Abstract
This study examined whether group III metabotropic glutamate (mGlu) receptor agonists injected into the globus pallidus (GP), substantia nigra pars reticulata (SNr) or intracerebroventricularly (i.c.v.) could reverse reserpine-induced akinesia in the rat.
Male Sprague–Dawley rats, cannulated above the GP, SNr or third ventricle, were rendered akinetic with reserpine (5 mg kg−1 s.c.). 18 h later, behavioural effects of the group III mGlu receptor agonists L-serine-O-phosphate (L-SOP) or L-(+)-2-amino-4-phosphonobutyric acid (L-AP4) were examined.
In reserpine-treated rats, unilateral injection of L-SOP (2000 and 2500 nmol in 2.5 μl) into the GP produced a significant increase in net contraversive rotations compared to vehicle, reaching a maximum of 83±21 rotations 120 min−1 (n=8). Pretreatment with the group III mGlu receptor antagonist methyl-serine-O-phosphate (M-SOP; 250 nmol in 2.5 μl) inhibited the response to L-SOP (2000 nmol) by 77%.
Unilateral injection of L-SOP (250-1000 nmol in 2.5 μl) into the SNr of reserpine-treated rats produced a dose-dependent increase in net contraversive rotations, reaching a maximum of 47±6 rotations 30 min−1 (n=6). M-SOP (50 nmol in 2.5 μl) inhibited the response to L-SOP (500 nmol) by 78%.
Following i.c.v. injection, L-SOP (2000–2500 nmol in 2.5 μl) or L-AP4 (0.5–100 nmol in 2 μl) produced a dose-dependent reversal of akinesia, attaining a maximum of 45±17 (n=8) and 72±3 (n=9) arbitrary locomotor units 30 min−1, respectively.
These studies indicate that injection of group III mGlu receptor agonists into the GP, SNr or cerebral ventricles reverses reserpine-induced akinesia, the mechanism for which remains to be established.
Keywords: Akinesia, group III metabotropic glutamate receptors, globus pallidus, in vivo, substantia nigra pars reticulata, rat, reserpine, Parkinson's disease
Introduction
Parkinson's disease (PD) is a debilitating movement disorder that results from degeneration of dopaminergic neurones in the nigrostriatal pathway. The ensuing striatal dopamine denervation leads to downstream changes in the activity of a number of pathways within the basal ganglia (BG) motor loop, which culminate in excessive activity in the output regions, the internal globus pallidus (GPi) and substantia nigra pars reticulata (SNr). Overactivity of the SNr/GPi in turn leads to inhibition of thalamocortical feedback, giving rise to the characteristic motor deficits of PD including bradykinesia, akinesia and postural instability (Obeso et al., 2000). Dopamine replacement strategies such as L-DOPA have traditionally been used as the first line of therapeutic intervention in PD. However, such treatments are associated with a reduced efficacy and the occurrence of debilitating dyskinesia after long-term use (Stocchi et al., 1997). For these reasons, attention has turned to seeking alternative nondopaminergic strategies aimed at correcting the abnormal BG circuitry downstream of the striatum.
Among the downstream changes, two pathways of the ‘indirect' circuit show a pronounced increase in activity under parkinsonian conditions of striatal dopamine depletion. The first of these is the GABAergic striatopallidal pathway, overactivity of which is evidenced by increased GABA release in the globus pallidus (GP; rodent homologue of the external globus pallidus, GPe) of parkinsonian rodents (Segovia et al., 1986). The second is the glutamatergic pathway from the subthalamic nucleus (STN) to the BG output regions, overactivity of which is shown by the elevation of glutamate release in the SNr in animal models of the disease (You et al., 1996). Previous attempts to surgically correct the activity of these pathways via subthalamotomy, pallidotomy or deep brain stimulation, to effectively switch off the overactive output nuclei, have proven successful in alleviating the motor deficits in PD patients and in counteracting some of the aforementioned side effects encountered with long-term use of conventional dopaminergic treatments (reviewed by Lozano, 2003). However, a pharmacological means of achieving similar correction of the BG motor circuits would clearly be a more attractive prospect to patients.
One pharmacological approach to correcting the abnormal BG circuitry could be to reduce the release of neurotransmitter from these overactive pathways of the indirect circuit. Previous studies from this group lend credence to this approach. For example, on the one hand, direct administration of either the group II metabotropic glutamate (mGlu) receptor agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl) glycine (DCG IV) (Dawson et al., 2000) or the GABAB receptor agonist baclofen (Johnston & Duty, 2003) into the SNr is able to reverse akinesia in the reserpine-treated rat model of PD. Since electrophysiological studies have shown that both group II mGlu receptor and GABAB receptor activation leads to inhibition of glutamate release from STN efferent terminals in the SNr (Shen & Johnson, 1997; Bradley et al., 2000), such a reduction in transmitter release most likely explains these anti-akinetic effects. On the other hand, reserpine-induced akinesia is also reversed following direct injection of the 5-HT1B receptor agonist CP-93129 into the rodent GP, where it brings about a reduction in depolarisation-evoked GABA release from presumed striatopallidal terminals (Chadha et al., 2000a).
Group III mGlu receptors offer an exciting avenue for further investigation of the potential of presynaptic receptor targeting in the treatment of PD. A combination of in situ hybridisation and immunocytochemical studies has revealed the presence of these receptors (specifically mGlu4,7 and 8) within the BG motor loop on both the glutamatergic STN efferent terminals in the SNr and the GABAergic striatopallidal terminals in the GP (Bradley et al., 1999a, 1999b; Kosinski et al., 1999; Messenger et al., 2002). Moreover, recent electrophysiological studies support a role for group III mGlu receptors as both autoreceptors, mediating the inhibition of glutamate release from STN efferent terminals in the SNr (Wittmann et al., 2002) and heteroreceptors, mediating the inhibition of GABA release from striatopallidal terminals (Valenti et al., 2003). Therefore, activation of group III mGlu receptors could offer a double-edged approach to correcting the abnormal BG circuitry in PD.
Given the strategic localisation of group III mGlu receptors within the BG motor loop, the aim of the present study was to examine whether injection of selective group III mGlu receptor agonists either discretely into the GP or SNr, or more diffusely into the cerebral ventricles, could reverse reserpine-induced akinesia in the rat. Some of this work has previously been published in abstract form (MacInnes & Duty, 2003; Messenger et al., 2003).
Methods
Intracerebral cannulation and induction of akinesia
Male Sprague–Dawley rats (270–300 g) were housed in a temperature- and humidity-controlled environment, on a 12 h light/dark cycle with free access to food and water. All procedures conformed to the U.K. Animals (Scientific Procedures) Act, 1986, and all efforts were made to minimise the animals' suffering and the number of animals used. Under general anaesthesia (4% Halothane in 95% O2, 5% CO2), animals were stereotaxically implanted with 23-gauge guide cannulae positioned 1 mm above either the third ventricle (4.0 mm posterior, 0 mm lateral and 4.0 mm ventral to bregma), GP (0.9 mm posterior, 3.0 mm lateral and 5.6 mm ventral to bregma) or SNr (5.3 mm posterior, 2.0 mm lateral and 7.4 mm ventral to bregma), according to the rat brain atlas of Paxinos & Watson (1986). After a minimum 4 days recovery, cannulated animals were then treated with either reserpine (5 mg kg−1 s.c. in a dose volume of 1 ml kg−1 of 0.8% glacial acetic acid) to deplete catecholamines and induce akinesia or reserpine vehicle (designated as non-reserpine-treated group hereafter). 18 h later, when a stable level of akinesia was established, the effects of group III mGlu receptor modulation were assessed.
Assessment of reversal of akinesia
Both intrapallidal and intranigral studies were performed in 40-cm diameter hemispheric, flat-bottomed bowls and the net number of contraversive 360° rotations was measured as an index of unilateral relief from akinesia. Intracerebroventricular (i.c.v.) studies were performed in rectangular cages with a 5-cm square grid pattern covering the base. Locomotor activity was measured in arbitrary locomotor units (ALUs), where one ALU is scored each time both front paws of an animal cross over a grid line. In all cases, responses were recorded on video for subsequent blinded scoring. Each experiment began with an initial 5-min acclimatisation period, followed by a 30-min baseline assessment, after which point the selective group III mGlu receptor agonists L-SOP or L-AP4 were administered. Each animal received a single dose of agonist dissolved in 2.5 (L-SOP) or 2.0 (L-AP4) μl phosphate-buffered saline (PBS; (mM); NaCl 137, KCl 2.7, KH2PO4 1.8, Na2HPO4 10) titrated with NaOH to pH 7.4. The need for larger volumes of injection compared to previous studies in this laboratory (Chadha et al., 2000a; Dawson et al., 2000; Johnston & Duty, 2003) reflected the solubility restraints of the drugs used. Injections (1 μl min−1) were made via a 10 μl Hamilton microsyringe attached with flexible portex tubing to a 21-gauge needle that extended 1 mm below the guide cannula tip either unilaterally into the GP or SNr or into the third ventricle. Following injection, behaviour was recorded for a further 30–120 min. Where receptor specificity was assessed, the selective group III mGlu receptor antagonist M-SOP (50 or 250 nmol in 2.5 μl PBS/NaOH, pH 7.4) (Thomas et al., 1996) was administered 30 min prior to L-SOP and behaviour recorded as above. Animals were used only once, except for non-reserpine-treated animals that received both vehicle and L-SOP injections at least 1 day apart. After the completion of behavioural assessments, animals were killed by sodium pentobarbitone overdose, followed by cervical dislocation, their brain removed and rapidly frozen on dry ice. Frozen brains were sectioned (30 μm) on a Bright OTF cryostat and microscopic assessment of these sections made to identify those animals in which the cannula was correctly placed. Only behavioural data from these animals were included in the data analyses.
Data analysis
In all cases, data were quantified per 10 min time bin. In reserpine-treated animals, the effects of treatment were analysed using a mixed-design two-way analysis of variance (ANOVA; where treatment was the between subjects factor and time was the within subjects factor) and independent post hoc Student–Newman–Keuls or t-tests (Statistical Package for the Social Sciences Version 10). The effects of treatment in non-reserpine-treated animals were analysed with a paired t-test.
Drugs
L-AP4, L-SOP and M-SOP were obtained from Tocris Cookson Ltd, U.K. and all other reagents were purchased from Sigma, U.K. Reserpine was prepared by dissolving 25 mg in 42.4 μl glacial acetic acid and making up to 5 ml with distilled H2O.
Results
Reversal of akinesia following intrapallidal drug administration
Correct positioning of the guide cannula over the GP was seen in 48 of the 60 rats tested. Animals that were incorrectly injected with L-SOP into regions adjacent to the GP did not produce any response and were not included in the subsequent data analyses.
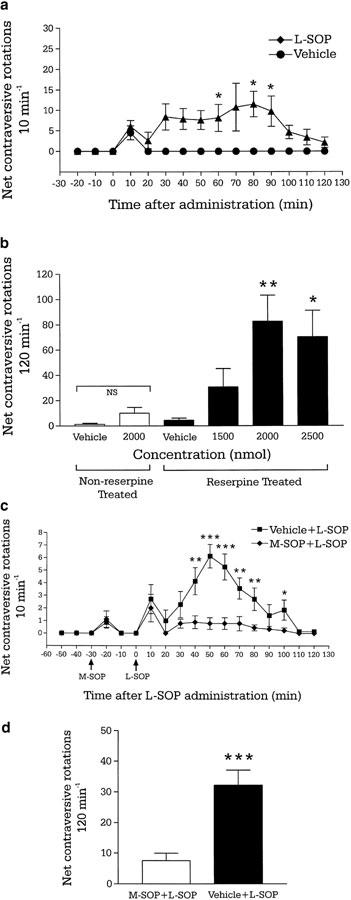
During the baseline period, reserpine-treated animals exhibited negligible activity (Figure 1a). Administration of L-SOP (1500–2500 nmol) produced an increase in net contraversive rotations, with a significant main effect for both treatment (F (3,264)=5.477, P<0.05) and time (F (11,264)=2.53, P<0.01; two-way ANOVA). Student–Newman–Keuls post hoc analysis indicated that the two highest doses of L-SOP tested (2000 and 2500 nmol) produced a significantly greater number of rotations in 200 min compared to vehicle (Figure 1b). Analysis of the time course revealed that, for the 2000 nmol dose, this increase was significant at 60, 80 and 90 min (Figure 1a), and by 120 min activity had returned to baseline levels. Administration of the group III mGlu receptor antagonist M-SOP (250 nmol) alone produced no significant increase in contraversive rotations compared to M-SOP vehicle (Figure 1c). However, the number of subsequent mean contraversive rotations produced by L-SOP (2000 nmol) was inhibited by 77% in the continuing presence of M-SOP compared to M-SOP vehicle (P<0.01; Figure 1d). Post hoc comparisons revealed this difference in locomotor activity to be significant over a large portion of the time course, as shown in Figure 1c. In non-reserpine-treated animals, administration of the optimal dose of L-SOP (2000 nmol) failed to evoke significantly different total net contraversive rotations compared to vehicle (P>0.05; paired t-test; Figure 1b).
Figure 1.
(a) Time course of locomotor activity induced by a maximally effective dose of the group III mGlu receptor agonist L-SOP (2000 nmol in 2.5 μl PBS) or vehicle (2.5 μl PBS) and (b) total locomotor effects of L-SOP (1500–2500 nmol in 2.5 μl PBS) or vehicle (2.5 μl PBS) following unilateral injection into the GP of reserpine-treated or non-reserpine-treated rats. Values represent mean±s.e.m. (n=6–8 animals per dose). *P<0.05, **P<0.01, indicate the significant differences from vehicle-treated reserpinised rats (two-way ANOVA with Student–Newman–Keuls post hoc test). NS indicates nonsignificant difference between vehicle and L-SOP in non-reserpine-treated rats (P>0.05; paired t-test). (c) Time course of locomotor activity and (d) total locomotor activity induced by unilateral intrapallidal injection of L-SOP (2000 nmol in 2.5 μl PBS) following 30 min pretreatment with either the group III mGlu receptor antagonist M-SOP (250 nmol in 2.5 μl PBS; n=9) or vehicle (2.5 μl PBS; n=7). Values represent mean ±s.e.m. *P<0.05, **P<0.01, ***P<0.001 indicate the significant differences between M-SOP and vehicle pretreated groups (unpaired t-test).
Reversal of akinesia following intranigral drug administration
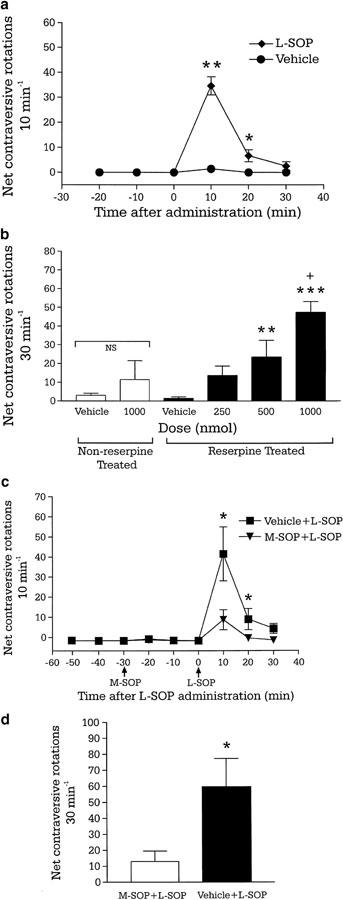
Histological verification of the guide cannula sites indicated that 46 of the 56 animals tested had received injections of L-SOP into the intended target, the SNr. Animals that had received injection of L-SOP in regions adjacent to the SNr failed to show any remission from akinesia and data from these animals were not included in the final analysis. All reserpine-treated animals exhibited virtually no activity over the baseline period (Figure 2a). Injection of L-SOP (250–1000 nmol) produced a dose-dependent increase in net contraversive rotations 30 min−1, with a significant main effect for treatment (F (3,44)=12.77, P<0.001) and time (F (2,44)=51.38, P<0.001) (Figure 2b). Analysis of the time course revealed that the increase in contraversive rotations with L-SOP (1000 nmol) was significant compared to vehicle after 10 and 20 min, with a return to vehicle levels after 30 min (Figure 2a). Administration of the group III mGlu receptor antagonist, M-SOP (50 nmol) alone produced no change in contraversive rotations compared to M-SOP vehicle (Figure 2c). However, in the continuing presence of M-SOP (50 nmol) compared to M-SOP vehicle, the subsequent number of net contraversive rotations produced by L-SOP (500 nmol) was significantly inhibited by 78% (P<0.001; Figure 2d). Post hoc comparisons revealed this difference in rotations to be significant over the entire time course of the L-SOP response (Figure 2c). In non-reserpine-treated animals, administration of the optimal dose of L-SOP (1000 nmol) failed to produce significantly different total net contraversive rotations compared to vehicle (P>0.05, paired t-test; Figure 2b).
Figure 2.
(a) Time course of locomotor activity induced by a maximally effective dose of the group III mGlu receptor agonist L-SOP (1000 nmol in 2.5 μl PBS) and (b) total locomotor effects of L-SOP (250–1000 nmol in 2.5 μl PBS) or vehicle (2.5 μl PBS) following unilateral injection into the substantia nigra pars reticulata in reserpine-treated or non-reserpine-treated rats. Values represent mean±s.e.m. (n=6–8 animals per dose). *P<0.05, **P<0.01, ***P<0.001 indicate the significant differences from vehicle-treated reserpinised rats and +P<0.01 versus lower doses of L-SOP (two-way ANOVA with Student–Newman–Keuls post hoc test). NS indicates nonsignificant difference between L-SOP and vehicle treatment in non-reserpine-treated rats (P>0.05; paired t-test). (c) Time course of locomotor activity and (d) total locomotor activity induced by unilateral intranigral injection of L-SOP (500 nmol in 2.5 μl PBS), following 30 min pretreatment with either the group III mGlu receptor antagonist M-SOP (50 nmol in 2.5 μl PBS; n=8) or vehicle (2.5 μl PBS; n=7). *P<0.05 indicates a significant difference between M-SOP and vehicle pretreatment (unpaired t-test).
Locomotor effects following i.c.v. drug administration
The correct positioning of cannulae over the dorsal tip of the third ventricle was noted in 65 of the 75 animals cannulated. Animals with aberrantly placed cannulae failed to show any remission from akinesia following L-AP4 or L-SOP injection, and were not included in the subsequent data analyses.
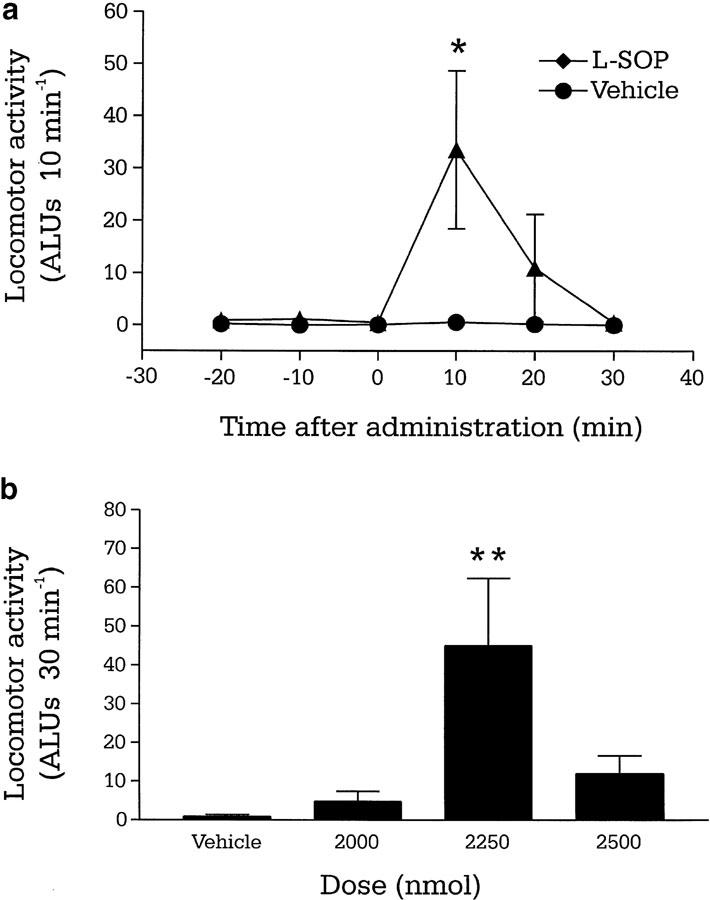
Reserpine-treated rats exhibited minimal baseline locomotor activity (Figure 3a). Third ventricular administration of L-SOP produced a significant increase in total locomotor activity for only the middle doses of L-SOP tested (2250 nmol) compared to vehicle, reaching 45±17 ALUs 30 min−1 (P<0.05; two-way ANOVA and Student–Newman–Keuls post hoc test) (Figure 3b). Analysis of the time course revealed that this significance was restricted to the first 10 min after administration (P<0.05; Figure 3a), with locomotor activity having returned to control levels after 30 min.
Figure 3.
(a) Time course of locomotor activity induced by a maximally effective dose of the group III mGlu receptor agonist L-SOP (2250 nmol in 2.5 μl PBS) or vehicle (2.5 μl PBS) and (b) total locomotor effects of L-SOP (2000–2500 nmol in 2.5 μl PBS) or vehicle (2.5 μl PBS), following i.c.v. injection in the reserpine-treated rat. Values represent mean±s.e.m. (n=6–8 animals per dose). *P<0.05, **P<0.01 indicate the significant differences from vehicle-treated animals (two-way ANOVA with Student–Newman–Keuls post hoc test).
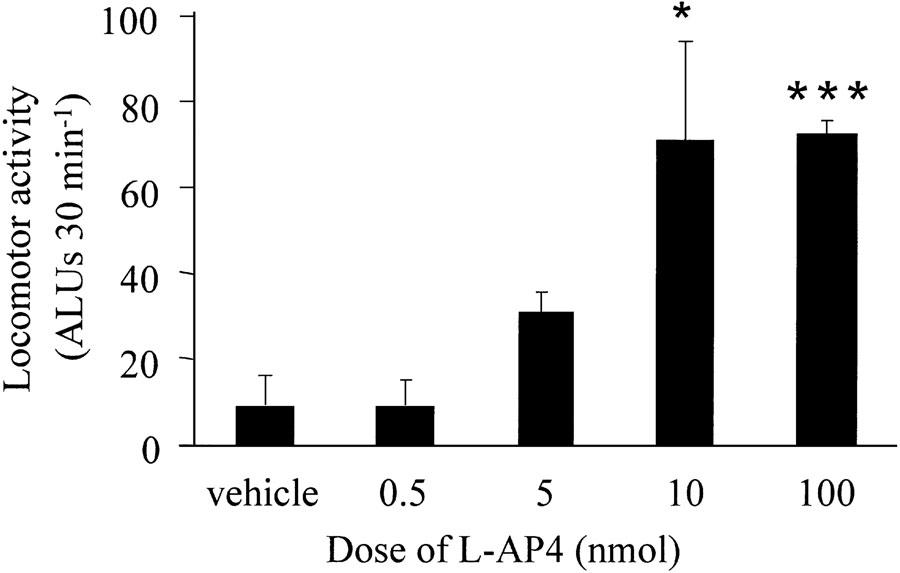
The third ventricular administration of a second group III agonist, L-AP4, produced a similar time course of locomotor activity to L-SOP, peaking at 10 min after administration and declining to baseline levels by 30 min. Over the entire 30 min recording period, L-AP4 (0.5–100 nmol) produced a significant dose-dependent increase in net contraversive rotations, reaching a maximum of 72±3 ALUs 30 min−1 at 100 nmol (P<0.05; two-way ANOVA and Student–Newman–Keuls post hoc test) (Figure 4).
Figure 4.
Total locomotor activity produced by L-AP4 (0.5–100 nmol in 2.0 μl PBS) or vehicle (2.0 μl PBS) following i.c.v. injection in the reserpine-treated rat. Values represent mean±s.e.m. (n=5–9 animals per dose). *P<0.05, ***P<0.001 indicate the significant differences from animals treated with vehicle and the two lowest doses of L-AP4 (two-way ANOVA with Student–Newman–Keuls post hoc test).
Discussion
This study has revealed that administration of the group III mGlu receptor agonists L-SOP or L-AP4 either directly into the GP and SNr or more diffusely into the third ventricle bring about relief of akinesia in the reserpine-treated rat. That no rotational behaviour was induced following injection of L-SOP into the GP or SNr of non-reserpine-treated rats indicates that the cellular mechanisms behind this reversal of akinesia most likely involve normalisation of the pathologically altered pathway activity present in the dopamine-depleted BG motor loop. The narrow effective dose range observed, regardless of the site of administration, is consistent with previous behavioural studies of this type using group II mGlu receptor agonists (Dawson et al., 2000) and related behavioural studies utilising both L-SOP (Yip et al., 2001) and L-AP4 (Valenti et al., 2003).
The reversal of akinesia seen following administration of group III mGlu receptor agonists via all the three routes of administration is qualitatively comparable, though modest in size, compared to that previously elicited by intranigral or i.c.v. injection of the group II mGlu receptor agonist DCG-IV (Dawson et al., 2000) or the GABAB receptor agonist baclofen (Johnston & Duty, 2003) or by intrapallidal injection of the 5-HT1B receptor agonist CP-93129 (Chadha et al., 2000a). However, none of the above agonists were capable of producing effects in both the desired targets (GP and SNr) so, in this way, the group III mGlu receptor agonists offer a different overall activity profile for consideration.
The response to L-SOP injection in both the GP and SNr appeared to be somewhat variable, as evidenced by the different magnitude of responses to identical doses between experiments (e.g. Figure 1b versus 1d and 2b versus 2d). While the reasons behind this variability are not known, since both the GP and SNr are anatomically and functionally heterogeneous (e.g. Parent & Hazrati, 1995a, 1995b), slight differences in cannula location within the nuclei may account for these differences between studies. However, since during the histological verification note was only taken of whether the cannula was positioned within the required nucleus and not of its exact location therein, such an explanation remains speculative at this stage.
The responses to L-SOP also varied depending on the site of administration, with intrapallidal injections producing a much more sustained locomotor response (up to 120 min versus 30 min for intranigral or i.c.v. injections). In addition, the doses of L-SOP and M-SOP required to elicit effects in the GP were four- to five-fold greater than those required in the SNr. These differences in sensitivity to the group III mGlu ligands may reflect either different pharmacokinetic profiles of the drug–receptor interactions or the involvement of different group III mGlu receptor subtypes with subtly different sensitivities to the same ligand in different target nuclei.
Reversal of akinesia following injection of L-SOP into the GP
An involvement of group III mGlu receptors in mediating the anti-akinetic response to intrapallidal L-SOP is supported by the marked inhibition (∼77%) afforded by the selective group III mGlu receptor antagonist M-SOP. The population of group III mGlu receptors most likely involved is that located on presynaptic elements of the striatofugal GABAergic neurones that synapse in the GP (Kosinski et al., 1999). As noted earlier, loss of dopamine D2-receptor-mediated inhibitory drive, following striatal dopamine depletion, renders the striatopallidal pathway markedly overactive, resulting in increased GABA release in the GP (Segovia et al., 1986; Levy et al., 1997). Group III mGlu receptors have been recently reported to function as heteroreceptors at the striatopallidal synapse, following the demonstration that L-AP4 inhibited GABA-evoked inhibitory post synaptic potentials (IPSPs) in the GP (Valenti et al., 2003). If a similar effect was produced by L-SOP, this could provide the necessary inhibition of GABA release from striatopallidal neurones required to normalise the BG motor loop. Of the three group III mGlu receptors believed to exist on striatopallidal terminals (mGlu4,7 and 8; Bradley et al., 1999a, b; Kosinski et al., 1999; Messenger et al., 2002), the mGlu4 receptor is the strongest contender for mediating the desired heteroreceptor role, as witnessed by the loss of L-AP4-mediated inhibition of GABA-mediated IPSPs in the GP of mGlu4 knockout mice (Valenti et al., 2003). Demonstration of a similar loss of the presently observed rotational behaviour in dopamine-deplete mGlu4 receptor knockout mice would help to clarify the involvement of mGlu4 in mediating this anti-akinetic effect.
Reversal of akinesia following injection of L-SOP into the substantia nigra pars reticulata
An involvement of group III mGlu receptors in mediating the dose-dependent reversal of akinesia seen following intranigral injection of L-SOP is also supported by the marked inhibitory effect (78%) of prior administration of M-SOP. Two potential populations of presynaptic group III mGlu receptor may be activated within the SNr; those localised on the GABAergic striatonigral terminals and those on glutamatergic subthalamonigral terminals (Bradley et al., 1999b; Kosinski et al., 1999). Electrophysiological studies have confirmed the functional significance of both these receptor populations in effecting presynaptic inhibition of neurotransmitter release. Thus, the group III mGlu receptor agonist L-AP4 reduces both excitatory glutamatergic transmission across the STN-SNr synapse and inhibitory GABAergic transmission across the striatonigral synapse (Wittmann et al., 2002). In deducing which, if either, of these populations most likely mediates the anti-akinetic response of L-SOP presently observed, it is necessary to reconsider the pathophysiology of BG circuitry in PD. Considering firstly the subthalamonigral pathway, this is known to be markedly overactive in PD, leading to increased glutamate-mediated excitation of the SNr and subsequent inhibition of thalamocortical feedback, leading to the generation of motor deficits (e.g. Blandini et al., 2000). Therefore, activation of group III mGlu receptors on subthalamonigral terminals to bring about a reduction in the release of glutamate in the SNr would help normalise the BG motor loop. In contrast, the striatonigral pathway is believed to be slightly underactive in the parkinsonian state, as witnessed by the compensatory increase in GABAA receptor binding in the SNr in animal models of PD (Pan et al., 1985; Chadha et al., 2000b). Therefore, a reduction of GABA release from these neurones, via activation of group III mGlu receptors residing on the striatonigral terminals, would be predicted to have little net effect on the activity of the SNr. Indeed, any further reduction of GABA transmission in this region would rather exacerbate the already overactive SNr and potentially worsen parkinsonian symptoms. Taken together, it is clear that, of the two populations, group III mGlu receptors on terminals of subthalamonigral neurones most likely underlie the present reversal of akinesia produced by L-SOP. Adding strength to this suggestion, the elegant electrophysiological studies of Wittmann et al. (2002) showed that while the autoreceptor role of group III mGlu receptors on subthalamonigral neurones remained functional in the reserpine-treated rat, the heteroreceptor role at the striatonigral synapse was lost.
The exact nature of the group III mGlu receptor implicated in this response remains to be established. However, since the doses of L-SOP required to elicit a response in the SNr were only four- to five-fold lower than those required in the GP, it is most likely that either mGlu4 (as suggested above for the GP) or mGlu8 receptors, which display similar agonist sensitivities, are implicated. Conversely, mGlu7 receptors that generally require an approximate 100-fold higher concentration of agonist for activation (Conn & Pin, 1997) are least likely to be involved.
Reversal of akinesia following i.c.v. injection of L-SOP and L-AP4
That the ability of L-SOP to reverse reserpine-induced akinesia was maintained when the drug was administered outside the potential sites of action (i.e. GP and SNr) holds promise for investigations with systemically active compounds, should these become available. However, following i.c.v. administration, the efficacy of L-SOP displayed a particularly narrow dose range. This may reflect the pharmacokinetic properties of the drug itself, with a combination of diffusion barriers and washout of the drug in the cerebrospinal fluid contributing to a restriction in the peak concentration reaching the desired target sites. In addition, the bell-shaped dose–response relationship generated via this route of administration may be indicative of the higher doses of L-SOP becoming nonspecific. This is supported by the presence of barrel rolling in animals that had been administered higher doses (data not shown).
From the preceding discussion, it is clear that both the GP and SNr could be potential sites of action mediating the desired reversal of akinesia in response to i.c.v. administration of L-SOP. However, actions of L-SOP in additional parts of the BG motor loop that could be potentially detrimental to the anti-akinetic efficacy cannot be fully ruled out at this stage. For example, activation of any group III mGlu receptors found on GP efferent terminals in the STN, to effect a reduction in GABA release, would exacerbate the already overactive STN. Similarly, activation of such receptors located on thalamocortical terminals leading to a reduction in glutamate release would clearly lessen thalamocortical motor drive and worsen akinesia. However, the former heteroreceptor action is unlikely to pose a real problem since mRNA encoding all the three group III mGlu receptors is very low in GP (Messenger et al., 2003) and there are no reports of immunoreactivity for group III mGlu receptors in STN. Conversely, the high levels of both mGlu4 and mGlu7 mRNA found in many thalamic relay nuclei (Messenger et al., 2003) add strength to the notion that group III mGlu receptors may exist on the less than ideal site of thalamocortical terminals. However, the lack of supporting immunohistochemical data confirming the existence of group III mGlu receptors on the thalamocortical terminals renders this argument purely hypo-thetical. Even if such an action was mediated by L-SOP, the fact that reversal of akinesia is observed following i.c.v. injection indicates that the overriding response dictating the net outcome of L-SOP on the BG motor loop is that mediated at the level of the GP and SNr.
That reversal of akinesia was also observed in the present study following i.c.v. administration of a second selective group III mGlu receptor agonist, L-AP4, adds strength to the implied involvement of group III receptors in mediating this anti-akinetic response. The response to L-AP4 followed a similar time course to that of L-SOP, but produced a more classical dose–response relationship indicating, perhaps, a ‘cleaner' mechanism of action of this agonist over the dose range examined. L-AP4 has also recently been shown by others to reverse reserpine-induced akinesia following i.c.v. administration (Valenti et al., 2003). However, given the relative crudeness of the reserpine-treated rat model that displays parkinsonian-like akinesia following depletion of all catecholamines, not just dopamine, caution must be placed when interpreting the relevance of these results in the overall context of PD. However, Valenti et al. (2003) also revealed the efficacy of L-AP4 in two additional rodent models of PD. They demonstrated that L-AP4, at doses similar to those used in the present study (50 and 100 nmol given i.c.v.), was able to reverse haloperidol-induced catalepsy in the rat and to lower forelimb asymmetry score in the 6-hydroxydopamine (6-OHDA)-lesioned rat. We have recently observed the production of contraversive rotations in 6-OHDA-lesioned rats following i.c.v. L-SOP, further supporting the efficacy of group III mGlu receptor agonists in this more chronic model of PD (MacInnes & Duty, unpublished observations). Therefore, the presently observed efficacy of group III mGlu receptor agonists appears to be common to all rodent models of PD examined thus far. Although no specific site-directed injections were performed by Valenti et al. (2003), the authors propose that the observed behavioural responses are caused by mGlu4 receptor-mediated inhibition of GABA release from striatopallidal neurones. Clearly the present data support this suggestion, but additionally identify the SNr as a second potential site of action that cannot be ignored. Overall, these complementary data add strength to the notion that activation of group III mGlu receptors may be of potential benefit for normalising the activity of the BG motor loop under parkinsonian conditions.
In conclusion, the data presented in this paper establish a role for group III mGlu receptor agonists in modulating the functional outcome of BG activity via actions within at least two specific nuclei of this motor circuit, the GP and SNr. These findings corroborate the current pharmacological model of the BG circuitry, as well as the many in vitro studies suggesting that group III mGlu receptors can serve to inhibit transmitter release from selected BG pathways. The demonstrated efficacy of L-SOP and L-AP4 following i.c.v. injection supports the notion that any future systemically active group III agonists may warrant investigation in a wider range of established rodent and primate models of PD.
Acknowledgments
This work was funded by Merck Sharp & Dohme and the Wellcome Trust. MJM was in receipt of an MRC studentship.
Abbreviations
- BG
basal ganglia
- GABA
γ-aminobutyric acid
- GP
globus pallidus
- L-AP4
L-(+)-2-amino-4-phosphonobutyric acid
- L-SOP
L-serine-O-phosphate
- mGlu
metabotropic glutamate
- M-SOP
methyl-serine-O-p hosphate
- PD
Parkinson's disease
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
References
- BLANDINI F., NAPPI G., TASSORELLI C., MARTIGNONI E. Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog. Neurobiol. 2000;62:63–88. doi: 10.1016/s0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- BRADLEY S.R., MARINO M.J., WITTMANN M., ROUSE S.T., AWAD H., LEVEY A.I., CONN P.J. Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia nigra pars reticulata. J. Neurosci. 2000;20:3085–3094. doi: 10.1523/JNEUROSCI.20-09-03085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY S.R., STANDAERT D.G., LEVEY A.I., CONN P.J. Distribution of group III mGluRs in rat basal ganglia with subtype-specific antibodies. Ann. NY Acad. Sci. 1999a;868:531–534. doi: 10.1111/j.1749-6632.1999.tb11322.x. [DOI] [PubMed] [Google Scholar]
- BRADLEY S.R., STANDAERT D.G., RHODES K.J., REES H.D., TESTA C.M., LEVEY A.I., CONN P.J. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J. Comp. Neurol. 1999b;407:33–46. [PubMed] [Google Scholar]
- CHADHA A., SUR C., ATACK J., DUTY S. The 5HT1B receptor agonist, CP-93129, inhibits [3H]-GABA release from rat globus pallidus slices and reverses akinesia following intrapallidal injection in the reserpine-treated rat. Br. J. Pharmacol. 2000a;130:1927–1932. doi: 10.1038/sj.bjp.0703526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHADHA A., HOWELL O., ATACK J., SUR C., DUTY S. Changes in [3H]zolpidem and [3H]Ro 15-788 binding in rat globus pallidus and substantia nigra pars reticulata following a nigrostriatal tract lesion. Brain Res. 2000b;862:280–283. doi: 10.1016/s0006-8993(00)02081-3. [DOI] [PubMed] [Google Scholar]
- CONN P.J., PIN J.P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- DAWSON L., CHADHA A., MEGALOU M., DUTY S. The group II metabotropic glutamate receptor agonist, DCG-IV, alleviates akinesia following intranigral or intraventricular administration in the reserpine-treated rat. Br. J. Pharmacol. 2000;129:541–546. doi: 10.1038/sj.bjp.0703105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON T., DUTY S. GABAB receptor agonists reverse akinesia following intranigral and intracerebroventricular injection in the reserpine-treated rat. Br. J. Pharmacol. 2003;139:1480–1486. doi: 10.1038/sj.bjp.0705372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSINSKI C.M., RISSO BRADLEY S., CONN P.J., LEVEY A.I., LANDWEHRMEYER G.B., PENNEY J.B., JR, YOUNG A.B., STANDAERT D.G. Localization of metabotropic glutamate receptor 7 mRNA and mGluR7a protein in the rat basal ganglia. J. Comp. Neurol. 1999;415:266–284. [PubMed] [Google Scholar]
- LEVY R., HAZRATI L.N., HERRERO M.T., VILA M., HASSANI O.K., MOUROUX M., RUBERG M., ASENSI H., AGID Y., FEGER J., OBESO J.A., PARENT A., HIRSCH E.C. Re-evaluation of the functional anatomy of the basal ganglia in normal and Parkinsonian states. Neuroscience. 1997;76:335–343. doi: 10.1016/s0306-4522(96)00409-5. [DOI] [PubMed] [Google Scholar]
- LOZANO A.M. Surgery for Parkinson's disease, the five W's: why, who, what, where, and when. Adv. Neurol. 2003;91:303–307. [PubMed] [Google Scholar]
- MACINNES N., DUTY S. Intraventricular and intrapallidial injections of the group III metabotropic glutamate receptor agonist L-serine-O-phosphate (L-SOP) alleviate reserpine-induced akinesia in the rat. Br. J. Pharmacol. 2003;138:226p. [Google Scholar]
- MESSENGER M., DAWSON L., DUTY S. Changes in metabotropic glutamate receptor 1-8 gene expression in the rodent basal ganglia motor loop following lesion of the nigrostriatal tract. Neuropharmacology. 2002;43:261–271. doi: 10.1016/s0028-3908(02)00090-4. [DOI] [PubMed] [Google Scholar]
- MESSENGER M.J., GREENWOOD K., DUTY S. The group III mGlu receptor agonist, L-AP4 reverses akinesia in the reserpine-treated rat and inhibits [3H]D-aspartate release from rat nigral slices. Br. J. Pharmacol. 2003;1:14p. [Google Scholar]
- OBESO J.A., RODRIGUEZ-OROZ M.C., RODRIGUEZ M., LANCIEGO J.L., ARTIEDA J., GONZALO N., OLANOW C.W. Pathophysiology of the basal ganglia in Parkinson's disease. Trends Neurosci. 2000;23:S8–S19. doi: 10.1016/s1471-1931(00)00028-8. [DOI] [PubMed] [Google Scholar]
- PAN H.S., PENNEY J.B., YOUNG A.B. Aminobutyric acid and benzodiazepine receptor changes induced by unilateral 6-hydroxydopamine lesions of the median forebrain bundle. J. Neurochem. 1985;45:1396–1404. doi: 10.1111/j.1471-4159.1985.tb07205.x. [DOI] [PubMed] [Google Scholar]
- PARENT A., HAZRATI L.N. Functional anatomy of the basal ganglia I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Brain Res. Rev. 1995a;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- PARENT A., HAZRATI L.N. Functional anatomy of the basal ganglia II. The place of the subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res. Brain Res.Rev. 1995b;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Co-Ordinates. UK: Academic Press; 1986. [Google Scholar]
- SEGOVIA J., TOSSMAN U., HERRERA-MARSCHITZ M., GARCIA-MUNOZ M., UNGERSTEDT U. Gamma-Aminobutyric acid release in the globus pallidus in vivo after a 6-hydroxydopamine lesion in the substantia nigra of the rat. Neurosci. Lett. 1986;70:364–368. doi: 10.1016/0304-3940(86)90580-x. [DOI] [PubMed] [Google Scholar]
- SHEN K.Z., JOHNSON S.W.Presynaptic GABAB and adenosine A1 receptors regulate synaptic transmission to rat substantia nigra reticulata neurones J. Physiol. (Lond). 1997505153–163.(Part 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCCHI F., NORDERA G., MARSDEN C.D. Strategies for treating patients with advanced Parkinson's disease with disastrous fluctuations and dyskinesias. Clin. Neuropharmacol. 1997;20:95–115. doi: 10.1097/00002826-199704000-00001. [DOI] [PubMed] [Google Scholar]
- THOMAS N.K., JANE D.E., TSE H.W., WATKINS J.C. Alpha-methyl derivatives of serine-O-phosphate as novel, selective competitive metabotopic glutamate receptor antagonists. Neuropharmacology. 1996;35:637–642. doi: 10.1016/0028-3908(96)84635-1. [DOI] [PubMed] [Google Scholar]
- VALENTI O., MARINO M.J., WITTMAN M., LIS E., DILELLA A.G., KINNEY G.G., CONN P.J. Group III metabotropic glutamate receptor-mediated modulation of the striatopallidal synapse. J. Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITTMANN M., MARINO M.J., CONN P.J. Dopamine modulates the function of group II and group III metabotropic glutamate receptors in the substantia nigra pars reticulata. J. Pharmacol. Exp. Ther. 2002;302:433–441. doi: 10.1124/jpet.102.033266. [DOI] [PubMed] [Google Scholar]
- YIP P.K., MELDRUM B.S., RATTRAY M. Elevated levels of group-III metabotropic glutamate receptors in the inferior colliculus of genetically epilepsy-prone rats following intracollicular administration of l-serine-O-phosphate. J. Neurochem. 2001;78:13–23. doi: 10.1046/j.1471-4159.2001.00418.x. [DOI] [PubMed] [Google Scholar]
- YOU Z.-B., HERRERA-MARSCHITZ M., PETTERSSON E., NYLANDER I., GOINY M., SHOU H.Z., KEHR J., GOUDUKHIN O., HOKFELT T., TERENIUS L., UNGERSTEDT U. Modulation of neurotransmitter release by cholecystokinin in the neostriatum and substantia nigra of the rat: regional and receptor specifity. Neuroscience. 1996;74:793–804. doi: 10.1016/0306-4522(96)00149-2. [DOI] [PubMed] [Google Scholar]