Abstract
The mechanisms and receptors involved in the vasoactive intestinal peptide (VIP)- and pituitary adenylate cyclase-activating polypeptide (PACAP)-induced relaxations of the pig intravesical ureter were investigated.
VIP, PACAP 38 and PACAP 27 concentration-dependently relaxed U46619-contracted ureteral strips with a similar potency. [Ala11,22,28]-VIP, a VPAC1 agonist, showed inconsistent relaxations.
The neuronal voltage-gated Ca2+ channel inhibitor, ω-conotoxin GVIA (ω-CgTX, 1 μM), reduced the VIP relaxations. Urothelium removal or blockade of capsaicin-sensitive primary afferents, nitric oxide (NO) synthase and guanylate cyclase with capsaicin (10 μM), NG-nitro-L-arginine (L-NOARG, 100 μM) and 1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 5 μM), respectively, did not change the VIP relaxations. However, the PACAP 38 relaxations were reduced by ω-CgTX, capsaicin, L-NOARG and ODQ.
The VIP and VIP/PACAP receptor antagonists, [Lys1, Pro2,5, Arg3,4, Tyr6]-VIP (1 μM) and PACAP (6–38) (0.4 μM), inhibited VIP and VIP and PACAP 38, respectively, relaxations.
The nonselective and large-conductance Ca2-activated K+ channel blockers, tetraethylammonium (3 mM) and charybdotoxin (0.1 μM), respectively, and neuropeptide Y (0.1 μM) did not modify the VIP relaxations. The small-conductance Ca2-activated K+ channel blocker apamin (1 μM) did not change the PACAP 27 relaxations.
The cAMP-dependent protein kinase A (PKA) blocker, 8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphorothioate (Rp-8-CPT-cAMPS, 100 μM), reduced VIP relaxations. The phosphodiesterase 4 inhibitor rolipram and the adenylate cyclase activator forskolin relaxed ureteral preparations. The rolipram relaxations were reduced by Rp-8-CPT-cAMPS. Forskolin (30 nM) evoked a potentiation of VIP relaxations.
These results suggest that VIP and PACAP relax the pig ureter through smooth muscle receptors, probably of the VPAC2 subtype, linked to a cAMP-PKA pathway. Neuronal VPAC receptors localized at motor nerves and PAC1 receptors placed at sensory nerves and coupled to NO release, seem also to be involved in the VIP and PACAP 38 relaxations.
Keywords: Vasoactive intestinal peptide, pituitary adenylate cyclase-activating polypeptide, neuronal VPAC and PAC1 receptors, smooth muscle VPAC2 receptors, nitric oxide, cAMP-dependent protein kinase A, K+ channels, pig intravesical ureter
Introduction
Vasoactive intestinal peptide (VIP) produces relaxation in the mammalian urinary tract either by a direct action on the smooth muscle cells or by indirect effects mediated by nitric oxide (NO) release from autonomic intramural neurons. Thus, it has been reported that VIP relaxes pig ureter (Godet et al., 1993), human (Uckert et al., 2002) and pig (Hosokawa & Kaseda, 1993) bladder and pig (Hosokawa & Kaseda, 1993) and guinea-pig (Werkström et al., 1998) urethra.
VIP and/or pituitary adenylate cyclase-activating polypeptide (PACAP) receptors belong to the family of the seven transmembrane-spanning G protein-coupled receptors. Cloning techniques have identified three major types of receptors that interact with VIP and PACAP denoted PAC1, VPAC1 and VPAC2. PAC1 receptors exhibit high affinity for PACAP and a low affinity for VIP, while VPAC1 and VPAC2 receptors have a similar affinity for both VIP and PACAP (Harmar et al., 1998; Vaudry et al., 2000). PAC1 receptors have been described on neurons and smooth muscle. Thus, neuronal PAC1 receptors placed on NO-synthesizing neurons exhibit a high affinity for PACAP 38 and its activation leads to an increased NO production, while smooth muscle PAC1 receptors preferentially bind PACAP 27 and are coupled to apamin (Apa)-sensitive K+ channels in the rat colon (Kishi et al., 1996; Ekblad, 1999). In addition to these receptors, PACAP-insensitive VIP receptors, coupled to charybdotoxin (ChTX)-sensitive K+ channels (Kishi et al., 1996), and VIP specific receptors regulated by neuropeptide Y (NPY) (Ekblad & Sundler, 1997) have been described in the rat colon and ileum smooth muscle, respectively.
PACAP receptors are coupled to several signal transduction pathways, i.e. activation of adenylate cyclase, stimulation of phospholipase C leading to inositol triphosphate (IP3)-mediated Ca2+ mobilization and Ca2+- and diacylglycerol-mediated PKC activation, or stimulation of a constitutive NO synthase (Makhlouf & Murthy, 1997; Hahm et al., 1998). A mechanism independent of both IP3 and cAMP has also been reported for the PACAP-induced Ca2+ release from ryanodine/caffeine stores (Tanaka et al., 1998).
The autonomic nervous system plays an essential role in the maintenance of ureteral motor activity. Thus, the classical neurotransmitters noradrenaline (NA) (Hernández et al., 1992) and acetylcholine (ACh) (Hernández et al., 1993) stimulate phasic activity and basal tone of the pig intravesical ureter through heterogeneous populations of adrenergic and muscarinic receptors, respectively. Autacoids such as 5-hydroxytryptamine (5-HT) essentially stimulate ureteral basal tone, these contractions being partly mediated via NA release from sympathetic nerves (Hernández et al., 2003). The role of nonpeptide, nonadrenergic, noncholinergic inhibitory neurotransmitters has also been elucidated in the intravesical ureter. Thus, NO (Hernández et al., 1995) relaxes the ureteral smooth muscle by a mechanism involving a soluble guanylate cyclase and the activation of glibenclamide-sensitive K+ channels (Hernández et al., 1997). Moreover, adenosine produces smooth muscle relaxation via A2B adenosine receptors and also modulates ureteral excitatory neurotransmission (Hernández et al., 1999).
Double label immunofluorescence techniques have revealed the colocalization of VIP and NO, and VIP and NPY in ureteral nerves (Smet et al., 1994). Thus, in the human lower ureter, VIP/NPY-like immunoreactive (VIP/NPY-LIR) and VIP/NOS-LIR nerve fibres supplying the submucosa and inner smooth muscle fascicles, and around blood vessels, have been described (Smet et al., 1994). NPY has been shown to enhance the adrenergic motor activity of the equine intravesical ureter and ureteral arteries (Prieto et al., 1997). In addition to the rich VIPergic motor innervation, in the rat ureter, a high density of PACAP immunoreactivity, essentially PACAP 38, has been localized to varicose nerve fibres associated with blood vessels, smooth muscle and subepithelium; PACAP was colocalized with calcitonin gene-related peptide (CGRP) in capsaicin-sensitive primary afferents (Fahrenkrug & Hannibal, 1998). Functional studies have described the involvement of tachykinins and CGRP, peptides localized in these afferents, in the ureteral NANC excitatory (Bustamante et al., 2000) and inhibitory (Maggi & Giuliani, 1991), respectively, neurotransmission. All these findings support a role for VIP and PACAP in the ureteral nonadrenergic noncholinergic (NANC) neurotransmission. However, there is no information about the subtype(s) of VIP/PACAP receptors as well as the underlying mechanisms involved in the ureteral VIP- and PACAP-induced relaxations. Therefore, the present study was undertaken to characterize pharmacologically the mechanisms and functionally active receptors involved in the relaxations evoked by VIP and PACAP in the pig intravesical ureter.
Methods
Adult pigs of either sex with no lesions in their urinary tract were selected from the local slaughterhouse. Urinary bladders with attached ureters were removed immediately after the animals were killed, and kept in chilled (4°C) physiological saline solution (PSS). The adjacent connective and fatty tissues were removed with care, and longitudinal preparations (4–6 mm long and 2–3 mm wide) of the intravesical ureter were isolated from the bladder by dissection, as previously described (Hernández et al., 1992). The ureteral strips were suspended horizontally with one end connected to an isometric transducer (Grass FT 03C) and the other one to a micrometer screw, which regulates the tension applied to the preparations, in 5 ml organ baths containing PSS gassed with 5% CO2 in O2, giving a final pH of 7.4. The signal was continuously recorded on a polygraph (Graphtec Multicorder MC 6621, Hugo Sachs Elektronik, Germany). Tension of 2 g (Hernández et al., 1992) was applied to the preparations and they were allowed to equilibrate for 60 min.
Experimental procedure
The contractile capacity of the samples was tested by exposing the ureteral strips to 124 mM potassium-enriched PSS (KPSS). The preparations were contracted with the thromboxane analogue U46619 (0.1 μM), and when the contraction was stable concentration–response relaxation curves were obtained to VIP, PACAP 38, PACAP 27, [Ala11,22,28]-VIP, CGRP, rolipram and forskolin.
The functional characterization of the VIP/PACAP receptors and the mechanisms involved in the VIP- and PACAP-induced relaxations were investigated in preparations contracted with 0.1 μM U46619. A first concentration–response curve was performed, the bath solution was changed every 20 min for a period of 80 min and the preparations were incubated for 30 min with the VIP receptor antagonists or the inhibitors of Ca2+-activated K+ channels, neuronal voltage-gated Ca2+ channels, NO synthase, guanylate cyclase or cAMP-dependent protein kinase A (PKA) and then a second relaxation curve was constructed. There were no significant differences in the U46619-induced tone in two consecutive relaxation curves, and this tone was maintained throughout the experiment. Control curves were run in parallel. The blockade of capsaicin-sensitive primary afferents was obtained by incubating ureteral preparations with 10 μM capsaicin for 1 h, washing every 20 min, this drug being present in the bath during the experiment. After the ureteral strips were incubated with the appropriate drug they were contracted to U46619 and a second concentration–response curve was obtained.
Drugs and solutions
The following drugs were used: Apa, CGRP (calcitonin gene-related peptide), capsaicin, ChTX, ω-CgTX (ω-conotoxin GVIA), forskolin, L-NOARG (NG-nitro-L-arginine), TEA (tetraethylammonium), U46619 (9,11-dideoxy-11α,9α-epoxy-methanoprostaglandin F2α), VIP (vasoactive intestinal peptide) and [Lys1, Pro2,5, Arg3,4, Tyr6]-VIP (all from Sigma, U.S.A.); Ala11,22,28]-VIP, ODQ (1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one) and rolipram (all from Tocris, U.K.); PACAP 38, PACAP 27 and PACAP (6–38) (all from Neosystem, France); and Rp-8-CPT-cAMPS (8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphorothioate) (Biolog, Germany).
U46619 and capsaicin were dissolved in 96% ethanol. ODQ, Rp-8-CPT-cAMPS, rolipram and forskolin were dissolved in dimethylsulphoxide. The other drugs were dissolved in distilled water. The solvents used had no effect on the ureteral activity.
The composition of PSS was (mM): NaCl 119, KCl 4.6, MgCl2 1.2, NaHCO3 24.9, glucose 11, CaCl2 1.5, KH2PO4 1.2, EDTA (ethylenediamine tetraacetic acid) 0.027. The solution was continuously gassed at 37°C with 95% O2 and 5% CO2, to maintain the pH at 7.4. KPSS was PSS with KCl exchanged for NaCl on an equimolar basis. Stock solutions were prepared daily in distilled water.
Calculations and statistics
For each concentration–response curve, the concentrations required to give half-maximal response (EC50) to VIP, PACAP analogues, rolipram and forskolin were estimated by computerized nonlinear regression analysis (GraphPad Prism, U.S.A.). The sensitivity of the drugs is expressed in terms of pD2, where pD2 is defined as the negative logarithm of EC50 (pD2=−log EC50 (M). Each parameter was determined from ureters of at least 4–6 different animals. Results are shown as percentage of 0.1 μM U46619-induced contraction. Statistical significance of differences was calculated by Student's t-test, for paired observations for individual concentrations and by variance analysis (ANOVA) for multiple comparisons, followed by an a posteriori Bonferroni test. Differences were considered significant with a probability level of P<0.05.
Results
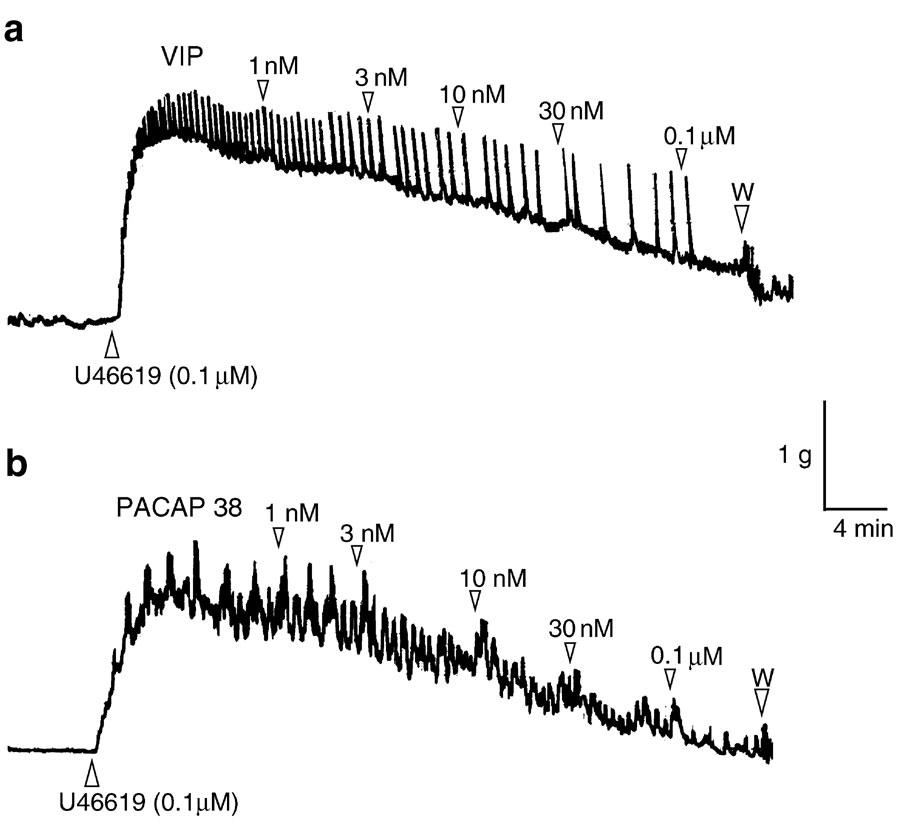
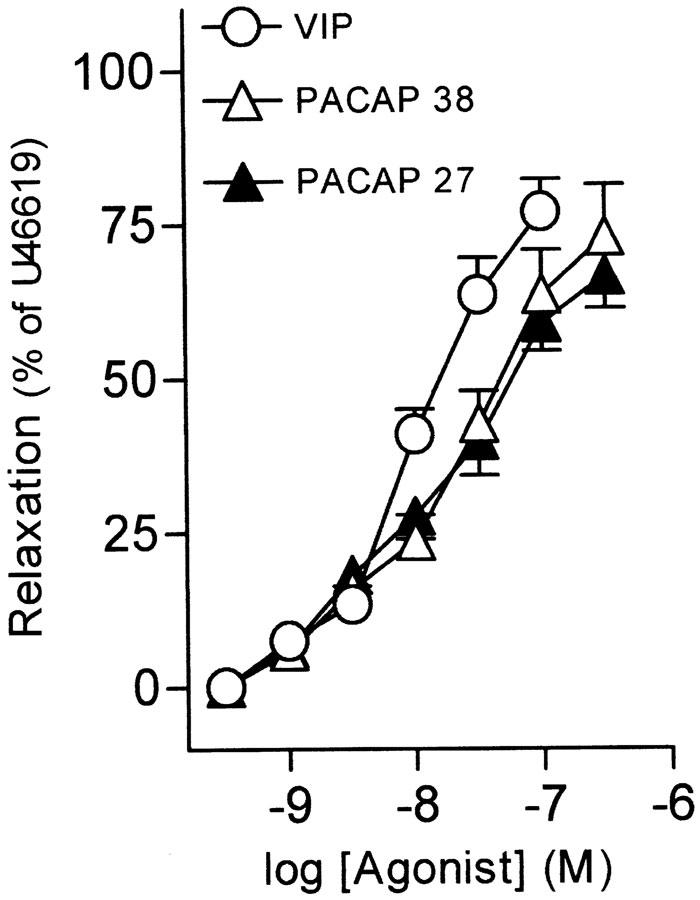
Pig intravesical ureteral strips were allowed to equilibrate to a passive tension of 1. 7±0.4 g (n=93). Under these conditions, the exposition of samples to KPSS evoked a contraction of 2.3±0.4 g (n=93). U46619 (0.1 μM) induced a sustained increase in tone above the baseline of 1.8±0.3 g (n=93). VIP (1 nM–0.1 μM), PACAP 38 (1 nM–0.3 μM), and PACAP 27 (1 nM–0.3 μM), evoked concentration-dependent slow relaxations of the U46619-contracted intravesical ureteral strips, with the order of potency being VIP=PACAP 27=PACAP 38 (Figures 1a, b, 2, Table 1). [Ala11,22,28]-VIP (1 nM–0.3 μM), a selective VPAC1 agonist, produced no response in eight out of 14 preparations studied, while it induced a poor (19.6±6.3% of U46619-induced contraction) relaxation in the other six strips. Calcitonin gene-related peptide (CGRP) induced a small relaxation of 12.1±3.9% (n=7), at a concentration of 0.1 μM.
Figure 1.
Isometric force recordings showing the relaxations to (a) VIP (1 nM–0.1 μM) and (b) PACAP 38 (1 nM–0.1μM) in U46619 (0.1 μM)-contracted pig intravesical ureteral strips. Vertical bar shows tension in g and horizontal bar time in min. Numbers indicate nano- or micromolar concentrations in the organ bath. W: wash out.
Figure 2.
Log concentration–response relaxation curves to VIP, PACAP 38 and PACAP 27 in pig intravesical ureter contracted to U46619 (0.1 μM). Relaxations are expressed as a percentage of the U46619-induced contraction. The results represent means and vertical lines s.e.mean of 7–12 preparations.
Table 1.
Relaxations induced by VIP, PACAP 38 and PACAP 27 in pig intravesical ureter
| Agonist | n | pD2 | Emax (%) |
|---|---|---|---|
| VIP | 12 | 7.91±0.04 | 77.0±5.3 |
| PACAP 38 | 7 | 7.69±0.09 | 72.9±8.5 |
| PACAP 27 | 9 | 7.83±0.10 | 66.6±5.2 |
The results are expressed as mean ± s.e.m. of n experiments. Differences were analysed by one-way analysis of variance (ANOVA) followed by an a posteriori Bonferroni t-test in case of significance. Emax is the maximal relaxation, expressed as a percentage of the U46619-induced contraction, obtained for each drug. pD2=−log EC50, where EC50 is the concentration of agonist producing 50% of the Emax.
Experiments to determine the localization of VIP/PACAP receptors and the role of NO on the relaxations to VIP and PACAP 38
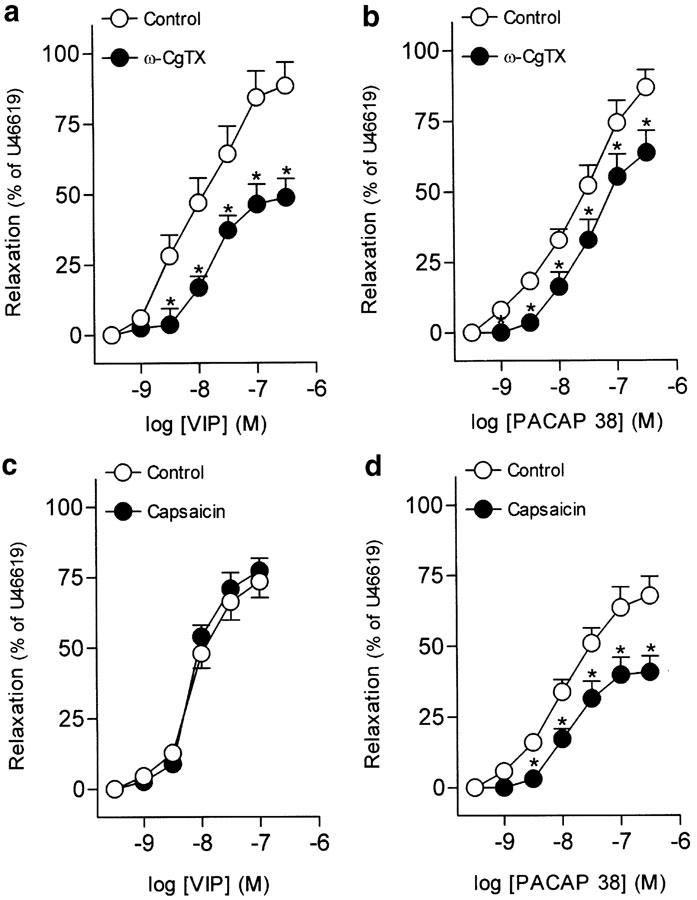
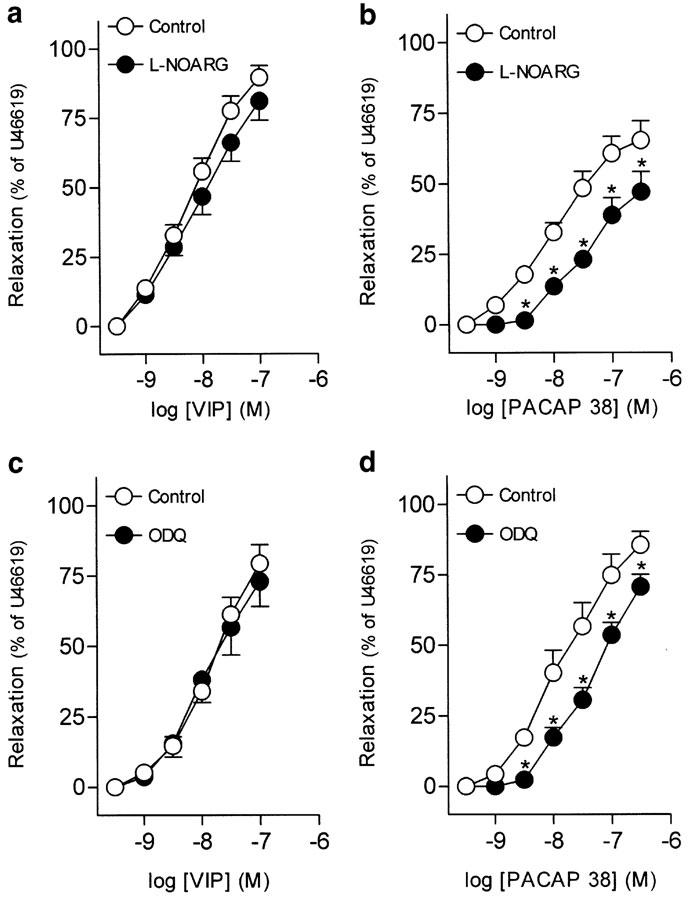
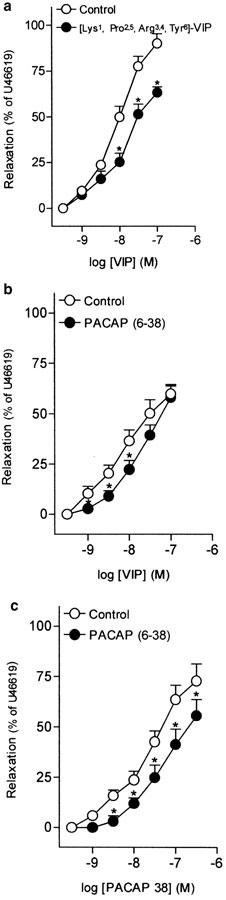
Urothelium removal failed to modify VIP-induced relaxations in the intravesical ureter (Table 2). The inhibitor of neuronal voltage-gated Ca2+ channels, ω-CgTX (1 μM), reduced the relaxations to VIP and PACAP 38 (Figures 3a, b, Tables 2, 3). Blockade of capsaicin-sensitive primary afferents, NO synthase or guanylate cyclase with capsaicin (10 μM), L-NOARG (100 μM) and ODQ (5 μM), respectively, did not change the relaxations to VIP (Figures 3c, 4a, c, Table 2). These blockers, however, reduced the PACAP 38-induced relaxations (Figures 3d, 4b, d, Table 3).
Table 2.
Effect of urothelium removal and neuronal-, NO synthase-, guanylate cyclase- and Ca2+-activated K+ channel blockers and NPY on the relaxations to VIP and PACAP 27
| n | pD2 | Emax (%) | |
|---|---|---|---|
| VIP | |||
| +Urothelium | 7 | 7.22±0.07 | 63.9±6.2 |
| −Urothelium | 7 | 7.19±0.09 | 68.9±6.3 |
| Control | 6 | 8.01±0.12 | 88.3±8.3 |
| ω-CgTX (1 μM) | 6 | — | 48.8±6.7* |
| Control | 6 | 8.14±0.11 | 73.4±5.6 |
| Capsaicin (10 μM) | 6 | 8.16±0.09 | 77.3±4.5 |
| Control | 7 | 8.01±0.11 | 89.5±4.4 |
| L-NOARG (100 μM) | 7 | 7.92±0.12 | 81.0±6.8 |
| Control | 8 | 7.91±0.07 | 79.2±6.9 |
| ODQ (5 μM) | 8 | 7.98±0.08 | 72.9±8.8 |
| Control | 7 | 7.78±0.09 | 76.3±4.9 |
| ChTX (0.1 μM) | 7 | 7.79±0.08 | 68.9±5.3 |
| Control | 6 | 7.76±0.10 | 77.8±4.3 |
| TEA (3 mM) | 6 | 7.78±0.09 | 79.1±5.1 |
| Control | 6 | 7.90±0.16 | 85.1±6.1 |
| NPY (0.1 μM) | 6 | 7.84±0.19 | 82.4±4.1 |
| PACAP 27 | |||
| Control | 6 | 7.85±0.10 | 83.6±5.1 |
| Apa (1 μM) | 6 | 7.80±0.11 | 83.3±7.5 |
Results are expressed as mean ± s.e.mean of n experiments.
Parameter significantly (P<0.05) different compared (paired t-test) to the control value. Emax is the maximal relaxation, expressed as a percentage of the U46619-induced contraction, obtained for each drug. pD2=−log EC50, where EC50 is the concentration of agonist producing 50% of the Emax.
Figure 3.
Log concentration–response relaxation curves to (a, c) VIP and (b, d) PACAP 38 in pig intravesical ureter contracted to U46619 (0.1 μM), in control conditions and in the presence of ω-CgTX (1 μM) (a, b) or capsaicin (10 μM) (c, d). Relaxations are expressed as a percentage of the U46619-induced contraction. The results represent means and vertical lines s.e.m. of 6–8 preparations. *P<0.05 versus control value (paired t-test).
Table 3.
Effect of blockers of neuronal voltage-gated Ca2+ channels, capsaicin-sensitive primary afferents, NO synthase and guanylate cyclase on the relaxations to PACAP 38
| n | pD2 | Emax (%) | |
|---|---|---|---|
| Control | 6 | 7.53±0.11 | 86.7±6.3 |
| ω-CgTX (1 μM) | 6 | — | 63.7±7.8* |
| Control | 8 | 7.95±0.08 | 67.7±6.9 |
| Capsaicin (10 μM) | 8 | — | 40.7±5.7* |
| Control | 6 | 7.96±0.12 | 65.2±7.0 |
| L-NOARG (100 μM) | 6 | — | 43.6±7.2* |
| Control | 6 | 7.68±0.20 | 85.5±4.7 |
| ODQ (5 μM) | 6 | — | 70.7±4.3* |
The results are expressed as mean ±s.e.m. of n experiments.
Parameter significantly (P<0.05) different compared (paired t-test) to the control value. Emax is the maximal relaxation, expressed as a percentage of the U46619-induced contraction, obtained for each drug. pD2=−log EC50, where EC50 is the concentration of agonist producing 50% of the Emax.
Figure 4.
Log concentration–response relaxation curves to (a, c) VIP and (b, d) PACAP 38 in pig intravesical ureter contracted to U46619 (0.1 μM), in control conditions and in the presence of L-NOARG (100 μM) (a, b) or ODQ (5 μM) (c, d). Relaxations are expressed as a percentage of the U46619-induced contraction. The results represent means and vertical lines s.e.m. of 6–8 preparations. *P<0.05 versus control value (paired t-test).
Experiments to determine the VIP/PACAP receptors involved in the relaxations to VIP and PACAP 38.
Treatment of ureteral strips with [Lys1, Pro2,5, Arg3,4, Tyr6]-VIP (1 μM), a VIP receptor antagonist, reduced the VIP maximal relaxation (Figure 5a, Table 4). PACAP (6–38) (0.4 μM), a VIP/PACAP receptor antagonist, produced a rightwards displacement of the concentration–response relaxation curve to VIP and a reduction of the PACAP 38 maximal relaxation (Figures 5b, c, Table 4).
Figure 5.
Log concentration–response relaxation curves to (a, b) VIP and (c) PACAP 38 in pig intravesical ureter contracted to U46619 (0.1 μM), in control conditions and in the presence of [Lys1, Pro2,5, Arg3,4, Tyr6]-VIP (1 μM) (a) or PACAP (6–38) (0.4 μM) (b, c). Relaxations are expressed as a percentage of the U46619-induced contraction. The results represent means and vertical lines s.e.m. of 6–7 preparations. *P<0.05 versus control value (paired t-test).
Table 4.
Effect of VIP and VIP/PACAP receptor antagonists on the relaxations to VIP and PACAP 38
| n | pD2 | Emax (%) | |
|---|---|---|---|
| VIP | |||
| Control | 7 | 8.08±0.07 | 90.1±5.3 |
| [Lys1, Pro2,5, Arg3,4,Tyr6]-VIP (1 μM) | 7 | — | 63.2±3.6* |
| Control | 6 | 8.11±0.12 | 59.9±4.6 |
| PACAP (6-38) (0.4 μM) | 6 | 7.74±0.08* | 58.2±5.7 |
| PACAP 38 | |||
| Control | 6 | 7.66±0.12 | 72.9±8.5 |
| PACAP (6–38) (0.4 μM) | 6 | — | 55.6±8.1* |
The results are expressed as mean±s.e.m. of n experiments.
Parameter significantly (P<0.05) different compared (paired t-test) to the control value. Emax is the maximal relaxation, expressed as a percentage of the U46619-induced contraction, obtained for each drug. pD2=−log EC50, where EC50 is the concentration of agonist producing 50% of the Emax.
Role of Ca2+-activated K+ channels and NPY in the relaxations to VIP
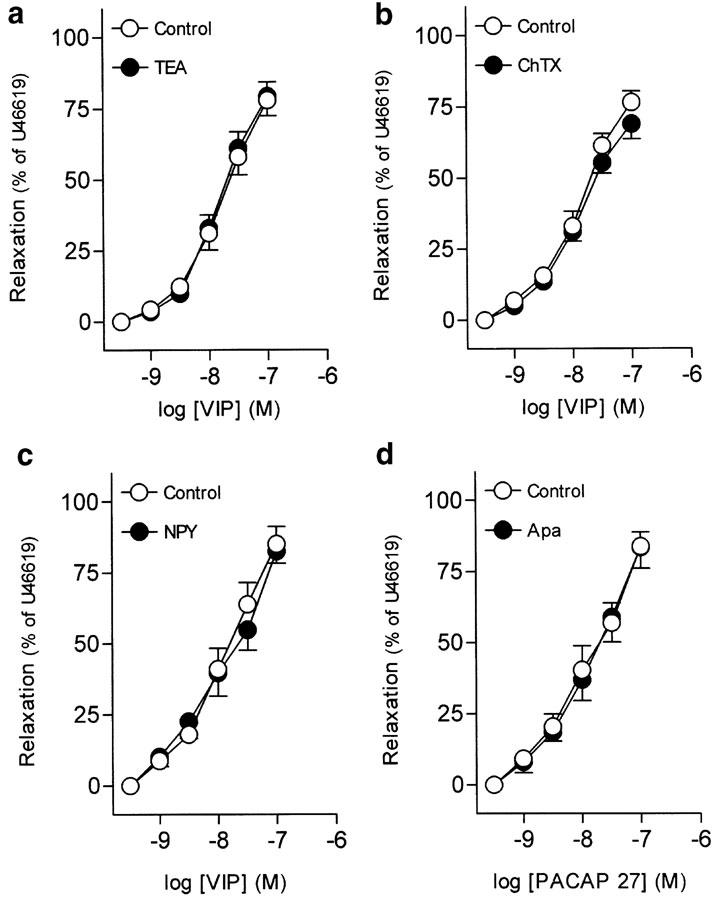
Pretreatment of ureteral strips with TEA (3 mM) and ChTX (0.1 μM),. non-selective and large-conductance Ca2+-activated K+ channel blockers, respectively, failed to modify the relaxations to VIP (Figures 6a, b, Table 2). NPY (0.1 μM) also did not alter the VIP-induced relaxations (Figure 6c, Table 2).
Figure 6.
Log concentration–response relaxation curves to (a–c) VIP and (d) PACAP 27 in pig intravesical ureter contracted to U46619 (0.1 μM), in control conditions and in the presence of TEA (3 mM) (a), ChTX (0.1 μM) (b), NPY (0.1 μM) (c) Apa (1 μM) (d). Relaxations are expressed as a percentage of the U46619-induced contraction. The results represent means and vertical lines s.e.m. of 6–7 preparations.
Experiments to investigate the smooth muscle PAC1 receptors involved in the relaxations to PACAP 27
The inhibitor of small-conductance Ca2+-activated K+ channels, Apa (1 μM), did not change the relaxations to PACAP 27 (Figure 6d, Table 2).
Experiments. to determine the involvement of the adenylate cyclase/cAMP pathway in the relaxations to VIP
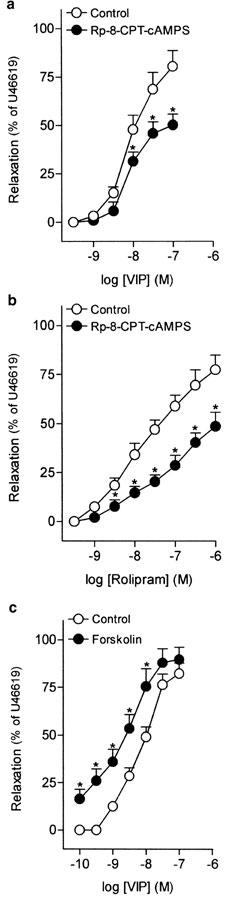
Rolipram (1 nM–1 μM) and forskolin (1 nM–10 μM), a phosphodiesterase type 4 (PDE 4) inhibitor and an adenylate cyclase activator, respectively, induced concentration-dependent relaxations of the intravesical ureteral strips (Table 5). The pD2 and maximal relaxation for forskolin were 6.26±0.16 and 94.5±3.19%, respectively (n=7). The inhibitor of cAMP-dependent PKA, Rp-8-CPT-cAMPS (100 μM), reduced the relaxations evoked by VIP and rolipram (Figures 7a, b, Table 5). In addition, 30 nM forskolin, which relaxed ureteral strips by 11.2±3.5% (n=7), evoked a leftwards displacement of the concentration–response relaxation curve to VIP (Figure 7c, Table 5).
Table 5.
Effect of inhibition of the cAMP-dependent PKA pathway on relaxations to VIP and rolipram and of activation of adenylate cyclase on the relaxations to VIP
| n | pD2 | Emax (%) | |
|---|---|---|---|
| VIP | |||
| Control | 7 | 8.06±0.06 | 80.6±7.1 |
| Rp-8-CPT-cAMPS (100 μM) | 7 | — | 50.3±5.6* |
| Control | 6 | 8.10±0.08 | 82.3±5.4 |
| Forskolin (30 nM) | 6 | 8.71±0.18* | 89.7±6.4 |
| Rolipram | |||
| Control | 6 | 7.64±0.12 | 78.5±7.5 |
| Rp-8-CPT-cAMPS (100 μM) | 6 | — | 48.5±7.2* |
The results are expressed as mean±s.e.m. of n experiments.
Parameter significantly (P<0.05) different compared (paired t-test) to the control value. Emax is the maximal relaxation, expressed as a percentage of the U46619-induced contraction, obtained for each drug. pD2=−log EC50, where EC50 is the concentration of agonist producing 50% of the Emax.
Figure 7.
Log concentration–response relaxation curves to (a, c) VIP and (b) rolipram in pig intravesical ureter contracted to U46619 (0.1 μM), in control conditions and in the presence of Rp-8-CPT-cAMPS (100 μM) (a, b) or forskolin (30 nM) (c). Relaxations are expressed as a percentage of the U46619-induced contraction. The results represent means and vertical lines s.e.m. of 6–7 preparations. *P<0.05 versus control value (paired t-test).
Discussion
The present study was designed to characterize the VIP/PACAP receptor(s) and the underlying mechanisms involved in the relaxations to VIP and PACAP of the pig intravesical ureteral smooth muscle. Our results suggest the existence of PAC1 receptors coupled to NO production on sensory nerves and NO-independent neuronal VPAC receptors. Smooth muscle VPAC receptors, probably of the VPAC2 subtype and linked to activation of the cAMP-dependent PKA pathway, also seem to be involved.
VIP and PACAP analogues produced a slow relaxation of the pig intravesical ureter, which agrees with that reported by Kishi et al. (1996) for rat intestine. These authors ascribed the delay of relaxing responses to VIP and PACAP to the slow spread of these peptides into the smooth muscle. However, the slow-starting long-lasting relaxations to VIP and PACAP may also be due to their indirect action by releasing other transmitters from nerve endings, and to the intracellular signalling pathways, that is, adenylate cyclase/cAMP, activated by these peptides in the smooth muscle. Removal of the urothelium did not modify the relaxations to VIP, thus indicating that these responses are mediated via receptors located in the smooth muscle. However, the mediation of receptors placed at ureteral nerves is also suggested by ω-CgTX inhibition of VIP and PACAP 38 relaxations.
Neuronal PACAP and VIP receptors comprise the PAC1 (Ekblad, 1999) and VPAC (Seebeck et al., 2002) subtypes, respectively. Neuronal PAC1 receptors are localized on NO-synthesizing neurons exhibiting a high affinity for PACAP 38 (Ekblad, 1999). In the rat ureter, numerous PACAP 38-positive nerve fibres have been reported in the adventitia, muscle and subepithelial layers (Fahrenkrug & Hannibal, 1998). PACAP 38 colocalizes with CGRP in capsaicin-sensitive primary afferents, suggesting a chemosensory or nociceptive role of PACAP 38 in the ureter, in addition to its efferent function in the regulation of blood flow and motility, according to its presence around blood vessels and in the smooth muscle (Fahrenkrug & Hannibal, 1998). Unlike the potent relaxation induced by PACAP 38 in the pig intravesical ureter, CGRP evoked a poor relaxation, which agrees with the scarce CGRP-IR innervation found in the smooth muscle (unpublished observations) and suggests a minor motor role for CGRP. Capsaicin did not modify the responses to VIP but reduced the PACAP 38-induced relaxations. These results, together with the inhibition elicited by ω-CgTX on PACAP 38 relaxations, suggest the existence of prejunctional PAC1 receptors in sensory nerves, whose activation partially mediates the relaxations to PACAP 38. Since these relaxations were also reduced by inhibitors of NOS and guanylate cyclase, the neuronal PAC1 receptors involved seem to be coupled to NO release. This finding agrees with that reported for rat colon (Ekblad, 1999) and basilar arteries (Seebeck et al., 2002), where a neuronal NO synthase expressed in intramural neurons partially mediates the PACAP-induced relaxation. In the distal ureter, NO is present in postganglionic parasympathetic nerves, with cell bodies in the major pelvic ganglia, and in sensory nerves with cell bodies in the lumbosacral dorsal root ganglia (Mohammed & Santer, 2001). Therefore, our data confirm the motor role for PACAP in the ureter suggested by Fahrenkrug & Hannibal (1998) and indicate that this role is in part related to the modulation of NO production from ureteral sensory nerves.
VIP may be released from nerve endings in parallel with NO or be linked to NO in such a way that NO regulates VIP release while VIP directly stimulates the production of NO in target cells by increasing NOS activity and thereby activating soluble guanylate cyclase (Grider et al.,1992; Murthy & Makhlouf, 1994). NO plays an essential role as a NANC inhibitory neurotransmitter in the intravesical ureter (Hernández et al., 1995). In the human ureter, VIP colocalizes with NOS in nerve fibres running along smooth muscle bundles beneath the epithelium and around blood vessels (Edyvane et al., 1992; Smet et al., 1994). On the basis of the colocalization of VIP and NO in ureteral nerves, it could be possible that part of the relaxation to VIP was indirectly produced by NO released from ureteral nerves, or mediated by NO produced within smooth muscle. However, the relaxations to VIP were not modified by L-NOARG or ODQ, which initially rules out an interaction between VIP and NO. These results agree with those found in the pig gastric fundus where a role for NO in the relaxations to VIP was excluded (Lefebvre et al., 1995). Nevertheless, the reduction caused by ω-CgTX on VIP-induced relaxations suggests the presence of neuronal VPAC receptors, whose activation causes an NO-independent relaxation of the pig intravesical ureter.
VIP- and PACAP-related peptides also act at smooth muscle receptors. VPAC1 receptors have been identified in several peripheral tissues of the rat such as the lung and small intestine (Usdin et al., 1994). The inconsistent relaxations shown by [Ala 11,22,28]-VIP, a selective VPAC1 receptor agonist (Nicole et al., 2000), and the inhibition produced by a PACAP (6–38) concentration (0.4 μM) selective for VPAC2 receptors (Dickinson & Fleetwood-Walker, 1999), indicate that these receptors may be involved in the VIP- and PACAP-induced relaxations of the intravesical ureter. However, 0.4 μM PACAP (6–38) also blocks PAC1 receptors (Dickinson & Fleetwood-Walker, 1999). Smooth muscle PAC1 receptors have been reported in rat colon (Kishi et al., 1996; Ekblad, 1999). In our preparation, the potency order exhibited by the agonists (VIP=PACAP 27=PACAP 38), the inhibition produced by [Lys1, Pro2,5, Arg3,4, Tyr6]-VIP on VIP relaxations, and the lack of effect of Apa on the relaxations to PACAP 27 rule out the mediation of smooth muscle PAC1 receptors and suggest the involvement of smooth muscle VPAC2 receptors. These results agree with those published in the human airways, where VIP-induced broncodilatation is mediated through VPAC2 receptors (Schmidt et al., 2001).
VIP relaxations in rat colon are mediated via receptors coupled to ChTX-sensitive K+ channels (Kishi et al., 1996). In the current study, ChTX and TEA did not modify the relaxations to VIP, which excludes the involvement of large- and medium-conductance Ca2+-activated K+ channels in the relaxations to VIP. A VIP-specific, NPY-regulated receptor has also been reported in the rat ileum (Ekblad & Sundler, 1997). In the lower ureter, a rich density of NPY-IR nerve fibres to the smooth muscle and blood vessels has been described (Smet et al., 1994; Prieto et al., 1997) and VIP colocalizes with NPY in these nerves (Smet et al., 1994). However, the lack of effect of NPY on VIP relaxations excludes a VIP-specific receptor regulated by NPY.
VPAC receptor stimulation initiates signalling cascades in smooth muscle that lead to cAMP generation (Vaudry et al., 2000). Smooth muscle cAMP synthesis and degradation are regulated by adenylate cyclase and by phosphodiesterase 4 (PDE 4), respectively (Ekholm et al., 1997; Murthy et al., 2001). In ureteral tissue, the presence of PDE 4 and the ability of PDE 4 inhibitors, such as rolipram, to relax the human ureter have been reported. (Taher et al., 1994; Kühn et al., 2000). In our study, rolipram evoked a potent relaxation, thus suggesting an enhancement of ureteral basal cAMP levels. These results agree with those observed in the human (Taher et al., 1994; Kühn et al., 2000) and rabbit (Becker et al., 1998) ureter. The specificity of the rolipram relaxant effect and the involvement of cAMP is confirmed by the inhibition caused by Rp-8-CPT-cAMPS. The fact that Rp-8-CPT-cAMPS reduced VIP relaxations to a lesser extent than those of rolipram is probably related to the involvement of neuronal mechanisms in VIP relaxations, as depicted from the inhibition produced by ω-CgTX. The adenylate cyclase activator forskolin relaxed the ureteral strips. In addition, a threshold concentration (30 nM) of forskolin evoked a potentiation of VIP relaxations. The enhancement of VIP responses by forskolin, and its inhibition by blockade of PKA suggest an involvement of the adenylate cyclase/cAMP pathway in the relaxations to VIP in the intravesical ureter.
In conclusion, the results of the present study suggest that the relaxations induced by VIP and PACAP in the pig intravesical ureter involve non-neuronal and neuronal mechanisms. Thus, VIP and PACAP analogues relax intravesical ureter probably through smooth muscle VPAC2 receptors. VIP-elicited relaxations seem to be partially mediated by the cAMP-dependent PKA pathway without apparently TEA-sensitive K+ channels being implicated. The relaxations to VIP also involve neuronal VPAC receptors placed at ureteral motor nerves and not linked to NO release. Finally, a part of the PACAP 38-induced relaxation is due to activation of neuronal PAC1 receptors coupled to NO release and located in capsaicin-sensitive primary afferents. Since intravesical ureter is an integral part of the ureterovesical junction, the potent relaxations exerted by VIP and PACAP would favour the urine bolus progression throughout the ureter and its discharge into the urinary bladder. On the other hand, the reported location of PACAP in sensory nerves would suggest a role for this agent by favouring the progression of ureteral calculi in the ureterovesical junction.
Acknowledgments
The authors thank Mr. Manuel Perales and Mr. Francisco Puente for their technical assistance. They also thank GIPISA slaughterhouse (Pozuelo de Alarcón, Madrid) for kindly donating the ureters.
Abbreviations
- Apa
apamin
- ChTX
charybdotoxin
- ω-CgTX
ω-conotoxin GVIA
- L-NOARG
NG-nitro-L-arginine
- ODQ
1H-[1,2,4]-oxadiazolo[4,3-a]quinoxalin-1-one
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PKA
cAMP-dependent protein kinase A
- Rp-8-CPT-cAMPS
8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphorothioate
- TEA
tetraethylammonium
- U46619
9,11-dideoxy-11α,9α-epoxy-methanoprostaglandin F2α
- VIP
vasoactive intestinal peptide
References
- BECKER A.J., STIEF C.G., MEYER M., TRUSS M.C., FORSSMANN W.G., JONAS U. The effect of the specific phosphodiesterase-IV-inhibitor rolipram on the ureteral peristalsis of the rabbit in vitro and in vivo. J. Urol. 1998;160:920–925. doi: 10.1016/S0022-5347(01)62833-7. [DOI] [PubMed] [Google Scholar]
- BUSTAMANTE S., ORENSANZ L.M., BARAHONA M.V., CONTRERAS J., GARCÍA-SACRISTÁN A., HERNÁNDEZ M. Tachykininergic excitatory neurotransmission in the pig intravesical ureter. J. Urol. 2000;164:1371–1375. [PubMed] [Google Scholar]
- DICKINSON T., FLEETWOOD-WALKER S.M. VIP and PACAP: very important in pain. Trends Pharmacol. Sci. 1999;20:324–329. doi: 10.1016/s0165-6147(99)01340-1. [DOI] [PubMed] [Google Scholar]
- EDYVANE K.A., TRUSSELL D.C., JONAVICIUS J., HENWOOD E.A., MARSHALL V.R. Presence and regional variation in peptide-containing nerves in the human ureter. J. Auton. Nerv. Syst. 1992;39:127–138. doi: 10.1016/0165-1838(92)90053-j. [DOI] [PubMed] [Google Scholar]
- EKBLAD E. Pharmacological evidence for both neuronal and smooth muscular PAC1 receptors and a VIP-specific receptor in rat colon. Regul. Pept. 1999;85:87–92. doi: 10.1016/s0167-0115(99)00080-4. [DOI] [PubMed] [Google Scholar]
- EKBLAD E., SUNDLER F. Distinct receptors mediate pituitary adenylate cyclase-activating peptide and vasoactive intestinal peptide-induced relaxation of rat ileal longitudinal muscle. Eur. J. Pharmacol. 1997;334:61–66. doi: 10.1016/s0014-2999(97)01144-8. [DOI] [PubMed] [Google Scholar]
- EKHOLM D., BELFRAGE P., MANGANIELLO V., DEGERMAN E. Protein kinase A-dependent activation of PDE4 (cAMP-specific cyclic nucleotide phospho diesterase) in cultured bovine vascular smooth muscle cells. Biochem. Biophys. Acta. 1997;1356:64–70. doi: 10.1016/s0167-4889(96)00159-0. [DOI] [PubMed] [Google Scholar]
- FAHRENKRUG J., HANNIBAL J. Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience. 1998;83:1261–1272. doi: 10.1016/s0306-4522(97)00474-0. [DOI] [PubMed] [Google Scholar]
- GODET A., HEIDGER P.M., JR., WINFIELD H.N. Peptidergic nerves in the ureter. J. Endourol. 1993;7:407–410. doi: 10.1089/end.1993.7.407. [DOI] [PubMed] [Google Scholar]
- GRIDER J.R., MURTHY K.S., JIN J.G., MAKHLOUF G.M. Stimulation of nitric oxide from muscle cells by VIP: prejunctional enhancement of VIP release. Am. J. Physiol. 1992;262:G774–G778. doi: 10.1152/ajpgi.1992.262.4.G774. [DOI] [PubMed] [Google Scholar]
- HAHM S.H., HSU C.M., EIDEN L.E. PACAP activates calcium influx-dependent and -independent pathways to couple met-enkephalin secretion and biosynthesis in chromaffin cells. J. Mol. Neurosci. 1998;11:43–56. doi: 10.1385/JMN:11:1:43. [DOI] [PubMed] [Google Scholar]
- HARMAR A.J., ARIMURA A., GOXES I., JOURNOT L., LABURTHE M., PISENGA J.R., RAWLINGS S.R., ROBBERECHT P., SAID S.I., SREEDHARAN S.P., WANK S.A., WASCHNEK J.A. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- HERNÁNDEZ M., BARAHONA M.V., BUSTAMANTE S., GARCÍA-SACRISTÁN A., ORENSANZ L.M. A2B adenosine receptors mediate relaxation of the pig intravesical ureter: adenosine modulation of non adrenergic non cholinergic excitatory neurotransmission. Br. J. Pharmacol. 1999;126:969–978. doi: 10.1038/sj.bjp.0702386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁNDEZ M., BARAHONA M.V., SIMONSEN U., RECIO P., RIVERA L., MARTÍNEZ A.C., GARCÍA-SACRISTÁN A., ORENSANZ L.M., PRIETO D. Characterization of the 5-hydroxytryptamine receptors mediating contraction in the pig isolated intravesical ureter. Br. J. Pharmacol. 2003;138:137–144. doi: 10.1038/sj.bjp.0705019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁNDEZ M., PRIETO D., ORENSANZ L.M., BARAHONA M.V., GARCÍA-SACRISTÁN A., SIMONSEN U. Nitric oxide is involved in the non-adrenergic non-cholinergic inhibitory neurotransmission of the pig intravesical ureter. Neurosci. Lett. 1995;186:33–36. doi: 10.1016/0304-3940(94)11275-n. [DOI] [PubMed] [Google Scholar]
- HERNÁNDEZ M., PRIETO D., ORENSANZ L.M., BARAHONA M.V., JIMÉNEZ-CIDRE M., RIVERA L., GARCÍA-SACRISTÁN A., SIMONSEN U. Involvement of a glibenclamide-sensitive mechanism in the nitrergic neurotransmission of the pig intravesical ureter. Br. J. Pharmacol. 1997;120:609–616. doi: 10.1038/sj.bjp.0700952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁNDEZ M., PRIETO D., SIMONSEN U., RIVERA L., BARAHONA M.V., GARCÍA-SACRISTÁN A. Noradrenaline modulates smooth muscle activity of the isolated intravesical ureter of the pig through different types of adrenoceptors. Br. J. Pharmacol. 1992;107:924–931. doi: 10.1111/j.1476-5381.1992.tb13387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNÁNDEZ M., SIMONSEN U., PRIETO D., RIVERA L., GARCÍA P., ORDAZ E., GARCÍA-SACRISTÁN A. Different muscarinic receptor subtypes mediating the phasic activity and basal tone of pig isolated intravesical ureter. Br. J. Pharmacol. 1993;110:1413–1420. doi: 10.1111/j.1476-5381.1993.tb13978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSOKAWA H., KASEDA M. Experimental studies on VIP as non-cholinergic and non-adrenergic neurotransmitter in bladder neck and posterior urethra. Nippon Hinyokika Gakkai Zasshi. 1993;84:440–449. doi: 10.5980/jpnjurol1989.84.440. [DOI] [PubMed] [Google Scholar]
- KISHI M., TAKEUCHI T., SUTHAMNATPONG N., ISHII T., NISHIO H., HATA F., TAKEWAKI T. VIP- and PACAP-mediated nonadrenergic, noncholinergic inhibition in longitudinal muscle of rat distal colon: involvement of activation of charybdotoxin- and apamin-sensitive K+ channels. Br. J. Pharmacol. 1996;119:623–630. doi: 10.1111/j.1476-5381.1996.tb15719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KÜHN R., ÜCKERT S., STIEF C.G., TRUSS M.C., LIETZ B., BISCHOFF E., SCHRAMM M., JONAS U. Relaxation of human ureteral smooth muscle in vitro by modulation of cyclic nucleotide-dependent pathways. Urol. Res. 2000;28:110–115. doi: 10.1007/s002400050147. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A., SMITHS G.J.M., TIMMERMANS J.P. Study of NO and VIP as non-adrenergic non-cholinergic neurotransmitter in the pig gastric fundus. Br. J. Pharmacol. 1995;116:2017–2026. doi: 10.1111/j.1476-5381.1995.tb16406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S. The neurotransmitter role of CGRP in the rat and guinea-pig ureter: effect of a CGRP antagonist and species-related differences in the action of omega conotoxin on CGRP release from primary afferents. Neuroscience. 1991;43:261–271. doi: 10.1016/0306-4522(91)90433-o. [DOI] [PubMed] [Google Scholar]
- MAKHLOUF G.M., MURTHY K.S. Signal transduction in gastrointestinal smooth muscle. Cell Signal. 1997;9:269–276. doi: 10.1016/s0898-6568(96)00180-5. [DOI] [PubMed] [Google Scholar]
- MOHAMMED H., SANTER R.M. Distribution and changes with age of nitric oxide synthase-immunoreactive nerves of the rat urinary bladder, ureter and in lumbosacral sensory neurons. Eur. J. Morphol. 2001;39:137–144. doi: 10.1076/ejom.39.3.137.4676. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., MAKHLOUF G.M. Vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide-dependent activation of membrane-bound NO synthase in smooth muscle mediated by pertussis toxin-sensitive Gi1-2. J. Biol. Chem. 1994;269:15977–15980. [PubMed] [Google Scholar]
- MURTHY K.S., ZHOU H., MAKHLOUF G.M. PKA-dependent activation of PDE3A and PDE4 and inhibition of adenylyl cyclase V/VI in smooth muscle. Am. J. Physiol. 2001;282:C508–C517. doi: 10.1152/ajpcell.00373.2001. [DOI] [PubMed] [Google Scholar]
- NICOLE P., LINS L., ROUYER-FESSARD C., DROUOT C., FULCRAND P., THOMAS A., COUVINEA A., MARTINEZ J., BRASSEUR R., LABURTHE M. Identification of key residues for interaction of vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors and development of a high selective VPAC1 receptor agonist. Alanine scanning and molecular modelling of the peptide. J. Biol. Chem. 2000;275:24003–24012. doi: 10.1074/jbc.M002325200. [DOI] [PubMed] [Google Scholar]
- PRIETO D., HERNÁNDEZ M., RIVERA L., GARCÍA-SACRISTÁN A., SIMONSEN U. Distribution and functional effects of neuropeptide Y on equine ureteral smooth muscle and resistance arteries. Regul. Pept. 1997;69:155–165. doi: 10.1016/s0167-0115(97)00003-7. [DOI] [PubMed] [Google Scholar]
- SCHMIDT D.T., RUHLMANN E., WALDECK B., BRANSCHEID D., LUTS A., SUNDLER F., RABE K.F. The effect of the vasoactive intestinal polypeptide agonist Ro 25-1553 on induced tone in isolated human airways and pulmonary artery. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:314–320. doi: 10.1007/s002100100458. [DOI] [PubMed] [Google Scholar]
- SEEBECK J., LÖWE M., KRUSE M.L., SCHMIDT W.E., MEHDORN H.M., ZIEGLER A., HEMPELMANN R.G. The vasorelaxant effect of pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in isolated rat basilar arteries is partially mediated by activation of nitrergic neurons. Regul. Pept. 2002;107:115–123. doi: 10.1016/s0167-0115(02)00072-1. [DOI] [PubMed] [Google Scholar]
- SMET P.J., EDYVANE K.A., JONAVICIUS J., MARSHALL V.R. Colocalization of nitric oxide synthase with vasoactive intestinal peptide, neuropeptide Y, and tyrosine hydroxylase in nerves supplying the human ureter. J. Urol. 1994;152:1292–1296. doi: 10.1016/s0022-5347(17)32570-3. [DOI] [PubMed] [Google Scholar]
- TAHER A., SCHULZ-KNAPPE P., MEYER M., TRUSS M., FORSSMANN W.G., STIEF C.G., JONAS U. Characterization of cyclic nucleotide phosphodiesterase isoenzymes in the human ureter and their functional role in vitro. World J. Urol. 1994;12:286–291. doi: 10.1007/BF00191209. [DOI] [PubMed] [Google Scholar]
- TANAKA K., SHIBUYA I., UEZONO Y., UETA Y., TOYOHIRA Y., YANAGIHARA N., IZUMI F., KANNO T., YAMASHITA H. Pituitary adenylate cyclase-activating polypeptide causes Ca2+ release from ryanodine/caffeine stores through a novel pathway independent of both inositol trisphosphates and cyclic AMP in bovine adrenal medullary cells. J. Neurochem. 1998;70:1652–1661. doi: 10.1046/j.1471-4159.1998.70041652.x. [DOI] [PubMed] [Google Scholar]
- UCKERT S., STIEF C.G., LIETZ B., BURMESTER M., JONAS U., MACHTENS S.A. Possible role of bioactives peptides in the regulation of human detrusor smooth muscle functional effects in vitro and immunohistochemical presence. World J. Urol. 2002;20:244–249. doi: 10.1007/s00345-002-0287-y. [DOI] [PubMed] [Google Scholar]
- USDIN T.B., BONNER T.I., MEZEY E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2668. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- VAUDRY D., GONZALEZ B.J., BASILLE M., YON L., FOURNIER A., VAUDRY H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol. Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- WERKSTRÖM V., ALM P., PERSSON K., ANDERSSON K.E. Inhibitory innervation of the guinea-pig urethra; roles of CO, NO and VIP. J. Auton. Nerv. Syst. 1998;74:33–42. doi: 10.1016/s0165-1838(98)00135-0. [DOI] [PubMed] [Google Scholar]