Abstract
The purpose of this study was to investigate whether a membrane-permeable superoxide dismutase mimetic, tempol, added either alone or in combination with the nitric oxide (NO) donor molsidomine, prevents the development of pulmonary hypertension (PH) in chronic hypoxic rats.
Chronic hypobaric hypoxia (10% oxygen) for 2 weeks increased the right ventricular systolic pressure (RVSP), right ventricle and lung wet weight. Relaxations evoked by acetylcholine (ACh) and the molsidomine metabolite SIN-1 were impaired in isolated proximal, but not distal pulmonary arteries, from chronic hypoxic rats.
Treatment with tempol (86 mg kg−1 day−1 in drinking water) normalized RVSP and reduced right ventricular hypertrophy, while systemic blood pressure, lung and liver weights, and blunted ACh relaxation of pulmonary arteries were unchanged.
Treatment with molsidomine (15 mg kg−1 day−1 in drinking water) had the same effects as tempol, except that liver weight was reduced, and potassium and U46619-evoked vasoconstrictions in pulmonary arteries were increased. Combining tempol and molsidomine did not have additional effects compared to tempol alone. ACh relaxation in pulmonary arteries was not normalized by these treatments.
The media to lumen diameter ratio of the pulmonary arteries was greater for the hypoxic rats compared to the normoxic rats, and was not reversed by treatment with tempol, molsidomine, or the combination of tempol and molsidomine.
We conclude that tempol, like molsidomine, is able to correct RVSP and reduce right ventricular weight in the rat hypoxic model. Functional and structural properties of pulmonary small arteries were little affected. The results support the possibility that superoxide dismutase mimetics may be a useful means for the treatment of PH.
Keywords: Molsidomine, tempol, chronic hypoxic pulmonary hypertension, proximal pulmonary arteries, distal resistance pulmonary arteries
Introduction
A growing body of evidence indicates that superoxide and other reactive oxygen species (ROS) contribute both to acute hypoxic vasoconstriction (Waypa et al., 2001) and to pulmonary hypertension (PH) associated with chronic hypoxia (Hoshikawa et al., 2001). Thus, in patients with (PH), increased lipid peroxidation was found (Cracowski et al., 2001), and antioxidant enzymes are decreased, while indicators of free radicals are increased in lungs of rats with PH (Nakanishi et al., 1995). Moreover, treatment with antioxidants such as N-acetylcysteine and dimethylthiourea attenuates PH and right ventricular hypertrophy in chronic hypoxic rats (Lai et al., 1998; Herget et al., 2000), and inhibits endothelin-1 (ET-1) ROS-mediated pulmonary smooth muscle cell proliferation (Wedgwood et al., 2001). In systemic hypertension, the level of superoxide production parallels changes in the vascular wall (Dobrian et al., 2001), and recently a superoxide dismutase mimetic, tempol, was shown to reduce systemic blood pressure, vascular resistance, and medial hypertrophy of systemic resistance arteries in different rat models of systemic hypertension (Schnackenberg et al., 1998). Tempol is membrane-permeable and an efficient scavenger of free radicals (Nilsson et al., 1989), and would therefore also be expected to be superior to other less-specific antioxidants for the treatment of PH.
Short-term NO inhalation reduces pulmonary arterial pressure in various forms of PH (Katayama et al., 1997; Roberts et al., 1997), whereas prolonged inhalation of NO attenuates pulmonary vascular remodelling induced by chronic hypoxia in rats (Roberts et al., 1995; 2000). However, the use of inhaled NO is hampered by the need for complicated delivery systems and possible toxic side effects (Weinberger et al., 2001), and NO donors could provide a means of improving NO treatment. Although the effect on pulmonary pressure disappeared after 3 weeks, the NO prodrug molsidomine, which can be administered orally, reduced pulmonary pressure in patients with chronic obstructive pulmonary disease (Lampert et al., 1991), and in three patients with PH, secondary to Takayasu's arteritis, 3 months treatment with molsidomine improved hemodynamic parameters (Lee et al., 2001). Molsidomine also lowered the pulmonary arterial pressure in rats with PH evoked by monocrotaline injection or normobaric hypoxia (Mathew et al., 1997), and reduced pulmonary vascular remodelling and ET-1 expression (Blumberg et al., 2001), but the effect was less pronounced after long-term treatment (Blumberg et al., 2001). In the liver, molsidomine is converted to the active metabolite, SIN-1, which in addition to NO also releases peroxynitrite (ONOO−), generated through the interaction between NO and superoxide (Feelisch et al., 1989; Megson, 2000). Therefore, we hypothesized that a free radical cell-permeable scavenger such as tempol has the same effect as molsidomine, and the combination would have a greater effect on PH in chronic hypoxic rats.
To test this hypothesis, we examined the effect of treating chronic hypoxic rats with tempol, added either alone or in combination with molsidomine on PH, right ventricular hypertrophy as well as structural and functional changes in both proximal and distal pulmonary arteries.
Methods
Animal model
Male Wistar rats (9–10 weeks) were placed in a hypobaric chamber, which was depressurized to 500 mbar, thus halving the oxygen partial pressure. The temperature in the chamber was maintained at 21–22°C and the chamber was ventilated with air at approximately 45 l min−1. Rats were maintained in these hypoxic, hypobaric conditions for 2 weeks and studied immediately after removal from the chamber. The hypoxic chambers were opened two to three times a week for approximately 30 min in order to clean the cages and provide water, food and medication. Age-matched controls were maintained in similar but normoxic, normobaric chambers. All rats had free access to rat chow.
The animals were divided into five groups: (1) normoxic control group, (2) chronic hypoxic control group, (3) chronic hypoxic group receiving the antioxidant tempol (86 mg kg−1 day−1 corresponding to 1 mmol l−1 drinking water), (4) chronic hypoxic group receiving the NO donor molsidomine (15 mg kg−1 day−1 in the drinking water), (5) chronic hypoxic group receiving tempol (1 mmol l−1) and molsidomine (15 mg kg−1 day−1).
The local animal welfare officer supervised the health status, and all experiments were performed according to Danish legislation.
Hemodynamic measurments
Prior to the experiment, and on the day of sacrifice, body weight and systemic blood pressure of the animals were measured. The systemic blood pressure was measured using the tail cuff method with a plethysmograph (LE 5000, Letica). Before measurements, rats were preheated for 20–30 min at 35°C in their cages. Rats were then moved to a small heated container, where they were trained to stay for periods up to 20 min. In each rat, three measurements were made and the mean values were used. The equipment was calibrated every day by comparison with a mercury column.
For measurements of right ventricular systolic pressure (RVSP), rats were anesthetized with an intraperitoneal injection of midazolam (0.413 mg per 100 g) and fentanyl 0.026 mg per 100 g plus fluanisone 0.825 mg per 100 g (Hypnorm). For maintenance, intraperitoneal injection of 100 μl Hypnorm was given every 30 min. A catheter (30 cm Tygon micropore) was inserted through the jugular vein and led into the right ventricle (RV), where RVSP was measured. If signs of excessive bleeding were detected during the pressure measurement, the pressure record was discarded. The pressure profile was registered via a pressure transducer (Gabarith PMSET 1DT-XX, Becton Dickinson), amplified (Simonsen and Weel 8000, device 8041) and recorded (Goertz Servogor 124). Once the catheter was in place, the pressure was left to stabilize over a period of approximately 10 min.
Assessment of right ventricular hypertrophy
The hearts were removed, cleaned of mediastinal fat and weighed. Atria were discarded, and the free wall of the RV was separated from the left ventricle and septum (LV+septum) and then weighed. The ratio of RV weight to LV+septum weight was calculated to assess right ventricular hypertrophy (Reid, 1979). RV and LV+septum weights and wet weights of isolated lung and liver are expressed in grams and as a percentage of the total body weight.
Dissection
The first and fourth intrapulmonary branches were isolated and mounted in PSS (see composition below). Segments of the pulmonary arteries (2 mm long) were mounted on two wires (diameter of 100 μm for first branch arteries and 40 μm for fourth order branches) in microvascular myographs for isometric tension recordings, as described earlier (Mulvany & Halpern, 1977). One wire was attached to an isometric force transducer and the other to a displacement unit, permitting control of the internal circumference of the preparation. The organ bath contained PSS gassed with 5% CO2 in air. The pulmonary arteries were allowed to equilibrate in PSS to 37°C, and stepwise stretched to 3.9 kPa of passive tension, which we found, in preliminary experiments, was optimal for maximal force development.
Functional measurements
The viability of the preparations was examined by initially exposing them twice to potassium-rich PSS (KPSS, 125 mM). Contraction was induced by exposing them to the thromboxane analogue 9,11-dideoxy-11α, 9α-epoxymethanoprostaglandin F2α (U46619, 100 nM), and, once contraction was stable, relaxation curves for acetylcholine (ACh; 10−9–10−5 M) and the NO donor SIN-1 (10−8–10−3 M) were performed with 30 min interval. A third concentration–response curve for ET-1 (3 × 10−10–3 × 10−8 M) was performed at resting tension. At the end of the protocol, the vessels were activated by U46619 (100 nM) in KPSS. Preparations where the final maximal contraction was less than the two initial KPSS induced contractions were discarded.
Histology
On completion of the mechanical experiments with the pulmonary arteries, the solution was changed to calcium-free PSS for 10 min to obtain complete relaxation. The vessels were then fixed, while mounted on the myograph and still at internal circumference corresponding to 3.9 kPa, using 4% formaldehyde in Sørensen buffer adjusted to pH 7.4 (Mulvany & Korsgaard, 1983). The segments were processed, embedded in paraffin, cut transversely on a microtome in 10 μm sections, stained with Giemsa, and examined in the light microscope for determination of adventitial, medial and endothelial thickness.
Drugs and solutions
The pulmonary arteries were dissected, mounted, and held relaxed in PSS of the following composition (mmol l−1): NaCl 119, KCl 4.7,. MgSO4 1.17, NaHCO3 25, KH2PO4 1.18, glucose 5.5, CaCl2 2.5, and ethylenediaminetetraacetic acid (EDTA) 0.026. KPSS was PSS in which NaCl was exchanged for KCl on an equimolar basis.
The following drugs were used: ACh hydrochloride, ET-1, molsidomine (Alexis Biochemicals, San Diego, CA, U.S.A.), 3-morpholinosydnonimine hydrochloride (SIN-1), 4 hydroxy-2, 2,6,6-tetramethylpiperidin-N-oxyl (tempol), 9,11-dideoxy-11α,9α-epoxymethanoprostaglandin F2α (U46619) (Sigma Aldrich, U.S.A.). All drugs were dissolved in water.
Data analysis
The mechanical responses were measured as force and expressed as wall tension, ΔT, which is the increase in measured force, ΔF, divided by twice the segment length (Mulvany & Halpern, 1977). Relaxations are expressed as a percentage of the response to U46619 just before construction of the concentration–relaxation curves. By using a computer program (Graph Pad Prism, San Diego, CA, U.S.A.), the concentration–response curves were fitted to the classical Hill equation, as earlier described (Simonsen et al., 1997). Sensitivity to the agonists is expressed in terms of pD2=−log (EC50), EC50 being the concentration (M) of agonist required to give half-maximal relaxation.
The results are expressed as mean±s.e.m. Means of multiple groups were compared by one-way analysis of variance (ANOVA) followed by Bonferroni post-tests. The concentration–response curves were evaluated by calculation of the area under curve followed by one-way ANOVA, and, in case of significance, t-tests were applied with Bonferroni correction for the number of comparisons. Probability levels less than 5% were considered significant.
Results
Basal characteristics
Body weight was significantly smaller in the hypoxic group than in the normoxic control group. Tempol did not change body weight compared to the hypoxic rats. However, low body weight was more pronounced in the groups treated with molsidomine or the combination of molsidomine and tempol. Lung wet weight was significantly larger in hypoxic than in normoxic control rats, and this was unchanged by tempol, molsidomine and the combination treatment. In addition to the altered weight, macroscopic inspection of sections of the lungs from hypoxic rats also revealed that the tissue was more solid and exhibited less elasticity when stretched compared to lungs from normoxic control rats. Liver weight was unchanged by hypoxia, but tended to be lower in the treated hypoxic rats and was significantly lower in the molsidomine-treated group (Table 1).
Table 1.
Parameters describing the systemic and pulmonary impact of hypoxia (H), treatment of hypoxic rats with tempol (HT), molsidomine (HM), and the combination of molsidomine and tempol (HTM) compared to a normoxic control group (N)
| Body weight (g) | Heart rate (min−1) | Lungs (% of body weight) | Liver (% of body weight) | |
|---|---|---|---|---|
| N | 336±7 | 351±6 | 0.57±0.03 | 4.28±0.15 |
| H | 291±8* | 364±7 | 0.83±0.04* | 4.36±0.15 |
| HT | 226±14* | 365±6 | 0.86±0.07* | 3.97±0.14 |
| HM | 226±15*# | 375±2 | 0.91±0.08* | 3.43±0.25*# |
| HTM | 247±17*# | 382±3 | 0.14±0.08* | 3.97±0.26 |
Values are mean±s.e.m. of 8-13 animals examined. Differences were evaluated by one way analysis of variance (ANOVA) followed by a posteriori Bonferroni test in case of significance: *P<0.05, parameter significantly different compared to N
P<0.05, parameter significantly different compared with H.
Blood pressures and heart weight
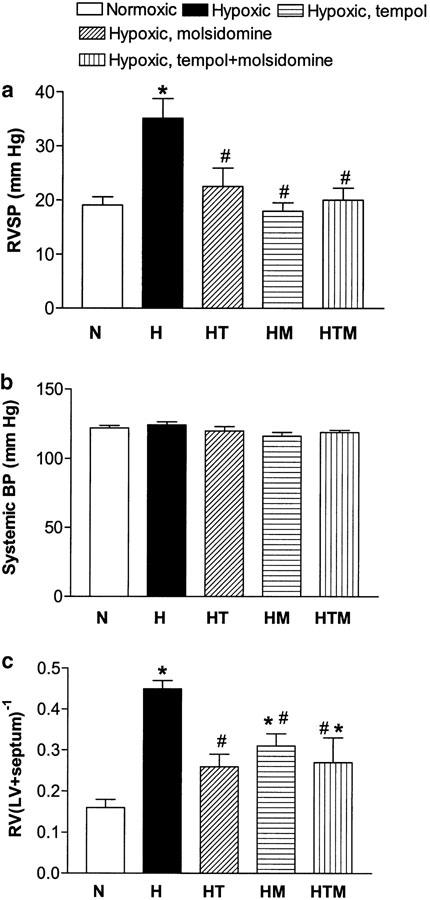
In the chronic hypoxic rats, RVSP was significantly increased compared to the normoxic control group. Treatment with tempol markedly prevented the increase in RVSP, while RVSP was normalized in the molsidomine and tempol plus molsidomine-treated groups (Figure 1a). Systemic blood pressure and heart rate were not changed by chronic hypoxia, and treatment with tempol, molsidomine, or the combination did not change these parameters (Figure 1b, Table 1).
Figure 1.
Long-term in vivo treatment with the NO donor molsidomine, the superoxide dismutase mimetic tempol and the combination of molsidomine and tempol reduces RVSP (a) without any effect on systemic blood pressure in chronic hypoxic rats (b). Right ventricular hypertrophy (RV(LV+septum)−1) was also reduced by the treatments (c), normoxic rats (N), hypoxic control rats (H), hypoxic tempol-treated rats (HT), molsidomine treated rats (HM) and tempol plus molsidomine-treated rats (HTM). Values are mean±s.e.m. of 6-13 measurements. Differences were evaluated by one-way ANOVA, followed by a posteriori Bonferroni test in case of significance: *P<0.05 versus N; #P<0.05 versus H.
Heart weight expressed as percentage of body weight was 0.33±0.02% (n=11) in the normoxic group and was increased to 0.43±0.03% (P<0.05, n=11) in the hypoxic group. Heart weight was also increased in the hypoxic rats treated with tempol, molsidomine, and the combination of tempol and molsidomine. Right ventricular hypertrophy was associated with chronic hypoxia as indicated by the significantly increased RV to LV+septum ratio in the hypoxic group compared to the normoxic group. This hypertrophy was significantly reduced by tempol and completely abolished by molsidomine treatment (Figure 1c). The combined treatment with tempol and molsidomine did not totally prevent an increased RV to LV+septum ratio (Figure 1c).
Functional responses in isolated pulmonary arteries
Proximal and distal pulmonary arteries with internal diameters of, respectively, 1083±71 μm (n=36) and 464±36 μm (n=39) were mounted. The internal diameter was less in proximal arteries isolated from vehicle, molsidomine and molsidomine plus tempol-treated hypoxic rats compared to arteries isolated from normoxic rats (Table 2). In case of distal arteries from molsidomine plus tempol-treated hypoxic rats, lumen diameter was smaller than arteries from normoxic rats, although it should be noted that the treatments appeared to change the overall structure of the lungs, so that it is not strictly possible to compare the vessel sizes between groups.
Table 2.
Contractile responses evoked by 125 mM K+-rich U46619 (100 nM), and endothelin-1 (ET-1, 30 nM) in proximal and distal pulmonary arteries from normoxic (N), vehicle-treated hypoxic (H), and hypoxic rats treated with tempol (HT), molsidomine (HM), and tempol plus molsidomine (HTM)
| N | H | HT | HM | HTM | |
|---|---|---|---|---|---|
| (n=8–9) | (n=8) | (n=5–8) | (n=5–7) | (n=6–8) | |
| Poximal | |||||
| Diameter (μm) | 1296±90 | 958±78* | 1200±51# | 835±41* | 818±61* |
| KPSS (N m−1) | 1.1±0.2 | 1.3±0.1 | 1.3±0.2 | 2.1±0.9 | 0.9±0.2 |
| U46619 (N m−1) | 1.1±0.2 | 1.5±0.1 | 1.2±0.1 | 2.3±0.8* | 1.1±0.1 |
| ET-1 (N m−1) | 0.9±0.2 | 0.7±0.2 | 1.7±0.2 | 2.8±1.1 | 1.8±0.8 |
| Distal | |||||
| Diameter (μm) | 660±85 | 515±70 | 430±53 | 549±45 | 305±77* |
| KPSS (N m−1) | 1.5±0.4 | 1.5±0.5 | 1.3±0.4 | 3.7±1.0* | 1.1±0.4 |
| U46619 (N m−1) | 1.5±0.3 | 1.3±0.4 | 0.3±0.3 | 3.0±0.7* | 1.0±0.4 |
| ET-1 (N m−1) | 1.7±0.3 | 1.7±0.5 | 1.0±0.2 | 2.6±0.7 | 1.3±0.5 |
Values are mean±s.e.m., where n indicates the number of animals. Differences were evaluated by one-way analysis of variance (ANOVA) followed by a posteriori Bonferroni test in case of significance: *P<0.05 versus arteries from normoxic animals
P<0.05 versus arteries from hypoxic rats.
Contraction evoked by KPSS (125 mM), U46619 (100 nM), or ET-1 (30 nM) was similar in proximal and distal arteries from normoxic and hypoxic rats, but in arteries from molsidomine-treated hypoxic rats contractions were markedly increased (Table 2).
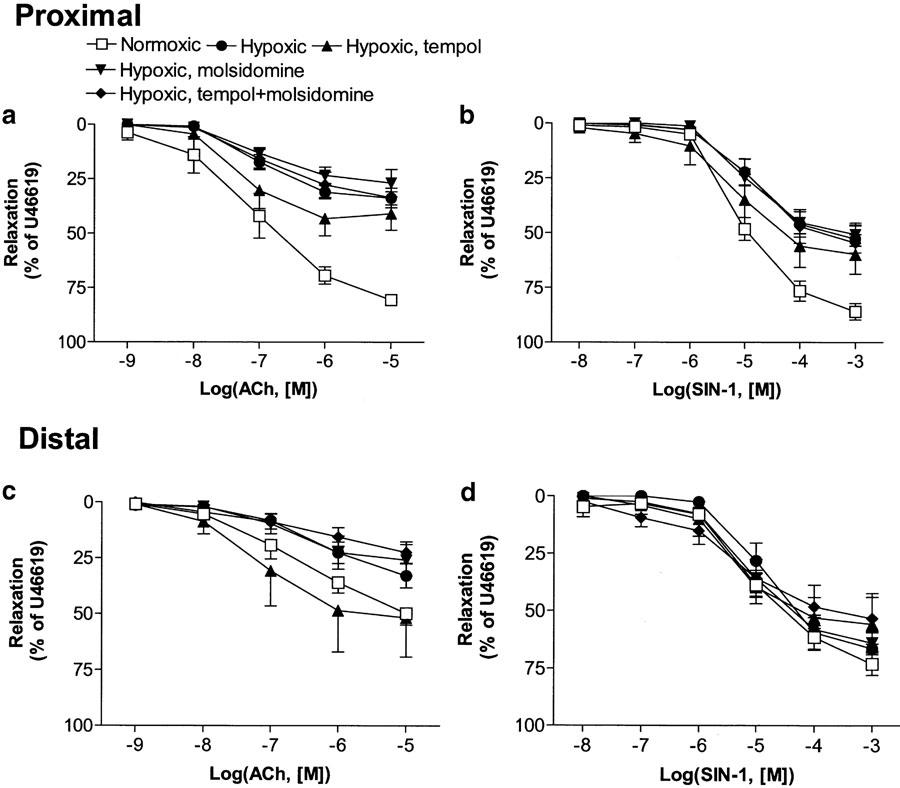
In pulmonary arteries contracted with U46619, ACh and SIN-1 evoked concentration-dependent relaxations, which were significantly impaired in proximal pulmonary arteries from hypoxic compared to normoxic control rats (Figure 2a, b; Table 3 ). ACh and SIN-1 relaxation was not restored by any of the in vivo treatments (Figure 2a, b; Table 3). In distal pulmonary arteries, ACh and SIN-1 relaxation was not impaired in arteries from vehicle-treated hypoxic rats, and neither of the in vivo treatments caused significant change from normoxic controls (Figure 2c, d). However, ACh relaxation in arteries from tempol-treated rats was significantly greater than in arteries from molsidomine-treated rats (Figure 2c).
Figure 2.
Concentration–relaxation curves for the endothelium-dependent vasorelaxant, ACh and endothelium-independent vasodilator SIN-1 in proximal (a, b) and distal (c, d) pulmonary arteries isolated from normoxic (N) and hypoxic rats treated with vehicle (H), tempol (HT), molsidomine (HM) and tempol plus molsidomine (HTM). The relaxations are expressed as a percentage of U46619-induced contraction. Results represent means±s.e.m. of 5-9 preparations. Differences in area under concentration–response curves were evaluated by one-way ANOVA followed by Bonferroni post-test. Concentration–response curves for ACh and SIN-1 in proximal pulmonary arteries from H, HT, HM and HTM were significantly (P<0.05) different from the corresponding concentration–response curves in normoxic controls (a, b). Moreover, ACh relaxation in arteries from tempol-treated rats was significantly greater than in arteries from molsidomine-treated rats (c).
Table 3.
Relaxations induced by acetylcholine and SIN-1 U46619 (10−7 M)-contracted proximal and distal pulmonary arteries isolated from normoxic (N) and hypoxic (H) rats, and hypoxic rats treated with tempol (HT), molsidomine (HM), and tempol plus molsidomine (HTM)
| Acetylcholine | SIN-1 | ||||
|---|---|---|---|---|---|
| n | pD2 | Max. relaxation (%) | pD2 | Max relaxation (%) | |
| Proximal | |||||
| N | 8 | 7.16±0.31 | 80.4±1.9 | 4.99±0.05 | 85.8±3.7 |
| H | 8 | 6.91±0.09 | 33.8±3.0* | 4.80±0.11 | 52.6±6.4* |
| HT | 8 | 7.33±0.18 | 40.9±7.6* | 4.91±0.10 | 59.7±9.0 |
| HM | 5 | 7.00±0.22 | 26.8±6.4* | 5.13±0.20 | 50.6±5.3* |
| HTM | 7 | 6.36±0.60 | 33.6±4.6* | 4.67±0.12 | 54.5±7.8* |
| Distal | |||||
| N | 9 | 6.23±0.34 | 49.8±5.2 | 4.86±0.16 | 73.1±4.9 |
| H | 8 | 6.29±0.21 | 32.9±5.4 | 4.79±0.15 | 66.4±7.1 |
| HT | 8 | 6.96±0.23 | 51.8±17.3 | 5.21±0.02 | 55.8±11.8 |
| HM | 7 | 6.50±0.23 | 25.9±7.0 | 5.09±0.08 | 63.9±5.1 |
| HTM | 7 | 6.33±0.58 | 22.4±4.8 | 5.25±0.28 | 53.3±10.9 |
Values are mean±s.e.m. of n number of preparations examined.
P<0.05, parameter significantly different from normoxic animal, pD2=−log EC50, where EC50 is the concentration of agonist producing half-maximal relaxation.
Morphometric characteristics of the vessel wall
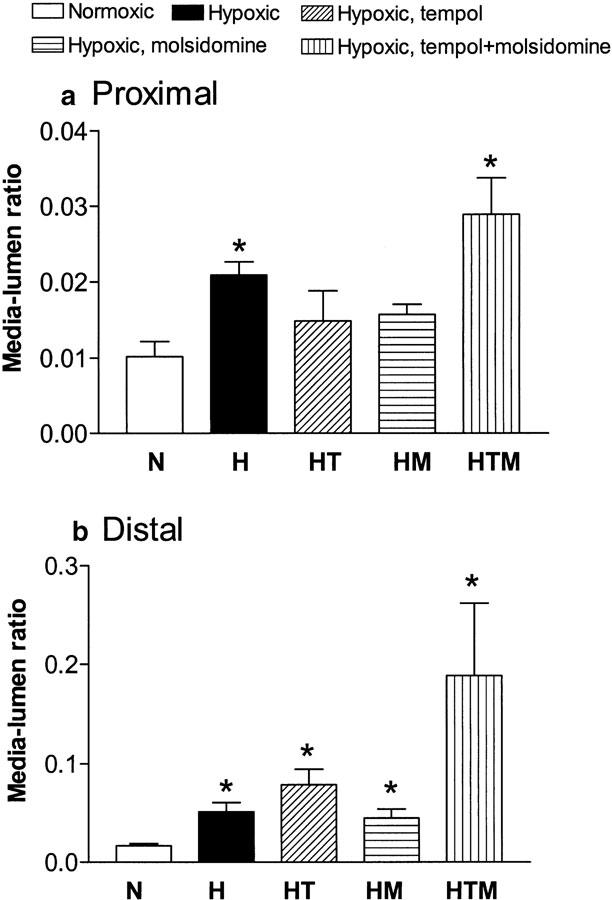
The media to lumen diameter ratio was significantly increased in both proximal and distal pulmonary arteries from vehicle-treated hypoxic rats compared to arteries from normoxic control rats (Figure 3). Treatment with tempol, molsidomine, or the combination did not affect the relation between media and lumen diameter significantly in pulmonary arteries from hypoxic rats (Figure 3).
Figure 3.
Media–lumen diameter ratio in (a) proximal and (b) distal pulmonary arteries isolated from normoxic (N) and hypoxic rats treated with vehicle (H), tempol (HT), molsidomine (HM) and tempol plus molsidomine (HTM). Results are mean±s.e.m. of 5-8 arteries. Differences were evaluated by one-way ANOVA, followed by a posteriori Bonferroni test in case of significance: *P<0.05 versus normoxic rats.
Discussion
The main finding of the present study is that treatment with the superoxide scavenger tempol in drinking water substantially suppressed the effects of chronic hypoxia on RVSP and hypertrophy, but the combination of tempol and the NO prodrug molsidomine did not have a greater effect compared to tempol alone.
The effect of the orally active NO donor molsidomine on RVSP and hypertrophy in the present study is comparable to previous studies showing the beneficial effect of chronic NO inhalation (Katayama et al., 1997) or prolonged administration of L-arginine, a precursor of endogenous NO production (Mitani et al., 1997), on PH in chronic hypoxic rats. Moreover, the present findings agree with other studies, which have shown that treatment with molsidomine lowers pulmonary arterial pressure in rats with PH evoked by monocrotaline injection or normobaric hypoxia (Blumberg et al., 2001). Molsidomine is converted in the liver to the active NO-releasing metabolite SIN-1, which evoked potent vasorelaxation in isolated pulmonary arteries in the present study. This suggests that the molsidomine-induced pulmonary arterial pressure reduction is mainly due to direct vasodilatation of the pulmonary arterial circulation. The conversion of molsidomine in the liver and metabolization in the lungs of SIN-1 probably confer selectivity for the pulmonary circulation, and explain the lack of changes in systemic blood pressure and heart rate in molsidomine-treated hypoxic rats.
Even though molsidomine and SIN-1 do not appear to induce tolerance and are not cross-tolerant with conventional nitrates (Sutsch et al., 1997; Megson, 2000), it has been suggested that long-term treatment with molsidomine either gradually wears off or the impact depends on the underlying pulmonary hypertensive disease. Thus, the effect of molsidomine was reported to disappear after 3 weeks of treatment in patients with chronic obstructive pulmonary disease (Lampert et al., 1991), and administration of molsidomine for 4 weeks in chronic hypoxic rats did not normalize the pulmonary arterial pressure (Blumberg et al., 2001). Although treatment with molsidomine had some effect on increased media area in the study by Blumberg et al. (2001), molsidomine did not normalize structure in the pulmonary arteries. In agreement with these observations, in the present study, we found that molsidomine did not prevent the increase in media to lumen diameter ratio of the pulmonary arteries. These morphological changes are likely to be more pronounced, and make a greater contribution to elevated pulmonary arterial pressure, with longer duration of hypoxia.
In addition to structural changes, increased vasoconstriction could play a role for disappearance of the effect of molsidomine. Molsidomine was described to lower ET-1 expression, but this effect is persistent throughout the study (Blumberg et al., 2001). Although endothelium-derived basal NO counteracts phenylephrine contraction in rat-isolated pulmonary arteries (Priest et al., 1998), prolonged administration of exogenous NO did not appear to alter the endogenous NO-cyclic GMP pathway in rats (Frank et al., 1998). However, cogeneration of superoxide is a major drawback of molsidomine and its metabolites (Megson, 2000). Therefore, a more likely explanation for enhanced vasoconstriction is that superoxide, which has previously been shown to cause pulmonary vasoconstriction (Wiklund et al., 1996) and is released from the molsidomine, causes enhanced vasoconstriction, as also found here. This is supported by the further observation in the present study that vasoconstriction is not enhanced in arteries from rats additionally treated with the superoxide scavenger tempol.
Several mechanisms of action could account for the lack of increased pulmonary arterial pressure in tempol-treated hypoxic rats in the present study. It has been suggested that ROS generation is a key component of acute hypoxic vasoconstriction in pulmonary arteries (Waypa et al., 2001). Tempol is a superoxide scavenger, and the possibility exists that tempol reduced hypoxic signalling and therefore decreased pulmonary arterial pressure and pressure load on the RV. This is also supported by the observations that ROS is increased in PH associated with chronic hypoxia (Cracowski et al., 2001), and application of antioxidants such as N-acetylcysteine and dimethylthiourea attenuates both PH and right ventricular hypertrophy in chronic hypoxic rats (Langleben et al., 1989). An alternative explanation is that tempol reduces remodelling of the pulmonary arteries. Thus, the level of superoxide production was found to parallel changes in vascular wall thickness in systemic hypertension (Dobrian et al., 2001), and ROS were suggested to be involved in the degradation of intracellular collagen, which in turn stimulates smooth muscle hypertrophy, synthesis and migration in the pulmonary circulation (Rabinovitch, 2001). In the present study even the combination of tempol and molsidomine did not prevent changes in remodelling of the pulmonary arteries, thus suggesting that direct pressure-lowering effects are more likely to play a role in the beneficial effects of tempol.
In systemic hypertension, increased bioavailability of kidney 20-HETE (Hoagland et al., 2003), reduced renal nerve sympathetic activity (Xu et al., 2001) and inhibition of angiotensin II-evoked vasoconstriction (Yu et al., 2002) were suggested to play a role in tempol-evoked reduction in blood pressure. In contrast to systemic arteries, 20-HETE evokes endothelium-dependent NO-mediated relaxation in pulmonary arteries (Yu et al., 2002), and increased bioavailability of 20-HETE could play a role in the lowering of pulmonary blood pressure observed in the present study. Inhibitors of NO synthase block the blood pressure-lowering effect in systemic hypertension (Schnackenberg et al., 1998) and inhibition of angiotensin II contraction by tempol (Shastri et al., 2002), which suggests that tempol also acts by increasing NO bioavailability. Tempol did not prevent the blunted endothelium-dependent vasorelaxation evoked by ACh in the present study, but these results do not exclude the possibility that basal NO production in the pulmonary circulation is enhanced by tempol and would lead to increased vasodilatation in the pulmonary circulation.
Tempol was found to lower the blood pressure in different rat models of systemic hypertension (Shokoji et al., 2003). High concentrations (3 mmol l−1) of tempol were also shown to lower the systemic blood pressure in normotensive rats (Shokoji et al., 2003), but in the present study lower concentrations (1 mmol l−1) of tempol did not change systemic blood pressure. These findings suggest that tempol, similar to molsidomine, has selectivity for the pulmonary circulation.
A surprising finding of the present study was that the treatments did not reduce lung weight, not even in chronic hypoxic rats treated with the combination of molsidomine and tempol. Both edema and altered structure of the lung can contribute to the enhanced lung weight in hypoxic rats (Meyrick & Reid, 1980). The media lumen ratio was increased in arteries from hypoxic animals and the treatments did not normalize the ratio (see Figure 3). These findings suggest that treatment with molsidomine and tempol is able to lower the pulmonary arterial pressure and blunt the increase in right ventricular hypertrophy, but is not able to prevent the morphological changes in the pulmonary arterial circulation.
In addition to increased pulmonary vascular resistance, increased pulmonary arterial pressure, right ventricular hypertrophy and arterial remodelling, mild to moderate PH is also characterized by a dysfunctional endothelium (Archer & Rich, 2000). In the present study, endothelium-dependent ACh-evoked relaxations were reduced in proximal, but not in distal pulmonary arteries compared to normoxic controls. These findings agree with other studies showing impaired endothelium-dependent relaxations in proximal pulmonary arteries (Oka et al., 1993), and conserved endothelium-dependent relaxation in distal pulmonary arteries (Priest et al., 1998). The expression of endothelial NO synthase, which is activated by ACh, is most abundant in proximal pulmonary arteries (Hampl & Herget, 2000), and agonist-evoked endothelium-dependent relaxations are considered NO-mediated in large arteries, while an endothelium-derived hyperpolarizing factor contributes to relaxation in smaller arteries (Busse et al., 2002). Therefore, the blunted ACh relaxation could be ascribed to either chronic hypoxia and/or PH interfering with the activation of endothelial NO synthase. However, in the present study, relaxations evoked by the NO donor SIN-1 were also impaired in proximal, but not in distal pulmonary arteries. Hypoxia increases free radical production in smooth muscle cells from rat main pulmonary artery (Killilea et al., 2000). Although scavenging of oxygen-derived free radicals cannot prevent all of the vascular injury caused by these radicals (Wiklund et al., 1996), it is unlikely that the impaired ACh and SIN-1 relaxation is alone due to decreased bioavailability of NO caused by enhanced superoxide production in chronic hypoxia, since treatment with the superoxide scavenger tempol did not restore the vasorelaxations by these agents. Moreover, basal as well as agonist-induced cyclic GMP synthesis was found reduced by 50–70% in rat main pulmonary artery by chronic hypoxia (Shaul et al., 1993). Therefore, in addition to increased superoxide production, changed activation of endothelial NO synthase and/or altered smooth muscle responsiveness can explain the blunted endothelium-dependent relaxation in proximal pulmonary arteries. These findings also suggest that other means, in addition to treatment with tempol, are necessary to prevent the changes taking place in pulmonary arteries from chronic hypoxic rats.
Perspectives
The present study has provided evidence that treatment of chronic hypoxic rats with the superoxide dismutase mimetic tempol or with the NO donor molsidomine prevents the development of PH and blunts right ventricular hypertrophy. However, the lack of effect of these drugs, even in combination on lung weight and endothelium-dependent relaxation, also suggests that pressure reduction is not enough to prevent the changes taking place in chronic hypoxic rats. This indicates a need for a better understanding of the mechanisms that allow normalization of right ventricular hypertrophy and endothelium-dependent relaxation of pulmonary arteries.
Acknowledgments
The Danish Heart Foundation, The Danish Lung Foundation, AGA AB Medical Research Fund and Novo Nordisk Foundation supported this study. We are grateful for the technical assistance of Mette Schandorff.
Abbreviations
- ACh
acetylcholine
- ET-1
endothelin-1
- LV
left ventricle
- NO
nitric oxide
- ONOO−
peroxynitrite
- PH
pulmonary hypertension
- ROS
reactive oxygen species
- RV
right ventricle
- RVSP
right ventricular systolic pressure
References
- ARCHER S., RICH S. Primary pulmonary hypertension: a vascular biology and translational research ‘Work in progress'. Circulation. 2000;102:2781–2791. doi: 10.1161/01.cir.102.22.2781. [DOI] [PubMed] [Google Scholar]
- BLUMBER G.F.C., WOLF K., SANDNER P., LORENZ C., RIEGGER G.A., PFEIFER M. The NO donor molsidomine reduces endothelin-1 gene expression in chronic hypoxic rat lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L258–L263. doi: 10.1152/ajplung.2001.280.2.L258. [DOI] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. EDHF: bringing the concepts together. Trends Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- CRACOWSKI J.L., CRACOWSKI C., BESSARD G., PEPIN J.L., BESSARD J., SCHWEBEL C., STANKE-LABESQUE F., PISON C. Increased lipid peroxidation in patients with pulmonary hypertension. Am. J. Resp. Crit. Care Med. 2001;164:1038–1042. doi: 10.1164/ajrccm.164.6.2104033. [DOI] [PubMed] [Google Scholar]
- DOBRIAN A.D., SCHRIVER S.D., PREWITT R.L. Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension. 2001;38:361–366. doi: 10.1161/01.hyp.38.3.361. [DOI] [PubMed] [Google Scholar]
- FEELISCH M., OSTROWSKI J., NOACK E. On the mechanism of NO release from sydnonimines. J. Cardiovasc. Pharmacol. 1989;14 Suppl 11:S13–S22. [PubMed] [Google Scholar]
- FRANK D.U., HORSTMAN D.J., MORRIS G.N., JOHNS R.A., RICH G.F. Regulation of the endogenous NO pathway by prolonged inhaled NO in rats. J. Appl. Physiol. 1998;85:1070–1078. doi: 10.1152/jappl.1998.85.3.1070. [DOI] [PubMed] [Google Scholar]
- HAMPL V., HERGET J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol. Rev. 2000;80:1337–1372. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- HERGET J., WILHELM J., NOVOTNA J., ECKHARDT A., VYTASEK R., MRAZKOVA L., OSTADAL M. A possible role of the oxidant tissue injury in the development of hypoxic pulmonary hypertension. Physiol. Res. 2000;49:493–501. [PubMed] [Google Scholar]
- HOAGLAND K.M., MAIER K.G., ROMAN R.J. Contributions of 20-HETE to the antihypertensive effects of tempol in Dahl salt-sensitive rats. Hypertension. 2003;41:697–702. doi: 10.1161/01.HYP.0000047881.15426.DC. [DOI] [PubMed] [Google Scholar]
- HOSHIKAWA Y., ONO S., SUZUKI S., TANITA T., CHIDA M., SONG C., NODA M., TABATA T., VOELKEL N.F., FUJIMURA S. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J. Appl. Physiol. 2001;90:1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- KATAYAMA Y., HIGENBOTTAM T.W., DIAZ DE ATAURI M.J., CREMONA G., AKAMINE S., BARBERA J.A., RODRIGUEZ-ROISIN R. Inhaled nitric oxide and arterial oxygen tension in patients with chronic obstructive pulmonary disease and severe pulmonary hypertension. Thorax. 1997;52:120–124. doi: 10.1136/thx.52.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLILEA D.W., HESTER R., BALCZON R., BABAL P., GILLESPIE M.N. Free radical production in hypoxic pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L408–L412. doi: 10.1152/ajplung.2000.279.2.L408. [DOI] [PubMed] [Google Scholar]
- LAI Y.L., WU H.D., CHEN C.F. Antioxidants attenuate chronic hypoxic pulmonary hypertension. J. Cardiovasc. Pharmacol. 1998;32:714–720. doi: 10.1097/00005344-199811000-00006. [DOI] [PubMed] [Google Scholar]
- LAMPERT E., TUO N., FRANS A., LONSDORFER J. Disappearance of molsidomine effects on pulmonary circulation of patients with chronic obstructive pulmonary disease after a three week treatment. Pathol. Biol. (Paris) 1991;39:29–33. [PubMed] [Google Scholar]
- LANGLEBEN D., FOX R.B., JONES R.C., REID L.M. Effects of dimethylthiourea on chronic hypoxia-induced pulmonary arterial remodelling and ventricular hypertrophy in rats. Clin. Invest. Med. 1989;12:235–240. [PubMed] [Google Scholar]
- LEE S.D., KIM D.S., SHIM T.S., LIM C.M., KOH Y., KIM W.S., KIM W.D. Nitric oxide and molsidomine in the management of pulmonary hypertension in Takayasu's arteritis. Chest. 2001;119:302–307. doi: 10.1378/chest.119.1.302. [DOI] [PubMed] [Google Scholar]
- MATHEW R., GLOSTER E.S., SUNDARARAJAN T., THOMPSON C.I., ZEBALLOS G.A., GEWITZ M.H. Role of inhibition of nitric oxide production in monocrotaline-induced pulmonary hypertension. J. Appl. Physiol. 1997;82:1493–1498. doi: 10.1152/jappl.1997.82.5.1493. [DOI] [PubMed] [Google Scholar]
- MEGSON I.L. Nitric oxide donor drugs. Drugs Future. 2000;25:701–715. [Google Scholar]
- MEYRICK B., REID L. Endothelial and subintimal changes in rat hilar pulmonary artery during recovery from hypoxia. A quantitative ultrastructural study. Lab. Invest. 1980;42:603–615. [PubMed] [Google Scholar]
- MITANI Y., MARUYAMA K., SAKURAI M. Prolonged administration of L-arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats. Circulation. 1997;96:689–697. [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., KORSGAARD N. Correlations and otherwise between blood pressure, cardiac mass and resistance vessel characteristics in hypertensive, normotensive and hypertensive/normotensive hybrid rats. J. Hypertens. 1983;1:235–244. doi: 10.1097/00004872-198310000-00007. [DOI] [PubMed] [Google Scholar]
- NAKANISHI I., TAJIMA F., NAKAMURA A., YAGURA S.Y., OOKAWARA T., YAMASHITA H., SUZUKI K., TANIGUCHI N., OHNO H. Effects of hypobaric hypoxia on antioxidant enzymes in rats. J. Physiol. 1995;489:869–876. doi: 10.1113/jphysiol.1995.sp021099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON U.A., OLSSON L.I., CARLIN G., BYLUNDFELLENIUS A.C. Inhibition of lipid-peroxidation by spin labels – relationships between structure and function. J. Biol. Chem. 1989;264:11131–11135. [PubMed] [Google Scholar]
- OKA M., HASUNUMA K., WEBB S.A., STELZNER T.J., RODMAN D.M., MCMURTRY I.F. EDRF suppresses an unidentified vasoconstrictor mechanism in hypertensive rat lungs. Am. J. Physiol. 1993;264:L587–L597. doi: 10.1152/ajplung.1993.264.6.L587. [DOI] [PubMed] [Google Scholar]
- PRIEST R.M., ROBERTSON T.P., LEACH R.M., WARD J.P. Membrane potential-dependent and -independent vasodilation in small pulmonary arteries from chronically hypoxic rats. J. Pharmacol. Exp. Ther. 1998;285:975–982. [PubMed] [Google Scholar]
- RABINOVITCH M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin. Chest. Med. 2001;22:433–449. doi: 10.1016/s0272-5231(05)70282-3. [DOI] [PubMed] [Google Scholar]
- REID L.M. The pulmonary circulation: remodeling in growth and disease. The 1978. J. Burns Amberson lecture. Am. Rev. Respir. Dis. 1979;119:531–546. doi: 10.1164/arrd.1979.119.4.531. [DOI] [PubMed] [Google Scholar]
- ROBERTS J.D., CHICHE J.D., WEIMANN J., STEUDEL W., ZAPOL W.M., BLOCH K.D. Nitric oxide inhalation decreases pulmonary artery remodeling in the injured lungs of rat pups. Circ. Res. 2000;87:140–145. doi: 10.1161/01.res.87.2.140. [DOI] [PubMed] [Google Scholar]
- ROBERTS J.D., JR, FINEMAN J.R., MORIN F.C., III, SHAUL P.W., RIMAR S., SCHREIBER M.D., POLIN R.A., ZWASS M.S., ZAYEK M.M., GROSS I., HEYMANN M.A., ZAPOL W.M. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N. Engl. J. Med. 1997;336:605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- ROBERTS J.D., ROBERTS C.T., JONES R.C., ZAPOL W.M., BLOCH K.D. Continuous nitric-oxide inhalation reduces pulmonary arterial structural-changes, right-ventricular hypertrophy, and growth-retardation in the hypoxic newborn rat. Circ. Res. 1995;76:215–222. doi: 10.1161/01.res.76.2.215. [DOI] [PubMed] [Google Scholar]
- SCHNACKENBERG C.G., WELCH W.J., WILCOX C.S. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- SHASTRI S., GOPALAKRISHNAN V., PODURI R., DI WANG H. Tempol selectively attenuates angiotensin II evoked vasoconstrictor responses in spontaneously hypertensive rats. J. Hypertens. 2002;20:1381–1391. doi: 10.1097/00004872-200207000-00025. [DOI] [PubMed] [Google Scholar]
- SHAUL P.W., WELLS L.B., HORNING K.M. Acute and prolonged hypoxia attenuate endothelial nitric oxide production in rat pulmonary arteries by different mechanisms. J. Cardiovasc. Pharmacol. 1993;22:819–827. doi: 10.1097/00005344-199312000-00007. [DOI] [PubMed] [Google Scholar]
- SHOKOJI T., NISHIYAMA A., FUJISAWA Y., HITOMI H., KIYOMOTO H., TAKAHASHI N., KIMURA S., KOHNO M., ABE Y. Renal sympathetic nerve responses to Tempol in spontaneously hypertensive rats. Hypertension. 2003;41:266–273. doi: 10.1161/01.hyp.0000049621.85474.cf. [DOI] [PubMed] [Google Scholar]
- SIMONSEN U., GARCIA-SACRISTAN A., PRIETO D. Apamin-sensitive K+ channels involved in the inhibition of acetylcholine-induced contractions in lamb coronary small arteries. Eur. J. Pharmacol. 1997;329:153–163. [PubMed] [Google Scholar]
- SUTSCH G., KIM J.H., BRACHT C., KIOWSKI W. Lack of cross-tolerance to short-term linsidomine in forearm resistance vessels and dorsal hand veins in subjects with nitroglycerin tolerance. Clin. Pharmacol. Therap. 1997;62:538–545. doi: 10.1016/S0009-9236(97)90049-7. [DOI] [PubMed] [Google Scholar]
- WAYPA G.B., CHANDEL N.S., SCHUMACKER P.T. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ. Res. 2001;88:1259–1266. doi: 10.1161/hh1201.091960. [DOI] [PubMed] [Google Scholar]
- WEDGWOOD S., DETTMAN R.W., BLACK S.M. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L1058–L1067. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- WEINBERGER B., LASKIN D.L., HECK D.E., LASKIN J.D. The toxicology of inhaled nitric oxide. Toxicol. Sci. 2001;59:5–16. doi: 10.1093/toxsci/59.1.5. [DOI] [PubMed] [Google Scholar]
- WIKLUND L., MCGREGOR C.G.A., MILLER V.M. Effects of prolonged exposure to oxygen-derived free radicals in canine pulmonary arteries. Am. J. Physiol. Heart Circ. Physiol. 1996;39:H2184–H2190. doi: 10.1152/ajpheart.1996.270.6.H2184. [DOI] [PubMed] [Google Scholar]
- XU H., FINK G.D., CHEN A., WATTS S., GALLIGAN J.J. Nitric oxide-independent effects of tempol on sympathetic nerve activity and blood pressure in normotensive rats. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H975–H980. doi: 10.1152/ajpheart.2001.281.2.H975. [DOI] [PubMed] [Google Scholar]
- YU M., MCANDREW R.P., AL SAGHIR R., MAIER K.G., MEDHORA M., ROMAN R.J., JACOBS E.R. Nitric oxide contributes to 20-HETE-induced relaxation of pulmonary arteries. J. Appl. Physiol. 2002;93:1391–1399. doi: 10.1152/japplphysiol.00247.2002. [DOI] [PubMed] [Google Scholar]