Abstract
The ORL1 agonists nociceptin and Ro 64-6198 were compared in their ability to modify spontaneous locomotor activity in male NMRI mice not habituated to the test environment.
Higher doses of nociceptin (>5 nmol i.c.v.) reduced whereas lower doses (<1 nmol i.c.v.) stimulated locomotor activity. Both effects were blocked by the putative ORL1 antagonists [NPhe1]nociceptin(1–13)NH2 (10 nmol i.c.v.) and UFP101 (10 nmol, i.c.v.). The effects were also blocked by naloxone benzoylhydrazone (1 mg kg−1 s.c.), but not by the nonselective opioid antagonist naloxone (1 mg kg−1 s.c.).
In contrast to nociceptin, the synthetic ORL1 agonist Ro 64-6198 (0.01–1.0 mg kg−1 i.p.) produced monophasic inhibition of locomotor activity, which was insensitive to the treatment with [NPhe1]nociceptin(1–13)NH2 or naloxone benzoylhydrazone. Treatment with UFP101 abolished the locomotor inhibition induced by Ro 64-6198 (1.0 mg kg−1), whereas naloxone (1.0 mg kg−1, s.c.) further increased the locomotor-inhibitory effects.
Naloxone benzoylhydrazone (0.3; 1.0 and 3.0 mg kg−1 s.c.) increased locomotor activity, although the effect was statistically significant only with the highest dose used.
Pretreatment with the tyrosine hydroxylase inhibitor H44-68 totally eliminated the motor-stimulatory effects of low doses of nociceptin, probably via dopamine depletion.
The results suggest that nociceptin stimulates locomotor activity at low doses if dopamine activity is intact. High doses of nociceptin and all the tested doses of Ro 64-6198 seem to interact with a functionally different subset of ORL1 receptors. In addition, the effects of Ro 64-6198 are modulated by tonic opioid receptor activity.
Keywords: Nociceptin, Ro 64-6198, mice, locomotion, ORL1 receptors
Introduction
Nociceptin (NC), a 17-amino-acid neuropeptide, has been found to be a natural ligand of the opioid receptor-like type 1 (ORL1) receptor (Meunier et al., 1995; Reinscheid et al., 1995), which is related to the opioid receptor family (Calo et al., 2000). Although the ORL1 receptor is highly homologous to the opioid receptors (Pan et al., 1996), traditional opioids have poor affinity for the ORL1 receptor. Conversely, the endogenous ligand for the ORL1 receptor NC displays no appreciable affinity for classical opioid receptors (Meunier et al., 1995; Reinscheid et al., 1995), and its pharmacological effects are not sensitive to naloxone (Jenck et al., 2000).
Reinscheid et al. (1995) were the first to show that i.c.v. administration of NC (1–10 nmol) to mice inhibits spontaneous locomotor activity, a finding later confirmed by several authors (Devine et al., 1996; Nishi et al., 1997; Noda et al., 1998; Calo et al., 1999; Kuzmin et al., 2003). The inhibitory action of NC was insensitive to naloxone, but reversed by naloxone benzoylhydrazone (NBZ) (Noda et al., 1998). When microinjected directly into the hippocampus or the ventromedial hypothalamus, but not the nucleus accumbens, high doses of NC (10–25 nmol) significantly decreased locomotor activity (Sandin et al., 1997; Stratford et al., 1997). Conversely, repeated i.c.v. injections of antisense oligodeoxynucleotides directed against pronociceptin mRNA produced significant hyperlocomotion in rats (Candeletti & Ferri, 2000). In contrast, Florin et al. (1996) observed a short-lasting increase in locomotor activity at low doses of NC, which was insensitive to naloxone treatment. This suggests that NC could have a biphasic effect on locomotor activity similar to its effects on pain mechanisms with low doses being hyperalgesic and antagonizing opioid analgesia (Meunier et al., 1995; Reinscheid et al., 1995), while higher doses are analgesic (Rossi et al., 1997; Kolesnikov & Pasternak, 1999). Paradoxically, opioid antagonists reversed the analgesic actions of NC, suggesting a link to classical opioid receptor functions, while its antiopioid and hyperalgesic actions were not sensitive to naloxone (Mogil & Pasternak, 2001).
Studies on the functional role of the ORL1 receptor have been hampered by the lack of potent and selective synthetic ligands. Recently, a nonpeptide ligand Ro 64-6198, which penetrates well into the brain after i.p. administration, was shown to be a full agonist with an affinity for the ORL1 receptor close to that of NC itself (Jenck et al., 2000; Wichmann et al., 2000). The pharmacological specificity of this drug was confirmed by the complete absence of behavioural effects in ORL1 receptor KO mice (Higgins et al., 2001).
Two peptide ligands selective for the ORL1 receptor have been described as antagonists: [NPhe1]nociceptin(1–13)NH2 (NNN, Calo et al., 2000; Guerrini et al., 2000) and [NPhe1, Arg14, Lys15]nociceptin(1–13)NH2 (UFP101, Calo et al., 2002). The antagonistic properties of both peptides have been confirmed in a variety of in vivo assays (Calo et al., 2002). NBZ is a chemical derivative of the nonselective opioid antagonist naloxone, and has been suggested to act as an antagonist for the ORL1 receptor in vivo (Bigoni et al., 2002).
The aims of the present work are two-fold: first, to examine the ability of a broad dose range of NC and Ro 64-6198 to modulate locomotor activity in mice. Secondly, to investigate whether the locomotor effects by NC and Ro 64-6198 are blocked by the putative ORL1 antagonists NNN, NBZ and UFP101 and the nonselective opioid antagonist naloxone.
Methods
Animals
Experiments were carried out in male NMRI mice (B&K Universal, AB, Sweden, 20–25 g). Animals were kept under standard laboratory conditions with free access to food pellets (low protein diet) and tap water. The animals were housed 4–6 per cage in a light-controlled room (12 h light/dark cycle, light on at 06:00 h) at 21°C and 60% humidity. All experiments were performed between 10:00 and 13.00 h.
Animal housing and all experimental procedures followed the provisions and general recommendations of Swedish animal protection legislation. The experiments were approved by a local Animal Ethics Committee (Permit Number 155/01).
Surgery and cannulation
The mice were anaesthetized with a combination of Ketalar® (ketamine hydrochloride 50 mg ml−1, 1 : 10, 0.2 ml, s.c., Parke Davis, Barcelona, Spain) and Hypnorm® (fluanisonum 10 mg ml−1+fentanylum 0.2 mg ml−1; 1 : 10, 0.2 ml, i.p., Janssen, Beerse, Belgium). The body temperature was maintained at 37°C using a thermostat regulated heat pad (CMA/105, CMA/Microdialysis, Stockholm, Sweden). Permanent steel guide cannulae with an outer diameter of 0.4 mm (Plastics One, Roanoke, VA, U.S.A.) were implanted into the right lateral ventricle using coordinates based on the stereotaxic plates (AP (Bregma) – 2.5 mm, L 3.0 mm, V 4.25 mm, mouse brain atlas: www.mbl.org). Cannulae were fixed to the scull by dental carboxylic cement (Durelon ESPE, Germany). The animals were allowed to recover for 4–5 days in the colony room (2–3 mice per cage) before the start of the experiment. After completion of the behavioural experiments, the position of the cannula was verified histologically by the injection of 2 μl of methylene blue.
Drugs
The following peptides and compounds were used: nociceptin (NC, peptide-free base, MW 1808.05, Tocris, Bristol, U.K.), [NPhe1]nociceptin(1–13)NH2 (NNN, MW 1380.60, Tocris, Bristol, U.K.), [NPhe1, Arg14, Lys15]nociceptin(1–13)NH2 (UFP-101, MW 1908.19, Tocris, Bristol, U.K.), Naloxone HCl (EndoLab, Wilmington, U.S.A.), Ro 64-6198 ((1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one hydrochloride, MW 438.017) a gift of Dr Juergen Wichmann (Hoffmann-La Roche Ltd, Pharma Preclinical Research, Basel, Switzerland), naloxone benzoylhydrazone (NBZ, ([5α]-4,5-epoxy-3,14-dihydroxy-17–morphinan-6-ylidene)hydrazide benzoic acid, Sigma-Aldrich Sweden AB, Stockholm). The tyrosine hydroxylase inhibitor H44-68 (α-methyl-p-tyrosine methyl ester, AstraZeneca, Sweden) was dissolved in saline and injected i.p. 2 h before the experiment. Other compounds were freshly dissolved in either artificial CSF (NC, NNN, UFP101) or saline (naloxone, Ro 64-6198, NBZ) just before the start of the experiment. Naloxone and NBZ were given s.c. (5 ml kg−1), while Ro 64-6198 was administered i.p. (5 ml kg−1). Peptides were infused i.c.v. in a volume of 2 μl mouse−1.
Artificial cerebrospinal fluid (CSF) contained (g l−1): NaCl (7.20), NaHCO3 (1.96), KCl (0.18), KH2PO4 (0.068), CaCl2 (0.16), MgCl2 × 6H2O (0.17), Na2SO4 (0.07), glucose (1.0). The pH of the solution was adjusted to 7.4 with 10 mM NaOH and HCl.
Locomotor activity
Mice were individually tested. They were removed from their home cages and placed in the middle of an activity monitor (standard transparent Macrolon® cage (42 × 26 × 20 cm3) with 50 ml of wood shavings on the floor) and the data-collecting system was immediately activated. The experiments were performed in nonhabituated animals, that is, mice were treated with the drug just before being placed in the activity box. The different parameters of motor activity were recorded during six 10-min intervals. In experiments with antagonists, they were administered i.c.v. or s.c. 3–4 min before administration of ORL1 agonists. Animals were placed in the motor activity monitor after administration of ORL1 agonists.
Motor activity was measured in eight animals simultaneously by means of a fully computerized multicage red and infrared-sensitive motion-detection system (Ögren et al., 1986). Horizontal locomotor activity was measured as all movements of a distance of 4 cm or more detected by 48 vertical photocells (red light-sensitive photocells in the floor of the apparatus), and represents a measure of general motility.
Dose-dependent effects of drugs on horizontal locomotor activity in mice were analysed by two-way ANOVA for repeated measures, the factors being treatment (drug doses and control) and time (6 × 10 min recording). This was followed when necessary by a post-hoc Newman–Keuls test for every time point separately. The whole study was designed as a between-subjects (independent groups) experiment (i.e. each animal was used only once).
Results
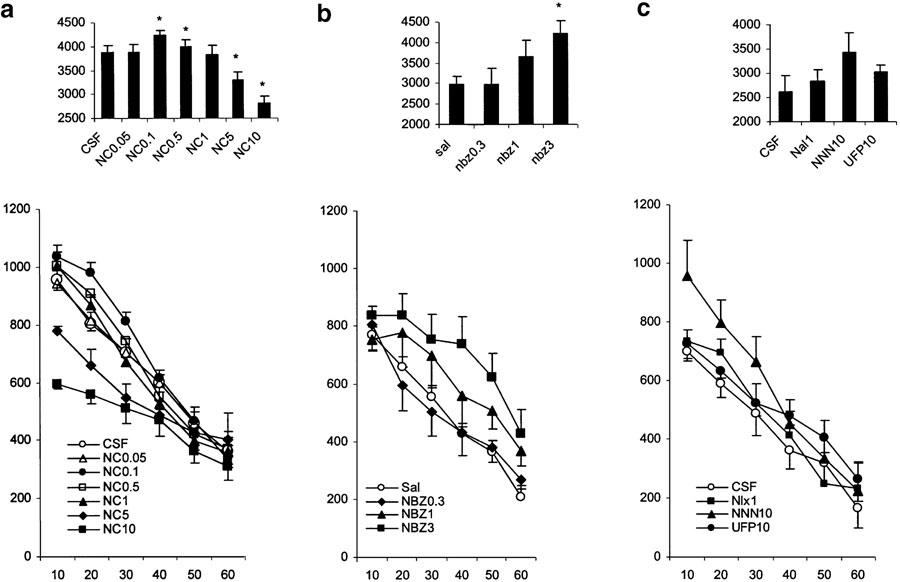
Effects of NC (Figure 1a)
Figure 1.
Effects of various treatments on motor activity in nonhabituated mice. The data represent the counts of motility in 10-min intervals (mean values and standard errors, n=8–12 per group). For statistical significance, see Results section. (a) Influence of NC infused i.c.v. just before placement of the animals in the activity cages. The numbers in the legends present the doses of the drugs used (nmol mouse−1). (b) Influence of NBZ injected s.c. just before placement of the animals in the activity cages. The numbers in the legends present the doses of the drugs used (mg kg−1). (c) Influence of NNN (10 nmol mouse−1 i.c.v.), UFP101 (10 nmol mouse−1 i.c.v.) and naloxone (1 mg kg−1 s.c.) on motor activity in nonhabituated mice. The bar graphs in the upper parts of the plates represent the cumulative motility counts for a test duration of 60 min. *P<0.05 (comparison with control group: CSF or Sal).
Animals treated with CSF demonstrated a gradual habituation to the test environment, resulting in a time-dependent decrease of locomotor activity. NC given i.c.v. (0.05, 0.l, 0.5, 1.0, 5.0 and 10.0 nmol mouse−1) produced a dose-dependent biphasic effect on locomotor activity with significant stimulation at the doses of 0.1 and 0.5 nmol mouse−1, and a significant inhibition at the doses 5 and 10 nmol mouse−1. There was a significant main effect of NC treatment on horizontal locomotor activity (F(6,51)=6.5, P<0.01), a significant time effect (F(5,255)=335.3, P<0.001) and a significant dose × time interaction (F(30,255)=4.6, P<0.01). The post-hoc Newman–Keuls test, analysed for each time point separately, revealed a significant (P<0.01) reduction in motility 10, 20 and 30 min after treatment with NC at the dose of 10 nmol mouse−1 (with respect to the CSF-treated group) and a significant (P<0.01) reduction in motility 10 and 20 min after treatment with the dose of 5 nmol mouse−1. In contrast, a significant (P<0.01) increase of locomotor activity was seen 10, 20 and 30 min after treatment with NC at the dose of 0.1 nmol mouse−1 (with respect to the CSF-treated group) and a significant (P<0.01) increase in locomotor activity 20 min after treatment with NC at the dose of 0.5 nmol mouse−1. This indicates that NC inhibits locomotor activity in mice from the 5 nmol mouse−1, while doses lower than 1 nmol mouse−1 stimulate locomotor activity. NC at doses of 1.0 and 0.05 nmol failed to significantly influence locomotor activity in mice.
Effects of NBZ (Figure 1b)
Animals treated with NBZ increased their locomotor activity. ANOVA revealed a significant effect of NBZ (0.3, 1.0 and 3.0 mg kg−1 s.c.) treatment (F(3,26)=4.4, P<0.05) and dose × time interaction (F(15,130)=3.9, P<0.01). The post-hoc test revealed a significantly (P<0.01) higher level of locomotor activity 10–30 min after treatment with NBZ at the dose of 3 mg kg−1.
Effects of NNN, UFP-101 and naloxone (Figure 1c)
Separate groups of mice were treated i.c.v. with CSF, NNN (10 nmol) or UFP-101 (10 nmol) or with naloxone (1 mg kg−1 s.c.). ANOVA failed to reveal a significant treatment effect as well as significant treatment × time interaction. However, a tendency for increased locomotor activity was seen 10–30 min after treatment in the group treated with NNN, while naloxone failed to change locomotor activity.
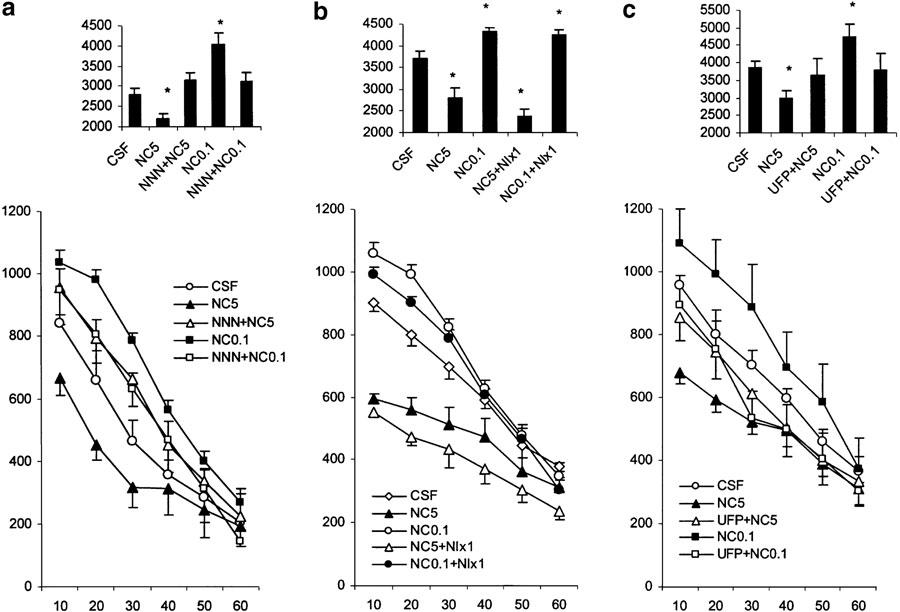
Influence of NNN on the effects of NC (Figure 2a)
Figure 2.
Effects of various treatments on motor activity in nonhabituated mice. The data represent the cumulative counts of motility in 10-min intervals (mean values and standard errors, n=8–10 per group). For statistical significance, see Results section. (a) Locomotor effects of NC (0.1 and 5.0 nmol mouse−1, i.c.v.) in mice pretreated with NNN (10 nmol mouse−1 i.c.v., 3–4 min before agonist treatment). (b) Locomotor effects of NC (0.1 and 5 nmol, i.c.v.) in mice pretreated with naloxone (1.0 mg kg−1 s.c., 3–4 min before agonist treatment). (c) Locomotor effects of nociceptin (0.1 and 5.0 nmol mouse−1, i.c.v.) in mice pretreated with UFP101 (10 nmol mouse−1 i.c.v., 3–4 min before agonist treatment). The bar graphs in the upper parts of the plates represent the cumulative motility counts for a test duration of 60 min. *P<0.05 (comparison with control group: CSF).
An overall ANOVA revealed a significant treatment effect (F(4,35)=5.6, P<0.01), a significant time effect (F(5,175)=335.3, P<0.01) and a significant treatment × time interaction (F(20,175)=4.6, P<0.01). The post-hoc Newman–Keuls test to analyse each time point separately revealed that there was a significant (P<0.01) reduction of locomotor activity 10, 20 and 30 min after treatment with NC at the dose of 5 nmol (with respect to the CSF-treated group), while the 0.1 nmol dose of NC significantly increased locomotor activity 10, 20 and 30 min after i.c.v. administration. Pretreatment with NNN (10 nmol) significantly blocked the motor stimulatory (0.1 nmol) and inhibitory (5 nmol) effects of NC. Interestingly, treatment with NNN reversed locomotor inhibition induced by NC (5 nmol) into a nonsignificant, but notable locomotor activation.
Influence of naloxone on the effects of NC (Figure 2b)
Naloxone (1 mg kg−1) failed to influence either stimulatory (0.1 nmol mouse−1) or inhibitory (5 nmol mouse−1) effects of NC on locomotor activity.
Influence of UFP-101 on the effects of NC (Figure 2c)
Treatment with UFP101 (10 nmol i.c.v.) failed to significantly modify locomotor activity (shown in Figure 1c), although a tendency to increased activity was observed. Combined treatment with UFP101 and NC showed that the antagonist could block both the motor-stimulatory and inhibitory effects of NC (treatment × time interaction F(10,120)=2.4, P<0.05 and F(10,145)=2.2, P<0.05, respectively). The ability of UFP101 to block the locomotor inhibitory effects of NC was significant at the 10- and 20-min intervals after NC administration (P<0.05), whereas it blocked the locomotor stimulation by the low dose of NC at all time intervals measured.
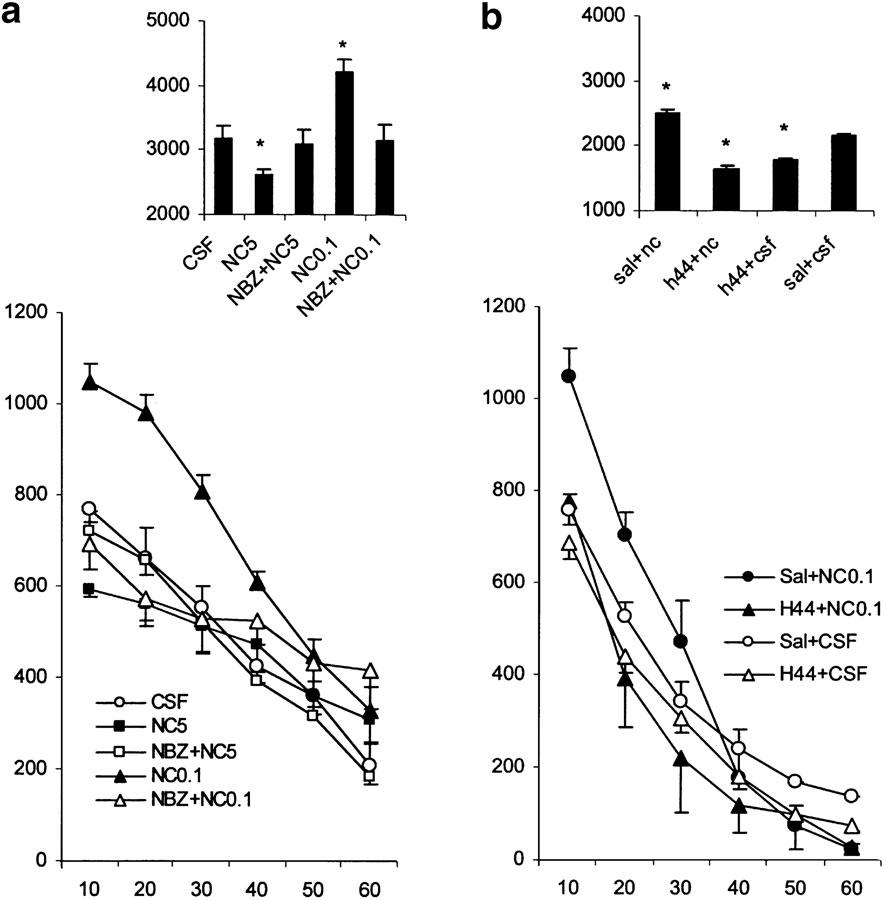
Influence of NBZ on the effects of NC (Figure 3a)
Figure 3.
Effects of various treatments on motor activity in nonhabituated mice. The data represent the counts of motility in 10-min intervals (mean values and standard errors, n=8–10 per group). For statistical significance, see Results section. (a) Locomotor effects of nociceptin (0.1 nmol mouse−1, i.c.v. 5.0 nmol mouse−1, i.c.v.) in mice pretreated with NBZ (1 mg kg−1, s.c., 3–4 min before agonist treatment). (b) Locomotor effects of nociceptin (0.1 nmol mouse−1, i.c.v.) in mice pretreated with the tyrosine hydroxylase inhibitor H44-68 (100 mg kg−1, i.p., 2 h before treatment with nociceptin). The bar graphs in the upper parts of the plates represent the cumulative motility counts for a test duration of 60 min. P<0.05 (comparison with control groups: CSF and Sal+CSF).
NBZ treatment (1 mg kg−1, s.c.) attenuated both locomotor stimulation produced by the low dose of NC (0.1 nmol) and locomotor inhibition caused by the high dose of NC (5 nmol) (treatment × time interaction F(5,70)=13.3, P<0.01 and F(5,60)=4.52, P<0.01, respectively). The stimulatory and inhibitory locomotor effects of NC were significantly blocked by NBZ treatment at the 10- and 20-min time intervals after NC administration, for example, in the time range locomotor activity was influenced by NC after i.c.v. administration.
Effect of pretreatment with the monoamine depletor H44-68 on the motor effects of NC (0.1 nmol) (Figure 3b)
Mice were pretreated with H44-68 (100 mg kg−1, i.p.) or saline 2 h prior to injection of NC (0.1 nmol i.c.v.) or CSF (totally four treatment groups, 9–12 mice per group). There was a significant treatment effect on motility (F(3,26)=4.4, P<0.01) and a significant treatment × time interaction (F(15,130)=3.9, P<0.01). The time effect was also significant (P<0.0001). The post-hoc comparison indicated a significant (P<0.01 vs Sal+NC group) decrease in motility at 10, 20 and 30 min in both groups with CSF infusion and in the H44+NC group. It is concluded that the motor-stimulatory effects of NC are fully blocked by pretreatment with H44/68.
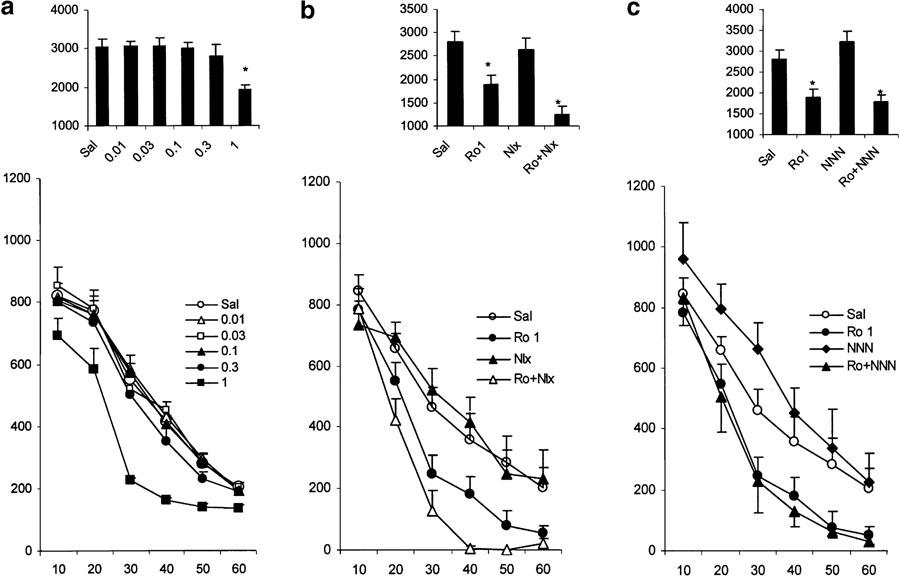
Effects of Ro 64-6198 (Figure 4a)
Figure 4.
Effects of various treatments on motor activity in nonhabituated mice. The data represent the counts of motility in 10-min intervals (mean values and standard errors, n=8–10 per group). For statistical significance, see Results section. (a) Influence of Ro 64-6198 infused i.p. just before placement of the animals in the activity cages. The numbers in the legends present the doses of the drugs used (nmol mouse−1). (b) Locomotor effects of Ro 64-6198(1.0 mg kg−1, i.p.) in mice pretreated with NNN (10 nmol mouse−1 i.c.v., 3–4 min before agonist treatment). (c) Locomotor effects of Ro 64-6198(1.0 mg kg−1, i.p.) in mice pretreated with naloxone (1.0 mg kg−1 s.c., 3–4 min before agonist treatment). The bar graphs in the upper parts of the plates represent the cumulative motility counts for a test duration of 60 min. *P<0.05 (comparison with control group: Sal).
In animals treated with vehicle (n=8), there was a gradual decrease of locomotor activity over time. There was a significant main effect of Ro 64-6198 (0.01, 0.03, 0.1, 0.3, 1.0 mg kg−1 i.p.) treatment (F(5,35)=6.4, P<0.01), a significant time effect (F(5,175)=220.2, P<0.01) and a significant dose × time interaction (F(25,175)=5.2, P<0.05). The post-hoc Newman–Keuls test, analysed for each time point separately, showed a significant (P<0.01) reduction of motility 20–40 min after treatment with Ro 64-6198 at the dose of 1 mg kg−1 (with respect to vehicle-treated group). Taken together, the data show that Ro 64-6198 dose-dependently decreases locomotor activity in mice.
Influence of naloxone on the effects of Ro64-6198 (Figure 4b)
Overall ANOVA reveals a significant treatment effect (F(3,32)=8.5, P<0.01), a significant time effect (F(5,160)=145.9, P<0.01) and a significant treatment × time interaction (F(15,160)=2.9, P<0.01). The post-hoc Newman–Keuls test indicated a significantly (P<0.01) lower level of locomotor activity 30–60 min after the 1 mg kg−1 dose of Ro 64-6198 (with respect to the vehicle-treated group). Surprisingly, the group treated with the combination Ro+naloxone exhibited significantly lower locomotor activity than the group treated with Ro 64-6198 only (40–60 min after injection, P<0.01). This suggests that naloxone further enhanced the locomotor-inhibitory effects of Ro 64-6198.
Influence of NNN on the effects of Ro 64-6198 (Figure 4c)
There were a significant treatment effect (F(3,34)=12.2, P<0.01), a significant time effect (F(5,170)=116.6, P<0.01) and a significant treatment × time interaction (F(15,170)=2.6, P<0.05). The post-hoc Newman–Keuls test, analysed for each time point separately, revealed that the 1 mg kg−1 dose of Ro 64-6198 significantly (P<0.01, with respect to vehicle-treated group) reduced locomotor activity 20, 30, 40, 50 and 60 min after injections. Treatment with NNN (10 nmol), which increased (P<0.01) locomotor activity 20 and 30 min after i.c.v. administration, failed to block the inhibitory effect of Ro 64-6198.
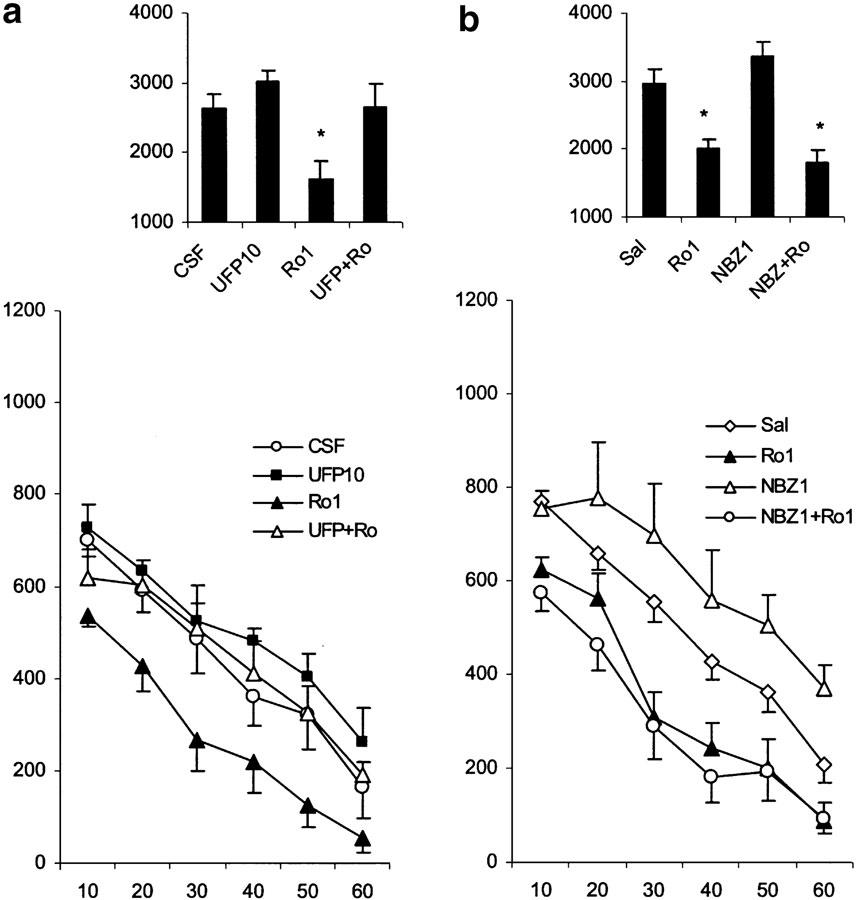
Influence of UFP101 on the effects of Ro 64-6198 (Figure 5a)
Figure 5.
Effects of various treatments on motor activity in nonhabituated mice. The data represent the counts of motility in 10-min intervals (mean values and standard errors, n=8–10 per group). For statistical significance, see Results section. (a) Locomotor effects of Ro 64-6198 (1.0 mg kg−1, i.p.) in mice pretreated with UFP101 (10 nmol mouse−1 i.c.v., 3–4 min before agonist treatment). (b) Locomotor effects of Ro 64-6198 (1.0 mg kg−1, i.p.) in mice pretreated with NBZ (1.0 mg kg−1 s.c., 3–4 min before agonist treatment). The bar graphs in the upper parts of the plates represent the cumulative motility counts for a test duration of 60 min. *P<0.05 (comparison with control group: CSF and Sal).
The 10 nmol dose of UFP101 did not alter locomotor activity, but blocked the motor-inhibitory effects of Ro 64-6198 (treatment × time interaction F(5,65)=2.37, P<0.05). The locomotor-inhibitory effects of Ro 64-6198 were significantly reduced at 10–30 min after UFP101 administration (P<0.05).
Influence of NBZ on the effects of Ro 64-6198 (Figure 5b)
NBZ (1 mg kg−1 s.c.) failed to significantly influence the locomotor inhibition induced by Ro 64-6198 (1 mg kg−1 i.p.) (treatment × time interaction F(5,70)=0.31, P=0.9).
Discussion
The present experiments have demonstrated that: (1) NC exerts a biphasic effect on locomotor activity in mice with stimulation at lower doses and inhibition at higher doses, whereas Ro 64-6198 produces a dose-dependent monophasic inhibitory effect. (2) The putative ORL1 antagonist NBZ (3 mg kg−1) significantly stimulates locomotor activity, whereas NNN tended to increase the locomotor activity. (3) The locomotor effects (both stimulatory and inhibitory) of NC are blocked by both NNN and NBZ, whereas the inhibitory effect of Ro 64-6198 is not. (4) The putative ORL1 antagonist UFP101 blocks the locomotor effects of both NC and Ro 64-6198. (5) Naloxone potentiates the motor-inhibitory effect of Ro 64-6198, but fails to influence the stimulatory or inhibitory effects of NC. (6) Monoamine depletion by the tyrosine hydroxylase inhibitor H44-68 fully eliminates the motor-stimulatory effects of low doses of NC. The results obtained with agonists are summarized in Table 1.
Table 1.
Summary of the effects of ORL1 ligands on spontaneous locomotor activity in mice
| Effect of putative ORL1 antagonists and naloxone | |||||
|---|---|---|---|---|---|
| ORL1 ligands | Effect | NNN | NBZ | UFP-101 | Naloxone |
| NC low doses | Stimulation | Blockade | Blockade | Blockade | 0 |
| NC high doses | Inhibition | Blockade | Blockade | Blockade | 0 |
| Ro 64-6198 | Inhibition | 0 | 0 | Blockade | Potentiation |
0–no significant effect.
Studies using a wide dose range revealed for the first time that the locomotor effects of NC given i.c.v. are biphasic. Previous studies have reported either stimulation or inhibition of locomotion depending on whether low or high doses of NC were used (Reinscheid et al., 1995; Florin et al., 1996; Jenck et al., 1997). In the present study, NC stimulated locomotor activity in the 0.05–0.5 nmol dose range, and inhibited it at higher doses (5 and 10 nmol). Both effects of NC were short lasting (up to 30 min), probably due to rapid enzymatic degradation.
Both the motor-stimulatory and motor-inhibitory effects of NC were blocked by NBZ, NNN and UFP101, suggesting that they are related to activation of the ORL1 receptor. This is in line with the observation that NC (10 nmol) failed to inhibit locomotion in ORL1 knockout mice (Nishi et al., 1997; Noda et al., 1998). Moreover, the lack of influence of naloxone (1 mg kg−1) on both the stimulatory and inhibitory effects of NC rules out the possibility that NC exerts the locomotor effects via kappa-opioid receptors, as suggested previously (Florin et al., 1996).
The mechanisms underlying the motor-stimulatory and -inhibitory effects of NC remain to be determined. The inhibitory effects on locomotor activity may be related to a reduction in mesolimbic dopamine neurotransmission. NC has been reported to decrease dopamine release in both the striatum and n.accumbens in the rat (Shieh & Pan, 2001) and mouse (Schlicker & Morari, 2000), as revealed by microdialysis studies in anaesthetized animals. NC administered i.c.v. at higher doses (16.6 and 166 nmol) also reduced DA release in the nucleus accumbens in anaesthetized animals (Murphy et al., 1996; Murphy & Maidment, 1999). This effect was blocked by the GABA-A antagonist bicuculline, suggesting mediation by GABAergic interneurons in the VTA, which are also influenced by opioids (Johnson & North, 1992).
The stimulatory effects of the low dose of NC appear to require an intact catecholamine system. Depletion of brain catecholamines by the tyrosine hydroxylase inhibitor H44-68 fully eliminated the stimulatory effects of the low doses of NC. At the dose used, H44-68 has been shown to reduce the motor stimulation induced by d-amphetamine in mice (Ögren & Ross, 1977). The increase in motor activity, produced by low doses of NC, was blocked by dopamine D1 and D2 receptor antagonists (Florin et al., 1996). Taken together, these results support the view that the stimulatory effects of NC are mediated by enhanced dopamine release. However, studies using lower doses of NC in freely moving animals have not shown any change in DA release from the n. accumbens or caudate nucleus (Di Giannuario et al., 1999; Di Giannuario & Pieretti, 2000).
Locomotor activity and other mesolimbic dopamine functions are under the influence of nondopaminergic (GABA ergic) neurons in the ventral tegmental area via direct contact with local dopamine neurons or via GABA ergic efferents that innervate nucleus accumbens and other mesolimbic structures (Carr & Sesack, 2000). Recent in vivo studies have shown that NC inhibits both GABAergic and dopaminergic neurons in the ventral tegmental area (Zheng et al., 2002). Thus, similar to opioids, which are known to hyperpolarize nondopaminergic neurons in the ventral tegmental area (Johnson & North, 1992), NC at low loses may disinhibit dopamine neurons by removing tonic inhibition by local GABA interneurons. However, at the same time, NC could hyperpolarize dopamine neurons (Zheng et al., 2002), indicating that the net effect on dopamine function will depend on a subtle balance between disinhibition and inhibition of GABAergic and dopaminergic neurons, respectively.
A glutamatergic mechanism for the stimulatory locomotor effects of ORL1 ligands is also possible. Perfusion with NC in the substantia nigra pars reticulata evoked an increase of local extracellular glutamate levels, which was insensitive to naloxone but prevented by the ORL1 receptor antagonist NNN (Marti et al., 2002). Thus, an increase in excitatory drive on nigra neurons might result either in a stimulation of nigra-cortical afferents or in a disinhibition of thalamic GABAergic neurons, resulting in activation of glutamatergic afferents to the motor cortex (Albin et al., 1989; Alexander & Crutcher, 1990).
A major finding of the present work is the observation that the synthetic ORL1 agonist Ro 64-6198 differs from NC. Ro 64-6198 lacked the stimulatory effect and dose-dependently reduced locomotor activity, in line with previous observations in rats (Jenck et al., 2000). Moreover, the ORL1 antagonists NNN and NBZ failed to block the inhibitory action of Ro 64-6198. This finding is difficult to explain, since Ro 64-6198 binds to ORL1 receptors with a potency comparable to that of NC (Rizzi et al., 2001). In contrast, the other proposed ORL1 receptor antagonist, UFP101, completely abolished the locomotor inhibition induced by Ro 64-6198. The mechanisms by which Ro 64-6198 inhibits the locomotor activity appear not to involve changes in catecholamine transmission, since recent in vivo microdialysis experiments failed to detect significant reductions in dopaminergic and noradrenergic transmission (Kawahara, personal communication). Tentatively, Ro 64-6198 may be selective for a subset of ORL-1 receptors (presumably inhibitory) from which it cannot be displaced by the antagonists NNN and NBZ, but is successfully displaced by UFP101. This subset of receptors is unmasked by naloxone, which may act on a tonically active opioid system. Naloxone may act via blockade of mu-opioid receptors located in the substantia nigra and ventral tegmental area (Dilts & Kalivas, 1989), by removing a tonic opioid inhibitory control in the GABAergic inhibitory interneurons.
It has been suggested that the ORL1 receptor system does not play a tonic role in the physiological regulation of locomotion since NBZ, at doses sufficient to prevent the inhibitory effect of NC, did not modulate spontaneous locomotor activity (Noda et al., 1998). Also, ORL1 knockout mice failed to display any evidence for enhanced basal locomotor activity (Nishi et al., 1997; Noda et al., 1998). However, in the present study, NBZ (3 mg kg−1) significantly stimulated locomotor activity, whereas NNN tended to do so. It should be noted that the doses of NBZ used in the present study were much lower than those used before (up to 50 mg kg−1), which might explain these discrepancies. The present findings suggest that brain NC systems may be tonically involved in the control of locomotor behaviour. The observation that repeated i.c.v. injections of antisense oligodeoxynucleotides directed against pronociceptin mRNA produced significant hyperlocomotion in rats (Candeletti & Ferri, 2000) supports a physiological role of the ORL1 receptor system.
Conclusion
The present results indicate that there exist several functional subtypes of ORL-1 receptors. The biphasic effects of NC may be due to its actions on subsets of receptors with different functions in subpopulations of dopaminergic and nondopaminergic neurons. The heterogeneity of the ORL1 receptor is also supported by the observation that shorter fragments of NC with negligible affinity for the receptor block forskolin-stimulated cAMP production (Mathis et al., 1997) and reduce NC-induced behavioural responses after intrathecal administration (Sakurada et al., 2000). The synthetic agent Ro 64-6198 seems to interact with a population of ORL1 receptor, which differs from that of NC. This subpopulation of ORL1 receptor appears to interact with an opioid system. The further exploration of the NC/ORL-1 system and its functional/therapeutic potential would greatly benefit by the access to more specific molecular probes.
Acknowledgments
We wish to thank Dr F. Jenck and Dr J. Wichmann at Hoffmann-La Roche Ltd for the generous gift of the synthetic nociceptin agonist Ro 64-6198. This project received financial support from The Marcus and Amalia Wallenberg Foundation, The Swedish Research Council, Karolinska Institutet and Systembolaget AB.
Abbreviations
- CSF
cerebrospinal fluid
- H44-68
α-methyl-p-tyrosine methyl ester
- NBZ
naloxone benzoylhydrazone
- NC
nociceptin
- NNN
[NPhe1]nociceptin(1–13)NH2
- ORL1
opioid receptor like type 1 receptor
- Ro 64-6198
((1S,3aS)-8-(2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one hydrochloride
- UFP101
[NPhe1, Arg14, Lys15]nociceptin(1–13)NH2
References
- ALBIN R.A., YOUNG A.B., PENNEY B. The functional anatomy of the basal ganglia disorders. TiNS. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- ALEXANDER G.E., CRUTCHER M.D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. TiNS. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- BIGONI R., CAO G., RIZZI A., OKAWA H., REGOLI D., SMART D., LAMBERT D.G. Effects of naloxone benzoylhydrazone on native and recombinant nociceptin/orphanin FQ receptors. Can. J. Physiol. Pharmacol. 2002;80:407–412. doi: 10.1139/y02-040. [DOI] [PubMed] [Google Scholar]
- CALO' G., GUERRINI R., RIZZI A., SALVADORI S., REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALO' G., BIGONI R., RIZZI A., MARZOLA G., GUERRINI R., SALVADORI S., REGOLI D., BIANCHI C. Characterization of nociceptin receptors modulating locomotor activity in mice. Fund. Clin. Pharmacol. 1999;13 S1:S27.6. [Google Scholar]
- CALO G., RIZZI A., RIZZI D., BIGONI R., GUERRINI R., MARZOLA G., MARTI M., MCDONALD J., MORARI M., LAMBERT D.G., SALVADORI S., REGOLI D. [Nphe1,Arg14,Lys15]nociceptin-NH2, a novel potent and selective antagonist of the nociceptin/orphanin FQ receptor. Br. J. Pharmacol. 2002;136:303–311. doi: 10.1038/sj.bjp.0704706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANDELETTI S., FERRI S. Effects of an antisense oligonucleotide to pronociceptin and long-term prevention of morphine actions by nociceptin. Peptides. 2000;21:1119–1124. doi: 10.1016/s0196-9781(00)00249-7. [DOI] [PubMed] [Google Scholar]
- CARR D.B., SESACK S.R. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- DEVINE D.P., TAYLOR L., REINSCHEID R.K., MONSMA F.J., CIVELLI O., AKIL H. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem. Res. 1996;21:1387–1396. doi: 10.1007/BF02532380. [DOI] [PubMed] [Google Scholar]
- DI GIANNUARIO A., PIERETTI S. Nociceptin differentially affects morphine induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- DI GIANNUARIO A., PIERETTI S., CATALANI A., LOIZZO A. Orphanin FQ reduces morphine-induced dopamine release in the nucleus accumbens: a microdialysis study in rats. Neurosci. Lett. 1999;272:183–186. doi: 10.1016/s0304-3940(99)00579-0. [DOI] [PubMed] [Google Scholar]
- DILTS R.P., KALIVAS P.W. Autoradiographic localization of mu-opioid and neurotensin receptors within the mesolimbic dopamine system. Brain Res. 1989;488:311–327. doi: 10.1016/0006-8993(89)90723-3. [DOI] [PubMed] [Google Scholar]
- FLORIN S., SUAUDEAU C., MEUNIER J.-C., COSTENTIN J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur. J. Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO' G., RIZZI A., BIGONI R., RIZZI D., REGOLI D., SALVADORI S. Structure–activity relationships of nociceptin and related peptides: comparison with dynorphin A. Peptides. 2000;21:923–933. doi: 10.1016/s0196-9781(00)00229-1. [DOI] [PubMed] [Google Scholar]
- HIGGINS G.A., GROTTICK A.J., BALLARD T.M., RICHARDS J.C., MESSER J., TAKE H., PAULY-EVERS M., JENK F., ADAM G., WICHMANN J. Influence of the selective ORL1 receptor agonist, Ro64-6198, on rodent neurological function. Neuropharmacology. 2001;41:97–107. doi: 10.1016/s0028-3908(01)00048-x. [DOI] [PubMed] [Google Scholar]
- JENCK F., MOREAU J.-L., MARTIN J.R., KILPATRICK G.J., REINSCHEID R.K., MONSMA F.J., NOTHACKER H.-P., CIVELLI O. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENCK F., WICHMANN J., DAUTZENBERG F.M., MOREAU J.-L., OUAGAZZAL A.M., MARTIN J.R., LUNDSTROM K., CESURA A.M., POLI S.M., ROEVER S., KOLCZEWSKI S., ADAM G., KILPATRICK G. A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4938–4943. doi: 10.1073/pnas.090514397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON S.W., NORTH R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLESNIKOV Y.A., PASTERNAK G.W. Peripheral orphanin FQ/nociceptin analgesia in the mouse. Life Sci. 1999;64:2021–2028. doi: 10.1016/s0024-3205(99)00149-6. [DOI] [PubMed] [Google Scholar]
- KUZMIN A., SANDIN J., TERENIUS L., OGREN S.O. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J. Pharmacol. Exp. Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- MARTI M., GUERRINI R., BEANI L., BIANCHI C., MORARI M. Nociceptin/orphanin FQ receptora modulate glutamate extracellular levels in the substantia nigra pars reticulata. A microdialysis study in the awake freely moving rats. Neuroscience. 2002;112:153–160. doi: 10.1016/s0306-4522(02)00050-7. [DOI] [PubMed] [Google Scholar]
- MATHIS J.P., RYAN–MORO J., CHANG A., HOM J.S., SCHEINBERG D.A., PASTERNAK G.W. Biochemical evidence for orphanin FQ/nociceptin receptor heterogeneity in mouse brain. Biochem. Biophys. Res. Commun. 1997;230:462–465. doi: 10.1006/bbrc.1996.5867. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.-C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.-L., GUILLEMOT J.-C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MOGIL J.S., PASTERNAK G.W. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol. Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- MURPHY N.P., LY H.T., MAIDMENT N.T. Intracerebroventricular orphanin FQ/nociceptin suppresses dopamine release in the nucleus accumbens of anaesthetized rats. Neuroscience. 1996;75:1–4. doi: 10.1016/0306-4522(96)00322-3. [DOI] [PubMed] [Google Scholar]
- MURPHY N.P., MAIDMENT N.T. Orphanin FQ/nociceptin modulation of mesolimbic dopamine transmission determined by microdialysis. J. Neurochem. 1999;73:179–186. doi: 10.1046/j.1471-4159.1999.0730179.x. [DOI] [PubMed] [Google Scholar]
- NISHI M., HOUTANI T., NODA Y., MAMIYA T., SATO K., DOI T., KUNO J., TAKESHIMA H., NUKADA T., NABESHIMA T., YAMASHITA T., NODA T., SUGIMOTO T. Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphanin FQ receptor. EMBO J. 1997;16:1858–1864. doi: 10.1093/emboj/16.8.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NODA Y., MAMIYA T., NABESHIMA T., NISHI M., HIGASHIOKA M., TAKESHIMA H. Loss of antinociception induced by naloxone benzoylhydrazone in nociceptin receptor-knockout mice. J. Biol. Chem. 1998;273:18047–18051. doi: 10.1074/jbc.273.29.18047. [DOI] [PubMed] [Google Scholar]
- ÖGREN S.O., HALL H., KÖHLER C., MAGNUSSON O., SJOSTRAND S.E. The selective dopamine D2 receptor antagonist raclopride discriminates between dopamine-mediated motor functions. Psychopharmacology. 1986;90:287–294. doi: 10.1007/BF00179179. [DOI] [PubMed] [Google Scholar]
- ÖGREN S.-O., ROSS S.B. Substituted amphetamine derivatives. II. Behavioral effects in mice related to monoaminergic neurones. Acta Pharmacol. Toxicol. 1977;41:353–368. doi: 10.1111/j.1600-0773.1977.tb02674.x. [DOI] [PubMed] [Google Scholar]
- PAN Y.X., XU J., PASTERNAK G.W. Cloning and expression of a cDNA encoding a mouse brain orphanin FQ/nociceptin precursor. Biochem. J. 1996;315:11–13. doi: 10.1042/bj3150011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.-P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., CIVELLI O. Orphanin FQ: a novel neuropeptide which is a natural ligand of an opioid-like G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- RIZZI D., BIGONI R., RIZZI A., JENCK F., WICHMANN J., GUERRINI R., REGOLI D., CALO G. Effects of Ro 64-6198 in nociceptin/orphanin FQ-sensitive isolated tissues. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:551–555. doi: 10.1007/s002100100399. [DOI] [PubMed] [Google Scholar]
- ROSSI G.C., LEVENTHAL L., BOLAN E., PASTERNAK G.W. Pharmacological characterization of orphanin FQ/nociceptin and its fragments. J. Pharmacol. Exp. Ther. 1997;282:858–865. [PubMed] [Google Scholar]
- SAKURADA T., SAKURADA S., KATSUYAMA S., SAKURADA C., TAN-NO K., TERENIUS L. Evidence that N-terminal fragments of nociceptin modulate nociceptin-induced scratching, biting and licking. Neurosci. Lett. 2000;279:61–64. doi: 10.1016/s0304-3940(99)00958-1. [DOI] [PubMed] [Google Scholar]
- SANDIN J., GEORGIEVA J., SCHOTT P.A., OGREN S.O., TERENIUS L. Nociceptin/orphanin FQ microinjected into hippocampus impairs spatial learning in rats. Eur. J. Neurosci. 1997;9:194–197. doi: 10.1111/j.1460-9568.1997.tb01367.x. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., MORARI M. Nociceptin/orphanin FQ and neurotransmitter release in the central nervous system. Peptides. 2000;21:1023–1029. doi: 10.1016/s0196-9781(00)00233-3. [DOI] [PubMed] [Google Scholar]
- SHIEH K.R., PAN J.T. Effects of orphanin FQ on central dopaminergic neuronal activities and prolactin secretion. Am. J. Physiol. Regulat. Integrat. Comp. Physiol. 2001;280:R705–R712. doi: 10.1152/ajpregu.2001.280.3.R705. [DOI] [PubMed] [Google Scholar]
- STRATFORD T.R., HOLAHAN M.R., KELLEY A.E. Injections of nociceptin into the nucleus accumbens shell or ventromedial hypothalamic nucleus increase food intake. Neuroreport. 1997;8:423–426. doi: 10.1097/00001756-199701200-00009. [DOI] [PubMed] [Google Scholar]
- WICHMANN J., ADAM G., ROVER S., HENNIG M., SCALONE M., CESURA A.M., DAUTZENBERG F.M., JENCK F. Synthesis of (1S,3aS)-8-(2,3,3a,4,5, 6-hexahydro-1H-phenalen-1-yl)-1-phenyl-1,3,8-triaza-spiro[4. 5]decan-4-one, a potent and selective orphanin FQ (OFQ) receptor agonist with anxiolytic-like properties. Eur. J. Med. Chem. 2000;35:839–851. doi: 10.1016/s0223-5234(00)00171-9. [DOI] [PubMed] [Google Scholar]
- ZHENG F., GRANDY D.K., JOHNSON S.W. Actions of orphanin FQ/nociceptin on rat ventral tegmental area neurons in vitro. Br. J. Pharmacol. 2002;136:1065–1071. doi: 10.1038/sj.bjp.0704806. [DOI] [PMC free article] [PubMed] [Google Scholar]