Abstract
The GABA-modulating and GABA-mimetic activities of the monoterpenoid thymol were explored on human GABAA and Drosophila melanogaster homomeric RDLac GABA receptors expressed in Xenopus laevis oocytes, voltage-clamped at −60 mV. The site of action of thymol was also investigated.
Thymol, 1–100 μM, resulted in a dose-dependent potentiation of the EC20 GABA response in oocytes injected with either α1β3γ2s GABAA subunit cDNAs or the RDLac subunit RNA. At 100 μM thymol, current amplitudes in response to GABA were 416±72 and 715±85% of controls, respectively. On both receptors, thymol, 100 μM, elicited small currents in the absence of GABA.
The EC50 for GABA at α1β3γ2s GABAA receptors was reduced by 50 μM thymol from 15±3 to 4±1 μM, and the Hill slope changed from 1.35±0.14 to 1.04±0.16; there was little effect on the maximum GABA response.
Thymol (1–100 μM) potentiation of responses to EC20 GABA for α1β1γ2s, α6β3γ2s and α1β3γ2s human GABAA receptors was almost identical, arguing against actions at benzodiazepine or loreclezole sites.
Neither flumazenil, 3-hydroxymethyl-β-carboline (3-HMC), nor 5α-pregnane-3α, 20α-diol (5α-pregnanediol) affected thymol potentiation of the GABA response at α1β3γ2s receptors, providing evidence against actions at the benzodiazepine/β-carboline or steroid sites. Thymol stimulated the agonist actions of pentobarbital and propofol on α1β3γ2s receptors, consistent with a mode of action distinct from that of either compound. These data suggest that thymol potentiates GABAA receptors through a previously unidentified binding site.
Keywords: Thymol, thyme essential oil, monoterpenoids, ionotropic GABA receptor, Drosophila melanogaster, allosteric modulation
Introduction
The rapid actions of the neurotransmitter GABA are mediated by ionotropic GABA receptors; these are pentameric transmembrane proteins with an integral, GABA-gated, anion channel (Moss & Smart, 2001). In vertebrates, all ionotropic GABA receptors are designated type A, and metabotropic receptors type B. Vertebrate GABAA receptors are confined to the nervous system (Sieghart, 1995), whereas insect ionotropic GABA receptors are present in the nervous system and on muscle cells (Sattelle, 1990). To date, 20 different vertebrate GABAA receptor subunit isoforms have been cloned: α(1–6), β(1–4), γ(1–3), δ(1), ɛ(1), π(1), θ(1) and ρ(1–3). Further diversity can arise due to alternative splicing of some subunit genes (for GABAA receptor classification see, for example, Korpi et al., 2002). The most common stoichiometry in mammalian brain is thought to be 2α2β1γ (Barnard et al., 1998), although there is potential for considerable diversity of subunit composition. No data are available on the subunit stoichiometry of insect ionotropic GABA receptors, although three different subunit candidates are known to be expressed, one of which, RDL, has four splice variants (Hosie et al., 1997). Subunit composition is an important determinant of the pharmacological and biophysical properties of recombinant GABAA receptors (Sigel et al., 1990; Rabow et al., 1995; Moss & Smart, 2001), and probably also of native insect GABA receptors (Hosie et al., 1996).
The many known GABA receptor ligands include agonists, antagonists and modulators; positive allosteric modulators, for example, potentiate the actions of GABA. In humans and other mammals, behavioural effects which are typical of positive allosteric modulators of GABAA receptors include anxiolysis, cessation of convulsions, sedation and general anaesthesia (Sieghart, 1995), although some of these effects may result from simultaneous action at other receptor types. Some positive GABAA receptor modulators can also act as agonists on the same receptors when tested at higher concentrations (Robertson, 1989; Franks & Lieb, 1994) and this activity may influence the spectrum of clinical effects observed (Sanna et al., 1999). Most GABAA receptor potentiating compounds, with the notable exception of the benzodiazepine clonazepam, also enhance the action of GABA at native and recombinant insect GABA receptors, although they are often less potent than on GABAA receptors and lack the agonist activity observed at some vertebrate GABAA receptors (Belelli et al., 1996; Hosie & Sattelle, 1996). Insect GABA receptors are targets for several pesticides, such as dieldrin, lindane, BIDN and fipronil, all of which are antagonists (Bloomquist, 1996). One insecticide analogue, δ-HCH, potentiates at GABA receptors, and this has been proposed to be acting via the barbiturate binding site on the receptor (Aspinwall et al., 1997).
Thymol is a monocyclic phenolic compound, the usual natural source being the essential oil of Thymus vulgaris (Lamiaceae). Its main therapeutic application is in dental preparations to kill odour-producing bacteria. It is also employed as a preservative on the strength of its antimicrobial (see, for example, Cosentino et al., 1999; Venturini et al., 2002) and antioxidant properties (Aeschbach et al., 1994). Thymol has molluscicidal (Singh et al., 1999) and insecticidal properties (Lee et al., 1997; Mansour et al., 2000; Hummelbrunner & Isman, 2001). In the mollusc Lymnaea acuminata, lethal doses of thymol affected the activity of key nervous tissue enzymes, and this was postulated to be the cause of toxicity (Singh et al., 1999). As yet, no mechanism of action has been identified for thymol lethality, or that of related monoterpenoids, towards insects.
Recently, thymol was shown to have a direct agonist effect on heterologously expressed human GABAA receptors resembling that of the anaesthetic propofol (Mohammadi et al., 2001). In this paper, we examine whether thymol, like propofol, also potentiates the activity of GABA at vertebrate GABAA receptors at lower concentrations than those required for agonist activity. We also test the actions of thymol at a recombinant insect GABA receptor, the Drosophila melanogaster RDLac subunit; this splice variant (one of four products of the Rdl gene) forms a functional homomeric GABA receptor and its pharmacology has been described in detail, including the actions of the insecticides dieldrin, lindane and fipronil (ffrench-Constant et al., 1991; Belelli et al., 1996; Hosie et al., 1997). We also examine whether the site of action of thymol is shared by any other known GABA receptor modulators (benzodiazepines, barbiturates, pregnane steroids, loreclezole and propofol).
Methods
Investigations on insect and human GABA receptors were carried out in different laboratories, and this is reflected in minor differences in the respective protocols, as detailed in this section.
GABA receptor subunit cDNAs and cRNAs
Previous publications have described the cloning and sequencing of cDNAs encoding α1, β1, β3, γ2 (Hadingham et al., 1993a, b) and α6 (Hadingham et al., 1996) human GABAA receptor subunits, and also the Drosophila RDLac GABA receptor (ffrench-Constant et al., 1991; 1993; Hosie et al., 1995). Human cDNAs, encoding α1, α6, β3, β1 and γ2s GABAA receptor subunits, were supplied by The Molecular Biology Department, Merck, Sharp and Dohme, Terling's Park, U.K. Wild-type Rdlac cDNA was a gift from Dr Richard Roush (Cornell University, U.S.A.); it had been inserted into the cloning vector pNB40 (Brown & Kafatos, 1988). The plasmid was subcloned following established methods (Hosie et al., 1995); subsequent extraction of pNB40 from E. coli was carried out using endotoxin-free, maxi-prep kits (Qiagen, U.K.). The plasmid was linearised with NotI restriction endonuclease to provide a transcription template, and RDLac cRNA was then synthesised with an SP6 RNA-polymerase and m7G(5′)ppp(5′)G capped using an ‘mMessage mMachine' (Ambion), following the manufacturer's protocol.
Receptor expression in Xenopus oocytes
Human GABA receptor subunit combinations were expressed in Xenopus laevis oocytes. Mature female Xenopus oocytes (Blades, U.K.) were anaesthetised by immersion in a 0.4% solution of 3-aminobenzoic acid ethylester for 30–40 min, or until completely unresponsive, and part of the ovary was excised via a small abdominal incision. The isolated ovaries were immersed in modified Barth's solution (MBS) of the following composition (mM): NaCl, 88; KCl, 1; NaHCO3, 2.4; HEPES, 10; MgSO4·7H2O, 0.82; Ca(NO3)2·4H2O, 0.33; CaCl2·2H2O, 0.91; pH 7.5 (adjusted with NaOH), and then transferred to a hypertonic isolation medium composed of (mM): NaCl, 108; KCl, 2; EDTA, 1.2; HEPES, 10; pH 7.9 (adjusted with NaOH), to aid subsequent manual defolliculation. Residual follicular cells were removed by incubating the oocytes in collagenase type IA (Sigma, U.K.), 0.5 mg ml−1 in MBS, for 6 min. A manual oocyte injection pipette (Drummond, U.K.) was used to administer 20 nl of GABA subunit cDNA mixture to each cell nucleus. Combinations of three human GABAA receptor cDNAs were injected, in the ratios of either 1 : 1 : 1 or 1 : 0.1 : 1 to optimise the expression of benzodiazepine-sensitive GABAA receptors. The concentration of total cDNA in each case was 20 ng ml−1, in an injection buffer consisting of (mM): NaCl, 88; KCl, 1; HEPES, 15; pH 7 (adjusted with NaOH). Following injection, the cells were transferred to MBS supplemented with gentamycin, 50 mg l−1; penicillin, 10,000 U l−1; streptomycin, 10 mg l−1; and sodium pyruvate, 2.5 mM. Oocytes were maintained at 19°C initially.
Insect GABA receptors were expressed in Xenopus oocytes by a similar method. In this case, before injection, the ovaries were washed and stored in standard oocyte saline (SOS), of the following composition (mM): NaCl, 100; KCl, 2; CaCl2, 1.8; MgCl2, 1; HEPES, 5; pH 7.6 (adjusted with NaOH). The cRNA encoding RDLac, 50 ng at 1 μgμl−1, was injected cytoplasmically using a Nanoject pipette (Drummond, U.K.); some cells were omitted for use in control experiments or injected with the same volume of dH2O. The incubation medium employed consisted of SOS supplemented with antibiotics and pyruvate, at the concentrations described previously, and horse serum at 10 ml l−1. In the 30 min following injection, oocytes were kept at 4°C to allow recovery. Cells were incubated at 16°C and transferred to fresh medium on a daily basis.
Batches of cells responding with large currents were transferred to 4°C to prevent receptor overexpression and prolong viability.
Electrophysiology and data analysis
To investigate GABA receptor responses, oocytes were secured by a ring of stainless-steel entomological pins embedded in the Sylgard floor of a Perspex bath. Fresh bathing solution was continually perfused through the chamber by a gravity-fed system. All drugs were applied dissolved in the bathing solution, although stock solutions of hydrophobic compounds were prepared in DMSO or acetone and diluted in bathing solution such that the concentration of organic solvent did not exceed 0.1%. Solutions of 0.1% acetone or DMSO had no effect on the current required to clamp injected oocyte membranes at −60 mV, nor did they affect responses to GABA. DMSO and acetone (Hosie et al., 1995), at these concentrations are used as solvents for drugs in oocyte electrophysiology.
Membrane currents recorded from oocytes expressing GABA receptors were measured by two-electrode voltage-clamp, with the membrane held at −60 mV, using 2 M KCl-filled electrodes with 1% agar in 2 M KCl at the tip for GABAA receptors, and 3 M KCL-filled electrodes for RDLac. Electrode resistance was maintained at 0.5–5 MΩ. Currents due to GABAA receptors were amplified using a GeneClamp 500 Amplifier (Axon Instruments, U.S.A.) and recorded on two outputs: electronically, using ‘Oocyte' for the Digitimer Digistore™ System (Digitimer Ltd, U.K.), and on chart paper with a Thermal Arraycorder (WR 8500 series, Graphtec, U.K.). Currents through RDLac homomers were amplified using an Oocyte Clamp OC-725C amplifier (Warner Instrument Corporation, U.S.A.) and displayed on a chart recorder. Each cell expressing GABAA receptors was challenged with 3 mM GABA to obtain the maximal response, and those with a maximal response of less than 100 nA were rejected. This was not necessary for RDLac receptors, as consistently large responses were generated. Only oocytes yielding stable responses were selected for experimental work. Uninjected or distilled water-injected (dH2O-injected) oocytes did not respond to GABA or thymol.
Responses to drugs were measured at peak current. Dose–response data were generated using increasing concentrations of the ligand of interest. Curves were fitted to the data, both for individual cells and also to the mean data points. GraphPad Prism (GraphPad Software, U.K.) was used to fit the four-parameter logistic equation below, which describes a sigmoid curve of variable slope, to the normalised data:
where ϕ is the normalised current induced by a given concentration of agonist, [A]; Imax and Imin are the maximal and minimal normalised agonist responses, respectively; EC50 is the concentration of agonist predicted to elicit half the maximal response and nH is the slope (Hill) coefficient. Results are presented as the mean±one standard error of the mean (±s.e.m.) of experiments on n cells. EC50 values given in the text for human GABAA receptors are mean values calculated from several EC50 values, each of which was estimated from the dose–response data obtained from an individual cell; for the insect RDLac receptor, the EC50 was estimated from dose–response data pooled from 14 cells. For the graphical presentation of data, all dose–response results were averaged before a single regression line was fitted. Differences between mean values were evaluated by unpaired or paired Student's t-test, or one-sample t-test, as appropriate, and considered significant if P<0.05.
Thymol dose–response experiments on human GABAA receptors were carried out by determining the GABA EC20 for each cell, and then applying the EC20 in conjunction with increasing doses of thymol after a 40 s preapplication with thymol alone. To estimate the GABA EC20 for RDLac homomers, dose–response data from 14 cells were pooled and equation (1) used to fit a curve to the averaged data. For thymol dose–response experiments, a set concentration of thymol was applied for 2 min, followed by coapplication of this concentration with EC20 GABA. This regime was repeated using increasing concentrations of thymol. Data were handled in the same manner as for the GABA dose–response curves, except that each response was normalised to the EC20 GABA response for each cell.
Further investigations were carried out on human GABAA receptors. The effects of thymol on the GABA dose–response curve were estimated by applying increasing concentrations of GABA to each cell, and then applying the same GABA doses together with 50 μM thymol, each after a 40 s thymol preapplication. To assess competitive interactions between thymol and GABA-inhibiting or -enhancing ligands, the GABA EC20 was applied in conjunction with the ligand in the presence and absence of 50 μM thymol. To assess competitive interactions between thymol and the agonist-like effects of the positive modulators pentobarbital and propofol, the ligand was applied until stable responses were obtained, and then it was coapplied with 50 μM thymol. In order to minimise desensitisation and rundown effects, washout periods between drug applications were 5 min after a maximal GABA response, and 3–10 min after other applications, depending on the drug and concentration applied.
Drugs
Propofol (2,6-diisopropylphenol, Aldrich), 3-HMC (Tocris), GABA (Sigma or Research Biochemicals Inc.), pentobarbital, 5α-pregnanediol, thymol (Sigma), flumazenil (synthesised by K. Moore in the Medicinal Chemistry Department, Merck, Sharp and Dohme, Terling's Park, U.K.).
Results
Potentiation of GABA action by thymol on human GABAA receptors
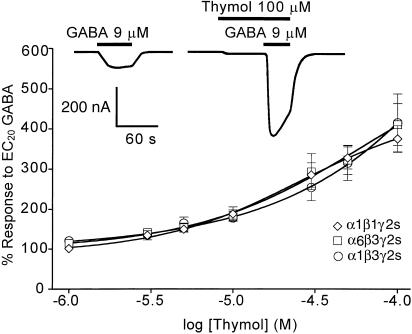
Initial studies were performed on human α1β3γ2s recombinant receptors as this subunit combination is abundant in the vertebrate CNS, and changing one or other of the subunits has a dramatic effect on the actions of modulators. Thymol, 1–100 μM, applied prior to (for 40 s) and during the application of EC20 GABA resulted in dose-dependent potentiation of the GABA response (Figure 1). Above 100 μM, thymol potentiation decreased with increasing thymol concentration. The maximal potentiation observed for the α1β3γ2s GABAA receptor was 416±72% (n=5) at 100 μM thymol.
Figure 1.
The dose-dependent potentiation of the EC20 GABA response by thymol at recombinant human GABAA receptors with the subunit combinations α1β1γ2s, α6β3γ2s and α1β3γ2s. The GABA response was potentiated about equally by thymol at all the three receptors. Each data point is a mean value±one s.e.m. (n=3–6). Recordings were obtained from Xenopus oocytes, voltage-clamped at −60 mV. The example trace was recorded from a single α1β3γ2s-expressing cell, and shows 100 μM thymol-induced potentiation of the control GABA current. The trace also illustrates how, at concentrations of 100 μM and above, thymol induces GABA receptor-mediated currents when applied alone during the 40 s preincubation time.
Intrinsic activity of thymol on human GABAA receptors
Cells expressing α1β3γ2s did not respond directly to 1–50 μM thymol (the change in membrane current over the course of the 40 s application of thymol was never greater than 9 nA in amplitude). Thymol at 100 μM and above generated responses, although these were extremely small in amplitude compared to the striking potentiation of the GABA-induced current by this same thymol concentration (as illustrated by the trace in Figure 1). Dose–response curves showing the agonist action of thymol were not examined because the concentration of acetone required to solubilise 300 μM and higher concentrations of thymol affected the current required to clamp the cell.
Thymol potentiates the GABA response mediated by recombinant, homomeric insect (Drosophila melanogaster) RDLac GABA receptors
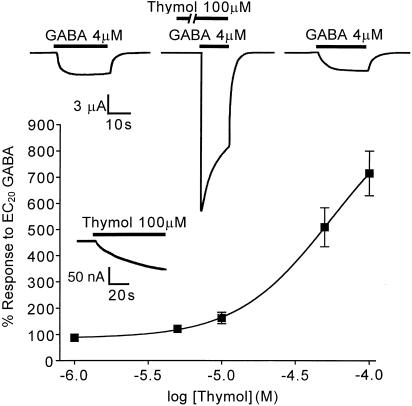
Of all the splice variants of the Rdl gene, the Rdlac gene product has been most extensively studied. The GABA dose–response curve obtained from oocytes expressing RDLac was used to estimate an EC20 value for GABA of 4 μM. This EC20 test concentration was then deployed in further experiments to investigate potentiation by thymol. The EC50 was 8.5 μM (95% confidence interval: 7.1–10.0 μM), which was close to previously published EC50 values for this receptor (Belelli et al., 1996).
Thymol, 1–100 μM, potentiated the EC20 GABA response when coapplied for 15–30 s, following a 2 min preapplication of thymol alone (Figure 2). The potentiation was fully reversible and dose-dependent. The degree of potentiation by thymol was also affected by the GABA concentration, thymol (100 μM) increasing the response to 10 μM GABA less than two-fold (data not shown), whereas the response to EC20 GABA (4 μM) was potentiated over seven-fold.
Figure 2.
Actions of thymol at an insect GABA receptor. Xenopus oocytes expressing the Drosophila GABA receptor subunit RDLac were voltage-clamped at −60 mV. The upper inset trace shows successive recordings from a single RDLac-expressing oocyte and demonstrates thymol-induced potentiation of the GABA-induced current through this receptor. The trace also shows that, after washout, the control GABA response is restored. The lower inset trace shows the current elicited by high concentrations of thymol, in this case 100 μM; this response was recorded during a preapplication period, where thymol was applied alone before being applied together with EC20 GABA, and is shown here at high amplification. The graph shows thymol-induced dose-dependent potentiation of the GABA response mediated by RDLac homomers. Currents are expressed as a percentage of the control response to GABA, shown here as 100%. Data points displayed are the mean±one s.e.m. (n=6–7).
Any possible potentiation by concentrations of thymol greater than 100 μM could not be investigated due to instability of the clamped current at these doses. Thymol at 100 μM was without effect when tested on uninjected oocytes (n=3, from three separate batches).
Thymol is a weak agonist on Drosophila melanogaster RDLac GABA receptors
A small-amplitude, direct agonist action of thymol was observed in oocytes expressing RDLac, but not in uninjected or dH2O-injected cells: Concentrations of 1–50 μM thymol and below had a negligible effect, eliciting changes in the membrane potential of 15 nA or less over a 2 min application. Thymol, at 100 μM, resulted in currents which were 57±22 nA (n=4) in amplitude at 2 min (Figure 2, lower inset trace). Responses to thymol alone began immediately upon application, but were slow to gain amplitude and failed to plateau throughout the 2 min application time.
Effects of thymol on the GABA dose–response curve of human α1β3γ2s GABAA receptors
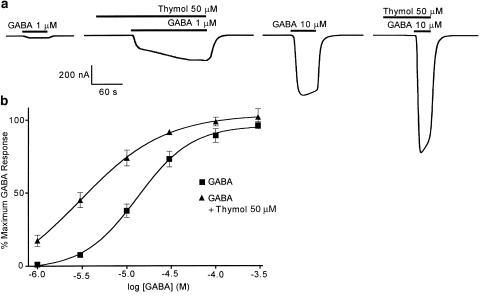
Thymol potentiates the response to concentrations of GABA below EC100, the largest effects being seen over the range EC5–EC50 (Figure 3a, b). The amplitudes of currents induced by 3 and 100 μM GABA were 7.6±1.7% (n=5) and 89.0±4.8% (n=5) of the maximum, respectively. In the presence of 50 μM thymol, these responses were potentiated to 45.0±5.2% (n=5) and 98.6±3.0% (n=5) of the maximum GABA response, respectively. The GABA dose–response curve was shifted to the left by 50 μM thymol (Figure 3b) and the mean EC50 for GABA was reduced significantly from 15±3 to 4±1 μM (paired t-test). The Hill slope was also reduced significantly (paired t-test) from 1.35±0.14 to 1.04±0.16. However, 50 μM thymol had little effect on the maximum GABA response (Figure 3b).
Figure 3.
Effects of thymol on the GABA dose–response relationship of a human GABAA receptor, heterologously expressed in Xenopus oocytes, voltage-clamped at −60 mV. The degree to which 50 μM thymol potentiates the GABA response of the α1β3γ2s GABAA receptor is dependent on GABA concentration. The example traces (a) show enhancement of the responses to 1 and 10 μM GABA. The graph (b) demonstrates that 50 μM thymol causes a significant leftward shift of the GABA dose–response curve. Data were normalised by expression as a percentage of the maximal GABA response, and are shown as the mean of five observations±one s.e.m.
Thymol potentiates the GABA responses mediated by human GABAA receptors of different subunit composition with similar potency
In the range 1–100 μM, thymol potentiated the EC20 GABA response of α1β1γ2s and α6β3γ2s vertebrate GABA receptors in a dose-dependent manner. The thymol dose-response curves obtained for α1β1γ2s, α6β3γ2s and α1β3γ2s receptors were almost identical: over the range of concentrations tested, GABA actions on the 3 subunit combinations were potentiated about equally by thymol (Figure 1, graph).
Thymol-induced potentiation of α1β3γ2s human GABAA receptors is not affected by the modulators flumazenil, 3-hydroxymethyl-β-carboline and 5α-pregnanediol
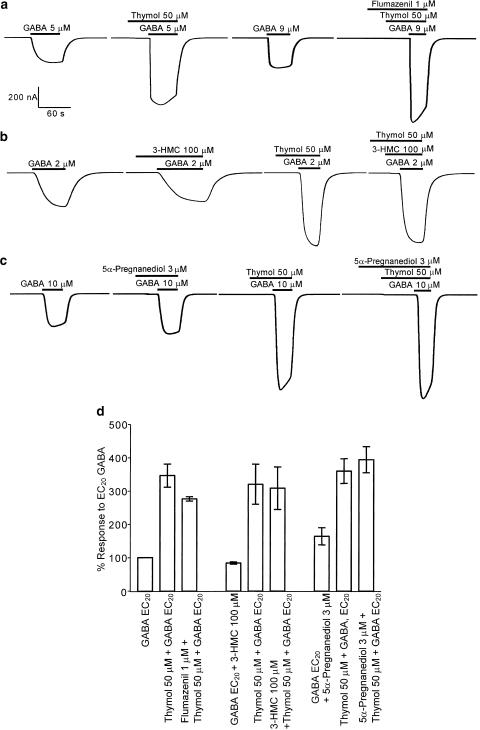
Flumazenil is a competitive antagonist at the benzodiazepine site; flumazenil is not expected to depress the GABA response under normal circumstances, but instead reduces the potentiation of GABA responses by other benzodiazepines. Coapplication of thymol and GABA, following a 40 s preincubation with thymol, potentiated the GABA response to 346.5±34.7% (n=4) (Figure 4a, d). Application of 50 μM thymol, EC20 GABA and 1 μM flumazenil to oocytes expressing α1β3γ2s receptors following preapplication of flumazenil (30 s) and thymol plus flumazenil (40 s) resulted in a mean increase of the EC20 GABA response to 276.3±6.6% (n=4) (Figure 4a, d). Mean values for thymol potentiation in the presence and absence of 1 μM flumazenil were not significantly different (unpaired t-test), suggesting that thymol is not acting via the benzodiazepine site.
Figure 4.
Benzodiazepine, β-carboline and steroid allosteric modulators have no effect on thymol-induced potentiation of GABA responses at human α1β3γ2s GABAA receptors heterologously expressed in Xenopus oocytes, voltage-clamped at −60 mV. This figure shows the enhancement of EC20 GABA responses elicited by thymol alone and in combination with either (a) flumazenil, (b) 3-hydroxymethyl-β-carboline (3-HMC), or (c) 5α-pregnane-3α, 20α-diol (5α-pregnanediol). The mean data from these experiments (d) are shown±one s.e.m. (n=3–4).
When applied to oocytes expressing α1β3γ2s, the β-carboline 3-HMC, 100 μM, caused a slight decrease in the EC20 GABA response, as expected, the amplitude being 83.7±3% (n=3) of control (Figure 4b, d). When challenged with 50 μM thymol and EC20 GABA in combination, potentiation of the GABA response to 320±60% (n=3) of control values was observed. Coapplication of 50 μM thymol, EC20 GABA and 100 μM 3-HMC together, following preapplications of thymol and 3-HMC, resulted in an augmentation of the EC20 GABA response to 308±64% (n=3). The difference in the degree of thymol potentiation in the presence and absence of 3-HMC was not significant using an unpaired t-test, suggesting a lack of competition between thymol and β-carbolines.
While there are no steroid site antagonists, the partial agonist 5α-pregnanediol, at 3 μM, applied with EC20 GABA, only increased the α1β3γ2s GABA response to a small degree. The amplitude of the potentiated response was 164±26% (n=4) of control (Figure 4c, d). The mean potentiation of the EC20 GABA response by 50 μM thymol in these cells was 360±37% (n=4) of control. Coappli-cation of thymol, GABA and 5α-pregnanediol, following preapplications of thymol and 5α-pregnanediol (40 s), enhanced the control response to 395±39% (n=4). As the enhancement produced by thymol and 5α-pregnanediol was not significantly lower than that of thymol alone (unpaired t-test), these data suggest that this steroid compound does not compete with thymol.
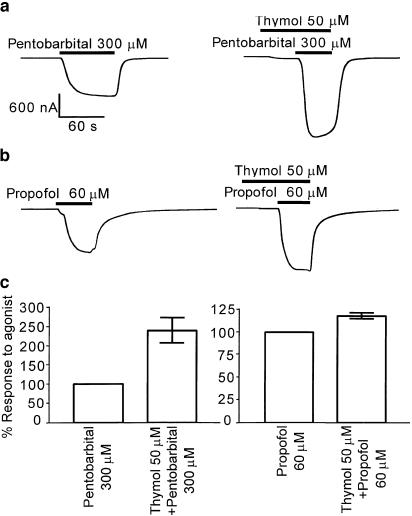
Actions of pentobarbital and propofol at α1β3γ2s human GABAA receptors are enhanced by thymol
Pentobarbital and propofol potentiate GABAA receptors, and at saturating concentrations (300 and 60 μM, respectively), both elicit inward currents in oocytes expressing the α1β3γ2s receptor. Thymol, 50 μM, applied for 40 s prior to coapplication with 300 μM pentobarbital, significantly potentiated responses to pentobarbital (in a one-sample t-test) to 241±36% (n=5) when expressed as a percentage of the pentobarbital current (Figure 5a, c). In a similar experiment, thymol, 50 μM, was applied for 40 s prior to coapplication with 60 μM propofol, a general anaesthetic with a similar structure to thymol. The response to propofol was also significantly enhanced in the presence of thymol (shown by one-sample t-test), but to a lesser degree than the pentobarbital response, showing 118±2.9% potentiation (n=4) (Figure 5b, c). These results suggest that thymol does not compete at the barbiturate- or propofol-binding sites.
Figure 5.
Thymol potentiates the agonist actions of pentobarbital and propofol at human α1β3γ2s GABAA receptors, expressed in Xenopus oocytes and voltage-clamped at −60 mV. Responses of the GABAA receptors to (a) pentobarbital or (b) propofol were enhanced by 50 μM thymol. The mean data from these experiments (c) are shown±one s.e.m., n=4 in each case. Both compounds show significant potentiation using a one-sample t-test.
Discussion
Thymol potentiates the actions of GABA at three recombinant human GABAA receptors of different subunit composition, and also at a recombinant insect ionotropic GABA receptor. Modulation of the human α1β3γ2s, α6β3γ2s and α1β1γ2s GABAA receptors and Drosophila RDLac homomers was dose-dependent over a similar concentration range, suggesting a non-subunit-selective action, although it appears that thymol is less potent on mammalian GABAA receptors than on the insect model ionotropic GABA receptor. At all subunit combinations, potentiation was observed at concentrations where thymol elicited either zero or minimal agonist activity, indicating that the enhanced response to GABA is likely to be the result of a positive allosteric action of thymol. A separate agonist action of thymol was seen at both mammalian and insect recombinant receptors between 100 and 500 μM, and at these concentrations thymol failed to generate currents in uninjected or dH2O-injected eggs, indicating that its effects were GABA receptor-mediated. Agonist actions of thymol have also recently been described at rat α1β2γ2 GABAA receptors expressed in HEK cells, although the potentiating effect was not investigated (Mohammadi et al., 2001).
The significant leftward shift of the GABA dose–response curve obtained from human α1β3γ2s-expressing oocytes demonstrates that thymol can decrease the apparent EC50 of GABA, but does not greatly affect the maximum response. This suggests that thymol is specifically facilitating the manner in which GABA binds to or activates the receptor, but cannot increase the current flow above the maximum possible achieved by GABA alone. Thymol also decreased the Hill coefficient (nH). In these respects, the actions of thymol on GABAA receptors are similar to that of the insecticide δ-HCH on RDLac homomers, but distinct from the action of δ-HCH on GABAA receptors. In the case of RDLac homomers, δ-HCH shifts the dose–response curve to the left, decreasing nH but without affecting the maximal response (Hosie & Sattelle, 1996), whereas on GABAA receptors δ-HCH shifts the dose–response curve to the left and also depresses the amplitude of the maximal response (Woodward et al., 1992).
Changing the receptor subunit composition of human GABAA receptors and observing the resulting impact on modulator potency is a rapid method of identifying specific subunits or subunit interfaces that are important for mediating the effects of a particular ligand. Such areas may form part of the modulator-binding site. Replacing α1 with α6 renders receptors otherwise sensitive to most benzodiazepines unresponsive to the majority of this class of compound, with the exception of certain benzodiazepine site ligands, such as the partial inverse agonist Ro 15-4513 (Wieland et al., 1992). The α1/α6 substitution was used previously in an attempt to characterise a novel positive allosteric modulator of GABAA receptors, where the activity of (+)-ROD188 was compared on benzodiazepine-sensitive (rat α1β2γ2s) and benzodiazepine-insensitive combinations (rat α1β2, rat α6β2γ2) of subunits. In fact, (+)-ROD188 was most active at receptors containing the α6 isoform in αxβ2γ2, where x=1, 2, 3, 5, 6 (Thomet et al., 2000). However, thymol showed no particular selectivity for α1 or α6 in terms of potency or maximal potentiation, suggesting that it does not act via the benzodiazepine-binding site, nor the (+)-ROD188 site. Loreclezole potentiates the actions of GABA at both RDLac and GABAA receptors, the determinants of potency at the loreclezole site on GABAA receptors being shared by the positive modulatory actions of etomidate and DMCM (Stevenson et al., 1995; Hill-Venning et al., 1997), but no competitive inhibitors have thus far been identified. Ligands binding to the loreclezole site are characterised by a reduced potency on β1-containing receptors in comparison to β3-containing receptors. Thymol potentiated the activity of GABA at recombinant α1βxγ2s GABAA receptors, where x=1 or 3, with no difference in potency, suggesting that thymol does not interact in the same manner as loreclezole.
Studying competitive interactions between ligands is another method of classifying allosteric sites. Most benzodiazepines are positive allosteric modulators of vertebrate GABAA and native insect receptors but, with the exception of 4′-chlorodiazepam (Hosie & Sattelle, 1996), are inactive or weakly active at RDLac homomers (Sattelle et al., 1988; Hosie & Sattelle, 1996), probably because activity depends on the presence of more than one subunit type, as it does in vertebrates (Peters et al., 1988). It is relatively difficult to perform competition studies between two compounds that both potentiate GABA responses; therefore, the potent GABAA receptor benzodiazepine site antagonists flumazenil and the β-carboline 3-HMC were chosen for study. Flumazenil (Ro15-1788), at 1 μM, suppressed potentiation of the rat recombinant α1β2γ2 GABAA receptor by the β-carboline ZK 91085, but had no effect on the activity of (+)-ROD188 (Thomet et al., 2000), suggesting that ZK 91085 has a mode of action in common with benzodiazepines, but (+)-ROD188 does not. At invertebrate GABA receptors, 3-HMC was reported to competitively antagonise the potentiating effects of 4′-chlorodiazepam at RDL homomeric receptors (Hosie & Sattelle, 1996). If thymol acted via the benzodiazepine β-carboline-binding site, it might be expected that flumazenil or 3-HMC would displace it and result in a substantial reduction in thymol activity; no significant reduction in thymol activity occurred, suggesting that thymol does not act through either binding site.
Functional competition studies where both agents enhance the effects of the agonist are more difficult to interpret; it is not possible to predict exactly how the potentiating activities of two different compounds, whether acting at the same binding site or not, will be integrated by the receptor in terms of overall conformational changes and subsequent effect on the response. Previous studies (Thomet et al., 2000) have used the rationale that an interaction between compounds would manifest as a potentiation by the dual combination less than the (theoretical) additive effect of the two compounds applied separately. The partial steroid agonist 5α-pregnanediol was used to investigate possible activity at the neurosteroid-binding site. The 5α-pregnanediol-induced potentiation of the GABA response was much lower than that of thymol, as expected. As 5α-pregnanediol is only weakly efficacious, competition between thymol and 5α-pregnanediol could be expected to result in a combined enhancement lower than that produced by thymol alone; instead, the combined enhancement was higher. This result suggests that thymol does not act via the steroid-binding site.
At GABAA receptors, barbiturates potentiate the effects of GABA and also have a direct agonist effect over a higher concentration range (Peters et al., 1988); since competition studies between two potentiating compounds yield complex results, we tested the ability of thymol to potentiate the agonist effects of pentobarbital and the anaesthetic propofol, ensuring saturation of the potentiating site. Like (+)-ROD188 (Thomet et al., 2000), thymol potentiated pentobarbital-induced currents, and, to a lesser degree, also those of propofol, indicating a lack of competitive interaction with these compounds. This was somewhat surprising since propofol and thymol are relatively similar in structure, and might be expected to share a site of action.
The evidence reported here suggests that thymol does not share sites of action with many of the most widely investigated allosteric modulators of GABAA activity, these being benzodiazepines, β-carbolines, barbiturates, propofol, loreclezole and steroids. It is still contentious as to whether the barbiturates and propofol act as agonists and positive modulators via the same or distinct sites. A range of GABA receptor subunit point mutations influence both agonist and modulator activities of pentobarbitone and/or propofol (Amin, 1999; Pistis et al., 1999), but this could reflect common transduction components rather than a single binding site (Belelli et al., 1999). The simplest conclusion is that thymol has a mode of action different from that of pentobarbital and propofol as GABA receptor agonists, and possibly also as modulators.
When the effects of one drug are suppressed by another, in the absence of binding data, it is not possible to classify the interaction as competitive or allosteric inhibition. Furthermore, variations in drug potency between receptors composed of different subunit combinations may reflect differential transduction rather than differential binding. The lack of effect on the thymol enhancement in all of our experiments suggests that neither the binding site nor transduction domains mediating thymol potentiation were affected in any of the test conditions. In this study, thymol has tentatively been termed a positive allosteric modulator of GABA receptors even though no putative allosteric site (as described in reviews such as Haefely, 1994; Changeux & Edelstein, 1998) has been defined. This term has previously been applied to ligands before a binding site on the receptor itself was confirmed, for example, (+)-ROD188 was described as a positive allosteric modulator of the GABA receptor, simply by the virtue that it stimulated GABA-induced currents at GABA receptors in a concentration-dependent fashion and induced negligible currents by itself (amplitude not given). No high-affinity binding sites were demonstrated for (+)-ROD188; a weak interaction with the benzodiazepine site was found, but was clearly not the site through which potentiation was mediated. Slight selectivity was shown for α6-containing receptors (Thomet et al., 2000), but this does not necessarily reflect binding preferences. Until a specific binding site on the receptor is described, an intermediary site of action cannot be ruled out.
Intermediary sites of action have previously been proposed for other enhancers of GABA function, such as anaesthetics. Many chemicals that are volatile and lipophilic, physicochemical properties shared by thymol, have anaesthetic effects in humans; furthermore, at therapeutic concentrations, ion channels are the principal targets of anaesthetics (Yamakura et al., 2001), but for many years this was thought to be an indirect action secondary to bilayer disordering, for example, detergents and free fatty acids affect receptor channel function in this way (Koenig & Martin, 1992). Thymol is known to affect plasma membrane properties such as stability (Singh, 1980; Manabe et al., 1987) and permeability to drugs, for example, piroxicam (Doliwa et al., 2001). In our study, thymol did not affect bilayer integrity at the concentrations required for potentiation, as negligible changes in the current required to clamp the membrane potential (<10 nA) occurred in uninjected oocytes.
The majority of evidence gathered recently supports anaesthetic action at discrete sites on the ligand-gated ion channels themselves. For example, although a huge diversity of structures enhance GABAA receptor function, within each chemical group there are strict structure–activity requirements and subunit preferences, and these observations are supported by competitive interactions between chemically related molecules in binding and functional studies (Belelli et al., 1999). Thus far, it is not possible to draw any conclusions about the purported thymol-binding site on the GABA receptor. There could be multiple sites mediating the effect of thymol, for example, the portions of the GABA receptor protein mediating potentiation might be separate from those through which the agonist effect occurs, as proposed for other ligands that elicit both effects (Mohammadi et al., 2001). As thymol does not appear to compete with other GABAergic ligands, it is possible that thymol enhancement occurs via a previously uncharacterised allosteric site on the GABAA receptor, which could represent a new avenue in therapeutic and/or pesticide research.
Acknowledgments
C.M.P. was supported by a School of Pharmacy Studentship. D.B.S. acknowledges the support of The Medical Research Council, U.K. We would like to thank Drs Emmanuel Culetto and Valerie Raymond for advice and assistance, and also Dr Sally Thompson for the provision of oocytes at Merck, Sharp and Dohme.
Abbreviations
- 3-HMC
3-hydroxymethyl-β-carboline
- MBS
modified Barth's solution
- SOS
standard oocyte saline
- 5α-pregnanediol
5α-pregnane-3α, 20α-diol
References
- AESCHBACH R., LOLIGER J., SCOTT B.C., MURCIA A., BUTLER J., HALLIWELL B., ARUOMA O.I. Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem. Toxicol. 1994;32:31–36. doi: 10.1016/0278-6915(84)90033-4. [DOI] [PubMed] [Google Scholar]
- AMIN J. A single hydrophobic residue confers barbiturate sensitivity to gamma-aminobutyric acid type C receptor. Mol. Pharmacol. 1999;55:411–423. [PubMed] [Google Scholar]
- ASPINWALL L.S., BERMUDEZ I., KING L.A., WAFFORD K.A. The interactions of hexachlorocyclohexane isomers with human GABAA receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1997;282:1557–1564. [PubMed] [Google Scholar]
- BARNARD E.A., SKOLNICK P., OLSEN R.W., MOHLER H., SIEGHART W., BIGGIO G., BRAESTRUP C., BATESON A.N., LANGER S.Z. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- BELELLI D., CALLACHAN H., HILL-VENNING C., PETERS J.A., LAMBERT J.J. Interaction of positive allosteric modulators with human and Drosophila recombinant GABA receptors expressed in Xenopus laevis oocytes. Br. J. Pharmacol. 1996;118:563–576. doi: 10.1111/j.1476-5381.1996.tb15439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELELLI D., PISTIS I., PETERS J.A., LAMBERT J.J. General anaesthetic action at transmitter-gated inhibitory amino acid receptors. Trends Pharmacol. Sci. 1999;20:496–502. doi: 10.1016/s0165-6147(99)01405-4. [DOI] [PubMed] [Google Scholar]
- BLOOMQUIST J.R. Ion channels as targets for insecticides. Annu. Rev. Entomol. 1996;41:163–190. doi: 10.1146/annurev.en.41.010196.001115. [DOI] [PubMed] [Google Scholar]
- BROWN N.H., KAFATOS F.C. Functional cDNA libraries from Drosophila embryos. J. Mol. Biol. 1988;203:425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- CHANGEUX J.P., EDELSTEIN S.J. Allosteric receptors after 30 years. Neuron. 1998;21:959–980. doi: 10.1016/s0896-6273(00)80616-9. [DOI] [PubMed] [Google Scholar]
- COSENTINO S., TUBEROSO C.I., PISANO B., SATTA M., MASCIA V., ARZEDI E., PALMAS F. In-vitro antimicrobial activity and chemical composition of Sardinian Thymus essential oils. Lett. Appl. Microbiol. 1999;29:130–135. doi: 10.1046/j.1472-765x.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- DOLIWA A., SANTOYO S., YGARTUA P. Effect of passive and iontophoretic skin pretreatments with terpenes on the in vitro skin transport of piroxicam. Int. J. Pharmacol. 2001;229:37–44. doi: 10.1016/s0378-5173(01)00849-3. [DOI] [PubMed] [Google Scholar]
- FFRENCH-CONSTANT R.H., MORTLOCK D.P., SHAFFER C.D., MACINTYRE R.J., ROUSH R.T. Molecular cloning and transformation of cyclodiene resistance in Drosophila: an invertebrate gamma-aminobutyric acid subtype A receptor locus. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FFRENCH-CONSTANT R.H., STEICHEN J.C., ROCHELEAU T.A., ARONSTEIN K., ROUSH R.T. A single-amino acid substitution in a γ-aminobutyric acid subtype A receptor locus is associated with cyclodiene insecticide resistance in Drosophila populations. Proc. Natl. Acad. Sci. U.S.A. 1993b;90:1957–1961. doi: 10.1073/pnas.90.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKS N.P., LIEB W.R. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- HADINGHAM K.L., GARRETT E.M., WAFFORD K.A., BAIN C., HEAVENS R.P., SIRINATHSINGHJI D.J., WHITING P.J. Cloning of cDNAs encoding the human gamma-aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors. Mol. Pharmacol. 1996;49:253–259. [PubMed] [Google Scholar]
- HADINGHAM K.L., WINGROVE P., LE BOURDELLES B., PALMER K.J., RAGAN C.I., WHITING P.J. Cloning of cDNA sequences encoding human α2 and α3 γ-aminobutyric acidA receptor subunits and characterisation of the benzodiazepine pharmacology of recombinant α1-, α2-, α3 and α5-containing human γ-aminobutyric acidA receptors. Mol. Pharmacol. 1993a;43:970–975. [PubMed] [Google Scholar]
- HADINGHAM K.L., WINGROVE P., WAFFORD K.A., BAIN C., KEMP J.A., PALMER K.J., WILSON A.W., WILCOX A.S., SIKELA J.M., RAGAN C.I., WHITING P.J. Role of the β subunit in determining the pharmacology of human γ-aminobutyric acid type A receptors. Mol. Pharmacol. 1993b;44:1211–1218. [PubMed] [Google Scholar]
- HAEFELY W.E.Allosteric modulation of the GABAA receptor channel: a mechanism for interaction with a multitude of central nervous system functions The Challenge of Neuropharmacology 1994Basel, Switzerland: Editiones Roche; 15–39.ed. Mohler, H. & Da Prada, M. pp [Google Scholar]
- HILL-VENNING C., BELELLI D., PETERS J.A., LAMBERT J.J. Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type A receptor. Br. J. Pharmacol. 1997;120:749–756. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSIE A.M., ARONSTEIN K., SATTELLE D.B., FFRENCH-CONSTANT R.H. Molecular biology of insect neuronal GABA receptors. Trends Neurosci. 1997;20:578–583. doi: 10.1016/s0166-2236(97)01127-2. [DOI] [PubMed] [Google Scholar]
- HOSIE A.M., BAYLIS H.A., BUCKINGHAM S.D., SATTELLE D.B. Actions of the insecticide fipronil, on dieldrin-sensitive and -resistant GABA receptors of Drosophila melanogaster. Br. J. Pharmacol. 1995;115:909–912. doi: 10.1111/j.1476-5381.1995.tb15896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSIE A.M., OZOE Y., KOIKE K., OHMOTO T., NIKAIDO T., SATTELLE D.B. Actions of picrodendrin antagonists on dieldrin-sensitive and -resistant Drosophila GABA receptors. Br. J. Pharmacol. 1996;119:1569–1576. doi: 10.1111/j.1476-5381.1996.tb16074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSIE A.M., SATTELLE D.B. Allosteric modulation of an expressed homo-oligomeric GABA-gated chloride channel of Drosophila melanogaster. Br. J. Pharmacol. 1996;117:1229–1237. doi: 10.1111/j.1476-5381.1996.tb16720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMELBRUNNER L.A., ISMAN M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae) J. Agric. Food. Chem. 2001;49:715–720. doi: 10.1021/jf000749t. [DOI] [PubMed] [Google Scholar]
- KOENIG J.A., MARTIN I.L. Effect of free fatty acids on GABAA receptor ligand binding. Biochem. Pharmacol. 1992;44:11–15. doi: 10.1016/0006-2952(92)90031-d. [DOI] [PubMed] [Google Scholar]
- KORPI E.R., GRUNDER G., LUDDENS H. Drug interactions at GABA(A) receptors. Prog. Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- LEE S., TSAO R., PETERSON C., COATS J.R. Insecticidal activity of monoterpenoids to western corn rootworm (Coleoptera:Chrysomelidae), twospotted spider mite (Acari: Tetranychidae), and house fly (Diptera: Muscidae) J. Econ. Entomol. 1997;90:883–892. doi: 10.1093/jee/90.4.883. [DOI] [PubMed] [Google Scholar]
- MANABE A., NAKAYAMA S., SAKAMOTO K. Effects of essential oils on erythrocytes and hepatocytes from rats and dipalmitoyl phosphatidylcholine-liposomes. Jpn. J. Pharmacol. 1987;44:77–84. doi: 10.1254/jjp.44.77. [DOI] [PubMed] [Google Scholar]
- MANSOUR S.A., MESSEHA S.S., EL-GENGAIHI S.E. Botanical biocides. 4. Mosquitocidal activity of certain Thymus capitatus constituents. J. Nat. Toxins. 2000;9:49–62. [PubMed] [Google Scholar]
- MOHAMMADI B., HAESELER G., LEUWER M., DENGLER R., KRAMPFL K., BUFLER J. Structural requirements of phenol derivatives for direct activation of chloride currents via GABA(A) receptors. Eur. J. Pharmacol. 2001;421:85–91. doi: 10.1016/s0014-2999(01)01033-0. [DOI] [PubMed] [Google Scholar]
- MOSS S.J., SMART T.G. Constructing inhibitory synapses. Nat. Rev. Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- PETERS J.A., KIRKNESS E.F., CALLACHAN H., LAMBERT J.J., TURNER A.J. Modulation of the GABAA receptor by depressant barbiturates and pregnane steroids. Br. J. Pharmacol. 1988;94:1257–1269. doi: 10.1111/j.1476-5381.1988.tb11646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PISTIS M., BELELLI D., MCGURK K., PETERS J.A., LAMBERT J.J. Complementary regulation of anaesthetic activation of human (α6β3γ2L) and Drosophila (RDL) GABA receptors by a single amino acid residue. J. Physiol. 1999;515:3–18. doi: 10.1111/j.1469-7793.1999.003ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABOW L.E., RUSSEK S.J., FARB D.H. From ion currents to genomic analyses: recent advances in GABAA receptor research. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- ROBERTSON B. Actions of anaesthetics and avermectin on GABAA chloride channels in mammalian dorsal root ganglion neurones. Br. J. Pharmacol. 1989;98:167–176. doi: 10.1111/j.1476-5381.1989.tb16878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANNA E., MOTZO C., USALA M., SERRA M., DAZZI L., MACIOCCO E., TRAPANI G., LATROFA A., LISO G., BIGGIO G. Characterization of the electrophysiological and pharmacological effects of 4-iodo-2,6-diisopropylphenol, a propofol analogue devoid of sedative–anaesthetic properties. Br. J. Pharmacol. 1999;126:1444–1454. doi: 10.1038/sj.bjp.0702449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATTELLE D.B. GABA receptors of insects. Adv. Insect Physiol. 1990;22:1–113. [Google Scholar]
- SATTELLE D.B., PINNOCK R.D., WAFFORD K.A., DAVID J.A. GABA receptors on the cell-body membrane of an identified insect motor neuron. Proc. R. Soc. Lond. B Biol. Sci. 1988;232:443–456. doi: 10.1098/rspb.1988.0006. [DOI] [PubMed] [Google Scholar]
- SIEGHART W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol. Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- SIGEL E., BAUR R., TRUBE G., MOHLER H., MALHERBE P. The effect of subunit composition of rat brain GABAA receptors on channel function. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- SINGH M. Amylase release from dissociated mouse pancreatic acinar cells stimulated by glucagon: effect of membrane stabilizers. J. Physiol. 1980;309:81–91. doi: 10.1113/jphysiol.1980.sp013495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGH V.K., SINGH S., SINGH D.K. Effect of active molluscicidal component of spices on different enzyme activities and biogenic amine levels in the nervous tissue of Lymnaea acuminata. Phytother. Res. 1999;13:649–654. doi: 10.1002/(sici)1099-1573(199912)13:8<649::aid-ptr518>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- STEVENSON A., WINGROVE P.B., WHITING P.J., WAFFORD K.A. beta-Carboline gamma-aminobutyric acidA receptor inverse agonists modulate gamma-aminobutyric acid via the loreclezole binding site as well as the benzodiazepine site. Mol. Pharmacol. 1995;48:965–969. [PubMed] [Google Scholar]
- THOMET U., BAUR R., RAZET R., DODD R.H., FURTMULLER R., SIEGHART W., SIGEL E. A novel positive allosteric modulator of the GABA(A) receptor: the action of (+)-ROD188. Br. J. Pharmacol. 2000;131:843–850. doi: 10.1038/sj.bjp.0703558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VENTURINI M.E., BLANCO D., ORIA R. In vitro antifungal activity of several antimicrobial compounds against Penicillium expansum. J. Food Prot. 2002;65:834–839. doi: 10.4315/0362-028x-65.5.834. [DOI] [PubMed] [Google Scholar]
- WIELAND H.A., LUDDENS H., SEEBURG P.H. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J. Biol. Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- WOODWARD R.M., POLENZANI L., MILEDI R. Effects of hexachlorocyclohexanes on gamma-aminobutyric acid receptors expressed in Xenopus oocytes by RNA from mammalian brain and retina. Mol. Pharmacol. 1992;41:1107–1115. [PubMed] [Google Scholar]
- YAMAKURA T., BERTACCINI E., TRUDELL J.R., HARRIS A.R. Anaesthetics and ion channels: molecular models and sites of action. Annu. Rev. Pharmacol. Toxicol. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]