Abstract
Growing evidence suggests that interleukin (IL)-13, a Th2-type cytokine, plays a critical role in the development of bronchial hyper-responsiveness (BHR), an essential feature of asthma, although the underlying mechanisms remain unknown. In the present study, we investigated whether IL-13 directly affects airway smooth muscle (ASM) function. In murine tracheal rings, IL-13 (100 ng ml−1, 24 h) significantly increased both the carbachol- and KCl-induced maximal force generation without affecting ASM sensitivity. In cultured human ASM cells, IL-13 (50 ng ml−1, 24 h) also augmented cytosolic calcium levels to bradykinin, histamine and carbachol by 60, 35 and 26%, respectively. The present study demonstrates that IL-13 may promote BHR by directly modulating ASM contractility, an effect that may be due to enhanced G protein-coupled receptor (GPCR)-associated calcium signaling.
Keywords: Asthma, isometric tension, airway smooth muscle, Th2 cytokine, calcium metabolism
Introduction
Studies now suggest that cytokine-induced modulation of airway smooth muscle (ASM), an important effector tissue regulating bronchomotor tone, may play an important role in the development of bronchial hyper-responsiveness (BHR) in chronic lung diseases such as asthma and chronic obstructive pulmonary disease (reviewed in Amrani & Panettieri, 2002). The mechanisms by which cytokines promote BHR have not been clearly established; however, reports using cultured ASM cells showed that TNFα or interleukin (IL)-1β, proinflammatory cytokines found in the bronchoalveolar lavage of subjects with asthma, directly regulates agonist-associated calcium signaling, a critical element regulating ASM contraction (Amrani et al., 1995; Deshpande et al., 2003; Hunter et al., 2003). In addition, evidence using animal models of asthma shows that the Th2-type cytokine IL-13 may also play a critical role in promoting BHR, although the exact mechanisms are unknown (Grunig et al., 1998; Wills-Karp et al., 1998; Walter et al., 2001; Akbari et al., 2003). Recent studies, however, showed that IL-13 may exert its deleterious effects in asthma by directly altering gene expression in airway resident cells such as epithelial or ASM cells (Laporte et al., 2001; Lee et al., 2001; Kuperman et al., 2002; Venkayya et al., 2002).
In this study, we report that IL-13 directly alters ASM responsiveness by enhancing contractile agonist-induced contractility and calcium signals. Further examination of the mechanisms by which IL-13 regulates ASM function may offer new thera-peutic approaches to treat BHR associated with asthma.
Methods
Measurement of isometric force generation
Measurements of force generation using murine cultured tracheal rings were performed as described previously (Chen et al., 2003). Tracheae were supported longitudinally by a plexiglas rod with a stainless-steel pin into the base of a double-jacketed, glass organ bath filled with 10 ml of Krebs–Henseleit (K–H) solution at 37°C. The K–H solution contained (mM): 118 NaCl, 4.7 KCl, 1.2 KH2PO4, 11.1 dextrose, 1.2 MgSO4, 2.8 CaCl2 and 25 NaHCO3, and was continuously aerated with a 5% CO2 and 95% O2 mixture; a pH of 7.40–7.45 was established for the duration of the experiments. The upper support was attached by a loop of silk thread to an FT03 isometric transducer (Astro-Med, Inc., West Warwirck, RI, U.S.A.) and changes in tension of the rings were measured. Concentration–response curves were synchronously recorded with an MP 100WS system (BIOPAC Systems, Inc., Santa Barbara, CA, U.S.A.) and displayed on a Macintosh computer. All initial tensions of tracheal rings were set at approximately 0.5 g and stimulated with agonists after attainment of steady-state tension.
RT–PCR analysis
RT–PCR analysis was performed as described previously (Chen et al., 2003). Briefly, total RNA was extracted from total murine tracheal rings by using the SV total RNA isolation system (Promega, Madison, WI, U.S.A.) according to the manufacturer's instructions. RT–PCR reactions were performed with the use of IL-13Rα1, IL-13Rα2 and β-actin primers for semiquantitative analysis as described previously (Zheng et al., 2003). The PCR products (IL-13Rα1, 549 bp; IL-13Rα2, 217 bp; and β-actin, 241 bp) were resolved on 1.8% agarose-gel electrophoresis, stained with ethidium bromide and photographed.
Measurement of intracellular calcium concentration
Calcium measurements were determined as described previously (Deshpande et al., 2003). Briefly, Fura-2-loaded human ASM cells grown on coverslips were mounted onto an open slide chamber, placed onto an inverted microscope and excited at 340 and 380 nm wavelength and emissions were collected at 510 nm wavelength using a CCD camera. The ratio of fluorescence intensities at 340 and 380 nm wavelength was determined and converted to the calcium concentrations using a standard curve. The net calcium responses to contractile agonists were calculated by subtracting the basal from that of the peak intracellular calcium concentration.
Data analysis
Tension was calculated as milligram tensions per milligram trachea weight (mg mg−1) and expressed as an individual percentage (%) of 10−5 M carbachol- or 100 mM KCl-evoked force of the cultured tracheal rings in the absence of IL-13 for contraction studies. The concentrations of agonists required to produce half-maximal contraction (pD2) were determined with log values of the EC50's. All values were expressed as means±s.e.m. Comparisons among groups with or without IL-13 were performed by a one-way analysis of variance followed by Student's unpaired t-test when appropriate. A P-value of less than 0.05 was considered significant.
Materials and reagents
Recombinant murine and human IL-13 were purchased from R&D Systems (Indianapolis, IN, U.S.A.). Carbachol, bradykinin, histamine and Fura2/AM were purchased from Sigma (St Louis, MO, U.S.A.).
Results
IL-13 potentiates carbachol- and KCl-evoked force generation
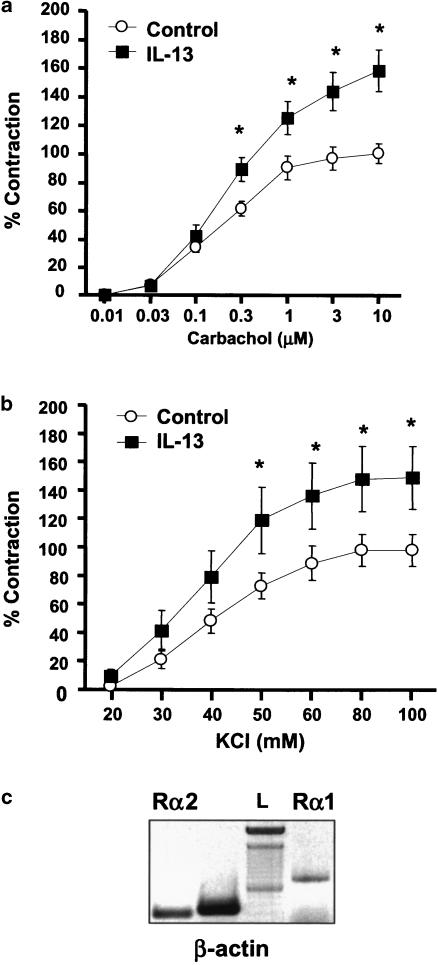
Cultured tracheal rings generate force in response to carbachol and KCl in a concentration-dependent manner with pD2 values of 6.62±0.05 (n=18) and 1.39±0.07 (n=7), respectively. In tracheal rings pretreated with 100 ng ml−1 of murine IL-13, there was a significant increase in force generation induced by both carbachol and KCl as compared to those obtained from rings treated with diluent alone (Figure 1a and b). In IL-13-treated rings, the maximal tensions to carbachol and KCl were increased by 58 and 51% (P<0.05), respectively. Neutralizing anti-IL-13 antibody (5 μg ml−1) completely prevented the IL-13-enhancing effect on agonist-evoked contraction (data not shown). In addition, the enhancing effect of IL-13 on the maximal force generation was not associated with changes in receptor affinity with pD2 values of 6.69±0.04 (carbachol, n=12) and 1.42±0.05 (KCl, n=6). RT–PCR analysis revealed that both IL-13 receptors (IL-13Rα1 and IL-13Rα2) are expressed in murine tracheal rings (Figure 1c). These data show that IL-13 enhances maximal force generation induced by carbachol and KCl without altering receptor affinity.
Figure 1.
IL-13 enhances carbachol- and KCl-induced contraction in cultured trachea. (a) Cumulative concentration–response curves to carbachol were completed in cultured control (n=18) or in the presence of IL-13 100 ng ml−1 (n=12). (b) Cumulative concentration–response curves to KCl were completed in the absence (n=7) and presence of IL-13 100 ng ml−1 (n=6). All tension measurements from groups were expressed as mean±s.e.m., *P<0.05 compared with rings treated with diluent alone. (c) Expression of IL-13 receptors in murine tracheal rings. Total RNA (1 μg) was subjected to RT–PCR with the primers for β-actin, IL-13Rα1 and IL-13Rα2 as described in Methods. PCR products were separated on 1.8% agarose gel and stained with ethidium bromide. L, 100 bp DNA ladder. Data are representative of two different tracheal rings.
IL-13 enhances agonist-evoked calcium signals
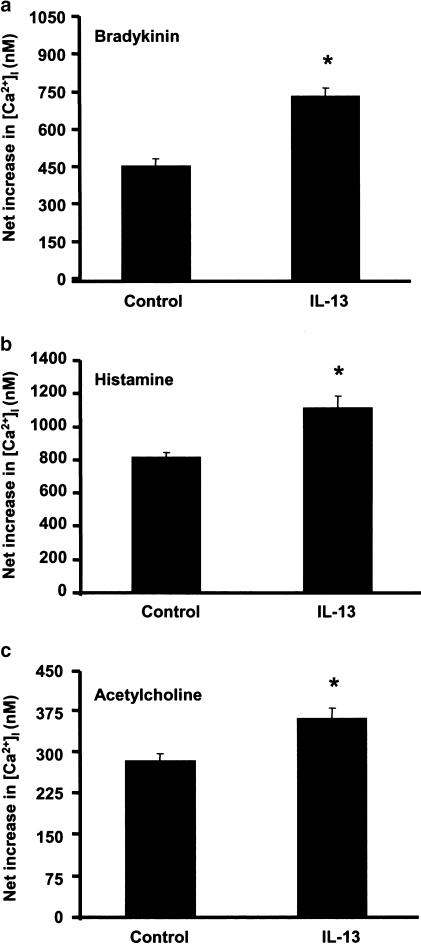
Since cytokines have the capacity to enhance calcium signaling to a variety of contractile agonists in ASM cells (reviewed in Amrani & Panettieri, 2002), we examined whether human IL-13 also modulates agonist-induced calcium signals in Fura-2-loaded human ASM cells. As shown in Figure 2, IL-13 (24 h, 50 ng ml−1) significantly increased calcium signals induced by effective concentrations of three different G protein-coupled receptor (GPCR) agonists. In control cells, the net increases in cytosolic-free calcium (in nM) to bradykinin, histamine and acetylcholine were 463±28 (n=49), 818±25 (n=113) and 283±12 (n=78), respectively. Interestingly, calcium responses to agonists were significantly (P<0.05) increased in ASM cells pretreated with 50 ng ml−1 IL-13 for 24 h by 60% (bradykinin, n=70), 35% (histamine, n=91) and 26% (acetylcholine, n=54). IL-13 alone has no effect on intracellular calcium levels (data not shown). These data demonstrate that IL-13 enhances GPCR agonist-associated calcium signals in human ASM cells.
Figure 2.
IL-13 enhances agonist-evoked calcium signals in cultured ASM cells. Human ASM cells treated with IL-13 (50 ng ml−1) for 24 h before calcium responses to 1 nM bradykinin (a), 50 μM histamine (b) and 10 μM acetylcholine (c) were performed in Fura-2-loaded cells. Results are expressed as the net increase in intracellular calcium concentration (in nM) at the peak and values are means±s.e.m. *P< 0.05, significantly different from cells treated with the diluent alone.
Discussion
Evidence now suggests that cytokines play an important role in the development of BHR, an exaggerated airway narrowing in response to a variety of stimuli including contractile agonists (Amrani & Panettieri, 2002). Recent studies using animal models of allergic asthma demonstrate that IL-13, a Th2-type cytokine, plays a major role in the regulation of BHR (Grunig et al., 1998; Wills-Karp et al., 1998; Walter et al., 2001; Akbari et al., 2003). In agreement with Grunstein et al. (2002), our study shows that IL-13 has the potential to increase the contractile responses to acetylcholine and KCl using an ex vivo model of airway reactivity (Chen et al., 2003) that expresses both IL-13 receptors, IL-13Rα1 and IL-13Rα2 (Figure 1c). Our study also supports the emerging evidence that IL-13 promotes BHR by directly modulating the function of resident airway cells, although the nature of the cell types remains unknown. A similar conclusion may be drawn from in vivo studies showing that IL-13 delivered directly to the airway, either intratracheally or intranasally, generates a BHR to muscarinic receptor stimulation (Kibe et al., 2003; Vargaftig & Singer, 2003). It is interesting to note that the induction of BHR by IL-13 even occurred in the absence of any sign of airway inflammation, such as inflammatory cell recruitment, cytokine production and mucus production (Yang et al., 2001; Venkayya et al., 2002). This inflammation-independent component of BHR by IL-13 may involve a direct effect on ASM, an essential effector tissue that regulates the bronchomotor tone (Amrani & Panettieri, 2003). Owing to the central role of intracellular calcium in regulating ASM contractility (Amrani & Panettieri, 2002), we investigated the effect of an effective concentration of IL-13 on the calcium signals generated by contractile agonists in cultured ASM cells that were shown to express both IL-13 receptors (Laporte et al., 2001). In a nonspecific manner, IL-13 potentiates the calcium responses induced by three different contractile agonists, supporting the novel hypothesis that IL-13 may increase ASM contractility and possibly BHR previously described in animal models of allergic asthma by altering calcium homeostasis in ASM cells. The ability of IL-13 to increase ASM responsiveness induced by high concentrations of KCl (100 mM) suggests the possible involvement of calcium-independent pathways. In that regard, IL-13 may increase ASM contractility by modulating the contractile machinery, either by enhancing the sensitivity of the myofilaments to calcium or by rearranging the cytoskeleton apparatus as previously shown with TNFα in human ASM cells (Hunter et al., 2003). As IL-13 also regulates the expression of various proinflammatory genes in ASM cells (Laporte et al., 2001; Hirst et al., 2002), collectively these data suggest that the interaction of IL-13 with ASM may play an important role in the pathogenesis of asthma.
Acknowledgments
We thank Mary McNichol for assistance in the preparation of the manuscript. This work was supported by NIH Grants 2R01-HL55301 (RAP), 1P50-HL67663 (RAP), HL057498 (MSK) and by an American Lung Association Grant RG-062-N (YA). Yassine Amrani is a Parker B. Francis Fellow in Pulmonary Research.
Abbreviations
- IL
interleukin
- BHR
bronchial hyper-responsiveness
- ASM
airway smooth muscle
- K–H
Krebs–Henseleit
- GPCR
G protein-coupled receptor
References
- AKBARI O., STOCK P., MEYER E., KRONENBERG M., SIDOBRE S., NAKAYAMA T., TANIGUCHI M., GRUSBY M.J., DEKRUYFF R.H., UMETSU D.T. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- AMRANI Y., MARTINET N., BRONNER C. Potentiation by tumour necrosis factor-alpha of calcium signals induced by bradykinin and carbachol in human tracheal smooth muscle cells. Br. J. Pharmacol. 1995;114:4–5. doi: 10.1111/j.1476-5381.1995.tb14896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMRANI Y., PANETTIERI R.A., JR Modulation of calcium homeostasis as a mechanism for altering smooth muscle responsiveness in asthma. Curr. Opin. Allergy Clin. Immunol. 2002;2:39–45. doi: 10.1097/00130832-200202000-00007. [DOI] [PubMed] [Google Scholar]
- AMRANI Y., PANETTIERI R.A. Airway smooth muscle: contraction and beyond. Int. J. Biochem. Cell. Biol. 2003;35:272–276. doi: 10.1016/s1357-2725(02)00259-5. [DOI] [PubMed] [Google Scholar]
- CHEN H., TLIBA O., VAN BESIEN C.R., PANETTIERI R.A., JR, AMRANI Y. Selected contribution: TNF-α modulates murine tracheal rings responsiveness to G-protein-coupled receptor agonists and KCl. J. Appl. Physiol. 2003;95:864–872. doi: 10.1152/japplphysiol.00140.2003. [DOI] [PubMed] [Google Scholar]
- DESHPANDE D.A., WALSETH T.F., PANETTIERI R.A., KANNAN M.S.CD38-cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyperresponsiveness FASEB J. 2003(FASEB Journal Express Article 10.1096/fj.02-0450fje) [DOI] [PubMed]
- GRUNIG G., WARNOCK M., WAKIL A.E., VENKAYYA R., BROMBACHER F., RENNICK D.M., SHEPPARD D., MOHRS M., DONALDSON D.D., LOCKSLEY R.M., CORRY D.B. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNSTEIN M.M., HAKONARSON H., LEITER J., CHEN M., WHELAN R., GRUNSTEIN J.S., CHUANG S. IL-13-dependent autocrine signaling mediates altered responsiveness of IgE-sensitized airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L520–L528. doi: 10.1152/ajplung.00343.2001. [DOI] [PubMed] [Google Scholar]
- HIRST S.J., HALLSWORTH M.P., PENG Q., LEE T.H. Selective induction of eotaxin release by interleukin-13 or interleukin-4 in human airway smooth muscle cells is synergistic with interleukin-1beta and is mediated by the interleukin-4 receptor alpha-chain. Am. J. Resp. Crit. Care Med. 2002;165:1161–1171. doi: 10.1164/ajrccm.165.8.2107158. [DOI] [PubMed] [Google Scholar]
- HUNTER I., COBBAN H.J., VANDENABEELE P., MACEWAN D.J., NIXON G.F. Tumor necrosis factor-alpha-induced activation of RhoA in airway smooth muscle cells: role in the Ca(2+) sensitization of myosin light chain(20) phosphorylation. Mol. Pharmacol. 2003;63:714–721. doi: 10.1124/mol.63.3.714. [DOI] [PubMed] [Google Scholar]
- KIBE A., INOUE H., FUKUYAMA S., MACHIDA K., MATSUMOTO K., KOTO H., IKEGAMI T., AIZAWA H., HARA N. Differential regulation by glucocorticoid of interleukin-13-induced eosinophilia, hyperresponsiveness, and goblet cell hyperplasia in mouse airways. Am. J. Respir. Crit. Care Med. 2003;167:50–56. doi: 10.1164/rccm.2110084. [DOI] [PubMed] [Google Scholar]
- KUPERMAN D.A., HUANG X., KOTH L.L., CHANG G.H., DOLGANOV G.M., ZHU Z., ELIAS J.A., SHEPPARD D., ERLE D.J. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat. Med. 2002;8:885–889. doi: 10.1038/nm734. [DOI] [PubMed] [Google Scholar]
- LAPORTE J.C., MOORE P.E., BARALDO S., JOUVIN M.H., CHURCH T.L., SCHWARTZMAN I.N., PANETTIERI R.A., JR, KINET J.P., SHORE S.A. Direct effects of interleukin-13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am. J. Respir. Crit. Care Med. 2001;164:141–148. doi: 10.1164/ajrccm.164.1.2008060. [DOI] [PubMed] [Google Scholar]
- LEE J.H., KAMINSKI N., DOLGANOV G., GRUNIG G., KOTH L., SOLOMON C., ERLE D.J., SHEPPARD D. Interleukin-13 induces dramatically different transcriptional programs in three human airway cell types. Am. J. Respir. Cell. Mol. Biol. 2001;25:474–485. doi: 10.1165/ajrcmb.25.4.4522. [DOI] [PubMed] [Google Scholar]
- VARGAFTIG B.B., SINGER M. Leukotrienes mediate murine bronchopulmonary hyperreactivity, inflammation, and part of mucosal metaplasia and tissue injury induced by recombinant murine interleukin-13. Am. J. Respir. Cell. Mol. Biol. 2003;28:410–419. doi: 10.1165/rcmb.2002-0032OC. [DOI] [PubMed] [Google Scholar]
- VENKAYYA R., LAM M., WILLKOM M., GRUNIG G., CORRY D.B., ERLE D.J. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am. J. Respir. Cell. Mol. Biol. 2002;26:202–208. doi: 10.1165/ajrcmb.26.2.4600. [DOI] [PubMed] [Google Scholar]
- WALTER D.M., MCINTIRE J.J., BERRY G., MCKENZIE A.N., DONALDSON D.D., DEKRUYFF R.H., UMETSU D.T. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J. Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- WILLS-KARP M., LUYIMBAZI J., XU X., SCHOFIELD B., NEBEN T.Y., KARP C.L., DONALDSON D.D. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- YANG M., HOGAN S.P., HENRY P.J., MATTHAEI K.I., MCKENZIE A.N., YOUNG I.G., ROTHENBERG M.E., FOSTER P.S. Interleukin-13 mediates airways hyperreactivity through the IL-4 receptor-alpha chain and STAT-6 independently of IL-5 and eotaxin. Am. J. Respir. Cell. Mol. Biol. 2001;25:522–530. doi: 10.1165/ajrcmb.25.4.4620. [DOI] [PubMed] [Google Scholar]
- ZHENG T., ZHU Z., LIU W., LEE C.G., CHEN Q., HOMER R.J., ELIAS J.A. Cytokine regulation of IL-13Ralpha2 and IL-13Ralpha1 in vivo and in vitro. J. Allergy Clin. Immunol. 2003;111:720–728. doi: 10.1067/mai.2003.1383. [DOI] [PubMed] [Google Scholar]