10.1053/jhep.2001.20677. [DOI] [PubMed] [Google Scholar]
- UEHATA M., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- VAN AELST L., D'SOUZA-SCHOREY C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- WANG P., BITAR K.N. RhoA regulates sustained smooth muscle contraction through cytoskeletal reorganization of HSP27. Am. J. Physiol. 1998;275:G1454–G1462. doi: 10.1152/ajpgi.1998.275.6.G1454. [DOI] [PubMed] [Google Scholar]
- WELSH C.F., ROOVERS K., VILLANUEVA J., LIU Y., SCHWARTZ M.A., ASSOIAN R.K. Timing of cyclin D1 expression within G1 phase is controlled by Rho. Nat. Cell Biol. 2001;3:950–957. doi: 10.1038/ncb1101-950. [DOI] [PubMed] [Google Scholar]
- YAMAKAWA T., TANAKA S., NUMAGUCHI K., YAMAKAWA Y., MOTLEY B.D., ICHIHARA S., INAGAMI T. Involvement of Rho-kinase in angiotensin II-induced hypertrophy of rat vascular smooth muscle cells. Hypertension. 2000;35:313–318. doi: 10.1161/01.hyp.35.1.313. [DOI] [PubMed] [Google Scholar]
- YEE H.F., JR Rho directs activation-associated changes in rat hepatic stellate cell morphology via regulation of the actin cytoskeleton. Hepatology. 1998;28:843–850. doi: 10.1002/hep.510280336. [DOI] [PubMed] [Google Scholar]