Abstract
C-type natriuretic peptide (CNP) and its receptor guanylyl cyclase (GC-B) are expressed in the heart and modulate cardiac contractility in a cGMP-dependent manner. Since the distal cellular signalling pathways remain unclear, we evaluated the peptide effects on cardiac function and calcium regulation in wild-type (WT) and transgenic mice with cardiac overexpression of cGMP-dependent protein kinase I (PKG ITG).
In isolated, perfused working WT hearts, CNP (10 nM) provoked an immediate increase in the maximal rates of contraction and relaxation, a small increase in the left ventricular systolic pressure and a decrease in the time of relaxation. These changes in cardiac function were accompanied by a marked increase in the levels of Ser16-phosphorylated phospholamban (PLB).
In PKG ITG hearts, the effects of CNP on cardiac contractility and relaxation as well as on PLB phosphorylation were markedly enhanced.
CNP increased cell shortening and systolic Cai2+ levels, and accelerated Cai2+ decay in isolated, Indo-1/AM-loaded WT cardiomyocytes, and these effects were enhanced in PKG I-overexpressing cardiomyocytes.
8-pCPT-cGMP, a membrane-permeable PKG activator, mimicked the contractile and molecular actions of CNP, the effects again being more pronounced in PKG ITG hearts. In contrast, the cardiac reponses to β-adrenergic stimulation were not different between genotypes.
Taken together, our data indicate that PKG I is a downstream target activated by the CNP/GC-B/cGMP-signalling pathway in cardiac myocytes. cGMP/PKG I-stimulated phosphorylation of PLB and subsequent activation of the sarcoplasmic reticulum Ca2+ pump appear to mediate the positive inotropic and lusitropic responses to CNP.
Keywords: C-type natriuretic peptide, cardiac contractile function, calcium regulation, cGMP-dependent protein kinase type I, transgenic mice
Introduction
The natriuretic peptide (NP) family consists of three structurally related peptides: atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP) (Anand-Srivastava & Trachte, 1993). Natriuretic peptides influence a variety of homeostatic processes by stimulating the intracellular accumulation of cyclic GMP (cGMP) through two different membrane-bound guanylyl cyclase (GC) receptors: GC-A (specific for ANP and BNP) and GC-B (specific for CNP) (Drewett & Garbers, 1994). ANP and BNP are cardiac hormones produced predominantly by the atrium and ventricle, respectively (De Bold et al., 2001), which act primarily through GC-A to regulate arterial blood pressure and volume status, and thus the maintenance of cardiovascular homeostasis (Anand-Srivastava & Trachte, 1993; Drewett & Garbers, 1994). On the other hand, CNP occurs in a wide variety of tissues, in which it may act locally via GC-B as an autocrine/paracrine regulator (Garbers, 1999).
All of the NPs and their receptors are expressed in the heart. As mentioned, ANP and BNP are secreted from cardiomyocytes; CNP is synthesized and secreted by the coronary endothelium and also by cardiac fibroblasts (Suga et al., 1992; Horio et al., 2003). GC-A and GC-B are located on cardiac myocytes and fibroblasts as well as in coronary vessels (Lin et al., 1995). It is postulated that, in addition to their endocrine actions on distant tissues, NPs modulate contractility and growth of cardiomyocytes and proliferation of cardiac fibroblasts in an autocrine/paracrine manner (Calderone et al., 1998; Tamura et al., 2000; Holtwick et al., 2003). Since the direct effects of NPs on cardiac contractility remain controversial, we recently further characterized this issue. We demonstrated that ANP does not directly affect the contractility of isolated perfused work-performing mouse hearts, but that CNP exerts marked cGMP-mediated positive inotropic and lusitropic effects (Pierkes et al., 2002).
In general, the increase in intracellular cGMP concentrations may lead to the activation of several downstream mediators such as cyclic nucleotide phosphodiesterases (PDEs), cGMP-regulated ion channels, and cGMP-dependent protein kinases (PKG) (Lohmann et al., 1991). At least two cGMP-regulated PDE isoforms (PDE2 and PDE3), which hydrolyze cAMP, are expressed in mammalian heart (Méry et al., 1993). Of the two known PKG isoforms (PKG I and PKG II), only the type I form is expressed in cardiomyocytes (Wollert et al., 2002). Our recent observations in isolated perfused murine hearts suggested that a putative mechanism of the immediate contractile responses to the CNP/GC-B system involves cGMP/PKG I-dependent phosphorylation of phospholamban (PLB) and subsequent activation of the sarcoplasmic reticulum Ca2+ pump (Pierkes et al., 2002). However, a direct role of PKG I as a downstream mediator of the CNP/GC-B/cGMP system in the heart was not definitively demonstrated.
To further investigate the role of PKG I as a potential mediator of the cardiac contractile effects of CNP, we generated a new transgenic mouse line with targeted overexpression of PKG I in cardiac myocytes (PKG ITG). Remarkably, the inotropic and lusitropic effects of CNP on isolated work-performing hearts, as well as CNP effects on Ca2+ transients and contractility in isolated ventricular cardiomyocytes from PKG ITG mice, were markedly enhanced. Additionally, the stimulatory actions of CNP on PLB phosphorylation were significantly increased in PKG ITG cardiomyocytes. 8-para-chlorophenylthio-cGMP (8-pCPT-cGMP), a membrane-permeable PKG activator, mimicked the contractile and molecular actions of CNP, the effects again being more pronounced in PKG ITG hearts. Taken together, these observations demonstrate that PKG I is the target activated by the CNP/GC-B/cGMP system, which mediates the cardiac contractile responses to CNP.
Methods
Generation of PKG I-transgenic mice
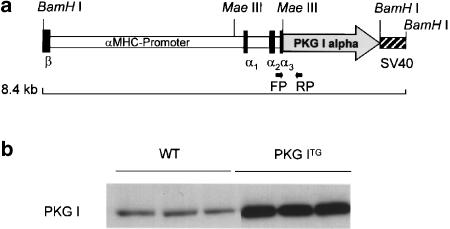
Transgenic mice expressing human PKG I alpha (Tamura et al., 1996) under the control of the 5.5 kb murine α-myosin heavy chain (αMHC) promoter (Gulick et al., 1991) were generated to drive transgene expression in adult atrial and ventricular cardiac myocytes. In brief, a BamHI site was introduced by PCR mutagenesis 14 bp upstream of the ATG start codon of human PKG I cDNA (Figure 1a). A 2149 bp PKG I cDNA fragment was then released by BamHI and ClaI digestion (ClaI site 116 bp downstream of the PKG I stop codon) and cloned into the MaeIII site in the third noncoding exon of the αMHC locus (Figure 1a). The transgene was linearized and pBluescript (Stratagene) vector sequences were removed by NotI digestion. Transgenic B6D2/F1/Crl founder mice were generated by standard procedures at the Center for Molecular Biology, University of Heidelberg, Germany. Founder mice were mated with wild-type C57BL/6 mice to establish three independent lines of PKG ITG mice. Transgenic mice were identified by genomic PCR: forward primer (CATAGGCTACGGTGTAAAAGAGGC) located in the αMHC gene locus (145 bp upstream of the PKG I start codon) and reverse primer(TACTCCACCGGGTACATACAATCC) located 368 bp downstream of the PKG I start codon (Figure 1a). PKG I overexpression in the heart was confirmed by Western blotting, using a polyclonal antiserum raised against recombinant human PKG I (Figure 1b) (Markert et al., 1995). Male PKG ITG mice, 5–6 months old, from transgenic line 2 and their nontransgenic, wild-type (WT) littermates were used in this study. The investigation conforms with the Guide for the Care and Use of Laboratory Animals, as published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996), and was approved by the local animal care committees.
Figure 1.
Generation of transgenic mice overexpressing PKG I alpha in cardiac myocytes. (a) Human PKG I cDNA was placed under the control of murine αMHC promoter. PKG ITG mice were identified by genomic PCR amplification of a 513 bp fragment. Forward (FP) and reverse primers (RP) are indicated. (b) Protein expression of PKG I (76 kDa) was determined in cardiac ventricles of WT and in PKG ITG mice by Western-blot analysis using specific antisera and a peroxidase-labelled goat anti-rabbit antibody in an ECL detection system. To obtain immunoreactive signals in the linear range, 20 μg protein from WT hearts and 2 μg protein from PKG ITG hearts were loaded. PKG I expression was significantly upregulated in PKG ITG compared to WT ventricles by ∼46-fold (mean±s.e.m., n=13 per genotype, *P<0.01 vs WT).
Measurement of contractile parameters in isolated work-performing heart preparations
Analysis of cardiac function was performed as described previously (Kuhn et al., 2002; Pierkes et al., 2002). In brief, isolated hearts from WT and PKG ITG mice were perfused with Krebs–Henseleit (KH) buffer through the pulmonary vein and left atrium, in an anterograde, fluid-ejecting mode. Fluid was ejected from an aortic cannula against a hydrostatic fluid column set at a height to yield a mean aortic pressure (afterload) of 50 mmHg. Aortic outflow and atrial inflow were continuously monitored via canulating ultrasonic flowprobes (Transonic Systems Inc., Ithaca, U.S.A.). Atrial inflow (venous return, preload) was adjusted to 5 ml min−1 using a roller pump (∼30 ml min−1 g−1 heart weight). Coronary flow was calculated as the difference between preload and aortic flow. Aortic pressure, left atrial pressure, and left intraventricular pressure were continuously measured via catheters filled with KH buffer, and connected to pressure transducers (Harvard Apparatus, Inc., distributed by FMI GmbH, Seeheim, Germany). All hearts were compared under exactly identical loading conditions: preload=5 ml min−1 (left atrial pressure=7±2 mm Hg) and afterload=50 mmHg and cardiac minute work of 250 ml min−1 mmHg. Heart rate, the first derivatives of left intraventricular pressure, +dP/dt and −dP/dt (in mmHg s−1), as well as the time to peak pressure and time to relaxation (in ms), were calculated (A Mon 2.1 program, Ingenieurbüro Jäckel, Hanau, Germany) (Kuhn et al., 2002; Pierkes et al., 2002).
Experimental protocols
After a 20 min equilibration period with KH buffer, either CNP (10 nM), 8-pCPT-cGMP (100 μM) or the β1-adrenoreceptor agonist isoproterenol (100 nM) were continuously infused via the coronary arteries for 50 min. Control hearts of each genotype were infused with KH buffer for the same time period (70 min). To study the effect of test agents on intracellular PLB phosphorylation, at the end of each experiment, the isolated perfused working hearts were quickly stopped by shock freezing both ventricles in liquid nitrogen. The frozen ventricles were then extracted for Western-blot analysis.
Western-blot analysis
To determine the expression level of PKG I and PLB, as well as the effect of CNP, 8-pCPT-cGMP, and isoproterenol on PLB phosphorylation, frozen ventricles from isolated hearts infused with these agents or with KH buffer (controls) were homogenized and analyzed by Western blot as described (Pierkes et al., 2002). The antibodies used were against PKG I (Markert et al., 1995), total PLB (PLB A-1 antibody), and PLB phosphorylated at serine-16 (PLB-PS-16 antibody) (Fluorescience Ltd, Leeds, U.K.) (Pierkes et al., 2002). Detection was with an ECL system (Amersham-Pharmacia, Freiburg, Germany) and results were quantitated by densitometry (ImageQuant software; Molecular Dynamics, Krefeld, Germany).
Isolation of ventricular cardiomyocytes for measurements of intracellular Ca2+ transients and single-cell contractility
Ventricular myocytes from WT and PKG ITG mouse hearts were isolated using an established protocol (Kirchhefer et al., 2001). Hearts were fixed to a Langendorff apparatus and were retrogradely perfused via the aorta for 5 min with a Ca2+-free solution (solution A) composed of (in mM) 140 NaCl, 5.8 KCl, 0.5 KH2PO4, 0.4 NaH2PO4, 0.9 MgSO4, 10 HEPES, and 11.1 glucose (pH 7.1), followed by perfusion for 30 min with solution A supplemented with 0.2 mg ml−1 collagenase (type 1, Cell Systems Biotechnologie, St Katharinen, Germany). The Ca2+ concentration was gradually increased during digestion to 100 μM. After enzymatic digestion, hearts were perfused for 10 min with solution A. Subsequently, cardiac ventricles were cut into several pieces and subjected to gentle agitation through a nylon mesh to separate the myocytes. Cardiomyocytes were then incubated for 5 min at room temperature with solution A containing in addition 2.5 mM CaCl2, 50 mg l−1 ascorbic acid (solution B), and 25 μM Indo-1/AM. The cells were then equilibrated with solution B without dye for 45 min before starting measurements. For measurements of Ca2+ transients and contractile parameters, cardiomyocytes were transferred to the well of a perfusion chamber located on the stage of an inverted microscope (Nikon Diaphot 200, Nikon, Tokyo, Japan) and constantly superfused with solution B. They were stimulated at 0.5 Hz with platinum electrodes placed on the sides of the experimental chamber. Indo-1 fluorescence was recorded from single myocytes at room temperature using a dual-emission microfluorescence system (Photon Technologies Inc., South Brunswick, NJ, U.S.A.). Excitation was at 365 nm, and the emitted fluorescence was recorded at 405 and 495 nm. The ratio of fluorescence at the two wavelengths was used as an index of cytosolic Ca2+ concentration. Simultaneously, cardiomyocyte dimensions and shortening were visualized on a monitor (SONY PVM 97, SONY, Tokyo, Japan) connected to a video edge detector (VED 105, Crescent Electronics, South East Sandy, UT, U.S.A.), which was interfaced to a video camera (SONY Camera Module XC-75, SONY, Tokyo, Japan) attached to the inverted microscope. After 15 min of baseline recording, the effects of CNP (0.3 μM) or the membrane-permeable cGMP analogue, 8-pCPT-cGMP (10 μM) were tested by continuous superfusion of the cells for 30 min. Initial experiments showed that lower CNP concentrations had no consistent effect on calcium handling, which is in accordance with published studies also evaluating CNP effects on isolated cardiomyocytes (Brusq et al., 1999). This might be related to the generally deleterious effects of enzymatic digestion on cell membrane receptors, as has been shown for other membrane and extracellular proteins (Oxhorn et al., 2002). Data were collected at 20 Hz, and acquisition and processing were supported by Felix 1.1 software (Photon Technologies Inc., South Brunswick, NJ, U.S.A.).
Chemicals
Human CNP (same sequence as rat) was obtained from Calbiochem-Novabiochem (Bad Soden, Germany), 8-pCPT-cGMP from Biolog (Bremen, Germany), and isoproterenol was from Sigma-Aldrich Chemie GmbH (Deisenhofen, Germany). Indo-1/AM was supplied by Molecular Probes (Leiden, The Netherlands).
Statistics
Results are presented as means±s.e.m. The agent-induced changes in cardiac contractile parameters were normalized by expression as % of baseline (before infusion of chemicals). Student's t-test was used for comparison of data, except that the serial changes in cardiac function measured during infusion of agents were tested with a repeated-measures ANOVA followed by Student–Newman–Keuls multiple comparisons test (GraphPad InStat software). Results were considered statistically significant in all analyses at P<0.05.
Results
Baseline contractile parameters are similar for WT and PKG ITG hearts
As shown by Western-blot analyses, PKG I protein was overexpressed by 46±3.7-fold in the cardiac ventricles of PKG ITG mice as compared to WT mice (Figure 1b). This did not affect heart weights and heart/body weight ratios, which were not different between the two genotypes (Table 1 ). Table 1 also shows the contractile parameters of isolated hearts from WT and PKG ITG mice in the work-performing preparation under baseline conditions. All basal parameters of cardiac function were similar for the two genotypes.
Table 1.
Baseline parameters of isolated hearts from WT and PKG ITG mice in the work-performing mode
| Baseline parameters | WT | PKG ITG |
|---|---|---|
| Heart weight (mg) | 179±2 | 182±2 |
| Heart/body weight ratio (mg g−1) | 6.3±0.2 | 6.4±0.3 |
| CF (ml min−1 g−1) | 16.8±1.0 | 16.9±0.5 |
| HR (b.p.m.) | 360±8 | 371±6 |
| Mean aortic pressure (mmHg) | 51.3±0.1 | 52.1±0.3 |
| Intraventricular pressure (mmHg) | ||
| Systolic | 87±1.4 | 87±1.5 |
| Diastolic | −0.9±0.8 | −0.3±0.9 |
| Time to peak pressure (ms) | 34.3±0.6 | 35.0±0.4 |
| Time to relaxation (ms) | 46.3±0.5 | 44.5±1.0 |
| +dP/dt (mmHg s−1) | 3067±21 | 3105±17 |
| −dP/dt (mmHg s−1) | 2559±74 | 2669±87 |
Perfusion conditions: preload of 30 ml min−1 g−1 (intra-atrial pressure of 7±2 mmHg), afterload of 50 mmHg aortic pressure. CF: coronary flow; HR: heart rate; +dP/dt, maximal rate of contraction; −dP/dt, maximal rate of relaxation. Values are means±s.e.m. (n=27 per genotype).
Enhanced contractile responses to CNP in isolated perfused PKG ITG hearts
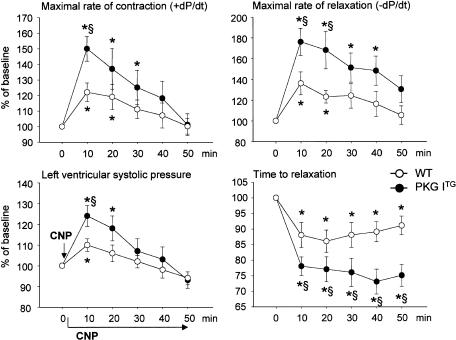
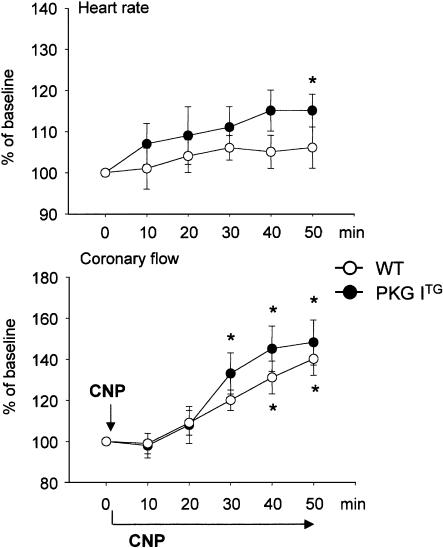
Infusion of 10 nM CNP into isolated WT working hearts during 50 min provoked an immediate increase in the maximal rates of contraction (+dP/dt) and relaxation (−dP/dt), a small increase in the left ventricular systolic pressure and a decrease in the time to relaxation (Figure 2). These effects started immediately after addition of CNP, reached their maximum at ∼10 min and then, except for the time to relaxation, slowly reversed to baseline at about 40 min. The time to relaxation was maximally shortened at 20 min of CNP infusion, and then started to increase but remained below baseline during the whole experimental period. The chronotropy of WT hearts did not significantly change during 50 min of infusion with CNP (Figure 3). Coronary flow was progressively increased by CNP to ∼140±8% of pretreatment values within 50 min (Figure 3).
Figure 2.
Enhanced inotropic and lusitropic effects of CNP in PKG ITG hearts. Time course of the effects of CNP (10 nM) on maximal rates of contraction (+dP/dt) and relaxation (−dP/dt), maximal left ventricular systolic pressure and time to relaxation in isolated working wild-type (WT, open circles) and PKG ITG (filled circles) hearts. CNP was added at time zero (arrow) and infused for 50 min in all panels. Data are expressed as % of baseline (at 0 min) and represent the mean±s.e.m. from eight experiments per genotype. *P<0.05 vs baseline (at 0 min), §P<0.05 vs WT.
Figure 3.
Time course of the effects of CNP (10 nM) on coronary flow and heart rate in isolated working WT (open circles) and PKG ITG (filled circles) hearts. CNP was added at time zero (arrow) and infused for 50 min in both panels. Data are expressed as % of baseline (at 0 min) and represent the mean±s.e.m. from eight experiments per genotype. *P<0.05 vs baseline (at 0 min).
In isolated PKG ITG hearts, the positive inotropic and, in particular, the positive lusitropic actions of CNP were markedly enhanced as compared to WT hearts (Figure 2). The inotropic effect was transient and reversed to baseline within 40–50 min. In contrast, the time to relaxation remained markedly shortened (positive lusitropism) during the whole experiment. These changes in contractility were accompanied by a slowly developing weak positive chronotropic effect (Figure 3). The CNP-induced increase in coronary flow was similar to that of WT hearts (Figure 3).
Control WT and PKG ITG hearts infused with vehicle (KH buffer) alone revealed no significant changes in contractile function over a 50 min observation time (n=5; data not shown), indicating that the reversal of the CNP effects does not reflect a spontaneous deterioration of heart function, but indeed represents specific actions of CNP.
Effects of 8-pCPT-cGMP and isoproterenol on cardiac contractile parameters
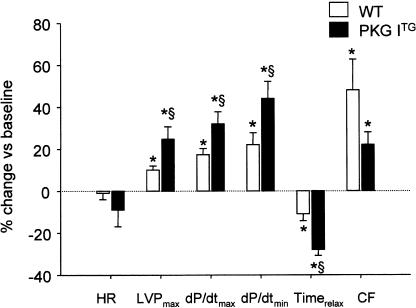
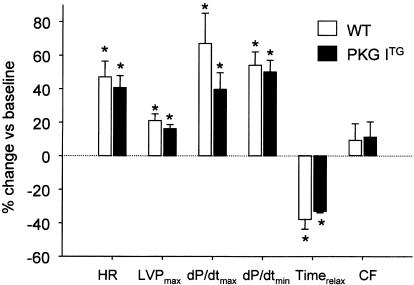
8-pCPT-cGMP, a membrane-permeable, PDE-resistant, selective activator of cGMP-dependent protein kinases (Butt et al., 1992), mimicked the effects of CNP on cardiac contractility and relaxation. As observed for CNP, the inotropic and lusitropic responses to 8-pCPT-cGMP (100 μM) were more pronounced in PKG ITG compared to WT hearts (Figure 4). These cardiac contractile responses started immediately after addition of 8-pCPT-cGMP and peaked earlier in PKG ITG (at 1 min) as compared to WT hearts (at 5 min). Thereafter, the positive inotropic and lusitropic effects slightly reversed but remained clearly over the baseline during the whole experimental period. The 8-pCPT-cGMP-induced increase in coronary flow was slightly but not significantly lower in PKG ITG hearts. Heart rates were not significantly affected in either genotype (Figure 4).
Figure 4.
Peak effects of 8-pCPT-cGMP (100 μM) on heart rate (HR), left ventricular systolic pressure (LVPmax), maximal rates of contraction (dP/dtmax) and relaxation (dP/dtmin), time to relaxation (Timerelax) and coronary flow (CF) in isolated working wild-type (WT) and PKG ITG (filled columns) hearts. Data are expressed as % change vs baseline and represent the mean±s.e.m. from nine experiments per genotype. *P<0.05 vs baseline (at 0 min), §P<0.05 vs WT.
To test whether the inotropic and lusitropic responses of PKG ITG hearts were indeed selectively enhanced in response to cGMP-elevating agents, the cAMP-mediated responses to the β1-adrenoreceptor agonist isoproterenol (100 nM) were tested. As shown in Figure 5, isoproterenol elicited marked positive chronotropic, inotropic, and lusitropic effects, which peaked within 5 min of infusion and were similar for both WT and PKG ITG hearts. These contractile changes were accompanied by a slight, nonsignificant increase in coronary flow (Figure 5).
Figure 5.
Peak effects of isoproterenol (100 nM) on heart rate (HR), left ventricular systolic pressure (LVPmax), maximal rates of contraction (dP/dtmax) and relaxation (dP/dtmin), time to relaxation (Timerelax) and coronary flow (CF) in isolated working WT and PKG ITG (filled columns) hearts. Data are expressed as % change vs baseline and represent the mean±s.e.m. from five experiments per genotype. *P<0.05 vs baseline (at 0 min).
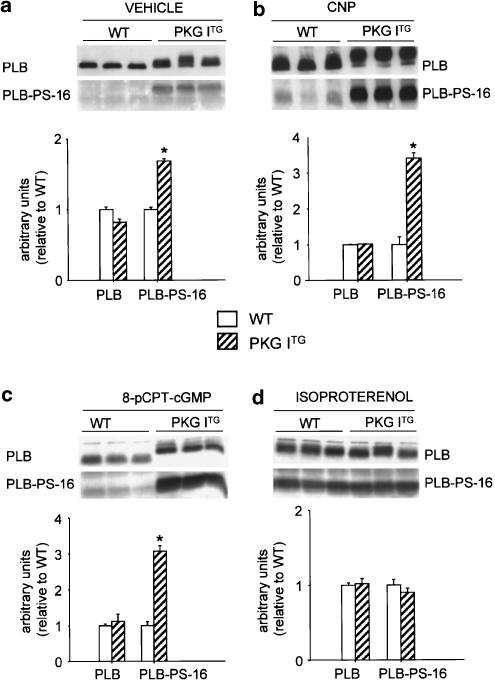
CNP and 8-pCPT-cGMP-induced phosphorylation of PLB is increased in PKG ITG ventricles
To investigate the mechanism of the inotropic and lusitropic effects of CNP, the expression and Ser16 phosphorylation (PLB-PS-16) of the sarcoplasmic reticulum (SR)-regulatory protein PLB were quantified in the isolated perfused hearts by Western-blot analysis. The expression levels of total PLB were not different in PKG ITG as compared to WT ventricles (Figure 6a–d). The PLB-Ser16 phosphorylation-specific antibody detected almost no signal in the ventricles of vehicle (KH)-treated WT hearts, and a weak but clearly more pronounced signal in vehicle-perfused PKG ITG hearts (Figure 6a). A strong increase in PLB-PS-16 was detected in isolated working hearts perfused with 10 nM CNP for 50 min (Figure 6b). Phosphorylation at Ser16 was 3.4-fold greater in PKG ITG hearts as compared to WT hearts treated with CNP (Figure 6b; *P<0.05). The cGMP-analog 8-pCPT-cGMP mimicked the effects of CNP, the responses being enhanced in PKG ITG ventricles (Figure 6c). Notably, CNP and 8-pCPT-cGMP-dependent phosphorylation also decreased the electromobility of total PLB in the PKG ITG as compared to WT hearts (Figures 6b, c), which is probably due to phosphorylation-evoked changes in protein conformation (Li et al., 1998). In contrast, the effects of isoproterenol on PLB phosphorylation and electromobility were not different between the two genotypes (Figure 6d).
Figure 6.
Enhanced baseline PLB phosphorylation as well as increased effects of CNP and 8-pCPT-cGMP on PLB phosphorylation at Ser16 (PLB-PS-16) in PKG ITG hearts. Top, Western blots showing the expression of total PLB as well as PLB-PS-16 in isolated working WT and PKG ITG ventricles treated with KH vehicle (a), 10 nM CNP (b), 100 μM 8-pCPT-cGMP (c) or 100 nM isoproterenol (d). Bottom, relative amount of total PLB and PLB-PS-16 expression (normalized to WT). In vehicle-perfused hearts, the basal signal for PLB-PS-16 was significantly increased in PKG ITG hearts (a; n=5). All test agents, CNP (b; n=8), 8-pCPT-cGMP (c; n=9), and isoproterenol (d, n=5), evoked a marked increase in PLB phosphorylation. The responses to CNP and 8-pCPT-cGMP but not to isoproterenol were significantly greater in PKG ITG ventricles (mean±s.e.m., *P<0.05 vs WT).
CNP increases Cai2+ transients and contractility of isolated adult ventricular cardiomyocytes
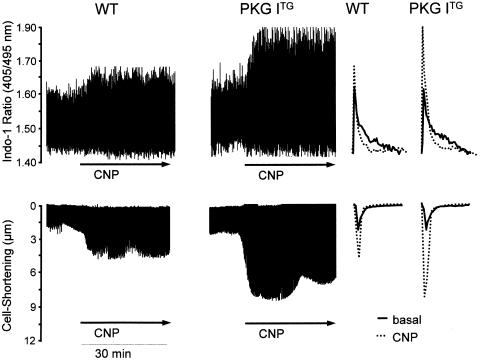
The stimulatory effects of CNP and 8-pCPT-cGMP on PLB phosphorylation and the observation that these effects were enhanced in PKG ITG hearts suggested a direct modulatory cGMP/PKG I-mediated effect of CNP on intracellular Ca2+ homeostasis. We therefore measured cytoplasmic Ca2+ transients and contractility in isolated ventricular myocytes from WT and PKG ITG hearts. Myocytes were loaded with Indo-1AM and stimulated at 0.5 Hz. Baseline diastolic Ca2+ levels were similar in cells from PKG ITG compared with WT mice (Tables 2 and 3). Also, the baseline values for the peak Ca2+-transient amplitude and the time to 50% decay were similar in both genotypes (Tables 2 and 3). CNP (0.3 μM) and 8-pCPT-cGMP (10 μM) did not affect diastolic Ca2+ levels, but provoked a significant increase in the peak amplitude of the Ca2+ transient, together with an accelerated Ca2+ decay in both WT and PKG ITG cardiomyocytes (Tables 2 and 3; Figure 7). These responses were observed immediately after addition of the test agents, reached their maximum at 10 min, and then remained stable for the whole experimental period (30 min) (Figure 7a). As shown in Tables 2 and 3, both effects were significantly enhanced in PKG ITG cardiomyocytes.
Table 2.
Effects of CNP (0.3 μM) on Cai2+ transients and contractile parameters of isolated cardiomyocytes
| Cai2+ transients | WT | PKG ITG |
|---|---|---|
| Baseline | ||
| Diastolic ratio (405/495 nm) | 1.43±0.02 | 1.43±0.02 |
| Cai2+ peak amplitude | 0.17±0.01 | 0.17±0.01 |
| Time to 50% decay (ms) | 141.0±10.8 | 153.4±21.4 |
| CNP | ||
| Diastolic ratio (405/495 nm) | 1.42±0.03 | 1.44±0.02 |
| Cai2+ peak amplitude | 0.22±0.03* | 0.28±0.03*§ |
| Time to 50% decay (ms) | 106.7±11.3* | 79±7.8*§ |
| Contractile parameters | ||
| Baseline | ||
| Lmax (μm) | 107.1±5.3 | 117.5±6.5 |
| Lmin (μm) | 104.7±5.4 | 113.4±6.5 |
| Cell shortening (%) | 2.29±0.3 | 3.3±0.6 |
| Time to 90% relaxation (ms) | 464±76 | 465±63 |
| CNP | ||
| Lmax (μm) | 106.0±5.5 | 115.2±7.2 |
| Lmin (μm) | 102.2±5.6 | 104.1±7.7 |
| Cell shortening (%) | 3.88±0.7* | 9.7±1.58*§ |
| Time to 90% relaxation (ms) | 217±34* | 124±13*§ |
Indo-1 signals were determined in isolated ventricular cardiomyocytes from WT and PKG ITG mice. The contractile measurements were performed by edge detection. Isolated myocytes were paced at 0.5 Hz. Lmax indicates the maximal myocyte length, and Lmin the minimal myocyte length. The maximal magnitude of contraction was normalized to Lmax and expressed as percentage of shortening. Values represent the mean±s.e.m. (n=13 cells from each of eight mice per genotype).
P<0.05 vs baseline,
P<0.05 vs WT.
Table 3.
Effects of 8p-CPT-cGMP (10 μM) on Cai2+ transients and contractile parameters of isolated cardiomyocytes
| Cai2+ transients | WT | PKG ITG |
|---|---|---|
| Baseline | ||
| Diastolic ratio (405/495 nm) | 1.44±0.03 | 1.35±0.03 |
| Cai2+ peak amplitude | 0.17±0.02 | 0.19±0.02 |
| Time to 50% decay (ms) | 150.0±13.8 | 157.4±23.4 |
| 8p-CPT-cGMP | ||
| Diastolic ratio (405/495 nm) | 1.43±0.04 | 1.33±0.04 |
| Cai2+ peak amplitude | 0.24±0.03* | 0.34±0.04*§ |
| Time to 50% decay (ms) | 124.7±5.3* | 84±8.2*§ |
| Contractile parameters | ||
| Baseline | ||
| Lmax (μm) | 129.8±6.3 | 120.5±7.8 |
| Lmin (μm) | 127.0±6.4 | 118.4±7.6 |
| Cell shortening (%) | 1.7±0.11 | 1.8±0.4 |
| Time to 90% relaxation (ms) | 593±146 | 557±71 |
| 8p-CPT-cGMP | ||
| Lmax (μm) | 129.0±6.4 | 119.5±7.6 |
| Lmin (μm) | 125.8±6.4 | 113.1±7.4 |
| Cell shortening (%) | 2.5±0.27* | 5.4±0.54*§ |
| Time to 90% relaxation (ms) | 405±136 | 201±37*§ |
Indo-1 signals were determined in isolated ventricular cardiomyocytes from WT and PKG ITG mice. The contractile measurements were performed by edge detection. Isolated myocytes were paced at 0.5 Hz. Lmax indicates the maximal myocyte length, and Lmin the minimal myocyte length. The maximal magnitude of contraction was normalized to Lmax and expressed as percentage of shortening. Values represent the mean±s.e.m. (n=6 cells from each of five mice per genotype).
P<0.05 vs baseline,
P<0.05 vs WT.
Figure 7.
Representatives examples of effects of CNP (0.3 μM) on intracellular Ca2+ transients (indo-1 405/495 ratio) (a) and simultaneous contraction (cell shortening) (b) in WT and PKG ITG cardiomyocytes. Right panels show respective single traces before (basal) and during CNP treatment.
To determine whether the increased [Ca2+]i responses of PKG ITG cardiomyocytes to CNP and 8-pCPT-cGMP influenced contractility, we simultaneously measured cardiomyocyte shortening using a video edge-detection system. As depicted in Tables 2 and 3, the resting maximal cell shortening and time to relaxation were similar for cardiomyocytes from both genotypes. CNP and 8-pCPT-cGMP significantly increased cell shortening and decreased the time of relaxation, and these responses were significantly enhanced in PKG ITG cardiomyocytes as compared to WT (Tables 2 and 3; Figure 7b). The time course of these contractile changes was parallel to Cai2+ changes (maximal at 10 min, then remained stable for the whole 30 min experimental period) (see Figure 7, original tracings).
Discussion
The present study extends previous observations of our own and other groups in isolated perfused ‘working hearts' and ventricular preparations (Beaulieu et al., 1997; Hirose et al., 1998; Brusq et al., 1999; Pierkes et al., 2002), showing that CNP directly increases contractility and accelerates the relaxation of isolated electrically paced adult mouse cardiomyocytes. These positive inotropic and lusitropic effects were concomitant with increased PLB phosphorylation, increased amplitude of Cai2+ transients, and an increased rate of Cai2+ decay, suggesting that acceleration of SR Ca2+ uptake and increased availability of Ca2+ for contraction, both secondary to PLB phosphorylation, are major mechanisms of the positive inotropic and lusitropic effects of CNP. Interestingly, the effects of CNP on contractility and Cai2+ homeostasis were mimicked by the PKG activator 8-pCPT-cGMP, and significantly enhanced in transgenic mice with cardiomyocyte-selective overexpression of PKG I, indicating that PKG I serves as a critical downstream target for CNP.
The CNP/GC-B-evoked elevation of intracellular cGMP in cardiac myocytes can potentially influence several different pathways stimulating contractility and relaxation in a calcium-dependent way, including the activation of PKG I, and inhibition of phosphodiesterase (PDE3) activity, with consequent increases in cAMP levels (Lohmann et al., 1991; Vandecasteele et al., 2001). It is also potentially feasible that high levels of cGMP could induce changes in contractility via direct crossactivation of cAMP-dependent protein kinase (PKA), as has been reported in smooth muscle cells (Ruiz-Velasco et al., 1998; Sausbier et al., 2000). To distinguish the effects of PKG I from those of other potential cGMP mediators, we created a new mouse model with targeted overexpression of PKG I in cardiomyocytes. As shown, the effects of CNP on contractility and relaxation, PLB phosphorylation, and intracellular Ca2+ regulation were markedly enhanced in PKG ITG cardiomyocytes, suggesting that PKG I can mediate these CNP effects. In support of this, 8-pCPT-cGMP, which direcly activates PKG I but lacks effects on PDEs (Butt et al., 1992), induced contractile and molecular changes similar to those evoked by CNP. Again, the effects of 8-pCPT-cGMP were more pronounced in PKG ITG as compared to WT hearts. In contrast, the contractile and molecular responses to cAMP-mediated β-adrenergic stimulation did not differ between WT and PKG I-overexpressing hearts, showing that the responses of the latter were selectively enhanced in response to agents increasing intracellular cGMP. As mentioned, the inotropic responses of isolated working hearts to CNP were reversible over time, in spite of continuous treatment with the peptide. Since the lusitropic and vasodilating responses to CNP were stable, desensitization of GC-B is unlikely to account for this observation. Future studies will be directed to elucidate the responsible mechanisms.
Notably, Ser16 phosphorylation of PLB was already increased in vehicle-perfused PKG ITG hearts as compared to WT, which is probably due to the endogenous release of some cGMP/PKG I-activating hormone (natriuretic peptides, NO) within the isolated, intact working hearts. In spite of this, the basal cardiac function of the two genotypes was similar, indicating either that this slight increase in PLB phosphorylation was not sufficient to alter SR calcium handling, or that compensatory changes in other cardiac regulatory pathways prevented consecutive changes in cardiac function. Phosphorylation of PLB by PKG I has been detected in isolated cardiac SR (Raeymaekers et al., 1988; Huggins et al., 1989). Moreover, a recent study in tracheal smooth muscle showed that the PKG I-signalling complex is directly associated with PLB, suggesting that these proteins interact specifically at the SR (Koller et al., 2003). However, the regulatory effects of PKG I on PLB phosphorylation and ultimately on Ca2+ uptake by the SR in vivo remain unclear and controversial (Huggins et al., 1989). Although PLB phosphorylation was observed after stimulation of intracellular cGMP synthesis with ANP in smooth muscle tissue (Sarcevic et al., 1989) and cultured neonatal rat cardiomyocytes (Sabine et al., 1995), or with CNP in rat and murine hearts (Brusq et al., 1999; Pierkes et al., 2002), the possibility that cGMP-dependent modulation of the cAMP/PKA pathway was involved was not definitively excluded in these previous reports. Our study provides strong evidence that CNP/cGMP-activated PKG I can phosphorylate PLB in intact hearts, leading to a marked stimulation of SR Ca2+ handling, cardiac contractility, and relaxation.
Our observations are in contrast to some studies reporting PKG I-mediated negative inotropic effects of synthetic cGMP analogs and cGMP-elevating agents such as nitric oxide (NO) (Layland et al., 2002; Wegener et al., 2002). The mechanism behind the negative inotropic action of PKG I may involve troponin I phosphorylation and a subsequent desensitization of myofilament Ca2+ responsiveness (Pfitzer et al., 1982; Shah et al., 1994; Kaye et al., 1999) and/or inhibition of L-type Ca2+ channel activity (Méry et al., 1991; Sumii & Sperelakis, 1995). The reasons for differing inotropic effects are unclear, but could involve differences in the species used, or the experimental protocol. It is also possible that CNP-stimulated endogenous cGMP in cardiomyocytes can stimulate PKG I localized in intracellular compartments (i.e. near to the SR), which are not always activated by the NO donors used in other studies. For instance, cardiomyocyte receptors for natriuretic peptides seem to be mostly confined to plasmalemmal caveolae (Doyle et al., 1997), suggesting that this close proximity to the SR–T-tubule connection could directly target CNP-stimulated cGMP to PKG I localized in or near to the SR. Interestingly, it was recently shown that the subcellular localization of different nitric oxide synthases (NOS) to distinct microdomains of cardiomyocytes (endothelial NOS near L-type Ca2+ channels, neuronal NOS near the SR) is responsible for the observation that NO signals can have dual stimulatory (nNOS) and inhibitory (eNOS) effects on cardiac contractility (Barouch et al., 2002). Thus, it is conceivable that spatial confinement of different GCs within distinct microdomains of cardiomyocytes allows cGMP to locally regulate different effector molecules and, ultimately, to have dual effects on cardiac contractile functions.
As shown, CNP not only affected cardiac contractility but also increased coronary flow in intact perfused murine hearts. Interestingly, recent studies have shown that CNP-induced vasodilation is mediated both through direct effects on the GC-B receptor expressed in vascular smooth muscle cells (Lin et al., 1995) and also by activation of Gi-coupled NPR-C receptors, which then lead to cellular hyperpolarization (Chauhan et al., 2003). In our study, the vasodilating effects of CNP were not different in hearts from WT and PKG ITG mice, whereas the effects on cardiac contractile functions were markedly enhanced in the latter. This demonstrates that the positive inotropic and lusitropic actions of CNP are directly related to the activation of the GC-B receptors and PKG I expressed in cardiomyocytes, and not indirectly provoked by any CNP-induced increases in myocardial perfusion flow. Furthermore, in a previous study, we demonstrated that the NPR-C specific ligand cANP(4–23) does not affect cardiac contractility and coronary flow in this experimental setup, ruling out CNP effects through this receptor (Pierkes et al., 2002).
Our results, together with those from other reports, indicate that cGMP can have both positive and negative inotropic effects, depending on the type of hormone and receptor GC involved in cGMP formation, the rate and cardiomyocyte microdomain in which cGMP is synthesized, and/or the presence of β-adrenergic stimulation. In particular, our studies indicate that CNP, which can be secreted by cardiac endothelial cells or fibroblasts, can increase cardiac SR calcium uptake and release in a cGMP/PKG I-mediated fashion, and thereby stimulate cardiac contractile and relaxing functions.
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Melanie Voβ. This work was supported by the Bundesministerium für Bildung und Forschung (BMBF 01EC9801) and the Deutsche Forschungsgemeinschaft (KU-1037/3-2, SFB 556, WO-552/2-2 and SFB 355).
Abbreviations
- ANP
atrial natriuretic peptide
- BNP
B-type natriuretic peptide
- cAMP, adenosine 3′
5′-cyclic monophosphate
- cGMP
guanosine 3′,5′-cyclic monophosphate
- CNP
C-type natriuretic peptide
- GC-A
guanylyl cyclase A
- GC-B
guanylyl cyclase B
- HEPES
4,(2-hydroxyethyl)-1-piperazine-ethanesulfonic acid)
- Indo-1/AM
Indo-1 acetoxymethyl ester
- KH buffer
Krebs–Henseleit buffer
- NO
nitric oxide
- 8-pCPT-cGMP
8-para-chlorophenylthio-cGMP
- PDE
phosphodiesterase
- PKA
cAMP-dependent protein kinase
- PKG I
cGMP-dependent protein kinase type I
- PLB
phospholamban
References
- ANAND-SRIVASTAVA M.B., TRACHTE G.J. Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol. Rev. 1993;45:455–497. [PubMed] [Google Scholar]
- BAROUCH L.A., HARRISON R.W., SKAF M.W., ROSAS G.O., CAPPOLA T.P., KOBEISSI Z.A., HOBAI I.A., LEMMON C.A., BURNETT A.L., O'ROURKE B., RODRIGUEZ E.R., HUANG P.L., LIMA J.A., BERKOWITZ D.E., HARE J.M. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–340. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- BEAULIEU P., CARDINAL R., PAGÉ P., FRANCOEUR F., TREMBLAY J., LAMBERT C. Positive chronotropic and inotropic effects of C-type natriuretic peptide in dogs. Am. J. Physiol. 1997;273:H1933–H1940. doi: 10.1152/ajpheart.1997.273.4.H1933. [DOI] [PubMed] [Google Scholar]
- BRUSQ J.-M., MAYOUX E., GUIGUI L., KIRILOVSKY J. Effects of C-type natriuretic peptide on rat cardiac contractility. Br. J. Pharmacol. 1999;128:206–212. doi: 10.1038/sj.bjp.0702766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTT E., NOLTE C., SCHULZ S., BELTMAN J., BEAVO J.A., JASTORFF B., WALTER U. Analysis of the functional role of cGMP-dependent protein kinase in intact human platelets using a specific activator 8-para-chlorophenylthio-cGMP. Biochem. Pharmacol. 1992;43:2591–2600. doi: 10.1016/0006-2952(92)90148-c. [DOI] [PubMed] [Google Scholar]
- CALDERONE A., THAIK C.M., TAKAHASHI N., CHANG D.L., COLUCCI W.S. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J. Clin. Invest. 1998;101:812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUHAN S.D., NILSSON H., AHLUWALIA A., HOBBS A.J. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE BOLD A.J., MA K.K., ZHANG Y., DE BOLD M.L., BENSIMON M., KHOSHBATEN A. The physiological and pathophysiological modulation of the endocrine function of the heart. Can. J. Physiol. Pharmacol. 2001;79:705–714. [PubMed] [Google Scholar]
- DOYLE D.D., AMBLER S.K., UPSHAW-EARLEY J., BASTWROUS A., GOING G.E., PAGE E. Type B atrial natriuretic peptide receptor in cardiac myocyte caveolae. Circ. Res. 1997;81:86–91. doi: 10.1161/01.res.81.1.86. [DOI] [PubMed] [Google Scholar]
- DREWETT J.G., GARBERS D.L. The family of guanylyl cyclase receptors and their ligands. Endocrine Rev. 1994;13:135–162. doi: 10.1210/edrv-15-2-135. [DOI] [PubMed] [Google Scholar]
- GARBERS D.L. The guanylyl cyclase receptors. Methods. 1999;19:477–484. doi: 10.1006/meth.1999.0890. [DOI] [PubMed] [Google Scholar]
- GULICK J., SUBRAMANIAM A., NEUMANN J., ROBBINS J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J. Biol. Chem. 1991;266:9180–9185. [PubMed] [Google Scholar]
- HIROSE M., FURUKAWA Y., KUROGOUCHI F., NAKAJIMA K., MIYASHITA Y., CHIBA S. C-Type natriuretic peptide increases myocardial contractility and sinus rate mediated by guanylyl cyclase-linked natriuretic peptide receptors in isolated, blood-perfused dog heart preparations. J. Pharmacol. Exp. Ther. 1998;28:70–76. [PubMed] [Google Scholar]
- HOLTWICK R., VAN EICKELS M., SKRYABIN B.V., BABA H.A., BUBIKAT A., BEGROW F., SCHNEIDER M.D., GARBERS D.L., KUHN M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J. Clin. Invest. 2003;111:1399–1407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIO T., TOKUDOME T., MAKI T., YOSHIHARA F., SUGA S.-I., NISHIKIMI T., KOJIMA M., KAWANO Y., KANGAWA K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144:2279–2284. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- HUGGINS J.P., COOK E.A., PIGGOTT J.R., MATTINSLEY T.J., ENGLAND P.J. Phospholamban is a good substrate for cyclic GMP-dependent protein kinase in vitro, but not in intact cardiac or smooth muscle. Biochem. J. 1989;260:829–835. doi: 10.1042/bj2600829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAYE D.M., WIVIOTT S.D., KELLY R. Activation of nitric oxide synthase (NOS3) by mechanical activity alters contractile activity in a Ca2+-independent manner in cardiac myocytes: role of troponin I phosphorylation. Biochem. Biophys. Res. Commun. 1999;256:398–403. doi: 10.1006/bbrc.1999.0346. [DOI] [PubMed] [Google Scholar]
- KIRCHHEFER U., NEUMANN J., BABA H.A., BEGROW F., KOBAYASHI Y.M., REINKE U., SCHMITZ W., JONES L.R. Cardiac hypertrophy and impaired relaxation in transgenic mice overexpressing triadin 1. J. Biol. Chem. 2001;276:4142–4149. doi: 10.1074/jbc.M006443200. [DOI] [PubMed] [Google Scholar]
- KOLLER A., SCHLOSSMANN J., ASHMAN K., UTTENWEILER-JOSEPH S., RUTH P., HOFMANN F. Association of phospholamban with a cGMP kinase signaling complex. Biochem. Biophys. Res. Commun. 2003;300:155–160. doi: 10.1016/s0006-291x(02)02799-7. [DOI] [PubMed] [Google Scholar]
- KUHN M., HOLTWICK R., BABA H.A., PERRIARD J.-C., SCHMITZ W., EHLER E. Progressive cardiac hypertrophy and dysfunction in atrial natriuretic peptide receptor (GC-A) deficient mice. Heart. 2002;87:368–374. doi: 10.1136/heart.87.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAYLAND J., LI J.-M., SHAH A.M. Role of cyclic GMP-dependent protein kinase in the contractile respose to exogenous nitric oxide in rat cardiac myocytes. J. Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI M., CORNEA R.L., AUTRY J.M., JONES L.R., THOMAS D.D. Phosphorylation-induced structural change in phospholamban and its mutants, detected by intrinsic fluorescence. Biochemistry. 1998;37:7869–7877. doi: 10.1021/bi9801053. [DOI] [PubMed] [Google Scholar]
- LIN X., HÄNZE J., HEESE F., SODMANN R., LANG R.E. Gene expression of natriuretic peptide receptors in myocardial cells. Circ. Res. 1995;77:750–758. doi: 10.1161/01.res.77.4.750. [DOI] [PubMed] [Google Scholar]
- LOHMANN S.M., FISCHMEISTER R., WALTER U. Signal transduction by cGMP in the heart. Basic Res. Cardiol. 1991;86:503–514. doi: 10.1007/BF02190700. [DOI] [PubMed] [Google Scholar]
- MARKERT T., VAANDRAGER A.B., GAMBARYAN S., POHLER D., HAUSLER C., WALTER U., DE JONGE H.R., JARCHAU T., LOHMANN S.M. Endogenous expression of type II cGMP-dependent protein kinase mRNA and protein in rat intestine. Implications for cystic fibrosis transmembrane conductance regulator. J. Clin. Invest. 1995;96:822–830. doi: 10.1172/JCI118128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÉRY P.F., LOHMANN S.M., WALTER U., FISCHMEISTER R. Ca2+ current is regulated by cyclic GMP-dependent protein kinase in mammalian cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 1991;88:1197–1201. doi: 10.1073/pnas.88.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÉRY P.F., PAVOINE C., BELHASSEN L., PECKER F., FISCHMEISTER R. Nitric oxide regulates Ca2+ current: involvement of cGMP-inhibited and cGMP-stimulated phosphodiesterases through guanylyl cyclase activation. J. Biol. Chem. 1993;268:26286–26295. [PubMed] [Google Scholar]
- OXHORN B.C., HIRZEL D.J., BUXTON I.L. Isolation and characterization of large numbers of endothelial cells for studies of cell signaling. Microvasc. Res. 2002;64:302–315. doi: 10.1006/mvre.2002.2436. [DOI] [PubMed] [Google Scholar]
- PFITZER G., RÜEGG J.C., FLOCKERZI V., HOFMANN F. cGMP-dependent protein kinase decreases calcium sensitivity of skinned cardiac fibres. FEBS Lett. 1982;149:171–175. doi: 10.1016/0014-5793(82)81095-8. [DOI] [PubMed] [Google Scholar]
- PIERKES M., GAMBARYAN S., BOKNÍK P., LOHMANN S.M., SCHMITZ W., POTTHAST R., HOLTWICK R., KUHN M. Increased effects of C-type natriuretic peptide on cardiac contractility in guanylyl cyclase A-deficient mice. Cardiovasc. Res. 2002;53:852–861. doi: 10.1016/s0008-6363(01)00543-0. [DOI] [PubMed] [Google Scholar]
- RAEYMAEKERS L., HOFMANN F., CASTEELS R. Cyclic GMP-dependent protein kinase phosphorylates phospholamban in isolated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem. J. 1988;252:269–273. doi: 10.1042/bj2520269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUIZ-VELASCO V., ZHONG J., HUME J.R., KEEF K.D. Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein. Circ. Res. 1998;82:557–565. doi: 10.1161/01.res.82.5.557. [DOI] [PubMed] [Google Scholar]
- SABINE B., WILLENBROCK R., HAASE H., KARCZEWSKI P., WALLUKAT G., DIETZ R., KRAUSE E.G. Cyclic GMP-mediated phospholamban phosphorylation in intact cardiomyocytes. Biochem. Biophys. Res. Commun. 1995;214:75–80. doi: 10.1006/bbrc.1995.2258. [DOI] [PubMed] [Google Scholar]
- SARCEVIC B., BROOKES V., MARTIN T.J., KEMP B.E., ROBINSON P.J. Atrial natriuretic peptide-dependent phosphorylation of smooth muscle cell particulate fraction proteins is mediated by cGMP-dependent protein kinase. J. Biol. Chem. 1989;264:20648–20654. [PubMed] [Google Scholar]
- SAUSBIER M., SCHUBERT R., VOIGT V., HIRNEISS C., PFEIFER A., KORTH M., KLEPPISCH T., RUTH P., HOFMANN F. Mechanisms of NO/cGMP-dependent vasorelaxation. Circ. Res. 2000;87:825–830. doi: 10.1161/01.res.87.9.825. [DOI] [PubMed] [Google Scholar]
- SHAH A.M., SPURGEON H.A., SOLLOTT S.J., TALO A., LAKATTA E.G. 8-Bromo-cGMP reduces the myofilament response to Ca2+ in intact cardiac myocytes. Circ. Res. 1994;74:970–978. doi: 10.1161/01.res.74.5.970. [DOI] [PubMed] [Google Scholar]
- SUGA S., NAKAO K., ITOH H., KOMATSU Y., OGAWA Y., HAMA N., IMURA H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor β. J. Clin. Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMII K., SPERELAKIS N. cGMP-dependent protein kinase regulation of the L-type Ca2+ current in rat ventricular myocytes. Circ. Res. 1995;77:803–812. doi: 10.1161/01.res.77.4.803. [DOI] [PubMed] [Google Scholar]
- TAMURA N., ITOH H., OGAWA Y., NAKAGAWA O., HARADA M., CHUN T.H., SUGA S., YOSHIMASA T., NAKAO K. cDNA cloning and gene expression of human type I alpha cGMP-dependent protein kinase. Hypertension. 1996;27:552–557. doi: 10.1161/01.hyp.27.3.552. [DOI] [PubMed] [Google Scholar]
- TAMURA N., OGAWA Y., CHUSHO H., NAKAMURA K., NAKAO K., SUDA M., KASAHARA M., HASHIMOTO R., KATSUURA G., MUKOYAMA M., ITOH H., SAITO Y., TANAKA I., OTANI H., KATSUKI M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDECASTEELE G., VERDE I., RÜCKER-MARTIN C., DONZEAU-GOUGE P., FISCHMEISTER R. Cyclic GMP regulation of the L-type Ca2+ channel current in human atrial myocytes. J. Physiol. 2001;533:329–340. doi: 10.1111/j.1469-7793.2001.0329a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEGENER J.W., NAWRATH H., WOLFSGRUBER W., KÜHBANDNER S., WERNER C., HOFMANN F., FEIL R. cGMP-dependent protein kinase I mediates the negative inotropic effect of cGMP in the murine myocardium. Circ. Res. 2002;90:18–20. doi: 10.1161/hh0102.103222. [DOI] [PubMed] [Google Scholar]
- WOLLERT K.C., FIEDLER B., GAMBARYAN S., SMOLENSKI A., HEINEKE J., BUTT E., TRAUTWEIN C., LOHMANN S.M., DREXLER H. Gene transfer of cGMP-dependent protein kinase I enhances the antihypertrophic effects of nitric oxide in cardiomyocytes. Hypertension. 2002;39:87–92. doi: 10.1161/hy1201.097292. [DOI] [PubMed] [Google Scholar]