Abstract
The effects of mefenamic acid on both membrane potential and K+ currents in pig urethral myocytes were investigated using patch-clamp techniques (conventional whole-cell, cell-attached, outside-out and inside-out configuration).
In the current-clamp mode, mefenamic acid caused a concentration-dependent hyperpolarization, which was inhibited by preapplication of 1 μM glibenclamide. In the voltage-clamp mode, mefenamic acid induced an outward current that was blocked by glibenclamide even in the presence of iberiotoxin (IbTX, 300 nM) at −50 mV.
ATP-sensitive K+ channels (KATP channels) could be activated in the same patch by mefenamic acid and levcromakalim, with the same unitary amplitude and the similar opening gating at −50 mV in cell-attached configuration.
In outside-out recording, external application of mefenamic acid activated intracellular Ca2+-activated IbTX-sensitive large-conductance K+ channels (BKCa channels).
Mefenamic acid (⩽30 μM) activated spontaneous transient outward currents (STOCs). In contrast, mefenamic acid (⩾100 μM) increased sustained outward currents, diminishing the activity of STOCs.
Over the whole voltage range, mefenamic acid caused opposite effects on the membrane currents in the absence and presence of 5 μM glibenclamide. In the presence of 10 mM 4-aminopyridine (4-AP), mefenamic acid only increased the outward currents.
These results indicate that mefenamic acid increases the channel activities of two distinct types of K+ channels (i.e. BKCa channels and KATP channels) and decreased 4-AP-sensitive K+ channels in pig urethral myocytes.
Keywords: ATP-sensitive K+ channels, KATP channel openers, BKCa channels, mefenamic acid, urethral myocytes
Introduction
Fenamates (mefenamic acid, flufenamic acid, niflumic acid, etc.) are often used clinically as nonsteroidal anti-inflammatory drugs. Mefenamic acid is one of the most commonly used fenamates, not only as an anti-inflammatory agent but also in the therapy of patent ductus arteriosus in preterm infants. Fenamates are known to affect various types of membrane channels. They inhibit nonselective cation channels (Gögelein et al., 1990; Jung et al., 1992; Siemer & Gögelein, 1992; Chen et al., 1993) and Cl− conductance (Bertrand & Galligan, 1994; Busch et al., 1994). Fenamates have emerged as potent activators of large-conductance K+ channels in the corneal epithelium (Rae & Farrugia, 1992). On the other hand, fenamates inhibit human neural voltage-gated K+ channels (hKv, Lee & Wang, 1999). In smooth muscle, mefenamic acid has been shown to activate Ca2+-independent delayed rectified K+ currents (canine jejunum, Farrugia et al., 1992; 1993), large-conductance Ca2+-activated K+ channels (pig coronary artery, Ottolia & Toro, 1994) and α1-adrenoceptor-activated nonselective cation currents (rabbit portal vein, Yamada et al., 1996). Additionally, in rabbit portal vein, Greenwood & Large (1995) reported that mefenamic acid inhibits spontaneous transient inward currents (STICs) and spontaneous transient outward currents (STOCs) in a concentration-dependent manner, and that noisy tetraethylammonium (TEA+)-sensitive outward currents were induced by the application of higher concentrations of fenamates (⩾100 μM). Despite its frequent use, the channels targeted by mefenamic acid, especially around the resting membrane potential, still remain to be directly identified in freshly dispersed smooth muscle cells by the use of single-channel recordings.
In this paper, we describe the use of single-channel recordings to demonstrate the presence of two distinct types of K+ channels, namely, an intracellular Ca2+-activated large-conductance K+ channel (BKCa channel) and a glibenclamide-sensitive 43 pS K+ channel (KATP channel) in pig proximal urethral smooth muscle cells. We investigated the effects of mefenamic acid not only on these two distinct K+ channels but also on STOCs. Furthermore, we studied the pharmacological properties of the mefenamic acid-sensitive membrane currents.
Methods
Cell preparation and recording procedure
Fresh female pig urethra with attached urinary bladder was collected from a local abattoir. The smooth muscle was dissected from the proximal urethra, 1–2 cm distal to the bladder neck. We employed the cell dispersion method (the gentle tapping method) previously described (Teramoto et al., 2000; 2001a, 2001b). The dispersed cells were normally used within 4–5 h for experiments and were stored at 4–6°C. The set-up of the patch-clamp experimental system used was essentially the same as described previously (Teramoto et al., 2000; 2001a, 2001b). All experiments were performed at room temperature (21–23°C).
Solutions and drugs
For whole-cell recordings, the ionic composition of the physiological salt solution (PSS) in the bath was (mM): Na+ 140, K+ 5, Mg2+ 1.2, Ca2+ 2, Cl− 151.4, glucose 10, HEPES 10, and was titrated to pH 7.35–7.40 with Tris base. High K+ pipette solution contained (mM): K+ 140, Cl− 140, glucose 5, EGTA 5 and HEPES 10/Tris (pH 7.35–7.40). The perforated-patch technique with nystatin was also occasionally used to record whole-cell currents (Teramoto & Brading, 1996). In short, nystatin was freshly dissolved in acidified methanol (1 N HCl to about pH 2) and the pH was adjusted to 7.4 with Tris base. This stock solution (10 mg ml−1) was diluted in the pipette solution at a final concentration of 50 μg ml−1 just before use. Whole-cell recording was performed with a pipette which was first dipped in normal pipette solution (nystatin-free) and then back-filled with nystatin-containing pipette solution. This resulted in chemical perforation of the membrane. In single-channel recordings, the composition of the bath solution was the same as the pipette solution in symmetrical 140 mM K+ conditions. For recording BKCa channels in outside-out patches, the pipette solution contained (mM): K+ 140, Ca2+ 0.75, Cl− 141.5, EGTA 5, glucose 5, HEPES 10/Tris (pH 7.35–7.40), estimating the free Ca2+ concentration ([Ca2+]) as approximately 60 nM, and the bath solution was PSS, 60 mM K+ solution, or 140 mM K+ solution (140 mM K+ solution was obtained by replacing 135 mM Na+ with equimolar K+ in PSS). To test the intracellular Ca2+ sensitivity of BKCa channels in inside-out patches, the pipette solution was 140 mM K+ solution and the bath solution contained initially (mM): K+ 140, Cl− 140, EGTA 5, glucose 5, HEPES 10/Tris (pH 7.35–7.40), with increasing free [Ca2+]. The concentrations of free ATP and free [Ca2+] were calculated by use of the commercial software ‘EQCAL' (Biosoft, Cambridge, U.K.). Cells were allowed to settle in the small experimental chamber (80 μl in volume). The bath solution was superfused by gravity throughout the experiments at a rate of 2 ml min−1. All chemicals were purchased from Sigma (Sigma Chemical K.K., Tokyo, Japan). The stock solution of mefenamic acid (500 mM) was made daily by dissolving it in dimethylsulphoxide (DMSO). This was diluted in the bath solution (final concentration, 500 μM), and was further ultrasonicated for about 15–25 min until it was dissolved (ultrasonicator, Bransonic, Branson Ultrasonic Corporation, U.S.A.). Both levcromakalim (kindly provided by SmithKline Beecham Pharmaceuticals, Harlow, U.K.) and glibenclamide were prepared daily as 500 mM stock solutions in DMSO. The final concentration of DMSO was less than 0.5%, not affecting membrane currents. Note that the pH of the bath solution was readjusted after addition of 4-AP with Tris (pH 7.35–7.40).
Data analysis
The whole-cell current data were low-pass filtered at 500 Hz (−3 dB) by an eight-pole Bessel filter (3611 multifunction filter, NF Electronic Instruments, Yokohama, Japan), sampled at 25 ms and analysed on a computer (Macintosh Quadra 610, Apple Computer U.K. Ltd, Uxbridge, U.K.), using the commercial software ‘Mac Lab 3.4.4' (ADInstruments Pty Ltd, Castle Hill, Australia). For the analysis of single-channel recordings, the stored data were low-pass filtered at 2 kHz (−3 dB) and sampled into the computer at an interval of 80 μs, using the ‘PAT' program (kindly provided by Dr Dempster, University of Strathclyde, U.K.). Single-channel events were detected using a half-amplitude criterion (Colquhoun & Sigworth, 1995) and inspected manually. Since we could not determine the total number of functioning channels in each patch, we only examined opening kinetics in the present studies (Teramoto et al., 1999). Open lifetime distribution of a 30 s recording was log-binned using the method of McManus et al. (1987). The conditional probability density function was fitted to the open lifetime distribution by the method of nonlinear regression fitting (Sigworth & Sine, 1987). However, events shorter than 200 μs were not included in the evaluation and no correction was made for missed events. The channel activity was calculated using the following equation from an all-point amplitude histogram and expressed as an NPo value (number of channels (N) × open-state probability (Po)).
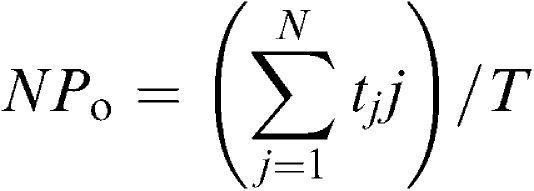 |
where tj is the time spent at each current level corresponding to j=0, 1, 2,…, N, and T is the duration of the recording (2 min). The all-point amplitude histogram was obtained from a continuous recording of 2 min and fitted with the Gaussian equation using a least-squares fitting. Continuous traces in the figures were obtained from records filtered at 500 Hz for presentation. However, the inset traces in both Figure 3b (expanded figures) are original traces (2 kHz filtration).
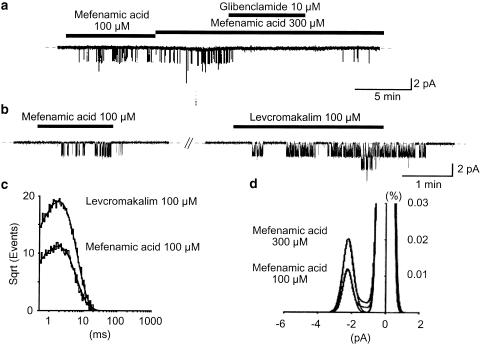
Figure 3.
Effects of mefenamic acid and levcromakalim on the same cell-attached patches in symmetrical 140 mM K+ conditions at −50 mV. Note that mefenamic acid, levcromakalim and glibenclamide were applied in the bath solution. (a)When mefenamic acid was applied in the bath, a small channel (2.14 pA at −50 mV) started to activate. Glibenclamide (10 μM) reversibly inhibited this channel. The dashed line indicates the current base line when the channel is not open. (b) The 2.14 pA channel was activated by mefenamic acid (100 μM) and 100 μM levcromakalim. Levcromakalim (100 μM) was applied 5 min after recovery from mefenamic acid. The dashed line indicates the current base line where the channel is not open. (c) The open lifetime in the presence of 100 μM mefenamic acid and 100 μM levcromakalim at −50 mV. The abscissa shows the logarithmic open lifetime (ms) and the ordinate shows the square root of the number of total events (mefenamic acid, 9382 events; levcromakalim, 16,008 events). The solid curve indicates a single exponential fitting using the least-squares method. The width of the bin was 0.2 ms. The time constant for the mean open lifetime was 1.7 ms (1.7±0.1 ms, n=6, 100 μM mefenamic acid) and 1.8 ms (1.7±0.1 ms, n=6, 100 μM levcromakalim). The data were obtained from 30 s recording and filtered at 2 kHz, with a sampling rate of 80 μs for analysis. (d) All-points amplitude histograms in the presence of 100 and 300 μM mefenamic acid. Continuous lines in the histograms are theoretical curves fitted with a Gaussian distribution function. The abscissa shows the amplitude of the current (pA), and the ordinate shows the percentage value of the probability density function (%).
Statistical analysis
Statistical analyses were performed with the analysis of variance (ANOVA) test (two-factor with replication). Changes were considered significant at P<0.01 (*). Data are expressed as mean with the standard deviation (s.d.).
Results
Effects of mefenamic acid on the resting membrane potential recorded in pig urethra
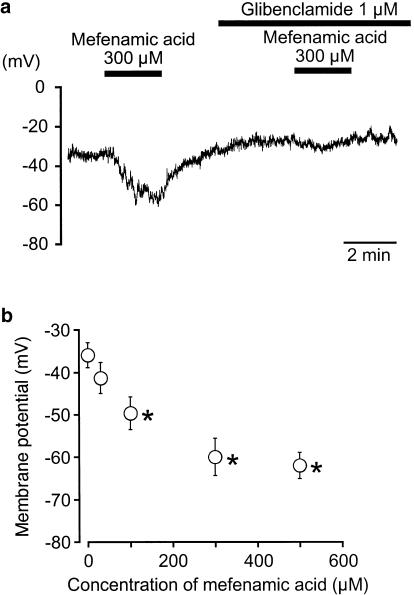
In a conventional whole-cell configuration, current-clamp experiments were performed to investigate the effects of mefenamic acid on the resting membrane potential in dispersed single smooth muscle cells from pig proximal urethra (pipette solution: 140 mM KCl containing 5 mM EGTA; the bath solution: PSS). In Figure 1a, the resting membrane potential in pig proximal urethral myocytes was approximately −36 mV, and the value was relatively stable (−37±5 mV, n=5). Application of 300 μM mefenamic acid caused a significant membrane hyperpolarization, which was inhibited by preapplication of 1 μM glibenclamide (Figure 1a). Reapplication of 300 μM mefenamic acid induced a similar amplitude of hyperpolarization, which was not significantly different from that of the first application (−61±4 mV, n=4). The hyperpolarization induced by mefenamic acid (⩽500 μM) was concentration-dependent (Figure 1b).
Figure 1.
Effects of mefenamic acid on single isolated smooth muscle cells of pig proximal urethra. The bath was superfused by PSS and the pipette solution was 140 mM KCl, containing 5 mM EGTA. (a) Under current-clamp conditions, 300 μM mefenamic acid hyperpolarized the membrane and its action was inhibited by 1 μM glibenclamide. (b) Concentration–response relationships. Control indicates the condition just before the application of mefenamic acid. Each symbol shows the mean of 4–5 observations with s.d. *Significantly different from the control (ANOVA, P<0.01).
Effects of mefenamic acid on KATP currents and KATP channels
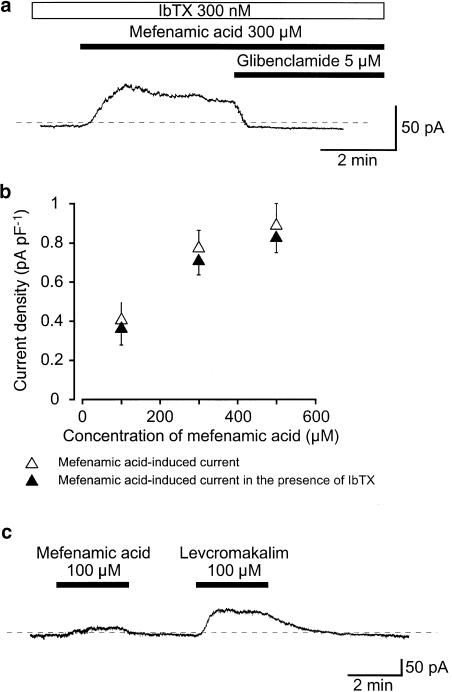
In the voltage-clamp mode, application of 300 μM mefenamic acid caused an outward membrane current that gradually decayed in amplitude at −50 mV. Figure 2a shows that this outward current persisted in the presence of 300 nM iberiotoxin (IbTX), a selective intracellular Ca2+-activated K+ channel (BKCa channel) blocker. IbTX did not significantly affect the amplitude of this outward current (in the presence of IbTX, this was 45±5 pA, n=5 vs 48±5 pA, n=5 in its absence). Similar results were observed with 500 μM mefenamic acid (Figure 2b, n=4). Additional application of 5 μM glibenclamide abolished the mefenamic acid-induced outward current, and slightly reduced the resting current. The peak amplitude of the mefenamic acid-induced outward membrane current increased in a concentration-dependent manner (Figure 2b). To compare the effects of mefenamic acid and levcromakalim, a well-known and potent ATP-sensitive K+ channel (KATP channel) opener, on the membrane currents, both drugs were applied on the same cells. Application of 100 μM mefenamic acid caused a small-amplitude outward current at −50 mV (Figure 2c). On washout, the basal current recovered to the control level. Approximately 2.5 min later, application of 100 μM levcromakalim caused a larger amplitude outward current, with a similar basal noise level at −50 mV to that of mefenamic acid.
Figure 2.
Effects of mefenamic acid on membrane currents in pig urethral myocytes. The bath was superfused by PSS and the pipette solution was 140 mM KCl, containing 5 mM EGTA in a conventional whole-cell recording. (a) Under voltage-clamp conditions, in the presence of bath-applied 300 nM IbTX, 300 μM mefenamic acid caused an outward current at −50 mV. Glibenclamide (5 μM) completely suppressed this current. The dashed line indicates the zero current level. (b) Relationships between the peak amplitude of the mefenamic acid-induced outward currents and the concentration of mefenamic acid in the absence or presence of 300 nM IbTX (the bath) at −50 mV. Each symbol indicates the mean of 5–8 observations with s.d. shown by vertical lines. (c) The comparative effects of 100 μM mefenamic acid and l00 μM levcromakalim on membrane current. The dashed line indicates the zero current level. Extracellular application of 100 μM mefenamic acid caused an outward membrane current at −50 mV. About 2.5 min later, levcromakalim caused a larger amplitude outward current with a similar basal noise level.
Cell-attached recordings were performed to study the channel openings of the smaller amplitude K+ channel in more detail at −50 mV. When the concentration of mefenamic acid was increased in a cumulative manner, the open probability of this 2.1 pA K+ channel was enhanced in a concentration-dependent manner. To determine the channel conductance, the membrane potential was stepped from −90 to 0 mV in 10 mV increments in the presence of 300 μM mefenamic acid, and the amplitude of the mefenamic acid-induced channel was measured at every membrane potential from an all-amplitude histogram fitted by the Gaussian distribution function. The conductance of the channel was 42.9±2.5 pS (n=6). This channel was reversibly suppressed by subsequent application of 10 μM glibenclamide (Figure 3a). We investigated the kinetics of the 2.1 pA K+ channel in order to determine whether or not mefenamic acid activated the same K+ channels as levcromakalim at −50 mV. Burst-like openings were observed when either mefenamic acid or levcromakalim were applied on the same patches, and the mean open lifetime of the channel calculated. Figure 3b shows the effect of sequential application of mefenamic acid and levcromakalim (100 μM) to the same membrane patch. Levcromakalim was applied after the mefenamic acid-induced K+ channel activity had disappeared for about 6 min. Both drugs activated the same amplitude K+ channel (n=6). The mean open lifetime in each condition was 1.7±0.1 ms (100 μM mefenamic acid, n=6) and 1.7±0.1 ms (100 μM levcromakalim, n=6), respectively, showing quite similar channel-opening kinetics (Figure 3c).
Effects of mefenamic acid on BKCa currents and channels
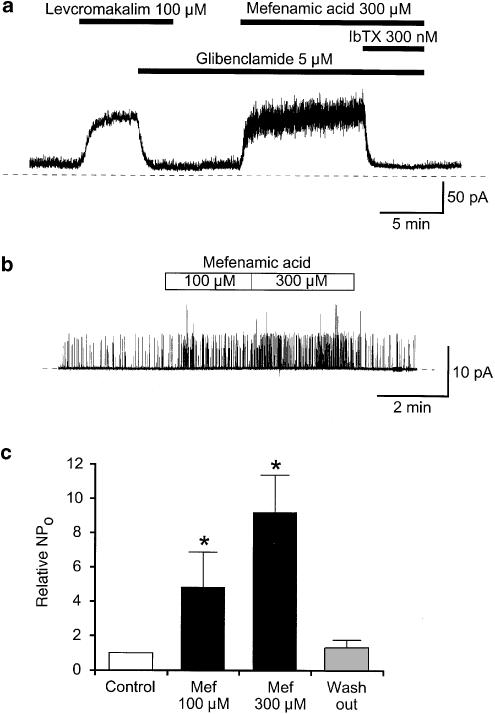
To investigate the effects of mefenamic acid on the membrane current further, experiments were performed at a holding potential of 0 mV (Figure 4a). After demonstrating the presence of a levcromakalim-induced membrane current, this was inhibited by additional application of glibenclamide (5 μM), after which application of 300 μM mefenamic acid caused an outward membrane current, which was exceedingly noisy when compared to the levcromakalim-induced outward current. The additional application of IbTX (300 nM) completely suppressed the mefenamic acid-induced noisy outward current at 0 mV.
Figure 4.
Effects of mefenamic acid on BKCa currents and BKCa channels in an outside-out patch. (a) At 0 mV, 100 μM levcromakalim caused an outward current that was blocked by 5 μM glibenclamide. In the presence of 5 μM glibenclamide, a much noisier outward current was evoked by the additional application of 300 μM mefenamic acid and was abolished by the extracellular application of 300 nM IbTX. (b) Extracellular application of mefenamic acid reversibly activated channel openings in a concentration-dependent manner at +20 mV. The pipette solution was 140 mM KCl containing a low free Ca2+ (< 10 nM). The bath solution was 5 mM K+ PSS. The dashed line indicates the current base line, where the channel is not open. (c) Mefenamic acid increased the relative NPo value of BKCa channel in a concentration-dependent manner (100 μM, 4.8±2.0, n=5; 300 μM, 9.2±2.2, n=5) when the NPo value in control was normalized as 1.0 (control, open column) in every patch (n=5). After washing out mefenamic aid, the NPo value recovered to near control level (grey-filled column, 1.32±0.4, n=5). The NPo value in each condition was obtained over 2 min recording.
Single-channel recordings were performed using the excised membrane patch (outside-out configuration) at +20 mV (140/5 mM K+ conditions, Figure 4b). It was found that BKCa channel sometimes opened even at a low free [Ca2+]i (<10 nM), as shown in Figure 4b. Extracellular application of mefenamic acid reversibly enhanced the activity of the channel in a concentration-dependent manner (Figure 4b, c, n=5). When the control NPo value of BKCa channel was normalized as 1.0 (n=5), external application of 300 nM IbTX caused a marked inhibition of the channel openings (0.007±1.4 × 10−5, n=5) in outside-out configuration at +30 mV (5 mM K+ PSS, bath/140 mM K+). On removal of IbTX, the channel activated again although it took 2–3 min to wash out the blocking effect even at 2 ml min−1 bath perfusion rate and recovery was not complete (0.61±0.1, n=5).
Effects of mefenamic acid on STOCs recorded using nystatin-perforated patches
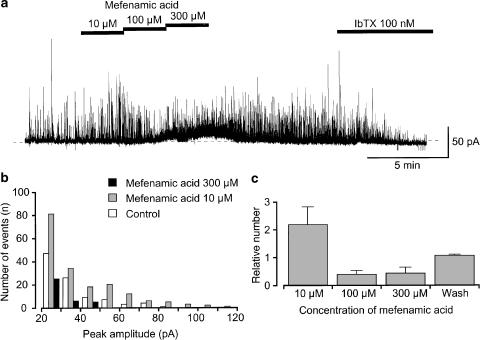
After establishment of the nystatin-perforated patch, spontaneous transient outward currents (STOCs) were observed at a holding membrane potential of −20 mV even in the presence of 5 μM glibenclamide (bath solution, PSS solution; pipette solution, 140 mM KCl solution containing 5 mM EGTA, Figure 5a). Application of 10 μM mefenamic acid enhanced STOCs, increasing the frequency (Figure 5b) and mean amplitude (Figure 5c). Subsequently, application of higher concentrations of mefenamic acid (100–300 μM) suppressed STOCs (Figure 5b, c) although a noisy outward current was induced. On removal of mefenamic acid, the mefenamic acid-induced noisy outward current gradually diminished and STOCs returned. STOCs were abolished by additional application of IbTX (100 nM) at −20 mV.
Figure 5.
Nystatin-perforated recording of the effects of mefenamic acid (10–300 μM) on the activity of STOCs at –20 mV in the presence of 5 μM glibenclamide. The bath solution was 5 mM K+ PSS, and the pipette solution was 140 mM KCl containing 5 mM EGTA. Note that 5 μM glibenclamide was present in the bath solution throughout the experimental protocol. (a) Current trace of STOCs in the absence and presence of mefenamic acid. Subsequent application of IbTX (100 nM) abolished STOCs. The dashed line indicates the zero current level. (b) Event histograms of STOCs were obtained from the results in (a) in the absence and presence of mefenamic acid (10 μM and 300 μM). The abscissa shows the amplitude of STOCs (pA) and the ordinate shows the number of total events of STOCs for a 2 min recording. Note that amplitudes of STOCs less than 20 pA were omitted from the evaluation. (c) Relative number of STOCs in each concentration of mefenamic acid. By normalizing the total number of STOCs for the 2 min recording just before application of mefenamic acid (control) as one, relative numbers of STOCs were obtained in each concentration of mefenamic acid. Each column indicates the mean of four observations with s.d. Note that amplitudes of STOCs less than 20 pA were omitted from the evaluation.
Effects of mefenamic acid on the membrane current in pig urethra
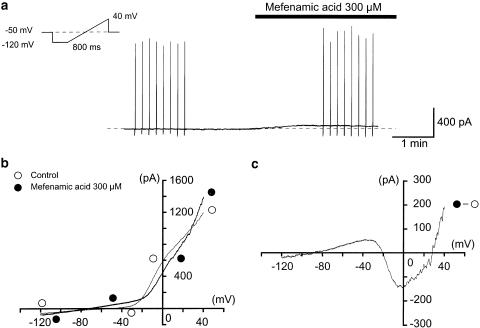
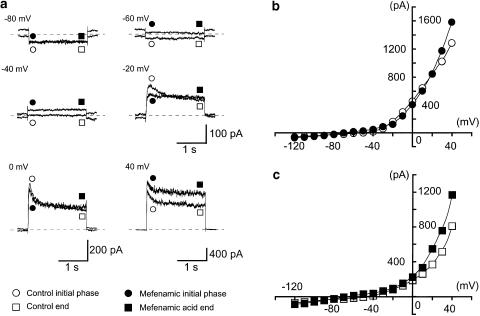
Figure 6a shows the overview of the experimental protocol. Applying 300 μM mefenamic acid in the bath, an outward membrane current was evoked. In order to obtain the current–voltage relationships, a ramp pulse (see the inset in Figure 6a) was applied in the absence and presence of mefenamic acid at −50 mV under quasi-physiological K+ conditions. Averaged currents from eight ramp pulses were obtained in each condition (Figure 6b). In the absence of mefenamic acid, the mean ramp membrane current changed sign at about −46 mV, showing an outward rectification. Mefenamic acid enhanced the amplitude of the ramp membrane currents (i.e. agonist action) in two voltage regions (from −120 to −20 mV and from +30 to +40 mV). However, in the voltage range from −20 to +30 mV, the current was reduced (i.e. antagonistic action) by mefenamic acid. The net membrane current induced by mefenamic acid was obtained by subtraction of the two ramp membrane currents before and during the application of mefenamic acid (Figure 6c). The membrane potentials of the mefenamic acid-induced current changed sign at −85±2, −21±2 and 28±2 mV (n=5). Using the same cell, when rectangular voltage step pulses (10 mV increment from −120 to +40 mV for 2 s duration, every 10 s) were applied in the absence or presence of 300 μM mefenamic acid, the dual effect was also observed (Figure 7). The peak amplitude of the membrane current during the initial 500 ms pulse duration was suppressed from −20 mV to +20 mV (antagonist action). On the other hand, the average current amplitude during the last 50 ms of the voltage step was enhanced by mefenamic acid (agonistic action) for the whole range of membrane potentials. Similar results were obtained in five other cells. In the presence of 10 μM glibenclamide, a similar dual action of mefenamic acid was observed (n=5).
Figure 6.
Effects of 300 μM mefenamic acid on the membrane current recorded from dispersed urethral smooth muscle cells at −50 mV. A conventional whole-cell record performed with 140 mM K+ containing 5 mM EGTA in the pipette solution. The holding membrane potential was −50 mV. The bath solution was 5 mM K+ PSS. (a) Effects of 300 μM mefenamic acid on the membrane current. The vertical deflections indicate ramp potential pulses (see inset). The dashed line indicates the zero current level. (b) Mean ramp currents induced by the eight ramp potential pulses from −120 to +40 mV for 800 ms, following a 300 ms conditioning pulse (−120 mV) applied every 10 s before and during the application of 300 μM mefenamic acid. In the absence of mefenamic acid (open circles), the membrane exhibited outward rectification. (c) Net membrane current evoked by mefenamic acid (300 μM). Net membrane current was obtained by subtraction of the two ramp membrane currents (shown in (b)) recorded before and during the application of 300 μM mefenamic acid, demonstrating a dual (agonistic and antagonistic) action.
Figure 7.
Effects of mefenamic acid (300 μM) on the membrane current evoked by application of rectangular pulses (duration 2 s) at 10 s intervals in conventional whole-cell configuration. The holding potential was −50 mV, the bath contained 5 mM K+ PSS, and the pipette solution was 140 mM KCl containing 5 mM EGTA. Data were obtained from the same cell as in Figure 6. (a) Current traces in the absence and presence of 300 μM mefenamic acid at the indicated membrane potentials. (b, c) Current-voltage relationships obtained in the absence (open circles) or presence (filled circles) of 300 μM mefenamic acid. The peak amplitude of the membrane current was measured as the peak value (b) within the initial 500 ms of the command pulses (from −120 to +40 mV). The end value (c) was measured as the mean amplitude within the last 50 ms of the rectangular pulses. The lines were drawn by eye.
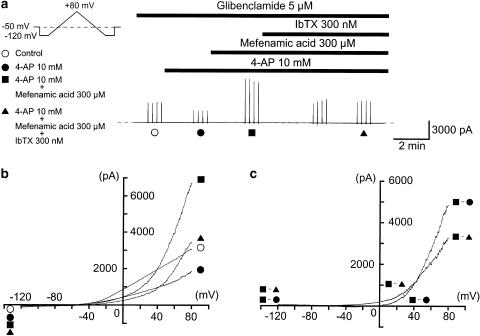
In order to characterize further the pharmacological properties of the mefenamic acid-sensitive membrane currents, 10 mM 4-aminopyridine (4-AP) was applied before application of 300 μM mefenamic acid. Figure 8a shows the overview of the experimental protocol at a holding potential of −50 mV when 5 μM glibenclamide was present throughout the experiments. Application of 4-AP reduced the membrane current. The current–voltage relationships were obtained by applying four ramp pulses (from −120 to +80 mV for 1 s duration, see inset in Figure 8a) every 15 s at −50 mV. Figure 8b shows the averaged membrane currents during the falling phase of the ramp pulses under various conditions of the experiment. The mefenamic acid-induced membrane current in the presence of 4-AP and glibenclamide was obtained by subtracting the mean membrane current in the presence of 300 μM mefenamic acid from the membrane current in its absence, demonstrating an outwardly rectifying property (Figure 8c, n=4). Additional application of IbTX further reduced the membrane currents.
Figure 8.
The effects of 300 μM mefenamic acid on the membrane currents in the presence of 10 mM 4-AP and 5 μM glibenclamide. The holding potential was −50 mV, the bath contained 5 mM K+ PSS, and the pipette solution was 140 mM KCl containing 5 mM EGTA. Note that 5 μM glibenclamide was present in the bath solution throughout the experimental protocol. (a) The vertical deflections indicate ramp potential pulses (see the inset). The dashed line indicates the zero current level. (b) Current–voltage curves measured from the negative-going limb (the falling phase) of the ramp pulse. Each symbol is the same as in the current trace (a). The lines are mean membrane currents from the four ramps in each condition. (c) Net membrane currents. The mefenamic acid-induced membrane current was obtained by subtraction of the mean ramp current recorded before, from that during application of 300 μM mefenamic acid in the presence of 10 mM 4-AP. The IbTX-sensitive membrane current was obtained by subtraction of the membrane currents in the absence and presence of IbTX when 300 μM mefenamic acid was present in the bath solution.
Discussion
In the present experiments in pig urethral myocytes, we have been able to demonstrate that mefenamic acid activates BKCa channels and KATP channels, and inhibits 4-AP-sensitive K+ currents and STOCs.
Effects of mefenamic acid on K+ channels
The first report of the effects of mefenamic acid on K+ current was by Farrugia et al. (1993), who showed that mefenamic acid increased the amplitude of delayed-rectifier K+ currents in canine jejunal smooth muscle, but the effects of glibenclamide were not reported. Greenwood & Large (1995) showed that fenamates induced outward currents in the rabbit portal vein, and concluded that they were Ca2+-activated K+ currents, since 10 μM glibenclamide did not affect them, although they were blocked by 1 mM TEACl at 0 mV. Thus, the target K+ channels for mefenamic acid in smooth muscle have been thought to be Ca2+-activated K+ channels although until now there have been no direct observations (such as single-channel recordings, efflux K+ studies, etc.). However, in the present experiments, glibenclamide (1 μM) suppressed the mefenamic acid-induced hyperpolarization and inhibited the mefenamic acid-induced outward current at a concentration of 10 μM. In cell-attached configuration, mefenamic acid (⩾100 μM) also activates KATP channels at −50 mV and levcromakalim activates the same-conductance K+ channels. These results taken together strongly suggest that mefenamic acid activates not only BKCa channels but also KATP channels in pig urethral myocytes, and that the hyperpolarization of the resting membrane potential caused by mefenamic acid is due to an outward current mainly carried by K+.
On the other hand, at potentials more positive than approximately −84 mV (i.e., the theoretical EK in quasi-physiological K+ conditions, 5/140 mM K+), mefenamic acid (300 μM) inhibited the membrane currents in the voltage range from −20 to +30 mV. Similar results were observed on the peak amplitude of the membrane currents (i.e. during the initial 500 ms of the pulse). In the presence of 10 mM 4-AP, mefenamic acid did not reduce the membrane currents. Given this, we suggest that mefenamic acid seems to inhibit the 4-AP- and voltage-sensitive membrane currents. Similarly, Lee & Wang (1999) reported that fenamates inhibited the voltage-dependent K+ channels. The presence of 4-AP- and voltage-sensitive K+ currents has been reported in a wide variety of smooth muscle (Bolton et al., 1999). In the present experiments, we were not able to detect the 4-AP-sensitive K+ channels by use of single-channel recordings, and further studies are needed to cast light upon the properties of 4-AP-sensitive K+ channels in pig urethra.
KATP channel openers and BKCa channel openers in smooth muscle
Quite a few classes of synthesized compounds have been introduced as potent K+ channel activators in smooth muscle. Surprisingly, KATP channel openers were initially reported to enhance the activity of BKCa channels (cromakalim: Gelband et al. (1990); cromakalim and pinacidil: Gelband et al. (1989), Gelband & McCullough (1993); cromakalim and levcromakalim ((−)-cromakalim: Carl et al. (1992)). Although these BKCa channels have an intracellular ATP sensitivity, they are not selectively inhibited by glibenclamide. Furthermore, in recombinant expressed studies, cromakalim had no effect on heterologously expressed BKCa channels (Gribkoff et al., 1996). Thus, it is generally believed that the target K+ channels for cromakalim are unlikely to be BKCa channels.
Recent molecular biological studies have revealed that KATP channels are composed of at least two subunits: an inwardly rectifying K+ channel (Kir) which forms the ion pore, and a sulphonylurea receptor (SUR), a member of the ATP-binding cassette superfamily (reviewed by Quayle et al., 1997). To identify these channels, it is essential to demonstrate that they possess glibenclamide sensitivity. Some K+ channel activators, such as cromakalim, levcromakalim and pinacidil, have been shown to open glibenclamide-sensitive KATP channels (30–50 pS in 140 mM K+ symmetrical conditions) but not BKCa channels in the smooth muscle (reviewed by Edwards & Weston, 1993). On the other hand, selective BKCa channel openers have also been reported in some vascular smooth muscle (NS1619; Holland et al., 1996). In order to avoid confusion regarding the designation of K+ channel activators, we suggest that openers of the glibenclamide-sensitive KATP channel should be referred to as KATP channel openers in smooth muscle, and the compounds that obviously activate BKCa channels as BKCa channel openers. Using these definitions, mefenamic acid, uniquely, is both an KATP channel opener and a BKCa channel opener, and is the only recorded compound which demonstrates such a clearly agonistic action on two distinctly different types of K+ channels which are revealed by single-channel recordings.
Effect of mefenamic acid on STOCs
In smooth muscle, STOCs are thought to be carried by K+, due to the opening of BKCa channels in response to a highly localized cytozolic elevation of Ca2+ released from intracellular Ca2+ stores (Benham & Bolton, 1986). In rabbit portal vein, Greenwood & Large (1995) reported that fenamates (⩾100 μM) inhibited STOCs in a concentration-dependent manner and induced TEA+-sensitive outward currents. However, in the present experiments, we have been able to demonstrate that mefenamic acid possesses a dual effect on STOCs, depending on its concentration. Namely, low concentrations of mefenamic acid (⩽30 μM) enhanced the amplitude and the frequency of STOCs. In contrast, high concentrations of mefenamic acid (⩾100 μM) induced an IbTX-sensitive sustained noisy outward current, associated with a decrease in STOC amplitude in a concentration-dependent manner. We suggest that the stimulatory effects of mefanemic acid on BKCa channels would be stronger than the activation of BKCa channels by cytosolic Ca2+. We suggest another possibility that mefanemic acid may also modulate Ca2+ release from intracellular Ca2+ stores, decreasing the amplitude and the frequency of STOCs. We are not certain whether or not the different effects of mefenamic acid on STOCs in rabbit portal vein and pig urethra are due to different experimental conditions (such as different recording modes, different membrane potentials, etc.), or are just characteristic of the type of smooth muscle cell. Further studies may elucidate the dual action of mefenamic acid on STOCs.
Clinical implication and good clues for synthesizing novel types of KATP channel openers
There is currently considerable interest in the potential of K+ channel openers for the treatment of urinary tract dysfunction (unstable bladder, bladder outflow obstruction, etc., reviewed by Andersson, 1993). Howe et al. (1995) reported that ZD6169, a novel KATP channel opener, has in vivo selectivity for the urinary bladder, without altering either the heart rate or the mean arterial blood pressure. Recently, we have demonstrated that ZD6169 possesses a dual effect on the activity of KATP channels in pig urethra, suggesting that K+ channel openers may possess a tissue selectivity in the clinical field (Teramoto et al., 2001a, 2001b). The classes of K+ channel openers that are currently classified as KATP channel openers are benzopyrans, pyridines, pyrimidines, benzothiadiazine and butenoic acid, although until further data on their mode/site of action are available, arbitrary (Edwards & Weston, 1993). In the present experiments, we indicate that mefenamic acid acts as an KATP channel opener and a BKCa channel opener in pig urethra. This might provide useful information for the synthesis of novel types of K+ channel opener and mefenamic acid may serve as a useful prototype for designing more selective compounds for KATP channels as well as BKCa channel openers. Since these channels can be activated under normal physiological conditions around the resting membrane potential (Teramoto et al., 1997), they represent a significant target for the treatment of various urinary tract dysfunctions such as urge incontinence in urinary bladder and bladder outflow obstruction in urethra.
In conclusion, we have been able to demonstrate that mefenamic acid not only induces the opening of KATP channel but also activates BKCa channels, using single-channel recordings in freshly dispersed pig urethral smooth muscle cells.
Acknowledgments
We are grateful to SmithKline Beecham Pharmaceuticals for the generous gift of levcromakalim. This work was supported by grants from the Sir Edward P. Abraham Foundation (Oxford, U.K.), Medical Research Council (U.K.), the Kaibara Morikazu Medical Science Promotion Foundation (Fukuoka, Japan) and AstraZeneca Research Grant 2001 (Osaka, Japan), awarded to Noriyoshi Teramoto, and from a Grant-in-Aid for Scientific Research by the Ministry of Education, Science, Sports and Culture of Japan (Grant Number 14540080).
Abbreviations
- 4-AP
4-aminopyridine
- BKCa channels
intracellular Ca2+-activated large-conductance K+ channels
- DMSO
dimethylsulphoxide
- EK
potassium equilibrium potential
- IbTX
iberiotoxin
- KATP channel
ATP-sensitive K+ channel
- NPo
channel open-state probability
- PSS
physiological salt solution
- STOCs
spontaneous transient outward currents
- TEACl
tetraethylammonium chloride
References
- ANDERSSON K.-E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- BENHAM C.D., BOLTON T.B. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J. Physiol. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTRAND P.P., GALLIGAN J.J. Contribution of chloride conductance increase to slow EPSC and tachykinin current in guinea-pig myenteric neurones. J. Physiol. 1994;481:47–60. doi: 10.1113/jphysiol.1994.sp020418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B., PRESTWICH S.A., ZHOLOS A.V., GORDIENKO D.V. Excitation–contraction coupling in gastro-intestinal and other smooth muscles. Annu. Rev. Physiol. 1999;61:85–115. doi: 10.1146/annurev.physiol.61.1.85. [DOI] [PubMed] [Google Scholar]
- BUSCH A.E., HERZER T., WAGNER C.A., SCHMIDT F., RABER G., WALDEGGER S., LANG F. Positive regulation by chloride channel blockers of ISK channels expressed in Xenopus oocytes. Mol. Pharmacol. 1994;46:750–753. [PubMed] [Google Scholar]
- CARL A., BOWEN S., GELBAND C.H., SANDERS K.M., HUME J.R. Cromakalim and lemakalim activate Ca2+-dependent K+ channels in canine colon. Pflügers Arch. 1992;421:67–76. doi: 10.1007/BF00374735. [DOI] [PubMed] [Google Scholar]
- CHEN S., INOUE R., ITO Y. Pharmacological characterization of muscarinic receptor-activated cation channels in guinea-pig ileum. Br. J. Pharmacol. 1993;109:793–801. doi: 10.1111/j.1476-5381.1993.tb13644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLQUHOUN D., SIGWORTH F.J.Fitting and statistical analysis of single channel records Single-Channel Recording 1995New York: Plenum Press; 483–587.2nd edn., Chapter 19, ed. Sakmann, B. & Neher, E. pp [Google Scholar]
- EDWARDS G., WESTON A.H. The pharmacology of ATP-sensitive potassium channels. Annu. Rev. Pharmacol. Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- FARRUGIA G., RAE J.L., SARR M.G., SZURSZEWSKI J.H. Initial characterization of a potassium delayed rectifier in canine and human isolated jejunal circular smooth muscle. Gastroenterology. 1992;103:1390. [Google Scholar]
- FARRUGIA G., RAE J.L., SZURSZEWSKI J.H. Characterization of an outward potassium current in canine jejunal circular smooth muscle and its activation by fenamates. J. Physiol. 1993;468:297–310. doi: 10.1113/jphysiol.1993.sp019772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELBAND C.H., LODGE N.J., VAN BREEMEN C. A Ca2+-activated K+ channel from rabbit aorta: modulation by cromakalim. Eur. J. Pharmacol. 1989;167:201–210. doi: 10.1016/0014-2999(89)90580-3. [DOI] [PubMed] [Google Scholar]
- GELBAND C.H., MCCULLOUGH J.R. Modulation of rabbit aortic Ca2+-activated K+ channels by pinacidil, cromakalim, and glibenclamide. Am. J. Physiol. 1993;264:C1119–C1127. doi: 10.1152/ajpcell.1993.264.5.C1119. [DOI] [PubMed] [Google Scholar]
- GELBAND C.H., SILBERBERG S.D., GROSCHNER K., VAN BREEMEN C. ATP inhibits smooth muscle Ca2+-activated K+ channels. Proc. R. Soc. Lond. B. 1990;242:23–28. doi: 10.1098/rspb.1990.0098. [DOI] [PubMed] [Google Scholar]
- GÖGELEIN H., DAHLEM D., ENGERT H.C., LANG H.J. Flufenamic acid, mefenamic acid, and niflumic acid inhibit single nonselective cation channels in the rat exocrine pancreas. FEBS Lett. 1990;268:79–82. doi: 10.1016/0014-5793(90)80977-q. [DOI] [PubMed] [Google Scholar]
- GREENWOOD I.A., LARGE W.A. Comparison of the effects of fenamates on Ca-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. Br. J. Pharmacol. 1995;116:2939–2948. doi: 10.1111/j.1476-5381.1995.tb15948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIBKOFF V.K., LUM-RAGAN J.T., BOISSARD C.G., POST-MUNSON D.J., MEANWELL N.A., STARRETT J.E., JR, KOZLOWSKI E.S., ROMINE J.L., TROJNACKI J.T., MCKAY M.C., ZHONG J., DWORETZKY S.I. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol. Pharmacol. 1996;50:206–217. [PubMed] [Google Scholar]
- HOLLAND M., LANGTON P.D., STANDEN N.B., BOYLE J.P. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br. J. Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWE B.B., HALTERMAN T.J., YOCHIM C.L., DO M.L., PETTINGER S.J., STOW R.B., OHNMACHT C.J., RUSSELL K., EMPFIELD J.R., TRAINOR D.A., BROWN F.J., KAU S.T. ZENECA ZD6169: a novel KATP channel opener with in vivo selectivity for urinary bladder. J. Pharmacol. Exp. Ther. 1995;274:884–890. [PubMed] [Google Scholar]
- JUNG F., SELVARAJ S., GARGUS J.J. Blockers of platelet-derived growth factor-activated nonselective cation channel inhibit cell proliferation. Am. J. Physiol. 1992;262:C1464–C1470. doi: 10.1152/ajpcell.1992.262.6.C1464. [DOI] [PubMed] [Google Scholar]
- LEE Y.T., WANG Q. Inhibition of hKv2.1, a major human neuronal voltage-gated K+ channel, by meclofenamic acid. Eur. J. Pharmacol. 1999;378:349–356. doi: 10.1016/s0014-2999(99)00485-9. [DOI] [PubMed] [Google Scholar]
- MCMANUS O.B., BLATZ A.L., MAGLEBY K.L. Sampling, log binning, fitting, and plotting durations of open and shut intervals from single channels and the effects of noise. Pflügers Arch. 1987;410:530–553. doi: 10.1007/BF00586537. [DOI] [PubMed] [Google Scholar]
- OTTOLIA M., TORO L. Potentiation of large conductance KCa channels by niflumic, flufenamic, and mefenamic acids. Biophys. J. 1994;67:2272–2279. doi: 10.1016/S0006-3495(94)80712-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE J.M., NELSON M.T., STANDEN N.B. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- RAE J.E., FARRUGIA G. Whole-cell potassium current in rabbit corneal epithelium activated by fenamates. J. Membr. Biol. 1992;129:81–97. doi: 10.1007/BF00232057. [DOI] [PubMed] [Google Scholar]
- SIEMER C., GÖGELEIN H. Activation of nonselective cation channels in the basolateral membrane of rat distal colon crypt cells by prostaglandin E2. Pflügers Arch. 1992;420:319–328. doi: 10.1007/BF00374465. [DOI] [PubMed] [Google Scholar]
- SIGWORTH F.J., SINE S.M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys. J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERAMOTO N., BRADING A.F. Activation by levcromakalim and metabolic inhibition of glibenclamide-sensitive K channels in smooth muscle cells of pig proximal urethra. Br. J. Pharmacol. 1996;118:635–642. doi: 10.1111/j.1476-5381.1996.tb15448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERAMOTO N., BRADING A.F., ITO Y. Glibenclamide-sensitive K+ channels underlying levcromakalim-induced relaxation of pig urethra. Eur. J. Pharmacol. 1999;365:291–300. doi: 10.1016/s0014-2999(98)00885-1. [DOI] [PubMed] [Google Scholar]
- TERAMOTO N., CREED K.E., BRADING A.F. Activity of glibenclamide-sensitive K+ channels under unstimulated conditions in smooth muscle cells of pig proximal urethra. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:418–424. doi: 10.1007/pl00005071. [DOI] [PubMed] [Google Scholar]
- TERAMOTO N., YUNOKI T., IKAWA S., TAKANO N., TANAKA K., SEKI N., NAITO S., ITO Y. The involvement of L-type Ca2+ channels in the relaxant effects of the ATP-sensitive K+ channel opener ZD6169 on pig urethral smooth muscle. Br. J. Pharmacol. 2001a;134:1505–1515. doi: 10.1038/sj.bjp.0704408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERAMOTO N., YUNOKI T., TAKANO M., YONEMITSU Y., MASAKI I., SUEISHI K., BRADING A.F., ITO Y. Dual action of ZD6169, a novel K+ channel opener, on ATP-sensitive K+ channels in pig urethral myocytes. Br. J. Pharmacol. 2001b;133:154–164. doi: 10.1038/sj.bjp.0704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERAMOTO N., YUNOKI T., TANAKA K., TAKANO M., MASAKI I., YONEMITSU Y., SUEISHI K., ITO Y. The effects of caffeine on ATP-sensitive K+ channels in smooth muscle cells from pig urethra. Br. J. Pharmacol. 2000;131:505–513. doi: 10.1038/sj.bjp.0703586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA K., WANIISHI Y., INOUE R., ITO Y. Fenamates potentiate the α1-adrenoceptor-activated nonselective cation channels in rabbit portal vein smooth muscle. Jpn. J. Pharmacol. 1996;70:81–84. doi: 10.1254/jjp.70.81. [DOI] [PubMed] [Google Scholar]