Abstract
In this study, we examined the role of mitogen-activated protein (MAP) kinases in the effects of verotoxins (VTs), from Escherichia coli O157:H7, upon both apoptosis and the release of tumour necrosis factor alpha (TNF-α) and granulocyte–macrophage colony-stimulated factor (GM-CSF) from human monocytes.
Both VT1 and VT2 stimulated a weak, transient increase in c-Jun-N-terminal kinase (JNK) activity and a strong activation of both p38 mitogen-activated protein kinase (MAP kinase) and extracellular-regulated kinase (ERK) activity in human monocytes, which was sustained in the case of p38 MAP kinase.
Stimulation of human monocytes with VT2 (100 ng ml−1) did not result in an increase in apoptosis; however, the toxin stimulated the release of both TNF-α and GM-CSF.
Pretreatment of human monocytes with the p38 MAP kinase inhibitor SB203580, at concentrations from 100 nM to 10 μM, significantly decreased the VT1- and VT2-induced TNF-α and GM-CSF release from monocytes. In contrast, inhibition of MEK1 with PD98059 only significantly decreased GM-CSF release.
Pretreatment of monocytes with SP600125 inhibited both GM-CSF and TNF-α production; however, significant effects upon p38 MAP kinase and ERK activation were observed.
Taken together, these results suggest a role for p38 MAP kinase and ERK in cytokine generation in response to the verotoxins. A role for JNK remains undetermined.
Keywords: E. coli O157:H7, verotoxins, c-Jun-N-terminal kinase, p38 MAP kinase, ERK, TNF-α, GM-CSF, apoptosis
Introduction
Verotoxigenic Escherichia coli (VTEC), principally the serotype O157:H7, is an extremely virulent foodborne pathogen in humans (Coia, 1998; Dundas & Todd, 1998) and has a high mortality rate, particularly among infants and the elderly, where complications such as haemolytic uraemic syndrome (HUS) can arise (Louise & Obrig, 1991; Hofman, 1993; Arbus, 1997; Murata et al., 1998). The major pathogenic components of E. coli O157:H7 are the verotoxins (VTs; also known as Shiga toxins; Stxs) (O'Brien & Holmes, 1987), which are classified into VT1 and VT2 families (O'Brien et al., 1992). The VTs mediate their effects by binding to globotriaoslyceramide (Gb3/CD77) receptors, expressed on cells such as the renal endothelium and epithelium and monocytes (Tesh & O'Brien, 1991; Karpman et al., 1998). Once bound, the VTs induce death through inhibition of protein synthesis and apoptosis (Tesh & O'brien, 1991; Kiyokawa et al., 1998).
Only relatively recently have a number of studies investigated the role of key signalling pathways in the effects of VT (Ikeda et al., 2000; Berin et al., 2002; Foster & Tesh, 2002; Yoshida et al., 2002; Smith et al., 2003). One intriguing aspect of cellular responsiveness to VT is that it is extremely cell-type specific. For example, Vero cells (African Green monkey kidney epithelial cells) and many endothelial and epithelial cells are exquisitely sensitive to the VTs cytotoxic effects (Inward et al., 1995; Karpman et al., 1998; Ohmi et al., 1998), while others such as peripheral blood monocytes have been reported to be resistant (Ramegowda & Tesh, 1996). Furthermore, in neutrophils, it appears that VT may prolong their survival by delaying spontaneous apoptosis (Liu et al., 1999,2002). These numerous cellular effects suggest differences in the mechanisms by which diverse cellular responses are initiated.
The mitogen-activated protein kinases (MAP kinases) are a family of threonine-directed kinases known to play an important role in cell function (Paul et al., 1997). They consist of several families, including the extracellular-regulated kinase (ERKs) and the stress-activated protein (SAP) kinases, C-Jun N terminal kinases (JNKs) and p38 MAP kinases. An increasing body of evidence has implicated a role for these kinases in the regulation of diverse cellular functions, including cytokine release (Swantek et al., 1997; Brinkman et al., 1999), cellular proliferation and apoptosis. Indeed, recent studies have implicated a role for JNK in both proapoptosis and cytoprotection, depending on the nature of the stimulus, kinetics of activation and cell type (Xia et al., 1995; Chen et al., 1996; Zanke et al., 1998; Harada & Sugimoto, 1999).
Recently, we have shown that VT, unlike a number of cytokines and certain agonists at G protein-coupled receptors (GPCRs), stimulates a sustained increase in JNK up until the point of cell death (Cameron et al., 2002). However, the roles of JNK and other related kinases, such as p38 MAP kinase and ERK, in the cellular effects of VT have not yet been elucidated. In this study, we examined the ability of VT1 and VT2 to stimulate JNK, p38 MAP kinase and ERK in human peripheral blood monocytes, which are reported to be resistant to the cytotoxic effects of the VTs and have been described to respond, instead, via the release of proinflammatory cytokines (Ramegowda & Tesh, 1996; van Setten et al., 1996).
Here, we describe the activation of JNK, p38 MAP kinase and ERK in response to VT1 and VT2 in human monocytes, with kinetics that differ markedly from those that we reported previously in Vero cells. We also show that PB monocytes do not undergo apoptosis, in response to VT, and that p38 MAP kinase and ERK play a role in tumour necrosis factor alpha (TNF-α) and granulocyte–macrophage colony-stimulated factor (GM-CSF) release. In contrast, a role for JNK in the regulation of TNF-α and GM-CSF release from human monocytes using a novel inhibitor, SP600125, remains unproven.
Methods
Materials
All chemicals and reagents were obtained from appropriate commercial sources. The E. coli expression plasmid for GST-c-Jun(5–89) was a kind gift from Professor C.J. Marshall, (Chester Beatty Laboratories, London, U.K.). VT1 and VT2 were purchased from Toxin Technology (FL, U.S.A.). All phosphorylation site-specific antibodies were purchased from Biosource (U.K., Ltd). Antibodies directed against the inactive forms of ERK and p38 MAP kinase were obtained from Santa Cruz (CA, U.S.A.). SB203580 and PD98059 were obtained from Calbiochem (Nottingham, U.K.) and SP600125 was a kind gift from Brydon Bennett (Signal Pharmaceuticals, San Diego, CA, U.S.A.).
Isolation of human peripheral blood monocytes
Venous blood (30–60 ml) from healthy volunteers was collected by the vacutainer® system (Greiner) utilising 19-gauge butterfly needles with holdexes® and 9 ml acid citrate dextran (ACD) collection vials. Blood was sedimented with 3% hydroxyethyl starch (Elo hespan; Fresenius Kabi, Ltd, Cheshire, U.K.) for 45 min and the resulting buffy coat supernatant was centrifuged at 450 × g for 10 min at 20°C. The cell pellet was gently resuspended in 2 ml 55% (v/v in PBS) Percoll and layered onto a Percoll gradient, comprising an 81% (v/v) layer (5 ml), followed by a 70% (v/v) layer (5 ml) placed on top. The Percoll gradients were then centrifuged at 500 × g for 25 min at 18°C. The mononuclear cells, visible at the 55 and 70% interface, were retained and washed three times in Hanks' balanced salt solution (HBSS), followed by centrifugation at 500 × g for 10 min at 20°C. The remaining cells were washed, counted and resuspended in RPMI 1640 (Dutch modification) containing 10% foetal calf serum (FCS), 2 mM L-glutamine, 100 U penicillin ml−1 and 100 μg streptomycin ml−1 in six- and 12-well plates (Corning Costar, Bucks, U.K.) in a humidified atmosphere containing 5% CO2 at 37°C. After a minimum of 90 min, plates containing the mononuclear cells were gently agitated and the lymphocytes were aspirated off. The remaining adherent monocytes were washed twice in HBSS and fresh medium was added.
Western blotting
The detection of phospho-p38 MAP kinase, phospho-ERK and phospho-JNK using SDS–PAGE was conducted as outlined previously (Laird et al., 1998). All antibodies were titred for optimum conditions and in all figures showing phosphoblots, equal loading by total ERK/p38/JNK was demonstrated.
Solid-phase JNK in vitro kinase assay
Following stimulation, the cells were washed in ice-cold phosphate-buffered saline (PBS), lysed in the appropriate solubilising buffer and precleared supernatants were added to the relevant substrate as described previously (Paul et al., 2000). Briefly, following solubilisation, lysates were clarified by centrifugation at 13,000 × g for 5 min at 4°C and supernatants retained. The precleared supernatants (10 μg protein) were added to 20 μl slurry of glutathione S-transferase (GST)–c Jun(5–89)/GSH–Sepharose beads and mixed for 3 h at 4°C. The precipitates were then resuspended in 25 μl of kinase buffer and the reaction was started by the addition of [γ-32P]ATP (1–2 μCi, 25 μM) and incubated for 20 min at 30°C. Adding 10 μl of 4 × Laemmli sample buffer terminated the reaction. Samples were boiled for 5 min, resolved on 11% SDS/PAGE gels and fixed in 20 ml fixer solution (20% (v/v) methanol/10% (v/v) acetic acid, 30 min). Gels were dried and subjected to autoradiography overnight.
MAPKAP kinase-2 assay
The reaction in VT-stimulated cells was stopped by washing twice with ice-cold PBS (750 μl) followed by lysis of cells in solubilising buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% (v/v) Triton X-100, 10% (v/v) glycerol, 2 mM EDTA, 10 mM β-glycerophosphate, 20 mM NaF, 0.1 mM Na3VO4, 2 μg ml−1 leupeptin, 2 μg ml−1 aprotinin, 0.1% (v/v) β-mercaptoethanol and 250 μM PMSF. Cells were scraped from the wells, placed in Eppendorf microfuge tubes, vortex mixed for 1 s and solubilised on ice for 30 min. Protein G–Sepharose beads (40 μl; Sigma, Dorset, U.K.) were washed twice in solubilising buffer and then anti-MAPKAP kinase-2 antibody (1 μg tube−1, final volume 40 μl; Upstate Biotechnology, Milton Keynes, U.K.) was coupled to the beads by incubation at 4°C with agitation for 1.5 h. The beads were then pelleted by centrifugation at 13,000 × g for 1 min, the precleared cell lysates (13,000 × g, 5 min) were added and MAPKAP kinase-2 was immunoaffinity isolated over 2.5 h by rotation at 4°C. After this period, beads were washed once more in solubilising buffer followed by kinase buffer, containing 25 mM HEPES-NaOH (pH 7.4), 20 mM MgCl2, 5 mM β-glycerophosphate, 0.1 mM Na3VO4 and 2 mM DTT. Beads were resuspended in kinase buffer containing 30 μM MAPKAPK-2 substrate peptide (Upstate Biotechnology), 2.5 μM PKA inhibitor peptide, 50 μM ATP/2 μCi [γ-32P]ATP/tube in a final volume of 30 μl and incubated with agitation for 30 min at 30°C. The reaction was terminated via the addition of 300 μM H3PO4 (10 μl). Samples were spotted onto P81 ion-exchanger paper (Merck) and washed twice with 75 mM H3PO4 over a 5-min period followed by two washes with distilled H2O over 5 min. Filters were dried and [γ-32P]phosphate incorporation into the substrate peptide was assessed by liquid scintillation counting.
Determination and quantification of apoptosis
Following incubation with VT, or any pharmacological agent, cells were incubated with phycoerythrin (PE)-conjugated Annexin V and 7-aminoactinomycin D (7-AAD), as described previously (Martin et al., 1995), and apoptosis was quantified by flow cytometry (FACScan, Becton Dickinson). Briefly, stimulated, adherent cells were scraped from the wells and washed twice with PBS. Cells were then resuspended in annexin V binding buffer (100 μl) containing 10 mM HEPES/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2, at a concentration of 1 × 106 cells ml−1, and incubated with Annexin V–PE (5 μl; Pharmingen) on ice in the dark for 15 min. Sample volumes were then increased to 500 μl in binding buffer and placed on ice in the dark and analysed within 1 h. Immediately prior to flow cytometric analysis, 7-AAD (5 μl) was added to each sample. Data were analysed with Win MDI 2.8 software.
Quantification of TNF-α and GM-CSF release
TNF-α release from human monocytes was quantified by indirect enzyme-linked immunosorbent assay (ELISA) employing antibodies and recombinant human TNF-α (rhTNF-α) from R & D Systems (Oxon, U.K.). A mouse anti-human TNF-α monoclonal antibody (100 μl well−1) was coated onto 96-well plates (F16 maxisorb immunomodules, Nunc, Scientific laboratory Supplies Ltd, Nottingham, U.K.) at a concentration of 4 μg ml−1 in PBS (pH 7.4) for 12 h at 4°C. Plates were then washed three times with PBS/0.05% (v/v) Tween-20 and the wells were blocked with PBS containing 1% (w/v) bovine serum albumin (BSA) and 5% (w/v) sucrose for 2 h at room temperature, before the plates were again washed. Unknown samples and rhTNF-α (at concentrations ranging from 16 pg ml−1 to 1 ng ml−1) were added at 100 μl per well and incubated at room temperature for 2 h. Plates were washed as before and a second biotinylated goat anti-human TNF-α antibody was added at a concentration of 200 ng ml−1, diluted from stock in an antibody buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% (w/v) BSA and 0.05% (v/v) Tween-20 at 100 μl per well for 2 h at room temperature. Following four further washes, a streptavidin–HRP conjugate (Cambridge Bioscience, Cambridge, U.K.) was diluted 1 : 4000 in antibody buffer and 100 μl added to each well for 20 min at room temperature. After a final washing stage, plates were developed with 100 μl 3,3′,5,5′-tetramethyl-benzidine (TMB) substrate solution (6 mg ml−1 TMB/DMSO (250 μl); 100 mM acetate/citrate buffer, pH 5.5 (25 ml); 0.1% (30%) H2O2) for 20 min in the dark at room temperature. TNF-α was measured colourimetrically at 450 nm and quantified by interpolation from a standard curve constructed to known concentrations of human recombinant TNF-α. The detection limit of this assay is 16 pg ml−1.
For quantification of GM-CSF release, 96-well round-bottom plates (Greiner Labortechnik Ltd, Dursley, Gloucestershire, U.K.) were coated with 50 μl of a rat, anti-human GM-CSF monoclonal antibody (Pharmingen/Cambridge Bioscience, Cambridge, U.K.) diluted 1 : 250 in buffer B (100 mM NaHCO3, 15 mM NaN3, pH 8.2) and left overnight at 4°C. Plates were subsequently washed in buffer B and immediately blocked with 200 μl foetal calf serum (FCS; 10% in buffer B) for 2 h at room temperature. After an additional wash with buffer B, 100 μl GM-CSF standards, quality controls and unknown samples, in supplemented Dutch-modified RPMI 1640, were added to the plates and left for 18 h at 4°C. Plates were washed in buffer B, incubated for 45 min at room temperature with 100 μl of a biotinylated rat, anti-human monoclonal GM-CSF antibody (Pharmingen/Cambridge Bioscience, Cambridge, U.K.), diluted 1 : 500 in buffer B, supplemented with 10% FCS, washed again, and then incubated for an additional 30 min at room temperature with 100 μl of avidin-peroxidase diluted 1 : 400 in buffer B supplemented with 10% FCS. Plates were washed again and developed with 100 μl 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) substrate solution (0.55 mM ABTS; 0.1 M citric acid, pH 4.35; 0.1% (30%) H2O2). GM-CSF was measured colourimetrically at 405 nm and quantified by interpolation from a standard curve constructed to known concentrations of human recombinant GM-CSF. The detection limit of this assay is 16 pg ml−1.
Data analysis
All data are expressed as means±standard deviation (s.d.) of the mean. Statistical analysis was performed by Students' t-test (two-tailed) and all data represent the mean of at least three independent experiments.
Results
VT induced an increase in JNK activity
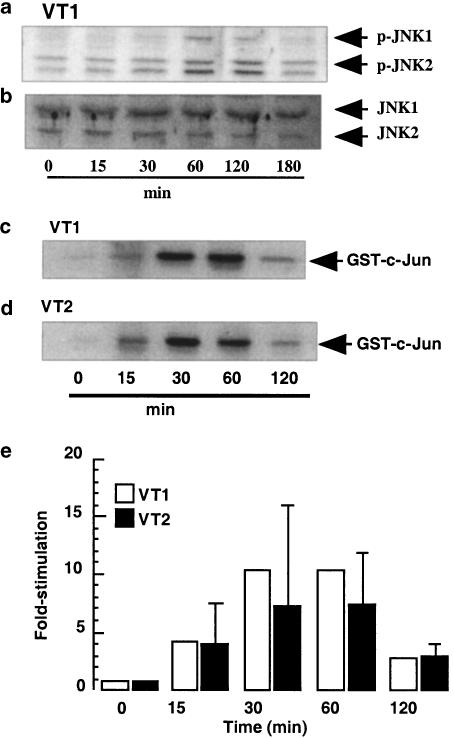
In Figure 1a, a small time-dependent increase in the level of p-JNK was observed and samples were also blotted for the inactive form of JNK to ensure equal protein loading (Figure 1b). However, since the activation of JNK was difficult to measure with phosphorylation site-specific antibodies, a solid-phase JNK assay was employed. Using this method, JNK was activated in response to stimulation with either VT1 or VT2 (both 3 μg ml−1; Figures 1c and d), reaching maximal levels at 30 min at 12.1- and 8.5±5-fold stimulation, over basal phosphorylation, respectively, and returning to basal by 120 min (Figure 1e).
Figure 1.
Effect of VT1 and VT2 on JNK activity in human monocytes. Human monocytes were treated with VT1 (3 ng ml−1) for the times indicated (a) and whole-cell extracts were blotted for phospho-JNK (n=3) as outlined in the Methods section. In (b and c), cells were treated with either VT1 or VT2 (both 3 ng ml−1) for the times indicated and whole-cell lysates were assayed for JNK activity by solid-phase phosphorylation of GST-c-Jun (n=3), as described in the Methods section. JNK activities were quantified by densitometry (d); each value represents the mean±s.d. of at least three experiments.
VT induced a sustained increase in p38 MAP kinase activity
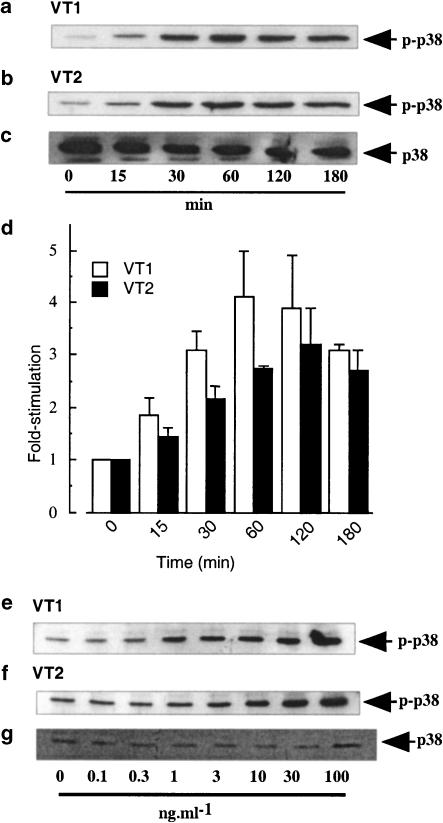
In Figure 2, a time-dependent increase in VT1- and VT2-stimulated phospho-p38 levels in human peripheral blood monocytes was observed, which was maximal at 60 min and remained sustained for up to 3 h (Figures 2a and b). VT1 induced at 4.1±0.9-fold increase in phospho-p38 activity and VT2 increased phospho-p38 levels by 2.8±0.1-fold (Figure 2d) over basal phosphorylation at 0 min. Samples were also blotted for the inactive form of p38 to ensure equal protein loading (Figure 2c). The increase in VT-induced p38 phosphorylation was also concentration dependent, reaching maximal levels with 3 and 10 ng ml−1 VT1 and VT2, respectively (Figures 2e and f). Samples were again blotted for the inactive form of p38 to ensure equal protein loading (Figure 2g).
Figure 2.
Effect of VT1 and VT2 on p38 MAP kinase activity in human monocytes. Human monocytes were treated with VT1 or VT2 (both 3 ng ml−1) for the times indicated (a and b) or with increasing concentrations of either VT1 or VT2 for 30 min (e and f). Whole-cell extracts were blotted for phospho-p38 (a, b, e and f) or total p38 MAP kinase (c) as outlined in the Methods section (n=3). Values obtained in (a and b) were quantified by scanning densitometry and the mean±s.d. of each value is represented in panel (d).
VT induced an increase in ERK activity
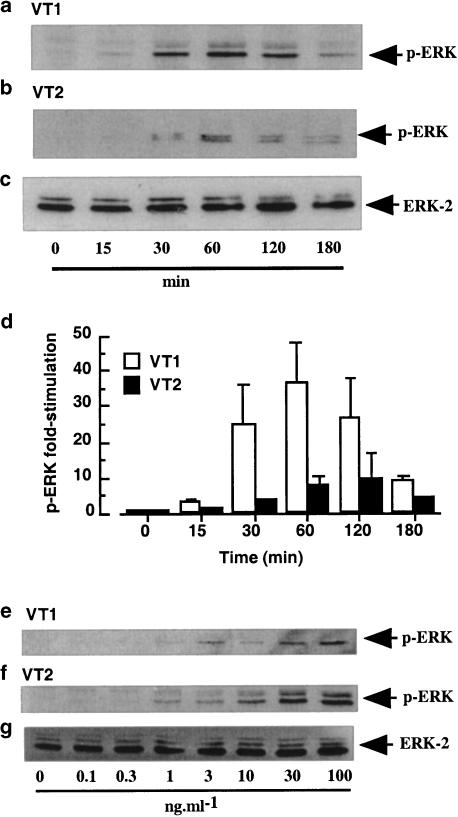
In Figure 3, a time-dependent increase in phosphorylation of ERK is shown for monocytes stimulated with VT1 and VT2 (both 3 ng ml−1; Figures 3a and b). This was maximal by 60 min with a 37±11.3- and 10±7-fold increase in phospho-ERK activity, following stimulation with VT1 and VT2, respectively (Figure 3d) and had returned to near basal levels by 3 h. Samples were also blotted for the inactive form of ERK to ensure equal protein loading (Figure 3c). The increase in ERK phosphorylation was also concentration dependent, reaching maximal levels with 3 and 10 ng ml−1 VT1 and VT2, respectively (Figures 3e and f). Samples were again blotted for the inactive form of ERK to ensure equal protein loading (Figure 3g).
Figure 3.
Effect of VT1 and VT2 on ERK activity in human monocytes. Human monocytes were treated with VT1 or VT2 (both 3 ng ml−1) for the times indicated (a and b) or with increasing concentrations of either VT1 or VT2 for 30 min (e and f). Whole-cell extracts were blotted for phospho-ERK (a, b, e–g) or ERK (c) as outlined in the Methods section (n=3). Values obtained in (a and b) were quantified by scanning densitometry and the mean±s.d. of each value is represented in (d).
Effect of VT2 on apoptosis in human monocytes and Vero cells
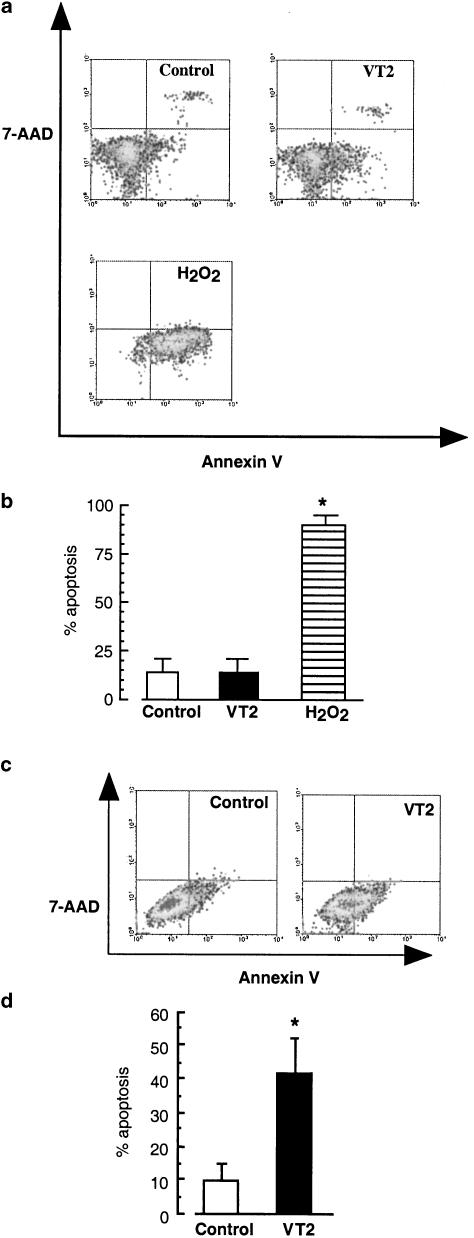
In Figures 4a and b, the basal level of apoptosis of untreated human peripheral blood monocytes was 14±10%, and this was not significantly increased following stimulation with VT2 (100 ng ml−1). Stimulation of monocytes with H2O2 (250 mM, 25 min) significantly increased the number of cells undergoing apoptosis to 90±5% (P<0.001). In order to confirm that VT was effective as a proapototic agent, a VT-susceptible cell line, Vero cells were examined. Following treatment for 18 h, VT2 significantly increased the number of cells undergoing apoptosis from 9.7±5% to 42±10% (P<0.05).
Figure 4.
Effect of VT2 on apoptosis in human monocytes and Vero cells. Human peripheral blood monocytes were stimulated for 18 h with VT2 (100 ng ml−1). Cells were then stained for apoptosis with annexin V, and counterstained with 7-AAD to exclude necrotic cells, before analysis by flow cytometry. In (a), cells in the lower left quadrant represent unstained, viable cells, the lower right quadrant are annexin V-positive cells, the upper right quadrant represent necrotic cells stained with both annexin V and 7-AAD, while the upper left quadrant is representative of necrotic cells stained with 7-AAD alone. (b) Represents the mean of % apoptosis±s.d., n=4. *P<0.001 compared to control. In (c), Vero cells were stimulated for 18 h with VT2 (100 ng ml−1), in the presence or absence of SP600125 (10 μM). Cells were then stained for apoptosis with annexin V, and counterstained with 7-AAD to exclude necrotic cells, before analysis by flow cytometry and (d) represents the mean of % apoptosis±s.d., n=3. *P<0.05 compared to untreated cells. **P<0.05 compared to VT2 stimulation.
VT1- and VT2-induced TNF-α release from monocytes – the roles of JNK, p38 MAP kinase and ERK
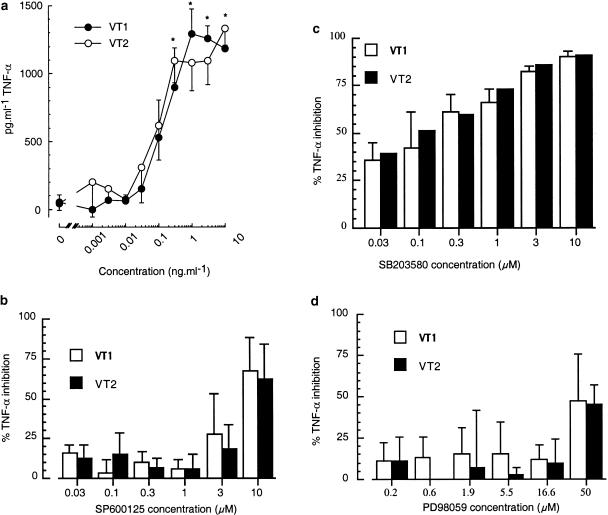
In contrast to the lack of effect upon monocyte apoptosis, both VT1 and VT2 were found to stimulate the release of TNF-α (Figure 5). The effects of VT1 and VT2 were concentration dependent, reaching a maximum at 3 ng ml−1, with EC50 values of 320±5 and 250±9 pg ml−1 TNF-α, respectively (Figure 5a). Figure 5 further illustrates the role of JNK, p38 MAP kinase and ERK in the release process. The JNK inhibitor, SP600125, had little effect on either VT1- or VT2-stimulated TNF-α release, significantly decreasing TNF-α release by 67±21 and 62±22%, respectively, only at a relatively high concentration of 10 μM (Figure 5b). However, the p38 MAP kinase inhibitor, SB203580, significantly reduced both VT1- and VT2-induced TNF-α release from monocytes in a concentration-dependent manner with respective IC50 values of 200±6 and 510±260 nM, reaching a maximum at 10 μM (Figure 5c). TNF-α release from VT-stimulated monocytes, pretreated with the MEK 1 inhibitor PD98059, was unaffected (Figure 5d).
Figure 5.
Effect of p38 MAP kinase, ERK and JNK inhibitors on TNF-α release from human monocytes. Monocytes were treated for 18 h with increasing concentrations of VT1 or VT2 (a), or were pretreated with SB203580 (10 μM), SP600125 (10 μM) or PD98059 (50 μM) for 45 min then stimulated with VT1 or VT2 (3 ng ml−1) for 18 h (b–d). After this period, cell supernatants were collected and TNF-α release was measured by ELISA as outlined in the methods. Each graph is representative of at least three independent experiments±s.d., n=3. *P<0.05 compared to control.
VT1- and VT2-induced GM-CSF release from monocytes – the role of JNK, p38 MAPK and ERK
Figure 6 shows the release of GM-CSF from monocytes after stimulation with VT1 and VT2, and the relative roles of JNK, p38 MAP kinase and ERK. GM-CSF was released from monocytes following stimulation with VT1 and VT2. This effect was concentration dependent, reaching a maximum at 3 ng ml−1 with EC50 values of 250±170 pg ml−1 and 300±130 pg ml−1 GM-CSF in response to VT1 and VT2, respectively (Figure 6a). SP600125 had a greater effect on both VT1- or VT2-stimulated GM-CSF release than on TNF-α release, seen in Figure 6b. GM-CSF levels were significantly reduced at concentrations of 1 and 10 μM inhibitor (Figure 6b). SB203580 also significantly reduced both VT1- and VT2-induced GM-CSF release from monocytes, reaching a maximum at 10 μM (Figure 6c). Pretreatment of monocytes with PD98059, prior to VT1 and VT2 stimulation, led to a significant, concentration-dependent reduction in GM-CSF, and reached a maximum at 50 μM with respective IC50 values of 1.23±0.73 and 1.51±0.71 μM (Figure 6d).
Figure 6.
Effect of p38 MAP kinase, ERK and JNK inhibitors on GM-CSF release from human monocytes. Human monocytes were treated for 18 h with increasing concentrations of VT1 or VT2 (a), or were pretreated with SB203580 (10 μM), SP600125 (10 μM) or PD98059 (50 μM) for 45 min then stimulated with VT1 or VT2 (3 ng ml−1) for 18 h (b–d). After this period, cell supernatants were collected and GM-CSF release was measured by ELISA as outlined in the Methods section. Each graph is representative of at least three independent experiments±s.d., n=3. *P<0.01, **P<0.001 compared to control.
Effect of SP600125, SB203580 and PD98059 on JNK, MAPKAPK-2 and ERK activities
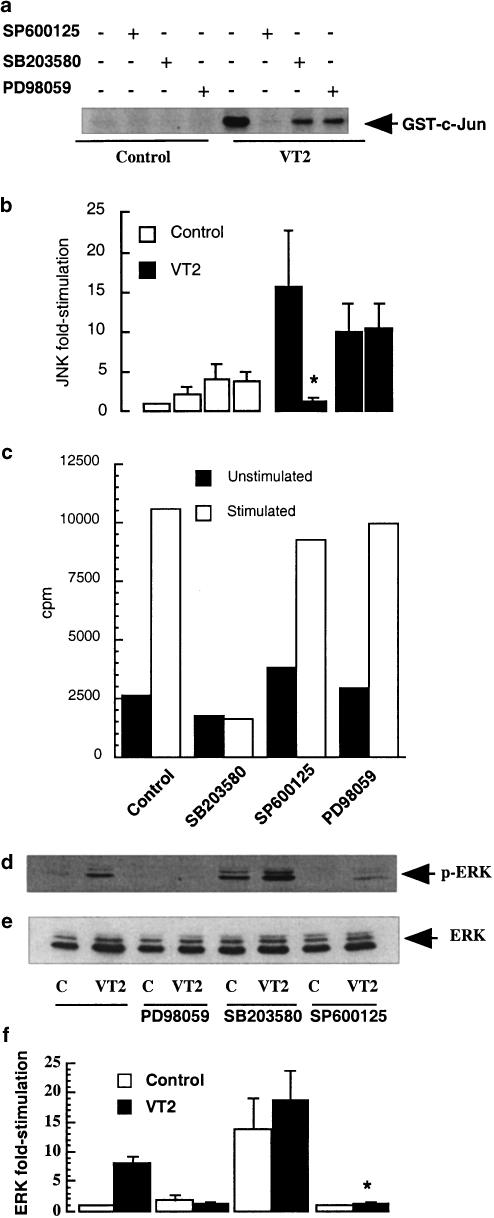
In order to confirm that pharmacological inhibition of TNF-α and GMCSF release accorded to appropriate inhibition of the cellular signalling pathways, the effects of SP600125, SB203580 and PD98059 upon JNK, p38 and ERK signalling was examined. VT2-induced activation of JNK in monocytes was completely abolished by the JNK inhibitor SP600125 (10 μM) with fold stimulation at 60 min falling from 16±9 to 1.6±0.6 (P<0.05) (Figure 7a). However, treatment of cells with the p38 MAP kinase and the MEK 1 (the upstream activator of ERK) inhibitors, SB203580 (10 μM) and PD98059 (50 μM), respectively, was without significant effect on JNK activity (Figures 7a and b).
Figure 7.
Effect of SP600125, SB203580 and PD98059 on JNK, MAPKAPK-2 and ERK activities. Cells were stimulated with VT2 (3 ng ml−1) in the presence or absence of SP600125 (10 μM), SB203580 (10 μM) and PD98059 (50 μM) and were assayed for JNK activity (a, n=4) by solid-phase phosphorylation of GST-c-Jun, for MAPKAPK-2 activity (c) or whole-cell extracts were blotted for phospho-ERK (d). In (b, d and e) each value represents the mean±s.d. of at least three experiments.
VT2-induced activation of MAPKAPK-2, a downstream target of p38 MAP kinase, was abolished by SB203580 (10 μM; Figure 7c) and the counts per minute, at 60 min, fell from 10,533 to 1601 (unstimulated control value=2615). There was no significant effect on MAPKAPK-2 phosphorylation when the cells were treated with SP600125 and PD98059. VT2-induced activation of ERK was abolished by PD98059 (50 μM; Figures 7d and f) with levels falling, at 30 min, from 8.05±1.05- to 1.85±0.85-fold activity. Surprisingly, preincubation of monocytes with SP600125 essentially abolished VT-induced ERK phosphorylation, while SB203580 significantly increased both unstimulated and VT2-stimulated phosphorylation. Samples were also blotted for the inactive form of ERK to ensure equal protein loading (Figure 7e).
Discussion
In this study, we examined the characteristics of MAP kinase activation by VT in relation to the induction of apoptosis and the synthesis of cytokines in human monocytes. In particular, we were interested in the kinetics of activation in relation to function since this has an important bearing on functional outcomes for the cell. We found that in contrast to Vero cells, where JNK activity was found to be sustained (i.e. up to 5–9 h poststimulation), VT induced a more transient increase in JNK activity in monocytes (Figure 1). This correlated well with a lack of apoptosis in response to VT challenge (Figure 4), signifying that the kinetics of JNK is relevant to its role in apoptosis (Roulston et al., 1998). In a previous study (Cameron et al., 2002), we found that serotypes of E. coli O157:H7, which lacked verotoxins and were unable to induce cell death, stimulated a similar transient increase in JNK. In agreement with this hypothesis, in preliminary studies we have shown that VT-induced death of Vero cells could be delayed using the JNK inhibitor, SP600125 (results not shown), demonstrating, as did a recent study (Smith et al., 2003), that JNK is involved in VT-induced apoptosis.
The activation of both p38 MAP kinase and ERK in monocytes, by VT1 and VT2, displayed similar kinetic profiles, slow in onset with activity being sustained for at least 3 h in the case of p38 MAP kinase and 2 h for ERK. These characteristics are dissimilar to that observed with agents that activate these pathways via well-characterised receptor-mediated effects, such as TNF-α and LPS (Jaworowski et al., 1996; Paul et al., 1996; Laird et al., 1998). For example, LPS generates a transient ERK signal, which is maximal at 15 min and returns to basal by 2 h (results not shown). Taken together, these kinetics suggest a different mechanism of activation than that observed for several other agents. The delayed kinetics of the responses suggest that internalisation of the toxins, bound to the receptor CD77, is likely to be a requirement for MAP kinase signalling, a phenomenon recognised to be essential for many VT-induced cellular responses (Obrig et al., 1987; Marcato et al., 2002). Nevertheless, whatever mechanism is involved, such a unique kinetic profile is likely to contribute to the functional effects of VT in this cell type.
Although JNK and p38 MAP kinase have previously been described to contribute to apoptosis of monocytes in response to an immunomodulating peptide (Oses-Prieto et al., 2000), a role for the MAP kinases in the resistance of apoptosis, following VT stimulation, could not be demonstrated in this study. We therefore sought to assess their role in cytokine release. We found that VT strongly increased not only TNF-α (Figure 5) but also GM-CSF release (Figure 6), and although the release of VT-induced TNF-α has been well described (Tesh et al., 1994; Ramegowda & Tesh, 1996; van Setten et al., 1996), the release of GM-CSF has not previously been observed for the verotoxin. TNF-α has been shown to upregulate the VT receptor, Gb3, on numerous endothelial cells (Louise & Obrig, 1991; van de Kar et al., 1992; van Setten et al., 1997), increasing their susceptibility to VT. Thus, TNF-α represents an important mediator of the cytotoxic actions of VT in other cell types. Neutrophils have previously been described to mediate endothelial cell damage in both human and in a mouse model of HUS (Forsyth et al., 1989; Fitzpatrick et al., 1992; Fernandez et al., 2000). Since GM-CSF can prolong the lifespan of neutrophils (Moulding et al., 1998), the release of this cytokine may indirectly enhance the inflammatory actions of the toxin.
Given that the major source of both TNF-α and GM-CSF are cells of the monocyte/macrophage lineage (Eskay et al., 1990), we sought to ascertain the role of the three MAP kinases by utilising specific cell-permeable inhibitors. This included the MEK 1 inhibitor PD98059 (Lazar et al., 1995), the p38 MAP kinase inhibitor SB203580 (Cuenda et al., 1995) and the novel JNK inhibitor SP600125 (Bennett et al., 2001; Shin et al., 2002). The use of SB203580 identified roles for p38 MAP kinase in VT-induced TNF-α and GM-CSF release (Figures 5c and 6c), in keeping with previous studies utilising the compound (Meja et al., 2000). Furthermore, studies using PD98059 implicated a major role for ERK in GM-CSF release (Figure 6d), comparable to that of p38 MAP kinase, but in contrast, its role in TNF-α release was minimal (Figure 5d).
Using SP600125, we identified a potential role for JNK in VT-induced GM-CSF (Figure 6d), and to a lesser extent, TNF-α release (Figure 5d). However, there are considerable difficulties with this interpretation. At a concentration of 10 μM, which inhibited JNK by approximately 90%, SP600125 completely inhibited ERK phosphorylation, comparable to that produced by PD98059, and conversely, promoted the activation of p38 MAP kinase (measured as phosphorylation of MAPKAPK-2). For GM-CSF, this is relevant since both p38 MAP kinase and ERK are implicated in the release of this cytokine in response to VT. Previously, this inhibitor was described to have 300-fold greater selectivity for JNK1, -2 and -3 than ERK or p38 MAP kinase (Bennett et al., 2001) with an IC50 of 5–10 μM for the inhibition of c-Jun. However, in our hands, concentrations of 1–10 μM were required for maximum inhibition of VT2-induced JNK activity. Therefore, a JNK inhibitor with greater selectivity is required for the true role of JNK in TNF-α and GM-CSF release following VT stimulation to be elucidated. However, it should be noted that SP600125 can be satisfactorily used in some systems. For example, in Vero cells, VT-induced activation of ERK is negligible, while JNK and p38 are very strong (Cameron et al., 2002). However, as p38 MAP kinase and ERK are not involved in apoptosis, a relative ‘selectivity' can be identified.
These data, together with our previous work with Vero cells (Cameron et al., 2002), show two very distinct signalling profiles in cell types with different responses to the VTs. In human monocytes, the VTs activate p38 MAP kinase and ERK but not JNK, while in Vero cells we demonstrated strong, sustained activation of JNK and p38 MAP kinase, with little activation of ERK. Furthermore, in Vero cells the VT-mediated sustained MAP kinase response, particularly JNK, is consistent with resulting apoptosis in the cells, while the more transient profile of MAP kinase signalling in monocytes has a role in generating inflammatory cytokine release.
Acknowledgments
We wish to acknowledge the kind gifts of constructs and reagents from various laboratories. We acknowledge the kind gift of SP600125 from Brydon Bennett of Signal Pharmaceuticals (bbennett@signalpharm.com). This work was sponsored by SHERT Grant No. RG6/01.
Abbreviations
- 7-AAD
7-amino actinomycin D
- E. coli
Escherichia coli
- EHEC
enterohaemorrhagic E. coli
- ERK
extracellular-regulated kinase
- GM-CSF
granulocyte–macrophage colony-stimulated factor
- GST
glutathione S-transferase
- JNK
c-Jun N-terminal kinase
- MAP kinase
mitogen-activated protein kinase
- MAPKAPK-2
mitogen-activated protein kinase-activated protein kinase-2
- MEK
MAP kinase/ERK kinase
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- rh (prefix)
recombinant human
- TNF-α
tumour necrosis factor alpha
- VT
verotoxin
- VTEC
verotoxigenic E. coli
References
- ARBUS G.S. Association of verotoxin-producing E. coli and verotoxin with hemolytic uremic syndrome. Kidney Int. 1997;51:S-91–S-96. [PubMed] [Google Scholar]
- BENNETT B.L., SASAKI D.T., MURRAY B.W., O'LEARY E.C., SAKATA S.T., XU W., LEISTEN J.C., MOTIWALA A., PIERCE S., SATOH Y., BHAGWAT S.S., MANNING A.M., ANDERSON D.W. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERIN M.C., DARFEUILLE-MICHAUD A., EGAN L.J., MIYAMOTO Y., KAGNOFF M.F. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-kappa B and MAP kinase pathways and the upregulated expression of interleukin 8. Cell Microbiol. 2002;4:635–648. doi: 10.1046/j.1462-5822.2002.00218.x. [DOI] [PubMed] [Google Scholar]
- BRINKMAN B.M.N., TELLIEZ J.-B., SCHIEVELLA A.R., LIN L.-L., GOLDFELD A.E. Engagement of tumor necrosis factor (TNF) receptor 1 leads to ATF-2- and p38 mitogen-activated protein kinase-dependent TNF-alpha gene expression. J. Biol. Chem. 1999;274:30882–30886. doi: 10.1074/jbc.274.43.30882. [DOI] [PubMed] [Google Scholar]
- CAMERON P., BINGHAM D., PAUL A., PAVELKA M., CAMERON S., ROTONDO D., PLEVIN R. Essential role for verotoxin in sustained stress-activated protein kinase and nuclear factor kappa B signaling, stimulated by Escherichia coli O157:H7 in Vero cells. Infect. Immunol. 2002;70:5370–5380. doi: 10.1128/IAI.70.10.5370-5380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Y.R., MEYER C.F., TAN T.H. Persistant activation of c-jun N-terminal kinase 1 (JNK1) in γ radiation-induced apoptosis. J. Biol. Chem. 1996;271:631–634. doi: 10.1074/jbc.271.2.631. [DOI] [PubMed] [Google Scholar]
- COIA J. Clinical, microbiological and epidemiological aspects of Escherichia coli O157 infection. FEMS Microbiol. Immunol. 1998;20:1–9. doi: 10.1111/j.1574-695X.1998.tb01105.x. [DOI] [PubMed] [Google Scholar]
- CUENDA A., ROUSE J., DOZA Y.N., MEIER R., COHEN P., GALLAGHER T.F., YOUNG P.R., LEE J.C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- DUNDAS S., TODD W.T.A. Escherichia coli O157 and human disease. Curr. Opin. Infect. Dis. 1998;11:171–175. doi: 10.1097/00001432-199804000-00015. [DOI] [PubMed] [Google Scholar]
- ESKAY R.L., GRINO M., CHEN H.T. Interleukins, signal transduction, and the immune system-mediated stress response. Adv. Exp. Med. Biol. 1990;274:331–343. doi: 10.1007/978-1-4684-5799-5_21. [DOI] [PubMed] [Google Scholar]
- FERNANDEZ G.C., RUBEL C., DRAN G., GOMEZ S., ISTURIZ M.A., PALERMO M.S. Shiga toxin-2 induces neutrophilia and neutrophil activation in a murine model of hemolytic uremic syndrome. Clin. Immunol. 2000;95:227–234. doi: 10.1006/clim.2000.4862. [DOI] [PubMed] [Google Scholar]
- FITZPATRICK M.M., SHAH V., FILLER G., DILLON M.J., BARRATT T.M. Neutrophil activation in the haemolytic uraemic syndrome: free and complexed elastase in plasma. Pediatr. Nephrol. 1992;6:50–53. doi: 10.1007/BF00856833. [DOI] [PubMed] [Google Scholar]
- FORSYTH K.D., SIMPSON A.C., FITZPATRICK M.M., BARRATT T.M., LEVINSKY R.J. Neutrophil-mediated endothelial injury in haemolytic uraemic syndrome. Lancet. 1989;2:411–414. doi: 10.1016/s0140-6736(89)90591-6. [DOI] [PubMed] [Google Scholar]
- FOSTER G.H., TESH V.L. Shiga toxin 1-induced activation of c-jun NH2-terminal kinase and p38 in the human monocytic cell line THP-1: possible involvement in the production of TNF-alpha. J. Leukocyte Biol. 2002;71:107–114. [PubMed] [Google Scholar]
- HARADA J., SUGIMOTO M. An inhibitor of p38 and JNK MAP kinases prevents activation of caspase and apoptosis of cultured cerebellar granule neurons. Jpn. J. Pharmacol. 1999;79:369–378. doi: 10.1254/jjp.79.369. [DOI] [PubMed] [Google Scholar]
- HOFMAN S. Southwestren Internal Medical Conference: Shiga-like toxins in hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Am. J. Med. Sci. 1993;306:398–406. doi: 10.1097/00000441-199312000-00009. [DOI] [PubMed] [Google Scholar]
- IKEDA M., GUNJI Y., YAMASAKI S., TAKEDA Y. Shiga toxin activates p38 MAP kinase through cellular Ca(2+) increase in Vero cells. FEBS Lett. 2000;485:94–98. doi: 10.1016/s0014-5793(00)02204-3. [DOI] [PubMed] [Google Scholar]
- INWARD C.D., WILLIAMS J., CHANT I., MILFORD D.V., ROSE P.E., TAYLOR C.M. Verocytotoxin-1 Induces apoptosis in Vero cells. J. Infect. 1995;30:213–218. doi: 10.1016/s0163-4453(95)90693-2. [DOI] [PubMed] [Google Scholar]
- JAWOROWSKI A., CHRISTY E., YUSOFF P., BYRNE R., HAMILTON J.A. Differences in the kinetics of activation of protein kinases and extracellular signal-related protein kinase 1 in colony-stimulating factor 1-stimulated and lipopolysaccharide-stimulated macrophages. Biochem. J. 1996;320:1011–1016. doi: 10.1042/bj3201011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARPMAN D., HAKANSSON A., PEREZ M.-T.R., ISAKSSON C., CARLEMALM E., CAPRIOLI A., SVANBORG C. Apoptosis of renal cortical cells in the hemolytic-uremic syndrome: in vivo and invitro studies. Infect. Immunol. 1998;66:636–644. doi: 10.1128/iai.66.2.636-644.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIYOKAWA N., TAGUCHI T., MORI T., UCHIDA H., SATO N., TAKEDA T., FUJIMOTO J. Induction of apoptosis in normal human renal tubular epithelial cells by Escherichia coli Shiga Toxins 1 and 2. J. Infect. Dis. 1998;178:178–184. doi: 10.1086/515592. [DOI] [PubMed] [Google Scholar]
- LAIRD S.M., GRAHAM A., PAUL A., GOULD G.W., KENNEDY C., PLEVIN R. Tumour necrosis factor stimulates stress-activated protein kinases and the inhibition of DNA synthesis in cultures of bovine aortic endothelial cells. Cell Signal. 1998;10:473–480. doi: 10.1016/s0898-6568(97)00173-3. [DOI] [PubMed] [Google Scholar]
- LAZAR D.F., WIESE R.J., BRADY M.J., MASTICK C.C., WATERS S.B., YAMAUCHI K., PESSIN J.E., CUATRECASAS P., SALTIEL A.R. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J. Biol. Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- LIU J., AKAHOSHI T., SASAHANA T., KITASATO H., NAMAI R., SASAKI T., INOUE M., KONDO H. Inhibition of neutrophil apoptosis by verotoxin 2 derived from Escherichia coli O157:H7. Infect. Immunol. 1999;67:6203–6205. doi: 10.1128/iai.67.11.6203-6205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU J., HE T., HE Y., ZHANG Z., AKAHOSHI T., KONDO H., ZHONG S. Prolongation of functional life-span of neutrophils by recombinant verotoxin 2. Chin. Med. J. (Engl.) 2002;115:900–903. [PubMed] [Google Scholar]
- LOUISE C.B., OBRIG T.G. Shiga toxin-associated hemolytic uremic syndrome: combined cytotoxic effect of shiga toxin, interleukin-1 and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect. Immunol. 1991;59:4173–4179. doi: 10.1128/iai.59.11.4173-4179.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCATO P., MULVEY G., ARMSTRONG G.D. Cloned Shiga toxin 2 B subunit induces apoptosis in Ramos Burkitt's lymphoma B cells. Infect Immunol. 2002;70:1279–1286. doi: 10.1128/IAI.70.3.1279-1286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- MARTIN S.J., REUTELINGSPERGER C.P., MCGAHON A.J., RADER J.A., VAN SCHIE R.C., LAFACE D.M., GREEN D.R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEJA K.K., SELDON P.M., NASUHARA Y., ITO K., BARNES P.J., LINDSAY M.A., GIEMBYCZ M.A. p38 MAP kinase and MKK-1 co-operate in the generation of GM-CSF from LPS-stimulated human monocytes by an NF-kappa B-independent mechanism. Br. J. Pharmacol. 2000;131:1143–1153. doi: 10.1038/sj.bjp.0703684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULDING D.A., QUAYLE J.A., HART C.A., EDWARDS S.W. Mcl-1 expression in human neutrophils: regulation by cytokines and correlation with cell survival. Blood. 1998;92:2495–2502. [PubMed] [Google Scholar]
- MURATA A., SHIMAZU T., YAMAMOTO T., TAENAKA N., NAGAYAMA K.-I., HONDA T., SUGIMOTO H., MONDEN M., MATSUURA N., OKADA S. Profiles of circulating inflammatory and anti-inflammatory cytokines in patients with hemolytic uremic syndrome due to E. coli O157 infection. Cytokine. 1998;10:544–548. doi: 10.1006/cyto.1997.0329. [DOI] [PubMed] [Google Scholar]
- O'BRIEN A.D., HOLMES R.K. Shiga and Shiga-like toxins. Microbiol. Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'BRIEN A.D., TESH V.L., DONOHUE-ROLFE A., JACKSON M.P., OLSNES S., SANDVIG K., LINDBERG A.A., KEUSCH G.T. Shiga toxin: biochemistry, genetics, mode of action and role in pathogenesis. Curr. Top. Microbiol. Immunol. 1992;180:65–94. doi: 10.1007/978-3-642-77238-2_4. [DOI] [PubMed] [Google Scholar]
- OBRIG T.G., MORAN T.P., BROWN J.E. The mode of action of Shiga toxin on peptide elongation of eukaryotic protein synthesis. Biochem. J. 1987;244:287–294. doi: 10.1042/bj2440287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHMI K., KIYOKAWA N., TAKEDA T., FUJIMOTO J. Human microvascular endothelial cells are strongly sensitive to Shiga toxins. Biochem. Biophys. Res. Commun. 1998;251:137–141. doi: 10.1006/bbrc.1998.9417. [DOI] [PubMed] [Google Scholar]
- OSES-PRIETO J.A., LOPEZ-MORATALLA N., SANTIAGO E., JAFFREZOU J.P., LOPEZ-ZABALZA M.J. Molecular mechanisms of apoptosis induced by an immunomodulating peptide on human monocytes. Arch. Biochem. Biophys. 2000;15:353–362. doi: 10.1006/abbi.2000.1875. [DOI] [PubMed] [Google Scholar]
- PAUL A., CUENDA A., BRYANT C.E., GOULD G.W., COHEN P., PLEVIN R. Dissociation of LPS-stimulated MAP kinase activation and nitric oxide synthase reduction in RAW 264.7 macrophages. Br. J. Pharmacol. 1996;118:7P. [Google Scholar]
- PAUL A., TORRIE L., MCLAREN G., KENNEDY C., PLEVIN R. P2Y-receptor mediated inhibition of tumour necrosis factor-α-stimulated stress-activated protein kinase in EAhy926 endothelial cells. J. Biol. Chem. 2000;275:13243–13249. doi: 10.1074/jbc.275.18.13243. [DOI] [PubMed] [Google Scholar]
- PAUL A., WILSON S., BELHAM C.M., ROBINSON C.J.M., SCOTT P.H., GOULD G.W., PLEVIN R. Stress-activated protein kinases: activation, regulation and function. Cell Signal. 1997;9:403–410. doi: 10.1016/s0898-6568(97)00042-9. [DOI] [PubMed] [Google Scholar]
- RAMEGOWDA B., TESH V.L. Differentiation-associated toxin receptor modulation, cytokine production and sensitivity to the shiga-like toxins in human monocytes and monocytic cell lines. Infect. Immunol. 1996;64:1173–1180. doi: 10.1128/iai.64.4.1173-1180.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROULSTON A., RIENHARDT C., AMIRI P., WILLIAMS L.T. Early activation of c-Jun N-terminal kinase and p38 kinase regulate cell survival in response to tumor necrosis factor α. J. Biol. Chem. 1998;273:10232–10239. doi: 10.1074/jbc.273.17.10232. [DOI] [PubMed] [Google Scholar]
- SHIN M., YAN C., BOYD D. An inhibitor of c-jun aminoterminal kinase (SP600125) represses c-Jun activation, DNA-binding and PMA-inducible 92-kDa type IV collagenase expression. Biochim. Biophys. Acta. 2002;1589:311–316. doi: 10.1016/s0167-4889(02)00195-7. [DOI] [PubMed] [Google Scholar]
- SMITH W.E., KANE A.V., CAMPBELL S.T., ACHESON D.W., COCHRAN B.H., THORPE C.M. Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells. Infect. Immunol. 2003;71:1497–1504. doi: 10.1128/IAI.71.3.1497-1504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWANTEK J.L., COBB M.H., GEPPERT T.D. Jun N-terminal/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol. Cell. Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESH V.L., O'BRIEN A.D. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol. Microbiol. 1991;5:1817–1822. doi: 10.1111/j.1365-2958.1991.tb00805.x. [DOI] [PubMed] [Google Scholar]
- TESH V.L., RAMEGOWDA B., SAMUEL J.E. Purified Shiga-like toxins induce expression of proinflammatory cytokines from murine macrophages. Infect. Immunol. 1994;62:5085–5094. doi: 10.1128/iai.62.11.5085-5094.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DE KAR N.C.A.J., MONNEMS L.A.H., KARMALI M.A., VAN HINSBERGH V.W.M. Tumour necrosis factor and interleukin-1 induce expression of the verocytotoxin receptor globotriaosylceramide on human endothelial cells: implications for the pathogenesis of the haemolytic uraemic syndrome. Blood. 1992;80:2755–2764. [PubMed] [Google Scholar]
- VAN SETTEN P.A., MONNENS L.A., VERSTRATEN R.G., VAN DEN HEUVEL L.P., VAN HINSBERGH V.W. Effects of verocytotoxin-1 on nonadherent human monocytes: binding characteristics, protein synthesis, and induction of cytokine release. Blood. 1996;88:174–183. [PubMed] [Google Scholar]
- VAN SETTEN P.A., VAN HINSBERGH V.W.M., VAN DER VELDEN T.J.A.N., VAN DE KAR N.C.A.J., VERMEER M., MAHAN J.D., ASSAMANN K.J.M., VAN DEN HEUVEL L.P.W.J., MONNENS L.A.H. Effects of TNFα on verocytotoxin cytotoxicity in purified human glomerular microvascular endothelial cells. Kidney Int. 1997;51:1245–1256. doi: 10.1038/ki.1997.170. [DOI] [PubMed] [Google Scholar]
- XIA Z., DICKENS M., RAINGEAUD J., DAVIS R.J., GREENBERG M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- YOSHIDA T., KOIDE N., SUGIYAMA T., MORI I., YOKOCHI T. A novel caspase dependent pathway is involved in apoptosis of human endothelial cells by Shiga toxins. Microbiol. Immunol. 2002;46:697–700. doi: 10.1111/j.1348-0421.2002.tb02753.x. [DOI] [PubMed] [Google Scholar]
- ZANKE B.W., LEE C., ARAB S., TANNOCK I.F. Death of tumor cells after intracellular acidification is dependant on stress-activated protein kinases (SAPK/JNK) pathway activation and cannot be inhibited by Bcl-2 expression or interleukin 1β-converting enzyme inhibition. Cancer Res. 1998;58:2801–2808. [PubMed] [Google Scholar]