Abstract
In vitro experiments were performed to investigate the actions of endothelin-1 (ET-1) on vasomotion and vasospasm in guinea-pig mesenteric lymphatics.
ET-1 modulated lymphatic vasomotion independent of the endothelium, with lower concentrations (⩽10 nM) increasing lymphatic vasomotion and higher concentrations (⩾100 nM) causing vasospasm.
ET-1-induced increases in vasomotion were accompanied by an increase in tonic [Ca2+]i.
These actions were inhibited by the ETA receptor antagonist BQ-123 (1 μM), the phospholipase C (PLC) inhibitor U73122 (5 μM), removal of extracellular Ca2+, chelation of intracellular Ca2+ with BAPTA/AM (10 μM), the store Ca2+-ATPase inhibitor thapsigargin (1 μM), caffeine (10 mM) and the inositol 1,4,5-trisphosphate (IP3) receptor blocker heparin and 2-APB (30 μM). In contrast, the ETB receptor antagonist BQ-788 (1 μM), ryanodine (1 & 20 μM), pertussis toxin (PTx) or Cs+ had no significant actions on vasomotion or the magnitude of increase in tonic [Ca2+]i.
ET-1-induced vasospasm was accompanied by a transient increase in smooth muscle [Ca2+]i followed by a sustained plateau, an action that was abolished by removal of extracellular Ca2+, but only marginally inhibited by nifedipine (1 μM).
Caffeine (10 mM), SKF 96165 (30 μM) or U73122 (5 μM) together with nifedipine (1 μM) abolished ET-1-induced vasospasm and increase in [Ca2+]i.
These results indicate that ET-1 increases lymphatic vasomotion by acting on smooth muscle ETA receptors and activation of G-protein-PLC-IP3 cascade, which is known to cause pacemaker Ca2+ release and resultant pacemaker potentials. High concentrations of ET-1 cause a failure in Ca2+ homeostasis causing vasospasm, triggered by excessive Ca2+ influx primarily through store-operated channels (SOCs) with L-Ca2+ voltage-operated channels (VOCs) also contributing, but to a much lesser extent.
Keywords: Endothelin-1, lymphatics, Ca2+ release, Ca2+ influx, vasomotion, vasospasm
Introduction
Many lymphatic and blood vessels exhibit vasomotion, a rhythmic constriction–dilation cycle of these vessels (Florey, 1927; Johnson, 1980). This mechanism is responsible for both the propulsion of lymph (each phasic constriction pumping lymph forward through unidirectional valves within lymphatic vessels; Mislin, 1983; Johnston & Elias, 1987; McHale, 1990), and for modulating the local vascular resistance and blood flow (see Haddock et al., 2002). Ranges of neurotransmitters and paracrine or hormonal substances including ET-1 modulate vasomotion. In contrast, excessive stimulation of the smooth muscle of vessels can lead to vasospasm, a pathological condition of sustained vasoconstriction, observed both in vivo (Kurihara et al., 1989; Wanebo et al., 1998) and in vitro (Willette et al., 1994; Kanashiro et al., 2000).
ET-1 is a 21-amino-acid polypeptide that is produced primarily by the endothelium of blood and lymphatic vessels (Yanagisawa et al., 1988; Reeder & Ferguson, 1996). It is a key smooth muscle activator known to either induce or enhance vasomotion, and at high concentrations cause vasospasm (Sakuma et al., 1995; Toribatake et al., 1997; Zhao & van Helden, 1997; Sakai et al., 1999). ET-1, after being released from vascular endothelium, is known to act in a paracrine manner on ETA and/or ETB2 receptors on smooth muscles and ETB1 receptors on the endothelium (Sokolovsky et al., 1992; Reeder & Ferguson, 1996; Ortega Mateo & de Artinano, 1997; Sakai et al., 1999). ET-1-induced constriction and/or vasomotion are primarily produced by stimulation of ETA and/or ETB2 receptors, which tends to swamp a counter, vasodilatory response resulting from stimulation of endothelial ETB1 receptors. The second messenger pathway has been well established for ETA receptors on vascular smooth muscle, where it is known that ETA receptor activation results in PLC-mediated hydrolysis of phosphatidylinositol 4,5-bisphosphate, producing IP3, which causes an increase in [Ca2+]i by releasing Ca2+ from intracellular stores (Marsden et al., 1989; Berridge, 1993; Neylon, 1999).
Various mechanisms have been proposed to explain ET-1-induced vasospasm. Research using eicosanoids on single smooth muscle cells isolated from porcine coronary arteries (Sirous et al., 2001), U46619 on pig skin flaps (Pang et al., 2001) and 5-hydroxytryptamine on rings from rabbit abdominal aorta (Consigny, 1990) have shown that ET-1 may induce vasospasm by amplifying the effects of other vasoactive substances. Others have demonstrated a role of the endothelium in ET-1-induced vasospasm (Coessens, 1994; Volpe & Cosentino, 2000). Recent research on canine cerebral and porcine coronary arteries (Sirous et al., 2001; Wickman et al., 2001) have shown that ET-1 may cause vasospasm by activating PKC. Studies on smooth muscle from small mesenteric arteries of rabbit indicate that there is an increased sensitivity of the contractile apparatus to Ca2+ during vasospasm (Nishimura et al., 1992). Other studies suggest that most of the effects of ET-1 on vascular tone can be accounted for by its potent ability to induce IP3 formation, which mobilises Ca2+ from intracellular stores and stimulates Ca2+ entry (Neylon, 1999). There have also been reports that ET-1 may directly or indirectly activate VOCs (Van Renterghem et al., 1988; Goto et al., 1989; Chen & Wagoner, 1991; Ota & Oku, 1997). However, the failure of VOC antagonists to prevent ET-1-induced vasospasm suggests that other Ca2+ influx pathways result from the activation of ET-1 receptors. One other such pathway is through SOCs that, as indicated from studies of the basilar artery of a double subarachnoid haemorrhage rabbit model, play a pivotal role in the pathogenesis of ET-1-induced vasospasm (Zuccarello et al., 1996b).
In the studies presented here, we examine the signal pathways underlying lymphatic vasomotion, demonstrating that ET-1 increases vasomotion by acting on ETA receptors to enhance the production of IP3, thus increasing pacemaker Ca2+ release and resultant vasomotion. Higher ET-1 concentrations herald an abrupt change from vasomotion to vasospasm, the latter arising by Ca2+ influx through both SOCs and VOCs. The most striking aspect of the data presented here is demonstration of a linkage between ET-1-induced vasomotion and vasospasm. We hypothesise that this transition represents a change from coordinated, synchronised store Ca2+ release to uncoordinated Ca2+ release arising through over stimulation. Some of these data have been presented previously in abstract form (Zhao & van Helden, 1997).
Methods
Lymphatic preparation
Young guinea-pigs (age <10 days) were euthanised by an overdose of the inhalation anaesthetic halothane (5–10% in air) followed by decapitation. Mesenteric lymphatic vessels were isolated from the ileal region of the intestine and pinned onto a sylgard-covered (Dow Corning) organ bath (volume 0.5 ml) mounted on an inverted microscope. Physiological saline solution of composition (mM): NaCl 120, KCl 5, CaCl2 2.5, MgCl2 2, NaHCO3 25, NaH2PO4 1, glucose 10, maintained at a pH of 7.2 by bubbling with a 95% : 5% O2 : CO2 gas mixture, was superfused over the tissue at a rapid rate (6 ml min−1) at 34–36°C. Two bath types were used: one with a volume of 0.5–1 ml and the other with a volume of ∼0.2 ml. A reasonably rapid change in solutions could be effected with the latter, with >95% bath changeover in <10 s. In some cases, vessels were luminally perfused (Rayner & van Helden, 1997) at a rate of about 3 μl min−1 to destroy the endothelium, as effected by briefly (5–10 s) passing air through the vessels some 5–6 times. The relative success of the lysing procedure was then tested (see von der Weid et al., 1996). Tissues were normally used within 1–4 h of isolation, and were stored at 4°C in physiological saline until used. Measurements were made by recording the constriction frequency and/or [Ca2+]i of the smooth muscle of individual chambers formed by adjacent valves, and termed lymphangions (Mislin, 1983).
Ratiometric measurement of [Ca2+]i with fura-2
[Ca2+]i was measured photometrically using the ratiometric Ca2+ indicator fura-2. This dye was loaded into the smooth muscle by luminally perfusing endothelium-denuded vessels at 35°C with 2 μM fura-2/AM and pluronic acid (0.2%, w v−1) for 30 min. Continuing vessel perfusion for a further 10 min washed out the extraneous dye. The tissue was then left for an equilibration time of at least 20 min, thus allowing the intracellular esterase to cleave fura-2/AM into active fura-2 (Goldman et al., 1990). The tissue was viewed with an inverted microscope using an × 40 oil-immersion objective (NA 1.3). A xenon lamp was used for illumination with the tissue alternately exposed to wavelengths of 340 and 380 nm for durations of 50 ms for each wavelength. The interval between wavelengths was 50 ms, with the cycle repeated at a frequency of 1–5 Hz (typically 5 Hz). Emission light was passed through a dichroic mirror (490 nm) and a bandpass filter (510 nm) and the light then recorded by a photomultiplier with the output responses, namely the fluorescence at 340 nm (F340) and 380 nm (F380), and the F340/F380 ratio captured by an analogue-to-digital converter attached to a PC computer. Autofluorescence in the fura-2-loaded vessels was minor, representing only 2±0.6% (n=3) of the total basal fura-2 fluorescence, and has not been further considered.
Measurement of vasomotion
Vasomotion of individual chambers was either monitored using a video camera attached to an inverted microscope or by monitoring the underlying Ca2+ transients during constriction. These experiments were made using lymphangions that exhibited spontaneous constrictions under control conditions. The former measurement was effected either by a computer-based edge-detection system that determined the vessel edge positions from the video images (Diamtrack), or by recording the output on videotape and analysing the constrictions visually. System limitations during Ca2+ photometry experiments prevented videoscopic edge detection. However, fura-2-based measurements at the isosbestic point made either directly at F360 (Furuya et al., 1994) or by calculating F360 from F340 and F380 data (Chiavaroli et al., 1994) allows the measurement of movement. We used the latter procedure and found that constriction-associated movement correlated with the brief Ca2+ transients regularly recorded throughout our experiments. These increases in [Ca2+]i, measured relatively as F340/F380 ratio, had a rapid rise phase of <0.5 s and duration at half amplitude between 0.5 and 1.0 s, values commensurate with independent measurements of vessel constriction (van Helden, 1993). Constriction was considered to have occurred when a Ca2+ transient of this wave shape presented with amplitude >1.5 standard deviations of the baseline noise. These transients were only used as a measure of vessel constriction and not of relative increases in [Ca2+]i. Should interpretation of the latter be of further interest to the reader, then inaccuracies in the relative [Ca2+]i increases during the brief rise phases of the transients are to be noted. These inaccuracies arose because the kinetics of the constriction (as based on typical constrictions measured by edge tracking) were sufficiently fast during the onset phase to cause movement of ∼25% of peak constriction between F340 and F380 measurements. However, such distortion was minimal during the remainder of the transient when movements between F340 and F380 measurements were less than ∼5%.
Heparin treatment of the tissue
Heparin (8 mg ml−1) was loaded into lymphatic smooth muscle using a reversible permeabilisation procedure (Kobayashi et al., 1988). Fluo-3 (1 μM) salt was simultaneously loaded with heparin to confirm that the tissue had been reversibly permeabilised, as confirmed using a confocal microscope laser-scanning system (Biorad MRC600) attached to an inverted microscope with an × 40 oil-immersion objective (NA 1.3). Fluo-3-loaded tissues were excited using an argon ion laser at 488 nm with a 490 nm dichroic mirror and 515 nm band pass filter.
Pertussis toxin incubation
Tissues were superfused for 4 h at 35°C with a physiological saline solution containing PTx (100 ng ml−1) (Burch et al., 1988). Experiments to determine PTx sensitivities were performed subsequent to this incubation, but in the absence of PTx in the bathing solution.
Experimental protocols
Constriction and calcium photometric experiments were made (except where noted) using a 15 min control period, a 5 min test period during which ET-1 was applied, followed by a 15–30 min washout period. Measurements were made by recording the constriction frequency of individual lymphangions or [Ca2+]i in the smooth muscle of these chambers for 4 min periods at the end of the initial control period, 1 min after application of the agonist and at the end of washout period. When pharmacological inhibitors were used, this protocol was repeated with the additional step of introducing the inhibitor, which was applied for a total of 20 min (15 min before and 5 min during the application of ET-1). In this case, constriction frequency or [Ca2+]i was also monitored for 4 min in the inhibitor, just before application of ET-1. There followed a 30 min recovery period with the tissue exposed to control solution before ET-1 was reapplied. Throughout the experiments, an interval of at least 30 min was used between applications of ET-1. This minimised any tachyphylaxis observed by repeated use of ET-1 at shorter intervals (e.g. 10 min intervals). Except for the concentration–response studies, ET-1 was used at a concentration of 1 nM for the vasomotion studies. Analysis was based on comparisons of the test response of individual chambers to that of the relevant control immediately preceding the response, both averaged over a 4 min period.
The same protocol was followed for the vasospasm experiments, except that the tissue was normally exposed to 100 nM ET-1 now for a period of only 1 min to minimise tachyphylaxis (except where noted otherwise). The smaller bath (0.2 ml) was used throughout in these experiments. Analysis was now based on comparing control responses taken over 4 min to the response during the 1 min application of ET-1, allowing for the bath changeover time of ∼10 s.
Chemicals
The special chemicals used were 2-aminoethoxydiphenyl borate (2-APB) from Calbiochem, Sydney, Australia; acetylcholine (ACh), adenosine 5′-trisphosphate (ATP), BAPTA/AM, BQ-123, BQ-788, caffeine, ET-1, heparin, ionomycin and thapsigargin from Sigma, Sydney, Australia; cyclopiazonic acid (CPA), 1-(6-((17-beta-methoxyestra-1,3,5(10)trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione (U73122), 1-[6-[[(17beta)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-2,5-pyrrolidinedione (U73343) from Biomol Pty Ltd, PA, U.S.A.; PTx and ryanodine from RBI and fura-2 acetoxymethylester (fura-2/AM) and fluo-3/AM from Molecular probes, OR, U.S.A. 2-APB, CPA, fura-2/AM, fluo-3/AM, ionomycin, ryanodine, thapsigargin, U73122 and U73343 were dissolved into dimethyl sulphoxide (DMSO), whereas BQ-123, BQ-788, caffeine, ET-1, heparin and PTx were dissolved in water. Stock solutions were stored at concentrations of 1–20 mM at −20°C. For experiments where the vehicle DMSO was used, the DMSO concentration was always less than 0.1%, a concentration which had no significant effect on lymphatic vasomotion or smooth muscle [Ca2+]i.
Statistical analysis
Diameter-based measures of constriction frequencies and F340/F380 fluorescence ratio-based amplitudes of Ca2+ transients, tonic [Ca2+]i and frequency of [Ca2+]i transients were normalised using the following equation:
 |
where fN is the normalised value, f is the actual value and fi is the initial value measured just before application of the test solution. In some cases, the frequency of oscillatory Ca2+ transients was expressed as either numbers of transients per minute or periods between two transients. The ET-1-induced changes in relative tone (ΔT) during vasomotion were measured as decreases in vessel diameter, normalised by the following relationship:
where D is the actual diastolic diameter and Di is the initial diastolic diameter measured just before application of the test solution.
Dose–response relation curves were analysed by a computer-based curve-fit program (GraphPad prism 2.01), with the EC50 measured as the concentration giving half-maximal response (see Zhao & van Helden, 2002).
All values are presented as the mean±standard error of mean (s.e.m.) with n, the number of experiments. Statistical comparisons were made using a two-tailed paired Student's t-test, with P<0.05 (*) significant and P<0.01 (**) highly significant.
Results
Effects of ET-1 on lymphatic vasomotion
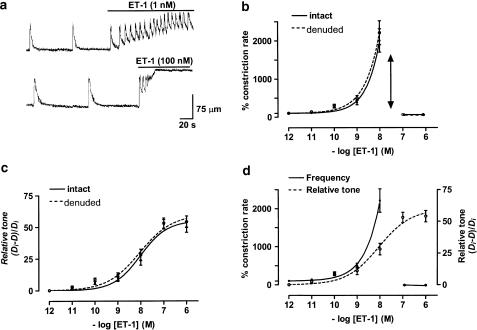
Lymphatic vessels under resting, nonperfused conditions were either quiescent or exhibited spontaneous constrictions at low frequency (e.g. 1–4 constrictions min−1). Analysis of 72 lymphangions (i.e. lymphatic chambers bounded by adjacent valves) provided a mean constriction rate of 1.5±0.2 constrictions min−1, corresponding to an average interval of 40±5.4 s. The effects of ET-1 on lymphatic vasomotion were dose-dependent, with lower concentrations (e.g. 1 nM) increasing vasomotion and higher concentrations (e.g. 100 nM), causing vasospasm (Figure 1a). The examples presented show that, at the two concentrations of ET-1 presented, there was a marked initial increase in the rate of vasomotion, which persisted in the 1 nM ET-1 solution but rapidly transformed into vasospasm in the 100 nM ET-1 solution. The concentration–response curve for the ET-1-induced increases in lymphatic vasomotion is presented in Figure 1b (solid curve, n=9). ET-1 concentrations as low as 0.1 nM tended to increase lymphatic vasomotion with maximal activation near 10 nM. Concentrations near 100 nM or higher abolished all vessel relaxation, resulting in sustained tonic contraction termed vasospasm.
Figure 1.
Effects of ET-1 on lymphatic vasomotion and vessel tone as measured from vessel diameter. (a) Sample traces indicating ET-1-induced lymphatic vasomotion (upper trace) and lymphatic vasospasm (lower trace; upward deflections indicate constrictions). (b, c) ET-1 concentration–response curves for lymphatic constriction rate and vessel tone (measured as (Di−D)/Di) in vessels with or without endothelium. The double-sided arrow in (b) is to highlight the abrupt change from vasomotion tovasospasm. (d) Comparison of concentration–response curves for ET-1 on the lymphatic constriction rate and tone. Data were normalised with respect to the corresponding control lymphangion constriction rate and diameter (Di), respectively, with n=9–12 lymphangions for all points.
ET-1 also acted to increase lymphatic tone in a dose-dependent manner, as estimated by measuring systolic lymphangion diameter with a maximum increase in estimated tone (i.e. (Di−D)/Di; Methods) of 52±3.7% (n=9, P<0.01) at a concentration of 1 μM (Figure 1c, solid curve). Comparison of the concentration–response curves for vasomotion frequency and tone is made in Figure 1d. There was a parallelism between the curves for vasomotion (solid curve) and tone at lower concentrations (ET-1), though at higher concentrations vasomotion showed steeper ET-1 concentration dependence. The greatest difference was at the inflection where vasomotion transformed to vasospasm, the concentration-dependent increase in tone continuing in a predictable manner and showing no obvious inflection over this same ET-1 concentration range.
ET-1 action is independent of the endothelium
The role of the endothelium in ET-1-induced enhancement of vasomotion and induction of vasospasm was tested by application of ET-1 to endothelium-denuded lymphatic vessels (Methods). The endothelium was not responsible for the ET-1 actions, as its absence caused no significant difference in the ET-1 concentration–response curves for vasomotion or vessel tone (Figures 1b, c, dashed curves, n=9, P>0.05 for each point between the + and − endothelium groups).
Effects of ET-1 on lymphatic smooth muscle [Ca2+]i
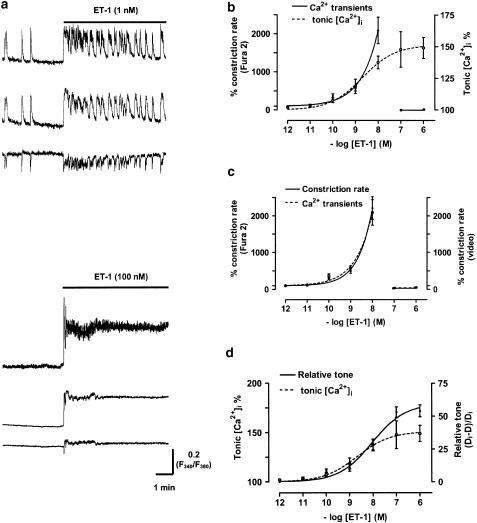
The mechanisms of ET-1 action were further investigated by measuring the effects of ET-1 on lymphatic smooth muscle [Ca2+]i, using the ratiometric Ca2+ indicator fura-2. These experiments were always performed on endothelium-denuded vessels to both facilitate loading of the calcium indicator fura-2/AM into the smooth muscle and to provide data specifically for the smooth muscle. System limitations during Ca2+ photometry experiments prevented videoscopic edge detection. However, by estimating the fluorescence of fura-2 at its isosbestic point (F360) using a linear combination of the F340 and F380 signal, it was possible to determine changes due to vessel movement (Methods). It was found that this movement correlated with the brief Ca2+ transients regularly recorded throughout the experiments, confirming that these transients reflected vasomotion (see also Zhao & van Helden, 2002). Examples of Ca2+ transients are presented in Figure 2a. The sample records presented here show the effects of application of 1 and 100 nM ET-1 on relative [Ca2+]i. The responses show similar characteristics to those of Figure 1a, obtained by measurement of lymphangion diameters, the Ca2+ transients reflecting vasomotion and the increase in tonic [Ca2+]i paralleling diameter-based measures of the increase in lymphangion tone.
Figure 2.
Effects of ET-1 on vasomotion (measured using Ca2+ transients) and relative tonic [Ca2+]i in lymphatic smooth muscle. (a) Sample traces showing fura-2 F340 and F380 fluorescence measurements with the F340/F380 ratio used to measure ET-1-induced increases in relative lymphatic smooth muscle [Ca2+]i in response to low (1 nM) and higher ET-1 concentrations (100 nM). (b) ET-1 concentration–response curves for the % change in constriction rate and tonic [Ca2+]i. (c) Comparison of ET-1 concentration–response curves for the % change in constriction rate measured either using fura-2-based Ca2+ transients or by video-recorded movement (i.e. the endothelium-denuded record of Figure 1b). (d) Comparison of ET-1 concentration–response curves for the % change in tonic [Ca2+]i and normalised change in vessel tone (i.e. (Di−D)/Di; endothelium-denuded record of Figure 1c). All data presented here are from endothelium-denuded vessels. Data were normalised with respect to the corresponding control tissues with n=6–9. Vertical lines denote s.e.m. with n=9–12 lymphangions for all points.
ET-1 increased the frequency of Ca2+ transients and hence vasomotion in a dose-dependent manner, with inhibition again occurring at higher [ET-1], commensurate with the onset of vasospasm (Figure 2b, solid curve). The concentration–response curve for the relative increase in tonic [Ca2+]i measured between Ca2+ transients is shown in Figure 2b (dashed curve). Comparison of the effects of ET-1 on the concentration-dependent action of ET-1 to increase Ca2+ transients with these then undergoing a vasospasm-associated inhibition parallels the findings made from measurements of vessel diameter (Figure 2c). The almost identical profiles of these curves both confirm the use of the Ca2+ transients as a measure of vasomotion, and indicates that loading vessels with the Ca2+ sensing fluorophore fura-2 or the acetoxymethylester carrier did not significantly alter the dependence of vasomotion and the transformation to vasospasm on [ET-1]. Comparisons of the ET-1 concentration–response curve for tonic [Ca2+]i and for diameter-based lymphangion tone are presented in Figure 2d. There was a reasonable correlation between lymphatic tone (Figure 2d, solid line) and tonic [Ca2+]i (Figure 2d, dashed line) up to an [ET-1] of ∼10 nM. However, higher [ET-1] produced steeper increases in lymphatic tone than in tonic [Ca2+]i. It is known that ET-1 can directly enhance smooth muscle contractility (Huang et al., 1990), and this may provide some explanation as to the difference in these curves.
Pharmacological properties of ET-1-induced vasomotion
Pharmacological properties of the mechanisms underlying lymphatic vasomotion were examined using an [ET-1] of 1 nM. ET-1-activated receptor and intracellular pathways underlying vasomotion were investigated with either fura-2-based ratiometric Ca2+ photometry of the smooth muscle of endothelium-denuded lymphatic vessels or videoscopic measurement of the diameters of endothelium-intact vessels.
ET-1 receptor subtypes
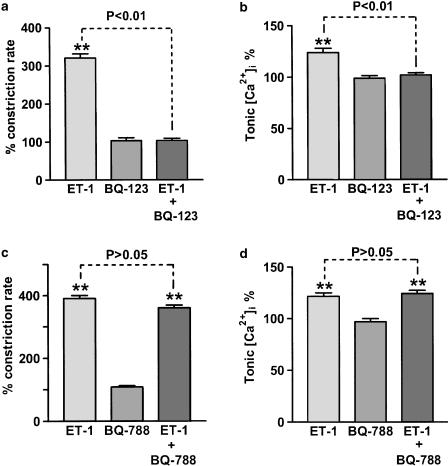
ET-1 actions were likely to be mediated by the ETA receptor subtype, as BQ-123 (1 μM), a specific antagonist of the ETA receptor (Eguchi et al., 1992; Ihara et al., 1992), abolished the effects of ET-1 (1 nM) both on the increase in frequency of the Ca2+ transients and on tonic [Ca2+]i (Figures 3a, b). In contrast, BQ-788 (1 μM), a specific antagonist of the ETB receptor (Ishikawa et al., 1994), had no significant effect on the ET-1-induced responses (Figures 3c, d). Neither BQ-123 (1 μM) nor BQ-788 (1 μM) had a direct effect on either the frequency of oscillatory Ca2+ transients or on tonic [Ca2+]i levels (Figure 3). Bath application of BQ-123 or BQ-788 also had no significant effects on the frequency and tone of lymphatic vasomotion, as measured by videoscopic vessel edge tracking (data not shown; P>0.05, n=6 for both).
Figure 3.
Effects of ET-1 receptor antagonists on ET-1-induced vasomotion and relative [Ca2+]i. BQ-123 (1 μM), an ETA receptor blocker, inhibited vasomotion induced by 1 nM ET-1 (a) and prevented the associated increase in [Ca2+]i (b). BQ-788 (1 μM), an ETB receptor blocker, did not significantly alter vasomotion induced by 1 nM ET-1 (c) or associated increase in [Ca2+]i (d). Direct application of the antagonists had no significant effects on either vasomotion or [Ca2+]i. Data were normalised with respect to the corresponding control lymphangions with n=6–9. Vertical lines denote s.e.m. **P<0.01.
Second messenger pathways
ET-1 receptors are known to be coupled to G-proteins. The possibility that this was of the Gi type was examined using PTx, an inhibitor of Gi-proteins (see Fields & Casey, 1997). Lymphatic vessels were superfused with physiological saline solution containing PTx (100 ng ml−1) for 4 h at 35°C, with the tissue then returned to control solution (Methods). PTx treatment of lymphatics had no significant effect on the 1 nM ET-1-induced increase in either vasomotion (342±20% of control; n=5, P>0.05) or tonic [Ca2+]i (125±2% of control; n=5, P>0.05).
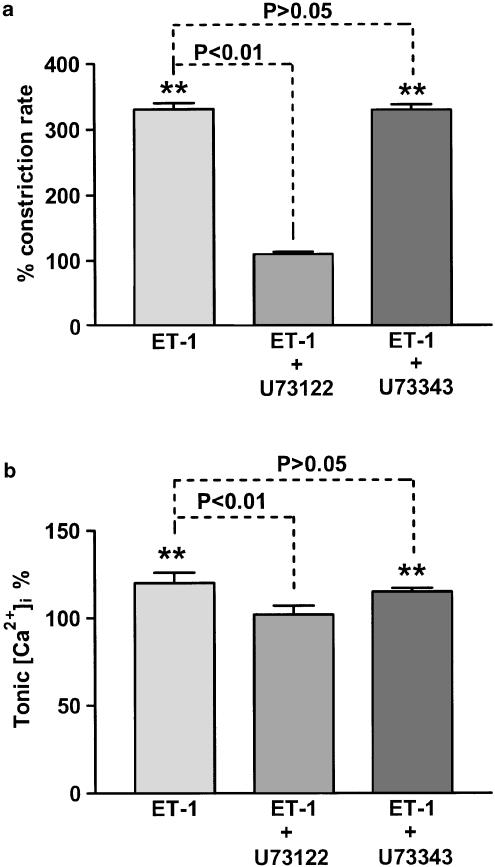
Application of the PLC inhibitor U73122 (5 μM) (Bleasdale et al., 1990; Thompson et al., 1991) prevented the increase in both vasomotion and resting [Ca2+]i induced by 1 nM ET-1 (Figures 4a, b). In contrast, U73343 (5 μM), an inactive analogue of U73122 (Bleasdale et al., 1990), had no measurable effect on these ET-1-induced increases (Figures 4a, b). U73122 itself had no significant effects on resting [Ca2+]i (100±2.6% of control, n=6, P>0.05), but inhibited spontaneous vasomotion (25±1.8% of control, n=6, P<0.01). U73343 had no significant effects on both vasomotion and resting [Ca2+]i (n=6, P>0.05, respectively).
Figure 4.
Application of the PLC inhibitor U73122 (5 μM) inhibited the increase in both vasomotion (a) and tonic [Ca2+]i (b) induced by 1 nM ET-1. In contrast, the inactive analogue U73343 (5 μM) had no significant effect. Data were normalised with respect to the corresponding control lymphangions with n=9–12. Vertical lines denote s.e.m. **P<0.01.
[Ca2+]i mobilisation and the role of the SR
Application of Ca2+-free solution (i.e. no added Ca2++1 mM EGTA) abolished spontaneous and 1 nM ET-1-induced vasomotion, as determined by measurement of the frequency of Ca2+ transients (n=8). The Ca2+-free solution did not alter resting [Ca2+]i (99±2.6% of control, n=8, P>0.05), but inhibited the increase in tonic [Ca2+]i (103±3.2% of control, n=8, P>0.05) induced by 1 nM ET-1. This indicates that extracellular Ca2+ is fundamental to ET-1 action.
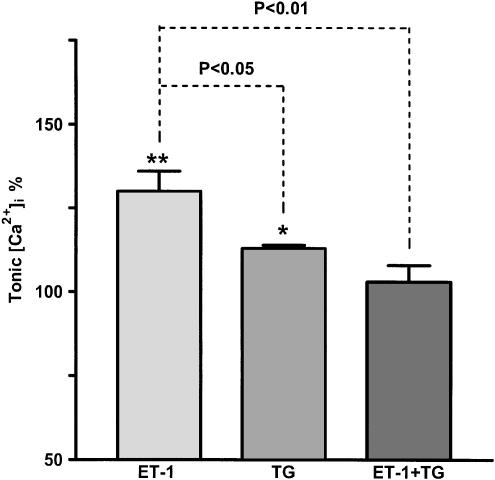
Evidence that ET-1 action occurred through activation of SR Ca2+ release was provided by experiments using BAPTA/AM to chelate intracellular Ca2+ and thapsigargin, an irreversible inhibitor of the SR Ca2+ pump (Ghosh et al., 1988; Takemura et al., 1990; Darby et al., 1993). BAPTA/AM (10 μM) applied for 6 min before application of 1 nM ET-1, now without BAPTA/AM present, abolished both spontaneous and ET-1-induced lymphatic vasomotion (n=3) and the increase in tonic [Ca2+]i (n=3). Thapsigargin (1 μM) caused a small but significant increase (P<0.05) in the resting [Ca2+]i of lymphatic smooth muscle (Figure 5), and inhibited spontaneous vasomotion in control conditions (n=4, P<0.01). Importantly, thapsigargin (1 μM) abolished the increase in both vasomotion and tonic [Ca2+]i induced by 1 nM ET-1 (Figure 5).
Figure 5.
Application of the store Ca2+ pump inhibitor thapsigargin (1 μM) inhibited the increase in both vasomotion (a) and tonic [Ca2+]i (b) induced by 1 nM ET-1. Data were normalised with respect to the corresponding control lymphangions with n=9–12. Vertical lines denote s.e.m. **P<0.01 and *P<0.05.
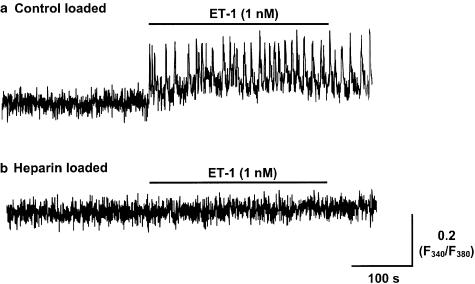
Evidence that ET-1 action occurred through activation of IP3Rs was provided by studies with heparin, a known blocker of IP3Rs (Ghosh et al., 1988; Kobayashi et al., 1988). Heparin, when introduced into the smooth muscle cells by reversible permeabilisation (Methods), inhibited both spontaneous and ET-1 (1 nM)-induced vasomotion (n=8; Figure 6b). In contrast, ‘control' tissues, exposed to the same loading procedure but in the absence of heparin, exhibited vasomotion of 2.0±0.5 transients min−1 before and 7.0±0.8 transients min−1 in the presence of 1 nM ET-1 (n=8, P<0.01; Figure 6a). Heparin-loaded tissues also showed no significant tonic increase in [Ca2+]i in response to 1 nM ET-1 (102±1.2% of control; n=8, P>0.05; Figure 6b). By comparison, application of 1 nM ET-1 to ‘control' tissues caused a significant increase in tonic [Ca2+]i of 119±3% (n=8, P<0.05; Figure 6a). Similar findings were made using 2-APB, which has been reported to block IP3Rs and/or store-dependent Ca2+ entry (Maruyama et al., 1997; Ascher-Landsberg et al., 1999), with application of 30 μM 2-APB abolishing both spontaneous vasomotion and the ET-1-induced enhancement of vasomotion (n=3).
Figure 6.
Intracellular heparin inhibits the increase in vasomotion and tonic [Ca2+]i induced by 1 nM ET-1. Lymphatic smooth muscle was preloaded with heparin (8 mg ml−1) by a reversible permeabilisation procedure. The records show the fura-2 F340/F380 fluorescence ratio response to ET-1 in lymphatic smooth muscle without (a) and with heparin loading (b).
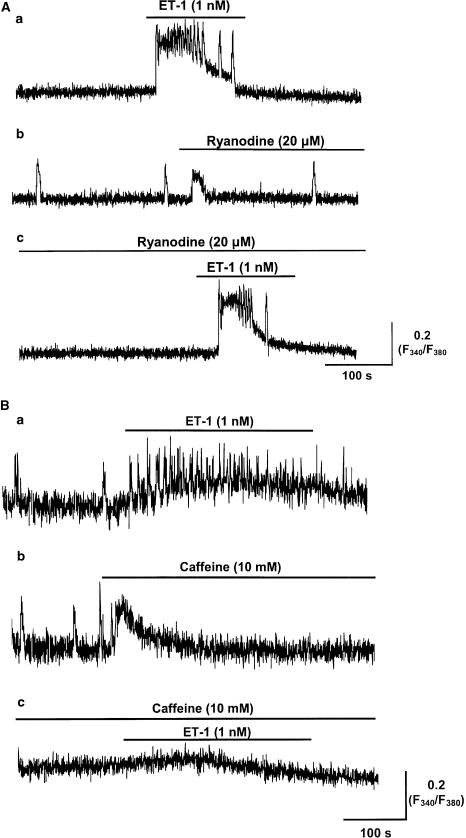
The role of ryanodine receptors (RyRs) was investigated by application of ryanodine. Ryanodine (1 and 20 μM) itself had no measurable effect on vasomotion, with frequencies of 2.5±0.6, 2.3±0.3 and 2.2±0.3 Ca2+ transients min−1 in control, 1 and 20 μM ryanodine, respectively (15 min exposure, n=5 for each, P>0.05), but caused a small but significant transient increase in [Ca2+]i (113±0.7 and 115±0.5%, P<0.05, n=3 and 5, respectively) (Figure 7Ab). Ryanodine (1 and 20 μM) had no significant effect on the enhancement of vasomotion and the magnitude of the increase in [Ca2+]i induced by 1 nM ET-1 (P>0.05, n=3 and 5 for both), but may have caused a decrease in duration of the ET-1-induced increase in relative [Ca2+]i (Figure 7Ac).
Figure 7.
Effects of ryanodine and caffeine on the increase in vasomotion and tonic [Ca2+]i induced by 1 nM ET-1. (A) The sample traces indicating the changes of the F340/F380 fluorescence ratio in the smooth muscle of the same lymphatic vessel exposed to 1 nM ET-1 (a), ryanodine (20 μM) (b), and ET-1 (1 nM), in the presence of ryanodine (20 μM) (c) are shown. Ryanodine did not prevent the responses to 1 nM ET-1. (B) Sample traces indicating the changes of the F340/F380 fluorescence ratio in the smooth muscle of a lymphatic vessel exposed to 1 nM ET-1 (a), 10 mM caffeine (b) and both 10 mM caffeine and 1 nM ET-1 (c) are shown. Caffeine inhibited the increase in vasomotion and tonic [Ca2+]i induced by 1 nM ET-1.
Caffeine (10 mM) abolished spontaneous vasomotion, despite causing a small but significant transient increase in [Ca2+]i of 113±0.2% (P<0.05, n=5, Figure 7Bb). This increase in [Ca2+]i was likely to be due to the activation of RyRs, as it was abolished following treatment of the tissue with 20 μM ryanodine (98±0.2%, P>0.05, n=6). Importantly, caffeine (10 mM) abolished spontaneous vasomotion and the enhancement of both vasomotion and tonic [Ca2+]i induced by 1 nM ET-1 (n=6; Figure 7Bc). This is a common action of caffeine, as observed for electrical rhythmicities in other smooth muscles (Hashitani et al., 1996; van Helden et al., 2000), and is likely to occur through its known actions to inhibit IP3R-mediated Ca2+ release (Ehrlich & Watras, 1988) and/or to increase cAMP (Beavo & Reifsnyder, 1990).
ET-1-induced vasospasm
The data of Figures 1 and 2 demonstrate that ET-1 at concentrations higher than 10 nM produced a maintained maximal, tonic vessel constriction and increase in [Ca2+]i. Therefore, subsequent experiments were performed using ET-1 at a concentration of 100 nM to examine the mechanisms underlying this vasospasm.
Role of extracellular Ca2+
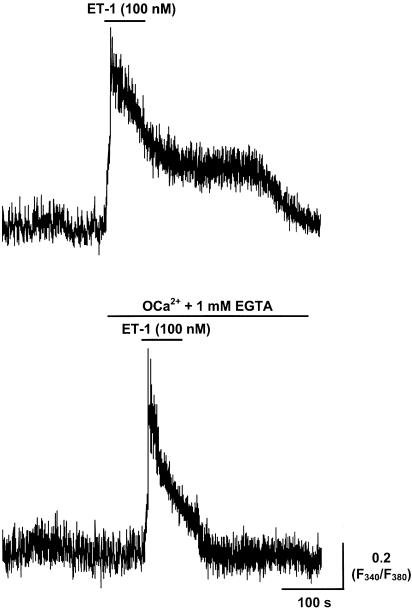
Application of ET-1 for 1 min at a concentration of 100 nM markedly increased vasomotion upon application, which rapidly changed to a sustained contracture and underlying maintained increase in smooth muscle [Ca2+]i (Figures 1a, 2a). Brief incubation of the tissue with Ca2+-free solution (i.e. 0 Ca2+, 1 mM EGTA solution) for 1 min (>95% bath changeover in 20 s) abolished all vasomotion and the sustained but not transient increase in [Ca2+]i induced by 1 min application of 100 nM ET-1 (n=5, Figure 8). These results suggest that the initial Ca2+ transient induced by ET-1 was primarily due to the release of Ca2+ from intracellular stores, while the sustained plateau was due to the influx of external Ca2+. The ET-1-induced transient increase in [Ca2+]i was abolished when ET-1 (100 nM) was applied to tissues pretreated for 3 min (and then maintained) in Ca2+-free solution (n=6). Commensurate with this, CPA (20 μM; n=3), while causing significant increases in [Ca2+]i (P<0.01) when applied after 1 min exposure to Ca2+-free solution, caused no response when applied after 3 min exposure. ATP (100 μM) also caused no response when applied after 3 min exposure, whereas we have previously reported a large response when ATP was applied upon changeover to Ca2+-free solution (Zhao & van Helden, 2002). This suggests that maintained exposure to the Ca2+-free solution rapidly depleted the SR stores. Significantly, application of the 100 nM ET-1 after 3 min exposure to the Ca2+-free solution prevented the induction of vasospasm, as measured by edge detection (n=4), confirming that Ca2+ influx from the extracellular space is required for ET-1-induced vasospasm.
Figure 8.
Effect of Ca2+-free solution on ET-1-induced increase in [Ca2+]i. Application of 100 nM ET-1 caused a large transient followed by sustained increase in [Ca2+]i measured as the fura-2 F340/F380 fluorescence ratio in lymphatic smooth muscle. The sustained increase in [Ca2+]i became transient when 100 nM ET-1 was applied in a Ca2+-free 1 mM EGTA-containing solution, with the tissue only briefly (i.e. ∼1 min) preincubated in this Ca2+-free solution.
Role of Ca2+ entry channels
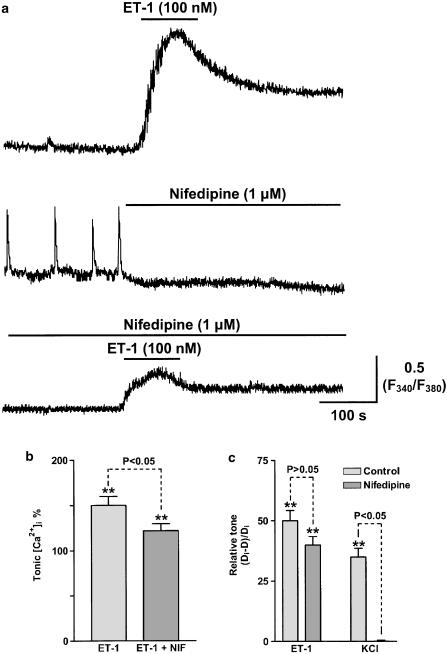
Voltage-operated, receptor-operated and/or store-operated Ca2+ channels have each been suggested as candidates for the Ca2+ influx pathway(s) underlying ET-1-induced vasospasm. The possibility of Ca2+ influx through VOCs, which in lymphatic smooth muscle are likely to be L-type Ca2+ channels (Ohhashi et al., 1978), was examined by applying nifedipine. Application of nifedipine (1 μM) abolished spontaneous and ET-1-induced vasomotion (Figure 9a). However, this was likely to be through blockade of the L-Ca2+ channel-mediated action potentials, and not through blockade of ET-1-induced Ca2+ entry, as nifedipine (1 μM) reduced, but did not abolish, the sustained increase in [Ca2+]i induced by 100 nM ET-1 (Figures 9a, b) and had no significant effect on vessel tone (Figure 9c). In contrast, nifedipine (1 μM) abolished the vasospasm induced by 100 mM KCl (Figure 9c). These results indicate that, while L-type Ca2+ channels are involved in the ET-1-induced vasospasm, their presence is not critical for vasospasm to occur.
Figure 9.
Effects of nifedipine on ET-1- and K+-induced vasospasm. (a) Sample traces indicating the changes in lymphatic smooth muscle [Ca2+]i measured as the F340/F380 fura-2 fluorescence ratio in response to 100 nM ET-1, 1 μM nifedipine and 100 nM ET-1 applied in the presence of 1 μM nifedipine. (b) Bar graph indicating the mean effect of 100 nM ET-1 on tonic [Ca2+]i before and in the presence of 1 μM nifedipine. (c) Bar graph showing the mean effects of both 100 nM ET-1 and 100 mM KCl solution on relative lymphangion tone (i.e. measured as (Di−D)/Di)) in the absence and presence of 1 μM nifedipine. Data bars in (b) and (c) were normalised with respect to the corresponding controls with n=5 lymphangions for each value. Vertical lines denote s.e.m. **P<0.01.
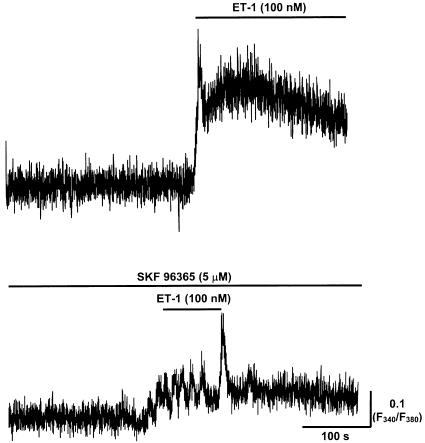
The role of voltage-independent Ca2+ entry in vasospasm was examined using SKF 96365, a compound that has been shown to inhibit Ca2+ entry through either SOCs (Merritt et al., 1990) or VOCs (Cuthbert et al., 1994). Importantly, SKF 96365 (30 μM) had no obvious effect on vasospasm induced by 100 mM KCl (n=6), but caused vasospasm induced by 100 nM ET-1 to revert back to vasomotion (rate of 10±2.5 constrictions min−1, n=6, Figure 10). This indicates that the ET-1 was now less effective, as the outcome parallels lowering the [ET-1] back into the range of inducing vasomotion. The effect of SKF 96365 was reversible, as, consequent to 10 min washout, reapplication of 100 nM ET-1 produced vasospasm with lymphangion diameter decreased to 50±2% of control (n=6).
Figure 10.
Effects of SKF 96365 on ET-1-induced vasospasm. Sample traces indicating the changes in lymphatic smooth muscle [Ca2+]i measured as the F340/F380 fura-2 fluorescence ratio in response to 100 nM ET-1 and both SKF 96365 (5 μM) and 100 nM ET-1.
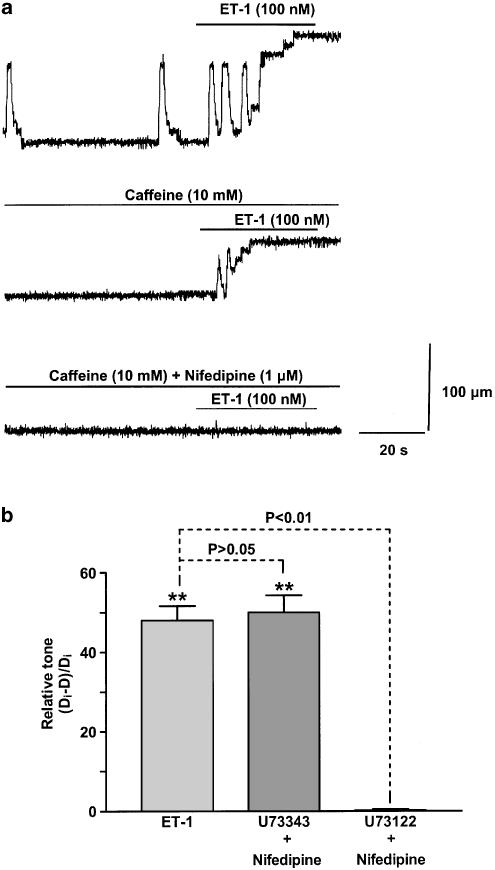
These results suggest that ET-1-induced vasospasm primarily involves SOCs with residual Ca2+ influx through L-type Ca2+ channels. Further evidence for a primary role of SOCs is provided by the finding that 2-APB (90 μM), an inhibitor of IP3 receptors and/or SOCs (Maruyama et al., 1997; Gregory et al., 2001), when applied in the presence of nifedipine (1 μM), abolished vasospasm induced by 100 nM ET-1 (n=6). However, it is possible that 2-APB at these high concentrations may have nonspecific effects. Therefore, we also investigated a role for SOCs using either caffeine or the PLC blocker U73122. Caffeine (10 mM) markedly blunted the action of 100 nM ET-1 to induce vasospasm, the relative tone now reduced from 51±2.2 to 33±1.0% of control diameter (n=6–9; Figure 11a). Further addition of nifedipine (1 μM) to the inhibitory 10 mM caffeine-containing solution abolished vasomotion (n=6; Figure 11a). Bath application of U73122 (5 μM) in the presence of 1 μM nifedipine abolished the vasospasm induced by 100 nM ET-1 (Figure 11b). In contrast, the same experiment made with nifedipine (1 μM) and U73343 (5 μM), an inactive analogue of U73122, had no significant effect on the ET-1-induced vasospasm (Figure 11b).
Figure 11.
Effects of caffeine and the PLC antagonist U73122 on ET-1-induced vasospasm. (a) The effects of 100 nM ET-1 on constriction of a lymphangion in control, caffeine (10 mM) and caffeine (10 mM) together with nifedipine (1 μM). (b) Bar graphs showing the effects of U73343 (5 μM) and U73122 (5 μM) on the relative tonic increase in lymphangion constriction in response to 100 nM ET-1 in the presence of nifedipine (1 μM). Data in (b) were normalised with respect to the corresponding control tissues with n=6 lymphangions for all points. Vertical lines denote s.e.m. **P<0.01.
Discussion
This project has investigated lymphatic vasomotion and vasospasm induced by ET-1. The data provide a detailed account of the receptor and intracellular pathways involved.
ET-1-induced vasomotion
The present study demonstrates that ET-1-induced enhancement of vasomotion occurs through activation of ETA receptors on lymphatic smooth muscle, which stimulates production of PLC by a PTx-insensitive G-protein, leading to synthesis of IP3 and release of Ca2+ from intracellular IP3R-operated stores. Evidence for this comes from the findings that ET-1-induced increases in vasomotion were blocked by (1) the ETAR antagonist BQ-123 but not the ETBR antagonist BQ-788, (2) the PLC inhibitor U73122 but not the inactive analogue U73343, (3) the Ca2+ store ATPase inhibitor thapsigargin, (4) removal of extracellular Ca2+, (5) the intracellular Ca2+ chelator BAPTA/AM and (6) the IP3R antagonists 2-APB and heparin. The ET-1-induced enhancement of vasomotion was also blocked by caffeine. This could result through direct inhibition of IP3 channels (Bezprozvanny et al., 1991), inhibition of PLC activity (Hamada et al., 1997) and/or phosphodiesterase-induced formation of cAMP (Beavo & Reifsnyder, 1990; Tsugeno et al., 1995). It did not occur through an action on RyRs, as RyRs while functionally present in the lymphatic smooth muscle did not abolish spontaneous or ET-1-induced vasomotion.
The finding that heparin abolished spontaneous vasomotion is consistent with the view that IP3-operated Ca2+ stores are required for vasomotion even under control conditions. Furthermore, 2-APB at its effective inhibitory concentrations (Maruyama et al., 1997) abolished spontaneous vasomotion, consistent with a general involvement of IP3R-operated stores.
There has been limited information about the role of endogenous ET-1 on lymphatic vasomotion both in vivo and in vitro. The fact that ET-1 antagonists BQ-123 and BQ-788 had no significant effects on spontaneous lymphatic vasomotion or smooth muscle [Ca2+]i, as studied here in vitro, suggests that, under these conditions, endogenous ET-1 does not modulate lymphatic vasomotion. Similar findings have been reported for blood vessels studied in vivo, where it was found that the concentration of circulating ET-1 was too low to produce any detectable vasoconstriction (Ortega Mateo & de Artinano, 1997). However, under some pathological conditions, it is likely that ET-1 concentrations become sufficiently elevated to either increase vasomotion or to cause vasospasm. Moderate enhancements of [ET-1] would increase lymphatic pumping and hence the propulsion of lymph. In contrast, high [ET-1] would cause vasospasm and interrupt the flow of lymph. The latter is unfortunately a common event for blood vessels where events such as haemorrhage can lead to vasospasm through marked enhancement of ET-1 production (Kwan et al., 2002). While vasospasm would be a less catastrophic event in lymphatic vessels, it remains a phenomenon of considerable interest, as the underlying mechanisms are likely to share the same properties as for blood vessels.
ET-1-induced vasospasm
It has been reported in bovine mesenteric lymphatic vessels that ET-1 at higher concentrations induces vasospasm (Sakai et al., 1999), but the underlying mechanisms were not elucidated. The present study provides the first evidence that ET-1 at concentrations greater than 10 nM causes vasospasm primarily by massive Ca2+ influx through SOCs, with a minor contribution by L-type Ca2+ channels.
ET-1 is known to induce depolarisation of vascular smooth muscle and associated biphasic elevation of [Ca2+]i and cell constriction (see Haynes & Webb, 1993). This elevation of [Ca2+]i consists of an initial rapid rise due to Ca2+ release from the SR, and is followed by a fall to plateau levels, which is maintained by the persistent influx of extracellular Ca2+ through membrane ion channels (Yanagisawa et al., 1988; Goto et al., 1989). Results from the present experiments parallel these findings with high [ET-1] (e.g. ⩾100 nM), causing an initial Ca2+ transient due to release from intracellular Ca2+ stores, followed by a sustained plateau due to Ca2+ influx from the extracellular space.
The extracellular Ca2+-dependent sustained component of the ET-1-induced [Ca2+]i changes associated with vasospasm resulted from activation of several Ca2+ permeant channels. L-Ca2+ channels played a minor role in this, as blockade of these channels by nifedipine caused no obvious change in vasospasm and only partially reduced [Ca2+]i. Studies with the nonspecific SOC blocker SKF 96365 (Chernaya et al., 1996) indicated that Ca2+ entry through either VOCs or SOCs was dominant, as application of SKF 96365 transformed ET-1-induced vasospasm to vasomotion. This action is equivalent to reducing the effective [ET-1] to less than 10 μM (Figure 1). Evidence that these channels were SOCs was provided by the finding that either the PLC inhibitor U73122 or the IP3R and/or SOC blocker 2APB, when applied together with nifedipine, abolished vasospasm. Significantly, application of caffeine also markedly reduced ET-1-induced vasospasm and abolished all constriction with the further addition of nifedipine.
These findings are consistent with observations on enzymatically isolated lymphatic smooth muscle cells, where it was found that maximum concentrations of agonists caused a biphasic Ca2+ signal composed of an early transient phase due to Ca2+ release from intracellular Ca2+ stores, and a following sustained plateau phase due to Ca2+ influx primarily through SOCs (Shuttleworth, 1999). The findings are also consistent with findings that ET-1-induced vasospasm in arteries of a two-haemorrhage rabbit model was due to Ca2+ influx through VOCs and SOCs (Zuccarello et al., 1996a).
Conclusions
Spontaneous lymphatic vasomotion occurs through a pacemaker mechanism dependent on Ca2+ release from intracellular IP3R-operated stores and largely independent of RyRs. ET-1 enhances lymphatic vasomotion through activation of smooth muscle ETA receptors, which stimulates the production of PLC by a PTx-insensitive G-protein, leading to synthesis of IP3 and release of Ca2+ from intracellular IP3R-operated stores.
A most striking observation of the present study was the abrupt transition from vasomotion to vasospasm. This occurred at [ET-1] in the range 10–100 nM, with supra maximal [ET-1] causing the frequency of vasomotion to increase to such an extent that reconstitution of [Ca2+]i could no longer occur. The consequence of this now uncontrolled Ca2+ homeostasis was a failure of vasomotion and the onset of vasospasm. Importantly, pharmacological agents such as SKF 96365, which reduce ET-1-induced increases in [Ca2+]i, allowed recovery of cellular Ca2+ homeostasis and now vessels behaved physiologically, undergoing vasomotion at [ET-1] that would normally cause vasospasm. We propose that ET-1-induced vasospasm primarily results through overstimulation of Ca2+ stores such that they can no longer refill, this abolishing normal function (e.g. vasomotion) and inducing massive Ca2+ entry through store-operated Ca2+ channels with L-type Ca2+ channels exerting a lesser role.
Acknowledgments
We gratefully acknowledge the assistance offered by Chris Katnik and Peter Dosen. The National Health and Medical Research Council of Australia and the Hunter Medical Research Institute supported the work.
Abbreviations
- ACh
acetylcholine
- 2-APB
2-aminoethoxydiphenyl borate
- ATP
adenosine 5′-trisphosphate
- BAPTA
1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid
- BAPTA/AM
BAPTA acetoxymethylester
- BQ-123
cyclic D-Trp-D-Asp-Pro-D-Val-Leu
- BQ-788,N
cis-2,6-dimethylpiperidinocarbonyl-L-methylleucyl-D-l-methoxycarbonyltryptophanyl-D-norleucine
- [Ca2+]i
intracellular Ca2+ concentration
- CPA
cyclopiazonic acid
- DMSO
dimethylsulphoxide
- EGTA
ethylene glycol-O,O′-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid
- ET-1
endothelin-1
- Fx
fluorescence at wavelength x
- fluo-3/AM
fluo-3 acetoxymethylester
- fura-2/AM
fura-2 acetoxymethylester
- IP3
inositol 1,4,5-trisphosphate
- IP3R
IP3 receptor
- lymphangion
lymphatic chamber between adjacent valves
- PLC
phospholipase C
- PTx
pertussis toxin
- RyR
ryanodine receptor
- SOC
store-operated channel
- U73122
1-(6-((17-beta-methoxyestra-1,3,5(10)trien-17-yl)amino)hexyl)-1H-pyrrole-2,5-dione
- U73343
1-[6-[[(17beta)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-2,5 pyrrolidinedione
- VOC
voltage-operated channel
References
- ASCHER-LANDSBERG J., SAUNDERS T., ELOVITZ M., PHILLIPPE M. The effects of 2-aminoethoxydiphenyl borate, a novel inositol 1,4,5-trisphosphate receptor modulator on myometrial contractions. Biochem. Biophys. Res. Commun. 1999;264:979–982. doi: 10.1006/bbrc.1999.1602. [DOI] [PubMed] [Google Scholar]
- BEAVO J.A., REIFSNYDER D.H. Primary sequence of cyclic nucleotide phosphodiesterase isozymes and the design of selective inhibitors. Trends Pharmacol. Sci. 1990;11:150–155. doi: 10.1016/0165-6147(90)90066-H. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- BEZPROZVANNY I., WATRAS J., EHRLICH B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- BLEASDALE J.E., THAKUR N.R., GREMBAN R.S., BUNDY G.L., FITZPATRICK F.A., SMITH R.J., BUNTING S. Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J. Pharmacol. Exp. Ther. 1990;255:756–768. [PubMed] [Google Scholar]
- BURCH R.M., JELSEMA C., AXELROD J. Cholera toxin and pertussis toxin stimulate prostaglandin E2 synthesis in a murine macrophage cell line. J. Pharmacol. Exp. Ther. 1988;244:765–773. [PubMed] [Google Scholar]
- CHEN C., WAGONER P.K. Endothelin induces a nonselective cation current in vascular smooth muscle cells. Circ. Res. 1991;69:447–454. doi: 10.1161/01.res.69.2.447. [DOI] [PubMed] [Google Scholar]
- CHERNAYA G., VAZQUEZ M., REEVES J.P. Sodium–calcium exchange and store-dependent calcium influx in transfected Chinese hamster ovary cells expressing the bovine cardiac sodium–calcium exchanger. Acceleration of exchange activity in thapsigargin-treated cells. J. Biol. Chem. 1996;271:5378–5385. doi: 10.1074/jbc.271.10.5378. [DOI] [PubMed] [Google Scholar]
- CHIAVAROLI C., BIRD G., PUTNEY J.W., JR Delayed ‘all-or-none' activation of inositol 1,4,5-trisphosphate-dependent calcium signaling in single rat hepatocytes. J. Biol. Chem. 1994;269:25570–25575. [PubMed] [Google Scholar]
- COESSENS B.C. Endothelin: an endothelium-derived vaso-active peptide. J. Reconstr. Microsurg. 1994;10:405–410. doi: 10.1055/s-2007-1006611. [DOI] [PubMed] [Google Scholar]
- CONSIGNY P.M. Endothelin-1 increases arterial sensitivity to 5-hydroxytryptamine. Eur. J. Pharmacol. 1990;186:239–245. doi: 10.1016/0014-2999(90)90439-d. [DOI] [PubMed] [Google Scholar]
- CUTHBERT N.J., GARDINER P.J., NASH K., POLL C.T. Roles of Ca2+ influx and intracellular Ca2+ release in agonist-induced contractions in guinea pig trachea. Am. J. Physiol. 1994;266:L620–L627. doi: 10.1152/ajplung.1994.266.6.L620. [DOI] [PubMed] [Google Scholar]
- DARBY P.J., KWAN C.Y., DANIEL E.E. Use of calcium pump inhibitors in the study of calcium regulation in smooth muscle. Biol. Signals. 1993;2:293–304. doi: 10.1159/000109510. [DOI] [PubMed] [Google Scholar]
- EGUCHI S., HIRATA Y., IHARA M., YANO M., MARUMO F. A novel ETA antagonist (BQ-123) inhibits endothelin-1-induced phosphoinositide breakdown and DNA synthesis in rat vascular smooth muscle cells. FEBS Lett. 1992;302:243–246. doi: 10.1016/0014-5793(92)80451-l. [DOI] [PubMed] [Google Scholar]
- EHRLICH B.E., WATRAS J. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 1988;336:583–586. doi: 10.1038/336583a0. [DOI] [PubMed] [Google Scholar]
- FIELDS T.A., CASEY P.J. Signalling functions and biochemical properties of pertussis toxin-resistant G-proteins. Biochem. J. 1997;321:561–571. doi: 10.1042/bj3210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOREY H.W. Observations on the contractility of lacteals. Part I. J. Physiol. (Lond.) 1927;62:1–18. doi: 10.1113/jphysiol.1927.sp002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUYA K., FURUYA S., YAMAGISHI S. Intracellular calcium responses and shape conversions induced by endothelin in cultured subepithelial fibroblasts of rat duodenal villi. Pflugers Arch. 1994;428:97–104. doi: 10.1007/BF00374846. [DOI] [PubMed] [Google Scholar]
- GHOSH T.K., EIS P.S., MULLANEY J.M., EBERT C.L., GILL D.L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J. Biol. Chem. 1988;263:11075–11079. [PubMed] [Google Scholar]
- GOLDMAN W.F., BOVA S., BLAUSTEIN M.P. Measurement of intracellular Ca2+ in cultured arterial smooth muscle cells using Fura-2 and digital imaging microscopy. Cell Calcium. 1990;11:221–231. doi: 10.1016/0143-4160(90)90073-4. [DOI] [PubMed] [Google Scholar]
- GOTO K., KASUYA Y., MATSUKI N., TAKUWA Y., KURIHARA H., ISHIKAWA T., KIMURA S., YANAGISAWA M., MASAKI T. Endothelin activates the dihydropyridine-sensitive, voltage-dependent Ca2+ channel in vascular smooth muscle. Proc. Natl. Acad. Sci. U.S.A. 1989;86:3915–3918. doi: 10.1073/pnas.86.10.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY R.B., RYCHKOV G., BARRITT G.J. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem. J. 2001;354:285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HADDOCK R.E., HIRST G.D., HILL C.E. Voltage independence of vasomotion in isolated irideal arterioles of the rat. J. Physiol. (Lond.) 2002;540:219–229. doi: 10.1113/jphysiol.2001.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMADA E., NAKAJIMA T., HATA Y., HAZAMA H., IWASAWA K., TAKAHASHI M., OTA S., OMATA M. Effect of caffeine on mucus secretion and agonist-dependent Ca2+ mobilization in human gastric mucus secreting cells. Biochim. Biophys. Acta. 1997;1356:198–206. doi: 10.1016/s0167-4889(96)00177-2. [DOI] [PubMed] [Google Scholar]
- HASHITANI H., VAN HELDEN D.F., SUZUKI H. Properties of spontaneous depolarizations in circular smooth muscle cells of rabbit urethra. Br. J. Pharmacol. 1996;118:1627–1632. doi: 10.1111/j.1476-5381.1996.tb15584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYNES W.G., WEBB D.J. The endothelin family of peptides: local hormones with diverse roles in health and disease. Clin. Sci. (Lond.) 1993;84:485–500. doi: 10.1042/cs0840485. [DOI] [PubMed] [Google Scholar]
- HUANG X.N., HISAYAMA T., TAKAYANAGI I. Endothelin-1 induced contraction of rat aorta: contributions made by Ca2+ influx and activation of contractile apparatus associated with no change in cytoplasmic Ca2+ level. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;341:80–87. doi: 10.1007/BF00195062. [DOI] [PubMed] [Google Scholar]
- IHARA M., ISHIKAWA K., FUKURODA T., SAEKI T., FUNABASHI K., FUKAMI T., SUDA H., YANO M. In vitro biological profile of a highly potent novel endothelin (ET) antagonist BQ-123 selective for the ETA receptor. J. Cardiovasc. Pharmacol. 1992;20:S11–S14. doi: 10.1097/00005344-199204002-00005. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA K., IHARA M., NOGUCHI K., MASE T., MINO N., SAEKI T., FUKURODA T., FUKAMI T., OZAKI S., NAGASE T., NISHIKIBE M., YANO M. Biochemical and pharmacological profile of a potent and selective endothelin B-receptor antagonist, BQ-788. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON P.C.The myogenic response Handbook of Physiology: The Cardiovascular System 1980Bethesda, MD: American Physiological Society; 400–442.ed. Geiger, S.R., Bohr, D.F., Somlyo, A.P. & Sparks, H.V. pp [Google Scholar]
- JOHNSTON M.G., ELIAS R. The regulation of lymphatic pumping. Lymphology. 1987;20:215–218. [PubMed] [Google Scholar]
- KANASHIRO C.A., ALTIRKAWI K.A., KHALIL R.A. Preconditioning of coronary artery against vasoconstriction by endothelin-1 and prostaglandin F2_ during repeated downregulation of epsilon-protein kinase C. J. Cardiovasc. Pharmacol. 2000;35:491–501. doi: 10.1097/00005344-200003000-00021. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI S., SOMLYO A.V., SOMLYO A.P. Heparin inhibits the inositol 1,4,5-trisphosphate-dependent, but not the independent, calcium release induced by guanine nucleotide in vascular smooth muscle. Biochem. Biophys. Res. Commun. 1988;153:625–631. doi: 10.1016/s0006-291x(88)81141-0. [DOI] [PubMed] [Google Scholar]
- KURIHARA H., YAMAOKI K., NAGAI R., YOSHIZUMI M., TAKAKU F., SATOH H., INUI J., YAZAKI Y. Endothelin: a potent vasoconstrictor associated with coronary vasospasm. Life Sci. 1989;44:1937–1943. doi: 10.1016/0024-3205(89)90406-2. [DOI] [PubMed] [Google Scholar]
- KWAN A.L., LIN C.L., CHANG C.Z., WINARDI D., YEN C.P., WU S.C., LEE K.S., KASSELL N.F., HOWNG S.L., SAVAGE P., JENG A.Y. Oral administration of an inhibitor of endothelin-converting enzyme attenuates cerebral vasospasm following experimental subarachnoid haemorrhage in rabbits. Clin. Sci. (Lond.) 2002;103:414S–417S. doi: 10.1042/CS103S414S. [DOI] [PubMed] [Google Scholar]
- MARSDEN P.A., DANTHULURI N.R., BRENNER B.M., BALLERMANN B.J., BROCK T.A. Endothelin action on vascular smooth muscle involves inositol trisphosphate and calcium mobilization. Biochem. Biophys. Res. Commun. 1989;158:86–93. doi: 10.1016/s0006-291x(89)80180-9. [DOI] [PubMed] [Google Scholar]
- MARUYAMA T., KANAJI T., NAKADE S., KANNO T., MIKOSHIBA K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. (Tokyo) 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- MCHALE N.G. Lymphatic innervation. Blood Vessels. 1990;27:127–136. doi: 10.1159/000158803. [DOI] [PubMed] [Google Scholar]
- MERRITT J.E., ARMSTRONG W.P., BENHAM C.D., HALLAM T.J., JACOB R., JAXA-CHAMIEC A., LEIGH B.K., MCCARTHY S.A., MOORES K.E., RINK T.J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISLIN H.The Lymphangion Lymphangiology 1983New York: F.K. Schattauer Verlag; 165–175.ed. Foldi, M. & Casley-Smith, J.R. pp [Google Scholar]
- NEYLON C.B. Vascular biology of endothelin signal transduction. Clin. Exp. Pharmacol. Physiol. 1999;26:149–153. doi: 10.1046/j.1440-1681.1999.03013.x. [DOI] [PubMed] [Google Scholar]
- NISHIMURA J., MORELAND S., AHN H.Y., KAWASE T., MORELAND R.S., VAN BREEMEN C. Endothelin increases myofilament Ca2+ sensitivity in alpha-toxin-permeabilized rabbit mesenteric artery. Circ. Res. 1992;71:951–959. doi: 10.1161/01.res.71.4.951. [DOI] [PubMed] [Google Scholar]
- OHHASHI T., AZUMA T., SAKAGUCHI M. Transmembrane potentials in bovine lymphatic smooth muscle. Proc. Soc. Exp. Biol. Med. 1978;159:350–352. doi: 10.3181/00379727-159-40346. [DOI] [PubMed] [Google Scholar]
- ORTEGA MATEO A., DE ARTINANO A.A. Highlights on endothelins: a review. Pharmacol. Res. 1997;36:339–351. doi: 10.1006/phrs.1997.0246. [DOI] [PubMed] [Google Scholar]
- OTA M., OKU H. Nicardipine modification of endothelin-1 effects on visual evoked potential. Jpn. J. Ophthalmol. 1997;41:38–42. doi: 10.1016/s0021-5155(96)00017-2. [DOI] [PubMed] [Google Scholar]
- PANG C.Y., XU H., HUANG N., FORREST C.R., PERREAULT T.M., NELIGAN P.C. Amplification effect and mechanism of action of ET-1 in U-46619-induced vasoconstriction in pig skin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R713–R720. doi: 10.1152/ajpregu.2001.280.3.R713. [DOI] [PubMed] [Google Scholar]
- RAYNER S.E., VAN HELDEN D.F. Evidence that the substance P-induced enhancement of pacemaking in lymphatics of the guinea-pig mesentery occurs through endothelial release of thromboxane A2. Br. J. Pharmacol. 1997;121:1589–1596. doi: 10.1038/sj.bjp.0701306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEDER L.B., FERGUSON M.K. Endothelin-1 synthesis and receptor-mediated activity in porcine lymph vessels. J. Surg. Res. 1996;63:215–219. doi: 10.1006/jsre.1996.0250. [DOI] [PubMed] [Google Scholar]
- SAKAI H., IKOMI F., OHHASHI T. Effects of endothelin on spontaneous contractions in lymph vessels. Am. J. Physiol. 1999;277:H459–H466. doi: 10.1152/ajpheart.1999.277.2.H459. [DOI] [PubMed] [Google Scholar]
- SAKUMA I., ASAJIMA H., FUKAO M., TOHSE N., TAMURA M., KITABATAKE A. Effects of BQ-485, a selective ETA antagonist, on endothelin-mediated vasomotion in rat coronary vascular beds. J. Cardiovasc. Pharmacol. 1995;26:S400–S403. [PubMed] [Google Scholar]
- SHUTTLEWORTH T.J. What drives calcium entry during [Ca2+]i oscillations? Challenging the capacitative model. Cell Calcium. 1999;25:237–246. doi: 10.1054/ceca.1999.0022. [DOI] [PubMed] [Google Scholar]
- SIROUS Z.N., FLEMING J.B., KHALIL R.A. Endothelin-1 enhances eicosanoids-induced coronary smooth muscle contraction by activating specific protein kinase C isoforms. Hypertension. 2001;37:497–504. doi: 10.1161/01.hyp.37.2.497. [DOI] [PubMed] [Google Scholar]
- SOKOLOVSKY M., AMBAR I., GALRON R. A novel subtype of endothelin receptors. J. Biol. Chem. 1992;267:20551–20554. [PubMed] [Google Scholar]
- TAKEMURA H., THASTRUP O., PUTNEY J.W., JR Calcium efflux across the plasma membrane of rat parotid acinar cells is unaffected by receptor activation or by the microsomal calcium ATPase inhibitor, thapsigargin. Cell Calcium. 1990;11:11–17. doi: 10.1016/0143-4160(90)90044-u. [DOI] [PubMed] [Google Scholar]
- THOMPSON A.K., MOSTAFAPOUR S.P., DENLINGER L.C., BLEASDALE J.E., FISHER S.K. The aminosteroid U-73122 inhibits muscarinic receptor sequestration and phosphoinositide hydrolysis in SK-N-SH neuroblastoma cells. A role for Gp in receptor compartmentation. J. Biol. Chem. 1991;266:23856–23862. [PubMed] [Google Scholar]
- TORIBATAKE Y., TOMITA K., KAWAHARA N., BABA H., OHNARI H., TANAKA S. Regulation of vasomotion of arterioles and capillaries in the cat spinal cord: role of alpha actin and endothelin-1. Spinal Cord. 1997;35:26–32. doi: 10.1038/sj.sc.3100348. [DOI] [PubMed] [Google Scholar]
- TSUGENO M., HUANG S.M., PANG Y.W., CHOWDHURY J.U., TOMITA T. Effects of phosphodiesterase inhibitors on spontaneous electrical activity (slow waves) in the guinea-pig gastric muscle. J. Physiol. (Lond.) 1995;485:493–502. doi: 10.1113/jphysiol.1995.sp020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HELDEN D.F. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J. Physiol. (Lond.) 1993;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN HELDEN D.F., IMTIAZ M.S., NURGALIYEVA K., VON DER WEID P., DOSEN P.J. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. J. Physiol. (Lond.) 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN RENTERGHEM C., VIGNE P., BARHANIN J., SCHMID-ALLIANA A., FRELIN C., LAZDUNSKI M. Molecular mechanism of action of the vasoconstrictor peptide endothelin. Biochem. Biophys. Res. Commun. 1988;157:977–985. doi: 10.1016/s0006-291x(88)80970-7. [DOI] [PubMed] [Google Scholar]
- VOLPE M., COSENTINO F. Abnormalities of endothelial function in the pathogenesis of stroke: the importance of endothelin. J. Cardiovasc. Pharmacol. 2000;35:S45–S48. doi: 10.1097/00005344-200000002-00011. [DOI] [PubMed] [Google Scholar]
- VON DER WEID P.Y., CROWE M.J., VAN HELDEN D.F. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J. Physiol. (Lond.) 1996;493:563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANEBO J.E., ARTHUR A.S., LOUIS H.G., WEST K., KASSELL N.F., LEE K.S., HELM G.A. Systemic administration of the endothelin-A receptor antagonist TBC 11251 attenuates cerebral vasospasm after experimental subarachnoid hemorrhage: dose study and review of endothelin-based therapies in the literature on cerebral vasospasm. Neurosurgery. 1998;43:1409–1417. [PubMed] [Google Scholar]
- WICKMAN G., NESSIM M.A., COOK D.A., VOLLRATH B. The polycationic aminoglycosides modulate the vasoconstrictive effects of endothelin: relevance to cerebral vasospasm. Br. J. Pharmacol. 2001;133:5–12. doi: 10.1038/sj.bjp.0704025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLETTE R.N., ZHANG H., MITCHELL M.P., SAUERMELCH C.F., OHLSTEIN E.H., SULPIZIO A.C. Nonpeptide endothelin antagonist. Cerebrovascular characterization and effects on delayed cerebral vasospasm. Stroke. 1994;25:2450–2455. doi: 10.1161/01.str.25.12.2450. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., INOUE A., ISHIKAWA T., KASUYA Y., KIMURA S., KUMAGAYE S., NAKAJIMA K., WATANABE T.X., SAKAKIBARA S., GOTO K., MASAKI T. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc. Natl. Acad. Sci. U.S.A. 1988;85:6964–6967. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO J., VAN HELDEN D.F. Role of endothelin-1 in modulation of lymphatic pacemaking. Proc. Austral. Physiol. Pharmacol. Soc. 1997;28:80. [Google Scholar]
- ZHAO J., VAN HELDEN D.F. ATP-induced endothelium-independent enhancement of lymphatic vasomotion in guinea-pig mesentery involves P2X and P2Y receptors. Br. J. Pharmacol. 2002;137:477–487. doi: 10.1038/sj.bjp.0704899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUCCARELLO M., BOCCALETTI R., TOSUN M., RAPOPORT R.M. Role of extracellular Ca2+ in subarachnoid hemorrhage-induced spasm of the rabbit basilar artery. Stroke. 1996a;27:1896–1902. doi: 10.1161/01.str.27.10.1896. [DOI] [PubMed] [Google Scholar]
- ZUCCARELLO M., SOATTIN G.B., LEWIS A.I., BREU V., HALLAK H., RAPOPORT R.M. Prevention of subarachnoid hemorrhage-induced cerebral vasospasm by oral administration of endothelin receptor antagonists. J. Neurosurg. 1996b;84:503–507. doi: 10.3171/jns.1996.84.3.0503. [DOI] [PubMed] [Google Scholar]