Abstract
The main aim of this investigation was to delineate the distribution of the 5-HT7 receptor in human brain. Autoradiographic studies in guinea-pig and rat brain were also carried out in order to revisit and compare the anatomical distribution of 5-HT7 receptors in different mammalian species.
Binding studies were performed in rat frontal cortex membranes using 10 nM [3H]mesulergine in the presence of raclopride (10 μM) and DOI (0.8 μM). Under these conditions, a binding site with pharmacological characteristics consistent with those of the 5-HT7 receptors was identified (rank order of binding affinity values: 5-CT>5-HT>5-MeOT>mesulergine ≈methiothepin>8-OH-DPAT=spiperone ≈(+)-butaclamol≫imipramine ≈(±)-pindolol≫ondansetron ≈clonidine ≈prazosin).
The autoradiographic studies revealed that the anatomical distribution of 5-HT7 receptors throughout the human brain was heterogenous. High densities were found over the caudate and putamen nuclei, the pyramidal layer of the CA2 field of the hippocampus, the centromedial thalamic nucleus, and the dorsal raphe nucleus. The inner layer of the frontal cortex, the dentate gyrus of the hippocampus, the subthalamic nucleus and superior colliculus, among others, presented intermediate concentrations of 5-HT7 receptors. A similar brain anatomical distribution of 5-HT7 receptors was observed in all three mammalian species studied.
By using [3H]mesulergine, we have mapped for the first time the anatomical distribution of 5-HT7 receptors in the human brain, overcoming the limitations previously found in radiometric studies with other radioligands, and also revisiting the distribution in guinea-pig and rat brain.
Keywords: 5-Hydroxytryptamine, 5-HT7 receptor, central nervous system, human, guinea-pig, rat, quantitative autoradiography
Introduction
Central nervous system serotonin (5-hidroxytryptamine, 5-HT) has been implicated in a vast array of behavioral and physiological processes in vertebrates (Baumgarten & Gothert, 1997). The presence of multiple 5-HT receptor subtypes with diverse modes of signal transduction and brain distribution contributes to the functional diversity attributed to 5-HT (Barnes & Sharp, 1999).
Among the seven distinct 5-HT receptor families known up to date, the 5-HT7 receptor is the most recently identified member of the family of G-protein-coupled 5-HT receptors (Vanhoenacker et al., 2000). Previously classified as belonging to the 5-HT1-like group and positively coupled to adenylate cyclase (Eglen et al., 1997), the 5-HT7 receptor has been cloned from rat, mouse, guinea-pig, Xaenopus laevis, and human (Bard et al., 1993; Lovenberg et al., 1993; Plassat et al., 1993; Shen et al., 1993; Tsou et al., 1994; Nelson et al., 1995). Recently, three receptor isoforms created by alternative splicing of the 5-HT7 receptor gene have been identified in both human and rat tissues (Heidmann et al., 1997; Jasper et al., 1997; Stam et al., 1997), and these isoforms are thought to have similar pharmacology and function (Vanhoenacker et al., 2000; Krobert et al., 2001). Competition-binding studies on cloned 5-HT7 receptors expressed in cell lines have yielded a unique pharmacological profile consistent across all tested species. The expressed 5-HT7 receptor has a high affinity for 5-carboxytryptamine (5-CT), 5-HT, 5-methoxytryptamine (5-MeOT), mesulergine, and (R)-3-(2-(2-(4-methylpiperidin-1-yl) ethyl)pyrrolidine-1-sulfonyl)-phenol) (SB-269970), moderate affinity for 8-hydroxy-2-(di-n-propylamino)tetraline (8-OH-DPAT), clozapine, methysergide, ritanserin, and spiperone, and low affinity for pindolol and sumatriptan (Eglen et al., 1997; Barnes and Sharp, 1999; Hagan et al., 2000).
Multiple roles have been suggested for 5-HT7 receptors present in the brain, including the regulation of the circadian rhythm via the suprachiasmatic nucleus of the hypothalamus (Lovenberg et al., 1993) and the modulation of activity of distinct brain neuronal populations (Bacon & Beck, 2000; Chapin & Andrade, 2001; Gill et al., 2002; Goaillard & Vincent, 2002). A number of reports have suggested that the regulation of 5-HT7 receptors may underlay the clinical efficacy of antidepressant drugs and/or the etiology of neuropsychiatric behavioral states such as depression (Barnes & Sharp, 1999). In addition, other reports have proposed that selective drugs for 5-HT7 receptor could constitute new therapeutics opportunities for the treatment of epilepsy, chronic pain, and learning and memory alterations (Bourson et al., 1997; Vanhoenacker et al., 2000; Meneses & Terron, 2001; Gill et al., 2002; Goaillard & Vincent, 2002).
Our present knowledge on the biological role of central 5-HT7 receptors is in part based on results from studies on the anatomical distribution of this receptor. Discrete regional distribution of the cell populations expressing the mRNA coding for 5-HT7 receptors in rat and guinea-pig brain tissue sections has been well described (To et al., 1995; Gustafson et al., 1996; Mengod et al., 1996; Heidmann et al., 1998; Kinsey et al., 2001; Neumaier et al., 2001). However, the study of the anatomical distribution of 5-HT7 receptor-binding sites in brain tissue sections has been hampered by the lack of a specific and selective radioligand and, more recently, by the low level of specific binding of a selective 5-HT7 radioligand (see below).
Several attempts have been made to label 5-HT7 receptor-binding sites in rat and guinea-pig brain tissue sections (To et al., 1995; Waeber & Moskowitz, 1995; Gustafson et al., 1996; Mengod et al., 1996). These autoradiographic studies used a relatively nonselective 5-HT7 agonist (i.e., [3H]5-CT) in the presence of drugs to mask other binding sites. In relative contrast with these autoradiographic studies, a relatively high abundance of 5-HT7 receptor mRNA or immunoreactivity has been reported in rat and guinea-pig brain (To et al., 1995; Gustafson et al., 1996; Mengod et al., 1996; Heidmann et al., 1998; Kinsey et al., 2001; Neumaier et al., 2001). In this regard, the authors concluded that masking drugs added in these autoradiographic studies occluded full visualization of the receptor. In addition, a further recognition site with high affinity for 5-CT has been identified and characterized in mammalian brain (Castro et al., 1997). In line with these results, a recent report indicates that, despite its high affinity, [3H]5-CT is not a good tracer for measuring 5-HT7-binding sites autoradiographically (Bonaventure et al., 2002).
[3H]-SB-269970 has been recently identified as a selective antagonist radioligand for 5-HT7 receptors (Thomas et al., 2000), though attempts made to study the anatomical distribution of 5-HT7-binding sites in brain tissue sections with [3H]-SB-269970 have been unsuccessful up to date (Thomas et al., 2002).
Owing to all the above mentioned facts, in the present study, the nonselective antagonist [3H]mesulergine was chosen as a radioligand, as it has a high affinity for 5-HT7 receptors but a low affinity for the atypical 5-HT-binding site reported by Castro et al. (1997). In addition, the use of an antagonist as radioligand allows for circumventing the complications that arise from the presence of multiple affinity states of the receptor in native tissues (Kenakin, 1997). Interestingly, recent studies using this binding strategy have proved to be successful for labeling 5-HT7-binding sites in the rat brain, guinea-pig, and mouse ileum homogenates (Hemedah et al., 1999; 2000).
In summary, the aim of the present study was to provide a detailed anatomical distribution of 5-HT7 receptor-binding sites in human brain. To this end, appropriate autoradiographic assay conditions were established by using [3H]mesulergine. In addition, autoradiographic studies in guinea-pig and rat brain tissue sections were undertaken to revisit and compare the anatomical distribution of 5-HT7 receptors in different mammalian species. Preliminary data from this study have been reported previously (Martín-Cora & Pazos, 2000).
Methods
Radioligand-binding experiments
Membrane preparations
Adult male Wistar rats (250–300 g; Harlam Interfauna, Barcelona, Spain) were decapitated and their brains were rapidly removed. The frontal cortex and striatum were dissected and membranes from those brain areas were prepared, as described by Pazos et al. (1984). Briefly, brain tissues were homogenized in 10 volumes of 0.32 M sucrose and subsequently centrifuged (70,000 × g, 15 min, 4°C). The pellet was resuspended in 50 mM Tris-HCl buffer (pH 7.5), incubated at 37°C for 15 min and centrifuged again. This final pellet was resuspended in 10 volumes of 50 mM Tris-HCl buffer (pH 7.7) containing 4 mM CaCl2 and 0.1% ascorbic acid and aliquots were stored at −80°C until used.
Binding assays
On the day of the experiment, the membrane homogenates were thawed and further diluted 1 : 4 (V : V) in the incubation buffer (Tris-HCl 50 mM, CaCl2 4 mM, 0.1% ascorbic acid, 10 μM pargyline, pH 7.7). For binding assays, 750 μl of tissue suspension was added to tubes containing 100 μl of [3H]mesulergine (see concentrations below) and 150 μl of different drugs. The samples were incubated for 30 min at 37°C and the experiment was terminated by vacuum filtration through GF/B glass fiber filters (Whatman, Maidstone, U.K.) presoaked in 0.1% polyethyleneimine. Filters were placed in plastic vials with 4 ml of Ecoscint A (National Diagnostics, Hessle Hull, U.K.) and filter radioactivity was measured in a scintillation counter (Beckman LS 6000IC, CA, U.S.A.) at least 14 h after the assay had been finished.
Saturation experiments were conducted in rat frontal cortex membranes using increasing (12–17) concentrations of [3H]mesulergine (0.5 pM–24 nM) with duplicate determinations per each concentration data point, and nonspecific binding was determined with 10 μM of 5-HT. Protein content per tube ranged from 1 to 1.6 mg of protein, as determined by the method of Lowry et al. (1951). All saturation experiments were performed in triplicates.
Competition studies were performed using [3H]mesulergine (10 nM) as radioligand, and raclopride (1 μM) and 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI; 0.8 μM) as masking drugs, unless otherwise specified (see results). Displacing drugs were assayed in 10–13 different concentrations (10−12–3.10−3 M), but for (±)-pindolol only six different concentrations (10−8–10−3 M) were used. Nonspecific binding was defined with 5-HT (10 μM). Competition experiments were carried out at least in triplicates, with duplicate determinations for each concentration data point.
Autoradiographic experiments
Brain tissues
Postmortem human brain tissues from 13 subjects (age=50±5 years; postmortem delay=24±4 h), who died without a history of neuropsychiatric disease, were obtained at necropsy. The characteristics of these cases are summarized in Table 1 . The brains were obtained and removed at the Department of Pathology, University Hospital ‘Marqués de Valdecilla'. The procedures of obtention and handling were approved by the Ethical Research Committee of this Institution. The brain was promptly removed, one hemibrain being fixed in 10% formalin for neuropathological examination and the other hemibrain cut into blocks and stored at −80°C. Sequential sections (20 μm thickness) of the human frontal cortex, striatum, hippocampus, thalamus, and midbrain were cut with a microtome-cryostat, thaw mounted in gelatinized slides and stored at −20°C. Not all human brain regions analyzed in this study were available from all the subjects. Five adult male Wistar rats (250–300 g) and seven male guinea-pigs (300–350 g) were decapitated, after which the brains were immediately removed, frozen and stored at −80°C. Sequential coronal rat and guinea-pig brain sections at five (rat) and seven (guinea-pig) representative levels were cut, thaw mounted, and stored until used.
Table 1.
Sources of human brain tissue
| Sex (female–male) | Age (years) | Postmortem delay (h) | Cause of death |
|---|---|---|---|
| F | 69 | 41 | Motor-vehicle accident |
| F | 34 | 24 | Motor-vehicle accident |
| F | 68 | 30 | Breast cancer |
| F | 43 | 12 | Pulmonary edema |
| F | 34 | 10 | Myocardial ischemia |
| M | 67 | 61 | Pneumonia |
| M | 22 | 14 | Motor-vehicle accident |
| M | 58 | 27 | Labor accident |
| M | 50 | 32 | Motor-vehicle accident |
| M | 41 | 20 | Labor accident |
| M | 74 | 21 | Motor-vehicle accident |
| F | 73 | 8 | Gastric cancer |
| M | 27 | 19 | Motor-vehicle accident |
Autoradiographic assay conditions
Tissue sections – in duplicate – were incubated in Coplin jars for 2 h at room temperature in 40 ml of incubation buffer containing [3H]mesulergine (10 nM) and masking drugs (see results). Nonspecific binding was determined in a consecutive section in the presence of 5-HT (10 μM). After incubation, the sections were washed in ice-cold (4°C) buffer twice for 10 min, followed by a quick immersion in cold distilled water to remove any salts. The sections were then dried in a stream of cool air, and exposed at 4°C for 75 days by apposition against a tritium-sensitive film (Hyperfilm-3H, Amersham Int., U.K.), together with the appropriate standards ([3H]Microscales, Amersham Int., U.K.). Films were developed with Kodak HC.110 and fixed with AGFA G350.
Data analysis
Binding assays
Analysis of both saturation and competition data was performed by nonlinear curve fitting using Prism 3.0 (GraphPad Software, San Diego, CA, U.S.A.). The equilibrium dissociation constant and the maximal binding density (KD, Bmax) were estimated using appropriate equations (Motulsky, 1999). Competition-binding data was analyzed for one- or two-sites models. The selection between models was made statistically by the extra sum of squares principle (F-test); if P-value was <0.05, the two-sites model was accepted. pKi values (−log of the inhibition constant) were calculated from IC50 (concentration of drug inhibiting specific binding by 50%), as described by Cheng & Prusoff (1973). Correlation plots were analyzed by linear regression, also using the computer program Prism 3.0. All results are reported as mean±standard error of the mean (s.e.m.).
Autoradiography
Digitized autoradiographs were analyzed with the aid of a computer program Scion Image β-3b (Scion Corp., MA, U.S.A.). Specific regions of interest were defined using standard rat, guinea-pig, and human brain atlases (Luparello, 1967; Paxinos & Watson, 1982; Paxinos, 1990). The optical density of each region was converted to fmol mg tissue−1, using a third-degree polynomial calibration curve generated from tritium standards. Bmax values were obtained by correcting the number of receptors bound in fmol mg tissue−1 (B) for full occupancy of the receptor, according to the equation: Bmax=B[1+KD/L], where KD was 10 nM and L the concentration of the radioactive ligand.
Drugs
The following drugs were used: (+)-butaclamol HCl, 5-CT maleate, DOI HCl, 5-HT creatinine sulfate, 5-MeOT HCl, (±)-8-OH-DPAT HBr, (±)-pindolol, raclopride l-tartrate and spiperone from Research Biochemicals International (Natick, MA, U.S.A.); clonidine HCl, imipramine HCl, pargyline HCl, and prazosin HCl from Sigma (Madrid, Spain); [3H]mesulergine (79–81 Ci mmol−1) from Amersham Int. (U.K.), ondansetron HCl from Glaxo (U.K.); methiothepin maleate from Tocris Cookson (Bristol, U.K.). Stock drug solutions were prepared fresh on the day of assay. Butaclamol, ondansetron, and spiperone were dissolved in ethanol and (±)-pindolol was dissolved in water acidified with a few drops of glacial acetic acid. Distilled water was used for further dilution up to the required final concentrations. All other drugs were dissolved in distilled water.
Results
Establishment of labeling conditions for 5-HT7 receptors
Based on published data, [3H]mesulergine, at 10 nM, was predicted to bind to 5-HT7 receptors, dopamine D2 receptors, 5-HT2A, and 5-HT2C receptors and, to a much lesser extent, α1/α2 adrenoceptors (Closse, 1983; Rinne, 1983; Pazos et al., 1985; Hoyer et al., 1994). To determine the appropriate concentrations of cold ligands required to mask the binding to dopamine D2 receptors, 5-HT2A, and 5-HT2C receptors, the competition profile of 10 nM [3H]mesulergine binding by raclopride and DOI was initially studied. These experiments yielded pKi values of 7.3±0.14 (n=4) for raclopride in rat striatal membranes and 8.2±0.06 (n=3) for DOI in rat frontal cortex membranes. Thus, concentrations of 1 μM of raclopride and 0.8 μM of DOI were chosen, so that there was at least 90% occupancy of dopamine D2, 5-HT2A, and 5-HT2C receptors, with little effect on 5-HT7 receptor binding (<9%).
As mentioned above, mesulergine presents a certain affinity for α1/α2-adrenoceptors; however, the specific binding of [3H]mesulergine defined in the presence of raclopride/DOI (1 and 0.8 μM, respectively) was not further inhibited by the addition of prazosin or clonidine at 0.1 μM concentrations. The lack of contribution of α1/α2-adrenoceptors to sites marked by [3H]mesulergine in the presence of raclopride and DOI was further substantiated in competition studies (n=3) that yielded pKi values lower than 4 for both prazosin and clonidine. In view of these results, drugs aimed to block the specific binding of [3H]mesulergine to α1/α2-adrenoceptors were not included in subsequent binding and autoradiographic assays.
[3H]mesulergine binding to 5-HT7 receptors in rat brain
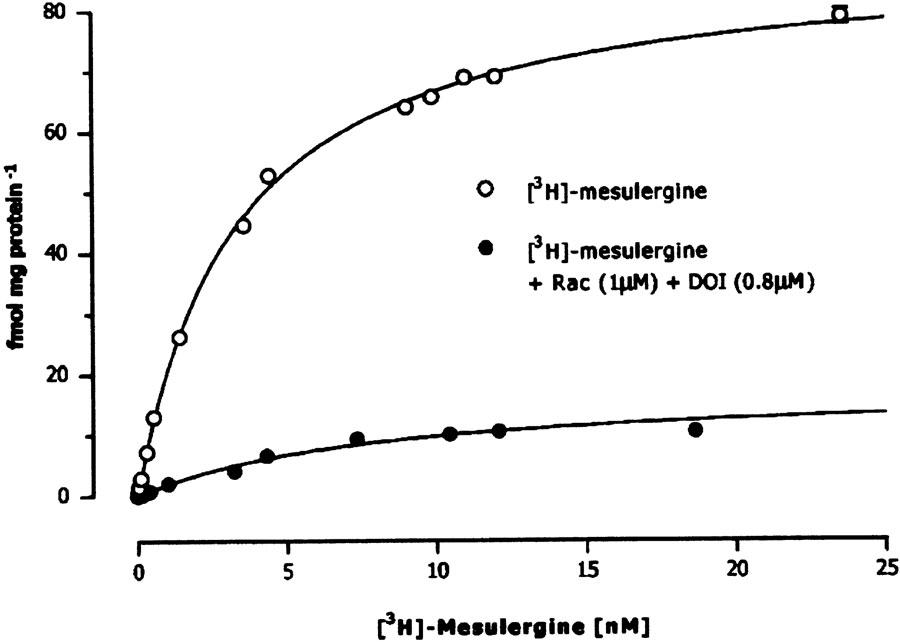
Saturation curves of [3H]mesulergine in rat frontal cortex membranes with and without the addition of raclopride and DOI are shown in Figure 1. The statistical analysis of saturation binding data favored a single site interaction with an estimated KD value of 8.1±0.1 nM and Bmax value of 17.9±1.2 fmol mg−1 (n=3) after the blockade of dopamine D2, 5-HT2A, and 5-HT2C receptors.
Figure 1.
Saturation curves of [3H]mesulergine binding in rat frontal cortex membranes in the absence and presence of raclopride and DOI. Data points show specific binding calculated by subtracting nonspecific binding (defined in the presence of 10 μM 5-HT) from total binding. Abscissa: concentration of [3H]mesulergine in nM; ordinate: fmol mg protein−1.
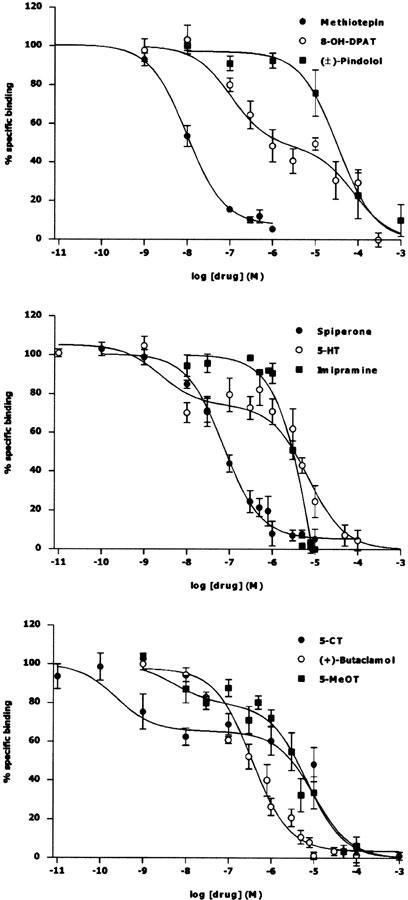
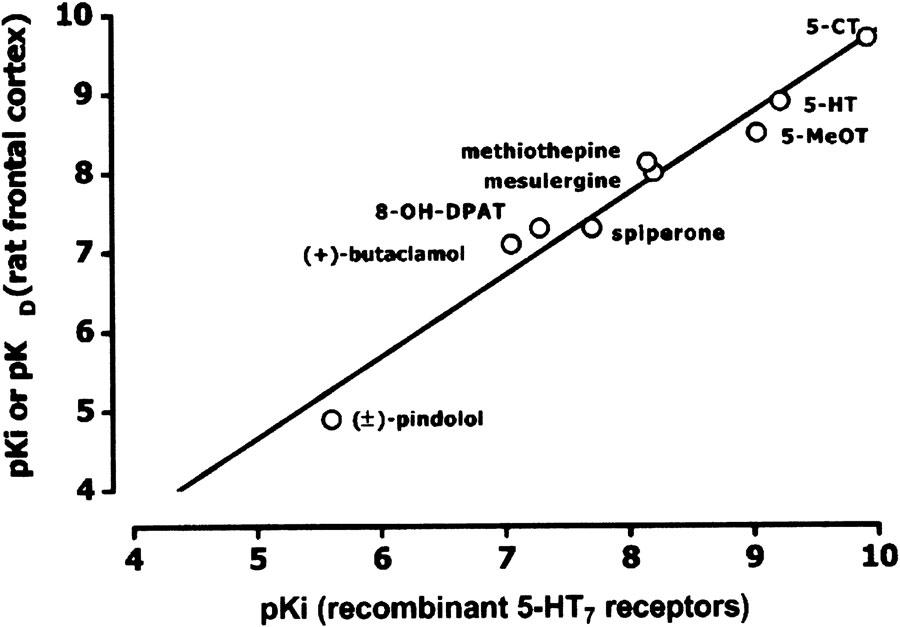
The pharmacological profile of [3H]mesulergine (10 nM) binding to rat frontal cortex membranes in the presence of raclopride and DOI was investigated using a range of competing drugs. Figure 2 shows the representative inhibition curves for displacement of [3H]mesulergine binding and Table 2 summarizes the affinity values. Some inhibiting drugs, namely 5-CT, 5-HT, 5-MeOT, and 8-OH-DPAT, competed for [3H]mesulergine-binding sites with a biphasic profile. In each case, binding to the high-affinity site constituted 22–52% of total specific receptor binding. When high-affinity pKi values for displacing drugs with a biphasic profile were considered, the rank order of potency of the displacing drugs was as follows: 5-CT>5-HT>5-MeOT⩾methiothepin>8-OH-DPAT, spiperone>butaclamol≫imipramine>(±)-pindolol>ondansetron, clonidine, and prazosin (Table 2). The correlation between pKi values obtained in rat frontal cortex membranes and previously published values for rat recombinant 5-HT7 receptors expressed in transfected cells was statistically significant (r2=0.965; P<0.001; Figure 3).
Figure 2.
Displacement of 10 nM [3H]mesulergine by selected compounds in rat frontal cortex membranes. Curves are representative of all compounds listed in Table 1, with the exception of ondansetron, prazosin, and clonidine, whose Ki values were higher than 0.1 mM (pKi1<4). All compounds displaced [3H]mesulergine in the presence of raclopride (1 μM) and DOI (0.8 μM) to the nonspecific level defined with 5-HT (10 μM). Abscissa: percent of specific binding in the absence of the displacer. Ordinate: −logarithm of molar concentrations of the displacing drug. Data points represent the mean±s.e.m. of at least three separate experiments, each performing duplicate determinations. Mean±s.e.m. of pKi values are listed in Table 2.
Table 2.
Comparison of drug effects on [3H]mesulergine binding in rat frontal cortex membranes
| Compound | pKi1 | % of site 1 | pKi2 | n |
|---|---|---|---|---|
| 5-CT | 9.7±0.30 | 35±7 | 5.1±0.2 | 6 |
| 5-HT | 8.9±0.02 | 30±4 | 5.4±0.1 | 3 |
| 5-MeOT | 8.5±0.07 | 22±5 | 5.6±0.2 | 3 |
| Methiothepin | 8.0±0.05 | 3 | ||
| 8-OH-DPAT | 7.3±0.13 | 52±9 | 4.4±0.2 | 3 |
| Spiperone | 7.3±0.15 | 3 | ||
| (+)-Butaclamol | 7.1±0.15 | 3 | ||
| Imipramine | 5.2±0.13 | 3 | ||
| (±)-Pindolol | 4.9±0.42 | 3 | ||
| Ondansetron | <4 | 3 | ||
| Clonidine | <4 | 3 | ||
| Prazosin | <4 | 3 |
High- and low-affinity values (pKi1 and pKi2; −log inhibition constant) and percentage for high-affinity sites are shown for those compounds displaying two-site competition. [3H]mesulergine binding in rat frontal cortex membranes was measured in the presence of raclopride (1 μM) and DOI (0.8 μM). Data are expressed as mean values±s.e.m. from at least three separate experiments (n), each performed using duplicate determinations.
Figure 3.
Correlation between binding affinity values (pKi) obtained in recombinant rat or mouse 5-HT7 receptors and expressed in transfected cells (Plassat et al., 1993; Ruat et al., 1993), and binding affinity values (pKi or pKD) obtained in rat frontal cortex membranes. High-affinity pKi values for drugs defining two-binding sites (see Table 2) were used in the analysis. Ondansetron, clonidine, prazosin, and imipramine were not included in the analysis because their exact pKi values were either not available from the literature or not accurately calculable. Plotted line represents the least-square linear regression y=1.03x−0.479; coefficient of determination (r2)=0.965; P<0.01.
Anatomical distribution of 5-HT7 receptors in the brain
The binding of [3H]mesulergine was specific and heterogeneous in all the three species studied (Figures 4, 5 and 6; Table 3). Following incubation with 10 nM [3H]mesulergine, in the presence of raclopride (10 μM) and DOI (0.8 μM), the specific-binding density transformed in Bmax, considering a KD value of 10 nM, ranged (in fmol mg−1 tissue) from 1.6 to 14.2, from 2.9 to 30.3, and from 3.8 to 61.5 in human, rat, and guinea-pig brain areas, respectively.
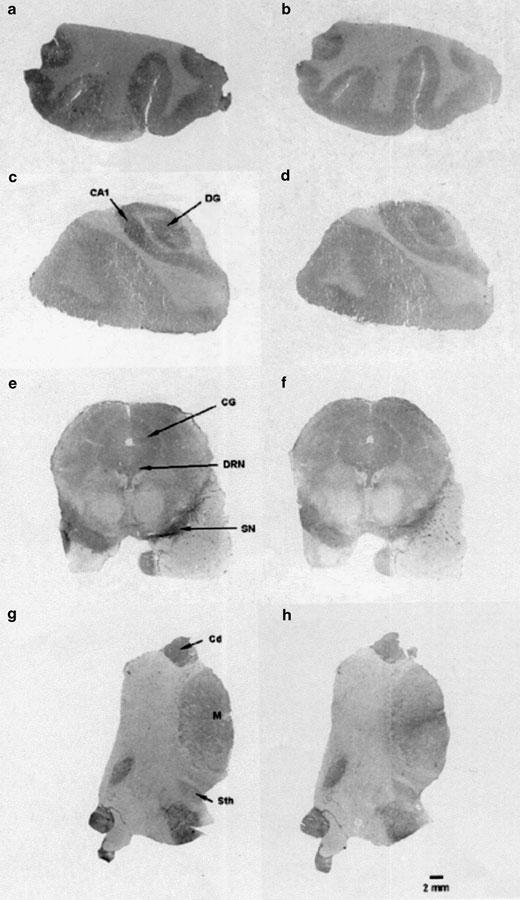
Figure 4.
Visualization of 5-HT7 receptors in the human brain with [3H]mesulergine. Autoradiograms at four coronal section levels, frontal cortex (a, b), hippocampus (c, d), mesencephalum (e, f), and thalamus (g, h). Binding observed with 10 nM of [3H]mesulergine in the presence of raclopride (1 μM) and DOI (0.8 μM) is depicted in (a, c, e, g). Nonspecific binding obtained in the presence of 10 μM of 5-HT is depicted in (b, d, f, h). Prominent binding remained in thalamic nuclei and mesencephalic nuclei, and moderate levels were detected in the cortical regions, hippocampus, and central gray. Abbreviations: Field 1 of Ammon's horn, CA1; caudate nucleus, Cd; central gray, CG; dentate gyrus, DG; dorsal raphe nucleus, DRN; medial thalamic nucleus, M; substantia nigra, SN; subthalamic nucleus, Sth.
Figure 5.
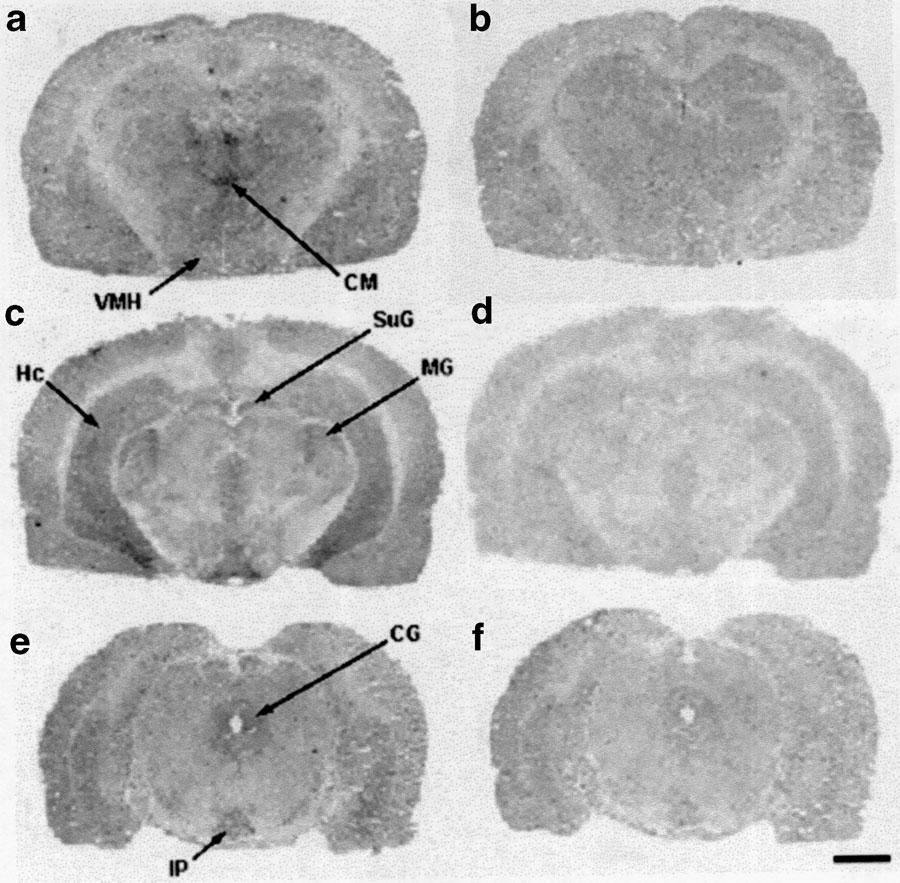
Rostro-caudal distribution of [3H]mesulergine-binding sites in rat brain by receptor autoradiography. Digitized pictures obtained from film autoradiograms at three coronal section levels: anterior thalamus and hypothalamus (a, b); hippocampus and posterior thalamus (c, d); mesencephalum (e, f). Binding observed with 10 nM of [3H]mesulergine in the presence of raclopride (1 μM) and DOI (0.8 μM) is shown in (a, c, e). Nonspecific binding defined with 10 μM of 5-HT is shown in (b, d, f). High levels of binding were found in thalamic and hypothalamic nuclei, moderate levels in the hippocampus and mesencephalum and low levels in the cortex layers. Abbreviations: central (periaqueductal) gray, CG; centromedial nucleus of the thalamus, CM; hippocampus, Hc; interpeduncular nucleus, IP; medial geniculate nucleus, MG; superficial gray layer of the superior colliculus, SuG; ventromedial nucleus of the hypothalamus, VMH. Bar=2 mm.
Figure 6.
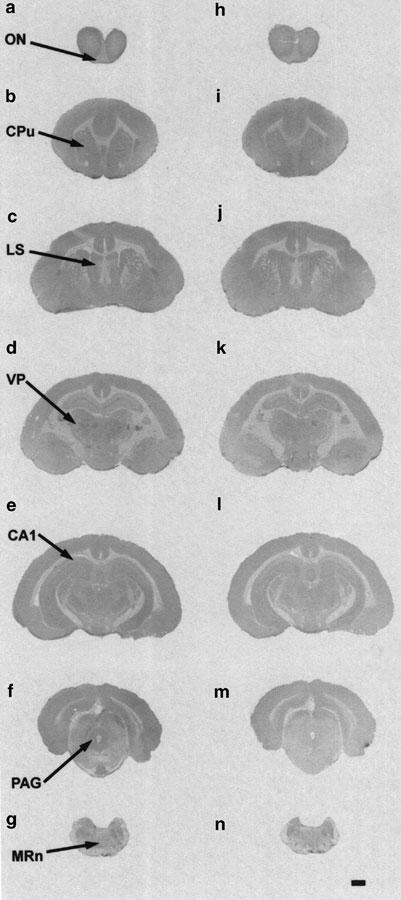
Rostro-caudal distribution of [3H]mesulergine-binding sites in guinea-pig brain by receptor autoradiography. Digitized pictures obtained from film autoradiograms at seven coronal section levels: olfactory bulb (a, h); anterior basal ganglia (b, i); posterior basal ganglia and septum (c, j); anterior thalamus and hypothalamus (d, k); hippocampus and posterior thalamus (e, l); mesencephalum (f, m); pons (g, n). Binding observed with 10 nM of [3H]mesulergine in the presence of raclopride (1 μM) and DOI (0.8 μM) is shown in (a–g). Nonspecific binding defined with 10 μM of 5-HT is shown in (h–n). High levels of binding were found in thalamic and hypothalamic nuclei, moderate levels in the hippocampus and mesencephalum, and low levels in cortex layers. Abbreviations: Field 1 of Ammon's horn, CA1; caudate putamen, Cpu; lateral septal nucleus, LS; olfactory nerve layer, ON; periaqueductal gray, PAG; medullary raphe nuclei, MRn; ventral postero-thalamic nuclei, VP. Bar=2 mm.
Table 3.
Autoradiographic distribution of [3H]mesulergine-binding sites corresponding to 5-HT7 receptors in human, rat and guinea-pig brain
| Specific binding* in fmol mg tissue−1 (n) | |||
|---|---|---|---|
| Human | Rat | Guinea-pig | |
| Olfactory bulb | |||
| Glomerular and external plexiform layers | 10.8±2.1 (6) | ||
| Cortex and claustrum | |||
| Frontal, outer layers (I–IV) | 2.1±0.4 (9) | 10.6±2.3 (4) | |
| Frontal, inner layers | 5.4±0.9 (11) | 7.7±2.8 (4) | |
| Frontoparietal, layers I–II | 8.5±1.1 (5) | ||
| Frontoparietal, layer III | 6.4±1.1 (5) | ||
| Frontoparietal, layer IV | 5.9±0.6 (5) | ||
| Frontoparietal, layer V | 5.7±1.2 (5) | ||
| Frontoparietal, layer VI | 2.9±1.7 (5) | ||
| Temporal, layers I–III | 7.2±2.2 (5) | 4.0±0.9 (5)a | |
| Temporal, layer IV | 7.5±1.3 (5) | ||
| Temporal, layer V | 6.8±1.3 (5) | 4.3±0.9 (5) | |
| Temporal, layer VI | 6.6±1.7 (5) | ||
| Occipital | 7.6±1.4 (6) | ||
| Entorhinal, layers I–III | 4.3±2.0 (5) | 11.1±2.3 (4) | 8.5±3.0 (4) |
| Entorhinal, layers IV–VI | 7.5±2.8 (5) | ||
| Primary olfactory | 17.2±2.5 (5) | ||
| Claustrum | 1.6±0.5 (5) | 10.3±1.1 (4) | 15.2±2.0 (7) |
| Basal ganglia and olfactory tubercle | |||
| Nucleus accumbens | 6.8±2.5 (6) | 8.6±1.6 (6) | |
| Internal capsule | 1.7±0.7 (6) | ||
| Caudate nucleus | 9.7±1.9 (11) | 7.1±1.8 (5)b | 9.0±2.9 (4)b |
| Putamen nucleus | 9.4±2.0 (9) | ||
| Globus pallidus | 7.2±1.7 (5) | 8.8±2.1 (6) | |
| Olfactory tubercle | 9.3±1.4 (4) | 42.9±10.7 (7) | |
| Hippocampus and septum | |||
| Field 1 of Ammon's horn, oriens layer | 4.0±1.6 (6) | 11.1±2.5 (5)c | 7.0±2.2 (7)c |
| Field 1 of Ammon's horn, pyramidal layer | 5.6±2.6 (6) | ||
| Field 1 of Ammon's horn, radial layer | 4.9±1.9 (7) | ||
| Field 2 of Ammon's horn, oriens layer | 3.6±2.0 (6) | 11.8±2.0 (5)c | 8.6±1.7 (5)c |
| Field 2 of Ammon's horn, pyramidal layer | 7.3±2.2 (7) | ||
| Field 2 of Ammon's horn, radial lacunosum-moleculare layer | 5.1±2.2 (8) | ||
| Field 3 of Ammon's horn | 13.1±1.8 (5) | ||
| Dentate gyrus, molecular layer | 6.6±2.1 (8) | 8.9±1.5 (5) | 13.8±2.4 (7) |
| Parasubiculum | 2.3±1.0 (7) | ||
| Presubiculum | 4.3±2.0 (6) | ||
| Subiculum dorsalis | 4.1±1.6 (8) | 8.2±0.8 (5) | |
| Subiculum ventralis | 25.6±2.6 (5) | 29.5±4.4 (4) | |
| Medial septal nucleus | 9.7±1.4 (7) | ||
| Lateral septal nucleus | 10.5±1.1 (4) | 6.6±2.0 (6) | |
| Thalamus | |||
| Anterior thalamic nucleus | 4.2±2.4 (6) | ||
| Reuniens nucleus | 25.3±2.7 (5) | 20.7±3.1 (6) | |
| Paratenial | 13.4±2.8 (6) | ||
| Paraventricular nucleus, posterior part | 30.3±5.2 (5) | 16.0±3.3 (7) | |
| Medial thalamic nucleus | 5.6±1.6 (5) | 14.0±3.0 (5)d | 17.4±2.1 (7)d |
| Centromedial nucleus | 11.6±0.6 (3) | 28.4±1.4 (5) | 17.5±2.7 (7) |
| Ventral anterior nucleus | 6.3±1.8 (7) | ||
| Ventroposterior nucleus, lateral part | 6.9±1.0 (5) | 12.6±3.1 (7) | |
| Ventromedial nucleus | 3.1±1.1 (4) | 9.8±0.6 (5) | 14.9±1.0 (3) |
| Lateroanterior thalamic nucleus | 2.2±1.1 (3) | ||
| Laterodorsal, ventrolateral | 13.2±2.3 (5) | 9.1±1.1 (6) | |
| Laterodorsal, dorsomedial part | 8.1±1.0 (6) | ||
| Posterior nuclear group | 11.7±1.8 (5) | 12.9±2.0 (6) | |
| Reticularis nucleus | 3.3±2.0 (5) | 11.7±3.2 (5) | |
| Subthalamic nucleus | 6.7±3.9 (4) | ||
| Anterior pretectal area | 8.3±1.6 (5) | 16.1±1.2 (4) | |
| Medial geniculate nucleus | 12.2±1.7 (5) | ||
| Ventrolateral geniculate nucleus | 15.0±3.2 (4) | ||
| Hypothalamus | |||
| Arcuate nucleus | 14.0±0.9 (5) | 15.4±2.5 (5) | |
| Anterior hypothalamic area | 13.2±1.4 (5) | ||
| Lateral area | 10.1±1.1 (5) | ||
| Medial preoptic area | 14.1±2.3 (5) | 9.3±2.1 (4) | |
| Ventromedial nucleus | 11.5±1.7 (5) | ||
| Dorsomedial nucleus | 14.9±1.6 (5) | ||
| Posterior hypothalamic area | 32.9±18.6 (5) | ||
| Mammillary nucleus | 56.9±16.0 (6) | ||
| Suprachiasmatic nucleus | 17.6±2.1 (4) | ||
| Midbrain, pons, and medulla | |||
| Superficial gray layer of the superior colliculus | 6.6±1.6 (4) | 17.8±1.6 (5) | 9.4±1.6 (6) |
| Optic nerve layer of the superior colliculus | 12.2±1.1 (5) | 7.1±1.5 (6) | |
| Intermediate gray layer of the superior colliculus | 12.8±2.2 (5) | 10.8±1.6 (6) | |
| Central (periaqueductal) gray | 7.9±1.7 (6) | 14.9±0.7 (5) | 17.0±2.8 (5) |
| Deep mesencephalic nucleus | 7.4±1.3 (4) | 11.4±1.2 (7) | |
| Caudate linearis nucleus | 6.3±2.0 (5) | ||
| Dorsal raphe nucleus | 9.7±3.3 (7) | 14.2±2.8 (4) | 16.9±3.3 (5) |
| Median raphe nucleus | 3.7±2.4 (3) | ||
| Medial lemniscus | 15.6±2.6 (5) | ||
| Red nucleus | 7.7±3.1 (6) | 9.2±2.0 (4) | |
| Interpeduncular nucleus | 15.0±3.2 (5) | 26.6±5.7 (7) | |
| Pontine nucleus | 3.8±0.4 (4) | ||
| Substantia nigra | 14.2±4.2 (7) | 5.4±1.5 (5) | 13.1±1.5 (6) |
Specific binding of 10 nm [3H]mesulergine in the presence of 1 μM raclopride and 0.8 μm DOI transformed in Bmax considering a KD value of 10 nm.
Outer layers.
Caudate-putamen.
All layers of the hippocampal field.
Mediodorsal nucleus.
In the human brain, areas with mean densities of 5-HT7 receptors (in fmol mg tissue−1) were arbitrarily considered to have low density (<3.8), intermediate density (⩾3.8<7.1) or high density (⩾7.1).
For rat brain structures, areas containing densities below 7.3 fmol mg tissue−1 were considered as ‘low receptor density' areas, while those with mean values between 7.3 and 14, or higher than 14, were reported to contain intermediate or high densities, respectively. Finally, guinea-pig brain areas with mean densities of 5-HT7 receptors were arbitrarily considered as having low density (<8.6), intermediate density (⩾8.6<16.5) or high density (⩾16.5).
The different cortical regions contained intermediate to low levels of 5-HT7 receptors in all the three species analyzed (Figures 4, 5 and 6; Table 3). In the human brain, intermediate densities of autoradiographic grains were observed in the inner layers (V–VI) of the frontal and entorhinal cortex, while low concentrations were found in the outer layers (I–IV) of the frontal cortex. The rat and guinea-pig cortex also contained intermediate densities of 5-HT7 receptors, the outer layers being richer than the inner ones. An exception to that was the primary olfactory cortex, which showed high concentrations of autoradiographic grains. Regarding the claustrum, the level of labeling ranged from intermediate (rat and guinea-pig) to low (human).
In the human basal ganglia, high concentrations of 5-HT7 receptors were present in the caudate and putamen nuclei, while moderate and low concentrations were found in the nucleus accumbens and the internal capsule, respectively. Receptor densities were lower in the other species analyzed, being in the low range over the rat caudate-putamen and globus pallidus and showing intemediate levels in the guinea-pig basal ganglia (Figures 5, 6; Table 3).
In the hippocampal formation of the human brain (Figure 4; Table 3), concentrations of 5-HT7 receptors in all layers of the field 1 of Ammon's horn (CA1), dentate gyrus, presubiculum, and subiculum were in the intermediate range. In the field 2 of Ammon's horn (CA2), high concentrations were present in the pyramidal layer and intermediate concentrations in the oriens and radial lacunosum-molecular layers. In a similar way, the rat hippocampus (Figure 5; Table 3) was moderately rich in receptors: all the different fields and layers contained intermediate densities of specific binding, with the exception of the ventral subiculum, where high concentrations of receptors were found. In contrast, the distribution of 5-HT7 receptors throughout the guinea-pig hippocampus was heterogenous (Figure 6 and Table 3) and the densities were found to be low in the field CA1, intermediate in the field CA2 and dentate gyrus, and high in the subiculum ventralis.
The thalamus was relatively rich in 5-HT7 receptors (Figures 4, 5 and 6; Table 3). The nuclei of the paraventricular (paraventricular, reuniens) and intralamellar (centromedial) formations contained high or very high levels of specific binding in the three species analyzed. Intermediate to high densities of autoradiographic grains were found over the medial and anterior nuclei. The different nuclei of the ventroposterior and laterodorsal groups presented levels of 5-HT7 receptors ranging from intermediate (rat, guinea-pig) to low (human). The autoradiographic densities over the human subthalamic nucleus and the geniculate nuclei (rat and guinea-pig) were in the intermediate range.
Similar to the thalamus, the hypothalamic nuclei contained intermediate to high densities of 5-HT7 receptors in both rat and guinea-pig brain (Figures 5, 6; Table 3). The dorsomedial, arcuate, and mamillary nuclei, as well as the posterior hypothalamic area, were among the brain areas with higher densities of autoradiographic grains. The rest of the examined hypothalamic nuclei/areas presented intermediate concentrations of 5-HT7 receptors.
Midbrain structures presenting high concentrations of 5-HT7 receptors in rat, guinea-pig, and human brain include the central gray and the dorsal raphe nucleus, while moderate receptor contents characterized the layers of the superior colliculus, the densities being higher in the rat brain. In contrast, the densities of autoradiographic grains in the substantia nigra varied upon the species analyzed, ranging from high (human) to intermediate (guinea-pig) and low (rat). Other areas with intermediate densities of 5-HT7 receptors included the interpeduncular and red nuclei (Figures 4, 5 and 6; Table 3).
Discussion
Although [3H]5-CT has been frequently used in the identification of 5-HT7 receptors, several results strongly suggest that this compound is not good enough to label this receptor (Castro et al., 1997; Bonaventure et al., 2002; see Introduction). The distribution of 5-HT7 receptors in the mouse brain has also been performed with [3H]8-OH-DPAT, but only in 5-HT1A knockout animals (Bonaventure et al., 2002). In addition, attempts to perform autoradiographic studies with the specific 5-HT7 antagonist [3H]SB-269970 have failed up to date. In the present study, we took advantage of nonselective pharmacological tools and the pharmacological profile of the 5-HT7 receptor-binding sites, in order to map for the first time the anatomical distribution of 5-HT7 receptors in human brain tissue sections by using [3H]mesulergine. In addition, the anatomical localization of this receptor in rat and guinea-pig brain tissue sections was revisited.
Receptor characterization
In view of the current unavailability of a selective and specific radioligand for 5-HT7 receptors, suitable for autoradiographic studies, the rank order of agonist and antagonist affinity was used to characterize the 5-HT7-binding site labeled with [3H]mesulergine. Mesulergine has an affinity for a number of receptors other than the 5-HT7, such as 5-HT2A, 5-HT2C, dopamine D2, and α1/α2-adrenoceptors (Closse, 1983; Rinne, 1983; Pazos et al., 1985; Hoyer et al., 1994). Raclopride and DOI were added at concentrations that would occupy 90% of their targeted receptor populations without inhibiting the binding of [3H]mesulergine to the 5-HT7 receptor (see Methods section). Under these assay conditions, saturation studies in rat brain rendered a pKD value of 8.1±0.1 for [3H]mesulergine, which is in agreement with previously reported pKi values at the 5-HT7 receptor (Hoyer et al., 1994; Thomas et al., 2002). The rank order of affinities herein reported from the inhibition of [3H]mesulergine binding to rat brain homogenates is in agreement with previous findings in cloned 5-HT7 receptors expressed in cell lines (Ruat et al., 1993; Thomas et al., 2002), as well as in guinea-pig brain homogenates following radiolabeling with [3H]-SB-269970, a recently developed selective 5-HT7 receptor antagonist (Thomas et al., 2002): 5-CT (pKi=9.7), 5-HT (8.9), 5-MeOT (8.5), methiothepin (8.0), 8-OH-DPAT (7.3), spiperone (7.3), (+)-butaclamol (7.1), and pindolol (4.9). The 5-HT7 agonists 5-CT, 5-HT, 5-MeOT, and 8-OH-DPAT displayed two-site displacement binding, which is not unusual, since agonists recognize both high- and low-affinity states of the receptor (Kenakin, 1984).
Mesulergine may bind to nonserotonergic receptors. [3H]mesulergine binding to dopamine D2 receptors was ruled out by the addition of raclopride as a masking drug, and by the use of specific agonists for serotonin receptors such as 5-CT, 5-HT, and 5-MeOT. In addition, mesulergine binding to α1/2-adrenoceptors was ruled out based on: (a) the lack of effect of prazosin or clonidine at 0.1 μM concentrations in inhibiting the specific binding of [3H]mesulergine in saturation studies, and (b) the micromolar affinities found for both clonidine and prazosin (see Table 2), while these compounds have nanomolar affinity at α1/α2-adrenoceptors (Rinne, 1983; Hoyer et al., 1994; Wood et al., 2000).
Mesulergine may also bind to serotonergic receptors other than the 5-HT7. The binding site identified in this study is unlikely to be of the 5-HT1A subclass, since pindolol has a nanomolar affinity for 5-HT1A (Ruat et al., 1993; Hoyer et al., 1994; Gustafson et al., 1996), while a micromolar affinity, consistent with 5-HT7 receptors, has been found in the present study. The 5-HT1B receptor was excluded, since it reportedly has shown higher affinity for pindolol but lower affinity for 8-OH-DPAT and spiperone than the one found in this study (Ruat et al., 1993; Hoyer et al., 1994; Gustafson et al., 1996). The contribution of 5-ht1E and 5-ht1F receptors is also unlikely as both 5-CT and methiothepin were found to have nanomolar affinity, while weaker affinities have been reported for these both compounds (Hoyer et al., 1994).
The receptor labeled with [3H]mesulergine is also unlikely to be 5-HT2A and 5-HT2C. First, these receptors were excluded by adding DOI. Second, the affinity of 5-CT for these subtypes is in the nanomolar range, while this drug showed a nanomolar affinity in this study (Hoyer et al., 1994). Finally, the affinity of 8-OH-DPAT was found to be much higher than previously reported values at 5-HT2A and 5-HT2C (Hoyer et al., 1994).
The affinities of 5-CT, 5-MeOT, and 8-OH-DPAT found in this study are not consistent with an involvement of 5-HT3 receptors, where these compounds are inactive (Hoyer et al., 1994). In addition, ondansetron was found to show a micromolar affinity in this study, while it reportedly has nanomolar affinity at the 5-HT3 receptor site (Hoyer et al., 1994).The use of a nanomolar concentration of [3H]mesulergine in the present study excludes the involvement of 5-HT4, 5-ht5, and 5-ht6 receptors, since mesulergine is inactive at 5-HT4 receptors and has a micromolar affinity at 5-ht5 and 5-ht6 receptors (Bard et al., 1993; Plassat et al., 1993; Hoyer et al., 1994). The affinity of 5-CT found at the identified site also argues against the involvement of those receptors (Bard et al., 1993; Hoyer et al., 1994). In addition, spiperone, which has shown high affinity under the conditions of the present study, has a micromolar affinity at 5-HT5A and does not recognize 5-HT5B receptors (Hoyer et al., 1994).
Albeit 5-CT has a high affinity at the atypical 5-HT site, probably related in some way to the 5-HT1A subclass, reported by Castro et al. (1997), it is unlikely to be detected here, considering the low affinity reported for mesulergine at this orphan site (Castro et al., 1997).
In conclusion, [3H]mesulergine, in the presence of DOI and raclopride, identifies a population of binding sites with pharmacological characteristics consistent with those of the 5-HT7 receptors. Therefore, this radioligand is a suitable tracer for autoradiographic mapping studies of 5-HT7 receptors in brain sections.
Distribution of 5-HT7 receptors in the human with [3H]mesulergine
To the best of our knowledge, in this study, we present for the first time a detailed anatomical distribution of 5-HT7 receptors in the human brain. The pattern of distribution of 5-HT7 receptors throughout the human brain was heterogeneous. Among other brain regions, high densities of 5-HT7 receptors were found over the CA2 field of the hippocampus, the caudate and putamen nuclei, the centromedial thalamic nucleus, the dorsal raphe nucleus, and the substantia nigra. More moderate concentrations of 5-HT7 sites were seen in most of the thalamic nuclei studied, some hippocampal areas and inner layers of the frontal cortex. The distribution of 5-HT7 receptors in the human brain is in general agreement with the results reported with the PCR analysis of distribution of the 5-HT7 mRNA in human CNS tissues (Hagan et al., 2000). For instance, Hagan et al. (2000) found high expression levels of 5-HT7 receptor mRNA in the thalamus, substantia nigra, and hippocampus. When these data are compared to the present results, some minor discrepancies are evident. For instance, we found that the distribution of 5-HT7 receptors in the hippocampus is heteregeneous, with the distinct layers presenting from low to high densities of binding sites. These apparent discrepancies probably arise from the lack of resolution of Taqman analyses, and support the advantage of autoradiographic techniques to describe the detailed anatomical distribution of neurotransmitter receptors.
The localization of 5-HT7 receptors in the human brain correlates well with that found in rat and guinea-pig brain in the present work as well as in previous studies (To et al., 1995; Gustafson et al., 1996; Mengod et al., 1996; Kinsey et al., 2001; Neumaier et al., 2001). For instance, the centromedial thalamic nucleus, CA2 field of the hippocampus, caudate nucleus, and dorsal raphe nucleus were enriched in these receptors in all the three species. However, some minor differences were noted. The densities of binding sites were in the high range in the inner layers of the frontal cortex and substantia nigra in the human brain, while only low to intermediate levels were found in rat and guinea-pig brain. In addition, the human brain displays, in general terms, markedly lower 5-HT7 receptor densities when compared to rat brain, and even lower when compared to guinea-pig brain. This is consistent with the lower 5-HT7 expression levels measured in rat cortex (Thomas et al., 2002) when compared to those in guinea-pig cortex (Thomas et al., 2000), and the very low levels of 5-HT7 receptors in human cortex, which could not even be accurately quantified in radioligand-binding studies (Thomas et al., 2002). The relevance of this apparent species differences remains to be established and, to our knowledge, there have been no reports showing species differences in the function of brain 5-HT7 receptors.
Using [3H]mesulergine, we have also revisited the brain distribution of 5-HT7 receptor binding sites in rat and guinea-pig. The brain regional distribution of 5-HT7 protein receptor and mRNA has been previously investigated in guinea-pig and rat using autoradiography, in situ hybridization histochemistry, and immunocytochemistry (To et al., 1995; Waeber & Moskowitz, 1995; Gustafson et al., 1996; Mengod et al., 1996; Kinsey et al., 2001; Neumaier et al., 2001). The anatomical distribution of the [3H]mesulergine-binding sites observed in rat and guinea-pig matched the distribution of 5-HT7 receptor mRNA and immunoreactivity reported in the literature. For instance, within the hippocampal formation, the distribution described in this study, that is, strong labeling of CA3 and lower in CA1, high densities of 5-HT7 receptor-binding sites throughout the hypothalamus (including the suprachiasmatic nucleus) and thalamic nuclei, parallels the findings described using 5-HT7 receptor-specific antibodies (Neumaier et al., 2001). It is noteworthy to mention that we have found high densities of 5-HT7 receptor-binding sites within the dorsal raphe nucleus in the three species studied. Consistent with this, similar findings have been recently reported using [3H]8-OH-DPAT for labeling brain 5-HT7 receptors in 5-HT1A/AB double-knockout mice (Bonaventure et al., 2002). This receptor localization is suggestive of a possible autoreceptor role for the 5-HT7 receptor, although a recent study has been unable to provide evidence supporting this function (Roberts et al., 2001).
Functional implications of 5-HT7 distribution
This study provides a comparative mapping analysis of the 5-HT7 receptor in human, guinea-pig, and rat brain. Our results concur with previously published localization data where available, and expand to human previous suggestions of the possible roles of this receptor.
Previous reports have suggested a role for 5-HT7 receptors in learning and memory (Meneses, 1999; Meneses & Terron, 2001). The presence of 5-HT7 receptors in the hippocampus and the entorhinal cortex, the major input to the hippocampus, supports a potential involvement of 5-HT7 receptors in this behavior.
The localization of 5-HT7 receptor in limbic areas suggests a role for this receptor in affective behavior. Several reports have suggested that the 5-HT7 receptor is implicated in the mechanisms of action of drug treatments for affective disorders (Sleight et al., 1995; Stowe & Barnes, 1998a; Mullins et al., 1999; Simmen et al., 1999). Several atypical antipsychotics also bind to 5-HT7 receptors with high affinity (Roth et al., 1994). It has been reported that chronic antidepressant treatment downregulates 5-HT7-mediated responses and receptor density in limbic areas (Sleight et al., 1995; Mullins et al., 1999). It is interesting to speculate that, since this receptor is affected by chronic treatment with antidepressants, it may have some role in the pathophysiology of some forms of depression. Studies are in progress in our laboratory to determine whether the level of expression of 5-HT7 receptors in the brain of patients diagnosed with major depression is altered.
Several reports have suggested that 5-HT7 receptors may be involved in circadian function (Lovenberg et al., 1993; Kawahara et al., 1994; Ying & Rusak, 1997; Mullins et al., 1999; Yu et al., 2001). 5-HT7 mRNA has been detected in the suprachiasmatic nucleus – the primary circadian clock in the mammalian brain – in some (Heidmann et al., 1998; Stowe & Barnes, 1998b; Neumaier et al., 2001) but not all studies (To et al., 1995; Gustafson et al., 1996; Kinsey et al., 2001). The strong labeling in the guinea-pig suprachiasmatic nucleus found in the present study parallels the findings described by immunocytochemistry in the rat (Neumaier et al., 2001) and by autoradiographic techniques in the mouse (Bonaventure et al., 2002), supporting an involvement of this receptor in circadian function. In addition, nuclei throughout the hypothalamus of rat and guinea-pig, other than the suprachiasmatic nucleus, were found to have high to moderate density of 5-HT7 receptors, suggesting a role for 5-HT7 in the control of neuroendocrine and homeostatic functions. In this regard, the presence of these receptors over the preoptic area support the recent findings of the hypothermic effects linked to activation of these sites in wild-type, but not in 5-HT7 knockout mice (Hedlund et al., 2003).
Together with other slow acting neurotransmitters, serotonin has been proposed to control the state of activity and excitability of thalamic neurons and thereby modulate the state of thalamocortical activity (McCormick, 1992). 5-HT7 receptors were densely distributed in several thalamic nuclei (i.e., intralaminar nuclei). Thus, these receptors may play a key role in mediating serotonin responses in thalamic neurons. In line with this idea, very recent functional studies have reported that the activation of 5-HT7 receptors strongly modulates the excitability of these neurons (Chapin & Andrade, 2001; Goaillard & Vincent, 2002). The presence of these receptors in different areas of the hippocampus, thalamus, hypothalamus, and brainstem could be related to their role in controlling sleep activity (Thomas et al., 2003).
In summary, after establishing appropriate labeling conditions for 5-HT7 receptors with [3H]mesulergine, in combination with masking drugs, we have been able to map for the first time the anatomical distribution of 5-HT7 receptors in the human brain using autoradiographic approaches, comparing it with that observed in guinea-pig and rat brain. The present results can be of interest from the point of view of identifying possible central therapeutic targets through the pharmacological manipulation of 5-HT7 receptors.
Acknowledgments
This study was supported by a grant from the Spanish Ministry of Science and Technology (CICYT) SAF98-0064-C02-01. FJM-C held a junior research position by the Spanish Ministry of Science and Technology. We thank M.J. Castillo and L. Lanza for their helpful technical assistance.
Abbreviations
- 5-CT
5-carboxytryptamine
- DOI
1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane
- 5-MeOT
5-methoxytryptamine
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetraline
- SB-269970
(R)-3-(2-(2–(4-methylpiperidin-1-yl) ethyl)pyrrolidine-1-sulfonyl)-phenol)
References
- BACON W.L., BECK S.G. 5-Hydroxytryptamine7 receptor activation decreases slow afterhyperpolarization amplitude in CA3 hippocampal pyramidal cells. J. Pharmacol. Exp. Ther. 2000;294:672–679. [PubMed] [Google Scholar]
- BARD J.A., ZGOMBICK J., ADHAM N., VAYSSE P., BRANCHEK T., WEINSHANK R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- BARNES N.M., SHARP T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- BAUMGARTEN H.G., GOTHERT M. Serotoninergic Neurons and 5-HT Receptors in the CNS. Berlin, Heidelberg: Springer-Verlag; 1997. [Google Scholar]
- BONAVENTURE P., NEPOMUCENO D., KWOK A., CHAI W., LANGLOIS X., HEN R., STARK K., CARRUTHERS N., LOVENBERG T.W. Reconsideration of 5-hydroxytryptamine (5-HT7) receptor distribution using [3H]5-carboxamidotryptamine and [3H]8-hydroxy-2-(di-n-propylamino)tetraline: analysis in brain of 5-HT1A knockout and 5-HT1A/1B double-knockout mice. J. Pharmacol. Exp. Ther. 2002;302:240–248. doi: 10.1124/jpet.302.1.240. [DOI] [PubMed] [Google Scholar]
- BOURSON A., KAPPS V., ZWEINGELSTEIN C., RUDLER A., BOESS F.G., SLEIGHT A.J. Correlation between 5-HT7 receptor affinity and protection against sound-induced seizures in DBA/2J mice. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:820–826. doi: 10.1007/pl00005123. [DOI] [PubMed] [Google Scholar]
- CASTRO M.E., ROMON T., CASTILLO M.J., Del OLMO E., PAZOS A., DEL ARCO C. Identification and characterization of a new serotonergic recognition site with high affinity for 5-carboxamidotryptamine in mammalian brain. J. Neurochem. 1997;69:2123–2131. doi: 10.1046/j.1471-4159.1997.69052123.x. [DOI] [PubMed] [Google Scholar]
- CHAPIN E.M., ANDRADE R. A 5-HT7 receptor-mediated depolarization in the anterodorsal thalamus. II. Involvement of the hyperpolarization-activated current Ih. J. Pharmacol. Exp. Ther. 2001;297:403–409. [PubMed] [Google Scholar]
- CHENG Y.C., PRUSOFF W.H. Relationship between the inhibitor constant (Ki) and the concentration of inhibitor which causes 50% inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CLOSSE A. 3H]Mesulergine, a selective ligand for serotonin-2 receptors. Life Sci. 1983;32:2485–2493. doi: 10.1016/0024-3205(83)90375-2. [DOI] [PubMed] [Google Scholar]
- EGLEN R., JASPER J., CHANG D., MARTIN G. The 5-HT7 receptor: orphan found. Trends Pharmacol. Sci. 1997;18:104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- GILL C.H., SOFFIN E.M., HAGAN J.J., DAVIES C.H. 5-HT7 receptors modulate synchronized network activity in rat hippocampus. Neuropharmacology. 2002;42:82–92. doi: 10.1016/s0028-3908(01)00149-6. [DOI] [PubMed] [Google Scholar]
- GOAILLARD J.-M., VINCENT P. Serotonin suppresses the slow afterhyperpolarization in rat intralaminar and midline thalamic neurones by activating 5-HT7 receptors. J. Physiol. 2002;541:453–465. doi: 10.1113/jphysiol.2001.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUSTAFSON E., DURKIN M., BARD J., ZGOMBICK J., BRANCHEK T. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGAN J., PRICE G., JEFFREY P., DEEKS N., STEAN T., PIPER D., SMITH M., UPTON N., MEDHURST A., MIDDLEMISS D., RILEY G., LOVELL P., BROMIDGE S., THOMAS D. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDLUND P.B., DANIELSON P.E., THOMAS E.A., SLANINA K., CARSON M.J., SUTCLIFFE J.G. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIDMANN D.E.A., METCALF M.A., KOHEN R., HAMBLIN M.W. Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron–exon organization. J. Neurochem. 1997;68:1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- HEIDMANN D.E.A., SZOT P., KOHEN R., HAMBLIN M.W. Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology. 1998;37:1621–1632. doi: 10.1016/s0028-3908(98)00070-7. [DOI] [PubMed] [Google Scholar]
- HEMEDAH M., COUPAR I.M., MITCHELSON F.J. 3H]-Mesulergine labels 5-HT7 sites in rat brain and guinea-pig ileum but not rat jejunum. Br. J. Pharmacol. 1999;126:179–188. doi: 10.1038/sj.bjp.0702293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEMEDAH M., COUPAR I.M., MITCHELSON F.J. Characterisation of a 5-HT7 binding site in mouse ileum. Eur. J. Pharmacol. 2000;387:265–272. doi: 10.1016/s0014-2999(99)00797-9. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P.A. International union of pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- JASPER J.R., KOSAKA A., TO Z.P., CHANG D.J., EGLEN R.M. Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7b) Br. J. Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAHARA F., SAITO H., KATSUKI H. Inhibition by 5-HT7 receptor stimulation of GABAA receptor-activated current in cultured rat suprachiasmatic neurones. J. Physiol. 1994;478:67–73. doi: 10.1113/jphysiol.1994.sp020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENAKIN T.P. The classification of drugs and drug receptors in isolated tissues. Pharmacol. Rev. 1984;36:165–222. [PubMed] [Google Scholar]
- KENAKIN T.P. Pharmacologic Analysis of Drug–Receptor Interaction. New York: Lippincot, Williams and Wilkins; 1997. [Google Scholar]
- KINSEY A.M., WAINWRIGHT A., HEAVENS R., SIRINATHSINGHJI D.J.S., OLIVER K.R. Distribution of 5-ht5A, 5-ht5B, 5-ht6 and 5-ht7 receptor mRNAs in the rat brain. Mol. Brain Res. 2001;2001:194–198. doi: 10.1016/s0169-328x(01)00034-1. [DOI] [PubMed] [Google Scholar]
- KROBERT K.A., BACH T., SYVERSVEEN T., KVINGEDAL A.M., LEVY F.O. The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- LOVENBERG T.W., BARON B.M., LECEA L., MILLER J.D., PROSSER R.A., REA M.A., FOYE P.E., RACKE M., SLONE A.L., SIEGEL B.W., DANIELSON P.E., SUTCLIFFE J.G., ERLANDER M.G. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.N., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- LUPARELLO T.J. Stereotaxic Atlas of the Forebrain of the Guinea Pig. Basel: Karger; 1967. [Google Scholar]
- MARTÍN-CORA F.J., PAZOS A. Characterization and distribution of 5-HT7 sites in human brain using [3H]mesulergine. Soc. Neurosci. Abstr. 2000;26:2116. [Google Scholar]
- MCCORMICK D.A. Neurotransmitter actions in the thalamus and cerebral cortex. J. Clin. Neurophysiol. 1992;9:212–223. doi: 10.1097/00004691-199204010-00004. [DOI] [PubMed] [Google Scholar]
- MENESES A. 5-HT system and cognition. Neurosci. Biobehav. Rev. 1999;23:1111–1125. doi: 10.1016/s0149-7634(99)00067-6. [DOI] [PubMed] [Google Scholar]
- MENESES A., TERRON J.A. Role of 5-HT1A and 5-HT7 receptors in the facilitatory response induced by 8-OH-DPAT on learning consolidation. Behav. Brain Res. 2001;21:21–28. doi: 10.1016/s0166-4328(00)00378-8. [DOI] [PubMed] [Google Scholar]
- MENGOD G., VILARO M.T., RAURICH A., LOPEZ-GIMENEZ J.F., CORTES R., PALACIOS J.M. 5-HT receptors in mammalian brain: receptor autoradiography and in situ hybridization studies of new ligands and newly identified receptors. Histochem. J. 1996;28:747–758. doi: 10.1007/BF02272148. [DOI] [PubMed] [Google Scholar]
- MOTULSKY H.J. Analyzing Data with GraphPad Prism. San Diego: GraphPad Software Inc; 1999. [Google Scholar]
- MULLINS U.L., GIANUTSOS G., EISON A.S. Effects of antidepressants on 5-HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacology. 1999;21:352–367. doi: 10.1016/S0893-133X(99)00041-X. [DOI] [PubMed] [Google Scholar]
- NELSON C.S., CONE R.D., ROBBINS L.S., ALLEN C.N., ADELMAN J.P. Cloning and expression of a 5HT7 receptor from Xenopus laevis. Receptors Channels. 1995;13:61–70. [PubMed] [Google Scholar]
- NEUMAIER J.F., SEXTON T.J., YRACHETA J., DIAZ A.M., BROWNFIELD M. Localization of 5-HT7 receptors in rat brain by immunocytochemistry, in situ hybridization, and agonist stimulated cFos expression. J. Chem. Neuroanat. 2001;21:63–73. doi: 10.1016/s0891-0618(00)00092-2. [DOI] [PubMed] [Google Scholar]
- PAXINOS G. The Human Nervous System. New York: Academic Press; 1990. [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1982. [Google Scholar]
- PAZOS A., HOYER D., PALACIOS J.-M. The binding of serotonergic ligands to the porcine choroid plexus: characterization of a new type of serotonin recognition site. Eur. J. Pharmacol. 1984;106:539–546. doi: 10.1016/0014-2999(84)90057-8. [DOI] [PubMed] [Google Scholar]
- PAZOS A., HOYER D., PALACIOS J.M. Mesulergine, a selective serotonin-2 receptor ligand in the rat cortex, does not label these receptors in porcine and human cortex: evidence for species differences in brain serotonin-2 receptors. Eur. J. Pharmacol. 1985;106:531–538. doi: 10.1016/0014-2999(84)90056-6. [DOI] [PubMed] [Google Scholar]
- PLASSAT J.-L., AMLAIKY N., HEN R. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. J. Pharmacol. Exp. Ther. 1993;44:229–236. [PubMed] [Google Scholar]
- RINNE U.K.New ergot derivatives in the treatment of Parkinson's disease Lisuride and Other Dopamine Agonists 1983New York: Raven Press; 431–442.Caine D.B. et al. (eds). pp [Google Scholar]
- ROBERTS C., ALLEN L., LANGMEAD C.J., HAGAN J.J., MIDDLEMISS D.N., PRICE G.W. The effect of SB-269970, a 5-HT(7) receptor antagonist, on 5-HT release from serotonergic terminals and cell bodies, Br. Br. J. Pharmacol. 2001;132:1574–1580. doi: 10.1038/sj.bjp.0703979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH B.l., CRAIGO S.C., CHOUDHARY M.S., ULUER A., MONSMA F.J., SHEN Y., MELTZER H.Y., SIBLEY D.R. Binding of typycal and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., LEURS R., TARDIVEL-LACOMBE J., DIAZ J., ARRANG J.-M., SCHWARTZ J.-C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN Y., MONSMA F.J.J., METCALF M.A., JOSEL P.A., HAMBLIN M.W., SIBLEY D.R. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- SIMMEN U., BURKARD W., BERGER K., SCHAFFENER W., LUNDSTROM K. Extracts and constituents of Hypericum perforatum inhibit the binding of various ligands to recombinant receptors expressed with the semliki forest virus system. J. Recept. Signal Transduct. Res. 1999;19:59–74. doi: 10.3109/10799899909036637. [DOI] [PubMed] [Google Scholar]
- SLEIGHT A.J., CAROLO C., PETIT N., ZWINGELSTEIN C., BOURSON A. Identification of 5-hydroxytryptamine7 receptor binding sites in rat hypothalamus: sensitivity to chronic antidepressant treatment. J. Pharmacol. Exp. Ther. 1995;47:99–103. [PubMed] [Google Scholar]
- STAM N.J., ROESINK C., DIJCKS F., GARRITSEN A., VAN HERPEN A., OLIJVE W. Human serotonin 5-HT7 receptor: cloning and pharmacological characterisation of two receptor variants. FEBS Lett. 1997;413:489–494. doi: 10.1016/s0014-5793(97)00964-2. [DOI] [PubMed] [Google Scholar]
- STOWE R.L., BARNES N.M. Selective labelling of 5-HT7 receptor recognition sites in rat brain using [3H]5-carboxamidotryptamine. Neuropharmacology. 1998a;37:1611–1619. doi: 10.1016/s0028-3908(98)00117-8. [DOI] [PubMed] [Google Scholar]
- STOWE R.L., BARNES N.M. Cellular distribution of 5-HT7 receptor mRNA in rat brain. Br. J. Pharmacol. 1998b;123:229. [Google Scholar]
- THOMAS D.R., ATKINSON P.J., HASTIE P.G., ROBERTS J.C., MIDDLEMISS D.N., PRICE G.W. 3H]-SB-269970 radiolabels 5-HT7 receptors in rodent, pig and primate brain tissues. Neuropharmacology. 2002;42:74–81. doi: 10.1016/s0028-3908(01)00151-4. [DOI] [PubMed] [Google Scholar]
- THOMAS D.R., ATKINSON P.J., HO M., BROMIDGE S.M., LOVELL P.J., VILLANI A.J., HAGAN J.J., MIDDLEMISS D.N., PRICE G.W. 3H]-SB-269970 – a selective antagonist radioligand for 5-HT7 receptors. Br. J. Pharmacol. 2000;130:409–417. doi: 10.1038/sj.bjp.0703318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS D.R., MELOTTO S., MASSAGRANDE M., GRIBBLE A.D., JEFREY P., STEVENS A.J., DEEKS N.J., EDDERSHAW P.J., FENWICH S.H., RILEY G., STEAN T SCOTT C.M., HILL M.J., MIDDLEMISS D.N., HAGAN J.J., PRICE G.W., FORBES I.T. SB-656104-A, a novel selective 5-HT7 receptor antagonist modulates REM sleep in rats. Br. J. Pharmacol. 2003;139:705–714. doi: 10.1038/sj.bjp.0705290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TO Z.P., BONHAUS D.W., EGLEN R.M., JAKEMAN L.B. Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br. J. Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSOU A.P., KOSAKA A., BACH C., ZUPPAN P., YEE C., TOM L., ALVAREZ R., RAMSEY S., BONHAUS D.W., STEFANICH E., JAKEMAN L., EGLEN R.M., CHAN H.W. Cloning and expression of a 5-hydroxytryptamine-7 receptor positively coupled to adenylyl cyclase. J. Neurochem. 1994;63:456–464. doi: 10.1046/j.1471-4159.1994.63020456.x. [DOI] [PubMed] [Google Scholar]
- VANHOENACKER P., HAEGEMAN G., LEYSEN J.E. 5-HT7 receptors: current knowledge and future prospects. Trends Pharmacol. Sci. 2000;21:70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- WAEBER C., MOSKOWITZ M.A. Autoradiographic visualisation of [3H]5-carboxamidotryptamine binding sites in the guinea pig and rat brain. Eur. J. Pharmacol. 1995;283:31–46. doi: 10.1016/0014-2999(95)00275-p. [DOI] [PubMed] [Google Scholar]
- WOOD M., CHAUBEY M., ATKINSON P., THOMAS D.R. Antagonist activity of meta-chlorophenylpiperazine and partial agonist activity of 8-OH-DPAT at the 5-HT7 receptor. Eur. J. Pharmacol. 2000;396:1–8. doi: 10.1016/s0014-2999(00)00213-2. [DOI] [PubMed] [Google Scholar]
- YING S.-W., RUSAK B. 5-HT7 receptors mediate serotonergic effects on light-sensitive suprachiasmatic nucleus neurons. Brain Res. 1997;755:246–254. doi: 10.1016/s0006-8993(97)00102-9. [DOI] [PubMed] [Google Scholar]
- YU G.-D., LIU Y.-L., JIANG X.-H., GUO S.-Y., YIN Q.-Z., HISAMITSU T. The inhibitory effect of serotonin on the spontaneous discharge of suprachiasmatic neurons in hypothalamic slice is mediated by 5-HT7 receptor. Brain Res. Bull. 2001;54:395–398. doi: 10.1016/s0361-9230(00)00462-7. [DOI] [PubMed] [Google Scholar]