Abstract
Increased circulating levels of adrenomedullin (ADM) cause peripheral vasodilatation and hypotension, accompanied by cardiac actions including tachycardia and increases in cardiac contractility, cardiac output, coronary conductance (CC) and coronary blood flow (CBF). It is unclear to what extent these cardiac effects are direct actions of ADM or secondary to the hypotension and altered cardiac loading.
The direct cardiac actions of ADM were examined in conscious sheep previously implanted with aortic and coronary flow probes, and an indwelling left coronary artery cannula. Responses to infusion of ADM (0.5 μg kg−1 h−1 for 1 h) into the left coronary artery or jugular vein were compared (n=6). The effect of blockade of nitric oxide (NO) synthase with intracoronary (i.c.) Nω-nitro-L-arginine (L-NNA; 1.5 mg kg−1 h−1), infused for 2 h before and during ADM infusion, was assessed to determine whether the responses to ADM were mediated by NO (n=5).
I.c. ADM caused large and sustained increases in CC (0.35±0.07–0.55±0.13 ml min−1 mmHg−1, P<0.05) and CBF (28±6–42±9 ml min−1, P<0.05), but had no effect on arterial pressure or indices of cardiac contractility (first differential of the upstroke of systole and peak aortic flow rate). Intravenous infusion of ADM had no effects.
I.c. L-NNA, at a dose that abolished the coronary vasodilator action of acetylcholine, blocked ADM-induced coronary vasodilatation.
In conclusion, ADM had a direct coronary vasodilator action that was mediated by release of endogenous NO and resulted in increased CBF. There was no evidence for a direct inotropic action of ADM.
Keywords: Cardiac output, chronotropic, inotropic, intracoronary, sheep
Introduction
Adrenomedullin (ADM) is an endogenous circulating peptide belonging to the calcitonin-gene-related peptide family. It is expressed in blood vessels in many tissues including the adrenal medulla, cardiac atria, lung, kidney and brain (Kitamura et al., 1993; Ueta et al., 1995), and its vasodilator properties have been well characterised both in vitro and in vivo (Kitamura et al., 1993; Parkes, 1995). It has been proposed that the vasodilator action of ADM is mediated by a number of mechanisms, including a direct action on vascular smooth muscle cells via cAMP and Ca2+ mobilisation (Ishizaka et al., 1994; Shimekake et al., 1995), and by stimulation of nitric oxide (NO). Inhibition of nitric oxide synthase (NOS) has been shown to prevent the vasodilator action of ADM in dog kidney (Majid et al., 1996), rat hindquarters (Feng et al., 1994) and in the pulmonary circulation of the rat but not the cat (Nossaman et al., 1996).
A paracrine role for ADM in the control of cardiac function is suggested by the identification of specific binding sites for ADM in the myocardium (Owji et al., 1995), the secretion of ADM by myocytes (Horio et al., 1998), the demonstration of immunoreactive ADM in both atrial and ventricular tissue and the localisation of ADM mRNA within the heart ventricles. The cardiac responses to ADM are controversial, with reports suggesting both positive and negative inotropic actions. A direct negative inotropic effect was demonstrated in isolated hearts (Perret et al., 1993) and also in rabbit isolated myocytes (Ikenouchi et al., 1997). In contrast, positive inotropic actions have been reported (Szokodi et al., 1996; Ihara et al., 2000), whereas other authors have found no effect of ADM on myocardial contractility (Stangl et al., 2000). Studies in intact conscious sheep have shown that ADM increases the heart rate (HR), maximum rate of change in aortic flow (an index of contractility), cardiac output (CO), coronary blood flow (CBF) and coronary conductance (CC) (Parkes, 1995). The ADM-induced increases in CO and contractility were not abolished by ganglion blockade (Parkes & May, 1997), demonstrating that these changes were not secondary to a baroreflex-mediated increase in cardiac sympathetic nerve activity. This did not exclude the possibility that altered loading conditions on the heart may have accounted for these changes. Recent studies in which contractility was measured by load insensitive measures reported that ADM had a positive inotropic action in patients (Nagaya et al., 2002), but not in dogs (Lainchbury et al., 2000).
The aim of the present study was to determine to what extent the cardiac actions of ADM result from a direct action on the heart. In conscious instrumented sheep, we examined the responses to intracoronary (i.c.) infusion of a dose of ADM that had no effects when given systemically. To determine whether any of the actions of ADM were mediated by NO, we studied the effect of blockade of NOS on the responses to ADM.
Methods
Animals
Mature merino ewes (40–50 kg body weight), oophorectomised and with carotid artery loops, were individually housed in metabolism cages. Animals were not used for at least 2 weeks after surgery and until they were accustomed to laboratory conditions and human contact. Sheep were fed a diet of oaten chaff (800 g day−1, containing 80–120 mmol kg−1 Na+ and 270–380 mmol kg−1 K+) and water was offered ad libitum. The Animal Experimentation Ethics Committee of the Howard Florey Institute approved all experiments.
Animal instrumentation and data collection
Flow probes were implanted in sheep for the measurement of CO and CBF, as previously described (Bednarik & May, 1995). Briefly, anaesthesia was induced with intravenous (i.v.) sodium thiopentone (15 mg kg−1). Following intubation, sheep were placed on a ventilator and maintained on 2% isoflurane in air/oxygen. An electromagnetic flow probe (In Vivo Metrics, Healdsburg, CA, U.S.A.) was implanted on the ascending aorta, and a transit-time flow probe (3 mm, Transonic Systems Inc., Ithaca, NY, U.S.A.) was implanted on the left circumflex artery. During the same operation, a silicone cannula (OD 1.19 mm, ID 0.64 mm) was inserted approximately 0.2 cm into the left coronary artery and held in place with a 5/0 silk purse string suture. The implanted i.c. cannulae were continually infused with heparinised saline (25 U ml−1 at 3 ml h−1), which maintained patency for at least 3 months.
The transit-time flow probe was connected to a Transonics T201CDS flow meter via a four-channel sequential scanner (TM04, Transonics Systems Inc, Ithaca, NY, U.S.A.), and the EMF probe was activated by a BL610 flowmeter (Biotronex, MD, U.S.A.). The output voltage of the EMF meter was reset to zero with an autozero circuit, during a portion of each diastole when blood flow in the ascending aorta is assumed to be zero. The autozero circuit also incorporated a separate circuit to measure the first differential of the upstroke of systole (maximum rate of increase of aortic flow (dF/dt)) at each beat. Approximately 4 weeks after implantation, electromagnetic flow probes were calibrated in vivo against thermodilution over a wide range of CO values (4–9 l min−1), achieved by infusion of dobutamine (Dobutrex, Eli Lilly, France) (Bednarik & May, 1995).
The following cardiovascular variables were recorded: mean arterial pressure (MAP), CO, HR (calculated from the aortic flow signal), dF/dt, peak aortic flow rate (Fmax), stroke volume (CO/HR), total peripheral conductance (TPC=CO/MAP), CBF and CC (=CBF/MAP). Analogue signals, MAP, CO, dF/dt and CBF, were collected using a PC-based data-acquisition system with custom-written software. Following analogue to digital conversion, data were collected at 100 Hz for 10 s at 5 min intervals.
Arterial pressure was measured via a Tygon cannula (1.0 mm ID, 1.5 mm OD) inserted 15 cm into a carotid artery loop. Patency was maintained by infusing heparinised saline (25 U ml−1 at 3 ml h−1). The cannula was connected to a pressure transducer (TDXIII, Cobe, CO) tied to the sheep's back. The pressure was corrected to compensate for the height of the transducer above heart level. Polyethylene cannulae were inserted in a jugular vein under local anaesthesia 2 days before experimentation, for i.v. administration of ADM and measurement of central venous pressure (CVP), which was measured at the heart level.
Experimental protocols
I.c. vs i.v. ADM
This study examined whether the cardiac actions of ADM resulted from a direct action on the heart or were secondary to peripheral changes. Experiments were performed on six standing conscious sheep. Following a 1-h control period, human ADM 1-52 (0.5 μg kg−1 h−1) (Bachem, Torrance, CA, U.S.A.) or vehicle (normal saline at 12 ml h−1) was infused i.v. or into the left coronary artery (i.c.) for 60 min. Cardiovascular variables were monitored every 5 min throughout the control, infusion and 5-h postinfusion periods. Data were averaged over 15-min periods. The order in which the four infusions were given was randomised and experiments were performed at least 6 days apart.
Effect of inhibition of NOS on the response to ADM
The second series of experiments was conducted on five conscious sheep, three of which were also used in the first study. The dose of i.c. Nω-nitro-L-arginine (L-NNA) required to inhibit NOS in the coronary arteries was determined by examining the ability of L-NNA to inhibit the coronary vasodilator action of i.c. acetylcholine. The responses to acetylcholine (250 μg h−1 for 10 min) were examined before and after 2 h infusion of L-NNA.
Following a 1-h control period, infusion of L-NNA (1.5 mg kg−1 h −1) or vehicle (saline 12 ml h−1) into the coronary artery was started, and 2 h later a simultaneous i.c. infusion of ADM (0.5 μg kg−1 h−1) or vehicle was begun. Both infusions were stopped 60 min later. Cardiovascular parameters were monitored every 5 min and then averaged over 30-min periods. The order of the four treatments was randomised and experiments were performed at least 6 days apart.
Statistics
Values are expressed as means±s.e.m. A one-way analysis of variance (ANOVA) was used to compare the resting values between treatment groups. A multifactor repeated ANOVA was used to determine the effect of drug treatment (i.c. ADM vs i.c. vehicle) and route of administration (i.c. ADM vs i.v. ADM) during the infusion period (i.e. 0 to +1 h). The between-animal sum of squares as well as the treatment sum of squares were removed from the total sum of squares to obtain a within-animal sum of squares (Snedecor & Cochran, 1980). The effect of L-NNA was tested using a two-way repeated-measures ANOVA for the infusion period from –2 to + 1 h. The interaction between L-NNA and ADM was collectively compared, during the ADM infusion, using repeated-measures ANOVA, with the Greenhouse–Geisser correction. Analysis of the effect of L-NNA on the response to i.c. ADM compared the response to infusions of L-NNA and ADM with the responses to vehicle infusion, and the interaction between L-NNA and ADM infusions. The effect of L-NNA on the response to i.c. acetylcholine was tested using the Student's t-test. Responses were considered significant at the level of P<0.05.
Results
I.c. vs i.v. ADM
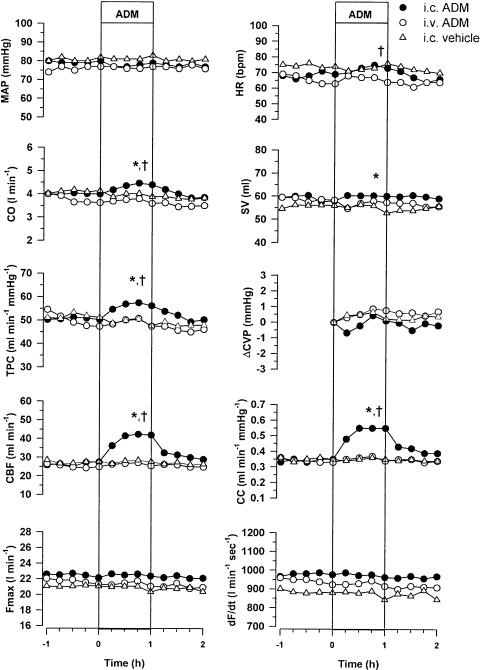
Resting levels of all variables were not significantly different between treatment groups (Figure 1). ADM infused directly into the coronary artery (0.5 μg kg−1 h−1 for 1 h) caused a large and sustained coronary vasodilatation. CC increased from 0.35±0.07 to 0.55±0.13 ml min−1 mmHg−1 (P<0.05, compared with i.c. vehicle), resulting in an increase in CBF from 28±6 to 42±9 ml min−1 (P<0.05, compared with i.c. vehicle) (Figure 1). In contrast, i.v. infusion of the same dose of ADM caused no changes in CBF (25±5–26±5 ml min−1) or CC (0.33±0.07–0.34±0.07 ml min−1 mmHg−1) (Figure 1). With i.c. infusion of ADM, there were small increases in CO (4.0±0.2–4.4±0.4 l min−1, P<0.05), HR (69.2±3.2–73.2±3.8 beats min−1, P<0.01) and TPC (50±4–56±5 ml min−1 mmHg−1, P<0.05), while with i.v. infusion of ADM these variables were unchanged (Figure 1). There were no significant differences in the responses of MAP, CVP, dF/dT and Fmax to i.c. infusion of ADM, when compared with i.c. infusion of vehicle or with i.v. infusion of ADM.
Figure 1.
Effect of i.c. ADM (0.5 μg kg−1 h−1), i.v. ADM (0.5 μg kg−1 h−1) and i.c. saline (12 ml h−1) on MAP, HR, CO, stroke volume (SV), TPC, change in central venous pressure (ΔCVP), CBF, CC, Fmax and dF/dt in conscious sheep (n=6). A multifactor repeated ANOVA was used to determine the effect of drug treatment (i.c. ADM vs i.c. vehicle) and route of administration (i.c. ADM vs i.v. ADM) during the infusion period (0 to +1 h). *Represents a significant effect of ADM (P<0.05). †Represents a significant effect of the route of administration of ADM (P<0.05).
Effect of inhibition of NOS on the response to i.c. ADM
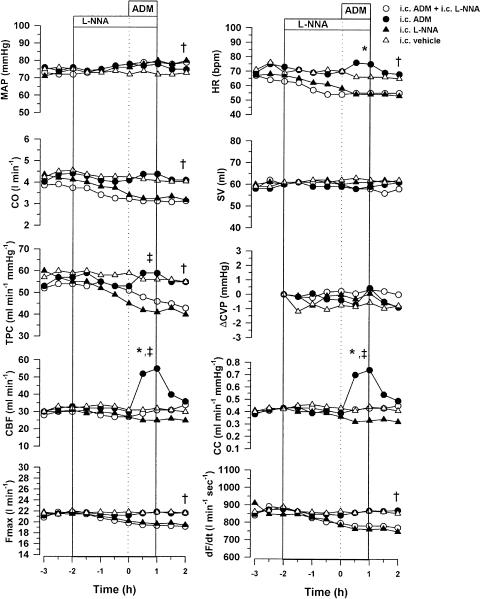
The ability of L-NNA to inhibit the coronary vasodilator action of i.c. acetylcholine was examined. I.c. infusion of acetylcholine (250 μg h−1 for 10 min) significantly increased CBF from 17±3 to 22±4 ml min−1 and CC from 0.23±0.04 to 0.30±0.06 ml min−1 mmHg−1. Following i.c. infusion of L-NNA (1.5 mg kg−1 h−1), the acetylcholine-induced increases in CBF (16±4–17±4 ml min−1) and CC (0.21±0.05–0.23±0.06 ml min−1 mmHg−1) were significantly attenuated.
As in the previous series of experiments, i.c. infusion of ADM increased CC (0.43±0.11–0.74±0.17 ml min−1 mmHg−1, P<0.01) and CBF (33±8–55±12 ml min−1, P<0.01) (Figure 2). In the presence of L-NNA, the coronary vasodilator action of ADM was abolished (P<0.05), and CBF (30±8–31±8 ml min−1) and CC (0.43±0.11–0.43±0.08 ml min−1 mmHg−1) were unchanged (Figure 2). The minor increases in CO, HR and TPC in response to i.c. ADM were also inhibited by treatment with L-NNA.
Figure 2.
Effect of i.c. infusions of ADM (0.5 μg kg−1 h−1), L-NNA (1.5 mg kg−1 h−1), ADM+L-NNA or vehicle on MAP, HR, CO, SV, TPC, ÄCVP, CBF, CC, Fmax and dF/dt in conscious sheep (n=5). The interaction between L-NNA and ADM was collectively compared using repeated-measures ANOVA for the infusion period 0 to +1 h. *Represents a significant effect of ADM (P<0.05). ‡ Represents a significant interaction between L-NNA and ADM (P<0.05). The effect of L-NNA infusion was tested using a two-way repeated-measures ANOVA for the infusion period from –2 to +1 h. †Represents significant effect of L-NNA.
Infusion of L-NNA (1.5 mg kg−1 h−1) into the left coronary artery for 3 h caused a significant increase in MAP (75±2–80±3 mmHg, P<0.01) due to peripheral vasoconstriction, as shown by the reduction in TPC (55±5–41±3 ml min−1 mmHg−1, P<0.001) (Figure 2). These changes were accompanied by reductions in HR (P<0.05), dF/dt (P<0.001) and Fmax (P<0.001), resulting in a significant reduction in CO (P<0.001). Both CC and CBF tended to decrease, but these changes did not reach significance.
Discussion
This study examined the responses to i.c. infusion of a low dose of ADM, which had no effects when given i.v., to determine the extent to which the cardiac effects of ADM are due to direct actions on the heart. The main finding was that in conscious, unstressed animals, i.c. infusion of ADM caused coronary vasodilatation that was due to a direct vascular effect of ADM and was mediated by release of endogenous NO. In contrast, i.c. ADM had no effect on dF/dt or Fmax, suggesting that the increases in these variables that we have demonstrated previously in response to i.v. ADM (100 μg over 1 h) (Parkes, 1995) were secondary to the haemodynamic changes.
The actions of ADM on the heart are of considerable interest because the levels of ADM are increased following myocardial infarction (Kobayashi et al., 1996b) and congestive heart failure (Jougasaki et al., 1995; Kato et al., 1996; Kobayashi et al., 1996a). Furthermore, there is evidence that exogenous administration of ADM is beneficial in heart failure (Rademaker et al., 1997; Nagaya et al., 2000) and following myocardial infarction (Nakamura et al., 2002), suggesting a possible role as a therapeutic agent. It is, therefore, important to fully understand its effects and mechanisms of action. At present, there is considerable controversy concerning the extent to which the cardiac effects (inotropic, chronotropic and coronary vasodilator) of ADM are due to direct actions on the heart rather than secondary to the peripheral vasodilatation and subsequent hypotension. The presence of abundant, specific binding sites for ADM in the myocardium (Owji et al., 1995) indicates that circulating ADM could have direct cardiac actions.
Studies of the action of ADM in vitro have resulted in contrasting findings with evidence for both positive (Szokodi et al., 1996; Ihara et al., 2000; Kinnunen et al., 2000) and negative inotropic (Perret et al., 1993; Ikenouchi et al., 1997) actions being reported. There are also contradictory findings from in vivo studies. In previous studies in conscious sheep, we found that ADM (100 μg h−1 i.v. for 1 h) caused peripheral vasodilatation and hypotension, accompanied by large increases in CO, HR, dF/dt and Fmax, together with coronary vasodilatation and an increase in CBF (Parkes, 1995). In a later study, we demonstrated that the chronotropic action of ADM was abolished by ganglion blockade, but that the increases in CO, dF/dt and coronary vasodilatation still occurred, indicating that these actions were not secondary to activation of the sympathetic nervous system (Parkes, 1995; Parkes & May, 1997). The increase in CO may, therefore, have been due either to the decrease in cardiac afterload or to a direct inotropic action of ADM. Following ganglion blockade, the increase in dF/dt was not abolished, but this variable is influenced by the loading conditions on the heart; so it remained unclear whether ADM had a direct inotropic action. Two recent studies, which have addressed this issue by using measures of ventricular contractility that are relatively load independent, have produced contrasting findings. In conscious dogs, with HRs fixed by pacing and beta-blockade, only the highest dose of ADM infused into the left ventricle, which increased plasma levels 30-fold, produced any evidence of an increase in left ventricular end-systolic elastance (Ees), a relatively load-independent measure of ventricular contractility (Lainchbury et al., 2000). The authors, however, attributed this increase in Ees to factors other than a direct inotropic response. In contrast, in a recent study in patients who had had myocardial infarctions, i.v. ADM increased Emax, an index of left ventricular contractility that is proposed to be practically independent of loading conditions (Nagaya et al., 2002). In the present study, in which ADM was infused directly into the left coronary artery, at a dose that had no effects on cardiac preload or afterload, there were no changes in dF/dt or Fmax. This finding indicates that ADM does not have a direct inotropic action and that the increases in stroke volume and CO seen following i.v. administration of ADM were due to the reduction in afterload.
With i.c. ADM (0.5 μg kg−1 h−1), the increases in CBF (57±11%) and CC (59±11%) were more than double those with i.v. ADM (100 μg h−1, equivalent to 2.5 μg kg−1 h −1) (Parkes, 1995; Parkes & May, 1997), while the increase in CO with i.c. ADM was only one-third of that with i.v. ADM. The substantially greater coronary vasodilatation, with a considerably smaller increase in CO, indicates that this vasodilator response is primarily due to a direct action of ADM rather than being secondary to increased metabolic demand due to increased cardiac work. This coronary vasodilator action of ADM in sheep is consistent with findings in other species, although a number of different mechanisms have been proposed to account for this effect. In isolated human coronary arteries, ADM-induced vasodilatation was attenuated by L-NAME and by removal of the endothelium, and blocked by high KCl (Terata et al., 2000). These findings suggested that the vasodilator action resulted in part through release of NO and in part through activation of K channels. In anaesthetised open-chested dogs, coronary vasodilatation in response to bolus injection of ADM, into the first diagonal branch of the left anterior descending coronary artery, was blocked by antagonists of adenosine receptors and KATP channels (Sabates et al., 1997). In porcine preconstricted coronary arteries in vitro, the ADM-induced vasodilatation was not blocked by removal of endothelium, and it was proposed that ADM acted directly on vascular smooth muscle cells to inhibit contraction (Yoshimoto et al., 1998). In the present study, we demonstrated that the coronary vasodilator action of ADM was totally prevented by inhibition of NOS. This complete prevention of the vasodilator response, in comparison with the partial prevention reported in other studies, may reflect a more complete inhibition of NOS by the 2 h pretreatment with L-NNA given directly into the coronary arteries in our study. Our observation that NOS inhibition prevented the vasodilator action of ADM in the coronary arteries of sheep is consistent with the reports that NOS inhibition blocks the effect of ADM in most, but not all, vascular beds (Feng et al., 1994; Majid et al., 1996; Nossaman et al., 1996).
In the present study, ADM (0.5 μg kg−1 h−1) infused i.c., but not i.v., caused a small increase in HR, suggesting that ADM has a direct chronotropic action. This is in contrast to our previous finding that ganglion blockade prevented the increase in HR in response to i.v. ADM (100 μg kg−1 h−1) (Parkes & May, 1997), suggesting that this effect was baroreflex mediated and not direct. The higher cardiac concentrations of ADM achieved with i.c. ADM (0.5 μg kg−1 h−1) compared with i.v. ADM (100 μg h−1) may account for this difference. With i.c. ADM, there was an increase in TPC that did not occur with the same dose-infused i.v., indicating that this was not due to a direct vasodilator action of ADM, but probably a reflex response to the increase in CO, which prevented a significant increase in MAP. Interestingly, the chronotropic action of i.c. ADM was prevented by treatment with L-NNA, as were the subsequent increases in CO and TPC.
The present study has provided new insights into the direct cardiac actions of ADM in conscious unstressed animals. I.c. infusion of ADM, at a dose that had no systemic actions, had a direct action on the coronary arteries causing vasodilatation and an increase in blood flow that was mediated by release of endogenous NO. There was no evidence to support a direct inotropic effect of ADM, although there was a mild chronotropic effect. It has been suggested that ADM plays a role as an autocrine or paracrine hormone in the heart, and the present data indicate that its main effect would be to cause coronary vasodilatation. This may be of importance following myocardial ischaemia, where it has been reported that that there is a substantial increase in ADM immunoreactivity in the microvascular endothelium of the ischaemic area, as well as increases in ADM-binding sites and ADM message in the failing ventricle (Oie et al., 2000).
Acknowledgments
This work was supported by an Institute Grant (No. 983001) from the National Health and Medical Research Council of Australia. We are grateful to Douglas McNestrie for excellent technical assistance.
Abbreviations
- ADM
adrenomedullin
- ANOVA
analysis of variance
- CBF
coronary blood flow
- CC
coronary conductance
- CO
cardiac output
- CVP
central venous pressure
- dF/dt
maximum rate of increase of aortic flow
- Fmax
peak aortic flow rate
- HR
heart rate
- i.c.
intracoronary
- i.v.
intravenous
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- L-NNA
Nω-nitro-L-arginine
- TPC
total peripheral conductance
References
- BEDNARIK J.A., MAY C.N. Evaluation of a transit-time system for the chronic measurement of blood flow in conscious sheep. J. Appl. Physiol. 1995;78:524–530. doi: 10.1152/jappl.1995.78.2.524. [DOI] [PubMed] [Google Scholar]
- FENG C., KANG B., KAYE A., KADOWITZ P., NOSSAMAN B. L-NAME modulates responses to adrenomedullin in the hindquarters vascular bed of the rat. Life Sci. 1994;55:PL433–PL438. doi: 10.1016/0024-3205(94)00347-5. [DOI] [PubMed] [Google Scholar]
- HORIO T., NISHIKIMI T., YOSHIHARA F., NAGAYA N., MATSUO H., TAKISHITA S., KANGAWA K. Production and secretion of adrenomedullin in cultured rat cardiac myocytes and nonmyocytes: stimulation by interleukin-1beta and tumor necrosis factor-alpha. Endocrinology. 1998;139:4576–4580. doi: 10.1210/endo.139.11.6306. [DOI] [PubMed] [Google Scholar]
- IHARA T., IKEDA U., TATE Y., ISHIBASHI S., SHIMADA K. Positive inotropic effects of adrenomedullin on rat papillary muscle. Eur. J. Pharmacol. 2000;390:167–172. doi: 10.1016/s0014-2999(00)00011-x. [DOI] [PubMed] [Google Scholar]
- IKENOUCHI H., KANGAWA K., MATSUO H., HIRATA Y. Negative inotropic effect of adrenomedullin in isolated adult rabbit cardiac ventricular myocytes. Circulation. 1997;95:2318–2324. doi: 10.1161/01.cir.95.9.2318. [DOI] [PubMed] [Google Scholar]
- ISHIZAKA Y., TANAKA M., KITAMURA K., KANGAWA K., MINAMINO N., MATSUO H., ETO T. Adrenomedullin stimulates cyclic AMP formation in rat vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1994;200:642–646. doi: 10.1006/bbrc.1994.1496. [DOI] [PubMed] [Google Scholar]
- JOUGASAKI M., WEI C.M., MCKINLEY L.J., BURNETT J.C., JR Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation. 1995;92:286–289. doi: 10.1161/01.cir.92.3.286. [DOI] [PubMed] [Google Scholar]
- KATO J., KOBAYASHI K., ETOH T., TANAKA M., KITAMURA K., IMAMURA T., KOIWAYA Y., KANGAWA K., ETO T. Plasma adrenomedullin concentration in patients with heart failure. J. Clin. Endocrinol. Metab. 1996;81:180–183. doi: 10.1210/jcem.81.1.8550749. [DOI] [PubMed] [Google Scholar]
- KINNUNEN P., SZOKODI I., NICHOLLS M.G., RUSKOAHO H. Impact of NO on ET-1- and AM-induced inotropic responses: potentiation by combined administration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R569–R575. doi: 10.1152/ajpregu.2000.279.2.R569. [DOI] [PubMed] [Google Scholar]
- KITAMURA K., KANGAWA K., KAWAMOTO M., ICHIKI Y., NAKAMURA S., MATSUO H., ETO T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI K., KITAMURA K., ETOH T., NAGATOMO Y., TAKENAGA M., ISHIKAWA T., IMAMURA T., KOIWAYA Y., ETO T. Increased plasma adrenomedullin levels in chronic congestive heart failure. Am. Heart J. 1996a;131:994–998. doi: 10.1016/s0002-8703(96)90185-4. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI K., KITAMURA K., HIRAYAMA N., DATE H., KASHIWAGI T., IKUSHIMA I., HANADA Y., NAGATOMO Y., TAKENAGA M., ISHIKAWA T., IMAMURA T., KOIWAYA Y., ETO T. Increased plasma adrenomedullin in acute myocardial infarction. Am. Heart J. 1996b;131:676–680. doi: 10.1016/s0002-8703(96)90270-7. [DOI] [PubMed] [Google Scholar]
- LAINCHBURY J.G., MEYER D.M., JOUGASAKI M., BURNETT J.C., JR, REDFIELD M.M. Effects of adrenomedullin on load and myocardial performance in normal and heart-failure dogs. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H1000–H1006. doi: 10.1152/ajpheart.2000.279.3.H1000. [DOI] [PubMed] [Google Scholar]
- MAJID D.S., KADOWITZ P.J., COY D.H., NAVAR L.G. Renal responses to intra-arterial administration of adrenomedullin in dogs. Am. J. Physiol. 1996;270:F200–F205. doi: 10.1152/ajprenal.1996.270.1.F200. [DOI] [PubMed] [Google Scholar]
- NAGAYA N., GOTO Y., SATOH T., SUMIDA H., KOJIMA S., MIYATAKE K., KANGAWA K. Intravenous adrenomedullin in myocardial function and energy metabolism in patients after myocardial infarction. J. Cardiovasc. Pharmacol. 2002;39:754–760. doi: 10.1097/00005344-200205000-00017. [DOI] [PubMed] [Google Scholar]
- NAGAYA N., SATOH T., NISHIKIMI T., UEMATSU M., FURUICHI S., SAKAMAKI F., OYA H., KYOTANI S., NAKANISHI N., GOTO Y., MASUDA Y., MIYATAKE K., KANGAWA K. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000;101:498–503. doi: 10.1161/01.cir.101.5.498. [DOI] [PubMed] [Google Scholar]
- NAKAMURA R., KATO J., KITAMURA K., ONITSUKA H., IMAMURA T., MARUTSUKA K., ASADA Y., KANGAWA K., ETO T. Beneficial effects of adrenomedullin on left ventricular remodeling after myocardial infarction in rats. Cardiovasc. Res. 2002;56:373–380. doi: 10.1016/s0008-6363(02)00594-1. [DOI] [PubMed] [Google Scholar]
- NOSSAMAN B.D., FENG C.J., KAYE A.D., DEWITT B., COY D.H., MURPHY W.A., KADOWITZ P.J. Pulmonary vasodilator responses to adrenomedullin are reduced by NOS inhibitors in rats but not in cats. Am. J. Physiol. 1996;270:L782–L789. doi: 10.1152/ajplung.1996.270.5.L782. [DOI] [PubMed] [Google Scholar]
- OIE E., VINGE L.E., YNDESTAD A., SANDBERG C., GROGAARD H.K., ATTRAMADAL H. Induction of a myocardial adrenomedullin signaling system during ischemic heart failure in rats. Circulation. 2000;101:415–422. doi: 10.1161/01.cir.101.4.415. [DOI] [PubMed] [Google Scholar]
- OWJI A.A., SMITH D.M., COPPOCK H.A., MORGAN D.G., BHOGAL R., GHATEI M.A., BLOOM S.R. An abundant and specific binding site for the novel vasodilator adrenomedullin in the rat. Endocrinology. 1995;136:2127–2134. doi: 10.1210/endo.136.5.7720662. [DOI] [PubMed] [Google Scholar]
- PARKES D. Cardiovascular actions of adrenomedullin in conscious sheep. Am. J. Physiol. 1995;268:H2574–H2578. doi: 10.1152/ajpheart.1995.268.6.H2574. [DOI] [PubMed] [Google Scholar]
- PARKES D.G., MAY C.N. Direct cardiac and vascular actions of adrenomedullin in conscious sheep. Br. J. Pharmacol. 1997;120:1179–1185. doi: 10.1038/sj.bjp.0701034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET M., BROUSSARD H., LEGROS T., BURNS A., CHANG J., SUMMER W., HYMAN A., LIPPTON H.The effect of adrenomedullin on the isolated heart Life Sci. 199353PL377–PL379.PL [DOI] [PubMed] [Google Scholar]
- RADEMAKER M.T., CHARLES C.J., LEWIS L.K., YANDLE T.G., COOPER G.J., COY D.H., RICHARDS A.M., NICHOLLS M.G. Beneficial hemodynamic and renal effects of adrenomedullin in an ovine model of heart failure. Circulation. 1997;96:1983–1990. doi: 10.1161/01.cir.96.6.1983. [DOI] [PubMed] [Google Scholar]
- SABATES B.L., PIGOTT J.D., CHOE E.U., CRUZ M.P., LIPPTON H.L., HYMAN A.L., FLINT L.M., FERRARA J.J. Adrenomedullin mediates coronary vasodilation through adenosine receptors and KATP channels. J. Surg. Res. 1997;67:163–168. doi: 10.1006/jsre.1996.4985. [DOI] [PubMed] [Google Scholar]
- SHIMEKAKE Y., NAGATA K., OHTA S., KAMBAYASHI Y., TERAOKA H., KITAMURA K., ETO T., KANGAWA K., MATSUO H. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J. Biol. Chem. 1995;270:4412–4417. doi: 10.1074/jbc.270.9.4412. [DOI] [PubMed] [Google Scholar]
- SNEDECOR G.W., COCHRAN W.G. Statistical Methods. Iowa: Iowa State University Press; 1980. [Google Scholar]
- STANGL V., DSCHIETZIG T., BRAMLAGE P., BOYE P., KINKEL H.T., STAUDT A., BAUMANN G., FELIX S.B., STANGL K. Adrenomedullin and myocardial contractility in the rat. Eur. J. Pharmacol. 2000;408:83–89. doi: 10.1016/s0014-2999(00)00765-2. [DOI] [PubMed] [Google Scholar]
- SZOKODI I., KINNUNEN P., RUSKOAHO H. Inotropic effect of adrenomedullin in the isolated perfused rat heart. Acta Physiol. Scand. 1996;156:151–152. doi: 10.1046/j.1365-201X.1996.454169000.x. [DOI] [PubMed] [Google Scholar]
- TERATA K., MIURA H., LIU Y., LOBERIZA F., GUTTERMAN D.D. Human coronary arteriolar dilation to adrenomedullin: role of nitric oxide and K(+) channels. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2620–H2626. doi: 10.1152/ajpheart.2000.279.6.H2620. [DOI] [PubMed] [Google Scholar]
- UETA Y., KITAMURA K., ISSE T., SHIBUYA I., KABASHIMA N., YAMAMOTO S., KANGAWA K., MATSUO H., ETO T., YAMASHITA H. Adrenomedullin-immunoreactive neurons in the paraventricular and supraoptic nuclei of the rat. Neurosci. Lett. 1995;202:37–40. doi: 10.1016/0304-3940(95)12204-4. [DOI] [PubMed] [Google Scholar]
- YOSHIMOTO R., MITSUI-SAITO M., OZAKI H., KARAKI H. Effects of adrenomedullin and calcitonin gene-related peptide on contractions of the rat aorta and porcine coronary artery. Br. J. Pharmacol. 1998;123:1645–1654. doi: 10.1038/sj.bjp.0701805. [DOI] [PMC free article] [PubMed] [Google Scholar]