Abstract
Docosahexaenoic acid (DHA) induced rapid (t1/2=33 s) and dose-dependent decreases in pHi in BCECF-loaded human (Jurkat) T-cells. Addition of 5-(N,N-dimethyl)-amiloride, an inhibitor of Na+/H+ exchanger, prolonged DHA-induced acidification as a function of time, indicating that the exchanger is implicated in pHi recovery.
Other fatty acids like oleic acid, arachidonic acid, eicosapentaenoic acid, but not palmitic acid, also induced a fall in pHi in these cells.
To assess the role of calcium in the DHA-induced acidification, we conducted experiments in Ca2+-free (0% Ca2+) and Ca2+-containing (100% Ca2+) buffer. We observed that there was no difference in the degree of DHA-induced transient acidification in both the experimental conditions, though pHi recovery was faster in 0% Ca2+ medium than that in 100% Ca2+ medium.
In the presence of BAPTA, a calcium chelator, a rapid recovery of DHA-induced acidosis was observed. Furthermore, addition of CaCl2 into 0% Ca2+ medium curtailed DHA-evoked rapid pHi recovery. In 0% Ca2+ medium, containing BAPTA, DHA did not evoke increases in [Ca2+]i, though this fatty acid still induced a rapid acidification in these cells. These observations suggest that calcium is implicated in the long-lasting DHA-induced acidosis.
DHA-induced rapid acidification may be due to its deprotonation in the plasma membrane (flip-flop model), as suggested by the following observations: (1) DHA with a –COOH group induced intracellular acidification, but this fatty acid with a –COOCH3 group failed to do so, and (2) DHA, but not propionic acid, -induced acidification was completely reversed by addition of fatty acid-free bovine serum albumin in these cells.
These results suggest that DHA induces acidosis via deprotonation and Ca2+ mobilization in human T-cells.
Keywords: Docosahexaenoic acid, Jurkat T-cells, intracellular pH, calcium
Introduction
Under physiological conditions, activation of T-cells is initiated by an interaction between an antigen-specific T-cell receptor (TCR) and a foreign antigen associated with a membrane-bound major histocompatibility complex molecule (Grinstein & Dixon, 1989). This event triggers a number of intracellular biochemical processes that assure T-cell cycle progression (Grinstein & Dixon, 1989; Germain, 1994). The early stages of T-cell activation, initiated within 1–100 s of T-cell engagement, involve mobilization of intracellular calcium, activation of protein kinase C (PKC), expression of immediate early genes, interleukin-2 synthesis and its secretion (Cantrell, 1996). The late events that assure transition of cells from S phase to the G2/M phase of the cell cycle are sustained responses, implicating the prolonged PKC activation and the expression of receptors for interleukin-2, IL-2R (Ward, 1996). Regulation of cytosolic pHi is crucial to many cellular functions, particularly for T-cell proliferation (Grinstein & Dixon, 1989). A rapid increase in intracellular pH is associated with the activation of a cascade of second messengers during T-cell proliferation (Gerson et al., 1982). Although changes in pHi and Ca2+ have been observed in many cell systems, it is not yet clear which of these ionic events, if any, are necessary steps in leading to DNA synthesis (Ives & Daniel, 1987).
Increase in cytosolic pH is reported to be due to the functioning of one of the membrane-bound transporters, the Na+/H+ exchanger (NHE), which mediates an electroneutral exchange between extracellular Na+ and intracellular H+, and plays a major role in the regulation of cell volume (Parker & Castranova, 1984) and pH homeostasis (Grinstein & Dixon, 1989) in several cell types. The exchanger is pHi dependent and activated allosterically by intracellular protons. Indeed, acidic intracellular pH activates it, but the exchanger remains relatively inactive at neutral pHi (Orlowski & Grinstein, 1997). NHE1 isoform has been identified in human T-cells and is one of the main mechanisms responsible for proton extrusion in these cells (Siczkowski et al., 1994). However, studies on pHi changes have yielded to conflicting results on whether Na+/H+ activation in stimulated T-cells was dependent on Ca2+ influx (Rosoff & Cantley, 1985).
During the recent past, there has been an upsurge of information on the role of polyunsaturated fatty acids (PUFAs) in the regulation of immune cell functions (Khan & Hichami, 2002). The PUFAs of n-3 series, eicosapentaenoic acid (EPA, 20 : 5 n-3) and docosahexaenoic acid (DHA, 22 : 6 n-3), have been considered as immunosuppressors, whereas PUFAs of n-6 series (viz arachidonic acid, AA, 20 : 4 n-6) have been reported to be immunostimulators (Calder, 1996). The n-3 PUFAs have been shown to exert protective effects against rheumatoid arthritis (Kremer et al., 1990), psoriasis (Bittiner et al., 1988) and multiple sclerosis (Bates et al., 1989). Moreover, nutritional interventions, conducted in mice which were fed an EPA- and DHA-rich diet, have shown that n-3 PUFAs diminished IL-2 secretion associated with a lesser expression of the α chain of the IL-2R in T-cells (Jolly et al., 1998). As far as pHi homeostasis is concerned, AA has been shown to induce a decrease in pHi in the cytoplasm and nucleoplasm of rat cerebellar granule cells (Chen et al., 2001), rat cardiac myocytes (Wu et al., 2000), platelets (Cavallini et al., 1996) and rat thymocytes (Astashkin et al., 1993). Nevertheless, no detailed information is available on the role of n-3 PUFAs in the modulation of intracellular pH in human T-cells. Therefore, the present study was designed in order to shed light on the role of DHA, an n-3 PUFA, in the modulation of pHi in (Jurkat)T-cells.
Methods
Chemicals
The culture medium RPMI-1640, L-glutamine, HEPES buffer, streptomycin/penicillin and fetal calf serum (FCS) were purchased from Biowhitaker, Belgium. 2′,7′-bis-(carboxyethyl-5(6′)-carboxyfluorescein (BCECF)/acetoxymethylester (AM) and Fura-2/AM were obtained from Molecular Probes, Eugene, OR, U.S.A. Unless otherwise stated, all other chemicals including DHA (22 : 6 n-3), DHA methyl ester, AA (20 : 4 n-6), EPA (20 : 5 n-3), oleic acid (OA,18 : 1 n-9), palmitic acid (16 : 0), nigericin, bumetanide, oligomycin and bafilomycin were purchased from Sigma (St Louis, MO, U.S.A.).
Cell culture and lipid analysis
The human (Jurkat) T-cells were routinely cultured in RPMI-1640 medium supplemented with 10% FCS, 2 mM L-glutamine, 50 μg ml−1 penicillin–streptomycin and 20 mM HEPES at 37°C, in a humidified chamber containing 95% air and 5% CO2. Cell viability was assessed by trypan blue exclusion test. Cell numbers were determined by a haemocytometer.
Fatty acid derivatives were analysed by using high-performance liquid chromatography (HPLC). Cells were incubated for 1 h in the presence of (14C)DHA (10 μM) and then washed three times in RPMI 1640 medium (0.2% bovine serum albumin (BSA)). Total lipids were extracted by chloroform/methanol/NaCl 2 M (1 : 1 : 0.9, v v−1) and neutral lipids were separated by thin-layer chromatography in the following solvent system: hexane/diethyl ether/acetic acid (90 : 30 : 1). Radio-labelled spots corresponding to fatty acids were scraped off and extracted by hexane. Fatty acid classes were separated by reverse-phase HPLC using a 5 μM LiChrosorb RP 18 and the following solvents: acetonitrile/water (95 : 5, v v−1). A volume of 1 ml min−1 fraction was collected, dried, and the radioactivity of each fraction was quantified. The retention times of fatty acids were determined using authentic standards.
Measurement of intracellular pH (pHi)
For these experiments, cells (2 × 106 ml−1) were loaded with the pH-sensitive fluorescent probe BCECF-AM (3 μM) for 30 min at 37°C under a 95% air–5% CO2 atmosphere, in a nominally bicarbonate-free medium containing: 140 mM NaCl; 5 mM KCl; 1 mM MgSO4; 1 mM CaCl2; 20 mM HEPES; 1 mM NaH2PO4 and 5.5 mM glucose, pH 7.4. After loading, the cells were washed three times (720 g × 10 min) to remove the extracellular dye, and made to remain suspended in the identical buffer. A sample (4 × 106 cells) was then transferred to a magnetically stirred cuvette in a PTI spectrofluorometer. BCECF fluorescence was detected in the ratio mode at the excitation wavelengths of 440 and 506 nm, and at an emission wavelength of 535 nm. Calibration of fluorescence vs pH was performed by equilibration of external and internal pH with nigericin (10 μM) in a high K+ buffer and by adding aliquots of propionic acid (PA; 0.1 M). The relative fluorescence ratio values were plotted against corresponding pHi values, which allowed determination of the unknown pHi.
For the experiments conducted in the absence of external calcium (0% Ca2+), CaCl2 was replaced by 1 mM EGTA in the buffer. All test molecules were added in small volumes with no interruption in recordings. Fatty acids were dissolved in ethanol (w v−1, 0.1%) and used immediately or kept at −20°C, tightly sealed under the stream of nitrogen.
Measurement of free intracellular Ca2+ concentrations, [Ca2+]i
The cells (2 × 106 ml−1) were washed with phosphate-buffered saline, pH 7.4, and then incubated with Fura-2/AM (1 μM) for 60 min at 37°C in a loading buffer containing: 110 mM NaCl; 5.5 mM KCl; 25 mM NaHCO3; 0.8 mM MgCl2; 0.4 mM KH2PO4; 0.33 mM Na2HPO4; 20 mM HEPES; 1.2 mM CaCl2, and the pH was adjusted to 7.4.
After loading, the cells were washed three times (720 g × 10 min) and made to remain suspended in the identical buffer. The fluorescence intensities were measured in the ratio mode in a PTI spectrofluorometer at 340 and 380 nm (excitation filters) and 510 nm (emission filters). The cells were continuously stirred throughout the experiment. The intracellular concentration of free Ca2+, [Ca2+]i, was calculated using the equation: [Ca2+]i=Kd × (R−Rmin)/(Fmax−F)(Sf2/Sb2). A value of 224 nM for Kd was added into the calculations. Rmax and Rmin values were obtained by addition of ionomycin (5 μM) and MnCl2 (2 mM), respectively.
Experiments were also conducted in Ca2+-free medium (0% Ca2+), where CaCl2 in the phosphate-buffered saline was replaced by EGTA (1.2 mM). The test molecule DHA was added into the cuvettes in small volumes, with no interruptions in the recordings.
Confocal microscopy
The cells (2 × 106 ml−1) were loaded with the pH-sensitive fluorescent probe BCECF-AM (3 μM), as described for pHi measurements. The cells were transferred to the microscopic slide that was examined by an oil-immersion objective (× 63) of Leica TCS 4D confocal microscope, equipped with a numerical aperture of 1.4. The cells were excited by the laser beam at 488 nm and the excited fluorescence was detected at 515 nm. The X−Y plane images (512 pixels × 512 pixels) were generated from fluorescence emission images collected with a band pass filter.
Statistical analysis
Results are shown as mean±s.e.m. for a given number of experiments (n). The data were analysed by using Statistica (4.1 version, Statsoft, Paris, France). The significance between mean values was determined by analysis of variance one way, followed by a least-significant-difference (LSD) test, with a value of P<0.05 being considered statistically significant.
Results
DHA induces a decrease in intracellular pH in a dose-dependent manner
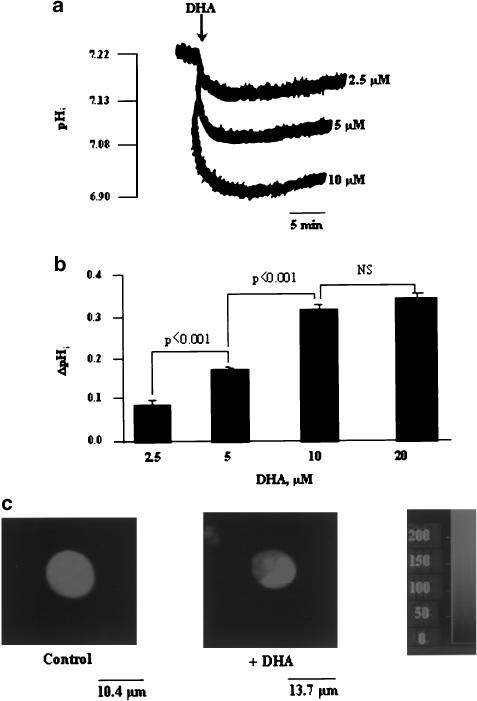
The resting level of pHi in (Jurkat) T-cells in the nominally bicarbonate-free medium was 7.22±0.03 (n=30). DHA induced a rapid (t1/2=33 s) and dose-dependent decrease in pHi that plateaued at the concentration of 10 μM. During the time course of the experiments (20 min), complete recovery of the resting pHi could not be observed. In the subsequent experiments, we used DHA at 10 μM (Figure 1a,b). Figure 1c shows that addition of DHA induces acidosis only in the cytoplasm, without significantly influencing the BCECF fluorescence in the nucleoplasm, of Jurkat T-cells.
Figure 1.
Effects of DHA on pHi. Cells (4 × 106 assay−1) were loaded with the fluorescent dye BCECF/AM, as described in Methods. (a) Intracellular acidosis induced by different concentrations of DHA, that is, 2.5, 5, 10 and 20 μM. (b) ΔpHi represents the amplitude of the intracellular acidification produced. Values are expressed as mean±s.e.m. of independent experiments (n=30). (c) Acidosis induced by DHA (10 μM), as visualized by confocal microscopy (n=6). NS=insignificant differences.
DHA exerts additive effects in the presence of a weak acid
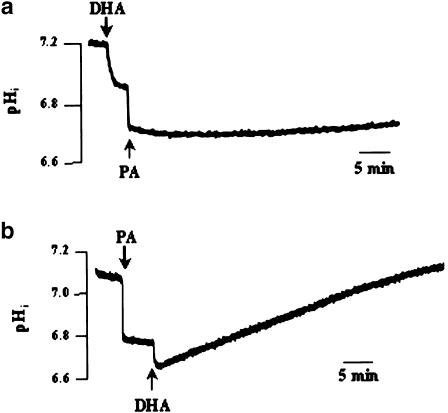
We employed PA, known to induce the cytosolic acid load by directly entering the cells and without accumulating in the phospholipid bilayer (Chen et al., 2001). Addition of PA evoked an intracellular acidification of 0.32±0.03 pH units (n=8) (Figure 2b). When DHA was added after PA, the former induced a slight acidification (0.13±0.02 pH units) that was rapidly recovered (0.0035±0.02 ΔpH min−1) (Figure 2b). When PA was applied after DHA, we observed an additive acidification of 0.16±0.02 pH units (Figure 2a).
Figure 2.
Effects of DHA and PA on pHi. Cells (4 × 106 assay−1) were loaded with the fluorescent dye BCECF/AM, as described in Methods. The arrowheads indicate the time when DHA (10 μM) and PA (2.5 mM) were added into the cuvette, without interruption in the recordings. The figure shows single traces of observations, which were reproduced independently (n=8). All experiments were performed in the bicarbonate-free medium, pH 7.4.
DHA induces an increase in [Ca2+]i: possible role of calcium in the DHA-induced intracellular acidification
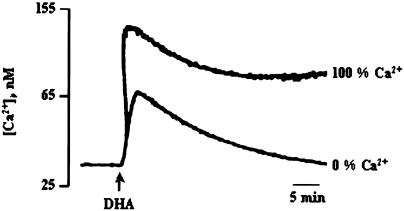
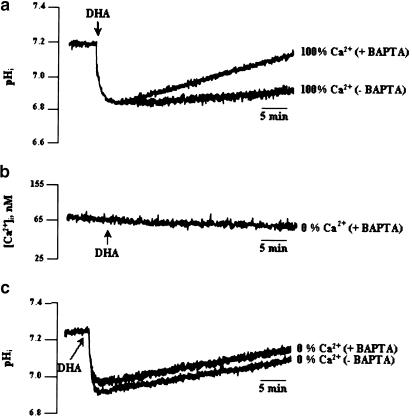
In order to determine the contribution of Ca2+ in the DHA-induced intracellular acidification, we first measured intracellular free calcium after treatment with DHA. Addition of DHA induced an increase in [Ca2+]i (97.63 nM±0.03) that was significantly curtailed when the experiments were conducted in 0% Ca2+ buffer (28.5 nM±0.03), as compared to that in 100% Ca2+ buffer (Figure 3). Indeed, the DHA-induced rise in [Ca2+]i was inhibited by 70.8±1.2% in 0% Ca2+ medium.
Figure 3.
Effects of DHA on (Ca2+)i. Cells (4 × 106 assay−1) were loaded with the fluorescent dye Fura-2/AM, as described in Methods, and then resuspended in the 100% or 0% Ca2+ buffers. DHA (10 μM) was added into the cuvette without interruptions in the recordings (n=4).
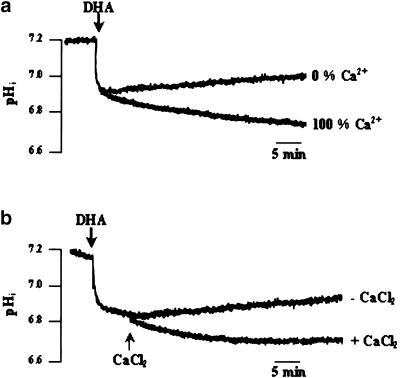
The role of calcium on the DHA-induced changes in pHi was examined by conducting experiments in 0% Ca2+ buffer. In the 0% Ca2+ medium, DHA induced intracellular acidification to the same extent as in the 100% Ca2+ medium (0.27±0.02 pH units); however, the recovery of the resting pHi was faster in the former (0.0093±0.01 ΔpH min−1) (Figure 4a). Then, we designed a Ca2+-free/Ca2+ reintroduction protocol that consisted of conducting the experiment in 0% Ca2+ buffer and adding, exogenously, first DHA and then CaCl2. Hence, we observed that addition of CaCl2 restored the DHA-induced prolonged decrease, as was observed in the 100% Ca2+ medium (Figure 4b). We, further, clamped intracellular free calcium, by using the calcium chelator bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetra-acetoxymethyl ester (BAPTA-AM). T-cells were preincubated with BAPTA-AM (50 μM) for 15 min, then washed and resuspended in the 100% Ca2+ medium. Figure 5a shows that when intracellular free calcium is chelated, the recovery of pHi is faster (0.01±0.02 ΔpH min−1) than that in the medium without BAPTA (0.003±0.01 ΔpH min−1). When BAPTA is added to 0% Ca2+ medium, DHA did not induce increases in [Ca2+], (Figure 5b), though this fatty acid was able to induce rapid acidification under such experimental conditions (Figure 5c).
Figure 4.
Role of external calcium in the DHA-induced decrease in pHi. Cells (4 × 106 assay−1) were loaded with the fluorescent dye BCECF/AM, as described in Methods. (a) Cells were resuspended in either 0% or 100% Ca2+ buffers. (b) Ca2+-free/Ca2+-reintroduction experiment in 0% Ca2+ medium: CaCl2 (2 mM) was applied after DHA (10 μM), as indicated in the figure. The figures show single traces of observations, which were reproduced independently (n=10).
Figure 5.
Effect of intracellular free calcium chelation on the DHA-induced decrease in pHi. Cells (4 × 106 assay−1) were loaded with the fluorescent dye BCECF/AM and preincubated with BAPTA/AM (50 μM, 15 min), as described in Methods. DHA (10 μM) was added at the end of the incubation. The figure shows single traces of observations, which were reproduced independently (n=6).
Effect of 5-(N,N-dimethyl)-amiloride (DMA), an NHE inhibitor, on DHA-induced intracellular acidification
NHEs are responsible for the regulation of intracellular pH and cell volume by extruding protons and taking up sodium ions into cells. To date, seven isoforms (NHE1–NHE7) have been identified and cloned. NHE6 and NHE7 are localized on recycling endosomes and the trans-Golgi network, respectively, whereas the other isoforms (NHE1–NHE5) are expressed in the cell plasma membrane (Grinstein et al., 1989). Though immunoblotting experiments in human T-cells have shown that NHE1 is mainly responsible for pHi regulation in these cells (Siczkowski et al., 1994), no information is available, to our knowledge, whether this NHE subtype is implicated in the pHi homeostasis in Jurkat T-cells. We, therefore, have termed it NHE.
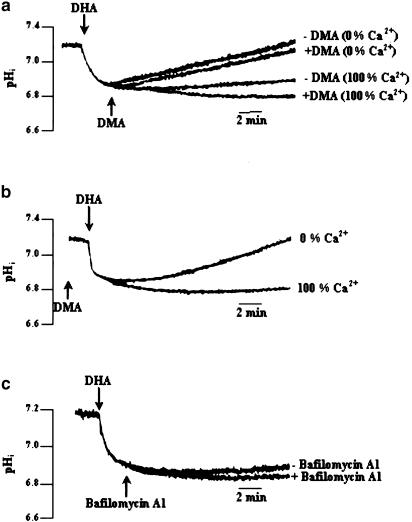
It was of interest to assess whether the NHE was activated to counter the acidification evoked by DHA. We, therefore, employed the NHE1 inhibitor, DMA, that has a high affinity for the exchanger, a low cell permeability and only exhibits nonspecific effects at concentrations 10–100-fold higher than that used in the present study (Kleyman & Cragoe, 1988). When DMA was applied before or after DHA, in 100% Ca2+ medium, the DHA-induced acidification was enhanced (0.11±0.04 pH units) and little recovery was observed (Figure 6a,b). However, incubation of cells with DMA in 0% Ca2+ medium did not affect the fast pHi recovery observed in these calcium depletion experiments (Figure 6a). We were also tempted to assess whether the vacuolar-type H+-ATPase (VPATPase) was implicated in proton extrusion in T-cells. VPATPase has been described in a variety of cell types, being responsible for the acidification of the intracellular compartments, such as lysosomes and endosomes (Forgac, 1989). In addition to their endomembrane distribution, they have also been detected in the plasma membranes of various cell types (i.e. macrophages), thereby maintaining the cytosolic pH by extruding protons out of the cells (Bidani & Brown, 1990). Figure 6c shows that addition of bafilomycin A1 (at 50 nM), a potent inhibitor of VPATPase, failed to prolonge the DHA-induced acidification (with bafilomycin 0.35±0.03 vs without bafilomycin 0.29±0.02 pH units).
Figure 6.
Effect of DMA and bafilomycin A1 on DHA-evoked decrease in pHi. Cells (4 × 106 assay−1) were loaded with the fluorescent dye BCECF/AM, as described in Methods. The arrowheads indicate the time when the test molecules DHA (10 μM), DMA (10 μM) and bafilomycin A1 (50 nM) were added into the cuvette, without interruptions in the recordings. The figures show single traces of observations that were reproduced independently (n=11). Experiments were performed both in the Ca2+-containing (100% Ca2+) and Ca2+-free (0% Ca2+) buffers.
DHA-induced acidosis: lack of involvement of the Na+–K+–2Cl− symport, free radical production, NADPH oxidase or mitochondrial H+ leakage
We investigated whether the Na+–K+–2Cl− symport, mainly responsible for chloride and sodium influx, was implicated in DHA-induced decrease in pHi. We, therefore, used a specific inhibitor of this symport, bumetanide. Table 1 shows that prior addition of bumetanide had no effect on the DHA-evoked intracellular acidosis (with bumetanide 0.28±0.05 pH units vs without bumetanide 0.31±0.02 pH units). The involvement of NADPH oxidase and free radical production was also tested. Prior addition of CdCl2 or ZnCl2, the inhibitors of the NADPH oxidase, and of superoxide dismutase (SOD) failed to influence DHA-induced intracellular acidification. Carbonyl cyanide m-chlorophenyl-hydrazone (mClCCP), which dissipates H+ gradient across the inner mitochondrial membrane and oligomycin, a F0F1-ATPase/H+ pump inhibitor, were used in order to determine the contribution of mitochondrial H+ in the DHA-induced response. When mClCCP or oligomycin were applied before DHA, no enhanced acidification was observed (Table 1). It is important to mention that any of the molecules tested modified neither the initial rate of acidification nor the resultant fall in pHi, induced by DHA.
Table 1.
Intracellular acidosis induced by the addition of DHA under various conditions
| Treatment | ΔpHi | N |
|---|---|---|
| DHA alone | 0.31±0.02 | 18 |
| CdCl2+DHA | 0.29±0.05 | 4 |
| ZnCl2+DHA | 0.27±0.03 | 4 |
| Bumetanide+DHA | 0.28±0.05 | 4 |
| SOD+DHA | 0.27±0.03 | 4 |
| Oligomycin+DHA | 0.28±0.02 | 4 |
| mClCCP+DHA | 0.28±0.02 | 4 |
SOD, superoxide dismutase (0.25 U ml–1); CdCl2 and ZnCl2 were used at 1 mM, bumetanide at 100 μM, oligomycin and mClCCP at 10 μM; n, number of experiments performed.The ΔpHi under the above conditions did not differ significantly (P>0.05) as compared to the control (DHA alone).
DHA-induced decrease in pHi is reversed by BSA
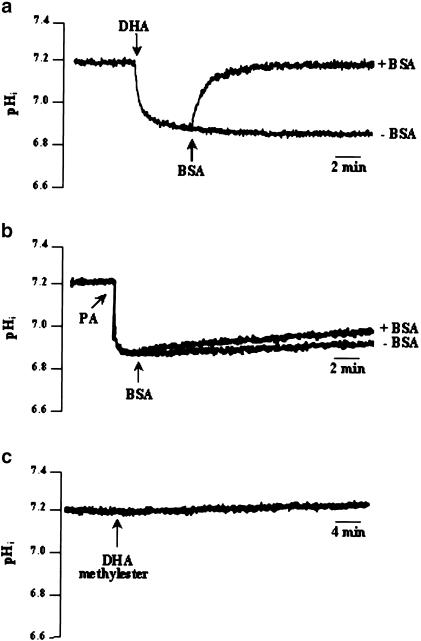
DHA-induced response could be a result of simple diffusion (flip-flop) of the fatty acid, demonstrated to occur in phospholipid bilayers and responsible for intracellular acidification (Kamp & Hamilton, 1992). Albumin has been shown to compete with PUFAs bound to the plasma membrane and to extract them from the lipid bilayer, thus decreasing the rate of intracellular proton influx (Kamp & Hamilton, 1993; Kamp et al., 1995; Hamilton, 1998). If flip-flop were to occur in our system, on addition of fatty acid-free BSA and in presence of DHA, a rise in pHi should be observed. When 0.2% fatty acid-free BSA was added after DHA, a rapid recovery (0.16±0.01 ΔpH min−1) of the pHi was observed (Figure 7a). This effect of BSA on pHi recovery was specific to the intracellular acidification evoked by DHA, since no effect was seen on the acidosis induced by PA (Figure 7b). Similarly, the addition of DHA methyl ester (–COOH group replaced by –COOCH3) had no effect on pHi (Figure 7c).
Figure 7.
Effect of BSA and DHA methyl ester on pHi. Cells (4 × 106 assay−1) were loaded with the fluorescent dye BCECF/AM, as described in Methods. The arrowheads indicate the time when the test molecules DHA (10 μM), BSA (0.2% w v−1), PA (2.5 mM) and DHA methyl ester (10 μM), were added into the cuvette, without interruptions in the recordings. The figures show single traces of observations that were reproduced independently (n=4). All experiments were performed in the bicarbonate-free medium, pH 7.4.
Effect of other fatty acids on intracellular pH
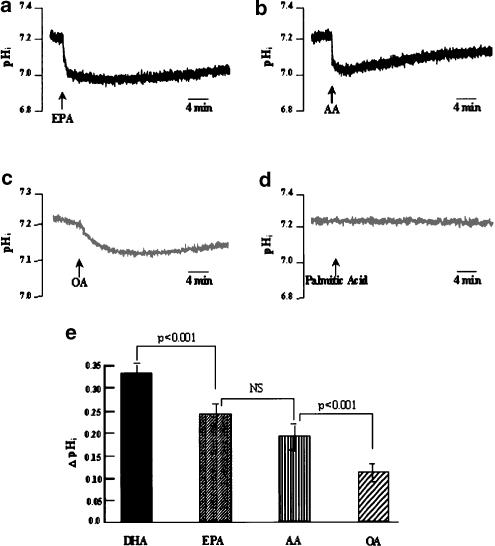
To get insight into the specificity of DHA on intracellular acidification, other fatty acids of the n-9, n-6 and n-3 families were tested. DHA and these fatty acids were used in the same batches of cells. OA (18 : 1 n-9), AA (20 : 4 n-6) and EPA (20 : 5 n-3) were all able to acidify the cytoplasm of Jurkat T-cells, when used at 10 μM (Figure 8a), the concentration that gave for each fatty acid an optimal response (Table 2 ). However, DHA-induced acidification (0.34±0.02 pH units) was significantly higher than that triggered by AA (0.22±0.03 pH units) or OA (0.13±0.04 pH units). EPA, the other important PUFA of the n-3 family, triggered a pHi fall (0.25±0.02 pH units) which was significantly lesser than the DHA-induced acidosis. EPA- and AA-induced responses were not significantly different (Figure 8a,b,e). In order to assess the implication of unsaturation of fatty acids, a saturated fatty acid was tested on pHi. Addition of palmitic acid (16 : 0; at 10 μM) to cells did not affect the intracellular pH. However, as palmitic acid has a low water solubility, we verified (not shown) using 14C-labelled palmitic acid that, under our experimental conditions, it did not precipitate, upon immediate addition, as the acid-soap in contact with the aqueous medium. This could have accounted for the palmitic acid inability to induce a pHi drop. We observed that palmitic acid at 10 μM and even at 20 μM was almost completely soluble in the bicarbonate-free buffer (not shown).
Figure 8.
Effect of different fatty acids on pHi. Cells (4 × 106 assay−1) were loaded with the fluorescent dye BCECF/AM, as described in Methods. (a–d) Intracellular acidosis induced by different fatty acids: AA, arachidonic acid, EPA, eicosapentaenoic acid, OA, oleic acid and palmitic acid. All fatty acids were used at 10 μM. (e) ΔpHi represents the amplitude of the intracellular acidification produced by these agents. All fatty acids, including DHA, were used in the same batches of cells. Values are expressed as mean±s.e.m. of independent experiments (n=5). NS=insignificant differences.
Table 2.
Intracellular acidosis (ΔpHi) induced by AA, EPA and OA at different concentrations
| Concentrations (μM) | |||
|---|---|---|---|
| Fatty acid | 5 | 10 | 20 |
| AA | 0.12±0.03 | 0.21±0.03a | 0.24±0.02b |
| EPA | 0.17±0.03 | 0.27±0.03a | 0.29±0.02b |
| OA | 0.05±0.03 | 0.14±0.03a | 0.18±0.02b |
Represents significant values (P<0.001), as compared to 5 μM concentration of the respective fatty acid.
Represents insignificant values, as compared to 10 μM concentration of the respective fatty acid (n=7).
Discussion
PUFAs of n-3 series, particularly DHA and EPA, have been shown to exert immunosuppressive effects (Calder, 1996); however, their mechanisms of action in T-cell activation have not been well elucidated. Intracellular pH being an important factor of T-cell proliferation, we undertook the present study to elucidate the role of an n-3 PUFA, DHA, on the modulation of pHi. DHA, the end product of the metabolism of α-linolenic acid (Lands, 1991), evoked an intracellular acidification. Since AA has been shown to induce acidosis both in the cytoplasm and nucleoplasm in rat brain cells (Chen et al., 2001), we used confocal microscopy to localize in situ acidification. DHA-induced acidification was only observed in the cytoplasmic area of Jurkat T-cells. In order to shed light on the mechanism(s) by which DHA could act, PA, a weak acid, was employed. When DHA was applied after PA or vice versa, additive effects were observed, indicating that these two agents act differently. Indeed, PA does not accumulate in the phospholipid bilayer and, hence, intracellular acid load depends on the amount of PA present in the extracellular medium and the amount able to enter and dissolve within the cell (Wu et al., 2000).
The role of free intracellular calcium, [Ca2+]i, in the DHA-induced decrease in pHi was also investigated. Hence, DHA induced increases in [Ca2+]i which were significantly curtailed when experiments were conducted in 0% Ca2+ medium. It has been recently shown that DHA-induced increases in [Ca2+]i are due to, in part, Ca2+ mobilization from reticulum endoplasmic stores, and the prolonged calcium response is contributed by calcium influx which takes place by opening of Ca2+ release-activated Ca2+ (CRAC) channels (Bonin & Khan, 2000). We observed that DHA-evoked intracellular acidification was modified when cells were resuspended in the 0% Ca2+ medium. Indeed, no significant difference in the amplitude of DHA-induced acidosis was seen between the two media of incubation (0 and 100% Ca2+ media); however, in the 0% Ca2+ buffer, the recovery of the resting pHi was faster than that in 100% Ca2+ medium. Preincubation of BCECF-loaded cells with the calcium chelator BAPTA/AM induced a rapid recovery of the pHi, as compared to that in 100% Ca2+ medium. Furthermore, addition of exogenous CaCl2 to 0% Ca2+ medium diminished the DHA-induced rapid recovery. These observations suggest that calcium influx may also be implicated in the prolonged phase of DHA-induced acidification. Hence, we can hypothesize that DHA-induced increases in [Ca2+]i could activate the Ca2+/H+ ATPase that drives Ca2+ out and H+ in (Berthe et al., 1991). Our hypothesis can be supported with the observations of Chien et al. (2001), who have demonstrated that in human peripheral T-cells, PMA-induced acidification occurs when intracellular concentrations of free calcium are increased, and this is due to Ca2+ efflux, followed by H+ influx. Similarly, N-methyl-D-aspartate (NMDA)-induced Ca2+-dependent intracellular acidosis is brought about by the activation of Ca2+/H+ ATPase in rat cerebellar granule cells (Wu et al., 1999). To sum up, we can say that calcium is implicated in the maintenance of the DHA-evoked sustained decrease in pHi. However, DHA-induced rapid acidification seems a calcium-independent phenomenon as in 0% medium, containing BAPTA/AM, DHA could not induce an increase in [Ca2+]i, though this fatty acid did rapidly acidify these cells under such conditions.
The role of DMA, a potent NHE1 inhibitor, in the DHA-induced acidosis was also investigated. Enhanced acidification was observed when DMA was applied before or after DHA and cells could not recover their diminished pHi. Thus, NHE is implicated in proton extrusion and it is unlikely that DHA acidify by inhibiting the antiport. If such a process had occurred in our system, prior addition of DMA would have abolished the response of DHA. Surprisingly, preincubation of cells with DMA or subsequent addition of DMA after DHA, in 0% Ca2+ medium, could not delay the fast pHi recovery observed after addition of DHA. In our study, the NHE1 isoform seems to be implicated, as Chambrey et al. (1997) have shown that NHE3 and NHE4 are amiloride-resistant isoforms, while NHE1 and NHE2 are the most sensitive isoforms to amiloride inhibition. The inhibition constants of DMA for these isoforms are as follows: NHE1 (Ki=0.023 μM), NHE2 (Ki=0.25 μM), NHE3 (Ki=14 μM) (for a review, see Masereel et al., 2003). Furthermore, NHE3 isoform is mainly expressed in high levels in the colon and small intestine, where it contributes to sodium absorption by the brush-border membrane in intestinal or renal epithelia (Masereel et al., 2003). Furthermore, DMA was able to significantly inhibit NHE activity in the 100% Ca2+ medium. Thus, the fast pHi recovery observed in the presence of DMA is not related to the existence of an amiloride-resistant NHE subtype in our cells.
The members of the NHE family display remarkable functionality. They are modulated by agents that primarily target tyrosine kinases, and also by agonists of Ser/Thr kinases like protein kinase A (PKA) and C (PKC). Bertrand et al. (1994) have demonstrated that calmodulin physically interacts with a particular subdomain of the NHE1 cytosolic region (Wakabayashi et al., 1994), thus resulting in the activation of NHE1 by increases in intracellular calcium. This calmodulin-binding regulatory box is sufficient to account for the rapid and transient activation of NHE1 in response to growth factors and other Ca2+-mobilizing agonists. An identical sequence is not found in NHE3 (Levine et al., 1995). Indeed, Maly et al. (2002) have shown, by chelating the cytoplasmic calcium by BAPTA/EGTA, that cytoplasmic free calcium concentrations in epidermal growth factor receptor-6 (EGFR6) were critical for the regulation of the NHE. However, the results of the study of Chien et al. (2001) imply that in phytohemagglutinin (PHA)-stimulated human peripheral T-cells, the second messengers, for example, PKC and Ca2+, may modulate pHi in a dual-antagonistic mechanism, that is, PKC-mediated alkalinization and Ca2+ influx-mediated acidification; hence, the latter observation corroborates our study. Bafilomycin A1, a potent and specific macrolide antibiotic that inhibits VPATPase (Crider et al., 1994), was used in order to determine the contribution of VPATPase in proton extrusion. Bafilomycin A1 did not significantly enhance DHA-induced acidification, indicating that VPATPase does not participate to proton extrusion in T-cells. It has been shown that activation of the plasma membrane NADPH oxidase is associated with a rapid depolarization of the membrane potential that is followed by a slight fall in pHi, and the generation of superoxide (O2−) (Henderson et al., 1987). A study conducted on human resting neutrophils has shown that AA induced a pHi fall as a result of the opening of the H+ channel by this fatty acid. As described for H+ conductance in snail neuron, the proton channel is inhibited by Cd2+ and Zn2+ ions (Henderson et al., 1988). We, therefore, used cadmium chloride (CdCl2) and zinc chloride (ZnCl2) to determine whether DHA activates the H+ channel. Addition of these cations failed to diminish the DHA-induced intracellular acidification; thus, we can rule out the possibility that this fatty acid could activate the NADPH oxidase-associated H+ channel. It also seemed unlikely that DHA could act by inhibiting the Na+–K+–2 Cl− symport, mainly responsible for Na+ and chloride cotransport, since prior addition of bumetanide, a specific inhibitor of this symport, did not affect DHA-induced acidification. Matsuyama et al. (2000) have recently shown that cytosol acidification is associated with mitochondrial-matrix alkalinization, as a result of H+ gradient dissipation across the inner mitochondrial membrane. We were tempted to elucidate if DHA-induced acidification was the result of mitochondrial-H+ leakage. We, therefore, performed prior addition of oligomycin, a F0F1-ATPase/H+ pump inhibitor, and carbonyl cyanide m-chlorophenyl-hydrazone (mClCCP), which dissipates H+ gradient across the inner mitochondrial membrane. Again, we rule out the eventual contribution of mitochondrial H+ in DHA-evoked response, since these two molecules failed to influence the decrease in pHi induced by this fatty acid.
As we have seen in the previous paragraph that Ca2+ does play a role in the DHA-induced prolonged acidification, the mechanisms of action of DHA in transient (t1/2=33 s) and immediate acidosis are not well understood. Hence, we would like to refer to the hypothesis of Kamp and Hamilton (Kamp & Hamilton, 1992; Kamp et al., 1995), who have demonstrated that, in phospholipid bilayers, fatty acids can produce free intracellular H+ by a simple diffusion. Indeed, the charged surface of the plasma membrane affects the ionization of fatty acids, thus, increasing their apparent pK (7.6) and the formation of nonionized fatty acids. As fatty acids have a high lipid solubility, all of them will bind to the lipid bilayer. At an extracellular pH of 7.4, fatty acids bind to the outer leaflet that consists of equal amounts of unionized fatty acids and ionized fatty acids. In all, 50% of the unionized fatty acid diffuses rapidly (within seconds) from the outer leaflet to the inner leaflet (flip) of the phospholipid bilayer, where half dissociates into an ionized form (FA−), generating cytosolic H+ (Kamp & Hamilton, 1992). The use of BSA has shown that such a mechanism is likely to occur in our system, as in the presence of albumin, the DHA-, but not the propionic-, induced acidification was totally reversed. The same has been observed for AA (Cavallini et al., 1996; Wu et al., 2000; Chen et al., 2001). Hence, BSA extracts fatty acids from the inner leaflet and allows their transport in the reverse direction (flop). The notion that DHA could acidify the intracellular medium by deprotonation as a result of simple diffusion mechanism is further supported by the use of a DHA derivative in which the carboxylic end of the molecule was replaced by a methyl ester group. DHA methyl ester failed to induce intracellular acidification, and this in agreement with the work of Chen et al. (2001), who also observed that AA methyl ester had no effect on pHi.
Long-chain fatty acids are important metabolic substrates for both energy production and lipid synthesis, as well as they participate in a variety of crucial cell-signalling cascades (Amri et al., 1994; Tebbey et al., 1994; Warnotte et al., 1994). Civelek et al. (1996) have shown in adipocytes that exposure to lipolytic agents or external free fatty acids (FFA) results in a rapid intracellular acidification that is reversed by metabolism of the FFA. As far as the metabolism/catabolism of DHA is concerned, we performed the analysis on the cells/assays during the time course of our study and we observed that this fatty acid was neither converted into docosapentaenoic acid or EPA nor acylated/esterified (results not shown). In fact, DHA is the end product of the metabolism of α-linolenic acid and it does not give rise to eicosanoids (Lands, 1991). Moreover, Jurkat T-cells do not possess the lipoxygenase and cyclooxygenase enzymes that account for lipid mediators (Kurland & Bockman, 1978; Goldyne et al., 1984). Moreover, Kamp et al. (2003) have recently shown both in vesicles prepared from the plasma membrane of adipocytes and in the intact adipocytes that OA metabolism was very little during the time course of the experiment and it was not essential for the decreases in pHi, observed after immediate addition of the fatty acid.
To determine whether the observed pHi changes were unique to DHA, we tested other fatty acids of the n-9, n-6 and n-3 families, respectively, OA (18 : 1 n-9), AA (20 : 4 n-6) and EPA (20 : 5 n-3). All of these fatty acids induced a drop in pHi, in the following order: DHA>EPA>AA>OA. The acidification induced by AA was lesser than that induced by EPA, though the difference was not significantly different. The dose-dependent experiments on AA, EPA and OA were performed for each fatty acid, in order to determine the concentration leading to an optimal response. Under our experimental conditions, all fatty acids exerted maximal decrease in pHi at 10 μM, thus underlying the specificity of the DHA-induced acidosis. Moreover, addition of palmitic acid (16 : 0) was without effect on pHi, suggesting that fatty acid-mediated intracellular acidosis is not a so common feature. It seems to depend not only on the degree of fatty acid unsaturation, but also on the carbon chain length. However, the above observations could be limited by the differences in the water solubility that may exist between fatty acids, particularly between palmitic acid or OA and the others. Indeed, it is generally assumed that, at physiological pH (pH=7.0–7.4), the fatty acids do not form soluble micelles and their equilibrium state is an insoluble aggregate, much like the phospholipid bilayer. In addition, water solubility increases with the degree of cis-unsaturation and carbon chain length of the fatty acids (Cistola et al., 1986; Hicks & Gebicki, 1997). The solubility limit of fatty acids is difficult to measure (Richieri et al., 1992; Vorum et al., 1992). However, we noticed that palmitic acid, even at 20 μM, was homogeneously distributed in the bicarbonate-free medium (pH 7.4); so, the observed effects of palmitic acid are not due to its aggregation. As also mentioned by Hamilton (1998), ethanolic solutions of fatty acids are generally used to achieve higher fatty acids concentrations for presentation to membranes. All fatty acids tested in the present study were dissolved in ethanol. We have not measured the amount of membrane-bound and unbound forms of each fatty acid, although one would expect, as indicated by the affinity constant, that almost all exogenously added free fatty acids adsorb quantitatively and rapidly to the plasma membrane phospholipid bilayer (Anel et al., 1993). Gamberucci et al. (1997) have shown in Erlich tumour cells, treated with OA, that this faty acid was taken up by cells at 1 min of incubation. In our system, at 10 μM, fatty acids are likely to follow the same kinetics; thus, differences observed in the magnitude of fatty acid-induced pHi fall might not be related to differences in the amount of fatty acid adsorption.
Though all of the unsaturated fatty acids induced a fall in pHi; DHA was the most potent inducer of acidification and this difference, in part, may contribute to its mechanisms of action. Our study is certainly of physiological relevance as, under pathophysiological conditions, large amounts of free fatty acids can be released, as it is the case, during cardiac ischaemia, of AA whose concentrations are increased up to 50 μM (Nakamura et al., 1989).
The present study has shown that treatment of human T cells with DHA, an n-3 PUFA, triggered a decrease in pHi, which was due, in part, to simple diffusion of the fatty acid across the phospholipid bilayer and that the long-lasting acidification was dependent on increases in [Ca2+]i. As DHA has been shown to diminish T-cell proliferation (Bonin & Khan, 2000) and to induce apoptosis (Diep et al., 2000), it would be of interest, in future, to determine whether DHA-induced long-term increase in intracellular H+ has a direct effect on immediate-early gene expression and apoptotic signalling pathways in human T cells, in normal or pathological conditions.
Acknowledgments
This work was supported by a contingent grant from Burgundy Region, Dijon. Thanks are due to Dr Claude Humbert, Centre for the Microscopy, for confocal microscopy.
Abbreviations
- AA
arachidonic acid (20 : 4 n-6)
- BAPTA-AM
bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetra-acetoxymethyl ester
- BSA
bovine serum albumin
- [Ca2+]i
free intracellular calcium concentration
- DHA
docosahexaenoic acid (22 : 6 n-3)
- DMA
5-(N,N-dimethyl)-amiloride
- EPA
eicosapentaenoic acid (20 : 5 n-3)
- NHE
Na+/H+ exchanger
- OA
oleic acid (18 : 1 n-9)
- PA
propionic acid
- pHi
intracellular pH
- PUFA
polyunsaturated fatty acid
- TCR
T-cell receptor
- VPATPase
vacuolar-type H+-ATPase
References
- AMRI E.Z., AILHAUD G., GRIMALDI P.A. Fatty acids as signal transducing molecules: involvement in the differentiation of preadipose to adipose cells. J. Lipid Res. 1994;35:930–937. [PubMed] [Google Scholar]
- ANEL A., RICHIERI G.V., KLEINFELD A.M. Membrane partition of fatty acids and inhibition of T cell function. Biochemistry. 1993;32:530–536. doi: 10.1021/bi00053a018. [DOI] [PubMed] [Google Scholar]
- ASTASHKIN E.I., KHODOROVA A.B., SURIN A.M. Arachidonic acid abolishes the mitogen-induced increase in cytosolic free Ca2+ and intracellular pHi in rat thymocytes. FEBS Lett. 1993;329:72–74. doi: 10.1016/0014-5793(93)80196-2. [DOI] [PubMed] [Google Scholar]
- BATES D., CARTLIDGE N.E.F., FRENCH J.M., JACKSON M.J., NIGHTINGALE S., SHAW D.A., SMITH S., WOO E., HAWKINS S.A., MILLAR J.H.D., BELIN J., CONROY D.M., GILL S.K., SIDEY M., SMITH A.D., THOMPSON R.H.S., ZILKHA K., GALE M., SINCLAIR H.M. A double-blind controlled trial of long-chain n-3 polyunsaturated fatty acids in the treatment of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 1989;52:18–22. doi: 10.1136/jnnp.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTHE P., COUSIN J.L., BREITTMAYER J.P. Intracellular Ca2+ regulation in CD3 stimulated Jurkat T cells involves H+ fluxes. Cell. Signal. 1991;3:453–459. doi: 10.1016/0898-6568(91)90075-6. [DOI] [PubMed] [Google Scholar]
- BERTRAND B., WAKABAYASHI S., IKEDA T., POUYSSEGUR J., SHIGEKAWA M. J. Biol. Chem. 1994. pp. 13703–13709. [PubMed]
- BIDANI A., BROWN S.E. ATP-dependent recovery in lung macrophages: evidence for plasma membrane H+-ATPase. Am. J. Physiol. 1990;259:C586–C598. doi: 10.1152/ajpcell.1990.259.4.C586. [DOI] [PubMed] [Google Scholar]
- BITTINER S.B., TUCKER W.F.G., CARTWRIGHT I., BLEEHEN S.S. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;I:378–380. doi: 10.1016/s0140-6736(88)91181-6. [DOI] [PubMed] [Google Scholar]
- BONIN A., KHAN N.A. Regulation of calcium signalling by docosahexaenoic acid in human T-cells: implication of CRAC channels. J. Lipid Res. 2000;41:277–284. [PubMed] [Google Scholar]
- CALDER P.C. Effects of fatty acids and dietary lipids on cells of the immune system. Proc. Nutr. Soc. 1996;55:127–150. doi: 10.1079/pns19960015. [DOI] [PubMed] [Google Scholar]
- CANTRELL D. T-cell antigen receptor signal transduction pathways. Annu. Rev. Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- CAVALLINI L., COASSIN M., BOREAN A., ALEXANDRE A. Arachidonic acid activates a proton conductance pathway and the Na+/H+ exchanger in platelets. Biochem. J. 1996;319:567–574. doi: 10.1042/bj3190567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBREY R., ACHARD J.M., WARNOCK D.G. Heterologous expression of rat NHE4: a highly amiloride-resistant Na+/H+ exchanger isoform. Am. J. Physiol. 1997;272:C90–C98. doi: 10.1152/ajpcell.1997.272.1.C90. [DOI] [PubMed] [Google Scholar]
- CHEN W.-H., CHEN C.-R., YANG K.-T., CHANG W.-L., SU M.-J., WU C.-C., WU M.-L. Arachidonic acid-induced H+ and Ca2+ increases in both the cytoplasm and nucleoplasm of rat cerebellar granule cells. J. Physiol. 2001;537:497–510. doi: 10.1111/j.1469-7793.2001.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIEN E.J., HSIEH D.J., WANG J.E.-C. Response of alkalinisation or acidification by phytohemagglutinin is dependent on the activity of protein kinase C in human peripheral T cells. J. Cell. Biochem. 2001;81:604–612. doi: 10.1002/jcb.1089. [DOI] [PubMed] [Google Scholar]
- CISTOLA D.P., ATKINSON D., HAMILTON J.A., SMALL D.M. Phase behavior and lipid bilayer properties of fatty acids: hydrated 1 : 1 acid soaps. Biochemistry. 1986;25:2804–2812. doi: 10.1021/bi00358a011. [DOI] [PubMed] [Google Scholar]
- CIVELEK V.N., HAMILTON J.A., TORNHEIM K., KELLY K.L., CORKEY B.E. Intracellular pH in adipocytes: effects of free fatty acid diffusion across the plasma membrane, lipolytic agonists, and insulin. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10139–10144. doi: 10.1073/pnas.93.19.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRIDER B.P., XIE X.S., STONE D.K. Bafilomycin inhibits proton flow through the H+-channel of vacuolar proton pumps. J. Biol. Chem. 1994;269:17379–17381. [PubMed] [Google Scholar]
- DIEP Q.N., TOUYZ M., SCHIFFRIN E.L. Docosahexaenoic acid, a peroxisome proliferator-activated receptor-α ligand, induces apoptosis in vascular smooth muscle cells by stimulation of p38 mitogen-activated protein kinase. Hypertension. 2000;36:851–855. doi: 10.1161/01.hyp.36.5.851. [DOI] [PubMed] [Google Scholar]
- FORGAC M. Structure and function of vacuolar class of ATP-driven proton pumps. Physiol. Rev. 1989;69:765–796. doi: 10.1152/physrev.1989.69.3.765. [DOI] [PubMed] [Google Scholar]
- GAMBERUCCI A., FUULCERI R., BENEDETTI A. Inhibition of store-dependent capacitative Ca2+ influx by unsaturated fatty acids. Cell Calcium. 1997;21:375–385. doi: 10.1016/s0143-4160(97)90031-2. [DOI] [PubMed] [Google Scholar]
- GERMAIN R.N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- GERSON D.F., KIEFER H., EUFE W. Intracellular pH of mitogen-stimulated lymphocytes. Science. 1982;216:1009–1010. doi: 10.1126/science.6281887. [DOI] [PubMed] [Google Scholar]
- GOLDYNE M.E., BURRISH G.E., PAUBELLE P., BORGEAT P. Arachidonic acid metabolism among human mononuclear leukocytes. Lipoxygenase-related pathways. J. Biol. Chem. 1984;259:8815–8819. [PubMed] [Google Scholar]
- GRINSTEIN S., DIXON S.J. Ion transport membrane potential, and cytoplasmic pH in lymphocytes: changes during activation. Physiol. Rev. U.S.A. 1989;69:417–481. doi: 10.1152/physrev.1989.69.2.417. [DOI] [PubMed] [Google Scholar]
- GRINSTEIN S., ROTIN D., MASON M.J. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim. Biophys. Acta. 1989;988:73–97. doi: 10.1016/0304-4157(89)90004-x. [DOI] [PubMed] [Google Scholar]
- HAMILTON J.A. Fatty acids transport: difficult or easy. J. Lipid Res. 1998;39:467–481. [PubMed] [Google Scholar]
- HENDERSON L.M., CHAPPELL J.B., JONES O.T.G. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDERSON L.M., CHAPPELL J.B., JONES O.T.G. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils. Further evidence for the presence of an H+ conducting channel. Biochem. J. 1988;251:563–567. doi: 10.1042/bj2510563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HICKS M., GEBICKI J.M. Microscopic studies of fatty acid vesicles. Chem. Phys. Lipids. 1997;20:143–252. [Google Scholar]
- IVES H.E., DANIEL T.O. Interrelationship between growth factor-induced pH changes and intracellular Ca2+ Proc. Natl. Acad. Sci. U.S.A. 1987;84:1950–1954. doi: 10.1073/pnas.84.7.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOLLY C.A., MC MURRAY D.N., CHAPKIN R.S. Effect of dietary n-3 fatty acids on interleukin-2 and interleukin-2 receptor alpha expression in activated murine lymphocytes. Prostagland. Leukotr. Essent. Fatty Acids. 1998;58:289–293. doi: 10.1016/s0952-3278(98)90038-2. [DOI] [PubMed] [Google Scholar]
- KAMP F., GUO W., SOUTO R., PILCH P.F., CORKEY B.E., HAMILTON J.A. Rapid flip-flop of oleic acid across the plasma membrane of adipocytes. J. Biol. Chem. 2003;278:7988–7995. doi: 10.1074/jbc.M206648200. [DOI] [PubMed] [Google Scholar]
- KAMP F., HAMILTON J.A. Gradients across phospholipid membrane caused by fast flip-flop of un-ionized fatty acids. Proc. Natl. Acad. Sci. U.S.A. 1992;89:11367–11370. doi: 10.1073/pnas.89.23.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMP F., HAMILTON J.A. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 1993;32:11074–11086. doi: 10.1021/bi00092a017. [DOI] [PubMed] [Google Scholar]
- KAMP F., ZAKIM D., ZHANG F., NOY N., HAMILTON J.A. Fatty acid flip-flop in phospholipid bilayers is extremely fast. Biochemistry. 1995;34:11928–11937. doi: 10.1021/bi00037a034. [DOI] [PubMed] [Google Scholar]
- KHAN N.A., HICHAMI A.Role of n-3 polyunsaturated fatty acids in the modulation of T-cell signalling Recent Research Developments in Lipids 2002Trivandrum, India: Transworld Publications; 65–78.ed. Pandalai, G., Vol. 6, pp [Google Scholar]
- KLEYMAN T.R., CRAGOE E.J. Amiloride and its analogs as tools in the study of ion transport. J. Membr. Biol. 1988;105:1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- KREMER J.M., LAWRENCE D.A., JUBIZ W., DI GIACOMO R., RYNES K., BARTOLOMEW L.E., SHERMAN M. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Arthrit. Rheum. 1990;33:810–820. doi: 10.1002/art.1780330607. [DOI] [PubMed] [Google Scholar]
- KURLAND J.L., BOCKMAN R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J. Exp. Med. 1978;147:952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDS W.E.M. Biosynthesis of prostaglandins. Annu. Rev. Nutr. 1991;11:41–60. doi: 10.1146/annurev.nu.11.070191.000353. [DOI] [PubMed] [Google Scholar]
- LEVINE S.A., NATH S.K., YUN C.H., YIP J.W., MONTROSE M., DONOWITZ M., TSE C.M. Separate C-terminal domains of the epithelial specific brush border Na+/H+ exchanger isoform NHE3 are involved in stimulation and inhibition by protein kinases/growth factors. J. Biol. Chem. 1995;269:13716–13725. doi: 10.1074/jbc.270.23.13716. [DOI] [PubMed] [Google Scholar]
- MALY K., STRESE K., KAMPFER S., UEBERALL F., BAIER G., GHAFFARI-TABRIZI N., GRUNICKE H.H., LEITGES M. Critical role of protein kinase Cα and calcium in growth factor induced activation of the Na+/H+ exchanger NHE1. FEBS Lett. 2002;521:205–210. doi: 10.1016/s0014-5793(02)02867-3. [DOI] [PubMed] [Google Scholar]
- MASEREEL B., POCHET L., LAEKMANN D. An overview of Na+/H+ exchanger. Eur. J. Med. Chem. 2003;38:547–554. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- MATSUYAMA S., LLOPIS J., DEVERAUX Q.L., TSIEN R.Y., REED J.C. Changes in mitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2000;2:318–325. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- NAKAMURA K., ICHIHARA K., ABIKO Y. Effect of propranolol on accumulation of NEFA in the ischemic perfused rat heart. Eur. J. Pharmacol. 1989;160:61–69. doi: 10.1016/0014-2999(89)90654-7. [DOI] [PubMed] [Google Scholar]
- ORLOWSKI J., GRINSTEIN S. Na+/H+ exchangers of mammalian cells. J. Biol. Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- PARKER J.C., CASTRANOVA V. Volume-responsive sodium and proton movements in dog red blood cells. J. Gen. Physiol. 1984;84:379–401. doi: 10.1085/jgp.84.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHIERI G.V., OGATA R.T., KLEINFELD A.M. A fluorescently labeled intestinal fatty acid binding protein. Interactions with fatty acids and its use in monitoring free fatty acids. J. Biol. Chem. 1992;267:23495–23501. [PubMed] [Google Scholar]
- ROSOFF P.M., CANTLEY L.C. Stimulation of the T3-T cell receptor-associated Ca2+ influx enhances the activity of the Na+/H+ exchanger in a leukemic human T cell line. J. Biol. Chem. 1985;260:14053–14059. [PubMed] [Google Scholar]
- SICZKOWSKI M., DAVIES J. Activity and density of the Na+/H+ antiporter in normal and transformed human lymphocytes and fibroblasts. Am. J. Physiol. 1994;267:C745–C752. doi: 10.1152/ajpcell.1994.267.3.C745. [DOI] [PubMed] [Google Scholar]
- TEBBEY P.W., MCGOWAN K.M., STEPHENS J.M., BUTTKE T.M., PEKALA P.H. Arachidonic acid down-regulates the insulin-dependent glucose transporter gene (GLUT4) in 3T3-L1 adipocytes by inhibiting transcription and enhancing mRNA turnover. J. Biol. Chem. 1994;269:639–644. [PubMed] [Google Scholar]
- VORUM H., BRODERSEN R., KRAGH-HANSEN U., PEDERSEN A.O. Solubility of long chain fatty acids in phosphate buffer at pH 7.4. Biochim. Biophys. Acta. 1992;1126:135–142. doi: 10.1016/0005-2760(92)90283-2. [DOI] [PubMed] [Google Scholar]
- WAKABAYASHI S., BERTRAND B., IKEDA T., POUYSSEGUR J., SHIGEKAWA M. Mutation of calmodulin-binding site renders the Na+/H+ exchanger (NHE1) highly H(+)-sensitive and Ca2+ regulation-defective. J. Biol. Chem. 1994;269:13710–13715. [PubMed] [Google Scholar]
- WARD G.W. CD28: a signalling perspective. Biochem. J. 1996;318:361–377. doi: 10.1042/bj3180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARNOTTE C., GILON P., NENQUIN M., HENQUIN J.-C. Mechanisms of the stimulation of insulin release by saturated fatty acids. A study of palmitate effects in mouse beta-cells. Diabetes. 1994;43:703–711. doi: 10.2337/diab.43.5.703. [DOI] [PubMed] [Google Scholar]
- WU M.-L., CHAN C.-C., SU M.-J. Possible mechanism(s) of arachidonic acid-induced intracellular acidosis in rat cardiac myocytes. Circ. Res. 2000;86:e55–e62. doi: 10.1161/01.res.86.3.e55. [DOI] [PubMed] [Google Scholar]
- WU M.-L., CHEN J.-H., CHEN W.-H., CHEN Y.-J., CHU K.-C. Novel role of the Ca2+-ATPase in NMDA-induced intracellular acidification. Cell. Physiol. 1999;46:C717–C727. doi: 10.1152/ajpcell.1999.277.4.C717. [DOI] [PubMed] [Google Scholar]