Abstract
We have recently reported that systemic delivery of A-317491, the first non-nucleotide antagonist that has high affinity and selectivity for blocking P2X3 homomeric and P2X2/3 heteromeric channels, is antinociceptive in rat models of chronic inflammatory and neuropathic pain. In an effort to further evaluate the role of P2X3/P2X2/3 receptors in nociceptive transmission, A-317491 was administered either intrathecally or into the hindpaw of a rat in several models of acute and chronic nociception.
Intraplantar (ED50=300 nmol) and intrathecal (ED50=30 nmol) injections of A-317491 produced dose-related antinociception in the CFA model of chronic thermal hyperalgesia. Administration of A-317491 by either route was much less effective to reduce thermal hyperalgesia in the carrageenan model of acute inflammatory hyperalgesia.
Intrathecal, but not intraplantar, delivery of A-317491 attenuated mechanical allodynia in both the chronic constriction injury and L5-L6 nerve ligation models of neuropathy (ED50=10 nmol for both models). Intrathecal injections of A-317491 did not impede locomotor performance.
Both routes of injection were effective in reducing the number of nocifensive events triggered by the injection of formalin into a hindpaw. Nocifensive behaviors were significantly reduced in both the first and second phases of the formalin assay (intrathecal ED50=10 nmol, intraplantar ED50>300 nmol). Nocifensive behaviors induced by the P2X receptor agonist α,β-meATP were also significantly reduced by intraplantar injection of A-317491.
These data indicate that both spinal and peripheral P2X3/P2X2/3 receptors have significant contributions to nociception in several animal models of nerve or tissue injury. Intrathecal administration of A-317491 appears to be more effective than intraplantar administration to reduce tactile allodynia following peripheral nerve injury.
Keywords: P2X3, ATP, neuropathic, allodynia, hyperalgesia, inflammation, formalin, intrathecal, intraplantar
Introduction
Adenosine 5′-triphosphate (ATP), an endogenous fast neurotransmitter, is thought to be a key mediator of nociception (Burnstock, 1996; Burnstock & Wood, 1996; Cook & McCleskey, 2002). ATP depolarizes primary sensory neurons (Cook et al., 1997; Grubb & Evans, 1999) as well as neurons in the dorsal horn of the spinal cord (Jahr & Jessell, 1985; Li & Perl, 1995). ATP triggers neuronal excitation through several different subtypes of P2 purinoceptors. ATP is a nonselective agonist with varied potencies for each of the seven ionotropic P2X and eight metabotropic P2Y purinoceptor subtypes currently identified (Ralevic & Burnstock, 1998; Bianchi et al., 1999; Jacobson et al., 2002; Abbracchio et al., 2003). Receptor localization and behavioral studies suggest that more than one of these receptor subtypes is involved in the transmission of peripheral and/or spinal nociceptive signals (Collo et al., 1996; Vulchanova et al., 1996; 1997; Cockayne et al., 2000; Souslova et al., 2000; Okada et al., 2002; Yoshida et al., 2002; Tsuda et al., 2003; Wismer et al., 2003).
Site-specific injections of nonselective P2 receptor agonists and antagonists have been shown to modulate nociceptive behaviors in rodents. Intraplantar administration of various P2 receptor agonists results in short-lasting licking, biting and lifting of the injured paw (Jarvis et al., 2001; Wismer et al., 2003), and can lead to mechanical allodynia (Tsuda et al., 2000). Peripheral injections of P2 receptor agonists can also enhance nociception in animal models of chemical (formalin) and inflammatory (carrageenan) pain (Sawynok & Reid, 1997; Hamilton et al., 2001). Intrathecal delivery of P2 receptor agonists increases sensitivity to mechanical stimulation in naive animals and induces thermal hyperalgesia (Tsuda et al., 1999a; Okada et al., 2002). Furthermore, intrathecal application of nonselective P2 receptor antagonists decreases the number of nociceptive events following intraplantar formalin, capsaicin, or bee venom (Driessen et al., 1994; Tsuda et al., 1999b; Zheng & Chen, 2000). Electrophysiologically, spinal infusion of P2 receptor antagonists inhibits C-fiber-evoked responses in carrageenan-inflamed animals (Stanfa et al., 2000).
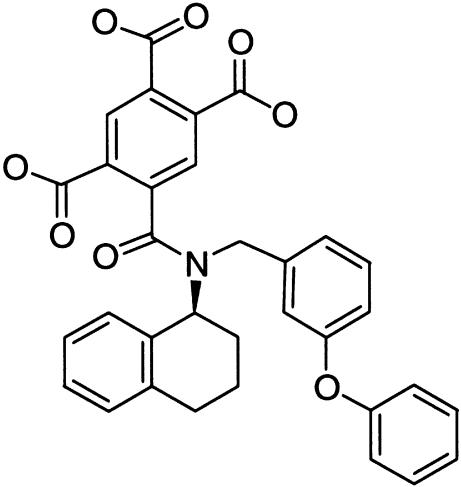
Determination of specific P2 receptor activation in different pain states has been hampered by the lack of useful receptor ligands for in vivo studies. Existing P2 receptor agonists nonselectively activate a variety of P2 receptor subtypes, and are metabolically labile (Jacobson et al., 2002). We have recently reported that systemic administration of A-317491 (Figure 1), the first non-nucleotide antagonist that has high affinity and selectivity for blocking P2X3 homomeric and P2X2/3 heteromeric channels, is antinociceptive in rat models of chronic inflammatory and neuropathic pain (Jarvis et al., 2002). Several lines of evidence suggest that P2X3 receptors have a substantial contribution to ATP-related nociception. These receptors are highly expressed in dorsal root ganglion neurons, particularly in small-diameter sensory neurons (Chen et al., 1995). Neuronal expression of the P2X3 receptor is elevated in both the dorsal root ganglion and the ipsilateral spinal cord following chronic constriction injury (CCI) of the sciatic nerve (Novakovic et al., 1999). Furthermore, P2X3 (−/−) gene-ablated mice show a significant attenuation in spontaneous pain behaviors following administration of ATP or formalin (Cockayne et al., 2000; Souslova et al., 2000), and animals treated with P2X3 antisense oligonucleotides exhibit reduced thermal hyperalgesia and mechanical allodynia (Barclay et al., 2002; Honore et al., 2002).
Figure 1.
Structure of A-317491.
In an effort to further evaluate the role of P2X3/P2X2/3 receptors in nociceptive transmission, the novel and selective antagonist A-317491 was administered either intrathecally or into the hindpaw of a rat in a variety of acute and chronic models of nociception.
Methods
Animal preparation
Male Sprague–Dawley rats (Charles River, MA, U.S.A., 260–350 g) were housed in a temperature-controlled room with a 12/12-h day/night cycle. Food and water were available ad libitum. Animals with implanted intrathecal catheters were housed one per cage following surgery. All animal-handling and experimental protocols were approved by Abbott's Institutional Animal Care and Use Committee (IACUC), and were conducted in accordance with the ethical principles for pain-related animal research of the American Pain Society.
Implantation of intrathecal catheters For spinal drug administration, animals were implanted with chronic indwelling catheters. Under halothane inhalation anesthesia, PE-5 catheters (external PE-10, Marsil Enterprises, CA, U.S.A.) were inserted through the cisternal membrane at the base of the skull down to the lumbar enlargement (8.5 cm). Rats were not tested for at least 7 days after surgery. Animals demonstrating motor dysfunction or dehydration immediately following surgery or at any point thereafter were euthanized.
Drug-administration procedures
A-317491 (a P2X3/P2X2/3 receptor antagonist synthesized at Abbott Laboratories, IL, U.S.A.) was dissolved in dimethylsulfoxide (10%), 2-hydroxypropyl-β-cyclodextrin (34%) and saline at physiological pH for intraplantar and intrathecal delivery. The drug administration procedures described below were followed for all behavioral paradigms. Each experimental group consisted of at least five animals.
A-317491 (1–500 nmol) or vehicle was injected directly into the lumbar spinal cord via indwelling intrathecal catheters or into the intraplantar region of a hindpaw. The hindpaw ipsilateral to the site of nerve or tissue injury was used for intraplantar injections. In a separate group of animals, the contralateral uninjured hindpaw was injected with A-317491 to explore the possible systemic actions of the drug following the intraplantar route of administration. Intrathecal and intraplantar deliveries of A-317491 were completed 5 and 30 min, respectively, prior to behavioral testing. Intrathecal infusions were conducted over a 2-min period. The volume of intrathecal injection was 10 μl, followed by a 10 μl sterile water flush. For intraplantar administration, A-317491 was injected in a volume of 100 μl.
Behavioral models and data analysis
Thermal nociception in naïve and hyperalgesic rats Thermal nociceptive thresholds were measured in three different groups of rats. Chronic inflammatory hyperalgesia was induced in one group of rats following the injection of complete Freund's adjuvant (CFA, 50%, 150 μl) into the plantar surface of the right hindpaw 48 h prior to testing. A second group of rats was injected with 1 mg of carrageenan (in 100 μl saline) into the right hindpaw 2 h before testing to induce acute inflammatory hyperalgesia. The final group of rats was uninjured prior to testing. All animals were placed in Plexiglas chambers (18 × 29 × 12.5 cm3) resting on a temperature-regulated (30°C) glass surface 30 min before thermal testing. Animals were removed from these chambers for drug or vehicle administration, as described below, and were then returned to their respective chambers. Thermal nociceptive thresholds were determined according to the method described by Hargreaves et al. (1988). Briefly, through the glass surface, a radiant heat source (8 V, 50 W projector bulb) was focused onto the plantar surface of the hindpaw. The rat's paw-withdrawal latency to this stimulus was recorded to the nearest 0.1 s. Each animal's latency score was an average of two trials, which were separated by at least 5 min. In hyperalgesic rats, both the injured and uninjured hindpaws were similarly tested, allowing direct comparisons between inflamed and noninflamed paws for each animal. Withdrawal latencies after injection of vehicle into a hindpaw did not differ from latencies observed in uninjected animals (unpublished observations).
The mean withdrawal latencies were compared within groups (inflamed vs noninflamed paws) and between drug- and vehicle-injected groups. ‘Reversal in hyperalgesia' scores for each animal were calculated by the following formula:
 |
In cases of negative values, the scores were designated as 0 (no reversal in hyperalgesia).
Neuropathic allodynia Under halothane inhalation anesthesia, rats received either a unilateral tight ligation of the L5 and L6 spinal nerves (Kim & Chung, 1992) or a chronic constriction injury to the left sciatic nerve (Bennett & Xie, 1988). Antiallodynic effects of A-317491 were evaluated at least 1 week after surgery. Tactile allodynia was determined by measuring paw withdrawal in response to stimulation, with a series of von Frey filaments (Stoelting, Wood Dale, IL, U.S.A.), using the up–down method described by Dixon (1980). Briefly, rats were placed on an elevated mesh bottom floor with a 1.27 × 1.27 cm2 grid to provide access to the ventral side of the hindpaws. An inverted, clear plastic cage (29 × 18 × 12 cm3) was placed over each rat. The von Frey filament was presented perpendicular to the plantar surface of the left hindpaw, and held in this position for approximately 8 s, with enough force to cause a slight buckle in the filament. Positive responses included sharp withdrawal or flinching behavior during stimulation. Each rat was tested in three sequential trials. Only those rats with a mean baseline threshold score of less than 4.5 g were used in this study.
Formalin- and α,β-meATP-induced nociception Experimentally naïve animals were placed in individual mirrored (45°) Plexiglas cages (26 × 22 × 16 cm3) and allowed to acclimate to the testing environment for 15 min. Formalin (5% in 50 μl) or the P2X receptor agonist α,β-meATP (1000 nmol in 50 μl) was then injected into the dorsal surface of the right hind paw using a 29.5-gauge needle. The number of nocifensive events (paw flinching, licking, guarding) for each animal was recorded during 1-min periods, with each period being separated by 5 min. The number of nociceptive events for the formalin and α,β-meATP tests were counted for periods of 50 and 15 min, respectively.
Locomotor activity Following intrathecal drug or vehicle administration, rats were placed in an open testing chamber (42 × 42 × 30 cm3) for 30 min. The chambers were located in a ventilated room with noise attenuation. During the 30-min testing period, the animal's horizontal movements were recorded with a Digiscan Animal Activity Monitor (16 beam – 1″ resolution, AccuScan Instruments, OH, U.S.A.). Locomotor activity was defined as the total number of horizontal beam interruptions over 30 min.
Statistics Statistical significance on group means was measured by an ANOVA, followed by a Fisher's PLSD post hoc analysis (P<0.05). ED50 values for all experiments were estimated using linear regression. Data are presented as mean+s.e.m.
Results
Thermal nociception in naïve and hyperalgesic rats
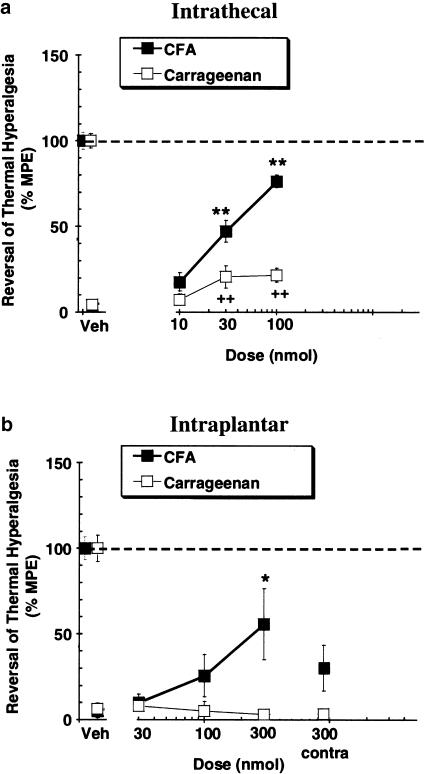
CFA-induced thermal hyperalgesia Significant CFA-induced hyperalgesia (P<0.01) was observed on the injured hindpaw. The withdrawal latencies were 4.73±0.38 and 12.16±0.62 s for inflamed and noninflamed paws, respectively, in animals that received vehicle by intrathecal administration. Indwelling intrathecal catheters and subsequent intrathecal administration of vehicle did not interfere with hindlimb withdrawal from noxious heat. Latencies after intraplantar injection of vehicle were 4.59±0.45 s (inflamed paw) and 10.73±0.81 s (noninflamed paw). Intrathecal administration of 30 and 100 nmol of A-317491 (Figure 2a) caused significant antihyperalgesia (P<0.01) in the inflamed paws without altering the withdrawal latencies of the noninflamed hindpaws. Significant antihyperalgesia (P<0.05) was also observed following intraplantar delivery of A-317491, but only at the 300 nmol dose (Figure 2b). Injection of A-317491 into the contralateral uninjured hindpaw did not significantly affect withdrawal latencies.
Figure 2.
Effects of (a) intrathecal and (b) intraplantar administration of A-317491 on thermal hyperalgesia induced by pretreatment with either CFA (48 h) or carrageenan (2 h). A-317491 was most effective following intrathecal administration in the CFA model of chronic inflammatory hyperalgesia. Mean ‘antihyperalgesic' scores following stimulation of inflamed paws are shown; a score of 100% indicates that there was a complete compound-related reversal of hyperalgesia. Withdrawal latencies after stimulation of noninflamed hindpaws did not differ between animals injected with either A-317491 (not shown) or vehicle (points shown at 100%) to the inflamed hindpaw. *(CFA) P<0.05; **(CFA), ++(carrageenan) P<0.01 vs vehicle control group, values are ±s.e.m.
Carrageenan-induced thermal hyperalgesia Significant hyperalgesia (P<0.01) was also observed in animals injected with carrageenan 2 h prior to testing. Withdrawal latencies were 2.83±0.32 and 10.3±0.44 s for inflamed and noninflamed paws, respectively, in animals that received vehicle by the intrathecal route. Latencies after intraplantar administration of vehicle were 3.52±0.49 s (inflamed paw) and 10.29±0.86 s (noninflamed paw). Significant, but weak, antihyperalgesia was observed after intrathecal infusion of 30 and 100 nmol of A-317491 to the carrageenan-treated animals (Figure 2a). Intraplantar administration of A-317491 did not have a significant effect in this model (Figure 2b).
Acute thermal nociception Intrathecal administration of A-317491 (up to a dose of 500 nmol) did not alter the hindpaw-withdrawal latencies to noxious heat in uninjured animals. The withdrawal latency in vehicle-treated animals was 11.64±0.18 s, whereas in A-317491-treated animals (500 nmol) the latency was 12.2±0.94 s.
Neuropathic allodynia
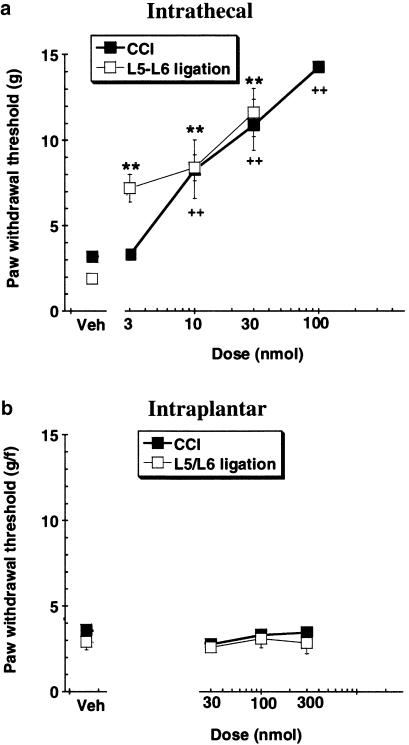
CCI and L5-L6 nerve ligation models of neuropathic allodynia In both models of neuropathic allodynia, vehicle-treated (intrathecal or intraplantar) animals exhibited paw withdrawal to von Frey hair stimulation of less than 4 g (Figure 3). Intrathecal delivery of A-317491 (10 and 30 nmol) resulted in withdrawal responses to von Frey hair stimulation that were significantly (P<0.01) higher, compared to vehicle-treated animals, in both models of neuropathic pain (Figure 3a). In the L5–L6 nerve ligation model, the antiallodynic actions of A-317491, at all doses, lasted for at least 2 h after the injection (data not shown). Mechanical allodynia was not significantly attenuated by intraplantar administration of A-317491 in either model of neuropathic pain (Figure 3b).
Figure 3.
Effects of (a) intrathecal and (b) intraplantar injection of A-317491 on mechanical allodynia in the CCI and L5–L6 nerve ligation models of neuropathic pain. A-317491 reduced allodynia in both models of neuropathy following intrathecal, but not intraplantar delivery. **(L5–L6) P<0.01; ++(CCI) P<0.01 vs vehicle control group, values are ±s.e.m. (n=5–6 per group).
Formalin- and α,β-meATP-induced nociception
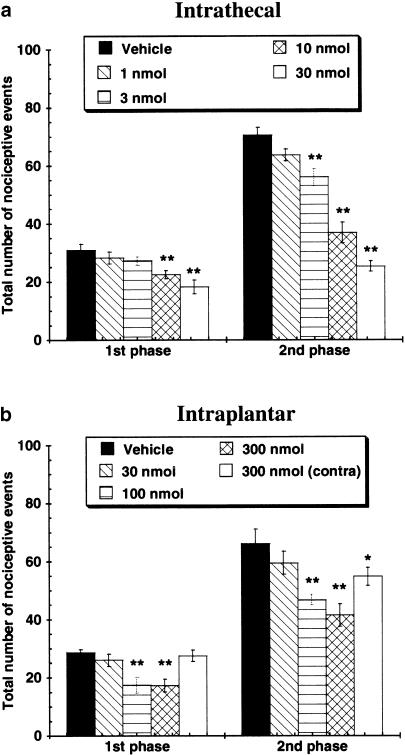
Formalin-induced nocifensive behaviors Injection of a 5% solution of formalin into a hindpaw caused licking, biting and lifting of the injured paw. Typical formalin-induced biphasic response patterns were observed in all animals. Intrathecal administration of A-317491 significantly reduced the number of nociceptive events evoked by formalin in both the first ‘acute' and second ‘tonic' phases of this assay compared to vehicle-treated animals (Figure 4a). At the highest dose tested (30 nmol), the decrease in nocifensive behaviors was more pronounced in the second phase (64% decrease from vehicle) compared to the first phase (41% decrease from vehicle).
Figure 4.
Effects of (a) intrathecal and (b) intraplantar administration of A-317491 on formalin-induced nocifensive behaviors. Following intradermal injections of formalin (5%) into the dorsal surface of the right hindpaw, the total number of nocifensive events (paw flinching, licking, guarding) was counted during the first (1–15 min) and second (30–50 min) phases of this model. Intrathecal and intraplantar injections of A-317491 were effective, in both phases, to reduce the number of formalin-induced nociceptive behaviors. *P<0.05, **P<0.01 vs vehicle control group, values are ±s.e.m. (n=5–6 per group).
Nocifensive behaviors in both phases of the formalin assay were also significantly reduced by intraplantar administration of 100 and 300 nmol of A-317491 (Figure 4b). A comparable effect was observed in the first (40% reduction from vehicle) and second (37% reduction from vehicle) phases after this route of administration. However, the number of nociceptive events in the second phase of the formalin assay was also somewhat reduced (17% from vehicle) following injection of 300 nmol of A-317491 into the contralateral, uninjured, hindpaw.
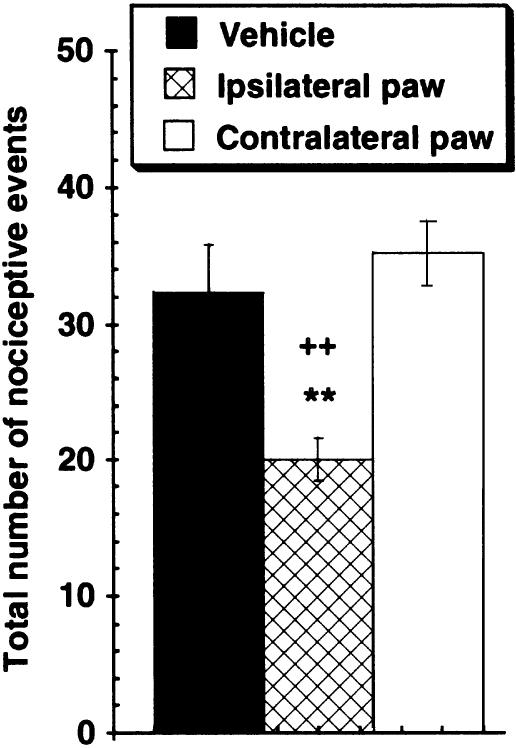
α,β-meATP-induced nocifensive behaviors Injection of 1000 nmol of the nonselective P2X receptor agonist α,β-meATP into a hindpaw also induced nocifensive behaviors such as licking, biting and lifting of the injured paw. The duration of these effects was short-lived, with the majority of the nocifensive behaviors occurring within the first 5 min. Intraplantar injection of A-317491 (300 nmol) into the injured hindpaw significantly reduced the number of nociceptive events triggered by α,β-meATP (Figure 5). Intraplantar injection of A-317491 into the contralateral uninjured hindpaw was without effect on α,β-meATP-evoked nocifensive behaviors.
Figure 5.
Intraplantar administration of 300 nmol of A-317491 reduced the number of nocifensive events produced by injection of the nonselective P2X agonist α,β-meATP (1000 nmol) into the same hindpaw. Injection of A-317491 into the contralateral hindpaw did not affect α,β-meATP-induced nociception. **P<0.01 vs vehicle control group, ++P<0.01 vs contralateral hindpaw, values are ±s.e.m. (n=6 per group).
Locomotor activity
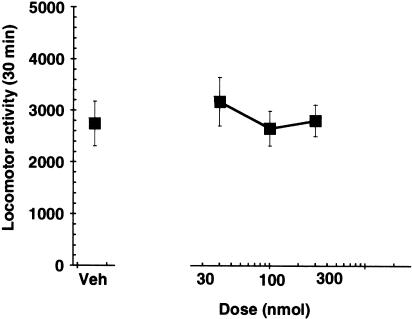
Intrathecal administration of A-317491 did not have a significant effect on animal locomotor activity (Figure 6).
Figure 6.
Intrathecal delivery of A-317491 did not affect locomotor activity (values are ±s.e.m., n=5 per group).
Table 1 summarizes the ED50 values for A-317491, following systemic (Jarvis et al., 2002), intrathecal and intraplantar routes of administration in each of the animal models tested.
Table 1.
Summary of the intrathecal, intraplantar, and systemic effects of A-317491 in various models of nociception
| Intrathecal | Intraplantar | Systemic (s.c.)a | ||||
|---|---|---|---|---|---|---|
| ED50 (nmol) | % Effect (30 nmol) | ED50 (nmol) | % Effect (300 nmol) | ED50 (μmol kg−1) | % Effect (100 μmol kg−1) | |
| Thermal nociception | ||||||
| CFA inflammation | 30 | 47%* | 300 | 56%* | 30 | 74%* |
| CARR inflammation | 100 | 21%* | >300 | 0% | 100 | 15% |
| Naive | >500 | 0% | — | — | 100 | 0% |
| Chemical nociception | ||||||
| Formalin first phase | >30 | 41%* | >300 | 40%* | — | — |
| Formalin second phase | 10 | 64%* | >300 | 37%* | 50 | 60%* |
| α,β-meATP | — | — | >300 | 38%* | — | — |
| Neuropathic nociception | ||||||
| L5–L6 ligation | 10 | 73%* | >300 | 0% | 100 | 50%* |
| CCI | 10 | 77%* | >300 | 0% | 15 | 100%* |
| Locomotor | 300 | 0% | — | — | >300 | 0% |
P<0.05; — not tested.
Discussion
Systemic delivery of A-317491, the first non-nucleotide, potent and selective, antagonist of the P2X3/P2X2/3 receptor has been shown to reduce both hyperalgesia and allodynia in animal models of chronic pain (Jarvis et al., 2002). The antinociceptive effectiveness of A-317491 in these and other pain models was replicated in the current study following localized injections. Spinal and intraplantar administration of A-317491 increased thermal thresholds in CFA-induced hyperalgesic rats and decreased spontaneous nocifensive behaviors induced by formalin. Intrathecal delivery of A-317491 also diminished tactile allodynia in two animal models of neuropathy. The spinal actions of A-317491 were not due to hypomobility, as intrathecal injections of A-317491 did not alter locomotor activity. Furthermore, as observed following systemic administration (Jarvis et al., 2002), the localized injections of A-317491 were also relatively ineffective in the carrageenan model of acute thermal hyperalgesia. Thus, the current data are consistent with the hypothesis that P2X3/P2X2/3 receptors contribute to several forms of ‘altered' nociception that are triggered by neuropathy or inflammation.
Spinal, but not peripheral, injection of A-317491 was very effective in reducing tactile allodynia caused by injuries to peripheral nerves. A-317491 was equally effective in the CCI and L5–L6 ligation models of neuropathic pain. This suggests that the antiallodynic action of systemically delivered A-317491 in both of these models depends on the compound entering the central nervous system. The site of this action is likely on the primary afferent terminals located in the superficial laminae of the dorsal horn (Cook et al., 1997; Vulchanova et al., 1997). Immunolabeling of the P2X3 receptor has been observed in the upper laminae of naïve animals, and is increased following CCI procedures (Novakovic et al., 1999). Despite a decrease in P2X3 immunoreactivity in the dorsal root ganglion of animals with L5–L6 ligations (Kage et al., 2002), the spinal effects of A-317491 in this model may be mediated via the spared dorsal horn terminals of L4 afferents, and/or by the terminals of IB4 negative large-diameter L5–L6 neurons (Kage et al., 2002).
The antihyperalgesic activity resulting from systemic administration of A-317491 in CFA-inflamed animals (Jarvis et al., 2002) was most likely due to interactions with both spinal and peripheral mechanisms, since both intrathecal and intraplantar injections of A-317491 were effective in this model of chronic hyperalgesia. However, the contributions from peripheral sites (ED50=300 nmol) may be relatively small compared to the contributions from spinal sites (ED50=30 nmol). The actions of A-317491 against thermal nociception were limited to animals with CFA-induced chronic hyperalgesia. Spinal administration of A-317491 was ineffective against noxious thermal stimulation in naïve animals. Furthermore, intrathecally injected A-317491 produced only a small reduction in nociceptive thresholds in carrageenan-hyperalgesic animals, and these effects were modest compared to those observed in CFA-inflamed rats. Similarly, animals treated with P2X3 antisense oligonucleotides have reduced thermal thresholds in chronic (CFA) but not acute (carrageenan) models of hyperalgesia (Honore et al., 2002). Taken together, these findings suggest that there are fundamental differences in the contributions of P2X3/P2X2/3 receptors to the mediation of thermal nociception in models of acute or chronic pain. To date, there are no reports of changes to the expression levels of P2X3/P2X2/3 receptors following carrageenan-induced inflammation. In contrast, higher levels of P2X3 receptors have been found in the dorsal root ganglion following CFA-induced inflammation (Xu & Huang, 2002). Thus, P2X3/P2X2/3 receptors may have a greater contribution to thermal nociception under conditions of chronic hyperalgesia. Although intrathecal administration of nonselective P2X receptor agonists has been shown to reduce C-fiber-evoked firing in dorsal horn neurons of carrageenan-inflamed animals (Stanfa et al., 2000), these effects were likely mediated through P2X receptors other than P2X3/P2X2/3 (Wismer et al., 2003).
Local injections of A-317491 were also antinociceptive in two models of chemogenic pain. The number of nocifensive behaviors induced by injections of a P2X receptor agonist, α,β-meATP, into a hindpaw was attenuated by the intraplantar administration of A-317491. Meanwhile, both intrathecal and intraplantar administration of A-317491 were effective against formalin-induced behaviors. Attenuation of similar behaviors has also been found in P2X3-deficient animals (Cockayne et al., 2000; Souslova et al., 2000). Taken together, these results demonstrate that both spinal and peripheral P2X3 receptors contribute to the transmission of nociception following a chemogenic injury. Furthermore, local injections of A-317491 reduced nocifensive behaviors over the entire time course of the formalin assay, indicating that P2X3/P2X2/3 receptors have a role in mediating both the acute (first) and tonic (second) phases of this model. A-317491 was most effective in reducing formalin-induced nociception following intrathecal delivery, particularly against the nociceptive behaviors observed in the tonic phase of this assay. Thus, P2X3/P2X2/3 receptors may be involved in the purported central sensitization that contributes to the tonic phase of the formalin model (Dickenson & Sullivan, 1987).
A-317491 represents an important pharmacological advance to help determine the functional role(s) of the P2X3/P2X2/3 receptor. The previously available tools include several nonspecific receptor antagonists such as suramin, pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) and 2′,3′-O-2,4,6-trinitrophenyl-ATP (TNP-ATP). Spinal and peripheral contributions of P2X receptors have been previously described for models of neuropathic (Fukuhara et al., 2000; Liu & Tracey, 2000; Stanfa et al., 2000; Tsuda et al., 2000), inflammatory (Tsuda et al., 1999b; Stanfa et al., 2000) and chemogenic (Driessen et al., 1994; Tsuda et al., 1999a; Zheng & Chen, 2000; Jarvis et al., 2001) nociception. Nonetheless, the contribution of specific subtype(s) of P2X receptors to peripheral and spinal transmission of the nociceptive signal could not be determined in these previous studies. The present data with A-317491 show clear roles for both spinal and peripheral P2X3/P2X2/3 receptors in mediating nociception. Furthermore, since spinal administration of A-317491 was more efficacious and affected a broader spectrum of pain models than intraplantar injections, the therapeutic effectiveness of a P2X3/P2X2/3 receptor antagonist may be optimized with penetration into the central nervous system.
However, the effectiveness of A-317491 across several models of pathological nociception does not imply that the compound acted on the same pathways or modulated the same downstream neurochemistry in each of the models. Indeed, it has been suggested that mechanical allodynia is mediated by P2X2/3 receptors on capsaicin-insensitive neurons with slow-desensitizing ATP currents, while thermal and spontaneous nocifensive behaviors are mediated by P2X3 receptors on capsaicin-sensitive neurons with fast-desensitizing ATP responses (Tsuda et al., 2000). Currently, there is only limited information regarding the endogenous substances that may be released into the spinal cord following P2X3/P2X2/3 receptor activation for in vivo models of altered nociception. While it is appreciated that both peripheral and spinal application of ATP can trigger the release of glutamate into the dorsal horn (Gu & MacDermott, 1997; Tsuda et al., 1999a; Wismer et al., 2003), other endogenous agents such as substance P, nitric oxide, GABA and opioids may also be involved (Bland-Ward & Humphrey, 1997; Fukuhara et al., 2000; Hugel & Schlichter, 2000; Ueda et al., 2000; Nakatsuka et al., 2001). Thus, even though a clear role for P2X3/P2X2/3 receptors has been demonstrated in many models of pathological nociception, the exact mechanisms for action in each of the models must be further explored.
In conclusion, intraplantar administration of the novel P2X3/P2X2/3 receptor antagonist, A-317491, attenuated CFA-induced hyperalgesia and formalin-induced nocifensive behaviors, demonstrating a clear role for peripheral P2X3/P2X2/3 receptors in these models of nociception. Intrathecal administration of A-317491 potently reduced allodynia in both the CCI and L5-L6 nerve ligation models of neuropathic pain, and was even more effective than intraplantar administration to reduce nociception in the CFA and formalin models. Thus, spinal P2X3/P2X2/3 receptors have significant contributions to neuropathic allodynia, chronic thermal hyperalgesia and chemogenic nociception. In contrast, there is likely only a minimal contribution from P2X3/P2X2/3 receptors (peripheral and spinal) to acute thermal nociception, since A-317491 was relatively ineffective in naïve and carrageenan-inflamed rats.
Abbreviations
- ATP
adenosine 5′-triphosphate
- CCI
chronic constriction injury
- CFA
complete Freund's adjuvant
References
- ABBRACCHIO M.P., BOEYNAEMS J.M., BARNARD E.A., BOYER J.L., KENNEDY C., MIRAS-PORTUGAL M.T., KING B.F., GACHET C., JACOBSON K.A., WEISMAN G.A., BURNSTOCK G. Characterization of the UDP-glucose receptor (re-named here the P2Y(14) receptor) adds diversity to the P2Y receptor family. Trends Pharmacol. Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARCLAY J., PATEL S., DORN G., WOTHERSPOON G., MOFFATT S., EUNSON L., ABDEL'AL S., NATT F., HALL J., WINTER J., BEVAN S., WISHART W., FOX A., GANJU P. Functional downregulation of P2X3 receptor subunit in rat sensory neurons reveals a significant role in chronic neuropathic and inflammatory pain. J. Neurosci. 2002;22:8139–8147. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT G.J., XIE Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- BIANCHI B.R., LYNCH K.J., TOUMA E., NIFORATOS W., BURGARD E.C., ALEXANDER K.M., PARK H.S., YU H., METZGER R., KOWALUK E., JARVIS M.F., VAN BIESEN T. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur. J. Pharmacol. 1999;376:127–138. doi: 10.1016/s0014-2999(99)00350-7. [DOI] [PubMed] [Google Scholar]
- BLAND-WARD P.A., HUMPHREY P.P. Acute nociception mediated by hindpaw P2X receptor activation in the rat. Br. J. Pharmacol. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., WOOD J.N. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr. Opin. Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- CHEN C.C., AKOPIAN A.N., SIVILOTTI L., COLQUHOUN D., BURNSTOCK G., WOOD J.N. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- COCKAYNE D.A., HAMILTON S.G., ZHU Q.M., DUNN P.M., ZHONG Y., NOVAKOVIC S., MALMBERG A.B., CAIN G., BERSON A., KASSOTAKIS L., HEDLEY L., LACHNIT W.G., BURNSTOCK G., MCMAHON S.B., FORD A.P. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOK S.P., MCCLESKEY E.W. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- COOK S.P., VULCHANOVA L., HARGREAVES K.M., ELDE R., MCCLESKEY E.W. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- DICKENSON A.H., SULLIVAN A.F. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987;30:349–360. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- DIXON W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- DRIESSEN B., REIMANN W., SELVE N., FRIDERICHS E., BULTMANN R. Antinociceptive effect of intrathecally administered P2-purinoceptor antagonists in rats. Brain Res. 1994;666:182–188. doi: 10.1016/0006-8993(94)90770-6. [DOI] [PubMed] [Google Scholar]
- FUKUHARA N., IMAI Y., SAKAKIBARA A., MORITA K., KITAYAMA S., TANNE K., DOHI T. Regulation of the development of allodynia by intrathecally administered P2 purinoceptor agonists and antagonists in mice. Neurosci. Lett. 2000;292:25–28. doi: 10.1016/s0304-3940(00)01427-0. [DOI] [PubMed] [Google Scholar]
- GRUBB B.D., EVANS R.J. Characterization of cultured dorsal root ganglion neuron P2X receptors. Eur. J. Neurosci. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- GU J.G., MACDERMOTT A.B. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- HAMILTON S.G., MCMAHON S.B., LEWIN G.R. Selective activation of nociceptors by P2X receptor agonists in normal and inflamed rat skin. J. Physiol. 2001;534:437–445. doi: 10.1111/j.1469-7793.2001.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARGREAVES K., DUBNER R., BROWN F., FLORES C., JORIS J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- HONORE P., KAGE K., MIKUSA J., WATT A.T., JOHNSTON J.F., WYATT J.R., FALTYNEK C.R., JARVIS M.F., LYNCH K. Analgesic profile of intrathecal P2X3 antisense oligonucleotide treatment in chronic inflammatory and neuropathic pain states in rats. Pain. 2002;99:11–19. doi: 10.1016/s0304-3959(02)00032-5. [DOI] [PubMed] [Google Scholar]
- HUGEL S., SCHLICHTER R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J. Neurosci. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A., JARVIS M.F., WILLIAMS M. Perspective: purine and pyrimidine (P2) receptors as drug targets. J. Med. Chem. 2002;45:4057–4093. doi: 10.1021/jm020046y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAHR C.E., JESSELL T.M. Synaptic transmission between dorsal root ganglion and dorsal horn neurons in culture: antagonism of monosynaptic excitatory postsynaptic potentials and glutamate excitation by kynurenate. J. Neurosci. 1985;5:2281–2289. doi: 10.1523/JNEUROSCI.05-08-02281.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARVIS M.F., BURGARD E.C., MCGARAUGHTY S., HONORE P., LYNCH K., BRENNAN T.J., SUBIETA A., VAN BIESEN T., CARTMELL J., BIANCHI B., NIFORATOS W., KAGE K., YU H., MIKUSA J., WISMER C.T., ZHU C.Z., CHU K., LEE C.H., STEWART A.O., POLAKOWSKI J., COX B.F., KOWALUK E., WILLIAMS M., SULLIVAN J., FALTYNEK C. A-317491, a novel potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors, reduces chronic inflammatory and neuropathic pain in the rat. Proc. Natl. Acad. Sci. U.S.A. 2002;99:17179–17184. doi: 10.1073/pnas.252537299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARVIS M.F., WISMER C.T., SCHWEITZER E., YU H., VAN BIESEN T., LYNCH K.J., BURGARD E.C., KOWALUK E.A. Modulation of BzATP and formalin induced nociception: attenuation by the P2X receptor antagonist, TNP-ATP and enhancement by the P2X(3) allosteric modulator, cibacron blue. Br. J. Pharmacol. 2001;132:259–269. doi: 10.1038/sj.bjp.0703793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGE K., NIFORATOS W., ZHU C.Z., LYNCH K.J, HONORE P., JARVIS M.F. Alteration of dorsal root ganglion P2X3 receptor expression and function following spinal nerve ligation in the rat. Exp. Brain Res. 2002;147:511–519. doi: 10.1007/s00221-002-1263-x. [DOI] [PubMed] [Google Scholar]
- KIM S.H., CHUNG J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- LI J., PERL E.R. ATP modulation of synaptic transmission in the spinal substantia gelatinosa. J. Neurosci. 1995;15:3357–3365. doi: 10.1523/JNEUROSCI.15-05-03357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU T., TRACEY D.J. ATP P2X receptors play little role in the maintenance of neuropathic hyperalgesia. Neuroreport. 2000;11:1669–1672. doi: 10.1097/00001756-200006050-00015. [DOI] [PubMed] [Google Scholar]
- NAKATSUKA T., MENA N., LINGM J., GUM J.G. Depletion of substance P from rat primary sensory neurons by ATP, an implication of P2X receptor-mediated release of substance P. Neuroscience. 2001;107:293–300. doi: 10.1016/s0306-4522(01)00342-6. [DOI] [PubMed] [Google Scholar]
- NOVAKOVIC S.D., KASSOTAKIS L.C., OGLESBY I.B., SMITH J.A., EGLEN R.M., FORD A.P., HUNTER J.C. Immuno-cytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80:273–282. doi: 10.1016/s0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- OKADA M., NAKAGAWA T., MINAMI M., SATOH M. Analgesic effects of intrathecal administration of P2Y nucleotide receptor agonists UTP and UDP in normal and neuropathic pain model rats. J. Pharmacol. Exp. Ther. 2002;303:66–73. doi: 10.1124/jpet.102.036079. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SAWYNOK J., REID A. Peripheral adenosine 5′-triphosphate enhances nociception in the formalin test via activation of a purinergic P2X receptor. Eur. J. Pharmacol. 1997;330:115–121. doi: 10.1016/s0014-2999(97)01001-7. [DOI] [PubMed] [Google Scholar]
- SOUSLOVA V., CESARE P., DING Y., AKOPIAN A.N., STANFA L., SUZUKI R., CARPENTER K., DICKENSON A.H., BOYCE S., HILL R., NEBENUIS-OOSTHUIZEN D., SMITH A.J., KIDD E.J., WOOD J.N. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- STANFA L.C., KONTINEN V.K., DICKENSON A.H. Effects of spinally administered P2X receptor agonists and antagonists on the responses of dorsal horn neurones recorded in normal, carrageenan-inflamed and neuropathic rats. Br. J. Pharmacol. 2000;129:351–359. doi: 10.1038/sj.bjp.0703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUDA M., KOIZUMI S., KITA A., SHIGEMOTO Y., UENO S., INOUE K. Mechanical allodynia caused by intraplantar injection of P2X receptor agonist in rats: involvement of heteromeric P2X2/3 receptor signaling in capsaicin-insensitive primary afferent neurons. J. Neurosci. 2000;20:RC90. doi: 10.1523/JNEUROSCI.20-15-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUDA M., SHIGEMOTO-MOGAMI Y., KOIZUMI S., MIZOKOSHI A., KOHSAKA S., SALTER M.W., INOUE K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- TSUDA M., UENO S., INOUE K. In vivo pathway of thermal hyperalgesia by intrathecal administration of alpha,beta-methylene ATP in mouse spinal cord: involvement of the glutamate-NMDA receptor system. Br. J. Pharmacol. 1999a;127:449–456. doi: 10.1038/sj.bjp.0702582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUDA M., UENO S., INOUE K. Evidence for the involvement of spinal endogenous ATP and P2X receptors in nociceptive responses caused by formalin and capsaicin in mice. Br. J. Pharmacol. 1999b;128:1497–1504. doi: 10.1038/sj.bjp.0702960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UEDA H., MATSUNAGA S., INOUE M., YAMAMOTO Y., HAZATO T. Complete inhibition of purinoceptor agonist-induced nociception by spinorphin, but not by morphine. Peptides. 2000;21:1215–1221. doi: 10.1016/s0196-9781(00)00262-x. [DOI] [PubMed] [Google Scholar]
- VULCHANOVA L., ARVIDSSON U., RIEDL M., WANG J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VULCHANOVA L., RIED M.S., SHUSTER S.J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- WISMER C.T., FALTYNEK C.R., JARVIS M.F., MCGARAUGHTY S. Distinct neurochemical mechanisms are activated following administration of different P2X receptor agonists into the hindpaw of a rat. Brain Res. 2003;965:187–193. doi: 10.1016/s0006-8993(02)04193-8. [DOI] [PubMed] [Google Scholar]
- XU G.Y., HUANG L.Y. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J. Neurosci. 2002;22:93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA K., NAKAGAWA T., KANEKO S., AKAIKE A., SATOH M. Adenosine 5′-triphosphate inhibits slow depolarization induced by repetitive dorsal root stimulation via P2Y purinoceptors in substantia gelatinosa neurons of the adult rat spinal cord slices with the dorsal root attached. Neurosci. Lett. 2002;320:121–124. doi: 10.1016/s0304-3940(02)00018-6. [DOI] [PubMed] [Google Scholar]
- ZHENG J.H., CHEN J. Modulatory roles of the adenosine triphosphate P2x-purinoceptor in generation of the persistent nociception induced by subcutaneous bee venom injection in the conscious rat. Neurosci. Lett. 2000;278:41–44. doi: 10.1016/s0304-3940(99)00896-4. [DOI] [PubMed] [Google Scholar]