Abstract
5-Hydroxytryptamine (5-HT) has been postulated to play a proarrhythmic role in the human atria via stimulation of 5-HT4 receptors.
The aims of this study were to examine the effects of 5-HT on the L-type Ca2+ current (ICaL) action potential duration (APD), the effective refractory period (ERP) and arrhythmic activity in human atrial cells, and to assess the effects of prior treatment with β-adrenoceptor antagonists.
Isolated myocytes, from the right atrial appendage of 27 consenting patients undergoing cardiac surgery who were in sinus rhythm, were studied using the whole-cell perforated patch-clamp technique at 37°C.
5-HT (1 nM–10 μM) caused a concentration-dependent increase in ICaL, which was potentiated in cells from β-blocked (maximum response to 5-HT, Emax=299±12% increase above control) compared to non-β-blocked patients (Emax=220±6%, P<0.05), but with no change in either the potency (log EC50: −7.09±0.07 vs −7.26±0.06) or Hill coefficient (nH: 1.5±0.6 vs 1.5±0.3) of the 5-HT concentration–response curve.
5-HT (10 μM) produced a greater increase in the APD at 50% repolarisation (APD50) in cells from β-blocked patients (of 37±10 ms, i.e. 589±197%) vs non-β-blocked patients (of 10±4 ms, i.e. 157±54%; P<0.05). Both the APD90 and the ERP were unaffected by 5-HT.
Arrhythmic activity was observed in response to 5-HT in five of 17 cells (29%) studied from β-blocked, compared to zero of 16 cells from the non-β-blocked patients (P<0.05).
In summary, the 5-HT-induced increase in calcium current was associated with a prolonged early plateau phase of repolarisation, but not late repolarisation or refractoriness, and the enhancement of these effects by chronic β-adrenoceptor blockade was associated with arrhythmic potential.
Keywords: Human atrium, isolated myocytes, 5-HT4 receptors, calcium current, action potential, refractory period β-adrenergic antagonists, arrhythmias (mechanisms)
Introduction
5-Hydroxytryptamine (5-HT) exerts a variety of effects in the heart, brain, adrenocortical cells, urinary bladder and alimentary canal via stimulation of the 5-HT4 receptor subtype (Hegde & Eglen, 1996). The 5-HT4 receptor is functionally present in the human atrium (Blondel et al., 1997), but not in the ventricle (Jahnel et al., 1992), which has stimulated interest in its possible roles in the occurrence of atrial arrhythmia. It has been postulated that 5-HT is involved in the progression of atrial fibrillation (AF) in patients due to its release from aggregating platelets within the fibrillating atria (Kaumann, 1994). In addition, there has been concern about the potential for atrial arrhythmia generation when using 5-HT4 receptor agonists as gastrokinetic agents (Medhurst & Kaumann, 1993; Tonini et al., 1999). In human atrial isolated muscle, arrhythmic contractions were induced by 5-HT, and abolished by a selective 5-HT4 receptor antagonist (Kaumann & Sanders, 1994). 5-HT has been shown to increase the magnitude of the L-type calcium current (ICaL) in human atrial myocytes (Ouadid et al., 1992; Jahnel et al., 1993), which may contribute to intracellular calcium overload and arrhythmic activity. However, in a study of a pig model of atrial flutter/AF (Rahme et al., 1999), the 5-HT4 receptor antagonist, RS-100302, was demonstrated to be antiarrhythmic, associated with prolongation of the atrial effective refractory period (ERP). This suggested that 5-HT may shorten the atrial ERP, which may predispose to AF by reducing the minimum path length required for re-entry. The importance of these potential arrhythmogenic effects of 5-HT, namely calcium overload and/or shortened refractoriness, have not yet been clarified in human atria.
One factor that may influence the arrhythmogenic potential of 5-HT is the prior treatment of the patient with β-adrenoceptor antagonists. The ability of 5-HT to cause rate-dependent arrhythmic contractions in strips of human atrial tissue was facilitated by chronic β-blockade (Kaumann & Sanders, 1994; Sanders et al., 1995). In addition, prior β-adrenoceptors antagonists (β-blocker) treatment increased the positive inotropic response of human atrial muscle to 5-HT (Sanders et al., 1995; Wangemann et al., 2003), but the influence of β-blockade on the effects of 5-HT on ICaL is unknown. We have recently reported that chronic β-blockade is associated with prolongation of the basal action potential duration (APD) and ERP in human atrial myocytes, and that this effect is not related to a change in ICaL (Workman et al., 2003). Such an increase in APD may result in increased contractile tension independent of ICaL (Bers, 2002), and this may underlie the increased basal force of contraction in atrial strips from patients pretreated with β1-adrenoceptor antagonists (Wangemann et al., 2003). However, the ionic mechanism by which chronic β-blockade enhances the effects of 5-HT on human atria, and in particular whether ICaL is involved, has not been proven.
The aims of this work were: (1) to study how the increase in ICaL by 5-HT may affect the action potential morphology, the cellular refractoriness and the occurrence of arrhythmic activity, and (2) to investigate the influence of chronic β-blocker therapy on the actions of 5-HT on ICaL, APD, ERP and arrhythmic mechanism in human atrial single myocytes.
Methods
Tissue and cell isolation
The procedures for the removal of human tissue were approved by the institutional research ethics committee of Glasgow Royal Infirmary, and each patient's informed consent was obtained. The investigation conforms to the principles outlined in the Declaration of Helsinki (World Medical Association, 1997). Specimens of the right atrial appendage were obtained from patients undergoing cardiac surgery. The excised tissue (weight: 0.45±0.06 g) was placed in Tyrode's solution and transported to the laboratory for processing within 5 min of removal. Atrial cells were isolated by enzymatic dissociation and mechanical disaggregation, using a modification of the chunk method, described in detail by Workman et al. (2001).
Electrical recording techniques
Action potentials and ion currents were recorded using the whole-cell patch-clamp technique, with an Axopatch-1D amplifier (Axon Instruments). Cells were superfused at 37°C at 1.5 ml min−1 (RC-24E fast exchange perfusion chamber, Warner) with a physiological salt solution containing (mM): NaCl (130.0), KCl (4.0), CaCl2 (2.0), MgCl2 (1.0), glucose (10.0), HEPES (10.0), pH 7.4. The perforated patch-clamp technique, with nystatin (184 μM), was used to prevent cell dialysis, prolonging recording and minimising current ‘run-down'. Microelectrodes were constructed from thin walled, filamented borosilicate glass (Clark Electromedical) using a micropipette puller (Narishige PP-83), and heat polished to resistances of 3–9 MΩ. To record calcium currents, electrodes were filled with a caesium-based solution (to eliminate outward K+ currents) containing (mM): CsCl (30.0), HEPES (5.0), MgCl2 (1.0), Cs methanesulphonic acid (100.0), NaCl (5.0). To record action potentials, an internal potassium-based solution was used, containing (mM): KCl (30.0), HEPES (5.0), MgCl2 (1.0), K methanesulphonic acid (100.0), NaCl (5.0). Using these solutions, a liquid junction potential of +5.0±0.2 mV (n=6) was measured (bath relative to pipette) and was compensated for prior to seal formation (Neher, 1992). Only single, elongated myocytes were selected for electrical recording. Following seal formation, a gradual reduction in the series resistance due to nystatin pore formation was observed, which stabilised (after ∼10 min) at 11.1±0.5 MΩ (n=84 cells). The mean cell capacity was 77±3 pF. Capacitative transients were subtracted electronically from the recordings. The voltage drop across the series resistance was routinely compensated for electronically, by 68–80%. The software program WinWCP (J Dempster, Strathclyde University) was used both to stimulate and record electrical activity. All currents were normalised to the cell's capacity. Current and voltage signals were low-pass filtered at 5 kHz and digitised (Digidata 1200 A-D converter, Axon Instruments) prior to storage on magnetic and compact discs.
Experimental protocols
ICaL was recorded under voltage-clamp conditions. The voltage dependency of this current was measured from a holding potential of −40 mV with depolarising pulses of 100 ms duration (0.33 Hz), increasing in steps of 10 mV, up to +40 mV. The time course of change in ICaL due to drugs was examined with repetitive voltage pulses, from −40 to +10 mV.
Action potentials were stimulated at 75 beats min−1 (bpm) using 5 ms current pulses of 1.2 × threshold strength, after current clamping resting cells at −75 to −80 mV, and keeping the holding current (<150 pA) constant thereafter. The stimulus threshold current amplitude was initially determined in each cell by stimulating repetitively with trains of three current pulses (5 ms duration), the 1st and 2nd being of equal, suprathreshold amplitude, and the 3rd pulse increasing progressively (from zero, in steps of 50 pA) until it produced a regenerative action potential. The stimulus strength was then kept constant throughout all recording protocols in each cell. The cell's ERP was measured using a standard S1–S2 stimulation protocol, with an 8-pulse conditioning train delivered at 75 bpm, and with S1 and S2 pulses of equal magnitude. The S1–S2 interval was shortened in 10 ms steps, and the ERP was defined as the longest S1–S2 interval which failed to elicit an S2 action potential of amplitude >80% of the preceding S1 action potential. The APD was calculated as the interval between the action potential upstroke and repolarisation to the level of 50% (APD50) and 90% (APD90) of the upstroke amplitude. ICaL and/or action potentials and the ERP were recorded before, and 90 s after, drug additions and again 180 s after removal of drugs.
Drugs
5-HT (Sigma, St Louis, MO, U.S.A.) was made up as a 10 mM stock solution in distilled water. The specific 5-HT4 antagonist GR-113808 (Kaumann, 1993) was kindly donated by Johnson & Johnson Pharmaceutical Research & Development and was prepared as a 10 mM stock solution in dimethyl sulphoxide.
Data analysis and statistics
Details of each patient's clinical characteristics and drug treatments were obtained from the case notes. The cardiac rhythm and heart rate were assessed from the preoperative electrocardiogram. Only those patients in sinus rhythm at the time of surgery were included. Cells were excluded from analysis if either the APD50 or peak ICaL decreased irreversibly during the protocol. Concentration–response data for the effect of 5-HT on ICaL were fitted iteratively (Prism software, Graphpad) with variable slope sigmoidal concentration–response curves, using the Hill equation: Y=Emin+[Emax−Emin]/[1+(x/EC50)nH], where Y=ICaL density (pA/pF, expressed as % above control), Emin=ICaL at 0 mM 5-HT (set to 0%), Emax=maximum ICaL response elicitable by 5-HT (% above control), x=[5-HT] (mM), EC50=[5-HT] producing 50% of Emax (mM) and nH=Hill coefficient (describing the steepness of the slope). The curves were fitted to mean ICaL values, obtained at five concentrations of 5-HT, within the range of 1 nM–10 μM. The use of the perforated patch technique maximised the duration of each experiment, but it was not possible to measure ICaL at every concentration studied in each cell. Curve fit values were compared using a two-tailed unpaired Student's t-test. Measurements taken from the action potentials were the resting potential (Vm), overshoot, amplitude and maximum upstroke velocity (Vmax), the APD50 and APD90, and the ERP. Data are expressed as mean±standard error of the mean (s.e.m.), with n being equal to the number of cells studied. The mean values were compared using two-tailed paired or unpaired Student's t-tests, as appropriate. A Chi-square test (χ2) was used to assess the level of significance of differences in the incidences of arrhythmic activity between groups. P<0.05 was regarded as statistically significant.
Results
Patients' characteristics
The majority of patients was male, underwent coronary artery bypass graft surgery, suffered from angina, and had normal left ventricular function (Table 1 ). In total 59% of the patients were taking β-adrenoceptor blockers, and 59% calcium channel blockers. Of the patients taking β-blockers, 50% were also receiving calcium channel blockers. All patients undergoing β-blockade were treated for >1 month with cardiac selective β1-adrenoceptor antagonists, namely atenolol (69%), bisoprolol (19%) or metoprolol (12%). No patient was administered sotalol (which has additional class III antiarrhythmic activity) except for one patient who was changed from sotalol to atenolol 1 week prior to surgery. Patients received their routine cardiac drugs on the day of surgery. Of the patients treated with calcium channel blockers, 44% were receiving diltiazem, a cardiac-acting drug, and the remaining 56% were taking dihydropyridines, with mainly vascular actions, namely amlodipine (38%), felodipine (12%) and nifedipine (6%). The heart rate was significantly reduced in β-blocked (62±4 bpm, n=16) compared to non-β-blocked patients (75±3 bpm, P<0.05, n=11), but was not altered by calcium channel blocker therapy (68±6 vs 66±3 bpm, for treated and nontreated patients, respectively).
Table 1.
Patients' preoperative clinical characteristics
| n | % | |
|---|---|---|
| Patients | 27 | |
| Male/female | 21/6 | 78/22 |
| Age | 66±2 | |
| Surgery | ||
| CABG | 22 | 82 |
| CABG+AVR | 5 | 18 |
| Drugs | ||
| Ca2+ channel blocker | 16 | 59 |
| β-Blocker | 16 | 59 |
| ACE inhibitor | 14 | 52 |
| Nitrate | 18 | 67 |
| Diuretic | 6 | 22 |
| Lipid lowering | 22 | 81 |
| Digoxin | 0 | 0 |
| Warfarin | 0 | 0 |
| Symptoms | ||
| Angina | 25 | 93 |
| Palpitations | 5 | 19 |
| Hypertension | 15 | 56 |
| Hyperlipidaemia | 21 | 78 |
| Previous history | ||
| MI | 10 | 37 |
| Diabetes | 2 | 7 |
| LV function | ||
| Normal | 21 | 78 |
| Mild–moderate | 5 | 18 |
| Severe | 1 | 4 |
Values are numbers of patients (n and % of total, respectively) with selected clinical characteristics, except for age (mean±s.e.m.). CABG=coronary artery bypass graft surgery; AVR=aortic valve replacement; ACE=angiotensin converting enzyme; MI=myocardial infarction; LV=left ventricular. All patients were in sinus rhythm at surgery.
Effects of 5-HT and GR-113808 on the calcium current in human atrial cells
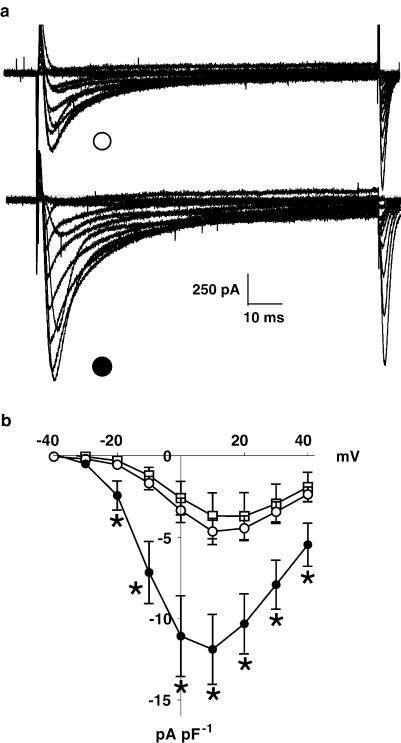
5-HT produced a substantial increase in the amplitude of ICaL, as shown by the original recordings in Figure 1a, and by the ICaL current density–voltage relationships in Figure 1b. 5-HT (10 μM) increased the mean magnitude of peak ICaL (recorded at +10 mV) from −4.7±0.8 to −11.9±2.2 pA pF−1 (P<0.05, n=7 cells from six patients), that is, by approximately 150%. This increase in ICaL occurred without any change in the voltage dependency of the current, and was shown to be reversible on washout of 5-HT (−3.7±1.4 pA pF−1, n=3 cells, three patients) (Figure 1b).
Figure 1.
Effect of 5-HT on ICaL current–voltage relationship in human atrial myocytes. (a) An example of original calcium current (ICaL) traces obtained from a human atrial cell (in this case from a β-blocked patient), during depolarising voltage-clamp pulses (100 ms, 0.33 Hz) from −40 to +40 mV, in 10 mV incremental steps, from a holding potential of −40 mV, under control conditions (open circle) and in the presence of 5-HT at 10 μM (closed circle). (b) Current–voltage relationships of ICaL expressed in terms of current density, pA pF−1 (n=7 cells, from six patients, treated and not treated with β-blockers). Values are means, with error bars denoting s.e.m., for control (open circles) 5-HT at 10 μM (closed circles) and after 3 min washout of 5-HT (open squares, n=3 cells, three patients). Asterisks indicate P<0.05 between control and 5-HT values at each voltage step (paired t-test).
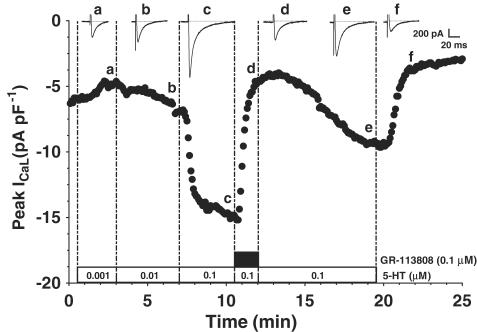
The time course of the effect of 5-HT on ICaL and its blockade by the specific 5-HT4 receptor antagonist, GR-113808, can be seen in Figure 2. 5-HT (0.1 μM) caused a stable increase in ICaL, which was completely antagonised by 0.1 μM GR-113808. The antagonism of the 5-HT-induced increase in ICaL by GR-113808 was seen in three cells from three patients. The effect of this antagonist was partially reversible upon its washout (Figure 2). GR-113808 (0.1 μM) had no direct effect on basal (unstimulated by 5-HT) ICaL (two cells from two patients).
Figure 2.
Effect of the specific 5-HT4 antagonist GR-113808 on ICaL stimulated by 5-HT in human atrial myocytes. An example of the time course of change in peak ICaL density (measured in this case in a cell from a β-blocked patient) plotted at 5 s resolution, in response to 0.001–0.1 μM 5-HT (open boxes), followed by the application of GR-113808 at 0.1 μM (solid box), and the subsequent washout of the antagonist and then of 5-HT. Inset traces show original currents recorded at the time points labelled.
Concentration–response relationship of the effect of 5-HT on ICaL
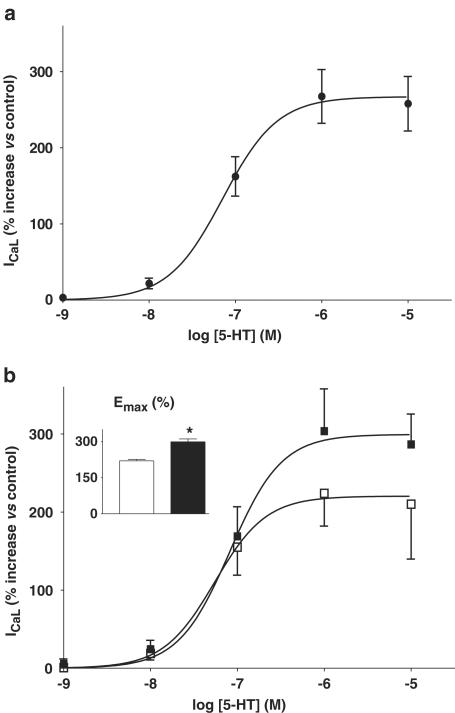
The effect of 5-HT on ICaL was concentration dependent. Figure 3a shows the concentration–response curve of the effect of 5-HT between 1 nM and 10 μM, fitted to the mean values obtained at each concentration. The log EC50 calculated from this curve was −7.15±0.05, and the nH was 1.37±0.28 (n=17–33 cells, 9–17 patients). The maximum response of ICaL to 5-HT (Emax) was observed to occur at around 1 μM 5-HT, with a value of 267±7%.
Figure 3.
Concentration-dependent effects of 5-HT on ICaL in human atrial myocytes. (a) Concentration–response relationship for 5-HT (0.001–10 μM) on peak ICaL in human atrial myocytes. Values are means±s.e.m. (n=17–33 cells, 9–17 patients). The increase in ICaL is expressed as a percentage of the control value before the addition of 5-HT. The mean data points were fitted by a variable slope sigmoidal curve using the Hill equation (see Methods). (b) Comparison of concentration–response curves for 5-HT on ICaL and (inset) of maximal ICaL response to 5-HT, Emax, between cells from patients not treated with β-blockers (open symbols; n=8–15 cells, 5–7 patients) and those from patients treated with β-blockers (closed symbols; n=9–18 cells, 4–11 patients). Asterisk denotes P<0.05 vs non-β-blocked group.
In Figure 3b, the 5-HT concentration–response data has been subdivided into those obtained from patients with and without prior treatment with β-blockers. The respective log EC50 (−7.09±0.07 and −7.26±0.06) and nH (1.5±0.6 and 1.5±0.3) values were not significantly different between these patient groups. By contrast, 5-HT caused a significantly greater increase in Emax in β-blocked (at 299±12%, n=9–18 cells, 4–11 patients) than in non-β-blocked patients (at 220±6%, n=8–15 cells, 5–7 patients; P<0.05) (Figure 3b). There was no significant difference in basal ICaL between non-β-blocked (−5.4±0.7 pA pF−1, n=30) and β-blocked patients (−4.2±0.3 pA pF−1, n=50).
Pretreatment with calcium channel blockers did not significantly alter the concentration–response curves to 5-HT on ICaL. Similarly, basal ICaL was not significantly different between cells from patients who had, and those from patients who had not received calcium channel blockers, at −4.8±0.6 (n=32) and −4.5±0.4 pA pF−1 (n=48), respectively.
Effect of 5-HT on action potentials and the refractory period
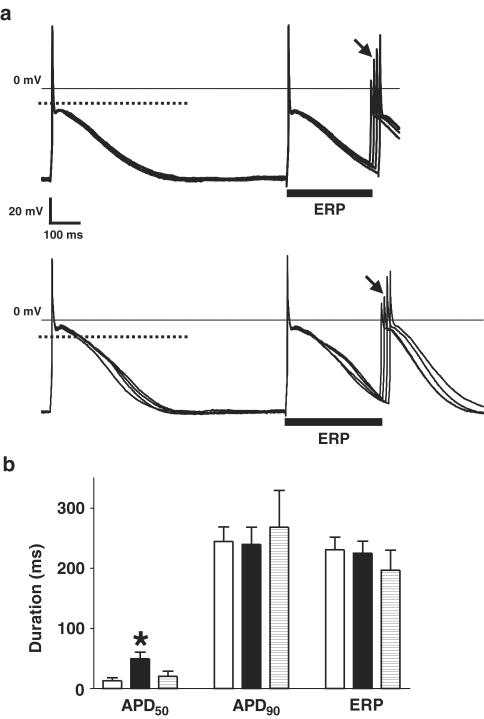
Figure 4a shows original action potentials and ERP measurement, from a single human atrial myocyte obtained from a patient who had not been treated with a β-blocker. 5-HT (10 μM) produced a small, but significant, prolongation in the APD50, but with little effect on the plateau amplitude, late repolarisation (APD90) or the ERP. The mean data confirmed these effects of 5-HT in the cells from nontreated patients (Figure 4b). 5-HT (10 μM) increased the APD50 from 6±1 to 16±5 ms (P<0.05), an effect that was fully reversible upon washout of 5-HT, but there was no significant or reversible effect of 5-HT on the APD90 (193±20 vs 208±24 ms, P>0.05) or ERP (178±23 vs 195±23 ms, P>0.05). Other action potential measurements, including Vmax (191±14 V s−1), overshoot (59±3 mV) and amplitude (139±2 mV), were unaffected by 5-HT.
Figure 4.
Effect of 5-HT on action potentials and refractoriness in single human atrial cells from patients not treated with β-blockers. (a) Representative examples of original action potential recordings obtained from a single human atrial myocyte, from a patient not treated with a β-blocker, before (upper panel) and in the presence of 10 μM 5-HT (lower panel). Cells were paced at 75 bpm. Dotted lines in bold show the level of 50% of the action potential amplitude. The majority of cells displayed type 3 action potentials, that is, with pronounced phase 1 and a plateau amplitude below the 50% level. The ERP, indicated by solid bars, was calculated as the longest S1–S2 interval failing to elicit an S2 response of amplitude >80% of the preceding S1 action potential. The S2 response used to measure this interval is labelled with an arrow. (b) Mean (±s.e.m.) APD (ms) measured at 50 and 90% repolarisation (APD50 and APD90, respectively; n=16 cells, eight patients) and ERP (n=12 cells, eight patients) in cells from patients not treated with β-blockers, in the absence (open bars), in the presence (closed bars) and after removal of 10 μM 5-HT (striped bars; n=11 cells, eight patients for APD and n=9 cells, seven patients for ERP). Asterisk denotes P<0.05 between control and 5-HT values (paired t-test).
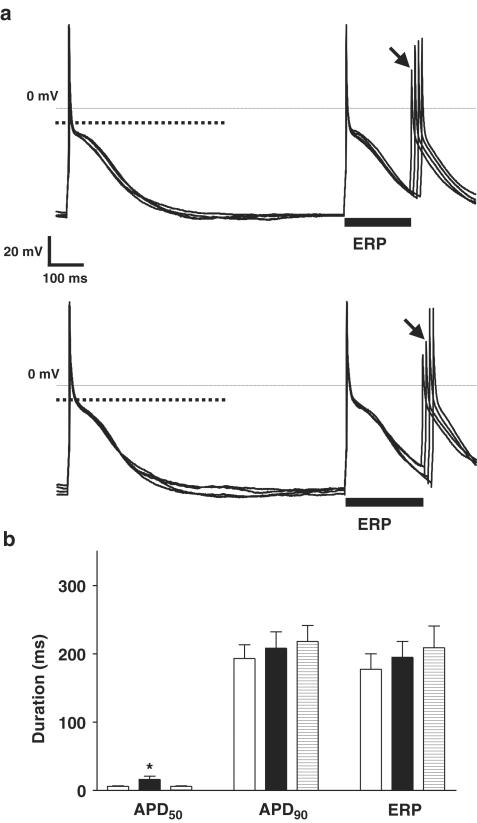
Chronic β-blocker treatment was associated with a greater prolongation of the early plateau phase of repolarisation by 5-HT than nontreatment. Figure 5a shows action potential traces and ERP measurements in a single cell from a β-blocked patient. 5-HT (10 μM) produced an elevation in the action potential plateau, with a consequent lengthening in the duration of the APD50. Figure 5b shows the mean data confirming this effect in the cells from β-blocked patients, with a marked and reversible increase in APD50 by 5-HT, from 13±5 to 50±11 ms (P<0.05). The mean prolongation in APD50 was 37±10 ms (P<0.05), representing an increase of 589±197% (P<0.05). This compares with a prolongation in APD50 by 5-HT in the cells from the non-β-blocked patients of 10±4 ms (P<0.05), or 157±54% (P<0.05). Both the absolute and percentage increase in APD50 by 5-HT were significantly greater in the cells from the patients treated with β-blockers, than in those from the nontreated patients (P<0.05 for each). The 5-HT-induced increase in the APD50 was abolished by the specific 5-HT4 antagonist GR-113808 (three cells from two patients, one with and one without prior β-blocker treatment), and this effect of the antagonist was partially reversible. In cells from β-blocked patients, 5-HT had no significant effect on the APD90 (245±24 vs 240±29 ms, P>0.05) or ERP (231±21 vs 225±20 ms, P>0.05), nor on the action potential Vmax, overshoot or amplitude. Prior treatment of patients with calcium channel blockers had no significant effect on basal APD50, APD90 or ERP, nor on the effects of 5-HT on these parameters. There were no significant differences in the capacity of cells obtained from patients treated and not treated with either β-blockers or calcium channel blockers.
Figure 5.
Effect of 5-HT on APD and refractoriness in human atrial cells from patients treated with β-blockers. (a) Representative examples of action potentials recorded from a single human atrial myocyte from a patient treated with a β-blocker, before (upper panel) and in the presence of 5-HT at 10 μM (lower panel). Cells were paced at 75 bpm. Dotted lines in bold show the level of 50% of the action potential amplitude. The ERP, indicated by solid bars, was defined and calculated as in the legend of Figure 4a. (b) Mean (±s.e.m.) APD (APD50 and APD90; n=17 cells, nine patients) and ERP (n=12 cells, nine patients) in cells from patients treated with β-blockers, in the absence (open bars), in the presence (closed bars) and after removal of 10 μM 5-HT (striped bars; n=7 cells, five patients for APD and n=3 cells, three patients for ERP). The asterisk denotes P<0.05 between control and 5-HT values (paired t-test).
Effects of 5-HT on arrhythmic activity in human atrial cells
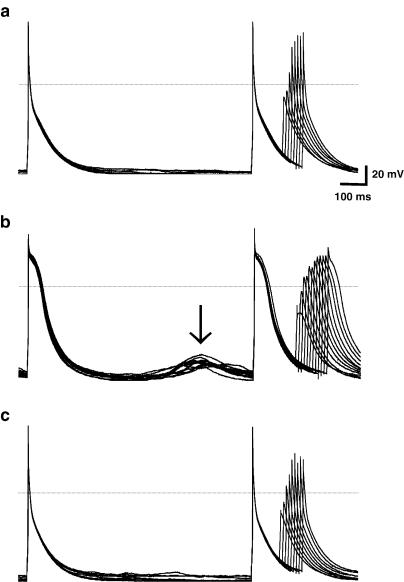
Chronic β-blockade was associated with an increased incidence of 5-HT-induced atrial cellular arrhythmic activity. Figure 6 shows an example of such activity, with the induction of delayed after depolarisations by 10 μM 5-HT, and their abolition following the removal of 5-HT, in a cell from a patient treated with a β-blocker. Abnormal depolarisations, including delayed after depolarisations and early after depolarisations were observed in five of 17 (29%) of the cells obtained from the patients who underwent chronic β-blockade in which action potentials were measured. By contrast, abnormal depolarisations were observed not to occur in response to 5-HT, in any of the 16 cells from the non-β-blocked patients in which action potentials were measured (P<0.05, χ2 test).
Figure 6.
Effect of 5-HT to promote abnormal depolarisations in cells from β-blocked patients. An example of original recordings of action potentials obtained from a single human atrial myocyte (75 bpm pacing) from a patient treated with a β-blocker, before (top panel), in the presence of 5-HT at 10 μM (middle panel), and then following washout of 5-HT (bottom panel). The arrow indicates the presence of abnormal depolarisations, which occurred only in the presence of 5-HT.
Discussion
The present work has demonstrated, for the first time to our knowledge, that the effects of 5-HT to increase the L-type Ca2+ current and to prolong the early plateau phase of action potential repolarisation in human atrial myocytes were each potentiated by prior treatment of patients with β-blockers, with evidence of arrhythmogenic activity. Since 5-HT had no effect on late repolarisation or refractoriness, it would not be expected to contribute to the shortening of re-entrant circuits, which stabilises AF. It is more likely that 5-HT may contribute to arrhythmia genesis, by causing after depolarisations via mechanisms involving increased ICaL and cellular calcium overload (Levy, 1989).
AF is known to be associated with platelet activation (Kamath et al., 2001), and it has been proposed that the consequent release of 5-HT may be involved in the progression of the arrhythmia (Kaumann, 1994). The concentration of free 5-HT in human whole blood has been reported to range from nanomolar, rising to nearly micromolar, levels after activation of platelets (Joseph et al., 1991). The present results confirm that within this concentration range 5-HT has important electrophysiological effects on human atrial myocytes. The novel finding of a prolongation by 5-HT of the early plateau phase of atrial action potential repolarisation, associated with plateau elevation, with no effect on late repolarisation or the ERP, is likely due to the observed increase in ICaL. While an involvement of the transient outward K+ current, ITO, which also contributes to early repolarisation, cannot be excluded, no reports of effects of 5-HT on ITO were found. Furthermore, a predominant contribution from ICaL is supported by a report that blockade of ICaL by nifedipine, a calcium channel blocker, markedly depressed the action potential plateau in human atrial cells, with a relatively small effect on late repolarisation and none on the ERP (Workman et al., 2001). The increase in ICaL may contribute to the intracellular calcium overload that occurs in AF, which is thought to contribute to subsequent electrophysiological changes (Nattel, 1999). The reported reduction in 5-HT4 receptor mRNA in chronically fibrillating human atria may represent an adaptive response in order to reduce calcium entry (Grammer et al., 2001).
There has been only one previous, nonquantitative, study of the effect of 5-HT on human atrial action potentials, and 5-HT had no effect on action potential morphology but increased tension in muscle strips, and increased ICaL in myocytes (Jahnel et al., 1993). In the present study, the majority of cells exhibited characteristic atrial type 3 action potentials (Dawodu et al., 1996), that is, with a pronounced phase 1, and a plateau below the level of 50% repolarisation, making APD50 more susceptible to changes in plateau amplitude, and hence inward currents. There are no previous reports of the effects of 5-HT on the ERP in the human atrium. In a pig model of AF, the 5-HT4 receptor antagonist, RS-100302, prolonged the ERP and was antiarrhythmic (Rahme et al., 1999). If that effect was a consequence of 5-HT4 receptor blockade in vivo, it suggested that 5-HT should reduce the ERP, which, by reducing the minimum path length required for re-entry, would promote AF (Moe, 1962). While this could represent a species-specific effect, or an additional direct action of the drug, it is also recognised that myocytes isolated by the ‘chunk' method may lack the delayed rectifier potassium current (Yue et al., 1996). Thus, even if 5-HT increased this current in vivo, shortening of late repolarisation and/or the ERP may not be observed in isolated cells. However, the report of an absence of effect of 5-HT on the action potential in intact human atrial muscle (Jahnel et al., 1993) makes this less likely, since ERP in human atrial myocytes is closely related to APD90 (Workman et al., 2001).
The concentration-dependent increase in ICaL, mediated via the 5-HT4 receptor subtype in the present report, with an EC50 of about 70 nM, is consistent with that reported for ICaL in human atrial myocytes (Ouadid et al., 1992) but is also of a similar potency to that reported for the positive inotropic effect of 5-HT in human atrial preparations (Jahnel et al., 1992). The present, novel observation of potentiation by β-blockade of the 5-HT effect on ICaL supports the likelihood that the reported positive inotropic action of 5-HT, which was also increased by β-blockade, is directly related to the increase in ICaL. Our finding of an increased efficacy, and no change in the EC50, of the effect of 5-HT on ICaL with β-blockade is in agreement with the increased maximal inotropic effect of 5-HT in human atrial strips, without change in potency, reported by Sanders et al. (1995). This differs from data on cell shortening in the same study (increased potency, no increase in maximal response), but is supported by a recent report of sensitisation by β-blockade of inotropic responses to 5-HT in human atrial strips (Wangemann et al., 2003). The reason for the discrepancy within the study of Sanders et al. (1995), and with our results is unknown, but it suggests that the potentiation of the effect of 5-HT on ICaL is correlated better with the maximal inotropic response than with myocyte shortening.
Chronic β-blockade has recently been shown to increase basal APD90 and ERP in human atrial myocytes, consistent with the trends in the present data, and this ‘pharmacological remodelling' was independent of an effect on ICaL (Workman et al., 2003). By contrast, the present effects of β-blockade, on responses to 5-HT, appear more likely to be related to changes in the phosphorylation state of the calcium channel. This may be due to intracellular cross-talk between Gs-coupled receptor populations, since chronic β-adrenoceptor blockade enhances inotropic effects mediated by β2-adrenoceptors, histamine H2 and 5-HT4 stimulation (Wangemann et al., 2003). It has been shown that the levels of mRNA encoding human atrial 5-HT4 receptors were unaffected by chronic β-blockade (Grammer et al., 2001), and the affinity of the β2-adrenoceptor was also unaffected (Hall et al., 1990). Thus, the increased efficacy of 5-HT with β-blockade that we, and others, have found is likely due to an enhancement of the intracellular biochemical cascade which links the receptor to calcium channels, rather than to changes in 5-HT4 receptor density or affinity. An alteration in the activity of G-proteins may be involved (Grimm et al., 1998; Wang et al., 1999) and while expression of atrial Gs and Gi was unaffected by chronic β-blockade (Jia et al., 1995), the activity of Gs was enhanced (Wang et al., 1999). Furthermore, 5-HT-induced increases in adenylyl cyclase activity (Wangemann et al., 2003) and intracellular cAMP (Sanders et al., 1995), leading to channel phosphorylation and increased ICaL availability, are potentiated in atrial tissue from β-blocked patients.
In conclusion, these data indicate that, in the human atrium, the 5-HT-induced increase in calcium current is associated with a prolonged early plateau phase of repolarisation, but not late repolarisation or refractoriness, and that the enhancement of these effects by chronic β-adrenoceptor blockade is associated with arrhythmic potential. They provide further support for the hypothesis that 5-HT released by aggregating platelets during AF, or 5-HT4 receptor agonists as therapeutic agents, may contribute to the origin and maintenance of atrial arrhythmia.
Acknowledgments
We thank the cardiac surgical staff of Glasgow Royal Infirmary for kindly providing access to human tissues, and Julie Russell for excellent technical support and for maintaining the patient database. This work was supported by Johnson & Johnson Pharmaceutical Research and Development.
Abbreviations
- APD
action potential duration
- β-blockers
β-adrenoceptors antagonists
- Emax
maximal ICaL response to 5-HT
- EC50
effective concentration causing 50% of maximal ICaL response
- ERP
effective refractory period
- 5-HT
5-hydroxytryptamine
- ICaL
L-type Ca2+ current
- nH
Hill coefficient
References
- BERS D.M. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- BLONDEL O., VANDECASTEELE G., GASTINEAU M., LECLERC S., DAHMOUNE Y., LANGLOIS M., FISCHMEISTER R. Molecular and functional characterization of a 5-HT4 receptor cloned from human atrium. FEBS Lett. 1997;412:465–474. doi: 10.1016/s0014-5793(97)00820-x. [DOI] [PubMed] [Google Scholar]
- DAWODU A.A., MONTI F., IWASHIRO K., SCHIARITI M., CHIAVARELLI R., PUDDU P.E. The shape of human atrial action potential accounts for different frequency-related changes in vitro. Int. J. Cardiol. 1996;54:237–249. doi: 10.1016/0167-5273(96)02605-8. [DOI] [PubMed] [Google Scholar]
- GRAMMER J.B., ZENG X., BOSCH R.F., KUHLKAMP V. Atrial L-type Ca2+-channel, β-adrenoreceptor, and 5-hydroxytryptamine type 4 receptor mRNAs in human atrial fibrillation. Basic Res. Cardiol. 2001;96:82–90. doi: 10.1007/s003950170081. [DOI] [PubMed] [Google Scholar]
- GRIMM M., GSELL S., MITTMANN C., NOSE M., SCHOLZ H., WEIL J., ESCHENHAGEN T. Inactivation of Giα proteins increases arrhythmogenic effects of β-adrenergic stimulation in the heart. J. Mol. Cell. Cardiol. 1998;30:1917–1928. doi: 10.1006/jmcc.1998.0769. [DOI] [PubMed] [Google Scholar]
- HALL J.A., KAUMANN A.J., BROWN M.J. Selective β1-adrenoceptor blockade enhances positive inotropic responses to endogenous catecholamines mediated through β2-adrenoceptors in human atrial myocardium. Circ. Res. 1990;66:1610–1623. doi: 10.1161/01.res.66.6.1610. [DOI] [PubMed] [Google Scholar]
- HEGDE S.S., EGLEN R.M. Peripheral 5-HT4 receptors. FASEB J. 1996;10:1398–1407. doi: 10.1096/fasebj.10.12.8903510. [DOI] [PubMed] [Google Scholar]
- JAHNEL U., NAWRATH H., RUPP J., OCHI R. L-type calcium channel activity in human atrial myocytes as influenced by 5-HT. Naunyn Schmiedebergs Arch. Pharmacol. 1993;348:396–402. doi: 10.1007/BF00171339. [DOI] [PubMed] [Google Scholar]
- JAHNEL U., RUPP J., ERTL R., NAWRATH H. Positive inotropic response to 5-HT in human atrial but not in ventricular heart muscle. Naunyn Schmiedebergs Arch. Pharmacol. 1992;346:482–485. doi: 10.1007/BF00169000. [DOI] [PubMed] [Google Scholar]
- JIA H., MONTEITH S., BROWN M.J. Expression of the α- and β-subunits of the stimulatory and inhibitory G-proteins in β1-adrenoceptor-blocked and non-β-adrenoceptor-blocked human atrium. Clin. Sci. (London) 1995;88:571–580. doi: 10.1042/cs0880571. [DOI] [PubMed] [Google Scholar]
- JOSEPH R., TSERING C., GRUNFELD S., WELCH K.M. Platelet secretory products may contribute to neuronal injury. Stroke. 1991;22:1448–1451. doi: 10.1161/01.str.22.11.1448. [DOI] [PubMed] [Google Scholar]
- KAMATH S., BLANN A.D., LIP G.Y.H. Platelets and atrial fibrillation. Eur. Heart J. 2001;22:2233–2242. doi: 10.1053/euhj.2001.2612. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Blockade of human atrial 5-HT4 receptors by GR 113808. Br. J. Pharmacol. 1993;110:1172–1174. doi: 10.1111/j.1476-5381.1993.tb13937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J. Do human atrial 5-HT4 receptors mediate arrhythmias. Trends Pharmacol. Sci. 1994;15:451–455. doi: 10.1016/0165-6147(94)90058-2. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., SANDERS L. 5-Hydroxytryptamine causes rate-dependent arrhythmias through 5-HT4 receptors in human atrium: facilitation by chronic β-adrenoceptor blockade. Naunyn Schmiedebergs Arch. Pharmacol. 1994;349:331–337. doi: 10.1007/BF00170877. [DOI] [PubMed] [Google Scholar]
- LEVY M.N. Role of calcium in arrhythmogenesis. Circulation. 1989;80:IV23–IV30. [PubMed] [Google Scholar]
- MEDHURST A.D., KAUMANN A.J. Characterization of the 5-HT4 receptor mediating tachycardia in piglet isolated right atrium. Br. J. Pharmacol. 1993;110:1023–1030. doi: 10.1111/j.1476-5381.1993.tb13916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOE G. On the multiple wavelet hypotesis of atrial fibrillation. Arch. Int. Pharmacodyn. Ther. 1962;140:183–188. [Google Scholar]
- NATTEL S. Atrial electrophysiological remodeling caused by rapid atrial activation: underlying mechanisms and clinical relevance to atrial fibrillation. Cardiovasc. Res. 1999;42:298–308. doi: 10.1016/s0008-6363(99)00022-x. [DOI] [PubMed] [Google Scholar]
- NEHER E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- OUADID H., SEGUIN J., DUMUIS A., BOCKAERT J., NARGEOT J. Serotonin increases calcium current in human atrial myocytes via the newly described 5-hydroxytryptamine4 receptors. Mol. Pharmacol. 1992;41:346–351. [PubMed] [Google Scholar]
- RAHME M.M., COTTER B., LEISTAD E., WADHWA M.K., MOHABIR R., FORD A.P.D.W., EGLEN R.M., FELD G.K. Electrophysiological and antiarrhythmic effects of the atrial selective 5-HT4 receptor antagonist RS-100302 in experimental atrial flutter and fibrillation. Circulation. 1999;100:2010–2017. doi: 10.1161/01.cir.100.19.2010. [DOI] [PubMed] [Google Scholar]
- SANDERS L., LYNHAM J.A., BOND B., DEL MONTE F., HARDING S.E., KAUMANN A.J. Sensitization of human atrial 5-HT4 receptors by chronic β-blocker treatment. Circulation. 1995;92:2526–2539. doi: 10.1161/01.cir.92.9.2526. [DOI] [PubMed] [Google Scholar]
- TONINI M., DE PONTI F., DI NUCCI A., CREMA F. Review article: cardiac adverse effects of gastrointestinal prokinetics. Aliment. Pharmacol. Ther. 1999;13:1585–1591. doi: 10.1046/j.1365-2036.1999.00655.x. [DOI] [PubMed] [Google Scholar]
- WANG T., PLUMPTON C., BROWN M.J. Selective β1-adrenoceptor blockade enhances the activity of the stimulatory G-protein in human atrial myocardium. Br. J. Pharmacol. 1999;128:135–141. doi: 10.1038/sj.bjp.0702750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANGEMANN T., GIESSLER C., WILLMY-MATTHES P., SILBER R.E., BRODDE O.E. The indirect negative inotropic effect of carbachol in β1-adrenoceptor antagonist-treated human right atria. Eur. J. Pharmacol. 2003;458:163–170. doi: 10.1016/s0014-2999(02)02763-2. [DOI] [PubMed] [Google Scholar]
- WORKMAN A.J., KANE K.A., RANKIN A.C. The contribution of ionic currents to changes in refractoriness of human atrial myocytes associated with chronic atrial fibrillation. Cardiovasc. Res. 2001;52:226–235. doi: 10.1016/s0008-6363(01)00380-7. [DOI] [PubMed] [Google Scholar]
- WORKMAN A.J., KANE K.A., RUSSELL J.A., NORRIE J., RANKIN A.C. Chronic beta-adrenoceptor blockade and human atrial cell electrophysiology: evidence of pharmacological remodelling. Cardiovasc. Res. 2003;58:518–525. doi: 10.1016/s0008-6363(03)00263-3. [DOI] [PubMed] [Google Scholar]
- WORLD MEDICAL ASSOCIATION World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc. Res. 1997;35:2–3. [PubMed] [Google Scholar]
- YUE L., FENG J., LI G.R., NATTEL S. Transient outward and delayed rectifier currents in canine atrium: properties and role of isolation methods. Am. J. Physiol. 1996;270:H2157–H2168. doi: 10.1152/ajpheart.1996.270.6.H2157. [DOI] [PubMed] [Google Scholar]