Abstract
The objective of the present study was to investigate the mechanism of the relaxant activity of marrubenol, a diterpenoid extracted from Marrubium vulgare. In rat aorta, marrubenol was a more potent inhibitor of the contraction evoked by 100 mM KCl (IC50: 11.8±0.3 μM, maximum relaxation: 93±0.6%) than of the contraction evoked by noradrenaline (maximum relaxation: 30±1.5%).
In fura-2-loaded aorta, marrubenol simultaneously inhibited the Ca2+ signal and the contraction evoked by 100 mM KCl, and decreased the quenching rate of fura-2 fluorescence by Mn2+.
Patch-clamp data obtained in aortic smooth muscle cells (A7r5) indicated that marrubenol inhibited Ba2+ inward current in a voltage-dependent manner (KD: 8±2 and 40±6 μM at holding potentials of −50 and −100 mV, respectively).
These results showed that marrubenol inhibits smooth muscle contraction by blocking L-type calcium channels.
Keywords: Marrubenol, fura-2, Ca2+ channel blocker, L-type Ca2+ channel
Introduction
The crude extract of Marrubium vulgare (Horehound, Lamiaceae) is widely used as antihypertensive treatment in traditional medicine. It has been shown to decrease systolic blood pressure in spontaneously hypertensive rats and to inhibit KCl-induced contraction in rat aorta (El Bardai et al., 2001). A pure compound with vasorelaxant activity has been isolated from the water extract of Marrubium vulgare. It has been identified and characterised as marrubenol (1,4-naphthalenediol, 1-[2-(3-furanyl)ethyl]decahydro-5-(hydroxymethyl)-2,5,8a-trimethyl,[1R-(1α, 2α, 4β, 4aα, 5β, 8aβ)] (El Bardai et al., 2003). Marrubenol is a diterpenoid that was first isolated from Marrubium vulgare by Fulke et al. (1968). It relaxes KCl-contracted artery in a concentration-dependent manner, with an IC50 value of 10 μM (El Bardai et al., 2003). However, its pharmacological properties have not been studied so far. Several diterpenes have been shown to have pronounced cardiovascular effects, for example, grayanotoxin I produces positive inotropic responses (Hotta et al., 1994), forskolin is a well-known activator of adenylate cyclase (Lebedinsky et al., 1992), eleganolone and 14-deoxyandrographolide exhibit vasorelaxant properties (Della Pieta et al., 1993; Zhang & Tan, 1998).
The present study deals with the characterisation of the relaxant effect of marrubenol, using functional studies on rat isolated aorta. The mechanism of the effect of marrubenol was first investigated in fura-2-loaded artery. The interaction of marrubenol with the Ca2+ channels was further examined in patch-clamp experiments. Results indicated that the relaxant effect of marrubenol may be attributed to its inhibitory interaction with L-type Ca2+ channels.
Methods
Marrubenol
Marrubenol was extracted from the dried aerial parts of Marrubium vulgare (60 g) with 400 ml of distilled water at 90°C for 15 min. The decoction was extracted three times with cyclohexane. The aqueous fraction was lyophilised and the cyclohexane fraction was evaporated under reduced pressure, yielding a greenish oily residue (180 mg) that was chromatographed on a medium pressure silica gel column (Lichoprep Si60 (15–25 μm), Merck), eluted with CH2Cl2–MeOH (99 : 1). Fractionation was monitored by thin layer chromatography (TLC), using CH2Cl2–MeOH (95 : 5) as the solvent system. Pure marrubenol (9.9 mg) was identified by TLC and NMR analysis, in comparison with marrubenol synthesised from marrubiin (El Bardai et al., 2003).
Measurement of the contractile response of rat aorta
Male Wistar rats (250–300 g) were used. Contractions of isolated aorta were measured as previously described (Morel & Godfraind, 1994). After removing the endothelium by gentle rubbing, aortic rings (2 mm wide) were suspended under a resting tension of 20 mN, in 12.5 ml organ baths filled with a physiological solution (composition (mM): NaCl, 122; KCl, 5.9; NaHCO3, 15; MgCl2, 1.25; CaCl2, 1.25; glucose, 11) bubbled with a gas mixture of 95% O2, 5% CO2 and maintained at 37°C. After an equilibration period, each preparation was contracted by changing the physiological solution in the bath to a depolarising 100 mM KCl solution (composition (mM): NaCl, 27; KCl, 100; NaHCO3, 15; MgCl2, 1.25; CaCl2, 1.25; glucose, 11), or by adding noradrenaline (1 μM) into the bath solution. After washing, the muscles were incubated for 30 min in the presence of marrubenol and a second contraction was evoked in the continuous presence of marrubenol. When the effect of marrubenol was tested on noradrenaline contraction in the presence of nimodipine, nimodipine was added to all solutions immediately after the KCl contraction. The absence of acetylcholine (1 μM)-induced relaxation in arteries contracted with noradrenaline (1 μM) was taken as an indicator that vessels were denuded successfully.
Measurement of contractile tension and cytosolic calcium concentration in aorta
Endothelium-denuded aortic rings were incubated for 4 h at room temperature in a physiological solution containing 5 μM fura-2 acetoxymethyl ester (fura-2 AM) and 0.05% cremophor EL (Ozaki et al., 1987). After the loading period, the rings were washed in physiological solution at 37°C for 30 min. They were mounted between two hooks under a tension of 20 mN in a 3 ml cuvette filled with physiological solution (composition as above) at 37°C, gassed with a 95–5% mixture of O2 and CO2. All solutions used in the fura-2 experiments contained Nω-nitro-L-arginine (L-NNA, 100 μM) to prevent the release of NO that could occur following induction of NO synthase during the long fura-2-loading procedure. The cuvette was part of a fluorimeter (CAF, JASCO, Tokyo), which allowed estimation of the calcium signal. The muscle tone was measured by an isometric force transducer. The Ca2+ signal was measured as previously reported (Ghisdal et al., 2000). The fluorescence signals at 340 and 380 nm, F340 and F380, were measured simultaneously with the contractile tension and recorded on a computer, by using the data acquisition hardware MacLab and data recording software Chart (AD Instruments Pty Ltd, Castle Hill, Australia). After being mounted in the cuvette, the artery segment was further incubated for 30 min in physiological solution. The artery was thereafter stimulated with 100 mM KCl solution. Marrubenol was added into the cuvette at the plateau of the contraction. At the end of the experiment, the fura-2-Ca2+ signal was calibrated. The maximal ratio (Rmax) was measured in calcium saturating medium by adding ionomycin (10 μM) in high-KCl solution, while the minimal ratio (Rmin) was obtained in calcium-free medium in the presence of EGTA (10 mM). The autofluorescence was measured at 340 and 380 nm by quenching the fura-2 fluorescence with MnCl2 (5 mM). Cytosolic calcium concentration ([Ca2+]cyt) was calculated as described previously (Salomone et al., 1995).
The Mn2+-induced quenching of fura-2 fluorescence was estimated at 363 nm excitation wavelength (F363), which represented the isosbestic wavelength in our system. Endothelium-denuded artery rings, loaded with fura-2 AM, were mounted in the cuvette and preincubated for 30 min in physiological solution with L-NNA. The physiological solution in the cuvette was then changed to a 100 mM KCl, Ca2+-free solution, containing the tested compounds when required. After 3 min, MnCl2 (0.1 mM) was added to the solution, which produced a quenching of the fluorescence. After 5 min, 10 mM MnCl2 was added to the cuvette to quench the remaining fluorescence. The minimum value recorded in the presence of 10 mM MnCl2 was considered as the background fluorescence (auto-fluorescence) and subtracted from all values. Experimental values of F363 were normalised to the fluorescence measured before the addition of 0.1 mM MnCl2.
Inhibition of Ba2+ current
Voltage-clamp experiments were performed at room temperature (20±2°C) in the whole-cell configuration of the patch-clamp technique. Aortic smooth muscle cells (A7r5) were used. Cells were cultured as described (Buryi et al., 1995). Pipettes (2–5 MΩ) were pulled and polished using a DMZ-universal puller (Zeitz Instrument Vertriebs GmbH, München, Germany) and connected to the head stage of a patch-clamp amplifier (List EPC-7, Darmstadt/Eberstadt, Germany). Programmed voltage-clamp sequences and data acquisition were performed by specific software (pClamp, V5-5-1, Axon Instruments, Foster City, CA, U.S.A.) through an A/D–D/A conversion board (Labmaster, Scientific solutions, Solon, U.S.A.). The protocol that was used to assess the effect of marrubenol on IBa consisted of recording the current elicited by depolarising pulses (200–500 ms) to 0 mV, applied every 10 s from a holding potential of −100 or −50 mV. The holding potential was then set back to −100 mV and marrubenol was added to the perfusion solution. The same protocol was thereafter applied in the continuous presence of marrubenol.
The pipette was filled with high Cs+, low EGTA solution containing (in mM): CsCl 140, MgCl2 5, adenosine 5′-triphosphate disodium salt (Na2ATP) 5, EGTA 0.1, HEPES 10 (pH 7.2) and the bath was continuously perfused with a physiological salt solution containing (in mM): NaCl 137, CsCl 6, BaCl2 6, glucose 10, HEPES 10 (pH 7.4) and supplemented with tetrodotoxin 1 μM. When needed, drugs were added to the perfusion solution.
Drugs
Noradrenaline bitartrate, L-NNA, verapamil, cremophor EL and ionomycin were from Sigma (Borhem, Belgium). Fura-2 AM was from Calbiochem (EuroBiochem, Bierges, Belgium). Nimodipine was a gift from Bayer (Leverkussen, Germany). Marrubenol was dissolved in ethanol as stock solutions at 30 mM, and further diluted in water as required before use. All experiments were performed under yellow light to prevent drug degradation.
Analysis
Inhibition of the contractions was calculated as a percentage of the contractile force measured before the addition of the drug, and was corrected for time-matched controls. The drug concentration inhibiting the contractile response by 50% (IC50) was determined by nonlinear regression of averaged data (PRISM, GraphPad). The dissociation constant (KD) of marrubenol to the Ca2+ channel was the concentration inhibiting IBa by 50%. It was calculated for each cell as described previously (Morel et al., 1998). Inactivation curves were fitted to the experimental data using the Boltzmann's equation 1/(1+exp(V−V0.5)/k) where V is the potential, V0.5 the midpoint of the curve and k the slope factor. Data are expressed as means±s.e.m. Comparisons were made using Student's t-test or by analysis of variance (one-way ANOVA) followed by a Bonferroni test, when more than two groups were involved in the comparison. P-values lower than 0.05 indicated the significant differences.
Results
Effect of marrubenol on aorta contractility
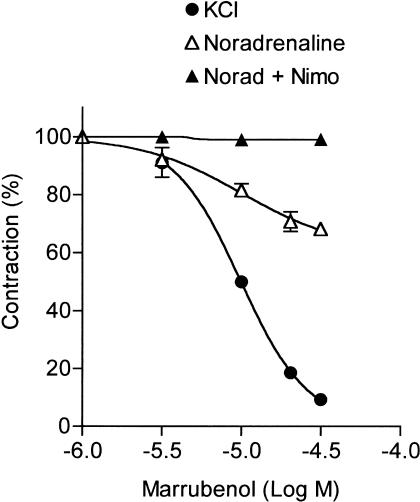
As previously reported, marrubenol relaxed the contraction evoked by KCl depolarisation in the rat aorta (El Bardai et al., 2003). The relaxing effect of marrubenol was unaffected by removal of the endothelium: IC50 (11.8±0.3 μM, n=5) and maximum relaxation (93.4±0.6% of the contraction) obtained in endothelium-denuded preparations (Figure 1) were not different from the values reported in endothelium-intact aorta (El Bardai et al., 2003). Marrubenol also inhibited the contraction induced by noradrenaline (1 μM) in aorta, but maximum inhibition obtained at the highest concentrations of marrubenol (30–50 μM) plateaued at 30±1.5% (n=5). Moreover, marrubenol was ineffective in aorta contracted by noradrenaline in the presence of 1 μM of the Ca2+ channel blocker nimodipine (Figure 1).
Figure 1.
Effect of marrubenol on the contraction of rat aorta. Inhibition of the contraction was measured in endothelium-denuded rat aortic rings, preincubated in the presence of different concentrations of marrubenol (1–30 μM) in the bath solution and contracted either by 100 mM KCl solution, or by noradrenaline (1 μM) in the absence or in the presence of 1 μM of the Ca2+ channel blocker nimodipine (Norad+Nimo). In control muscles, equivalent amounts of ethanol were added. Contractions are expressed as a percentage of the contraction measured before the addition of marrubenol. Data are means from four to five determinations. Vertical bars indicate the s.e.m. values. Concentration–effect curve of marrubenol in endothelium-denuded KCl-contracted preparation was identical to the curve obtained in endothelium-intact aorta (El Bardai et al., 2003).
Effect of marrubenol on cytoplasmic Ca2+ level and on Mn2+ influx in fura-2-loaded aorta
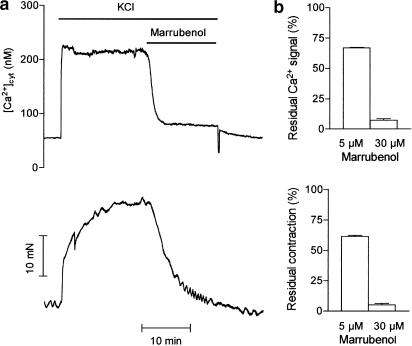
Depolarisation of aortic smooth muscle cells by the high KCl solution simultaneously increased the cytosolic Ca2+ concentration from 79±10 to 266±47 nM (n=9) and the contractile tension by 16.6.±1.9 mN (n=9). The addition of marrubenol into the bathing solution when responses were at steady-state simultaneously decreased the cytosolic Ca2+ concentration and the contraction of the preparation in a concentration-dependent manner (Figure 2).
Figure 2.
Effect of marrubenol on the cytosolic calcium concentration and contractile tension in a fura-2-loaded aortic ring. (a) Typical traces of Ca2+ signal (upper trace) and contraction (lower trace). The artery was stimulated with 100 mM KCl solution. Marrubenol (30 μM) was added into the cuvette at the plateau of the contraction (as indicated). (b) Bar graphs representing the mean values (n=5) of the effect of marrubenol 5 and 30 μM on the Ca2+ signal (upper panel) and the contraction (lower panel). Data were expressed as percentages of the responses measured immediately before the addition of marrubenol, and were corrected for the decrease in responses measured in time-matched controls.
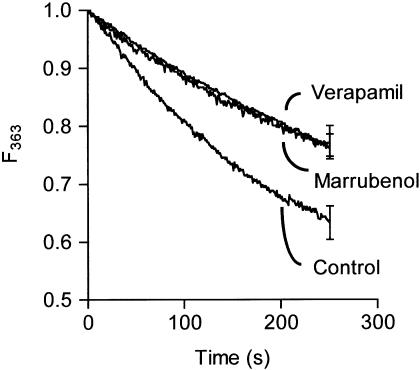
The influx of Ca2+ can be estimated by measuring the quenching rate of fura-2 by Mn2+. Indeed, Mn2+ ions can traverse Ca2+ channels, whereas they are not taken up by the Ca2+ pumps or exchangers of the sarcoplasmic reticulum and of the plasma membrane (Missiaen et al., 1990). In the cytosol, Mn2+ rapidly binds fura-2 and quenches the fluorescence (Chen & Rembold, 1992). Figure 3 shows the quenching of the fura-2 fluorescence F363 in fura-2-loaded aortas bathed in 100 mM KCl solution after the addition of MnCl2. In the presence of marrubenol (30 μM) or verapamil (10 μM), the quenching rate was significantly decreased (Figure 3). The negative slope values (× 1000) of the initial (0–60 s) rate of fluorescence quenching by MnCl2 were 2.29±0.28 s−1 in controls (n=3), 1.32±0.13 s−1 in the presence of marrubenol (n=3, P<0.05 vs control) and 1.16±0.09 s−1 in the presence of verapamil (n=3, P<0.05 vs control, not significantly different from marrubenol).
Figure 3.
Effect of marrubenol and verapamil on the rate of Mn2+-induced quenching of fura-2 fluorescence. Fura-2-loaded aortic rings were incubated with marrubenol (30 μM), verapamil (10 μM), or an equivalent amount of solvent for 30 min. They were incubated for 2 min in 100 mM KCl Ca2+-free solution before addition of MnCl2 0.1 mM (time 0). Experimental values, corrected for the autofluorescence, were normalised to the fluorescence measured before the addition of 0.1 mM MnCl2. Data are means from three determinations. Vertical bars indicate the s.e.m. values.
Effect of marrubenol on Ba2+ current
In A7r5 aortic smooth muscle cells, a slowly inactivating inward Ba2+ current (IBa) was observed, in agreement with a previous report (Buryi et al., 1995). The amplitude of IBa was maximal at a test potential around 0 mV. Changing the holding potential from −100 to −50 mV led to the partial inactivation of the current elicited by a test pulse to 0 mV.
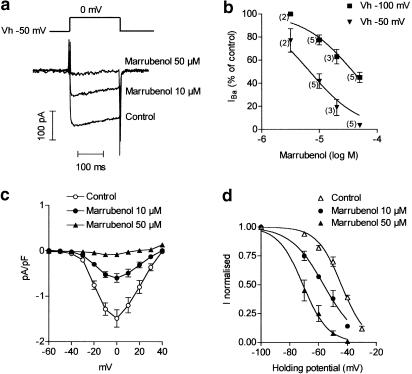
Marrubenol (3–50 μM) inhibited IBa in a concentration-dependent manner, but was more potent at depolarised holding potentials: mean KD values at −50 and −100 mV were 8±2 μM (n=10 cells) and 40±6 μM (n=13 cells), respectively (Figure 4a, b). Current-voltage relations of IBa were established by applying pulses to voltages varying from −60 to +40 mV from a holding potential of −50 mV. The I–V curve was depressed in the presence of marrubenol, but the position of the maximum and the threshold potential were not modified (Figure 4c). The steady-state inactivation of IBa was measured after applying the conditioning potential for 2 min. The half-maximal inactivation obtained by fitting normalised IBa values to Boltzmann's function was −44±0.8 mV in control cells (n=4 cells). In the presence of marrubenol, the steady-state availability curve was shifted to the left; voltages of half-inactivation were −57±0.9 and −71±1 mV in the presence of marrubenol 10 μM (n=3 cells, P<0.05 compared to control) and 50 μM (n=4 cells, P<0.05 compared to control), respectively (Figure 4d). The slope factors were not significantly different (8.4±0.8, 11±1 and 8.7±1 mV in control cells and 10 and 50 μM marrubenol-treated cells).
Figure 4.
Effect of marrubenol on Ba2+ current in A7r5 cells. (a) Typical record of the Ba2+ current evoked by test pulses to 0 mV from a holding potential of −50 mV, before the perfusion with marrubenol (control) and after perfusion with 10 and 50 μM marrubenol. (b) Influence of the holding potential on the inhibition of Ba2+ current (IBa) by marrubenol. Current was evoked by pulses to 0 mV from a holding potential (Vh) of −50 or −100 mV. Data are mean values±s.e.m. The number of cells is indicated between parentheses. Curves were fitted to the experimental data using the equation IBa (%)=100/(1+10̂(log IC50−log[Marr])), [Marr] being the concentration of marrubenol. Log IC50 fitted values were −4.41±0.06 and −5.16±0.09 at −100 and −50 mV, respectively. (c) Effect of marrubenol (10–50 μM) on the current–voltage relationship of the Ba2+ current evoked by 200 ms pulses from a holding potential of −50 mV. Data are mean values±s.e.m. from five cells. (d) Steady-state inactivation curves of Ba2+ current in control cells and after equilibration of the cells in the presence of marrubenol. Curves were drawn according to the Boltzmann's equation. Ba2+ current was normalised to its value at −100 mV. Data are means from four cells (control and marrubenol 50 μM) or three cells (marrubenol 10 μM).
Discussion
Functional data reported in the present study indicated that: (1) inhibition of the KCl contraction of the aorta by marrubenol was endothelium-independent; (2) marrubenol was more potent on the contractile response induced by KCl than by noradrenaline; (3) inhibition of the noradrenaline contraction was prevented by pretreatment of the aorta with a Ca2+ channel blocker; (4) relaxation of the contraction was associated with a decrease in cytosolic Ca2+ concentration.
It is well known that high-K+-induced contraction in smooth muscle is mediated by cell membrane depolarisation and an increase in calcium influx through voltage-gated calcium channels (Godfraind & Kaba, 1969; Somlyo & Somlyo, 1994). The contraction generated by noradrenaline in the rat aorta is less dependent upon Ca2+ influx through voltage-operated Ca2+ channels, as indicated by its partial resistance to organic Ca2+ channel blockers (Morel & Godfraind, 1991). Several reports have shown that noradrenaline contraction is the complex result of the mobilisation of both intracellular and extracellular Ca2+, and of the Ca2+ sensitisation of the contractile machinery (Kitazawa et al., 1991; Karaki et al., 1997). The selective inhibition of the contraction and the increase in cytosolic Ca2+ concentration evoked by high KCl solution by marrubenol thus suggests that this compound could act as a blocker of voltage-gated Ca2+ channel.
This was corroborated by experiments measuring the entry of Mn2+ in fura-2-loaded arteries. The presence of marrubenol or of verapamil in the bathing solution indeed decreased the quenching rate of the fura-2 fluorescence by Mn2+ significantly and to a similar extent, suggesting that the inhibition of the Ca2+ signal by marrubenol in KCl-depolarised artery was caused by the inhibition of the Ca2+ entry stimulated by depolarisation.
The interaction of marrubenol with voltage-dependent Ca2+ channels was confirmed by recording the inward current through the Ca2+ channel. In aortic A7r5 cells, marrubenol inhibited IBa in a concentration-dependent manner. Inhibition of Ca2+ channel current by verapamil or dihydropyridine derivatives like nisoldipine or nifedipine has been reported to be dependent on the voltage (Sanguinetti & Kass, 1984; Morel et al., 1998). Similar property was observed with marrubenol in A7r5 cells, where marrubenol exhibited a five-fold smaller KD value at a holding potential of −50 mV than at −100 mV. Such a ratio is similar to that of nifedipine, which shows a four- to 13-fold reduction in KD when membrane potential is changed from −100 to −50 mV (Méry et al., 1996; Morel et al., 1998), but it is lower than the voltage-dependency of verapamil, for which ratios of KD values at holding potentials of −100 and −50 mV between 25 and 30 have been reported (Sanguinetti & Kass, 1984; Morel et al., 1998). Voltage-dependence was confirmed by the shift in the availability curves (Bean et al., 1983). The KD value of marrubenol, which was calculated from the inhibition of IBa at a holding potential near the physiological value (−50 mV, 8±2 μM), was close to the IC50 value (10–12 μM) of the compound on the contraction or the Ca2+ signal evoked by KCl.
The voltage-dependency of marrubenol activity may be the cause of its higher potency in smooth muscle compared to the heart, where contraction was unaffected by marrubenol concentrations up to 100 μM (data not shown). The vascular selectivity of marrubenol is similar to that reported for several Ca2+ channel blockers (Wibo et al., 1988) and is likely to be advantageous in relation to the therapeutic properties of Marrubium extract in hypertensive patients.
The selective inhibition of L-type Ca2+ channels by marrubenol was confirmed by the observation that it did not affect the transient, mibefradil-sensitive T-type current (Mehrke et al., 1994), recorded in N1E-115 neuroblastoma cells (unpublished data).
In conclusion, these data indicate that the relaxant activity of marrubenol may be attributed to its interaction with L-type Ca2+ channels.
Acknowledgments
This work was supported by a grant from the Ministère de l'Education et de la Recherche Scientifique (Action Concertée no. 00/05-260) and from the FRSM (grant no. 3.4534.98). S. El Bardai was supported by a fellowship from the Commissariat Général aux Relations Internationales de la Communauté française de Belgique.
Abbreviations
- EGTA
ethylene glycol-bis(β-aminoethyl ether)N,N,N′,N′-tetraacetic acid
- fura-2 AM
fura-2 acetoxymethyl ester
- IBa
barium current
- L-NNA
Nω-nitro-L-arginine
- NMR
nuclear magnetic resonance
- TLC
thin layer chromatography
References
- BEAN B.P., COHEN C.J., TSIEN R.W. Lidocaine block of cardiac sodium channels. J. Gen. Physiol. 1983;81:613–642. doi: 10.1085/jgp.81.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURYI V., MOREL N., SALOMONE S., KERGER S., GODFRAIND T. Evidence for a direct interaction of thapsigargin with voltage-dependent Ca2+ channel. Naunyn Schmiedeberg's Arch. Pharmacol. 1995;351:40–45. doi: 10.1007/BF00169062. [DOI] [PubMed] [Google Scholar]
- CHEN X.L., REMBOLD C.M. Cyclic nucleotide-dependent regulation of Mn2+ influx, [Ca2+]i, and arterial smooth muscle relaxation. Am. J. Physiol. 1992;263:C468–C473. doi: 10.1152/ajpcell.1992.263.2.C468. [DOI] [PubMed] [Google Scholar]
- DELLA PIETA F., BILIA A.R., BRESCHI M.C., CINELLI F., MORELLI I., SCATIZZI R. Crude extracts and two linear diterpenes from Cystoseira balearica and their activity. Planta Med. 1993;59:135–138. doi: 10.1055/s-2006-959628. [DOI] [PubMed] [Google Scholar]
- EL BARDAI S., LYOUSSI B., WIBO M., MOREL N. Pharmacological evidence of hypotensive activity of Marrubium vulgare and Foeniculum vulgare in spontaneously hypertensive rat. Clin. Exp. Hypertens. 2001;23:329–343. doi: 10.1081/ceh-100102671. [DOI] [PubMed] [Google Scholar]
- EL BARDAI S., MOREL N., WIBO M., FABRE N., LLABRES G., LYOUSSI B., QUETIN-LECLERCQ J. The vasorelaxant activity of Marrubenol and Marrubiin from Marrubium vulgare. Planta Med. 2003;69:75–77. doi: 10.1055/s-2003-37042. [DOI] [PubMed] [Google Scholar]
- FULKE J.W.B., HENDERSON M.S., MCCRINDLE R. Reactions of the diterpene marrubiin and its congeners. J. Chem. Soc. C. 1968;7:807–810. [Google Scholar]
- GHISDAL P., GOMEZ J.P., MOREL N. Action of a NO donor on the excitation–contraction pathway activated by noradrenaline in rat superior mesenteric artery. J. Physiol. 2000;522:83–96. doi: 10.1111/j.1469-7793.2000.t01-3-00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODFRAIND T., KABA A. Blockade or reversal of the contraction induced by calcium and adrenaline in depolarized arterial smooth muscle. Br. J. Pharmacol. 1969;36:549–560. doi: 10.1111/j.1476-5381.1969.tb08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOTTA Y., ANDO H., TAKEYA K., SAKAKIBARA J. Direct measurement of increased myocardial cellular 23Na NMR signals in perfused guinea-pig heart induced by dihydroouabain and grayanotoxin-I. Mol. Cell. Biochem. 1994;139:59–70. doi: 10.1007/BF00944204. [DOI] [PubMed] [Google Scholar]
- KARAKI H., OZAKI H., HORI M., MITSUI-SAITO M., AMANO K., HARADA K., MIYAMOTO S., NAKAZAWA H., WON K.J., SATO K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- KITAZAWA T., GAYLINN B.D., DENNEY G.H., SOMLYO A.P. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J. Biol. Chem. 1991;266:1708–1715. [PubMed] [Google Scholar]
- LEBEDINSKY Y., NORDSTROM S.T., ASCHOFF S.E., KAPPLES J.F., O'MALLEY G.J., KOSLEY R.W., JR, FIELDING S., HUBBARD J.W. Cardiotonic and coronary vasodilator responses to milrinone, forskolin, and analog P87-7692 in the anesthetized dog. J. Cardiovasc. Pharmacol. 1992;19:779–789. [PubMed] [Google Scholar]
- MEHRKE G., ZONG X.G., FLOCKERZI V., HOFMANN F. The Ca2+-channel blocker Ro 40-5967 blocks differently T-type and L-type Ca2+ channels. J. Pharmacol. Exp. Ther. 1994;271:1483–1488. [PubMed] [Google Scholar]
- MÉRY P.F., HOVE-MADSEN L., MAZET J.L., HANF R., FISCHMEISTER R. Binding constants determined from Ca2+ current responses to rapid applications and washouts of nifedipine in frog cardiac myocytes. J. Physiol. 1996;494:105–120. doi: 10.1113/jphysiol.1996.sp021479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISSIAEN L., DECLERCK I., DROOGMANS G., PLESSERS L., DE SMEDT H., RAEYMAEKERS L., CASTEELS R. Agonist-dependent Ca2+ and Mn2+ entry dependent on state of filling of Ca2+ stores in aortic smooth muscle cells of the rat. J. Physiol. 1990;427:171–186. doi: 10.1113/jphysiol.1990.sp018166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREL N., BURYI V., FERON O., GOMEZ J.P., CHRISTEN M.O., GODFRAIND T. The action of calcium channel blockers on recombinant L-type calcium channel alpha1-subunits. Br. J. Pharmacol. 1998;125:1005–1012. doi: 10.1038/sj.bjp.0702162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREL N., GODFRAIND T. Characterization in rat aorta of the binding sites responsible for blockade of noradrenaline-evoked calcium entry by nisoldipine. Br. J. Pharmacol. 1991;102:467–477. doi: 10.1111/j.1476-5381.1991.tb12196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOREL N., GODFRAIND T. Selective interaction of the calcium antagonist amlodipine with calcium channels in arteries of spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 1994;24:524–533. doi: 10.1097/00005344-199410000-00002. [DOI] [PubMed] [Google Scholar]
- OZAKI H., SATO K., SATOH T., KARAKI H. Simultaneous recordings of calcium signals and mechanical activity using fluorescent dye fura 2 in isolated strips of vascular smooth muscle. Jpn. J. Pharmacol. 1987;45:429–433. doi: 10.1254/jjp.45.429. [DOI] [PubMed] [Google Scholar]
- SALOMONE S., MOREL N., GODFRAIND T. Effects of 8-bromo cyclic GMP and verapamil on depolarization-evoked Ca2+ signal and contraction in rat aorta. Br. J. Pharmacol. 1995;114:1731–1737. doi: 10.1111/j.1476-5381.1995.tb14964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGUINETTI M.C., KASS R.S. Voltage-dependent block of calcium channel current in the calf cardiac Purkinje fiber by dihydropyridine calcium channel antagonists. Circ. Res. 1984;55:336–348. doi: 10.1161/01.res.55.3.336. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- WIBO M., DEROTH L., GODFRAIND T. Pharmacologic relevance of dihydropyridine binding sites in membranes from rat aorta: kinetic and equilibrium studies. Circ. Res. 1988;62:91–96. doi: 10.1161/01.res.62.1.91. [DOI] [PubMed] [Google Scholar]
- ZHANG C.Y., TAN B.K. Vasorelaxation of rat thoracic aorta caused by 14-deoxyandrographolide. Clin. Exp. Pharmacol. Physiol. 1998;25:424–429. doi: 10.1111/j.1440-1681.1998.tb02226.x. [DOI] [PubMed] [Google Scholar]